Abstract
Purpose
Epigallocatechin-3-gallate (EGCG) is an antioxidant agent derived from green tea. Because it has chemopreventive and anti-invasive effect against various cancer cells, EGCG can be used to inhibit proliferation and invasion of cholangiocarcinoma (CCA) cells.
Methods
The anticancer effects of EGCG were studied using human CCA cells (HuCC-T1). Apoptosis was analyzed by Western blotting. Invasion and migration of cancer cells were assessed with Matrigel® and wound healing assays. An animal tumor xenograft model of HuCC-T1 was used to study the in vivo antitumor activities of EGCG.
Results
EGCG effectively inhibited the growth of HuCC-T1 cells with no adverse effects on the viability of 293T cells. EGCG induced apoptotic cell death at 5 µg/mL concentration. It inhibited the expression of mutant p53 and induced apoptotic molecular signals such as Bax/Bcl-2, Caspase, and cytochrome C. Furthermore, EGCG dose-dependently inhibited the activity of matrix metalloproteinase (MMP)-2/9, invasion, and migration. In the animal tumor xenograft model of HuCC-T1 cells, EGCG was subcutaneously administered beside the tumor for local treatment. EGCG efficiently inhibited growth of the tumor and suppressed carcinogenic molecular signals such as Notch1, MMP-2/9, and proliferating cell nuclear antigen.
Conclusion
EGCG induced apoptosis of cancer cells without adverse effects on normal cells. EGCG inhibited growth, invasion, and migration of HuCC-T1 cells. We suggest EGCG as a promising candidate for local treatment of CCA.
Keywords: epigallocatechin-3-gallate, cholangiocarcinoma, matrix metalloproteinases-2, invasion, thermosensitive hydrogel
Introduction
Epigallocatechin-3-gallate (EGCG), which is the ester form of epigallocatechin/gallic acid, is the most plentiful catechin of green tea.1 Due to its anti-oxidant activity, EGCG has been extensively investigated in various kinds of cancer.1–6 In particular, EGCG has no adverse effects against normal cells and tissues, while it has anti-proliferative, anti-invasive, and chemopreventive effects against various cancer cells.2–8 EGCG inhibits expression of Janus-like kinase/signal transduction and transcription of cholangiocarcinoma (CCA) cells.8 Senggunprai et al also reported that EGCG was involved in the suppression of growth and cytokine-induced migration of CCA cells by regulation of inducible nitric oxide synthase and intracellular adhesion molecule-1.8 EGCG is also known to regulate various carcinogenic signal expressions such as vascular endothelial growth factors, matrix metalloproteinases (MMPs), insulin-like growth factors, epidermal growth factor receptors, and cell cycle regulatory proteins, and inhibit nuclear factor-κB, PI3-K/Akt, Ras/Raf/mitogen-activated protein kinase, and activator protein 1 signaling pathways.1 Furthermore, EGCG has been shown to have anti-invasive and anti-metastatic effects on cancer cells by several investigators.9–13 For example, Ramadass et al reported that EGCG in a co-delivery system with paclitaxel synergistically inhibits the activities of MMP-2/9 and invasive potential of MDA-MB 231 human breast carcinoma cells.10 Farabegoli et al also reported the downregulation of epidermal growth factor receptor and MMP-2/9 of drug-resistant breast cancer cells.11 Furthermore, EGCG induced apoptosis of glioma cells via laminin receptor and then inhibited their invasion/proliferation.14
CCA, which is a malignant transformation of epithelial cells in the bile duct region, is related to poor prognosis/high mortality and its incidence rate has also increased worldwide.15–17 CCA is frequently diagnosed at a malignant stage due to difficulties in its diagnosis, and then its surgical removal for curative treatment is practically impossible.16–17 To prolong patient survivability, palliative treatment, such as stent displacement, chemotherapy, radiotherapy, photodynamic therapy, and immunotherapy are considered.16,18–21 However, systemic treatment using chemotherapeutic agents or radiotherapy is not always successful because CCA is physiologically/biologically different compared to systemic cancer and has a low response rate to such treatment options. Therefore, a novel treatment strategy is needed in consideration of progression characteristics of CCA.
In this study, we assessed the anticancer activities of EGCG against human CCA cells (HuCC-T1) in vitro and in vivo. Because EGCG has shown anti-cancer activities in various cancer cells, it could inhibit migration, invasion, and proliferation of CCA cells. We studied the anti-carcinogenic effects of EGCG against various apoptosis signals, migration/spreading potentials, and invasion of HuCC-T1 cells in vitro and in vivo.
Material and methods
Materials
EGCG and dimethylsulfoxide were purchased from Sigma Aldrich. Co. (St Louis, MO, USA). Cell culture materials such as Roswell Park Memorial Institute (RPMI) 1640 media, fetal bovine serum (FBS), and antibiotics were purchased from Life Technologies (Grand Island, NY, USA). All reagents used in this experiment were of extra-pure grade.
Cell cultures
HuCC-T1 cells (Health Science Research Resources Bank, Osaka, Japan) and human embryonic kidney 293 T (HEK293T) cell line (Korean Cell Line Bank Co. Ltd., Seoul, Korea) were maintained in RPMI1640 medium supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator.
Trypan blue exclusion assay (inhibition of cell growth and cytotoxicity assay)
A total of 3×104 or 3×105 HuCC-T1 cells were seeded into 24-well plates for growth inhibition and the cytotoxicity assay, respectively, and incubated overnight in a CO2 incubator. The cells were treated with EGCG in RPMI1640 media for 24 hours. Serum-free media were used for the cytotoxicity assay. To measure viability of cells, the trypsinized cells were harvested by centrifugation, resuspended in phosphate-buffered saline (PBS), and trypan blue was added. The number of cells was counted using the Countess™ Automated Cell Counter (Invitrogen, Carlsbad, CA, USA).
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Apoptosis of cancer cells was observed by fragmented DNA using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). HuCC-T1 cells were treated with EGCG for 24 hours and then the cells were washed with PBS twice following fixation with 4% paraformaldehyde solution. After that, the cells were stained with an in situ apoptosis detection kit (Millipore, Billerica, MA, USA). The staining procedure was as follows: for blocking of endogenous peroxidase activity, the cells on the slides were incubated for 10 minutes in 3% H2O2. The slides were further incubated for 1 hour with the terminal deoxynucleotidyl transferase (TdT) enzyme and then incubated for 30 minutes with a blocking reagent in a humidified chamber (37°C). Following this, slides were incubated for 30 minutes with anti-digoiygenin–biotin (1:100) at 37°C and then the streptavidin–biotin complex (1:100) was applied to the slides at 37°C for 30 minutes. An Alexa488 secondary antibody was used to visualize immunoreactivity. For negative control, distilled water was used in place of the TdT solution. Apoptotic cells can be detected by the nuclear staining.
Apoptosis/necrosis assay
A total of 1×106 HuCC-T1 cells seeded into 6-well plates were treated with EGCG for 24 hours. After that, the trypsinized cells were washed with PBS and resuspended in 100 µL binding buffer. Fluorescein isothiocyanate-conjugated Annexin V and propidium iodide were used to stain cells for apoptosis and necrosis analysis, respectively. The extent of apoptosis or necrosis of cancer cells was assessed with flow cytometry.
Western blot analysis
Cells treated with EGCG for 24 hours were harvested and washed with cold PBS. Then, the cells collected by centrifugation were lysed with lysis buffer containing protease inhibitors (50 mM Tris, 150 mM NaCl, 1% 4-nonylphenyl-polyethylene glycol P-40 (NP-40), 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate [SDS]) with phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). Then, the cell lysate was centrifuged at 14,000× g for 30 minutes at 4°C and the supernatant or cell lysate was collected. Protein concentration was assayed with BCA Protein Assay kit (Pierce, Rockford, IL, USA).
Western blotting was carried out using proteins from cell lysates with SDS-poly acrylamide gel electrophoresis (SDS-PAGE). Fifty micrograms of protein was transferred to a polyvinylidene fluoride membrane and blocked with 5% skim milk in tris buffered saline with Tween® 20 (TBS-T). This was probed with a primary antibody followed by secondary horseradish peroxidase-conjugated antibody and then the proteins were detected by chemiluminescence. Blots were stripped and re-probed with anti-β-actin primary antibody followed by an appropriate secondary antibody for chemiluminescence detection. Quantification of proteins was performed with digital analyses of the protein bands using the Image-J software program.
Primary antibodies used for analysis of Western blotting were as follows: anti-wild type (wt)-p53 antibody (OP33) and anti-mutant type (mut)-p53 antibody (OP29), obtained from Calbiochem Co., Billerica, MA, USA. Anti-Lamin B antibody, (SC-373918), anti-Bax antibody (SC-7480), anti-Caspase-9 antibody (SC-17784), anti-poly adenosine diphosphate ribose polymerase (PARP) antibody (SC-7150), anti-Bcl-2 antibody (SC-7382), and anti-cytochrome C antibody (SC-13560) were obtained from Santa Cruz Biotech. Inc., Dallas, TX, USA. Anti-Bad antibody was obtained from Cell Signaling Tech. Inc., Danvers, MA, USA. Anti-Caspase-3 antibody was obtained from Enzo Life Sciences, ALX-804-305, Seoul, South Korea.
Gelatin zymography
A total of 1×106 HuCC-T1 cells in 6-well plates were treated with EGCG for 24 hours. Then, media were used to measure MMP activity of cancer cells. The conditioned medium was developed with substrate gel electrophoresis using SDS-PAGE containing 10% gelatin. Conditioned cell culture media having equal protein contents were mixed with Laemmli buffer (Bio-Rad Lab. Co., Hercules, CA, USA) and loaded onto the gel followed by separation by electrophoresis. To remove SDS, the gels were soaked three times for 30 minutes at room temperature in Triton buffer (2.5% Triton X-100 in PBS). After that, the gels were incubated for 24 hours at 37°C and then stained with 0.1% Coomassie Brilliant Blue R-250. The gels were destained to obtain clear bands. Quantitative results of the assays were obtained by densitometry.
Cell invasion assay
For the invasion assay of cancer cells, transwell chambers in 24-well plates were used: 20 µL Matrigel (1 mg/mL; BD Bioscience, San Jose, CA, USA) was placed onto the upper chamber to coat the membrane. Then, 2×104 HuCC-T1 cells in 100 µL serum-free medium were seeded on the upper chamber of the Transwell chamber into the 24-well plate and then 600 µL of RPMI1640 containing 10% FBS was added to the lower chamber following incubation for 24 hours at 37°C in a CO2 incubator. After that, the cells on the lower surface of the membrane were fixed with methanol and stained with hematoxylin and eosin (H&E). The cells on the lower surface of the membrane were photographed and counted using a computerized video image analyzing system.
Wound healing assay
A wound healing assay of HuCC-T1 cells using ibidi Culture-Inserts (ibidi GmbH, Planegg/Martinsried, Germany) was performed to measure the migration potential of cancer cells after EGCG treatment. A total of 5×105 HuCC-T1 cells were seeded into 6-well plates and treated with EGCG, at 37°C and 5% CO2, for 24 hours. The trypsinized cells were then washed twice with PBS. Then 5×104 cells in serum free media were introduced into the culture inserts and further incubated for 24 hours. The field of wound healing and cell migration was observed using light microscopy.
In vivo xenograft model of HuCC-T1 cells
To study the antitumor activities of EGCG, the tumor xenograft model of HuCC-T1 cells was prepared as follows: 1×107 HuCC-T1 cells were subcutaneously implanted into the backs of nude mice (5 weeks old male mice, 20–25 g in weight; Orient, Seongnam, South Korea). All animal experiments were carried out under supervision of the Institutional Animal Care and Use Committee of Pusan National University (PNU-IACUC), Korea. When the diameter of solid tumor reached about 4–5 mm, EGCG in the vehicle (thermosensitive gels, Guardix-SG; Genewel Co. Ltd., Seoul, South Korea) was injected subcutaneously beside the solid tumor. A vehicle without EGCG was also injected as a control. For EGCG treatment, 1 mg EGCG in 1 mL gels was used. Four to five mice were used for each group. Tumor diameter and body weight were measured twice a week. Two perpendicular diameters of the tumor were measured to calculate tumor volume (V = (a × [b]2)/2, a: largest diameter, b: smallest diameter). All animal experiments were carried out according to the guidelines of the Institutional Animal Care and Use Committee of Pusan National University, Korea.
Immunohistochemistry
For immunohistochemical analysis, isolated tumor tissues were fixed in 4% formamide, paraffin-embedded and sliced for H&E staining or immunohistochemical staining. For immunohistochemical staining, anti-Notch1 antibody (Enzo Life Sciences Inc, ADI-905-897, Farmingdale, NY, USA), anti-MMP-2 antibody (SC-10736; Santacruz Biotech. Inc.), anti-MMP-9 antibody (SC-21733; Santacruz Biotech. Inc.), and anti-proliferating cell nuclear antigen (PCNA) antibody (SC-25280; Santacruz Biotech. Inc.) were used at a dilution of 1:100 and the Envision kit (Life Technologies, Carlsbad, CA, USA) was used for the staining procedure.
Statistical analysis
Statistical analyses were performed using Student’s t-test. A P-value <0.05 was considered significant (* represents statistical significance compared to the vehicle-treated control).
Results
Anticancer activities of EGCG against CCA cells in vitro
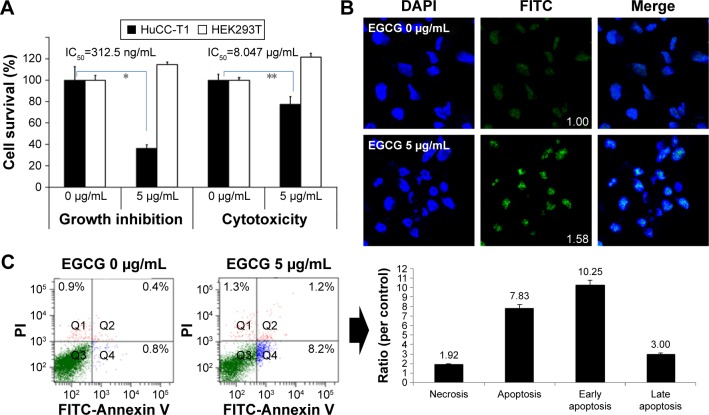
Anticancer activities of EGCG against HuCC-T1 cells are shown in Figures 1 and 2. Growth inhibition and anticancer effect of EGCG were studied using HuCC-T1 and HEK293T cells and are shown in Figure 1. As shown in Figure 1A, EGCG inhibited the growth of HuCC-T1 cells while growth of HEK293T cells was not inhibited. Furthermore, EGCG did not affect the viability of HEK293T cells while it suppressed the viability of HuCC-T1 cells in a cytotoxicity study. TUNEL assay and flow cytometry analysis also showed apoptosis of HuCC-T1 cells with 5 µg/mL EGCG (Figure 1B and C). When HuCC-T1 cells were treated with 5 µg/mL EGCG, cells revealed strong green fluorescence and apoptosis peaks were significantly increased. These results indicated that EGCG effectively suppresses growth and viability of cancer cells with absence of intrinsic toxicity against normal cells.
Figure 1.
The anticancer activities of EGCG against HuCC-T1 cells.
Notes: (A) The effects of EGCG on the viability of cancer cells. A total of 3×104 cells were used to measure the anticancer effect and 3×103 cells were used to test growth inhibition. RPMI1640 media supplemented with 10% FBS was used to assess tumor cell growth inhibition and serum-free media was used to assess the anti-cancer effects. (B) TUNEL staining. Images were observed at 400×. (Numbers in the boxes indicate intensities of FITC.) (C) Flow cytometric analysis of cancer cells. FITC Annexin V and PI were used for apoptosis and necrosis analysis of tumor cells, respectively. *,**P<0.01.
Abbreviations: IC50, half maximal inhibitory concentration; RPMI1640, Roswell Park Memorial Institute 1640; EGCG, epigallocatechin-3-gallate; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; HuCC-T1, human cholangiocellular carcinoma cell line; HEK 293T, human embryonic kidney 293 T; PI, propidium iodide; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
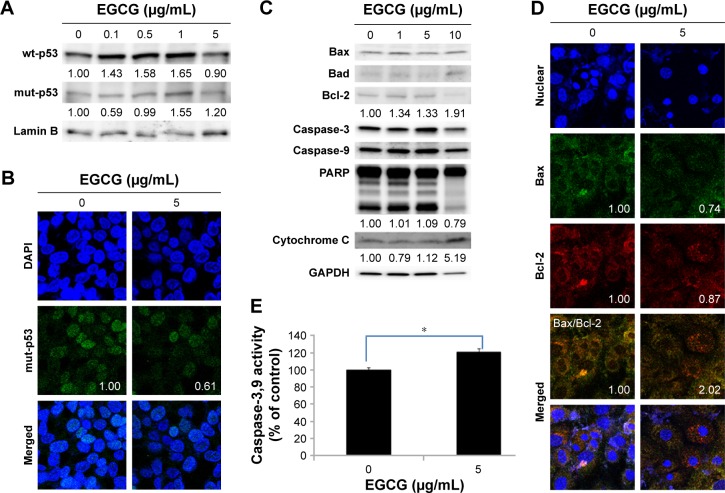
Figure 2.
The apoptosis and necrosis of HuCC-T1 cells by treatment of EGCG.
Notes: Western blot assay: (A) wt-p53, mut-p53; (C) Bax, Bad, Bcl-2, Caspase-3, Caspase-9, and PARP expression. Fluorescence microscopic observation: (B) mut-p53 (Numbers in the boxes indicate intensities of green fluorescence.); (D) Bax, Bcl-2, Caspase-3, Caspase-9, and PARP expression. (E) The extent of Caspase-3,9 activities. Images were observed at 400×. *P<0.01.
Abbreviations: wt, wild-type; mut, mutant; EGCG, epigallocatechin-3-gallate; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HuCC-T1, human cholangiocellular carcinoma cell line, PARP, poly adenosine diphosphate ribose polymerase.
Molecular signals regarding apoptosis were investigated, as shown in Figure 2. As shown in Figure 2A and B, mut-p53 expression of HuCC-T1 cells was also decreased by EGCG treatment. The expression ratio of Bax/Bcl-2 of HuCC-T1 cells was doubled, indicating that EGCG accelerates apoptosis of HuCC-T1 cells (Figure 2D). Furthermore, proteins for DNA repair/programmed cell death such as PARP were also decreased by treatment with EGCG (Figure 2C). The activities of Caspase-3 and 9 were increased by treatment with EGCG (Figure 2C and E). These results indicated that EGCG accelerated apoptosis of CCA cells.
The effects of EGCG on the migration/invasion capacity and MMP activity of HuCC-T1 cells
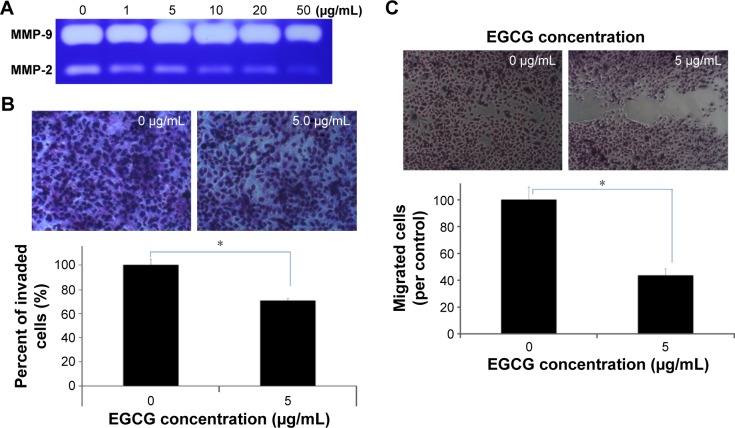
Figure 3 shows the effects of EGCG on the MMP activity, invasion capacity, and migration of HuCC-T1 cells. The MMP activity of HuCC-T1 cells was decreased dose-dependently by treatment with EGCG, as shown in Figure 3A. Because MMP-2 and -9 have a critical role in cancer cell invasion and migration through basement membrane degradation, the changes of cancer invasion/migration can be predicted by changes in MMP activity. Invasion and migration assays were performed using a Boyden chamber coated with Matrigel® and wound healing method (Figure 3B). Results showed that EGCG inhibited invasive capacity of cancer cells, indicating that invaded HuCC-T1 cells decreased 30% compared to control. Figure 3C shows the wound healing assay of HuCC-T1 cells. Migration of HuCC-T1 cells was decreased >50% compared to control. These results indicated that EGCG has anti-invasive and anti-migration capacity against HuCC-T1 cells.
Figure 3.
The effects of EGCG on the MMP activity, invasion and migration capacity of HuCC-T1 cells.
Notes: (A) Gelatin zymography: MMP-2 and -9 expressions. (B) Matrigel® invasion assay. (C) Wound healing assay for cancer cell migration. Images were observed at 400×. *P<0.001.
Abbreviations: EGCG, epigallocatechin-3-gallate; HuCC-T1, human cholangiocellular carcinoma cell line; MMP, matrix metalloproteinase.
Anticancer activities of EGCG against in vivo mouse tumor xenograft model of HuCC-T1 cells
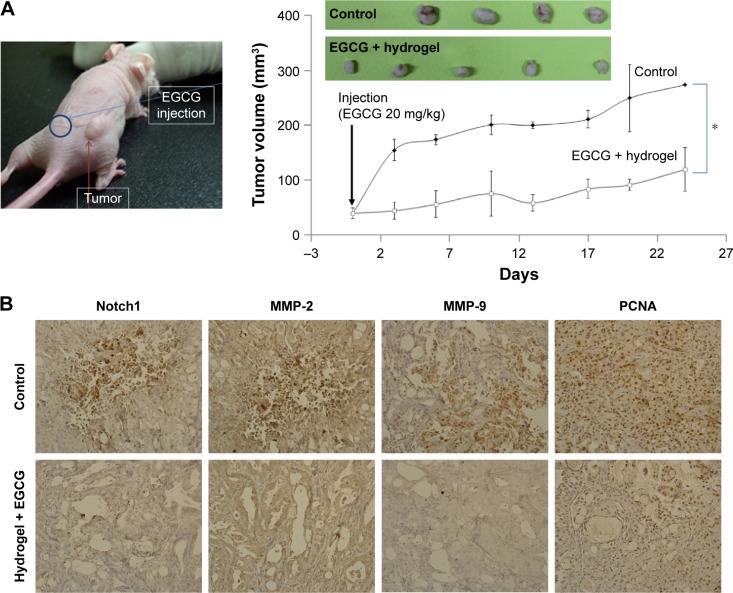
Figure 4 shows the anticancer activities of EGCG against HuCC-T1 tumor-bearing mice. For the in vivo study, EGCG was dissolved in thermosensitive hydrogel and subcutaneously injected beside the solid tumor. For control treatment, hydrogel was solely injected. As shown in Figure 4A, the tumor volume increased gradually. However, EGCG treatment significantly inhibited the growth of tumor volume, indicating that EGCG has anti-tumor activity against the in vivo tumor xenograft model of HuCC-T1 cells. Furthermore, carcinogenic proteins also decreased, as shown in Figure 4B, ie, Notch1, a precursor of the MMP protein, was distinctly decreased by EGCG treatment. As expected, the expression of MMP-2/9 was also decreased. Furthermore, PCNA, a proliferating-related protein, was also decreased. These results indicated that EGCG effectively inhibited MMP activity, expression of Notch and PCNA protein, and then inhibited the growth of solid tumor.
Figure 4.
Antitumor activities of EGCG against HuCC-T1 tumor xenograft mice model.
Notes: (A) Tumor growth. 1×107 cells were subcutaneously injected to the back of BALB/c nude mice. When the diameter of the solid tumor reached about 4 mm, EGCG in a vehicle (hydrogel) was injected subcutaneously beside the solid tumor (dose: 20 mg EGCG/kg). For comparison, the vehicle as a control was injected into the back of the mice. Growth of the tumor was calculated using the formula V = (a × [b]2)/2, with a being the largest and b being the smallest diameter. For immunohistochemistry of tumor tissues, tumors were isolated and fixed with 4% formamide 25 days after the injection. (B) Immunohistochemistry (400×) of tumor tissues. Notch 1, MMP-2 and -9, and PCNA antibodies were used for staining tumor tissues. *P<0.01.
Abbreviations: EGCG, epigallocatechin-3-gallate; HuCC-T1, human cholangiocellular carcinoma cell line; MMP, matrix metalloproteinase; PCNA, proliferating cell nuclear antigen.
Discussion
Antioxidants such as EGCG should be extensively investigated because of their promising activity in inhibition of invasion, metastasis, and angiogenesis of cancer cells.7–13 EGCG induces not only apoptotic cell death but also inhibits invasion of cancer cells through suppression of MMP-2 and -9.9–12 Especially, CCA cells are frequently known to have low responsiveness against conventional chemotherapeutic agents.18,22,23 One of the reasons for this problem seems to be physiological properties of CCA cells, ie, CCA cells spread and invade through the bile duct region.18,22,24 Furthermore, systemic administration of anticancer agents for CCA is known to have little survival benefit.25–27 An antioxidant such as EGCG has chemopreventive and anti-invasive effects against cancer cells with negligible harmful effects against normal cells. We have shown that EGCG has no anti-proliferative or cytotoxic effects against normal cells, with promising anti-proliferative effects against HuCC-T1 cells (Figure 1). EGCG induces apoptotic cell death in low concentrations (Figure 1B and C) and apoptotic molecular signals (Figure 2). Furthermore, it dose-dependently inhibits MMP-2 activity, which is a major enzyme for the invasion process, and migration of HuCC-T1 cells as shown in Figure 3. Zhang et al also reported that EGCG efficiently inhibits MMP-2/9 activity and invasion of hepatocellular carcinoma cells.28,29 Furthermore, they also reported that EGCG has no adverse effects against non-cancerous liver cells.
Perineural invasion of CCA frequently appears at an early stage and this aggressiveness of CCA cells has a strong relationship with short survival time.24,30 Survival time and quality of life can be improved by inhibition of invasion of CCA cells. As shown in our study, EGCG definitely inhibits invasive-related molecular signals and enzymes in vivo, ie, Notch1, MMP-2/9 were significantly reduced by treatment with EGCG and growth of tumor volume was also inhibited more than twofold (Figure 4). Several investigators also demonstrated the anti-cancer effects of EGCG in the in vitro cell culture and in vivo mouse tumor xenograft model.31,32 Lang et al reported that EGCG has beneficial effects when used for chemotherapy on human CCA cell-bearing mice.33 They demonstrated that EGCG increased the sensitivity to gemcitabine of Mz-ChA-1 cell xenografts in nude mice and effectively suppressed tumor growth. In our study, we locally administered EGCG by a subcutaneous injection beside the tumor because local application must be more effective than a systemic approach due to the physiological properties of CCA.24,27 We also proved that EGCG efficiently suppressed the growth of tumor volume with suppression of carcinogenic molecular signals (Figure 4).
In conclusion, we studied chemoprevention effects of EGCG against HuCC-T1 cells in vitro and in vivo. EGCG effectively inhibits and kills HuCC-T1 cells with no adverse effects against normal cells. EGCG induces apoptotic cell death and apoptotic molecular signals. Furthermore, EGCG also suppresses MMP-2/9 activity, and invasion and migration of cancer cells. Also, it inhibits growth of tumor and suppresses carcinogenic molecular signals.
Acknowledgments
This study was supported by a grant of the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (Project No HI14C2220).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shankar S, Ganapathy S, Srivastava RK. Green tea polyphenols: biology and therapeutic implications in cancer. Front Biosci. 2007;12:4881–4899. doi: 10.2741/2435. [DOI] [PubMed] [Google Scholar]
- 2.Liao S, Umekita Y, Guo J, Kokontis JM, Hiipakka RA. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995;96(2):239–243. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- 3.Thangapazham RL, Singh AK, Sharma A, Warren J, Gaddipati JP, Maheshwari RK. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007;245(1–2):232–241. doi: 10.1016/j.canlet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116(1):164–176. doi: 10.1002/cncr.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiao Y, Cao J, Xie L, Shi X. Cell growth inhibition and gene expression regulation by (−)-epigallocatechin-3-gallate in human cervical cancer cells. Arch Pharm Res. 2009;32(9):1309–1315. doi: 10.1007/s12272-009-1917-3. [DOI] [PubMed] [Google Scholar]
- 6.Philips BJ, Coyle CH, Morrisroe SN, Chancellor MB, Yoshimura N. Induction of apoptosis in human bladder cancer cells by green tea catechins. Biomed Res. 2009;30(4):207–215. doi: 10.2220/biomedres.30.207. [DOI] [PubMed] [Google Scholar]
- 7.Min NY, Kim JH, Choi JH, et al. Selective death of cancer cells by preferential induction of reactive oxygen species in response to (−)-epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2012;421(1):91–97. doi: 10.1016/j.bbrc.2012.03.120. [DOI] [PubMed] [Google Scholar]
- 8.Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2014;28(6):841–848. doi: 10.1002/ptr.5061. [DOI] [PubMed] [Google Scholar]
- 9.Sartor L, Pezzato E, Donà M, et al. Prostate carcinoma and green tea: (−)epigallocatechin-3-gallate inhibits inflammation-triggered MMP-2 activation and invasion in murine TRAMP model. Int J Cancer. 2004;112(5):823–829. doi: 10.1002/ijc.20496. [DOI] [PubMed] [Google Scholar]
- 10.Ramadass SK, Anantharaman NV, Subramanian S, Sivasubramanian S, Madhan B. Paclitaxel/epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells. Colloids Surf B Biointerfaces. 2015;125:65–72. doi: 10.1016/j.colsurfb.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Farabegoli F, Papi A, Orlandi M. (−)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9 and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant cells. Biosci Rep. 2011;31(2):99–108. doi: 10.1042/BSR20090143. [DOI] [PubMed] [Google Scholar]
- 12.Benelli R, Venè R, Bisacchi D, Garbisa S, Albini A. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem. 2002;383(1):101–105. doi: 10.1515/BC.2002.010. [DOI] [PubMed] [Google Scholar]
- 13.Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin Cancer Res. 2005;11(5):1918–1927. doi: 10.1158/1078-0432.CCR-04-1976. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Li Z, Xu YM, et al. Epigallocatechin-3-gallate induces apoptosis, inhibits proliferation and decreases invasion of glioma cell. Neurosci Bull. 2014;30(1):67–73. doi: 10.1007/s12264-013-1394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ustundag Y, Bayraktar Y. Cholangiocarcinoma: a compact review of the literature. World J Gastroenterol. 2008;14(42):6458–6466. doi: 10.3748/wjg.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandhu DS, Roberts LR. Diagnosis and management of cholangiocarcinoma. Curr Gastroenterol Rep. 2008;10(1):43–52. doi: 10.1007/s11894-008-0008-9. [DOI] [PubMed] [Google Scholar]
- 17.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 18.Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003;181(3):819–827. doi: 10.2214/ajr.181.3.1810819. [DOI] [PubMed] [Google Scholar]
- 19.Singh P, Patel T. Advances in the diagnosis, evaluation and management of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22(3):294–299. doi: 10.1097/01.mog.0000218967.60633.64. [DOI] [PubMed] [Google Scholar]
- 20.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 21.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 22.Bhuiya MR, Nimura Y, Kamiya J, et al. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg. 1992;215(4):344–349. doi: 10.1097/00000658-199204000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3(1):18–34. doi: 10.3978/j.issn.2304-3881.2014.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen FZ, Zhang BY, Feng YJ, et al. Current research in perineural invasion of cholangiocarcinoma. J Exp Clin Cancer Res. 2010;29:24. doi: 10.1186/1756-9966-29-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas MB. Biological characteristics of cancers in the gallbladder and biliary tract and targeted therapy. Crit Rev Oncol Hematol. 2007;61(1):44–51. doi: 10.1016/j.critrevonc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Thomas MB. Systemic and targeted therapy for biliary tract tumors and primary liver tumors. Surg Oncol Clin N Am. 2014;23(2):369–381. doi: 10.1016/j.soc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Kuhlmann JB, Blum HE. Locoregional therapy for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29(3):324–328. doi: 10.1097/MOG.0b013e32835d9dea. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Owusu L, Duan W, et al. Anti-metastatic and differential effects on protein expression of epigallocatechin-3-gallate in HCCLM6 hepatocellular carcinoma cells. Int J Mol Med. 2013;32(4):959–964. doi: 10.3892/ijmm.2013.1446. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Duan W, Owusu L, Wu D, Xin Y. Epigallocatechin-3-gallate induces the apoptosis of hepatocellular carcinoma LM6 cells but not non-cancerous liver cells. Int J Mol Med. 2015;35(1):117–124. doi: 10.3892/ijmm.2014.1988. [DOI] [PubMed] [Google Scholar]
- 30.Hai S, Kubo S, Uenishi T, et al. Postoperative survival in intrahepatic cholangiocarcinoma showing intraductal growth. Hepatogastroenterology. 2005;52(62):374–377. [PubMed] [Google Scholar]
- 31.Khan N, Bharali DJ, Adhami VM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2014;35(2):415–423. doi: 10.1093/carcin/bgt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujiki H, Sueoka E, Watanabe T, Suganuma M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J Cancer Res Clin Oncol. 2015;141(9):1511–1522. doi: 10.1007/s00432-014-1899-5. [DOI] [PubMed] [Google Scholar]
- 33.Lang M, Henson R, Braconi C, Patel T. Epigallocatechin-gallate modulates chemotherapy-induced apoptosis in human cholangiocarcinoma cells. Liver Int. 2009;29(5):670–677. [Google Scholar]