Abstract
Introduction
High-dose melphalan and autologous stem cell transplant (HDM/SCT) is an effective treatment modality for immunoglobulin light-chain (AL) amyloidosis, however, its application remains restricted to patients with good performance status and limited organ involvement. In recent years, the paradigm for AL amyloidosis has changed with the introduction of novel agents such as immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs). We hypothesized that use of novel agent induction regimens has improved outcomes for patients with AL amyloidosis undergoing HDM/SCT at our center.
Methods
All patients with AL amyloidosis, age ≥ 18 years who underwent HDM/SCT between 2001 and 2014 at the Fred Hutchinson Cancer Research Center and University of Washington Medical Center were included in this study. Any regimen administered within 6 months prior to HDM/SCT including an IMiD or a PI was considered a novel induction regimen. Use of induction regimen was evaluated in a Cox proportional hazard model for association with progression-free (PFS) and overall survival (OS).
Results
Forty-five patients with AL amyloidosis underwent HDM/SCT. The median age was 57.2 years (range, 39 – 74.4), 15 (33.3%) were women. The median number of organs involved was 2 (range 1 – 5), with 20 patients having only 1 (44.4%), 10 patients having 2 (22.2%), and 15 patients (33.3%) having ≥ 3 organs involved. Novel agent induction regimens were used prior to HDM/SCT in 21 patients (46.7%); these comprised PI in 13/21 (57.1%), IMiD alone in 6/21 (28.6%), PI and cyclophosphamide (CyBorD) in 3/21 (14.3%), and IMiD and PI in 3/21 (14.3%). Use of a novel agent induction regimen was associated with improved progressive-free survival (PFS), but not overall survival (OS). The 3-year PFS for patients who received a novel agent induction was 79%, while for those who did not was 53% (Hazard ratio [HR] = 0.317, p = 0.048). The 3-year OS for patients who received novel agent induction regimens was 95%, while for those who did not was 71% (HR = 0.454, p = 0.247).
Discussion
Our data suggest that use of a novel agent induction regimen including an IMiD or PI prior to HDM/SCT for patients with AL amyloidosis could improve outcomes, with improvement in PFS. Although these results are limited by sample size and lack of randomization, these results support possible further investigation of novel agent induction regimens in the context of a prospective clinical trial.
Keywords: Amyloidosis, Autologous transplant, Bortezomib, Lenalidomide, Induction therapy
Introduction
Primary systemic immunoglobulin light-chain (AL) amyloidosis is an uncommon plasma cell dyscrasia characterized by deposition of amyloidogenic light chains as beta-pleated sheets, also termed amyloid fibrils [1, 2]. In contrast to other more common plasma cell dyscrasias, AL amyloidosis is typified by damage of vital organs due to amyloid deposition leading to organ failure, such as heart or kidney failure. Left untreated, AL amyloidosis leads rapidly to disability and death in most cases.
Treatment for AL amyloidosis follows regimens used for the more common plasma cell dyscrasia, multiple myeloma, with novel agents such as immunomodulatory drugs (iMIDs) or proteasome inhibitors (PIs), and alkylating agents [3, 4]. The goal of treatment is reduction or elimination of the underlying plasma cell clone, with resultant reduction in serum levels of amyloidogenic light-chains. For eligible patients, upfront treatment with high-dose melphalan and autologous stem cell transplantation (HDM/SCT), typically without induction chemotherapy – in contrast to multiple myeloma – has proven to be an effective therapy with durable remission durations [5]. Despite promising results from many prospective studies, the role of HDM/SCT in AL amyloidosis continues to be a controversial treatment due to concerns regarding selection bias, as well as the results of a randomized trial by the Intergroupe Francophone due Myèlome which did not show a survival advantage for HDM/SCT compared to standard oral chemotherapy [6].
Unfortunately, many patients are ineligible due to advanced organ involvement or poor performance status at the time of diagnosis, and are unable to undergo HDM/SCT [5, 7]. Induction therapy prior to HDM/SCT has the potential to result in either major or minor organ responses, increasing the proportion of patients eligible for HDM/SCT, and improving the progression free and overall survival of patient undergoing HDM/SCT. There are limited data to support this practice – but with some early promising results using bortezomib-based induction therapy as part of HDM/SCT conditioning regimen [3, 8–10]. However, induction therapy also has the potential in some patients to result in worsened performance status due to development of treatment related toxicity and/or progression of organ involvement.
Herein, we report on a retrospective analysis of patients with primary AL amyloidosis who underwent HDM/SCT at the Fred Hutchinson Cancer Research Center (FHCRC) and the University of Washington Medical Center. The aim of this report is to determine the impact of novel agent induction regimens on progression free and overall survival.
Methods
Patients
Sequential patients older than 18 years of age with a confirmed diagnosis of primary AL amyloidosis who underwent HDM/SCT between December 2001 and July 2014 at the Fred Hutchinson Cancer Research Center and University of Washington Medical Center were eligible. Institutional approval was obtained to gather retrospective data from patient records and databases. Patients treated on an investigational study had signed a consent form authorized by the human subjects committee of the University of Washington and/or the institutional review board of the Fred Hutchinson Cancer Research Center in accordance with the Declaration of Helsinki.
A diagnosis of AL amyloidosis required a biopsy with Congo red staining for amyloid fibrils, and evidence for a clonal plasma cell dyscrasia, per standard criteria [11]. Patients with concomitant active multiple myeloma, as defined by standard criteria, were not included [12]. We also excluded patients with a diagnosis of AL amyloidosis who had bone marrow plasmacytosis greater than 30%. We defined hematologic response criteria for AL amyloidosis as per the most recent published guidelines for clinical trials [13]. Organ involvement was defined by the criteria published from the 10th International Symposium on Amyloid and Amyloidosis [11].
Transplantation methods
Our center has specific criteria determining which patients with AL amyloidosis are able to undergo HDM/SCT. We do not offer HDM/SCT to patients with a left ventricular echocardiogram ejection fraction < 40%, room air oxygen saturation < 95%, supine blood pressure ≤ 90 mmHg, or performance status ≥ 3, based on the eligibility criteria established by Boston University [10].
Melphalan dose is determined by clinical criteria. Our practice is to consider melphalan 140 mg/m2 in patients > 61 years old, patients with left ventricular ejection fraction between 40–44% by echocardiogram, or poorer performance status. We routinely give 140 mg/m2 in patients over the age of 70 years, or in renal failure with calculated creatinine clearance < 40 mL/min. All other patients receive melphalan 200 mg/m2.
Study variables
Baseline demographic data and patient features were collected at the time of HDM/SCT, including age, number and type of treatments received before HDM/SCT, initial Congo red positive biopsy, extent of amyloid organ involvement, and dose of melphalan received as conditioning regimen. We considered an induction regimen as a novel regimen if it included either an immunomodulatory drug or proteasome inhibitor, or both, and was administered within 6 months of undergoing HDM/SCT.
Statistical considerations
We used descriptive statistics to summarize demographics and patient characteristics. Groups were compared using chi-squared tests for categorical variables and t-tests for comparing continuous variables. Survival analysis using Kaplan-Meier curves were calculated to estimate the OS and PFS from the time of HDM/SCT. Induction regimen pre-transplant was evaluated in an adjusted Cox proportional hazards regression model for associations with PFS and OS. A landmark analysis, excluding all patients who had progression or death before day 100, was also performed on both OS and PFS. All statistical analyses were performed in R 3.2.3 [14].
Results
Patient characteristics
A total of 45 patients with documented primary AL amyloidosis underwent HDM/SCT at the FHCRC and SCCA from 2001 – 2014. Patient characteristics are summarized in Table 1. The median age at the time of transplant was 57.2 years (range, 39 – 74.4 years). A minority of patients were women, 15 (33.3%), and multi-organ involvement, defined as greater than 2 organs involved, was evident in 15 patients (33.3%). The majority of patients had renal involvement, 39 (86.7%), followed by cardiac in 18 (40%), gastrointestinal in 17 (37.8%), and nervous system in 14 (31.1%). The majority of patients had lambda clonality, 37 (82.2%), and 8 patients had kappa disease (17.8%). Baseline creatinine at the time of HDM/SCT was 1.0 mg/dL (range, 0.58 – 7.4 mg/dL), baseline 24-hour urine total protein was 4.27 g (range, 0 – 22.7 g), brain natriuretic peptide (BNP) was 119 pg/mL (range, 17 – 3398 pg/mL), and bone marrow plasmacytosis was 8% (range, 0 – 30%).
Table 1.
Demographics and Patient Characteristics
| Characteristic | All patients (n = 45) | Novel agent induction (n = 21) | No novel agent induction (n = 24) | p values* |
|---|---|---|---|---|
| Age, median (range) | 57.2 (39 – 74.4) | 57.2 (46.3 – 69.4) | 57.6 (39 – 74.9) | |
| Women | 15 (33.3) | 5 (25) | 9 (37.5) | 0.751 |
| >2 Organs involved | 15 (33.3) | 9 (42.9) | 6 (28.5) | 0.341 |
| Diagnosis to transplant (months) | 6 (1 – 33) | 8 (1 – 33) | 4 (2 – 15) | 0.008** |
| CD34+ cells/kg infused (x106) | 5.46 (2.33 – 12.77) | 5.04 (2.33 – 8.67) | 5.98 (3.06 – 12.77) | 0.126 |
| Melphalan dose | 0.875 | |||
| 100 | 10 (22.2) | 4 (19) | 6 (25) | |
| 140 | 15 (33.3) | 7 (33.3) | 8 (33.3) | |
| 200 | 20 (44.4) | 10 (47.6) | 10 (41.7) | |
| Clonality | 0.855 | |||
| Kappa | 8 (17.8) | 4 (14.3) | 5 (20.8) | |
| Lambda | 37 (82.2) | 18 (85.7) | 19 (79.2) | |
| Treatment related mortality | 3 (6.7) | 0 (0) | 3 (6.7) | |
| Organ involvement | ||||
| Renal | 39 (86.7) | 18 (85.7) | 21 (87.5) | 1.000 |
| Cardiac | 18 (40) | 10 (47.6) | 8 (33.3) | 0.502 |
| Hepatic | 4 (8.9) | 3 (14.3) | 1 (4.2) | 0.506 |
| Gastrointestinal | 17 (37.8) | 10 (47.6) | 7 (29.2) | 0.334 |
| Nervous | 14 (31.1) | 7 (33.3) | 7 (29.2) | 1.000 |
| Baseline laboratory values (median [range]) | ||||
| Serum creatinine, mg/dL | 1.0 (0.58 – 7.4) | 1 (0.7 – 3.8) | 1.83 (0.58 – 7.4) | 0.148 |
| 24 hour urine protein, g | 4.27 (0 – 22.7) | 4.19 (0 – 16.63) | 4.27 (0.1 – 22.7) | 0.442 |
| B type natriuretic peptide, pg/mL | 119 (17 – 3398) | 132 (17 – 3398) | 94.5 (23 – 1432) | 0.678 |
| Bone marrow plasmacytosis at ASCT, % | 8 (0 – 30) | 6 (0 – 20) | 8 (1 – 30) | 0.282 |
| Bone marrow plasmacytosis at diagnosis, % | 8.2 (1 – 30) | 8.4 (1.4 – 19) | 8 (1 – 30) | 0.261 |
Data presented are n (%), unless otherwise indicated
P values were calculated using the Chi Square analysis for categorical tests, and the Student’s T Test for continuous variables
Denotes statistically significant (defined as p < 0.05) p values
We used granulocyte colony stimulation factor (GCSF) as the stem cell mobilization regimen at a dose of 10 – 32 mcg/kg for 4 days. All patients received melphalan conditioning prior to autologous stem cell reinfusion. A total of 20 patients received a dose of 200 mg/m2 (44.4%), 15 patients received a dose of 140 mg/m2 (33.3%), and 10 patients received a dose of 100 mg/m2 (22.2%). Of the patients who received 100 mg/m2 dose, 9 (90%) had been treated on the protocol SWOG 0115-1, a study of tandem autologous transplants, with 2 cycles of melphalan 100 mg/m2 [15]. Treatment-related mortality (TRM), defined as death prior to day 100 post HDM/SCT, occurred in 3 patients (6.75%).
Novel agent induction patients
A total of 21 patients received induction therapy with novel agents (46.7%) (Table 1). The median number of regimens these patients received was 1 (range, 1 – 3). Patients who received induction therapy underwent HDM/SCT between January 2006 and May 2014. The most common regimen was bortezomib and dexamethasone in 13 patients (61.9%), for a median of 3 cycles (range, 1 – 6 cycles). Bortezomib, cyclophosphamide, and dexamethasone (CyBorD) was the next most common regimen administered, in 3 patients (14.2%), for a median of 3 cycles (range, 2 – 4 cycles). Other regimens included lenalidomide and dexamethasone in 4 (19%), bortezomib, lenalidomide, and dexamethasone in 2 patients (9.5%), thalidomide and dexamethasone in 2 (9.5%), and bortezomib, thalidomide, and dexamethasone in 1 (4.8%).
The median age of these patients was 57.2 years (range, 46.3 – 69.4 years) (Table 1). Five of these patients were women (25%), and 9 had greater than 2 organs involved (42.9%). The most common organ systems involved was renal in 18 (85.7%), cardiac in 10 (47.6%), gastrointestinal in 10 (47.6%), nervous in 7 (33.3%), and hepatic in 3 (14.3%). Conditioning regimens comprised melphalan 200 mg/m2 in 10 (47.6%), 140 mg/m2 in 7 (33.3%), and 100 mg/m2 in 4 (19%). There was no TRM among patients who received novel agent induction undergoing transplant. The overall response rate at 1 year post HDM/SCT among response evaluable patients (n=14) was 9/14 (64.3%), with 7/14 in CR, 1/14 in VGPR, and 1/14 in PR.
Regarding induction therapy, response data was present for 14 patients; the other 7 patients underwent induction therapy prior to transplant but insufficient data were available to determine responses. Of evaluable patients, the overall response rate after induction therapy was 9/14 (64.3%), with 1/14 in CR, 7/14 in VGPR, and 1/14 in PR. Only 10 patients had documentation as to the rationale for induction; most commonly, the reasons for induction were either for reduction of free light chain levels, or the desire by the clinician to see improvement in organ function or performance status prior to consideration of HDM/SCT. Of the 7 patients who received more than 1 regimen prior to transplant, the most common rationale given for multiple regimens was progressive disease to the first regimen in 4/7 patients. In 2/7 patients, the rationale was neuropathy from bortezomib; in 1 patient, the reason given was that a better response was desired.
Baseline serum creatinine among these patients was 1 mg/dL (0.7 – 3.8 mg/dL), 24-hour urine total protein was 4.19 g (range, 0 – 16.6 g), BNP was 132 pg/mL (17 – 229 pg/mL), and bone marrow plasma cells at the time of autologous transplant of 6% (range, 0 – 20%). The median bone marrow plasmacytosis at the time of diagnosis was 8.4% (range, 1.4 – 19%).
Upfront HDM/SCT patients
Twenty-four patients did not receive any pretransplant novel-agent induction therapy (53.3%) (Table 1). The median age of these patients was 57.6 years (range, 39 – 74.4 years), and there were 9 women (37.5%). Only 6 patients (28.5%) had greater than 2 organ systems involved. The range of years during which transplant occurred for these patients was December 2001 through January 2014. The most common organ systems involved were renal in 21 (87.5%), cardiac in 8 (33.3%), gastrointestinal in 7 (29.2%), nervous system in 7 (29.2%), and hepatic in 1 (4.2%). The most common conditioning regimen was melphalan 200 mg/m2 in 10 (41.7%), 140 mg/m2 in 8 (33.3%), and 100 mg/m2 in 6 (25%). The overall response rate at 1 year post HDM/SCT, in evaluable patients (n=7), was 5/7 (71.4%), with 2/7 in CR, 1/7 in VGPR, and 2/7 in PR. Three patients experienced TRM (6.7%). Of the patients experiencing TRM, the causes of death were asystole in 1 and respiratory failure in 2.
The baseline serum creatinine level was 1.83 mg/dL (0.58 – 7.4), 24-hour urine total protein was 4.27 g (0.1 – 22.7 g), BNP was 94.5 pg/mL (23 – 1432), and bone marrow plasma cells were 8% (1 – 30%). The median bone marrow plasmacytosis at time of diagnosis for these patients was 8% (range, 1 – 30%).
Survival analysis and proportional hazards model
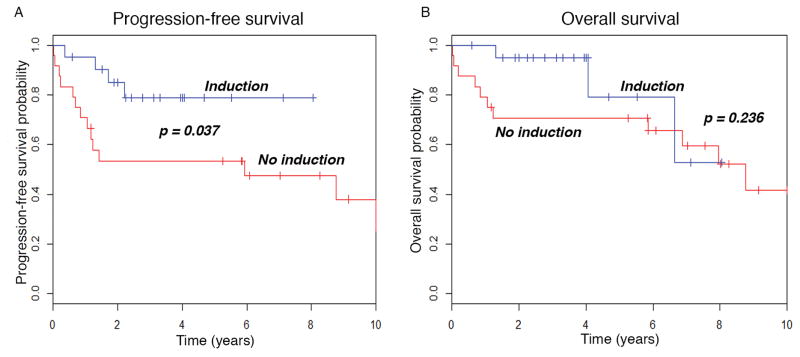
Receipt of induction therapy with a novel agent was significantly associated with PFS, but not OS. The 3-year PFS for patients who received a novel agent induction was 79%, while for those who did not was 53% (P = 0.037, as calculated by the log-rank analysis). The 3-year OS for patients who received novel agent induction regimen was 95%, while for those who did not was 71% (P = 0.236, as calculated by log-rank analysis). The Kaplan-Meier plots from these analyses are depicted in Figure 1. We also performed a Cox-proportional hazards model to adjust for the impact of induction therapy on PFS and OS (Table 2). We were unable to add additional covariates to the model given the limited number of events and degrees of freedom. There was a 3-fold lower risk of progression or death in patients who received a novel-agent induction therapy (hazard ratio [HR] = 0.317, 95% confidence interval [CI], 0.101 – 0.992, P = 0.048). There was a non-statistically significant 2-fold lower risk of relapse or death in patients who received a novel agent induction regimen (HR = 0.454, 95% CI, 0.119 – 1.728, P = 0.247).
Figure 1.
Pretransplant novel agent induction predicts PFS in patients with AL amyloidosis undergoing HDM/SCT. Kaplan-Meier plots for A) progression-free survival and B) overall survival.
Table 2.
Cox-proportional hazards model for association of induction regimen with PFS and OS
| Outcome | HR (95% CI) | P Value |
|---|---|---|
| PFS | ||
| Novel agent induction | 0.317 (0.101–0.992) | 0.048 |
| OS | ||
| Novel agent induction | 0.454 (0.119 – 1.728) | 0.247 |
Landmark analysis
Given the occurrence of three TRM events in the non-induction therapy group, and none in the group receiving induction, we were concerned about the impact of this imbalance on our reported outcomes. Therefore, a landmark analysis of both OS and PFS was performed. We performed a Cox-proportional hazards model on the adjusted data. In contrast to the original model, there was a non-statistically significant 2 fold lower risk of progression or death in patients who received a novel-agent induction regimen (HR = 0.462, 95% CI, 0.137 – 1.559, P = 0.213). There was a non-statistically significant1.3-fold lower risk of mortality in patients who received a novel-agent induction therapy (HR = 0.761, 95% CI, 0.181 – 3.207, P = 0.71).
Discussion
In this retrospective analysis of patients with AL amyloidosis undergoing HDM/SCT at a tertiary referral center, we have shown that use of induction therapy incorporating novel agents can be impactful, with improvement in PFS. There was no impact on OS, and the TRM did not differ significantly between the groups. The primary documented rationale for administration of most patients who received induction therapy prior to HDM/SCT related to concerns regarding performance status or reduction in the involved serum free light chain assay. While it is conceivable that patients saw improvement in performance status and organ involvement with induction therapy, thus enabling HDM/SCT, we did not have sufficient data to make any definitive conclusions regarding this question – a very common rationale for administration of induction treatments. The majority of patients who received induction novel agent therapy saw a benefit, with a 64.3% overall response rate. Interestingly, the landmark analysis of PFS and OS showed a non-statistically significant reduction in risk of progression or death, suggesting that the high rate of TRM in the group not receiving induction therapy may have affected our analysis, to some degree.
Our analysis has some limitations. We were limited by the relatively low numbers of patients with AL amyloidosis who underwent HDM/SCT. Moreover, there is significant concern for selection bias, as we did not directly compare outcomes to patients who began induction therapy with a novel agent but did not make it to transplant. Finally, we were unable to include more than one variable in our Cox proportional hazards model given the low number of events, so a multivariate analysis was not feasible, and we were unable to control for confounders. As such, our results should be taken with a caveat.
Although induction therapy with combination of an IMiD and PI are commonly recommended for patients with multiple myeloma prior to consolidation with HDM/SCT, this practice has not been thoroughly studied for patients with AL amyloidosis. Part of the rationale for induction therapy in multiple myeloma is to reduce the burden of disease prior to HDM/SCT –there are good data to support this, with the principle finding that depth of response is associated with improved outcomes after HDM/SCT [16].
In contrast to multiple myeloma, where reduction of disease burden is more critical, in AL amyloidosis, the primary insult is from amyloid-related vital organ damage due to amyloidogenic light chains. The plasma cell burden is often much lower, in the 5–10% range, as one would see with a monoclonal gammopathy. Thus, the use of induction therapy has often been limited to those patients with higher levels of plasmacytosis. Recently, the value of induction therapy in patients with plasmacytosis > 10% has been examined, and no impact on outcomes was seen. However, in contrast to this larger analysis, which included induction regimens with melphalan, we only included patients who were treated with novel-agent based induction regimens [17]. There is also concern that induction therapy will result in delay of, or complications with definitive therapy – potentially resulting in previously eligible patients becoming no longer capable of undergoing HDM/SCT due to disability from treatment-related toxicity or progression of amyloid-related organ dysfunction.
However, there may be some rationale for use of novel agent induction therapy in some patients, even in the absence of marked plasmacytosis. Classically, amyloid related organ damage has been primarily thought to be due to infiltration from amyloid fibril deposition [2]. However, other mechanisms of amyloid-related organ damage have also been demonstrated, including direct organ toxicity exerted by the light chains, unrelated to fibril deposition. Direct impairment of cardiac myocytes has been shown in multiple preclinical studies, with potential mechanisms including induction of apoptosis, oxidative stress, and cellular dysfunction [18–21]. The soluble forms of the light-chain variable domain proteins have also been shown to be directly toxic to cardiac myocytes in cell culture [22]. Thus, theoretically, using induction chemotherapy to reduce the toxic amyloid light chains while preparing a patient for HDM/SCT could benefit patients, by making previously transplant ineligible patients better able to tolerate the procedure. Although this was commonly given as a rationale for induction in our study, to definitively answer the question of whether induction novel agent therapy could result in previously ineligible patients becoming eligible would require a prospective study. Unfortunately, we do not know how many patients began induction with intent for HDM/SCT but declined rapidly and were subsequently unable to undergo transplant.
We found in our cohort a TRM rate of 3 (6.7%) in the non-induction receiving group, while a TRM of 0 (0%) was found in the patients with receipt of induction therapy. Given our small sample size, it is impossible to know for certain whether the receipt of novel agent induction therapy affected TRM in any way, but these findings warrant further investigation. Perhaps, as suggested previously, the reduction in toxic circulating light chains before transplant could theoretically result in a safer transplant, though this cannot be proven with our data.
These preclinical findings have been translated into clinical trials using novel agents as induction before HDM/SCT. Building on the results of a pilot study, the group at Boston University demonstrated in a phase 2 study that using bortezomib induction prior to HDM/SCT resulted in high rates of hematologic responses, with an acceptable safety profile and no negative impact on stem cell mobilization and collection [3]. A potential risk of this approach, highlighted by the experience of this study, was that some patients who were initially eligible for HDM/SCT may become ineligible during treatment with bortezomib. In the aforementioned study, 5 patients were unable to proceed with HDM/SCT after bortezomib induction due to decline in clinical status. A single center, randomized study comparing induction with bortezomib prior to HDM/SCT, versus HDM/SCT alone, demonstrated improved rates of hematologic responses, and improved survival rates for the group undergoing induction therapy [8]. In contrast to the promising results seen with use of bortezomib-based induction therapies, induction therapy with either intravenous melphalan, or vincristine, doxorubicin, and dexamethasone (VAD) was not shown to be beneficial [9, 10].
Conclusion
In summary, we have shown, in a retrospective analysis of patients with AL amyloidosis undergoing HDM/SCT at a tertiary referral center, an improvement in PFS, but no statistically significant impact on OS or TRM. Our results should be taken with caution, given the use of retrospective data, but are suggestive of a potential benefit of novel agent induction therapy, at least for some patients. Moving ahead, it is possible that a risk adapted approach to use of induction therapy would be better suited to patients with AL amyloidosis – reserving induction therapy for those who are borderline eligible or with declining performance status – and proceeding to directly to transplant for those with good performance status and minimal amyloid related organ involvement. In light of recent conflicting studies, however, more prospective studies are needed to further define the benefits and risks, as well as the population of patients most likely to benefit from novel agent induction therapy prior to HDM/SCT in AL amyloidosis.
Abbreviations
- CR
complete response
- GCSF
granulocyte colony stimulating factor
- HDM/SCT
high dose melphalan and autologous stem cell transplant
- iMIDs
immunomodulatory drugs
- OS
overall survival
- PFS
progression-free survival
- PIs
proteasome inhibitors
- PR
partial response
- TRM
treatment-related mortality
- VGPR
very good partial response
Footnotes
Declaration of interest
The authors report no conflicts of interest. This work was supported by research funding from National Institutes of Health [grant number K24CA184039] and philanthropic gifts from Frank and Betty Vandermeer.
References
- 1.Comenzo RL. How I treat amyloidosis. Blood. 2009;114(15):3147–57. doi: 10.1182/blood-2009-04-202879. [DOI] [PubMed] [Google Scholar]
- 2.Obici L, Perfetti V, Palladini G, Moratti R, Merlini G. Clinical aspects of systemic amyloid diseases. Biochim Biophysica Acta. 2005;1753:11–22. doi: 10.1016/j.bbapap.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Sanchorawala V, Brauneis D, Shelton AC, Lo S, Sun F, Sloan JM, Quillen K, et al. Induction therapy with bortezomib followed by bortezomib-high dose melphalan and stem cell transplantation for light chain amyloidosis: results of a prospective clinical trial. Biol Blood Marrow Transplant. 2015;21:1445–51. doi: 10.1016/j.bbmt.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Sanchorawala V, Wright DG, Rosenzweig M, Finn KT, Fennessey S, Zeldis JB, Skinner M, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109:492–6. doi: 10.1182/blood-2006-07-030544. [DOI] [PubMed] [Google Scholar]
- 5.Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM, Segal A, et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118:4346–52. doi: 10.1182/blood-2011-01-330738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, Recher C, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083–93. doi: 10.1056/NEJMoa070484. [DOI] [PubMed] [Google Scholar]
- 7.Skinner M, Sanchorawala V, Seldin DC, Dember LM, Falk RH, Berk JL, Anderson JJ, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85–93. doi: 10.7326/0003-4819-140-2-200401200-00008. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Wang Q, Chen W, Zeng C, Chen Z, Gong D, Zhang H, et al. Induction therapy with bortezomib and dexamethasone followed by autologous stem cell transplantation versus autologous stem cell transplantation alone in the treatment of renal AL amyloidosis: a randomized controlled trial. BMC Med. 2014;12:2. doi: 10.1186/1741-7015-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perz JB, Schonland SO, Hundemer M, Kristen AV, Dengler TJ, Zeier M, Linke RP, et al. High-dose melphalan with autologous stem cell transplantation after VAD induction chemotherapy for treatment of amyloid light chain amyloidosis: a single centre prospective phase II study. Br J Haematol. 2004;127:543–51. doi: 10.1111/j.1365-2141.2004.05232.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanchorawala V, Wright DG, Seldin DC, Falk RH, Finn KT, Dember LM, Berk JL, et al. High-dose intravenous melphalan and autologous stem cell transplantation as initial therapy or following two cycles of oral chemotherapy for the treatment of AL amyloidosis: results of a prospective randomized trial. Bone marrow transplant. 2004;33:381–8. doi: 10.1038/sj.bmt.1704346. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA, Comenzo R, Falk RH, Fermand JP, Hazenberg BP, Hawkins PN, Merlini G, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol. 2005;79:319–28. doi: 10.1002/ajh.20381. [DOI] [PubMed] [Google Scholar]
- 12.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 13.Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H, Falk R, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26:2317–25. doi: 10.1038/leu.2012.100. [DOI] [PubMed] [Google Scholar]
- 14.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 15.Sanchorawala V, Hoering A, Seldin DC, Finn KT, Fennessey SA, Sexton R, Mattar B, et al. Modified high-dose melphalan and autologous SCT for AL amyloidosis or high-risk myeloma: analysis of SWOG trial S0115. Bone Marrow transplant. 2013;48:1537–42. doi: 10.1038/bmt.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harousseau JL, Avet-Loiseau H, Attal M, Charbonnel C, Garban F, Hulin C, Michallet M, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. J Clin Oncol. 2009;27:5720–6. doi: 10.1200/JCO.2008.21.1060. [DOI] [PubMed] [Google Scholar]
- 17.Dittus C, Uwumugambi N, Sun F, Sloan JM, Sanchorawala V. The effect of bone marrow plasma cell burden on survival in patients with AL amyloidosis undergoing high dose melphalan and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2016;22:1729–32. doi: 10.1016/j.bbmt.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Trinkaus-Randall V, Walsh MT, Steeves S, Monis G, Connors LH, Skinner M. Cellular response of cardiac fibroblasts to amyloidogenic light chains. Am J Pathol. 2005;166:197–208. doi: 10.1016/S0002-9440(10)62244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci USA. 2010;107:4188–93. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migrino RQ, Hari P, Gutterman DD, Bright M, Truran S, Schlundt B, Phillips SA. Systemic and microvascular oxidative stress induced by light chain amyloidosis. Int J Cardiol. 2010;145:67–8. doi: 10.1016/j.ijcard.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, Apstein CS, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circulation Res. 2004;94:1008–10. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 22.Sikkink LA, Ramirez-Alvarado M. Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture. Cell Death Dis. 2010;1:e98. doi: 10.1038/cddis.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]