Abstract
The use of Polypodium leucotomos, a species of fern, has been reported to be beneficial in the treatment of atopic dermatitis, vitiligo, and psoriasis, and for prevention of polymorphic light eruption, sunburn, and squamous cell carcinoma. We review the in vivo animal, in vitro human, and human clinical studies performed to help elucidate the actions of and biologic pathways affected by P. leucotomos. These results serve as the scientific rationale and basis for the protection and effectiveness afforded by P. leucotomos in cutaneous diseases.
INTRODUCTION
Polypodium leucotomos is a species of fern in the family Polypodiaceae. The South American P. leucotomos species is known locally as "calaguala" and extracts of this fern are called "anapsos." P. leucotomos extracts have been used for the treatment of psoriasis in South America and Spain1 and growing clinical scientific evidence suggests P. leucotomos is also beneficial for the treatment of various skin disorders and conditions including atopic dermatitis2, vitiligo3 and, because it affords sun protection from ultraviolet radiation, prevention of polymorphic light eruption4. Its antitumor effects were alluded to nearly 50 years ago5. Phenolic components of P. leucotomos extract include chlorogenic acid, coumaric acid, vanillic acid, caffeic acid and ferulic acid, the latter two being the most potent inhibitors of oxidation in vitro 6,7. We review the in vivo animal, in vitro human, and human clinical studies performed to help elucidate the actions of and pathways affected by PL.
Experimental Evidence
In Vivo Animal Studies
Strains of hairless albino mice have been developed for studying the harmful effects of UV radiation on the skin8,9; using these animals, numerous studies have demonstrated the beneficial effects of P. leucotomos following skin exposure to ultraviolet light.
In 1999, the antioxidant and photoprotective properties of P. leucotomos extract were demonstrated when applied topically to hairless mice exposed to UVB radiation10. Compared to untreated mice, P. leucotomos-treated mice showed a significant reduction in the sunburn response and diminished histologic evidence of photoaging damage, including reduced dermal elastosis. Eight weeks after stopping UV exposure, P. leucotomos-treated mice also showed a reduction in the development of skin tumors compared to untreated UV-exposed mice.
A subsequent study showed that P. leucotomos also has beneficial effects when administered orally in a similar hairless mouse model11. These animals were fed ~300mg/kg/day of P. leucotomos extract or vehicle in their drinking water for 10 days and then exposed to UV radiation and evaluated for cyclooxygenase-2 (Cox-2) expression by Western blot analysis. Upregulation of Cox-2 is associated with UV-induced skin cancer12,13. Cox-2 levels were 4-times lower in P. leucotomos-fed mice at 48 hours and 5-times lower at 72 hours, compared to vehicle fed mice. Other beneficial effects of feeding P. leucotomos included a significant decrease in UV-induced inflammation including a 60% decrease in neutrophil infiltration into the skin at 24 hours and a 50% decrease macrophages in the skin at 24 and 48 hours11.
Other studies have assessed the ability of P. leucotomos to counteract UV radiation-associated skin cancers. In one study, animals received an oral extract of P. leucotomos (~300 mg/kg) for 5 days prior to UVB (equivalent to 1–1.5 times the minimal erythema dose [MED] and for 2 days afterward. The number of cells positive for the tumor suppressor p53 increased by 63%, proliferating cells decreased by 13% in P. leucotomos treated mice compared to mice that were UV-irradiated but did not receive P. leucotomos. They also had a reinforced network of dermal elastic fibers14. These animals were observed to have an enhanced the antioxidant plasma capacity that was 30% greater than controls. Because there was no change in the levels of antioxidant enzymes on Western blots, this beneficial effect is likely due to the direct antioxidant properties of P. leucotomos.
There is abundant evidence that UVB radiation-induced immunosuppression is a major contributing factor to UVB-induced skin cancers in mice15. Investigators sought to determine the effect of P. leucotomos on the immune system. They explored whether oral P. leucotomos placed in the drinking water would reverse direct immunosuppressive effect of UVB that occurs locally in the skin or the general immunosuppression that acts systemically following UVB exposure, or both.16 To test for the local effects of P. leucotomos on skin exposed to UVB, mice were exposed to UVB radiation daily for 4 days and were then sensitized by topical application of the experimental contact allergen oxazolone directly on the radiated site. To test for effects of P. leucotomos on UVB-induced systemic immunosuppression, a separate group of mice was exposed to 10,000 J/cm2 UVB radiation once and 3 days later were sensitized with oxazolone at a site not exposed to UVB. Compared to mice receiving plain drinking water, animals that had received P. leucotomos exhibited significantly less local and systemic UVB radiation-induced immune suppression‥ Thus, oral administration of P. leucotomos reverses UVB immunosuppression regardless whether it is local or systemic. In ther studies, P. leucotomos also inhibited UV mediated depletion of Langerhans cells.17 While recent observations indicate that the primary role of epidermal Langerhans cells may be to generate regulatory T-cells, many studies have shown a clear association between UVB-induced depletion of Langerhans cells and local UVB-induced immunosuppression.
In addition to affecting the immune system, UV radiation has also been shown to produce other adverse systemic effects including oxidative stress18. 8-oxo-dG is a pre-mutagenic marker of oxidative damage to DNA and is caused by the UV-induced generation of reactive oxygen species. 8-oxo-dG positive cells were reduced by approximately 59% at 24 hours and by 79% at 48 hours in P. leucotomos-treated animals compared to control animals. These findings support the concept that P. leucotomos reduces oxidative DNA damage. Two weeks after UV exposure, mutations in P. leucotomos-fed-mice were approximately 25% less than those from mice treated with UV alone11. In other studies, when hairless rats were pretreated with an oral extract of P. leucotomos (30 mg/kg) each day for 7 days prior to UV exposure, less glutathione oxidation in both blood and epidermis was observed, providing evidence for a potent systemic antioxidant effect19.
In Vitro Human Studies
While many of the studies performed on mice cannot be conducted in humans, many in vitro studies using human cell cultures have demonstrated the photoprotective and beneficial effects of P. leucotomos.
One study demonstrated that pretreatment of human keratinocytes with P. leucotomos inhibited solar-simulator-UV-mediated increase of tumor necrosis factor-alpha and nitric oxide production and prevented nitric oxide synthase induction20. Other effects observed were suppression of solar simulated radiation induced transcriptional activation of nuclear factor-kappaB (NFκB) and activator protein-1 (AP-1), both of which have been implicated in UVB-induced skin carcinogenesis. Pretreatment with P. leucotomos was cytoprotective against UV-induced damage resulting in increased cell survival.
P. leucotomos extract has been shown to be photoprotective against UV-induced cell damage. Fibroblasts obtained from healthy volunteers were treated with an extract of P. leucotomos and then exposed to UV-A and UV-B radiation21. P. leucotomos maintained fibroblast survival and proliferative capacity in a dose-dependent manner. P. leucotomos treatment of human fibroblasts had a protective effect on the morphological changes that occur following UV exposure. Specifically, there was less disorganization of F-actin-based cytoskeletal structures and a reduction in the coalescence of the tubulin cytoskeleton. Moreover, there was a more regular distribution of integrin and cadherin adhesion molecules in UV irradiated cells following UV exposure21. In that study, the protective effects of P. leucotomos following UV exposure in terms of proliferation and survival was also observed with the human HaCaT keratinocyte cell line. The ability of P. leucotomos treatment to protect against UV-induced death of human fibroblasts has been demonstrated by others as well22.
A major UV-absorbing chromophore in the stratum corneum is trans-urocanic acid (t-UCA). When the skin is exposed to ultraviolet radiation, trans-urocanic acid undergoes photoisomerization to its cis- conformation. Cis-urocanic acid is a well-studied mediator of UVB-induced immune suppression.23 In vitro studies demonstrated that P. leucotomos was also to inhibits UV-radiation-induced photoisomerization of t-UCA to cis-UCA. Trans-UCA will decompose in the presence of such oxidizing agents as H2O2, and titanium dioxide (TiO2). P. leucotomos treatment blocked that effect22.
Interestingly, an extract from the related fern Polypodium decumanum has been used to treat psoriasis and related immunological disorders. Using human neutrophils, the extract demonstrated inhibitory activity against platelet activating factor24, which is a known mediator of UVB-induced immunosuppression.25 The specific compound responsible for this effect has been identified as 1,2-di-O-palmitoyl-3-O-(6-sulpho-alpha-D-quinovopyranosyl)-glycerol26. In an in vitro model using human leukocytes P. decumanum was had the ability to inhibit the formation of the inflammatory mediator leukotriene B4, which is present in abnormally high quantities in psoriatic skin27.
Clinical Studies
The photoprotective effect of oral P. leucotomos extract has been studied in healthy human volunteers28. Participants with Fitzpatrick skin types II to III (N=9) were exposed to gradually increasing doses of UV radiation from a xenon arc lamp emitting wavelengths between 305 and 400 nm, in a manner similar to that used to determine the MED. Exposures were then repeated after oral administration of two doses of P. leucotomos capsules (7.5 mg/kg each). There was a significant decrease in the mean erythema response at 24 hours in P. leucotomos-treated patients (p<0.01). Consistent with its photoprotective effect there were significantly fewer sunburn cell numbers (P < .05), cyclobutane pyrimidine dimers (P < .001), proliferating epidermal cells (P < .001), dermal mast cell infiltration in biopsy specimens (P < .05) compared to those of untreated patients.
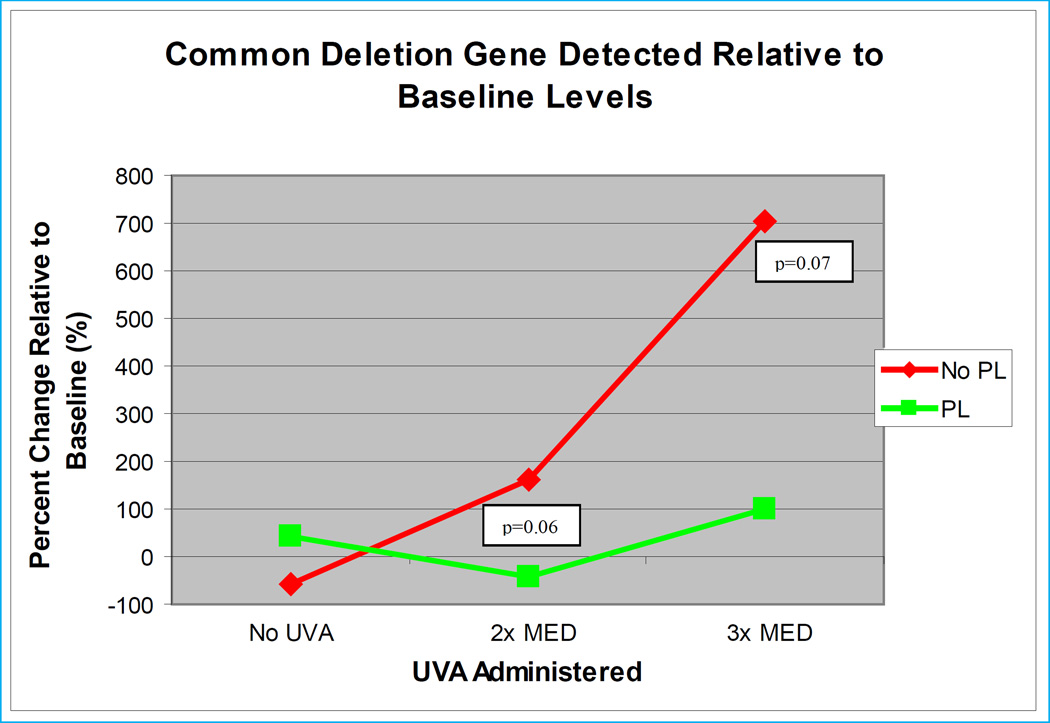
A marker of chronic UVA radiation in fibroblasts and keratinocytes is a mitochondrial DNA deletion known as the common deletion29. In a study designed to assess the effects of P. leucotomos on the common deletion in human subjects exposed to UVA30, ten healthy adult volunteers were then exposed to doses of UVA radiation ranging from 10–35 J/m2 from an artificial UVA light source. One week later subjects were randomized to either receive or not receive oral P. leucotomos, 240 mg given 8 and 2 hours prior to UVA exposure. An adjacent area of skin was shielded from exposure. Skin biopsies taken twenty-four hours after irradiation, showed that mean common deletion values in the untreated group increased by 160% over baseline while the mean common deletion values in the P. leucotomos-treated group decreased by 42% (p=0.06). At three times the MED for UVA, values of the common deletion showed increases of 703% and 102%, respectively (p=0.07). These results are graphically displayed in the Figure.
Figure.
Ten healthy adult volunteers initially underwent a punch biopsy for baseline common deletion measurement (26). One week later the volunteers were exposed to 2 and 3 times their MED with an area of skin shielded from exposure. Subjects were randomized to receive oral P. leucotomos 240 mg administered 8 and 2 hours before UV exposure. Twenty-four hours after UV, each subject underwent a second and a third punch biopsy of the irradiated areas and a fourth biopsy from the shielded area site and common deletion determinations were performed on all four biopsy specimens. At two times the MED, mean common deletion values in the untreated group increased by 159.7% over baseline while the mean common deletion values in the P. leucotomos-treated decreased by 42.3% (p=0.06). At three times the MED, those values increased 702.9% and 101.6%, respectively (p=0.07).
Two studies have assessed the beneficial effects of P. leucotomos extract in patients with polymorphic light eruption (PLE). In the first study, patients with PLE and 2 subjects with solar urticaria were treated with oral P. leucotomos 480 mg daily31. Of the 25 evaluable PLE participants, 80% improved; 31% reported that their response to sunlight normalized, 13% had clear improvement, and 36% had slight improvement. The improvement was statistically significant by Χ2 analysis (p<0.05). P. leucotomos was not effective in the two solar urticarial patients. The only adverse event was a worsening of irritable bowel syndrome in one patient. Otherwise oral P. leucotomos was well tolerated.
The objective of the second study also was to determine whether P. leucotomos extract could prevent or delay polymorphous light eruption lesions. In these experiments, an artificial light sources were employed for more detailed examination and quantitation of the wavelength bands affected by P. leucotomos4. Subjects with long-standing polymorphous light eruption were enrolled. Thirty patients were identified in whom PLE lesions could be reproduced by repeated UVA exposure. In 18 of those 30 patients, PLE could also be provoked by UVB. There were no subjects in whom PLE could be induced by UVB only. These patients then began treatment with oral P. leucotomos based on body weight: patients weighing ≤ 55 kg received 720 mg daily, those weighing 56–70 kg received 960 mg daily and those weighing > 70 kg received 1200 mg daily of P. leucotomos. After two weeks of P. leucotomos treatment, repeat photoprovocation studies were performed. P. leucotomos was continued during the photoprovocation testing. Of the 30 subjects, P. leucotomos completely blocked PLE lesion development in 9 following UVA photoprovocation (i.e. 30%), and in 5 of the 18 subjects following UVB photoprovocation (28%). Of those patients who were not completely protected with P. leucotomos, partial protection occurred. Specifically, the mean number exposures required to produce PLE lesions increased from 1.95 to 2.62 for UVA (p=0.05) and from 2.38 to 2.92 for UVB (p=0.047).
A randomized, double-blind study was designed to determine whether the use of P. leucotomos extract could reduce the use of topical corticosteroids in children and adolescents with atopic dermatitis2. The study enrolled 105 patients who were receiving topical corticosteroids at the time of entry into the study to treat moderate atopic dermatitis. In addition to their standard treatment, patients were randomized to receive P. leucotomos extract capsules or placebo for 6 months. Although the use of P. leucotomos did not significantly reduce the mean percentage of days on which topical corticosteroids were used, there was a significant reduction in median percentage of days of oral antihistamine use (4.5% for P. leucotomos vs. 13.6% for placebo; p=0.038).
A recent study was undertaken to determine the safety of capsules containing a carefully controlled extract of P. leucotomos (Heliocare, IFC, Spain) extract (240 mg) taken orally twice daily based on clinical history, physical findings and clinical laboratory parameters and to determine its ability to increase the minimal erythema dose or to reduce ultraviolet-associated erythema. In the randomized, double-blind, placebo-controlled study healthy subjects with Fitzpatrick skin types I-IV were randomized to receive oral PL extract (240 mg) twice daily at 8 AM and 2 PM for 2 months (N=20) and the other group received a placebo capsule twice daily for 2 months (N=20). Overall safety was assessed in both groups on Day 0 and Days 14, 28, and 56. Safety assessments included vital signs, complete blood count (CBC), a comprehensive metabolic panel, PT-PTT and any adverse events. The MED and UVB-associated erythema were assessed in 12 subjects from each group on Days 0, 14 and 28. These measures included MED, sunburn history and the number of hours of sun exposure.
All 40 subjects completed the study. No treatment-related serious adverse events were reported during 2 months of treatment and there were no significant changes in physical examinations, clinical laboratory parameters or vital signs. Four subjects treated with P. leucotomos extract reported mild episodic fatigue, bloating, and headaches while one placebo-treated subject reported fatigue. There were no significant between-group differences in sun exposure before or during the study, however, subjects in the placebo group showed a six-fold greater chance of experiencing at least one sunburn during the study than did subjects taking the P. leucotomos extract (p=0.04). Subjects in the PLE group had a 22-fold greater incidence (odds ratio) of an increase in UVB MED compared to the Placebo group after 28 days of treatment (p=0.01)32
CONCLUSION
In vitro and in vivo animal and human studies have demonstrated the beneficial effects of Polypodium leucotomos. Together, these data indicate that extracts of this unique plant utilize multiple mechanisms for providing photoprotection and therapeutic activity that include reducing UV-induced cell damage, reducing oxidative stress and DNA damage, blocking UV radiation-induced immune suppression, and inhibiting the release of UV-induced levels of cyclooxygenase-2 and inflammatory cytokines. The in vivo studies show that the effects of P. leucotomos extract are not just theoretical; indeed, benefit has been demonstrated in animals and in humans. Thus, this natural plant extract has the potential to complement sunscreens and other methods of photoprotection. This agent which can be taken orally and has not been noted to have serious adverse reactions, offers unique advantages in that it can be given orally, thus avoiding patient resistance to topically applied sunscreens.
Acknowledgments
The authors gratefully acknowledge the assistance of Dr. Carl Hornfeldt during the preparation of this manuscript.
Footnotes
Supported by NIH Grant AR050948
Relevant Conflicts of Interest: Dr. Berman is and has served on Advisory Boards and as investigator for Ferndale. Dr. Ellis serves as a consultant to Ferndale Healthcare, Inc. and to companies that market products for the treatment or prevention of photoaging, psoriasis, and atopic dermatitis. Dr. Elmets is a consultant for Ferndale Healthcare, Inc.
REFERENCES
- 1.Padilla HC, Laínez H, Pacheco JA. A new agent (hydrophilic fraction of Polypodium leucotomos) for management of psoriasis. Int J Dermatol. 1974;13:276–282. doi: 10.1111/j.1365-4362.1974.tb05081.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramírez-Bosca A, Zapater P, Betlloch I, et al. Polypodium leucotomos extract in atopic dermatitis: a randomized, double-blind, placebo-controlled, multicenter trial. Actas Dermosifiliogr. 2012;103:599–607. doi: 10.1016/j.ad.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Middelkamp-Hup MA, Bos JD, Rius-Diaz F, et al. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2007;21:942–950. doi: 10.1111/j.1468-3083.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanew A, Radakovic S, Gonzalez S, et al. Oral administration of a hydrophilic extract of Polypodium leucotomos for the prevention of polymorphic light eruption. J Am Acad Dermatol. 2012;66:58–62. doi: 10.1016/j.jaad.2010.09.773. [DOI] [PubMed] [Google Scholar]
- 5.Horvath A, Alvarado F, Szöcs J, et al. Metabolic effects of calagualine, an antitumoral saponine of Polypodium leucotomos. Nature. 1967;214:1256–1258. doi: 10.1038/2141256a0. [DOI] [PubMed] [Google Scholar]
- 6.Garcia F, Pivel JP, Guerrero A, et al. Phenolic components and antioxidant activity of Fernblock, an aqueous extract of the aerial parts of the fern Polypodium leucotomos. Methods Find Exp Clin Pharmacol. 2006;28:157–160. doi: 10.1358/mf.2006.28.3.985227. [DOI] [PubMed] [Google Scholar]
- 7.Gombau L, García F, Lahoz A, et al. Polypodium leucotomos extract: antioxidant activity and disposition. Toxicol In Vitro. 2006;20:464–471. doi: 10.1016/j.tiv.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 8.van Kranen HJ, de Gruijl FR. Mutations in cancer genes of UV-induced skin tumors of hairless mice. J Epidemiol. 1999;9:S58–S65. doi: 10.2188/jea.9.6sup_58. [DOI] [PubMed] [Google Scholar]
- 9.Perez C, Parker-Thornburg J, Mikulec C, et al. SKHIN/Sprd, a new genetically defined inbred hairless mouse strain for UV-induced skin carcinogenesis studies. Exp Dermatol. 2012;21:217–220. doi: 10.1111/j.1600-0625.2011.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alcaraz MV, Pathak MA, Rius F, et al. An extract of Polypodium leucotomos appears to minimize certain photoaging changes in a hairless albino mouse animal model. A pilot study. Photodermatol Photoimmunol Photomed. 1999;15:120–126. doi: 10.1111/j.1600-0781.1999.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 11.Zattra E, Coleman C, Arad S, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175:1952–1961. doi: 10.2353/ajpath.2009.090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmets CA, Viner JL, Pentland AP, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: A randomized, double-blind, placebo-controlled trial. J Natl Cancer I. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An KP, Athar M, Tang X, et al. Cyclooxygenase-2 expression in murine and human nonmelanoma skin cancers: implications for therapeutic approaches. Photochem Photobiol. 2002;76:73–80. doi: 10.1562/0031-8655(2002)076<0073:ceimah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Yanes E, Juarranz Á, Cuevas J, et al. Polypodium leucotomos decreases UV-induced epidermal cell proliferation and enhances p53 expression and plasma antioxidant capacity in hairless mice. Exp Dermatol. 2012;21:638–640. doi: 10.1111/j.1600-0625.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- 15.Elmets CA, Cala CM, Xu H. Photoimmunology. Dermatol Clin. 2014;32(3):277–290. vii. doi: 10.1016/j.det.2014.03.005. PubMed PMID: 24891051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siscovick JR, Zapolanski T, Magro C, et al. Polypodium leucotomos inhibits ultraviolet B radiation-induced immunosuppression. Photodermatol Photoimmunol Photomed. 2008;24:134–141. doi: 10.1111/j.1600-0781.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- 17.Mulero M, Rodriguez-Yanes E, Nogues MR, Giralt M, Romeu M, Gonzalez S, Mallol J. Polypodium leucotomos extract inhibits glutathione oxidation and prevents Langerhans cell depletion induced by UVB/UVA radiation in a hairless rat model. Exp Dermatol. 2008;17(8):653–658. doi: 10.1111/j.1600-0625.2007.00684.x. PubMed PMID: 18312382. [DOI] [PubMed] [Google Scholar]
- 18.Mulero M, Romeu M, Giralt M, et al. Oxidative stress-related markers and langerhans cells in a hairless rat model exposed to UV radiation. J Toxicol Environ Health A. 2006;69:1371–1385. doi: 10.1080/15287390500471187. [DOI] [PubMed] [Google Scholar]
- 19.Mulero M, Rodríguez-Yanes E, Nogués MR, et al. Polypodium leucotomos extract inhibits glutathione oxidation and prevents Langerhans cell depletion induced by UVB/UVA radiation in a hairless rat model. Exp Dermatol. 2008;17:653–658. doi: 10.1111/j.1600-0625.2007.00684.x. [DOI] [PubMed] [Google Scholar]
- 20.Jańczyk A, Garcia-Lopez MA, Fernandez-Peñas P, et al. A Polypodium leucotomos extract inhibits solar-simulated radiation-induced TNF-alpha and iNOS expression, transcriptional activation and apoptosis. Exp Dermatol. 2007;16:823–829. doi: 10.1111/j.1600-0625.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 21.Alonso-Lebrero JL, Domínguez-Jiménez C, Tejedor R, et al. Photoprotective properties of a hydrophilic extract of the fern Polypodium leucotomos on human skin cells. J Photochem Photobiol B. 2003;70:31–37. doi: 10.1016/s1011-1344(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 22.Capote R, Alonso-Lebrero JL, García F, et al. Polypodium leucotomos extract inhibits trans-urocanic acid photoisomerization and photodecomposition. J Photochem Photobiol B. 2006;82:173–179. doi: 10.1016/j.jphotobiol.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs NK, Tye J, Norval M. Recent advances in urocanic acid photochemistry, photobiology and photoimmunology. Photochem Photobiol Sci. 2008;7(6):655–667. doi: 10.1039/b717398a. PubMed PMID: 18528548. [DOI] [PubMed] [Google Scholar]
- 24.Tuominen M, Bohlin L, Rolfsen W. Effects of Calaguala and an active principle, adenosine, on platelet activating factor. Planta Med. 1992;58:306–310. doi: 10.1055/s-2006-961472. [DOI] [PubMed] [Google Scholar]
- 25.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195(2):171–179. doi: 10.1084/jem.20011450. PubMed PMID: 11805144; PubMed Central PMCID: PMC2193612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasänge M, Rolfsen W, Bohlin L. A sulphonoglycolipid from the fern Polypodium decumanum and its effect on the platelet activating-factor receptor in human neutrophils. J Pharm Pharmacol. 1997;49:562–566. doi: 10.1111/j.2042-7158.1997.tb06842.x. [DOI] [PubMed] [Google Scholar]
- 27.Vasänge-Tuominen M, Perera-Ivarsson P, Shen J, et al. The fern Polypodium decumanum, used in the treatment of psoriasis, and its fatty acid constituents as inhibitors of leukotriene B4 formation. Prostaglandins Leukot Essent Fatty Acids. 1994;50:279–284. doi: 10.1016/0952-3278(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 28.Middelkamp-Hup MA, Pathak MA, Parrado C, et al. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51:910–918. doi: 10.1016/j.jaad.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 29.Koch H, Wittern KP, Bergemann J. In human keratinocytes the common deletion reflects donor variabilities rather than chronologic aging and can be induced by ultraviolet A irradiation. J Invest Dermatol. 2001;117:892–897. doi: 10.1046/j.0022-202x.2001.01513.x. [DOI] [PubMed] [Google Scholar]
- 30.Villa A, Viera MH, Amini S, et al. Decrease of ultraviolet A light-induced "common deletion" in healthy volunteers after oral Polypodium leucotomos extract supplement in a randomized clinical trial. J Am Acad Dermatol. 2010;62:511–513. doi: 10.1016/j.jaad.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 31.Caccialanza M, Percivalle S, Piccinno R, et al. Photoprotective activity of oral Polypodium leucotomos extract in 25 patients with idiopathic photodermatoses. Photodermatol Photoimmunol Photomed. 2007;23:46–47. doi: 10.1111/j.1600-0781.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- 32.Nestor MS, Berman B. A Safety and Efficacy Study of Oral Heliocare® (Polypodium leucotomos Extract) in Healthy Adult Subjects. Poster at Practical Dermatol Dermatopath Symposium; Aug. 14, 2014; Vail, Co. [Google Scholar]