Abstract
A method for the synthesis of dihydrobenzofurans by a direct aryl C-O bond formation is described. A mechanistic pathway for the reaction, distinct from previously described similar transformations, allows for mild reaction conditions that are expected to be compatible with functionalized substrates.
Keywords: cyclization, iodonium salts, copper, oxygen heterocycle, fused-ring system
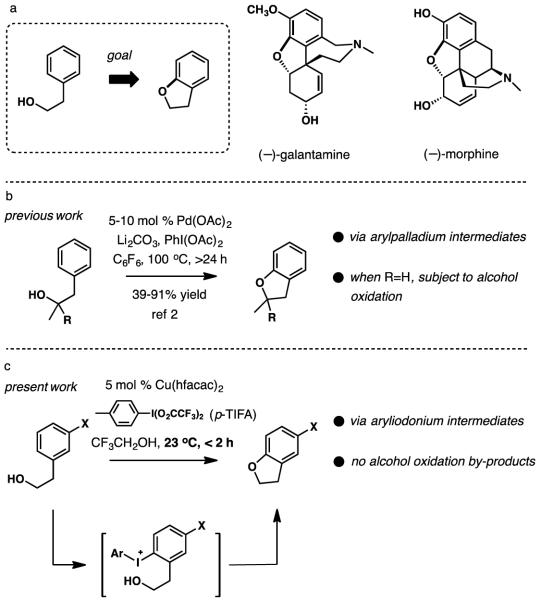
C—H Functionalization has emerged as an increasingly feasible strategic disconnection for the synthesis of complex natural products.[1] Among an expanding set of methods, the direct cyclization of tethered heteroatoms onto C—H bonds provides an attractive point of functionalization in synthesis design. In connection with our interest in developing a concise approach to Amaryllidaceae alkaloids such as galantamine, we required a method for the assembly of dihydrobenzofurans by direct aryl C—O bond formation as illustrated in Scheme 1a.
Scheme 1.
Our goals included specific emphasis on mildness of reaction conditions compatible with functionalized substrates, as many reported methods for C—H functionalization require rather high temperatures and prolonged reaction times. Recent work from the laboratories of Yu and Davies described an effective synthesis of dihydrobenzofurans by cyclization of secondary benzyllic and tertiary alcohols (Scheme 1b).[2] This method provides an attractive entry to substituted dihydrobenzofurans. In an attempt to adopt this reaction in an approach to galantamine, we discovered that competitive oxidation of a primary or secondary hydroxy group[3] posed a significant problem. Herein we describe an alternative method for the synthesis of functionalized benzofurans and dihydrobenzofurans by direct intramolecular aryl C—H bond functionalization under mild conditions that minimize oxidation of alcohols.
In considering various strategies, we became intrigued by diaryliodonium derivatives as intermediates for the synthesis of dihydrobenzofurans, as outlined in Scheme 1c.[4] Previously, the groups of Olofsson,[5] Gaunt,[6] and Stuart[7] described catalyst-free diaryl and alkyl aryl ether synthesis by intermolecular O-arylation of phenols or alkanols under basic conditions. In a mechanistically distinct process, Kita and co-workers reported the synthesis of dihydrobenzopyrans (chromans) by oxidative C—H cyclization of 3-hydroxypropyl phenols with hypervalent iodine oxidants in 1,1,1,3,3,3-hexafluoro-2-isopropanol.[8] Notably, this method was found to be unsuitable for the synthesis of five-membered analogs, dihydrobenzofurans.
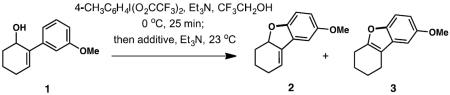
The closest precedent for our strategy came from the work of Onomura and co-workers, who recently described a Cu(OTf)2-catalyzed mono-arylation of 1,2- and 1,3-diols with symmetrical diaryliodonium triflates.[9] According to our reaction design, a mild Cu-catalyzed intramolecular C—H arylation of alcohols[10] affording dihydrobenzofurans will be accomplished via the intermediacy of non-symmetric diaryliodonium salts, which were to be prepared in situ and processed further without isolation. The choice of the prototypical substrate, 2-(3-methoxyphenyl)-2-cyclohexen-1-ol (1, Table 1) was influenced by its relevance to eventual synthesis targets (galantamine, morphine). We found that no established protocol was compatible with our model having observed only oxidation, decomposition or recovery of 1. Initial control experiments were carried out with PhI(O2CCF3) (PIFA) as the oxidant with no metal additive, and revealed that only partial cyclization to benzofuran products took place in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP), giving a 1:7 mixture of double bond isomers 2 and 3 in a combined 14% yield (Table 1, entry 1). In addition to 2 and 3, by-products from alkene oxidation and allylic rearrangement were formed. To attenuate the acidity and polarity of the reaction media, the solvent was changed to 2,2,2-trifluoroethanol (TFE) and 0.25 equiv of Et3N were added (entry 2). Although no cyclization of 1 was observed with PIFA under these conditions, an unsymmetrical diaryl-λ3-iodane product was isolated instead in 46% yield (vide infra).
Table 1.
| entry[a] | oxidan | additive (equiv) | time | 2:3 | yield[b] |
|---|---|---|---|---|---|
| 1 | PIFA | - | 14 h | 1:7 | 14%[c,d] |
| 2 | PIFA | - | 14 h | - | 0%[d] |
| 3 | PIFA | Cu(OTf)2 (1.0) | 8 h | 0:1 | 52%[d,e] |
| 4 | PIFA | Cu(OTf)2 (1.0) | 8 h | 1:0 | 62%[d] |
| 5 | TIFA | Cu(OTf)2 (1.0) | 5 h | 1:0 | 69% |
| 6 | TIFA | Cu(CO2CF3)2 (1.0) | 4 h | 1:0 | 67% |
| 7 | TIFA | Cu(tfacac)2 (1.0) | 2 h | 1:0 | 73% |
| 8 | TIFA | Cu(hfacac)2 (1.0) | 10 min | 1:0 | 75% |
| 9 | TIFA | CuCl2 (1.0) | 4 h | 1:0 | 54% |
| 10 | TIFA | CuI (1.0) | 12 h | 1:0 | 40% |
| 11 | TIFA | CuBr•Me2S (1.0) | 4 h | 1:0 | 48% |
| 12 | TIFA | CuCl (1.0) | 3 h | 1:0 | 55% |
| 13 | TIFA | Cu(OTf)•PhMe (1.0) | 2 h | 1:0 | 38% |
| 14 | TIFA | Cu(CuCN)4BF4 (1.0) | 30 min | 1:0 | 58% |
| 15 | TIFA | Cu(hfacac)2 (0.2) | 40 min | 1:0 | 75% |
| 16 | TIFA | Cu(hfacac)2 (0.05) | 5 h | 1:0 | 73% |
Unless otherwise noted reaction conditions were as follows: 1 (0.1 mmol), 4-CH3C6H4I(O2CCF3)2 (0.11 mmol), Et3N (0.025 mmol), CF3CH2OH (0.05 M) followed by additive and Et3N (0.4 mmol, 4 equiv), monitored by TLC analysis until consumption of diaryliodonium salt intermediate.
Isolated yield.
HFIP as solvent, no Et3N, isolated as 7:1 mixture of 3/2 product.
Consumption of 1 in 1.5 h.
no Et3N, isolated as 3.
Isolation of the diaryl-λ3-iodane intermediate suggested the use of copper salt additives for further optimization.[9] Indeed, addition of 1.0 equiv Cu(OTf)2 to the preformed diaryl-λ3-iodane (PIFA, TFE, 0 °C, 25 min) afforded 3 exclusively in 52% yield (Table 1, entry 3). To suppress alkene isomerization in the initially formed benzofuran 2, presumably acid-mediated, another 4 equiv of Et3N were added prior to the addition of copper catalyst, which delivered 2 in 62% yield (entry 4). No alkene isomerization to 3 was detected in this case. Further marginal improvement was observed when PIFA was replaced with p-tolueneiodonium bis(trifluoroacetate) (TIFA), which also reduced the reaction time from 8 to 5 h (entry 5). A further screen of copper compounds identified more effective reagents. In particular, soluble additives displayed a substantially increased reactivity as well as the yield (entries 6–16). Among these, copper bis(hexafluoroacetylacetonate) Cu(hfacac)2 was especially reactive, leading to a complete reaction within 10 min at 23 °C and affording 2 in 75% isolated yield (entry 8). Subsequent experimentation revealed that the copper additive was just as effective in substoichiometric amounts (20 mol % or 5 mol %) with virtually identical yields but at the expense of reaction time (entries 15 and 16).
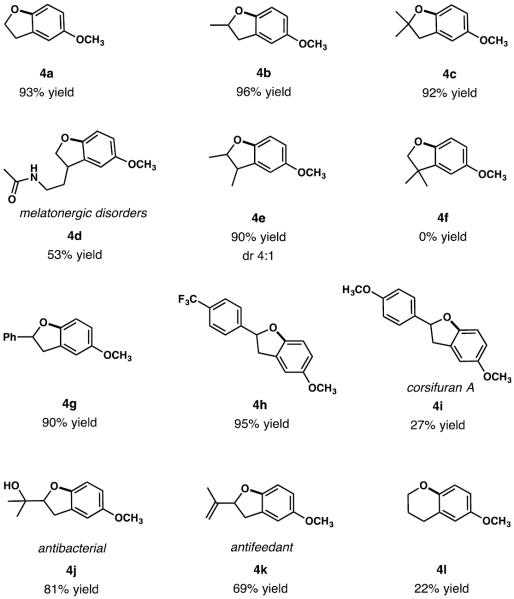
For the scope studies, we selected the conditions requiring 20 mol % of Cu(hfacac)2 and 1.1 equiv of TIFA for an optimal balance between the catalyst loading and reaction time. The scope of alcohols was examined first, starting by evaluation of primary, secondary, and tertiary alcohols with our reaction conditions, and resulting in formation of the corresponding dihydrobenzofurans (4a–c) in high yields (>90%). Substrates monosubstituted at the benzylic position with either alkyl (4e), alkenyl (4k) or aryl groups (4g, 4h) are also suitable substrates for dihydrobenzofurans formation, with the exception of the pharmaceutical intermediate 4d, which was obtained in a slightly lower yield (52%). The lower yield is probably due to the presence of the acetamido group. Disubstitution at the benzylic position results in no formation of dihydrobenzofuran 4f with full recovery of the starting material. Natural product corsifuran A (4i) was obtained in a low yield of 27%. This result can be explained by the presence of two electron rich aromatic rings, which can lead to competitive oxidation at the undesired site. Indeed, previous work by Kita and coworkers showed that 4-OMe substituted arenes react with PIFA through a radical cation pathway, while 3-OMe-substituted arenes afford iodonium salts.[11] Antibacterial compound 4j was obtained in a good yield, demonstrating selectivity for the dihydrobenzofuran versus chroman formation. In fact, chroman formation was not efficient using these reaction conditions as compound 4l was obtained with only 22% yield, in addition to tolyl group-transfer by-product.
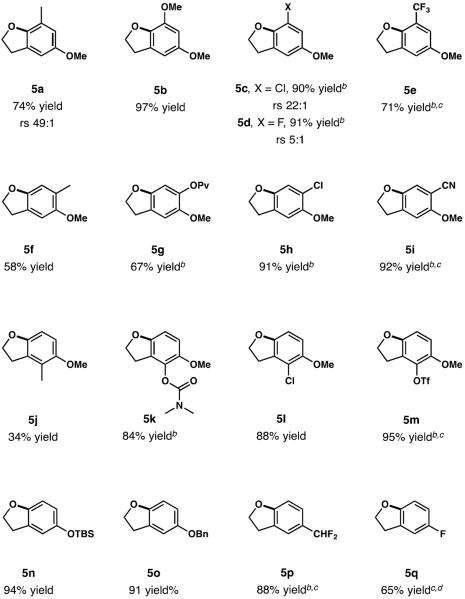
The scope of arene was then investigated using substrates with a primary hydroxy group (Table 3). These substrates often required extended reaction times (12–16 h) to reach completion. Ortho-Substitution relative to the newly formed C—O bond was well tolerated using both electron donating (5a, 5b) or electron withdrawing substituents (5c–e). It is important to note that strongly deactivating groups such as CF3 require the use of a stronger oxidant, [hydroxy(tosyloxy)iodo]benzene, still providing dihydrobenzofuran 5e in good yields (71%). The same pattern of reactivity was observed when a variety of substituents were introduced at the ortho position to the directing group. In fact, substrates with pivalate (5g, 67% yield), cyano (5i, 92% yield), and dimethyl carbamate (5k, 87% yield) substituents all afforded good to excellent yields of the products. Notably, chlorine (5h, 91% yield; 5l, 83% yield) and triflate (5m, 95% yield) functionalities were well tolerated and provide an excellent handle for subsequent synthetic derivatization. Surprisingly Me-substituted dihydrobenzofurans 5f and 5j were obtained in lower yields of 58% and 34%, respectively, presumably due to low stability of the products in the air. In our screening efforts, we placed an emphasis on the use of different directing groups in order to illustrate the versatility of the method. Thus –OTBS, -OBn and –OCHF2 functionalities have been evaluated, providing dihydrobenzofurans 5n–5p in excellent yields. Interestingly, fluorine was found to be a suitable directing group, affording dihydrobenzofuran 5q in 65% yield.
Table 3.
Scope studies: arene variation.[a]
Unless otherwise noted, reaction conditions include: substrate (0.5 mmol), 4-CH3C6H4I(O2CCF3)2 (0.55 mmol), Et3N (0.125 mmol), CF3CH2OH, 0 °C, followed by Cu(hfacac)2 (0.1 mmol) and Et3N (2 mmol), 23 °C; monitored by TLC analysis until consumption of the substrate, then diaryliodonium salt.
50 °C for iodonium salt formation.
3 equiv of hydroxyl(tosyloxy)iodobenzene as oxidant.
hexafluoroisopropanol as solvent, 23 °C for iodonium salt formation.
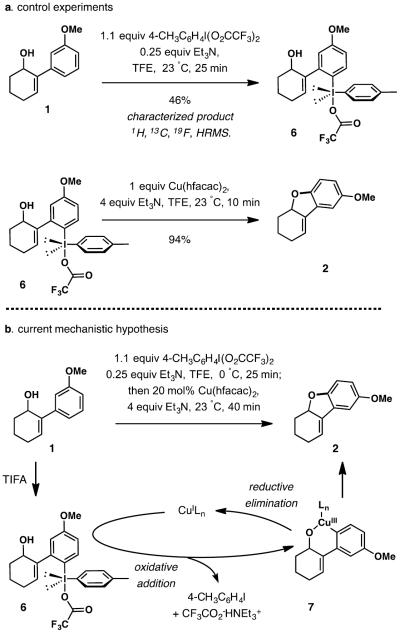
To gain initial insight into the mechanism of the process, we carried out the reaction with substrate 1 in the absence of a copper additive (scheme 2a). After 25 min at room temperature, diaryl-λ3-iodane 6 could be isolated in 46% yield and characterized by mass-spectroscopy and 1H, 13C, and 19F NMR spectroscopy. No cyclization of the intermediate to dihydrobenzofuran 2 was observed even after 1 week in the absence of a copper additive. In contrast, a rapid and nearly quantitative cyclization to dihydrobenzofuran 2 occurred when diaryl-λ3-iodane 6 was treated with 1 equiv of Cu(hfacac)2 and triethylamine in TFE at room temperature for 10 min. Based on these results, a tentative mechanism for the conversion of 1 to dihydrobenzofuran 2 is outlined in Scheme 2a. In the presence of an activating and directing group like MeO, the substrate is rapidly converted to the diaryl-λ3-iodane intermediate upon the initial exposure to an iodine(III) reagent. Subsequent addition of the soluble Cu catalysts results in a rapid oxidative insertion into the C-I bond,[12] affording a putative Cu(III) intermediate 7.[13] Reductive elimination affords the dihydrobenzofuran product.[14] Triethylamine serves as a buffer to minimize acid-mediated side reactions at various stages of the process.
Scheme 2.
Preliminary mechanistic studies and mechanistic hypothesis.
In summary, we have described an alternative Cu-catalyzed C—H functionalization reaction for the synthesis of functionalized dihydrobenzofurnans. An important feature of the reaction is mildness of the reaction conditions that avoid extremes in temperature and reaction times, strong bases, and strongly oxidizing reagents. We expect the mildness of the reaction conditions to be especially relevant in the synthesis of complex functionalized molecules. In the present study the utility of the method was illustrated by the synthesis of several simple bioactive compounds.
Experimental Section
[Bis(trifluoroacetoxy)iodo]p-toluene (0.25 g, 0.55 mmol, 1.1 equiv) or [hydroxy(tosyloxy)iodo]benzene (0.59 g, 1.5 mmol, 3 equiv) was added as one portion to a solution of alcohol substrate (0.5 mmol) and triethylamine (17 μL, 0.13 mmol, 0.25 equiv) in trifluoroethanol (10 mL, 0.05 M) at 0 °C under argon. The mixture was stirred until the alcohol was consumed as indicated by TLC analysis (typically accompanied with a color change to yellow or orange). Triethylamine (0.28 mL, 2.0 mmol, 4.0 equiv) was then added at 0 °C followed immediately by copper(II)hexafluoroacetylacetonate (0.05 g, 0.1 mmol, 20 mol%). The mixture was warmed to 23 °C and stirred until iodonium salt intermediate was consumed as indicated by TLC analysis. To the resulting blue green solution, saturated aqueous NaHCO3 was added and the mixture was extracted with CH2Cl2 (2 × 10 mL). The combined organic phases were dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography to give corresponding dihydrobenzofuran.
Supplementary Material
Table 2.
Scope studies: alcohol variation.[a]
Unless otherwise noted, reaction conditions include: substrate (0.5 mmol), 4-CH3C6H4I(O2CCF3)2 (0.55 mmol), Et3N (0.125 mmol), CF3CH2OH, 0 °C, followed by Cu(hfacac)2 (0.1 mmol) and Et3N (2 mmol), 23 °C; monitored by TLC analysis until consumption of the substrate, then diaryliodonium salt.
Acknowledgements
This work is supported by the NSF CHE-1463819, Amgen, Eli Lilly and the NIH Shared Instrumentation Grant (SIG) 1S10OD012077-01A1.
References
- [1].a) Lu P, Gu Z, Zakarian A. J. Am. Chem. Soc. 2013;135:14552–14552. doi: 10.1021/ja408231t. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lu P, Mailyan A, Gu Z, Guptil D, Wang H, Davies HML, Zakarian A. J. Am. Chem. Soc. 2014;136:17738–17749. doi: 10.1021/ja510573v. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gutekunst WR, Baran PS. J. Am. Chem. Soc. 2011;133:19076–19079. doi: 10.1021/ja209205x. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Pitts A, O'Hara F, Snell R, Gaunt M. Angew. Chem., Int. Ed. 2015;54:5451–5455. doi: 10.1002/anie.201500067. [DOI] [PubMed] [Google Scholar]; e) See Y, Herrmann AT, Aihara Y, Baran PS. J. Am. Chem. Soc. 2015;137:13776–13779. doi: 10.1021/jacs.5b09463. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Hong B, Li C, Wang Z, Chen J, Li H, Lei X. J. Am. Chem. Soc. 2015;137:11946–11949. doi: 10.1021/jacs.5b08551. [DOI] [PubMed] [Google Scholar]; g) Zhao L, Tsukano C, Kwon E, Takemoto Y, Hirama M. Angew. Chem., Int. Ed. 2012;52:1722–1725. doi: 10.1002/anie.201208297. [DOI] [PubMed] [Google Scholar]; h) Wang DH, Yu J-Q. J. Am. Chem. Soc. 2011;133:5767–5769. doi: 10.1021/ja2010225. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Yamaguchi A, Chepiga K, Yamaguchi J, Itami K, Davies HML. J. Am. Chem. Soc. 2015;137:644–647. doi: 10.1021/ja512059d. [DOI] [PubMed] [Google Scholar]; j) Daugulis O, Roane J, Tran LD. Acc. Chem. Res. 2015;48:1053–1064. doi: 10.1021/ar5004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Wang X, Lu Y, Dai H, Yu J-Q. J. Am. Chem. Soc. 2010;132:12203–12205. doi: 10.1021/ja105366u. [DOI] [PubMed] [Google Scholar]; b) Wang H, Li G, Engle K, Yu J-Q, Davies HML. J. Am. Chem. Soc. 2013;135:6774–6777. doi: 10.1021/ja401731d. [DOI] [PubMed] [Google Scholar]
- [3].Oxidation as a significant reaction pathway during palladium-catalyzed C-O bond formation was previously reported: Torraca KE, Kuwabe S-I, Buchwald SL. J. Am. Chem. Soc. 2000;122:12907–12908. doi: 10.1021/ja012046d. Vorogushin AV, Huang X, Buchwald SL. J. Am. Chem. Soc. 2005;127:8146–8149. doi: 10.1021/ja050471r. Also see ref 2.
- [4].a) Merritt E, Olofsson B. Angew. Chem., Int. Ed. 2009;48:9052–9070. doi: 10.1002/anie.200904689. Reviews: [DOI] [PubMed] [Google Scholar]; b) Zhdankin VV, Stang PJ. Chem. Rev. 2002;102:2523–2584. doi: 10.1021/cr010003+. [DOI] [PubMed] [Google Scholar]; c) Zhdankin VV, Stang PJ. Chem. Rev. 2008;108:5299–5358. doi: 10.1021/cr800332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].a) Jalalian N, Ishikawa EE, Silva LF, Jr., Olofsson B. Org. Lett. 2011;13:1552–1555. doi: 10.1021/ol200265t. [DOI] [PubMed] [Google Scholar]; b) Lindstedt E, Ghosh R, Olofsson B. Org. Lett. 2013;15:6070–6073. doi: 10.1021/ol402960f. [DOI] [PubMed] [Google Scholar]
- [6].Chan L, McNally A, Toh QY, Mendoza A, Gaunt M. Chem. Sci. 2015;6:1277–1281. doi: 10.1039/c4sc02856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sundalam S, Stuart D. J. Org. Chem. 2015;3:54–57. doi: 10.1021/acs.joc.5b00907. [DOI] [PubMed] [Google Scholar]
- [8].Hata K, Hamamoto H, Shiozaki Y, Cammerer S, Kita Y. Tetrahedron. 2007;63:4052–4060. [Google Scholar]
- [9].Kuriyama M, Hamaguchi N, Onomura O. Chem. Eur. J. 2012;18:1591–1594. doi: 10.1002/chem.201102770. [DOI] [PubMed] [Google Scholar]
- [10].Sokolovs I, Suna E. J. Org. Chem. 2016;81:371–379. doi: 10.1021/acs.joc.5b02728. [DOI] [PubMed] [Google Scholar]
- [11].a) Kita Y, Tohma H, Hatanaka K, Takada T, Fujita S, Mitoh S, Sakurai H, Oka S. J. Am. Chem. Soc. 1994;116:3684–3691. [Google Scholar]; b) Arisawa M, Ramesh N, Nakajima M, Tohma H, Kita Y. J. Org. Chem. 2001;66:59–65. doi: 10.1021/jo000953f. [DOI] [PubMed] [Google Scholar]
- [12].a) Lockhart TP. J. Am. Chem. Soc. 1983;105:1940–1946. [Google Scholar]; b) Crivello JV, Lee JL. J. Polym. Sci., Polym. Chem. Ed. 1981;19:539–548. [Google Scholar]; c) Varoglis A. The Organic Chemistry of Polycoordinated Iodine. VCH Publishers; New York: 1992. [Google Scholar]
- [13].a) King A, Huffman L, Casitas A, Costas M, Ribas X, Stahl SS. J. Am. Chem. Soc. 2010;132:12068–12073. doi: 10.1021/ja1045378. [DOI] [PubMed] [Google Scholar]; b) Casitas A, King A, Parella T, Costas M, Stahl SS, Ribas X. Chem. Sci. 2010;1:326–330. [Google Scholar]; c) Su X, Chen C, Wang Y, Chen J, Lou Z, Li M. Chem. Commun. 2013;49:6752–6754. doi: 10.1039/c3cc43216e. [DOI] [PubMed] [Google Scholar]
- [14].a) Huffman L, Casitas A, Font M, Canta M, Costas M, Ribas X, Stahl SS. Chem. Eur. J. 2011;17:10643–10650. doi: 10.1002/chem.201100608. [DOI] [PubMed] [Google Scholar]; b) Casitas A, Ioannidis N, Mitrikas G, Costas M, Ribas X. Dalton Trans. 2011;40:8796–8799. doi: 10.1039/c1dt10428d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.