Abstract
The SUR1-TRPM4 channel is a critical determinant of edema and hemorrhagic transformation after focal ischemia. Blockade of this channel by the small molecule glyburide results in improved survival and neurological outcome in multiple preclinical models of ischemic stroke. A robust, compelling body of evidence suggests that an intravenous (IV) formulation of glyburide, RP-1127, can prevent swelling and improve outcome in patients with stroke. Retrospective studies of diabetic stroke patients show improved outcomes in patients who are continued on sulfonylureas after stroke onset. Early phase II study of MRI and plasma biomarkers support the conclusion that RP-1127 may decrease swelling and hemorrhagic transformation. Finally, the ongoing phase II RP-1127 development program has demonstrated continued safety as well as feasibility of enrollment and tolerability of the intervention. Continued efforts to complete the ongoing phase IIb study and definitive efficacy studies are urgently needed to bring a candidate pharmacotherapy to a population of severe stroke patients that currently have no alternative.
Introduction
Ischemic stroke afflicts approximately 700,000 people in the United States annually1. Malignant infarction, characterized by the syndrome resulting from brain swelling due to rapidly accumulating cerebral edema, occurs in 10% of ischemic stroke patients2. Swelling in these patients very often results in case fatality rates as high as 60–80%3. A significant barrier to progress in managing these patients with a large stroke is identifying safe and effective pharmacotherapy to prevent brain swelling and the resulting morbidity.
IV rtPA administered within three hours of symptom onset is the only FDA approved treatment, yet this drug reaches a minority of eligible patients1. In the United States, rtPA is not FDA approved for use within 3–4.5 hours. Efforts directed at improving outcome through endovascular reperfusion approaches have also been unsuccessful4. In patients with large territory infarction, reperfusion may even result in worse outcome, because of swelling or hemorrhagic transformation5. Decompressive craniectomy (DC) is the only proven therapy for swelling after ischemia. However, numerous factors limit the usefulness of DC, including limited eligibility for those who have serious comorbidities, and DC itself has a high rate of complications6,7.
It is clear that a preventative approach, rather than a reactive one, would be more preferrable8. Recently, an inducible ion channel, SUR1-TRMP4, was discovered to be transcriptionally upregulated by all cell types in the neurovascular unit following ischemia9. Channel blockade reduces edema and hemorrhage, as well as necrotic cell death10. Small molecule therapy using glyburide, a well-known drug with an excellent safety profile is effective in blocking this channel9. Multiple preclinical models of ischemic stroke with a similar pattern of findings suggest clinically relevant effects of glyburide11. Importantly, the strong, consistent body of preclinical work conforms to all of the major components of the Stroke Treatment Academic Industry Roundtable (STAIR) criteria11,12.
The ongoing translation of glyburide to human stroke patients has been informed not only by robust basic science data from cell culture to preclinical models, but also by pertinent human observations including randomized clinical trials. In this paper, we summarize the human data available supporting the ongoing translation of glyburide for injection, or RP-1127, in patients with ischemic stroke.
Retrospective Studies of Stroke
The population level administration of oral glyburide for diabetes for many years facilitates comparative outcome analyses between patients who have been administered sulfonylureas such as glyburide and those who have not. Geographic differences in practice also facilitate yet another perspective that relates to the underlying biology of the target channel. In the United States, when admitted with an ischemic stroke, patients often receive insulin infusions to maintain euglycemia in the hospital, and baseline diabetes medications are withheld. In parts of Europe and Canada, stroke units continue home diabetes medications in the hospital, after the stroke. Because SUR1 is not transcriptionally expressed prior to the onset of ischemia, one would not expect pre-stroke administration of glyburide to be associated with any neurological benefit13. In contrast, when sulfonylureas are continued immediately post-stroke onset, channel blockade and neurovascular protection is very much a possibility.
Two relevant studies were designed to test the association between sulfonylureas and outcome when the medication was given at any time in relation to stroke. The first was by Favilla et al14. using the Virtual International Stroke Trials Archive (VISTA) where 1050 patients with diabetes were analyzed, 298 of whom were taking sulfonylureas. In this study, there was no association between sulfonylurea administration and improved outcome in the overall cohort. Interestingly, in the 28 patients for whom sulfonylureas were continued following stroke, the point estimate favored sulfonylureas. Of note, the stroke subtypes were not known, which is important since patients with lacunar stroke may not receive the same anti-swelling benefit from glyburide. A Danish study using a large administrative database suggested that the 30 day mortality was higher in patients with sulfonylureas15. Not only were patients discontinued on sulfonylureas after stroke onset, but the difference in mortality rate did not adjust for stroke severity and was absent at one year.
Analyses of post-stroke administration of sulfonylureas, consistent with the de novo expression of the target after the onset of ischemia, produced very different results. A review of diabetics hospitalized within 24 hours of acute ischemic stroke at Charité Hospital, Berlin evaluated those patients who were continued on their diabetes medications16. The primary outcome, a decrease in the National Institutes of Health Stroke Scale (NIHSS) score of ≥ 4 points from admission to discharge or a discharge NIHSS score = 0, was reached by 36.4% of patients in the sulfonylurea group and 7.1% in the control group (p=0.007). In addition, a separate retrospective study examined the incidence of symptomatic hemorrhagic transformation in diabetic patients with ischemic stroke17. Compared to diabetic patients with non-sulfonylurea regimens, those with sulfonylurea exposure had significantly decreased mortality. After matching for baseline imbalances, there was decreased symptomatic hemorrhage (0% vs 11%, p=0.016) in patients taking sulfonylureas. These studies, which carefully adjusted for baseline variables, suggested that glyburide administration after ischemia results in improved outcomes.
Finally, the presence of relevant expression of SUR1 has been explored in brain autopsies of patients with ischemic stroke18. In a series of patients with focal infarct, up to 31 days after ischemic stroke, elevated levels of SUR1 were demonstrated in neurons, glial cells, and capillary endothelium.
Phase I and Phase IIa Study of RP-1127 (Glyburide for injection) in Human Stroke
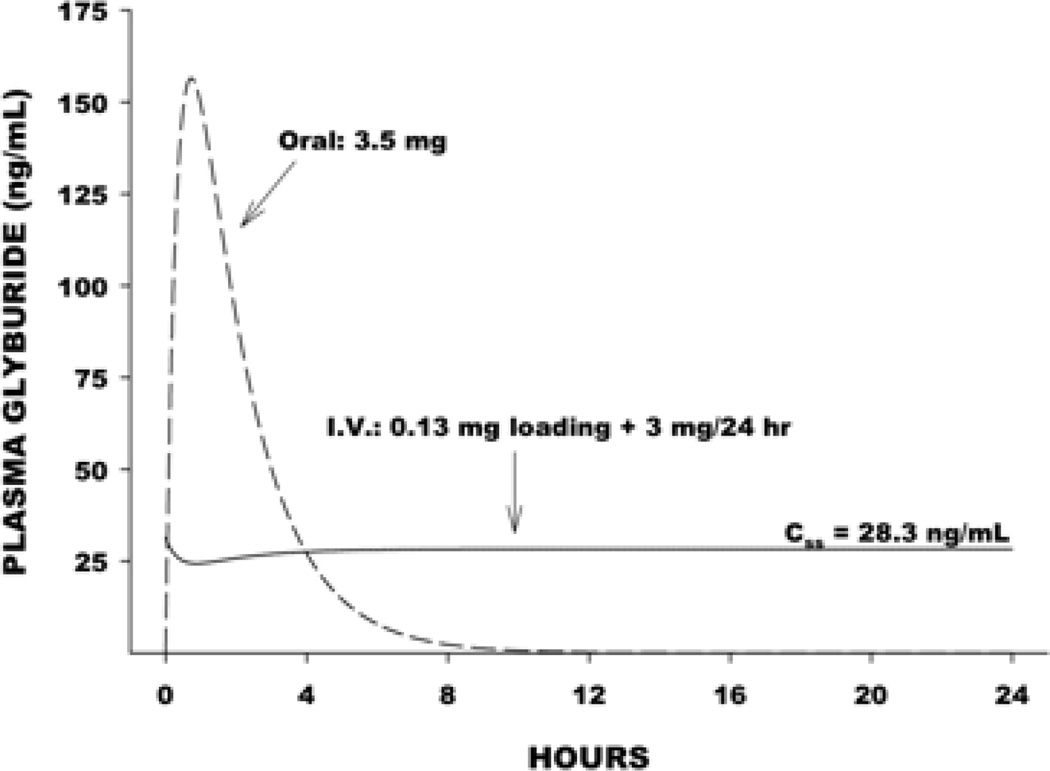
Oral glyburide has been used for decades in the treatment of diabetes mellitus. Because it was used for patients after consumption of a meal, the drug was developed so that plasma levels would have a rapid peak level, in order to lower post-prandial blood glucose, and then rapidly clear from the plasma (Figure 1). This is not the desired exposure after acute brain injury, where persistent channel blockade is the goal. Intravenous administration of the drug would allow for sustained, controlled plasma levels aimed at achieving a safe, durable drug effect. The need for achieving rapid steady state levels and maintaining receptor occupancy was demonstrated in preclinical models as well.
Figure 1.
Time course of plasma glyburide level following oral compared with intravenous bolus followed by 72 h infusion at 3 mg/day dose administration.
Using an IV formulation of glyburide (RP-1127), a phase I trial in health human volunteers (ClinicalTrials.gov identifier: NCT01132703) evaluated the safety, tolerability, and pharmacokinetics of RP-1127. A total of 34 patients were tested at the following doses: 0.4, 3.0 6.0, and 10.0 mg/day doses. Two patients (one each at 6 mg/day and 10 mg/day) discontinued the study because of persistent hypoglycemia. The incidence of blood glucose levels above 80 mg/dL was more frequent in the placebo group than in the 3 mg/day group, while there was no difference in the incidence of blood glucose levels below 70 mg/dL, implying a reduction in blood glucose at the 3 mg/day dose without hypoglycemia. There were no serious adverse events. Steady state plasma glyburide levels were determined to be 27.3 ng/mL. In preclinical experiments, a glyburide dosing regimen that was twice the effective dose average resulted in steady state plasma glyburide levels of 16 ng/mL. These observations suggest that the maximum tolerated dose of 3 mg/day dose results in human plasma levels that exceed the effective dose in rats.
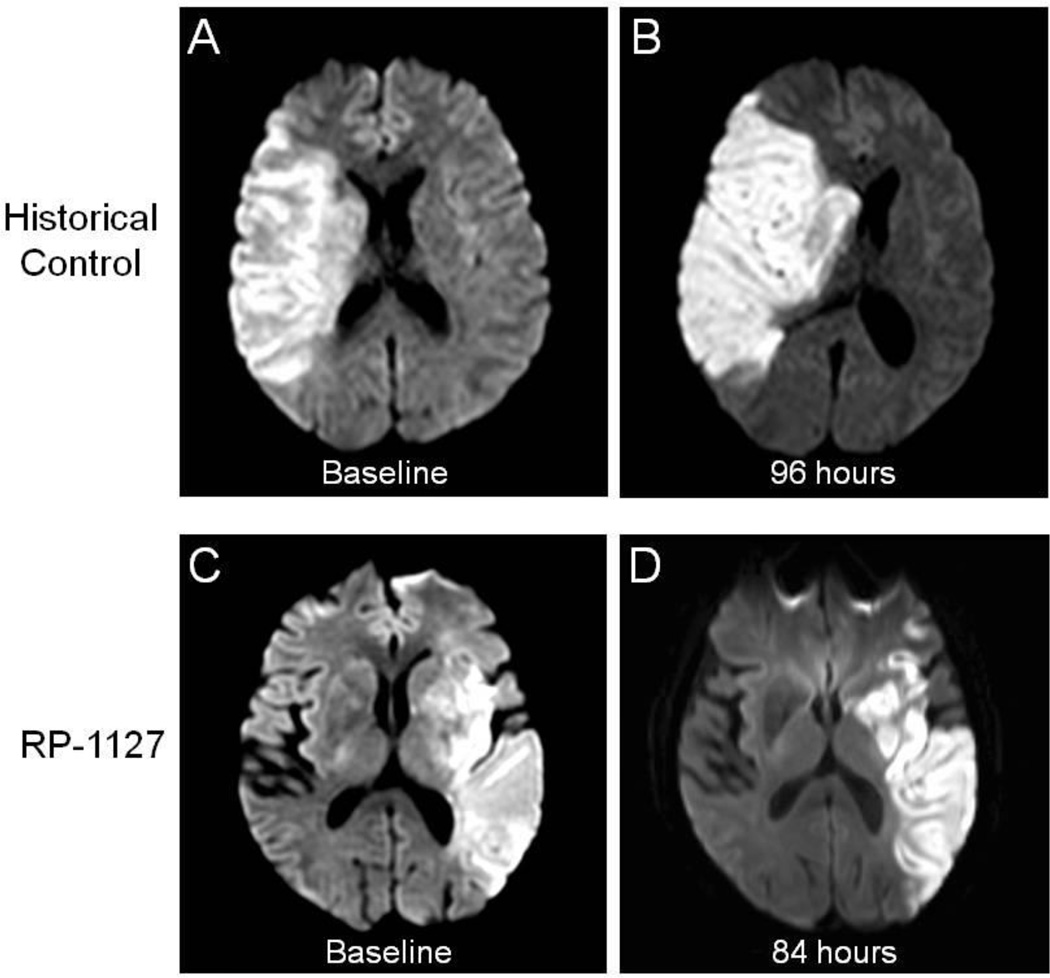
The Glyburide Advantage in Malignant Edema and Stroke (GAMES) Pilot study19,20 (ClinicalTrials.gov identifier: NCT01268683) evaluated the safety and feasibility of administering a 3 mg dose of RP-1127 in patients with severe stroke at high risk for swelling. The primary objective of this prospective, open-label, phase IIa study was to assess the feasibility of enrolling, evaluating, and treating with RP-1127 (bolus followed by 72 hour infusion at 3 mg/day dose) patients with severe ischemic stroke, whether or not they were treated with IV rtPA. Patients were enrolled at two centers, and the major inclusion criteria were as follows: clinical diagnosis of anterior circulation of ischemic stroke, baseline magnetic resonance imaging (MRI) diffusion weighted imaging (DWI) volume of 82–210 cm3, age 18–70 years of age, and start of drug infusion ≤ 10 hours from symptom onset. The central inclusion criteria is a DWI lesion volume ≥ 82 cm3, chosen because of the high specificity with which it predicts malignant edema21. Patients with endovascular treatment for stroke, pre-existing evidence of swelling, and recent use of sulfonylureas were excluded. In addition to the baseline MRI, patients underwent follow up MRI at 72 hours following drug infusion and clinical outcome assessment by modified Rankin Scale at 90 days. The mean age of enrolled patients was 51, the median baseline National Institutes of Health Stroke Scale (NIHSS) score was 18, and the mean baseline DWI lesion volume was 102 cm3. There were no serious adverse events related to the drug, including hypoglycemia. Figure 2 displays representative MRI brain images from a patient treated with RP-1127 (Panel A) and from an untreated historical patient (Panel B) at admission and 72–96 hours. The edema and mass effect expected at 72 hours are largely absent in the patient treated with RP-1127. Of the 10 patients, 1 patient had a neurological death, even after DC, and only 1 other patient required DC. Furthermore, 8/10 patients did not require any osmotherapy, intubation or DC. In addition, there were no PH1/PH2 hemorrhages, despite 9/10 patients receiving IV rtPA. Neurological outcome at three months, overall mortality, need for DC, and hemorrhagic transformation were all improved compared to historical, matched patients19,20. GAMES-Pilot was a non-randomized, unblinded study with a small sample size; however, safety at the target dose of 3 mg/day and preliminary signals of efficacy provided the motivation for a randomized, double-blind phase IIb study.
Figure 2.
Magnetic resonance diffusion weighted imaging at baseline (a, c) and at subacute follow up after stroke (b, d). The historical control (top panels) is from the EPITHET study. The bottom panels correspond to a GAMES-Pilot subject treated with RP-1127.
Effect of RP-1127 on Intermediate Markers of Swelling
Previously, reliable, validated neuroimaging measures of brain swelling after stroke had not been established. Since the treatment effect of glyburide results, in part, from decreased edema formation, the application of a neuroimaging marker of edema is critical to assessing biological effect of the drug.
Our group initially investigated MRI-based ipsilateral hemisphere volumetric determination in stroke patients22. Serial MRI studies from stroke patients in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET)23 with initial DWI lesion volume ≥ 82 cm3 were analyzed (baseline and day 3–5). The concordance correlation coefficient between readers was 0.90, and hemisphere volume correlated with early neurological deterioration (Area Under the Curve 0.83; p=0.04) Using this method, GAMES-Pilot subjects had an increase of 50 ± 33 cm3 compared to historical controls of 72 ± 27 cm3.
While serial change in hemisphere volume is a reasonable surrogate of swelling after ischemia, additional markers of cytotoxic and vasogenic injury can provide insight into the mechanism of action of RP-1127. MRI apparent diffusion coefficient (ADC) sequences vary depending on the restricted diffusion of water between the extracellular and intracellular compartments24. T2-based fluid attenuated inversion recovery sequences (FLAIR) may be sensitive to ischemic injury, which depends, in part, on the contribution of swelling from the vasculature25. In order to explore the potential effects of RP-1127 on these MRI signatures, serial MRI from GAMES-Pilot patients were compared with serial MRI from the control arm of the Normobaric Oxygen Therapy in Acute Ischemic Stroke Trial (NBO). Using a case-control design, ADC, FLAIR, and their respective signal intensity ratios (SIR) were obtained by normalizing the value within the stroke ROI to the contralateral hemisphere.
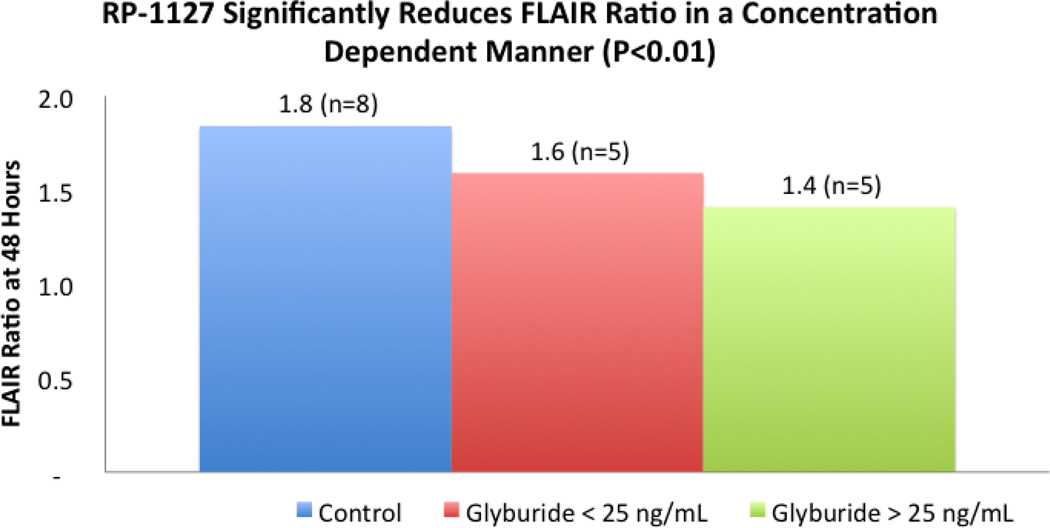
The baseline characteristics for both groups were the same except more NBO subjects were male and higher proportion of GAMES Pilot subjects underwent thrombolysis. There were no differences between ADC values or relative intensities in GAMES Pilot versus NBO subjects at baseline or day one. In contrast, the FLAIR SIR in the NBO cohort was significantly higher as early as 24 hours compared to GAMES Pilot subjects. The diminished increase in FLAIR SIR in RP-1127 treated GAMES Pilot subjects appeared to be a durable signal that lasted through the period of drug infusion, and there appeared to be a dose response relationship between the FLAIR ratio and RP-1127 level (p=0.014). When patients were dichotomized at a plasma RP-1127 concentration that was associated with mild glucose lowering in the phase I study (25 ng/mL), the FLAIR SIR was lower (p<0.01) in the group with high RP-1127 plasma levels (Figure 3).
Figure 3.
Dose dependent relationship between RP-1127 and FLAIR Ratio
A recent analysis of FLAIR SIR in a cohort of acute stroke subjects suggested that FLAIR SIR was associated with symptomatic hemorrhagic transformation, as well as matrix metalloproteinase-9 (MMP-9), an enzyme known to be associated with degradation of the blood-brain-barrier, edema, and hemorrhagic transformation26. Increased levels of MMP-9 have also been associated with the administration of IV rtPA27. When MMP-9 levels were compared between RP-1127 treated patients and control large-stroke patients, glyburide treatment was associated with lower levels of MMP-928. There were no significant differences in the baseline characteristics, including exposure to rtPA.
These results have several important implications for RP-1127 translation. First, there are several candidate MRI signatures that may be modified by glyburide administration. In addition, plasma levels of MMP-9 may also decrease in association to RP-1127 exposure. Further, these changes may have a dose-response relationship. The potential biological response of these markers to RP-1127 is even more important considering their prior, established association with brain swelling and hemorrhagic transformation after ischemia, each of which RP-1127 proposes to reduce. The relationship between MRI signatures, MMP-9 levels and RP-1127 will be further investigated in the ongoing GAMES-RP study; however, there is also an urgent need to further validate these relationships in independent populations of stroke patients, across a broad range of infarct volumes.
Phase II Randomized Trial
The robust preclinical and human data above directly led to the initiation of GAMES-RP (Clinicaltrials.gov identifier: NCT01794182), a randomized, multi-center prospective, double-blind phase IIb trial in patients with a severe anterior circulation ischemic stroke who are likely to develop malignant edema. The study population consists of subjects with a clinical diagnosis of acute stroke with a baseline DWI lesion volume of 82–300 cm3, age 18–80 years, and time of symptom onset to start of drug infusion of ≤ 10 hours. The primary objectives are to demonstrate the safety and efficacy of RP-1127 compared to placebo in patients with a severe anterior circulation ischemic stroke. The primary efficacy objective will be addressed by comparing the proportion of RP-1127 and placebo-treated patients with a day 90 modified Rankin Scale score ≤ 4 without decompressive craniectomy. The primary safety objective will be measured by comparing the frequency of adverse and severe adverse events in RP-1127 and placebo treated patients. Subjects are randomized to either RP-1127 or placebo delivered as an IV bolus followed by an IV infusion for 72 hours using the same dosing regimen as in GAMES-Pilot. In addition to a baseline MRI, subjects will undergo a 72–96 hour follow up MRI, and serial clinical assessments through one year, aimed at assessing functional outcome, mood, and quality of life. The primary efficacy endpoint is at 90 days. A multi-disciplinary team established clinical standardization guidelines, largely based on the American Heart Association guidelines for the management of patients with severe stroke3,29,30. This standardization of emergency and intensive care aims to make uniform practices surrounding sedation, use of osmotherapy and sodium management, fluid and insulin administration, and selection of patients for decompressive craniectomy. As of September 23, 2014, 52 subjects across 17 US hospitals have been enrolled, meeting the projected enrollment targets.
Conclusion
The discovery that SUR-TRPM4 channel may have a crucial role in the development of swelling and hemorrhagic transformation has led to an ongoing effort to translate these findings into an innovative therapy for patients with stroke. RP-1127 has been extensively studied in preclinical rodent models of stroke. Recently, several independent lines of inquiry in human stroke now suggest that RP-1127 is a safe, effective neurovascular protectant. Successful translation of this therapy may result in effective prevention of brain swelling and improvement in the recovery of stroke patients.
Bibliography
- 1.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. doi: 10.1161/STR.0b013e318284056a; 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol. 1996;53(4):309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 3.Wijdicks EF, Sheth KN, Carter BS, et al. Recommendations for the management of cerebral and cerebellar infarction with swelling: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45(4):1222–1238. doi: 10.1161/01.str.0000441965.15164.d6. [doi] [DOI] [PubMed] [Google Scholar]
- 4.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903. doi: 10.1056/NEJMoa1214300. doi: 10.1056/NEJMoa1214300; 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mlynash M, Lansberg MG, Straka M, et al. The malignant MRI profile: Implications for endovascular therapy. Stroke. 2012;43(A53) [Google Scholar]
- 6.Molina CA, Selim MH. Decompressive hemicraniectomy in elderly patients with malignant hemispheric infarction: Open questions remain beyond DESTINY. Stroke. 2011;42(3):847–848. doi: 10.1161/STROKEAHA.110.603613. [DOI] [PubMed] [Google Scholar]
- 7.Juttler E, Bosel J, Amiri H, et al. DESTINY II: DEcompressive surgery for the treatment of malignant INfarction of the middle cerebral arterY II. Int J Stroke. 2011;6(1):79–86. doi: 10.1111/j.1747-4949.2010.00544.x. doi: 10.1111/j.1747-4949.2010.00544.x; 10.1111/j.1747-4949.2010.00544.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheth KN. Novel approaches to the primary prevention of edema after ischemia. Stroke. 2013;44(6 Suppl 1):S136. doi: 10.1161/STROKEAHA.113.001821. doi: 10.1161/STROKEAHA.113.001821; 10.1161/STROKEAHA.113.001821. [DOI] [PubMed] [Google Scholar]
- 9.Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12(4):433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010;41(3):531–537. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simard JM, Sheth KN, Kimberly WT, et al. Glibenclamide in cerebral ischemia and stroke. Neurocrit Care. 2013 doi: 10.1007/s12028-013-9923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simard JM, Kent TA, Kunte H. Letter by simard et al regarding article, "sulfonylurea use before stroke does not influence outcome". Stroke. 2011 doi: 10.1161/STROKEAHA.111.620666. [DOI] [PubMed] [Google Scholar]
- 14.Favilla CG, Mullen MT, Ali M, Higgins P, Kasner SE. Virtual International Stroke Trials Archive (VISTA) Collaboration. Sulfonylurea use before stroke does not influence outcome. Stroke. 2011;42(3):710–715. doi: 10.1161/STROKEAHA.110.599274. [DOI] [PubMed] [Google Scholar]
- 15.Horsdal HT, Mehnert F, Rungby J, Johnsen SP. Type of preadmission antidiabetic treatment and outcome among patients with ischemic stroke: A nationwide follow-up study. J Stroke Cerebrovasc Dis. 2012;21(8):717–725. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.007. [doi] [DOI] [PubMed] [Google Scholar]
- 16.Kunte H, Schmidt S, Eliasziw M, et al. Sulfonylureas improve outcome in patients with type 2 diabetes and acute ischemic stroke. Stroke. 2007;38(9):2526–2530. doi: 10.1161/STROKEAHA.107.482216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunte H, Busch M, Trostdorf K, et al. Hemorrhagic transformation of ischemic stroke in diabeticson sulfonylureas. Ann Neurol. 2012;(72):799–806. doi: 10.1002/ana.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta RI, Ivanova S, Tosun C, Castellani RJ, Gerzanich V, Simard JM. Sulfonylurea receptor 1 expression in human cerebral infarcts. J Neuropathol Exp Neurol. 2013;72(9):871–883. doi: 10.1097/NEN.0b013e3182a32e40. doi: 10.1097/NEN.0b013e3182a32e40; 10.1097/NEN.0b013e3182a32e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheth KN, Taylor Kimberly W, Elm JJ, et al. Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocrit Care. 2014;21(1):43–51. doi: 10.1007/s12028-014-9970-2. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheth KN, Kimberly WT, Elm JJ, et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke. 2013 doi: 10.1161/STROKEAHA.113.003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomalla G, Hartmann F, Juettler E, et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann Neurol. 2010;68(4):435–445. doi: 10.1002/ana.22125. [DOI] [PubMed] [Google Scholar]
- 22.Yoo AJ, Sheth KN, Kimberly WT, et al. Validating imaging biomarkers of cerebral edema in patients with severe ischemic stroke. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (EPITHET): A placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 24.Todd NV, Picozzi P, Crockard A, Russell RW. Duration of ischemia influences the development and resolution of ischemic brain edema. Stroke. 1986;17(3):466–471. doi: 10.1161/01.str.17.3.466. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer PW. Diffusion-weighted imaging as a problem-solving tool in the evaluation of patients with acute strokelike syndromes. Top Magn Reson Imaging. 2000;11(5):300–309. doi: 10.1097/00002142-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Jha R, Battey TW, Pham L, et al. Fluid-attenuated inversion recovery hyperintensity correlates with matrix metalloproteinase-9 level and hemorrhagic transformation in acute ischemic stroke. Stroke. 2014;45(4):1040–1045. doi: 10.1161/STROKEAHA.113.004627. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castellanos M, Leira R, Serena J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34(1):40–46. [PubMed] [Google Scholar]
- 28.Kimberly WT, Battey TW, Pham L, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2013 doi: 10.1007/s12028-013-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimberly WT, Sheth KN. Approach to severe hemispheric stroke. Neurology. 2011;76(7 Suppl 2):S50–S56. doi: 10.1212/WNL.0b013e31820c35f4. [DOI] [PubMed] [Google Scholar]
- 30.Simard JM, Sahuquillo J, Sheth KN, Kahle KT, Walcott BP. Managing malignant cerebral infarction. Curr Treat Options Neurol. 2011;13(2):217–229. doi: 10.1007/s11940-010-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]