Abstract
Background
Endometriosis is a disabling disease of reproductive-age women. Dysmenorrhea, dyspareunia, and pelvic pain are the main symptoms of endometriosis. Its etiology is not clear. Endometriosis may have various causes, including vitamin D deficiency, but its effect is controversial.
Material/Methods
In this double-blind clinical trial, we enrolled patients with endometriosis diagnosed and treated by laparoscopy, with scores of at least 3 for of dysmenorrhea and/or pelvic pain at 8 weeks after surgical treatment. They were randomly prescribed vitamin D (50 000 IU weekly for 12 weeks) or placebo. Severity of pain in the 2 groups (placebo and treatment) was compared by VAS test at 24 weeks after surgical treatment.
Results
There were 19 patients in the vitamin D group and 20 in the placebo group. Baseline characteristics in the 2 groups were similar. Following the treatment with vitamin D or placebo, we did not find significant differences in severity of pelvic pain (p=0.24) and dysmenorrhea (p=0.45) between the 2 groups. Mean pelvic pain at 24 weeks after laparoscopy in the vitamin D group was 0.84±1.74 and in placebo group it was 0.68±1.70 (p=0.513). Mean dysmenorrhea was 2.10±2.33 in the vitamin D group and 2.73±2.84 in the placebo group (p=0.45).
Conclusions
After ablative surgery for endometriosis, vitamin D treatment did not have a significant effect in reducing dysmenorrhea and/or pelvic pain.
MeSH Keywords: Dysmenorrhea, Endometriosis, Laparoscopy, Vitamin D
Background
Endometriosis is defined as growth of endometrial glands and stroma outside the uterine cavity. It affects at least 10% of reproductive-age women [1,2]. Endometriosis is a prevalent cause of infertility, pelvic pain, dysmenorrhea, and dyspareunia in reproductive-age women. Its diagnosis is by inspection of the pelvis during laparoscopy [1]. In women with pelvic pain and infertility, the prevalence of endometriosis is as high as 90% [3,4]. Pain and infertility can greatly impair quality of life in affected women.
Endometriosis mimics some autoimmune and malignant diseases, including familial occurrence and immunological abnormalities in B and T cells, increased angiogenesis, invasion of endometrial cells to adjacent organs (e.g., bladder and bowels), and need for repeat surgeries due to recurrence [1,5–7]. Multiple mechanisms for etiology and improvement of endometriosis are suggested and the treatments are based on these unclear mechanisms. Therefore, progestin, GNRH agonists and antagonists [1], and drugs related to lipid metabolism (e.g., Simvastatin) are used for treatment [8,9]. It has been shown that inflammation is important in the pathogenesis of endometriosis [10], so endometriosis treatment should not be different from that of other inflammatory disorders [11]. There may be a correlation between vitamin D levels and the risk of polycystic ovarian disease, endometriosis, breast and ovarian cancer, increased arterial stiffness in older patients, and myasthenia gravis [12–18].
It has been found that vitamin D has a role in normal cellular growth regulation [19]. Vitamin D has immune regulatory effects in chronic inflammatory responses [20]. Vitamin D increases anti-inflammatory cytokines production and decreases pro-inflammatory cytokines [19–22]. Vitamin D induces apoptosis and suppression of angiogenesis in vitro and in vivo [25–28]. Although an indirect relationship between vitamin D and endometriosis has been reported in multiple studies, here has been no published randomized clinical trial on endometriosis and vitamin D treatment in women.
We explored the relationship between vitamin D and endometriosis in a double-blind, randomized clinical trial looking at the effect of vitamin D supplementation on cessation of pain in proven endometriosis after laparoscopic diagnosis and treatment.
Material and Methods
This randomized, double-blind clinical trial was performed in a single tertiary university hospital from Nov 2014 to Feb 2016.
To find patients with endometriosis, we did laparoscopy for various indications, including ovarian cyst, infertility, pelvic pain, and dysmenorrhea. Laparoscopy was performed under general anesthesia, using the triple-puncture technique. Using laparoscopy, the surgeons diagnosed patients with endometriosis and tried to excise or ablate all diseased tissue. The day before laparoscopy, a data collection form was completed by a physician, including the reason for laparoscopy, and the severity of pelvic pain and dysmenorrhea were estimated using a visual analogue scale test (VAS test), with a score of 0 being no pain and a score 10 being the worst pain ever experienced. The laparoscopies were done by 2 gynecologic laparoscopic surgeons; both were involved in each operation and they recorded the severity of endometriosis according to the revised American Society for Reproductive Medicine (ASRM) classification [1].
In the patients with endometriosis in the second menses after laparoscopic diagnosis and treatment, the VAS test was repeated. Patients with VAS scores of at least 3 for dysmenorrhea and/or pelvic pain were invited to participate in this clinical trial, and after consultation we asked them to sign the informed consent. The dyspareunia score was not a criterion for entering the study because some of the patients were not married and had not had intercourse.
Inclusion criteria
Women aged 15–40 years with proven endometriosis by laparoscopy and a VAS test score of 3 or more for dysmenorrhea and/or pelvic pain at second menses after operative laparoscopy.
Exclusion criteria
Patients with vitamin D treatment in the last 6 months prior to surgery;
Patients with known systemic diseases (e.g., hypertension, diabetes, coronary, renal, and hepatic diseases);
Patients with known malignancy;
Menopausal women;
Patients with hormonal treatment, including oral contraceptive pills, in the last 6 months.
After authorization by the university Ethics Committee, eligible patients were assigned by simple randomization to receive either vitamin D or placebo. In the vitamin D group (D group), we prescribed oral vitamin D 50 000 iu/weekly for 12 weeks (capsule D-Vigel, vitamin D3 50 000 iu, Daana Pharma Co. Tabriz-Iran) and in the placebo group (P group) we prescribed 1 capsule of placebo (Daana Pharma Co. Tabriz-Iran) weekly for 12 weeks. Four weeks after the end of the intervention (24 weeks after surgical treatment), the VAS test was repeated for the 2 groups.
Statistical analysis
We analyzed the data using SPSS 18. We used the KS test (one-sample Kolmogorov-Smirnov test) for normality of data distribution, Levine’s test for equality of variances and independent samples, and the t test for equality of means for comparing quantitative normal data between the 2 groups. We used the paired-samples t test for comparing quantitative normal data between before and after treatment in each group and the Pearson chi-square test for matching and comparing categorical variables between the 2 groups. Due to the small sample size, we conducted Mann-Whitney non-parametric analysis between the 2 groups and Wilcoxon signed ranks test for comparing before and after treatment data in each group.
This study was funded and supported by Iran University of Medical Sciences (IUMS), grant no. 93–02–140–24388, IRCT code IRCT2013021912151N3.
Results
We did 146 laparoscopies for different indications in gynecologic patients, and endometriosis was diagnosed in 75. At the second menses after diagnostic and therapeutic laparoscopy, 40 cases met the inclusion criteria for our study. One did not signed the informed consent. The remaining 39 cases were randomly assigned in vitamin D (n=19) or placebo treatment (n=20) groups. One patient in the placebo group became pregnant at the third month after the operation and discontinued placebo treatment. At the end of the study, we had 19 patients in each group (Figure 1).
Figure 1.
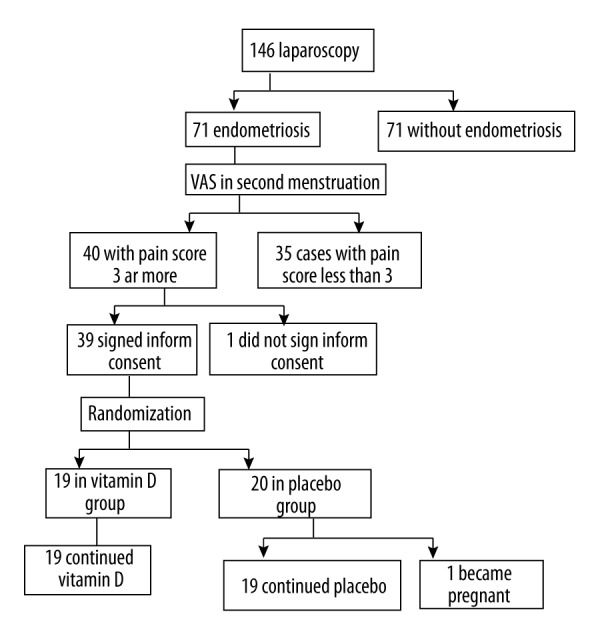
Flow diagram.
Table 1 shows a baseline comparison between the 2 study groups for general characteristics, reason for laparoscopy, severity of endometriosis, and severity of dysmenorrhea and pelvic pain.
Table 1.
Baseline comparison.
| Vitamin D group | Placebo group | Total | P value | ||
|---|---|---|---|---|---|
| Number of subjects | 19 | 19 | 38 | ||
| Mean age (SD) | 30.84 (5.79) | 28.95 (4.71) | 29.89 (5.30) | P=0.276 | |
| Marital status | P=0.179 | ||||
| Not married | 5 (26.32%) | 9 (47.37%) | 14 (37%) | ||
| Married | 14 (73.68%) | 10 (52.63%) | 24 (63%) | ||
| Total | 19 (100%) | 19 (100%) | 38 (100%) | ||
| Indications for laparoscopy, infertility | P=0.252 | ||||
| Absent | 13 (68.42%) | 16 (84.21%) | 29 (76%) | ||
| Present | 6 (31.58%) | 3 (15.79%) | 9 (24%) | ||
| Total | 19 (100%) | 19 (100%) | 38 (100%) | ||
| Indications for laparoscopy, pelvic pain | P=1 | ||||
| Absent | 10 (52.63%) | 9 (47.37%) | 19 (50%) | ||
| Present | 9 (47.37%) | 10 (52.63%) | 19 (50%) | ||
| Total | 19 (100%) | 19 (100%) | 38 (100%) | ||
| Indications for laparoscopy, dysmenorrhea | P=1 | ||||
| Absent | 5 (13.15%) | 5 (13.15%) | 10 (26.31%) | ||
| Present | 14 (36.84%) | 14 (36.84%) | 28 (73.68%) | ||
| Total | 19 (100%) | 19 (100%) | 38 (100%) | ||
| Indications for laparoscopy, ovarian cyst | P=0.5 | ||||
| Absent | 7 (18.42%) | 8 (21.05%) | 15 (39.47%) | ||
| Present | 12 (31.57%) | 11 (57.89%) | 23 (60.52%) | ||
| Total | 19 (100%) | 19 (100%) | 38 (100%) | ||
| Severity of endometriosis | P=0.626 | ||||
| Minimal | 0 (0.00%) | 1 (5.26%) | 1 (3%) | ||
| Mild | 1 (5.26%) | 1 (5.26%) | 2 (5%) | ||
| Moderate | 10 (52.63%) | 7 (36.84%) | 17 (45%) | ||
| Sever | 8 (42.11%) | 10 (52.63%) | 18 (47%) | ||
| Total | 19 (100%) | 19 (100%) | 38 (100%) | ||
| Pelvic pain before laparoscopy (median and interquartile range) | 5 (0–7) | 5 (0–9) | 5 (0–8) | P=0.513* | |
| Dysmenorrhea pain score before laparoscopy (median and interquartile range) | 8 (6–10) | 6 (5–10) | 14 (5–10) | P=0.325* | |
| Mean BMI | 22.46 | 23 | P=0.257 | ||
| Mean pelvic pain before laparoscopy (SD) | 4.05 (3.45) | 4.82 (4.1) | 4.45 (3.76) | P=0.513* | |
| Mean dysmenorrhea pain score before laparoscopy (SD) | 7.37 (2.61) | 6.42 (3.04) | 6.89 (2.84) | P=0.325* | |
Mann-Whitney U test.
The mean age of study participants was 29.89±5.30 years. Causes of laparoscopy in women with endometriosis diagnosed by laparoscopy were dysmenorrhea (n=28), ovarian cyst (n=23), chronic pelvic pain (n=19), and infertility (n=9). In some patients, there was more than 1 reason for laparoscopy. Severity of endometriosis in 45% was moderate (n=17) and 47% had severe endometriosis (n=18). Before laparoscopy, the mean pelvic pain score in the vitamin D group was 4.05±3.45 and 4.82±4.1(p=0.513) in the placebo group. Before laparoscopy, the mean dysmenorrhea pain score in the vitamin D group was 7.37±2.61 and in placebo group it was 6.42±3.04 (p=0.325).
Table 2 shows a comparison between the 2 groups for severity of pelvic pain and/or dysmenorrhea at different time points (before laparoscopy, in second menses after laparoscopy, and at 24 weeks after laparoscopy). At the second menses after laparoscopy, there was no significant difference between the 2 groups for pelvic pain (p=0.583) and dysmenorrhea (p=0.365), and at 24 weeks after laparoscopy there was no significant difference between mean pain scores in the 2 groups. Mean pelvic pain at 24 weeks after laparoscopy in the vitamin D group was 0.84±1.74 and in placebo group it was 0.68±1.70 (p=0.513). Mean dysmenorrhea was 2.10±2.33 in the vitamin D group and 2.73±2.84 in the placebo group (p=0.45).
Table 2.
Comparison between two groups for mean scores of severity of pain (VAS score).
| Vitamin D group | Placebo group | P value | ||
|---|---|---|---|---|
| Pain before laparoscopy | Mean pelvic pain (SD) | 4.05 (3.45) | 4.82 (4.1) | 0.513* |
| Dysmenorrhea pain (SD) Mean | 7.37 (2.61) | 6.42 (3.04) | 0.325* | |
| Pain at second menses | Mean pelvic pain (SD) | 1.53 (1.54) | 1.89 (2.40) | 0.583 |
| Dysmenorrhea pain (SD) Mean | 3.84 (2) | 4.42 (2.65) | 0.365 | |
| Pain after medical intervention (24 weeks after laparoscopy)) | Mean pelvic pain (SD) | 0.84 (1.74) | 0.68 (1.70) | 0.24 |
| Dysmenorrhea pain (SD) Mean | 2.10 (2.33) | 2.73 (2.84) | 0.45 | |
Mann-Whitney U test.
All of the patients had good cooperation for follow-up and continued their treatment until the end of the study.
Discussion
In this double-blind, randomized clinical trial, at 24 weeks after laparoscopic treatment of endometriosis there was no significant difference between effect of vitamin D3 (cholecalciferol) and placebo on severity of dysmenorrhea and/or pelvic pain.
This is the first clinical trial on women with endometriosis to explore the possible relationship between vitamin D treatment and relief of endometriosis-related pain. Our literature search found only studies that were indirectly related to vitamin D and endometriosis [27–43].
The etiology of endometriosis is poorly understood, and many etiologic factors have been suggested, including the serum level of 1, 25-dihydroxy vitamin D3. 1, 25-dihydroxy vitamin D3 is a fat-soluble vitamin with an unclear role in endometriosis. It is suggested that nuclear vitamin D receptors can have a regulatory role in inflammation and can be used as an index of cellular metabolic health [20]. A study on vitamin D receptor gene polymorphism in endometriosis compared 132 infertile women with endometriosis with 132 fertile women, reporting no significant difference and suggesting that vitamin D receptor gene polymorphism does not play an important role in the pathogenesis of endometriosis [29].
In some studies, higher plasma levels of 1, 25-dihydroxy vitamin D3 and higher intake of dairy foods was associated with lower risk of endometriosis [30,31]. Conversely, another study compared serum vitamin D levels of 87 women with endometriosis with 53 women without endometriosis; the mean serum levels of 1, 25-dihydroxy vitamin D3 in women with and without endometriosis were 24.9±14.8 ng/ml and 20.4±11.8, respectively (P=0.05) and the study concluded that endometriosis is associated with higher serum levels of vitamin D [32]. A systematic review of 10 case-control studies and 1 cohort study on women’s diet found that women with endometriosis had lower consumption of vegetables and omega-3, and reported a significant association between diet and endometriosis [33].
Vitamin D binding protein (DBP) is a plasma glycoprotein that modulates immune and inflammatory responses and also controls transport of vitamin D metabolites and bone development [34]. In a study comparing 13 ectopic endometrial tissues and 6 normal endometrial tissues, vitamin D binding protein was significantly higher in the ectopic endometrial tissues (P<0.05) [35]. A systematic review of research from 1946 to 2013 on vitamin D and endometriosis reported that women with endometriosis had higher serum levels of vitamin D binding protein [36]. Another study compared serum and peritoneal levels of DBP in 26 women with endometriosis and 17 women with other benign gynecological conditions and reported that women with endometriosis had higher serum levels of DBP than in the control group [37]. A study comparing urinary levels of DBP in 57 women with endometriosis with levels in 38 controls found that the urinary level of DBP was significantly higher in patients with endometriosis [38].
A study using a rat model of endometriosis reported that treatment with vitamin D3 produced fibrosis and apoptosis in the stroma of tissues with endometriosis [28]. A study on induced endometriosis in adult Balb female mice reported that administration of 100 μg/kg/day Elocalcitol (a vitamin D receptor agonist) for 3 weeks reduced total lesion weight [39].
Because a relationship between vitamin D and endometriosis has been suggested by multiple studies, and since there has been no randomized clinical trial on endometriosis and vitamin D treatment in women, we decided to explore this relationship. In the present study on endometriosis-related pain, we found no significant difference in results of vitamin D treatment vs. placebo at 24 weeks after surgical diagnosis and treatment.
Vitamin D is a fat-soluble vitamin mainly produced in the body from food and supplements and cutaneous sun exposure. Vitamin D deficiency is defined as serum 1.25-dihydroxy vitamin D3 levels under 20 ng/ml. Vitamin D deficiency is prevalent worldwide. In the USA, it is reported in 52% of black and Hispanic adolescents in Boston and in 48% of girls in Maine, and it is seen in 40–100% of elderly men and women in the USA and Europe [44]. A study in Germany found that 57% of people 18–79 years old were vitamin D deficient [45]. The prevalence of vitamin D deficiency was reported to be 90% in healthy subjects in Delhi, India [46]. In a systematic review of 195 studies in 44 countries found that 37.3% of studies found that the mean serum vitamin D levels were less than 20 ng/ml [44]. The prevalence in pregnant Turkish women was 81.4% [47]. In a study of high school students in Iran, the serum mean vitamin D level was 14.7±9.4 ng/ml [48]. In another study in university students in Shiraz, Iran, 51.2% of female students had low serum levels of vitamin D [49].
Because the incidence of vitamin D deficiency in Iran is high and we did not check the serum vitamin D levels in samples before intervention, it is possible that this dose and duration of vitamin D prescription was beneficial only for treatment of vitamin D deficiency and not endometriosis.
In 1072 women attending an infertility center, the prevalence of low serum vitamin D3 levels was 89%. Vitamin D3 levels were reported to be positively associated with height and endometriosis history [40].
An in vitro study compared the effect of vitamin D3 on 25 human endometriosis stromal cell cultures (ovarian endometrioma) with the effect of vitamin D3 on culture of 20 endometrial samples of non-endometriosis women; vitamin D3 inhibited proliferation, invasion, and pro-inflammatory cytokine production in endometriosis and reduced production of interleukin 6 and other inflammatory cytokines that stimulate adhesion of endometrial cells to the peritoneal cavity [41].
A study on in vitro effects of vitamin D3 on human endometriosis stromal cells found that vitamin D3 significantly reduced interleukin 1β and tumor necrotizing factor-α inflammatory responses, and also reported fewer endometrial stromal cells and reduced DNA synthesis. The study found significantly lower serum vitamin D3 levels in severe endometriosis compared to normal controls and patients with mild endometriosis [42].
A study in Italy investigated the effect of vitamin D on primary dysmenorrhea. The samples were 40 women aged 18–40 years old with 4 consecutive painful periods in the past 6 months. They measured the serum levels of 25 hydroxy vitamin D with high-performance liquid chromatography. Then the women were randomized into a group of 20 women who received a single oral dose of 300 000 IU vitamin D (cholecalciferol) at 5 days before their next menstrual cycle and another group of 20 women received placebo. There was a negative correlation between the baseline dysmenorrhea pain score and the level of 25 hydroxy vitamin D3 (r=0.36, p=0.2). The researchers found a significant reduction of dysmenorrhea pain in the vitamin D group in comparison with the placebo group in the next 2 menstruations (p<0.01). They suggested this significant reduction of dysmenorrhea pain in vitamin D prescription group was due to decreased levels of pro-inflammatory cytokines and decreased the biological activity of prostaglandins [43]. The samples were not assessed for existing endometriosis. Because low levels of serum vitamin D are common in healthy Italian pre-menopausal women [43], these results only show that vitamin D was effective in relieving dysmenorrhea at least for 2 months after treatment in women with or without endometriosis and also in women with or without vitamin D deficiency.
Conclusions
There may be a relationship between vitamin D and pathogenesis of endometriosis, but in our study vitamin D was not effective in treatment of endometriosis-related pain. Larger clinical trials are needed to determine the possible effects of vitamin D supplementation in endometriosis treatment.
Study limitations
The first limitation of this study is the high prevalence of vitamin D deficiency in our country and worldwide, meaning that a high percentage of our samples may have had vitamin D deficiency, which may have affected the results of our research. The second limitation is the small sample size of our study.
Further clinical trials are needed on the role of vitamin D treatment for endometriosis-related pain. Future studies should assess the serum levels of vitamin D before enrolling study subjects, and those with vitamin D deficiency should be excluded. Clinical trials with larger sample sizes will be able to produce more reliable results.
Footnotes
Source of support: Departmental sources
References
- 1.D’Hooghe TM. Endometriosis. In: Berek JS, editor. Berek & Novak’s gynecology. 15th ed. Lippincott Williams & Wlilkins; 2012. pp. 505–57. [Google Scholar]
- 2.Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: Epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaeco. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Koninckx PR, Meuleman C, Demeyere S, et al. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Sterill. 1991;55:759–65. doi: 10.1016/s0015-0282(16)54244-7. [DOI] [PubMed] [Google Scholar]
- 4.Meuleman C, Vandenabeele B, Fieuws S, et al. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steri. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 5.Esfandiari N, Khazaei M, Ai J, et al. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2007;87(2):257–62. doi: 10.1016/j.fertnstert.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 6.Varma R, Rollason T, Gupta JK, Maher ER. Endometriosis and the neoplastic process. Reproduction. 2004;127(3):293–304. doi: 10.1530/rep.1.00020. [DOI] [PubMed] [Google Scholar]
- 7.Agic A, Xu H, Altgassen C, et al. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci. 2007;14(5):486–97. doi: 10.1177/1933719107304565. [DOI] [PubMed] [Google Scholar]
- 8.Almassinokiani F, Mehdizadeh A, Sariri E, et al. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit. 2013;19:534–39. doi: 10.12659/MSM.883967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kargar M, Hadjibabaie M, Gholami K. Simvastatin versus triptorelin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit. 2013;19:858. doi: 10.12659/MSM.889682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olovsson M. Immunological aspects of endometriosis: An update. Am J Reprod Immunol. 2011;66(Suppl 1):101–4. doi: 10.1111/j.1600-0897.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- 11.Taylor RN, Hummelshoj L, Stratton P, Vercellini P. Pain and endometriosis: Etiology, impact, and therapeutics. Middle East Fertil Soc J. 2012;17(4):221–25. doi: 10.1016/j.mefs.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerchbaum E, Rabe T. Vitamin D and female fertility. Curr Opin Obstet Gynecol. 2014;26(3):145–50. doi: 10.1097/GCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 13.Colonese F, Laganà AS, Colonese E, et al. The pleiotropic effects of vitamin D in gynaecological and obstetric diseases: An overview on a hot topic. Biomed Res Int. 2015;2015:986281. doi: 10.1155/2015/986281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahrokhi SZ, Ghaffari F, Kazerouni F. Role of vitamin D in female reproduction. Clin Chim Acta. 2016;455:33–38. doi: 10.1016/j.cca.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Triunfo S, Lanzone A. Potential impact of maternal vitamin D status on obstetric well-being. J Endocrinol Invest. 2016;39(1):37–44. doi: 10.1007/s40618-015-0330-7. [DOI] [PubMed] [Google Scholar]
- 16.Nandi A, Sinha N, Ong E, et al. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Investig. 2016;25(1):15–28. doi: 10.1515/hmbci-2015-0051. [DOI] [PubMed] [Google Scholar]
- 17.Chang J, Ye XG, Hou YP, et al. Vitamin D level is associated with increased left ventricular mass and arterial stiffness in older patients with impaired renal function. Med Sci Monit. 2015;21:3993–99. doi: 10.12659/MSM.896559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadegiani FA. Remission of severe myasthenia gravis after massive-dose vitamin D treatment. Am J Case Rep. 2016;17:51–54. doi: 10.12659/AJCR.894849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger U, Wilson P, McClelland RA, et al. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J Clin Endocrinol Metab. 1988;67(3):607–13. doi: 10.1210/jcem-67-3-607. [DOI] [PubMed] [Google Scholar]
- 20.Calton EK, Keane KN, Soares MJ. The potential regulatory role of vitamin D in the bioenergetics of inflammation. Curr Opin Clin Nutr Metab Care. 2015;18(4):367–73. doi: 10.1097/MCO.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 21.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89(5):922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 22.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Nonn L, Peng L, Feldman D, Peehl DM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. 2006;66(8):4516–24. doi: 10.1158/0008-5472.CAN-05-3796. [DOI] [PubMed] [Google Scholar]
- 24.Moreno J, Krishnan AV, Swami S, et al. Regulation of prostaglandin metabolism by calcitriol attenuates growth stimulation in prostate cancer cells. Cancer Res. 2005;65(17):7917–25. doi: 10.1158/0008-5472.CAN-05-1435. [DOI] [PubMed] [Google Scholar]
- 25.Trump DL, Hershberger PA, Bernardi RJ, et al. Anti-tumor activity of calcitriol: Pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004;89–90(1–5):519–26. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Yu WD, Trump DL, Johnson CS. 1,25D3 enhances antitumor activity of gemcitabine and cisplatin in human bladder cancer models. Cancer. 2010;116(13):3294–303. doi: 10.1002/cncr.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J, Li W, Ma J, et al. Vitamin D – pivotal nutraceutical in the regulation of cancer metastasis and angiogenesis. Curr Med Chem. 2013;20(33):4109–20. doi: 10.2174/09298673113209990194. [DOI] [PubMed] [Google Scholar]
- 28.Abbas MA, Taha MO, Disi AM, Shomaf M. Regression of endometrial implants treated with vitamin D3 in a rat model of endometriosis. Eur J Pharmacol. 2013;715(1–3):72–75. doi: 10.1016/j.ejphar.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Vilarino FL, Bianco B, Lerner TG, et al. Analysis of vitamin D receptor gene polymorphisms in women with and without endometriosis. Hum Immunol. 2011;72(4):359–63. doi: 10.1016/j.humimm.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Harris HR, Chavarro JE, Malspeis S, et al. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: A prospective cohort study. Am J Epidemiol. 2013;177(5):420–30. doi: 10.1093/aje/kws247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mesrine S, Clavel-Chapelon F, Boutron-Ruault MC. Re: “Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: A prospective cohort study”. Am J Epidemiol. 2013;178(4):664–65. doi: 10.1093/aje/kwt150. [DOI] [PubMed] [Google Scholar]
- 32.Somigliana E, Panina-Bordignon P, Murone S, et al. Vitamin D reserve is higher in women with endometriosis. Hum Reprod. 2007;22(8):2273–78. doi: 10.1093/humrep/dem142. [DOI] [PubMed] [Google Scholar]
- 33.Parazzini F, Viganò P, Candiani M, Fedele L. Diet and endometriosis risk: A literature review. Reprod Biomed Online. 2013;26(4):323–36. doi: 10.1016/j.rbmo.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Speeckaert MM, Speeckaert R, van Geel N, Delanghe JR. Vitamin D binding protein: A multifunctional protein of clinical importance. Adv Clin Chem. 2014;63:1–57. doi: 10.1016/b978-0-12-800094-6.00001-7. [DOI] [PubMed] [Google Scholar]
- 35.Hwang JH, Wang T, Lee KS, et al. Vitamin D binding protein plays an important role in the progression of endometriosis. Int J Mol Med. 2013;32(6):1394–400. doi: 10.3892/ijmm.2013.1506. [DOI] [PubMed] [Google Scholar]
- 36.Sayegh L, Fuleihan Gel-H, Nassar AH. Vitamin D in endometriosis: A causative or confounding factor? Metabolism. 2014;63(1):32–41. doi: 10.1016/j.metabol.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Borkowski J, Gmyrek GB, Madej JP, et al. Serum and peritoneal evaluation of vitamin D-binding protein in women with endometriosis. Postepy Hig Med Dosw (Online) 2008;62:103–9. [PubMed] [Google Scholar]
- 38.Cho S, Choi YS, Yim SY, et al. Urinary vitamin D-binding protein is elevated in patients with endometriosis. Hum Reprod. 2012;27(2):515–22. doi: 10.1093/humrep/der345. [DOI] [PubMed] [Google Scholar]
- 39.Mariani M, Viganò P, Gentilini D, et al. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum Reprod. 2012;27(7):2010–19. doi: 10.1093/humrep/des150. [DOI] [PubMed] [Google Scholar]
- 40.Pagliardini L, Vigano’ P, Molgora M, et al. High prevalence of vitamin D deficiency in infertile women referring for assisted reproduction. Nutrients. 2015;7(12):9972–84. doi: 10.3390/nu7125516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delbandi AA, Mahmoudi M, Shervin A, Zarnani AH. 1,25-dihydroxy vitamin D3 modulates endometriosis-related features of human endometriotic stromal cells. Am J Reprod Immunol. 2016;75(4):461–73. doi: 10.1111/aji.12463. [DOI] [PubMed] [Google Scholar]
- 42.Miyashita M, Koga K, Izumi G, et al. Effects of 1,25-dihydroxy vitamin D3 on endometriosis. J Clin Endocrinol Metab. 2016;101(6):2371–79. doi: 10.1210/jc.2016-1515. [DOI] [PubMed] [Google Scholar]
- 43.Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: Results of a randomized, double-blind, placebo-controlled study. Arch Intern Med. 2012;172(4):366–67. doi: 10.1001/archinternmed.2011.715. [DOI] [PubMed] [Google Scholar]
- 44.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 45.Hintzpeter B, Mensink GB, Thierfelder W, et al. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62(9):1079–89. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 46.Goswami R, Mishra SK, Kochupillai N. Prevalence & potential significance of vitamin D deficiency in Asian Indians. Indian J Med Res. 2008;127(3):229–38. [PubMed] [Google Scholar]
- 47.Ergür AT, Berberoğlu M, Atasay B, et al. Vitamin D deficiency in Turkish mothers and their neonates and in women of reproductive age. J Clin Res Pediatr Endocrinol. 2009;1(6):266–69. doi: 10.4274/jcrpe.v1i6.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebrahimi M, Khashayar P, Keshtkar A, et al. Prevalence of vitamin D deficiency among Iranian adolescents. J Pediatr Endocrinol Metab. 2014;27(7–8):595–602. doi: 10.1515/jpem-2013-0428. [DOI] [PubMed] [Google Scholar]
- 49.Faghih S, Abdolahzadeh M, Mohammadi M, Hasanzadeh J. Prevalence of vitamin d deficiency and its related factors among university students in Shiraz, Iran. Int J Prev Med. 2014;5(6):796–99. [PMC free article] [PubMed] [Google Scholar]


