Abstract
Background
The aim of this study was to explore the regulating effects of Substance P (SP) on the collagen synthesis of rat myocardial fibroblasts (CFBs) induced by angiotensin II (Ang II) and its potential mechanism.
Material/Methods
The CFBs of a neonatal SD rat were separately cultured and divided into the control group, Ang II treatment group, and treatment groups with different concentrations of SP, Ang II +; each group was given corresponding treatment respectively.
Results
Ang II successfully induced the collagen synthesis of CFBs. Compared with the control group, the phosphorylation levels of TGF-β, erk, and smad2/3 were higher (p<0.05). Different concentrations of SP had an effect on Ang II-induced CFBs, reduced the collagen synthesis of CFBs, and increased the expressions of SP receptors, accompanied by lowering TGF-β protein, erk protein phosphorylation level, and smad2/3 protein phosphorylation level (p<0.05). Moreover, the higher the concentrations of SP, the more obvious of an effect it exerted. Treating the Ang II + SP group with aprepitant reduced the inhibiting effects of SP on collagen synthesis. The expression changes of collagen I and collagen III detected by immunocytochemistry were exactly in accordance with the results of qPCR and Western blotting.
Conclusions
SP can inhibit collagen synthesis of CFBs after Ang II inducing which may adjust the downstream signaling pathways associated protein including TGF-β, erk and smad2/3. SP can block the progress of myocardial fibrosis and is dose dependent, which is expected to be a promising target for the treatment of myocardial fibrosis.
MeSH Keywords: Collagen Type I, Collagen Type III, Substance P
Background
Myocardial fibrosis refers to the decline of myocardial metabolism, conduction, and function caused by the metabolic disorder of extracellular matrix, the excessive deposition of collagen and other factors, and the increasing myocardial stiffness and myocardial remodeling resulting from abnormal proliferation of myocardial fibroblasts under the effects of various pathogenic factors. The occurrence of many kinds of cardiac diseases such as cardiac failure, myocardial infarction, and other heart diseases are said to be associated with myocardial fibrosis. Moreover, the number of such diseases is on the rise year by year [1]. As a member of the neurotransmitter tachykinin family, substance P (SP) is widely distributed in the nervous system and peripheral organs and tissues and is involved in pain reaction, immune regulation, inflammatory reaction, and wound healing [2–5]. SP can effectively combat the elevation of blood pressure caused by norepinephrine and angiotensin II (Ang II) and also can lower blood pressure, relax blood vessels, and increase blood flow volume in a short time [6]. Studies have indicated that SP could inhibit the damaging effect of norepinephrine on myocardial cells in acute myocardial ischemia reperfusion, while its effect on myocardial remodeling and its role in the process of fibrosis are not entirely clear [7]. This experiment studied the effects on cardiac fibroblasts (CFBs), from a neonatal SD rat, that were induced by Ang II and the studied the potential mechanism to reveal the role of SP in myocardial fibrosis.
Material and Methods
Materials and reagents
Materials and reagents included: 1–3 day old neonatal SD rats (Vital River Laboratory Animal Co., Ltd.); DMEM culture medium (America Gibco); trypsin (America Sigma); Fetal bovine serum (America Gibco); Ang II (America Sigma); poly-L-lysine coated cover slip (America Corning); rabbit anti-rat vimentin monoclonal antibody (Wuhan Boster); rabbit anti-rat vascular smooth muscle protein monoclonal antibody (Wuhan Boster); goat anti-rabbit second antibody (Wuhan Boster); DAB chromogenic reagent kit (Zhongshan Jinqiao); Trizol reagent (America Invitrion); Rll purchased from Santa Cruz Company; horseradish peroxidase labelled rabbit anti-rat second reverse transcriptase reagent kit (Nanjing Vazyme); protein lysis solution (Changsha Beyotime); fluorescent quantitative PCR mix (Japan ToYoBo); primer synthesis (Shanghai Biological Engineering Co., Ltd.); rabbit anti-rat collagen I, collagen III, SP receptor, TGF-β, erkprotein phosphorylation, smad2/3 protein phosphorylation, and the monoclonal antibody of internal reference protein GAPDH were all purchased from the Santa Cruz Company; horseradish peroxidase-labeled rabbit anti-rat secondary antibody (Wuhan Boster); FITC labelled rabbit anti-rat second antibody (Wuhan Boster).
CFBs isolated culture
A 1–3 day neonatal SD rat was sacrificed and its chest skin sterilized with 75% ethyl alcohol. The chest was aseptically opened in on ultra-clean work platform and its cardiac ventricle removed and rinsed with PBS solution to remove the red blood cells. The tissue was cut into pieces, trypsinized with 0.25% trypsin several times, the extract centrifuged and the supernatant removed after centrifugalization. The centrifuged supernatant was filtered using a 70 μm cell strainer and cells were placed into DMEM medium with 20% fetal bovine serum. Cells were then incubated at 37°C and 5% of CO2. The myocardial cells were removed by differential adhesion for 60 minutes to 90 minutes. Afterwards the cells were cultured until the confluence reached 85% to 90%, and then subcultured at the ratio of 1:2.
CFBs identification
Immunohistochemical method was used to determine the expressions of vimentin and vascular smooth muscle actin in separately cultured cells. The status and shapes of the adherent cells was observed.
The CFBs suspension was evenly inoculated into a 6-well-plate covered by poly-L-lysine coated cover slips, and incubated at 37°C and 5% of CO2 to make the cell slides. After the cells were fixed by 4% of paraformaldehyde, 5% of bovine serum albumin (BSA) was added and cells were incubated for 20 minutes at room temperature. Diluted primary antibody working solution (mice/rabbit anti-rat vimentin and vascular smooth muscle actin) was added. PBS and BSA were used to make blank and negative control slides at the same time. After which all slides were incubated at 37°C for one hour and rinsed with PBS three times, two minutes per rinse. Diluted biotinylated goat anti-rabbit/rat secondary antibody IgG was added. Then slides were incubated in a wet box at 37°C for 30 minutes and rinsed with PBS four times, 5 minutes per rinse. Water was removed from the slides, DAB coloration was added, and the color effects were observed. The coloration was terminated by running water, and hematoxylin was added for 30 seconds. The positive staining indicated fibroblasts.
Cell treatment
The cells were divide into six groups, including the control group, Ang II (1 μM) treatment group, Ang II + different concentrations of SP (0.1 μM, 1 μM, 10 μM) treatment groups, and Ang II + SP (10 μM) + aprepitant (1 nM) treatment group. Aprepitant is known to antagonize the effect of SP by combining with the NK-1 receptors (SP receptors) of the central and peripheral nervous system. Thus this could be used to verify the effects of SP. After treating with trypsin, the cell concentration was adjusted to 1~2×105/mL, 2 mL/well, and then cultured overnight for 24 hours. The cells were then cultured in serum-free DMEM for 24 hours. Each group was delivered drugs for three repetitions according to the group’s protocol. The cells were then cultured for another 24 hours for subsequent experiments.
RT-PCR detection
The cells in each group were drug-treated until the cell confluence reaching 80% to 90%. Then, the culture medium in the wells was discarded and the wells were washed with PBS three times. Each well had added 1 mL Trizol reagent to extract the total RNA from the cells. An ultraviolet spectrophotometer was used to measure the concentration and purity of RNA. Then 1% agarose gel electrophoresis was used to measure the integrity of the RNA. Reverse transcribe 1 μg of RNA into cDNA using M-MLV reverse transcriptase and 20 μL system according to the instruction provided by Reverse Transcription Kit. Fluorescent quantitative PCR was employed to detected the response of the newly reverse-transcribed cDNA, with the total reaction system of 10 μL, of which the cDNA template accounted for 1 μL while the upstream and downstream accounted for 0.25 μL, respectively. The reaction conditions were as follows: pre-degeneration at 95°C for 2 minutes, degeneration at 95°C for 30 seconds, annealing at 60°C for 15 seconds, extension at 72°C for 15 seconds, for 40 cycles, then 2% agarose gel electrophoresis was used to detect specificity. Taking GAPDH as the internal reference gene, the relative expressions of the genes were calculated. The upstream and downstream primer sequences of collagen I, collagen III, SP receptor, TGF-β, erk, smad2/3, and internal reference gene GAPD are as shown in Table 1.
Table 1.
Primer sequences of qPCR’ genes.
| Genes | Forward primer | Reverse primer | Length |
|---|---|---|---|
| Collagen I | 5′ ACCTGCTCTGTGCGATTTGA3′ | 5′GGAATTCCGGGGTATCCGTC3′ | 211 bp |
| CollagenIII | 5′CCTTCGACTTCTCTCCAGCC3′ | 5′TTTCGTGCAACCATCCTCCA3′ | 192 bp |
| SP receptor | 5′CTAACACCTCGGAACCCAATC3′ | 5′CCACAATGACCGTGTAGGCAG3′ | 81 bp |
| TGF-β | 5′GGCCAGATCCTGTCCAAGC3′ | 5′GTGGGTTTCCACCATTAGCAC3′ | 201 bp |
| GAPDH | 5′GGAGCGAGATCCCTCCAAAAT3′ | 5′GGCTGTTGTCATACTTCTCATGG3′ | 197 bp |
Western blotting
The cells in each group were drug-treated until the cell confluence reaching 80% to 90%. Then the culture medium in the wells was discarded and the wells washed with PBS three times. Each well was given 1 mL RIPA buffer for cell lysis. The cells were scraped off by a cell scraper and centrifuged at 4°C, at 12,000 rev/minute for 15 minutes. The supernatant was removed and measured by BCA method for supernatant protein concentration. Then 1 μg of protein was used for SDS-PAGE gel electrophoresis to separate the protein, which was transferred onto a nitrocellulose membrane. The PVDF membrane was soaked into 5% skim milk powder (containing PBS with 0.05% Tween-20) and sealed at 37°C for 2 hours. Then diluted primary antibodies of mice/rabbit anti-rat specificity, including collagen I, collagen III, SP receptor, TGF-β, erk protein phosphorylation, smad2/3 protein phosphorylation, and monoclonal antibodies of internal reference protein GAPDH, were applied to the membrane and incubated overnight on a shaker. The membrane was rinsed with TPBS three times followed by the addition of the secondary antibody consisting of diluted horseradish peroxidase labeled anti-rabbit IgG, incubated at 37°C for two hours. The membrane was then rinsed with TPBS three times and then rinsed with TPBS free of Tween-20 once. Chemiluminescence was used for color development, with GAPDH used as an internal reference, and x-ray photographic images were developed. Kodak Digital Science 1D210 image analysis software was used for a semi-quantitative analysis of the seven proteins.
Immunocytochemical staining
The CFBs suspension was evenly inoculated into a 6-well-plate covered by poly-L-lysine coated cover slips and then cultured in the incubator at 37°C and 5% CO2 to produce cell slides. The cell slides were rinsed three times and fixed in 1% Triton X-100 for 5 minutes, and then soaked in 1% BSA for 20 minutes. A 1:100 dilution in 1× PBS of monoclonal antibodies of mouse-anti-human collagen I and collagen III was added and incubated overnight at 4°C. The slides were then rinsed with 1×PBS solution three times, 5 minutes each rinse. Then a 1:100 dilution in 1× PBS of FITC labelled rabbit anti-rat secondary antibody was added, slides coverslipped, incubated at room temperate for one hour, and then rinse with PBS three times, five minutes each rinse. Slides were observed under fluorescence microscope after mounting them with fluorescence mounting reagent.
Statistical analysis
All data were analyzed by using SPSS 17.0 statistical software. All numeric variable information and data were expressed in mean ± standard deviation (x±s); t-test was used to make comparison of the mean between two groups. One-way ANOVA variance analysis was used to make comparison of difference significance among multiple groups while SNK-q was used for the test between groups. Each experiment was repeated for three times. When p<0.05, it was considered to be statistically significant.
Results
CFBs isolated culture and immunofluorescence staining
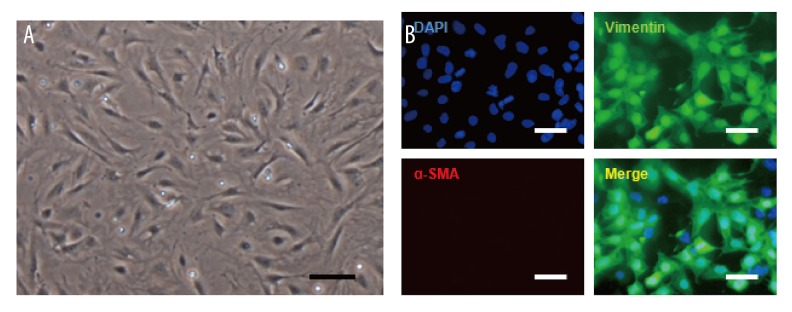
Under an inverted microscope, the cell density reached 60% to 70% after two or three days. CFBs adherence presented irregular shapes, most of which were polygonal or spindle-shaped. The cytoplasm was transparent and oval with a large nucleus. Through immunofluorescence staining, vimentin staining was found positive while the vascular smooth muscle protein staining was negative, which confirmed the staining characteristics of CFBs (Figure 1).
Figure 1.
Identification of CFBs in vitro. (A) Observation of cell state and morphology in inverted microscope. (B) Immunofluorescence staining detected vimentin (green) and vascular smooth muscle actin (red) in isolation and culture CFBs. Scale bar=100 μm.
The effect of SP on expression changes of CFBs collagen induced by AngII
Fluorescent quantitative PCR (qPCR) test indicated that the mRNA expression of cells collagen I and collagen III of Ang II treatment group was significantly higher compared with the control group (p<0.01). Compared with Ang II treatment group, the mRNA expressions of collagen I and collagen III in the three groups of different concentrations of Ang II+SP showed a dramatic decline. Higher SP concentration meant stronger inhibiting effects on the mRNA expressions of collagen I and collagen III (p<0.01). However, the expressions of collagen I and collagen III of Ang II + SP (10 μM) + aprepitant (1 nM) treatment group obviously recovered (p<0.01) compared with Ang II +SP (10 μM) treatment group (Figure 2A, 2B).
Figure 2.
The expression of collagen I, collagen III in each treatment group. qRT-PCR analyzed mRNA levels of collagen I (A) and collagen III (B) in each treatment group. (C) The expression of collagen I and collagen III was detected by Western blotting. (D) Complication of immunoblot analysis results of collagen I. (E) Complication of immunoblot analysis results of collagen III. n=3, * p<0.05, *** p<0.01.
The results of Western blotting were consistent with those of qPCR. At the protein level, in the Ang II treatment group, the expressions of collagen I and collagen III was significantly higher than the control group (p<0.01), which indicated that Ang II successfully induced the expressions of CFBs collagen. The expression of collagen I and collagen III in the three groups of different concentrations of Ang II+SP was significantly inhibited compared with the Ang II treatment group. Higher SP concentration means stronger inhibiting effects. However, aprepitant (1 nM) could recover as shown in that the expression of collagen I and collagen III induced by SP was lowered. In AngII +SP (10 μM) + aprepitant (1 nM) treatment group, the difference between collagen I, collagen III, and Ang II +SP (10 μM) showed statistical significance (p<0.01), which was consistent with that of qPCR (Figure 2C–2E).
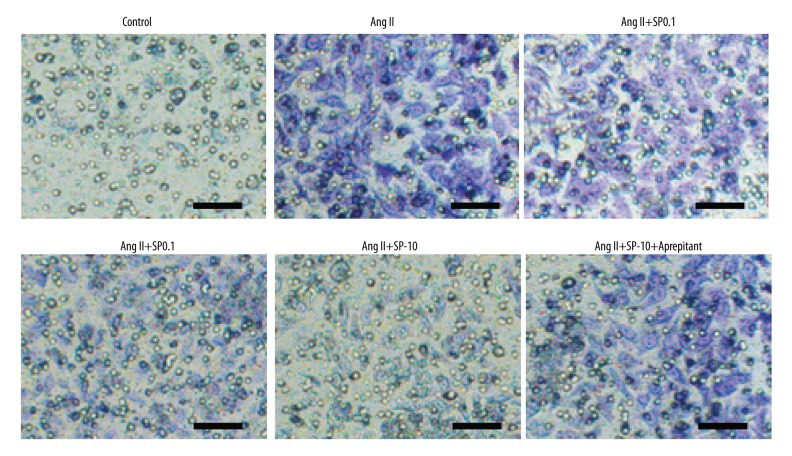
In addition, immunocytochemical staining was used to detect intracellular expressions in situ of collagen I. We found that collagen I staining in the Ang II treatment group was significantly deeper than in the control group and the green fluorescent intensity of FITC in the nucleus of Ang II treatment group. The effects of different concentrations of SP, its staining showed it was gradually weakened. However, collagen I staining was significantly recovered after adding in aprepitant (1 nM). This was in accordance with the aforementioned results that different concentrations of SP could inhibit mRNA and protein expressions of collagen I and collagen III, which further proved that the expression changes of cell collagen were induced by SP (Figure 3).
Figure 3.
Immunocytochemical staining detected intracellular expressions in situ of collagen I in each treatment group. Scale bar=100 μm.
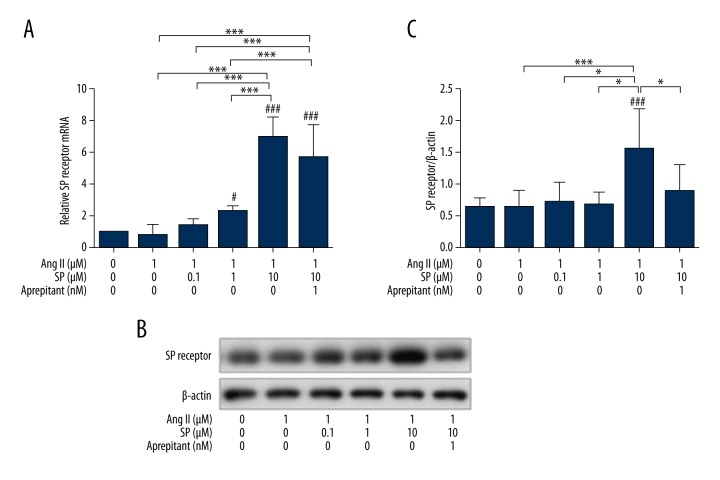
The upregulation of SP receptor expressions induced by high concentrations of SP
The qPCR showed no significant difference in the mRNA expression of SP receptor between the Ang II treatment group, the low and middle concentrations of Ang II + SP treatment group, and the control group. However, in the Ang II + SP-10 μM treatment group, SP receptor expression was significantly higher (p<0.01), and the addition of aprepitant had little effect on the induction (Figure 4A). Western blot analysis showed a similar result (Figure 4B). Thus, this shows that Ang II had no impact on SP receptor expression. However, the high concentrations of SP stimulated the expression of SP receptor significantly.
Figure 4.
The expression of SP receptor in each treatment group. (A) qRT-PCR analysis relative mRNA levels of SP receptor in each treatment group. (B) The expression of SP receptor was detected by Western blotting. (C) Complication and analysis of immunoblot results of SP receptor in each group, date were normalized to a housekeeping gene index. n=3, * p<0.05, *** p<0.01.
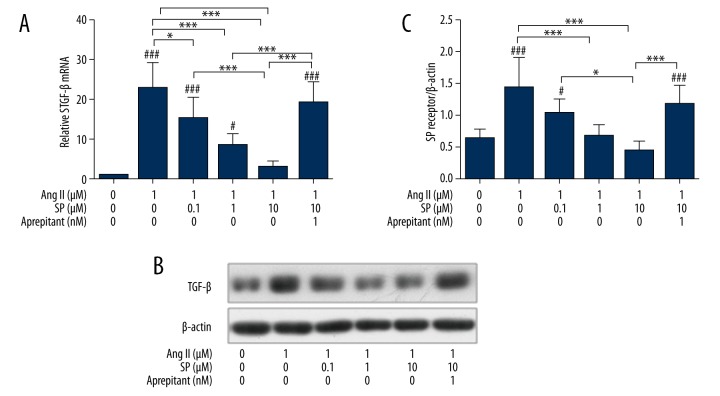
The effects of SP on the expression of TGF-β in CFBs induced by Ang II
The mRNA expression of TGF-β was detected by qPCR, which found that compared with the control group, the Ang II treatment group could significantly improve the expression of TGF-β (p<0.05). Cells treated by Ang II + different concentrations of SP showed a significantly lowered TGF-β mRNA expression level (p<0.05). When aprepitant (1 nM) was added to the Ang II + SP (10 μM) treatment group, the effects of SP were abolished and TGF-β mRNA expressions were recovered significantly more than the Ang II + SP (10 μM) group (p<0.05).
Western blotting was used to detect the expression of TGF-β protein in each treatment group, the results showed that the expression of TGF-β protein in the Ang II treatment group was significantly elevated compared with the control group (p<0.01). When treated with different concentrations of SP, the expressions of TGF-β protein induced by Ang II were significantly decreased (p<0.05). Aprepitant could inhibit the decline of TGF-β protein expression induced by SP (p<0.05). This indicated that SP could significantly inhibit the overexpression of TGF-β induced by Ang II in a dose-dependent manner. However, the NK-1 receptor antagonist aprepitant could inhibit the decline of TGF-β expression caused by SP. This further confirmed that SP had an effect on the expression changes of TGF-β (Figure 5).
Figure 5.
Detection and comparison of the expression of TGF-β in each treatment group. (A) qRT-PCR analysis relative mRNA levels of TGF-β in each treatment group. (B) The expression of TGF-β was detected by Western blotting. (C) Complication and analysis immunoblot results of TGF-β in each group, date were normalized to a housekeeping gene index. n=3, * p<0.05, *** p<0.01.
SP’s effect on erk protein phosphorylation level and smad2/3 protein phosphorylation level induced by Ang II
Western blotting was used to detect the erk and smad2/3 protein phosphorylation levels among the different groups. We found that the erk and smad2/3 protein phosphorylation levels in the Ang II treatment group were significantly higher than those in the control group (p<0.01). After treating with different concentrations of SP, the erk and smad2/3 protein phosphorylation levels decreased gradually, significantly corresponding with the SP concentration increases. The 10 μM SP treatment group was the most obvious one, and the 0.1 μM SP treatment group was the lowest one. In the Ang II + SP (10 μM) + aprepitant (1 nM) treatment group, erk and smad 2/3 protein phosphorylation levels were higher compared with the Ang II + SP (10 μM) treatment group (p<0.01). This shows that Ang II can strengthen the activation of erk and smad 2/3, while presenting no effect on their expression levels. Thus, SP could significantly inhibit the high phosphorylation level of erk protein and smad2/3 protein induced by Ang II treatment in a dose-dependent manner. When aprepitant was added to the Ang II + SP (10 μM) treatment group, the inhibition effect of SP on its activity was resumed. This confirmed the impact of SP on the activation of erk and smad2/3 (Figure 6).
Figure 6.
The expression and phosphorylation levels of erk and smad2/3 protein in each treatment group. (A) Western blotting detected erk protein phosphorylation levels in each treatment group. (B) Western blotting detected smad2/3 protein phosphorylation levels in each treatment group. (C) Complication and analysis immunoblot results of erk protein phosphorylation levels in each group, date were normalized to a housekeeping gene index. (D) Complication and analysis immunoblot results of smad2/3 protein phosphorylation levels in each group, date were normalized to a housekeeping gene index. n=3, * p<0.05, *** p<0.01.
Discussion
SP is a straight-chain polypeptide consisting of 11 amino acids, the first discovered neuropeptides, which plays an important role in regulating physiological functions of human body [8]. There is abundant neurotransmitter SP in the sensory nervous system around cardiac coronary vessels. Under short-time high pressure in blood vessels, SP can dilate blood vessels, lower blood pressure, and play a protective role. From the isolated fibroblasts separated from hypertensive mouse model, researchers have found that SP could be released quickly to regulate the adhesion between cells and matrix and genes related to cellular matrix, and become involved in myocardial remodeling by binding with NK1 receptors without changing the function of fibroblasts [9]. Our study successfully induced the collagen-secreting function of CFBs by Ang II. However, the collagen-secreting function of CFBs was inhibited after treated with SP. This result was similar to the results of the other studies.
Jing et al. [10] found that if the levels of elevated creatinine kinase (creatinine kinase CK) and troponin I (cardiac troponin I, cTnI) were reduced when cardiac ischemia occurred in diabetic mice, myocardial injury could be protected. But, this protection could be eliminated by inhibiting the expression of calcitonin gene-related peptide (calcitonin gene-related peptide, CGRP) and SP receptors. This indicates that the elevated CGRP and SP receptors could lower CK and cTnI levels, and SP could play a role in the prevention of myocardial damage and improve cardiac function by SP receptors. SP and its receptors (NK-1 receptors) have very high affinity. SP has been shown to play an important role in neuronal survival, pain, cell motility, inflammatory reaction, and tumorigenesis by binding with SP receptors [11,12]. As an antagonist of SP receptors, aprepitant could significantly inhibit the binding of SP, and it has been used to treat various tumors [13–15]. This is consistent with the role of SP and SP receptors after CFBs were given different treatment in our study. After cells were treated with aprepitant, the function of SP was weakened, which suggests that a higher expression of SP receptors could enhance the function of SP and SP could play its inhibiting effect on collagen synthesis by SP receptors. In the acute ischemic process, the concentration of norepinephrine was significantly elevated in regional myocardium, and myocardial cells treated with norepinephrine of high concentration significantly increase their apoptosis [16]. Myocardial apoptosis might be a leading cause of cardiac failure and eventual death, which could cause ventricular remodeling [17]. Long-term use of noradrenergic receptor blockers could reduce patient mortality and inhibit ventricular remodeling [18]. Studies have indicated that SP could inhibit myocardial cell apoptosis caused by norepinephrine and further block the deterioration of myocardial function [19,20]. Crossman et al. [21] found that blood vessels of epicardium were relaxed in a dose-dependent manner by injecting SP into patients with coronary arteriosclerosis. The aforementioned studies suggest that SP played a protective role in myocardial ischemia-induced damage and could improve cardiac remodeling caused by myocardial ischemia, with myocardial fibrosis a part of cardiac remodeling. We found by using different concentrations of SP on cells treated with Ang II that SP could inhibit the collagen synthesis of CFBs induced by Ang II, and that the expressions of TGF-β1, erk protein phosphorylation level, and smad2/3protein phosphorylation level associated with myocardial fibrosis were correspondingly lowered in a dose-dependent inhibition. This also indicated that SP played the role of protecting the heart by inhibiting myocardial fibrosis via the complex signaling pathways in myocardial fibrosis, and can further fix the formation of myocardial scar.
Angiotensin II (angiotensin II, Ang II) is an important activity factor in the RAS system and it can induce cardiomyocyte hypertrophy, cardiac fibroblast proliferation, and myocardial fibrosis through ATI receptors [22]. Ang II could regulate the formation of TGF-β1 in cardiomyocytes and stimulate their synthesis and release [23,24]. Elevated TGF-β1 could activate ERK signaling pathway via Raf/Ras pathway and could also phosphorylate the joint section of smad2/3 and activate TGF-β1/smad signaling pathway. ERK signaling pathways could regulate with each other and their abnormal activation could be an important cause of cardiovascular remodeling and myocardial fibrosis [25,26]. In this study, Ang II was used to induce isolated CFBs of the neonatal mice. By detecting the expressions of collagen I and collagen III, CFBs were activated and the cells successfully underwent fibrosis compared with the control group. TGF-β1 and its downstream SMAD signaling pathway and ERK signaling pathway were significantly elevated. This further indicates that Ang II might activate the aforementioned downstream signaling pathway genes by acting on TGF-β1 and thereby promoting the activation of the genes related to collagen synthesis in CFBs. Other studies showed that the protective effect of the combined SP and NK1 receptors on injured myocardial cells in myocardial ischemia might be realized by AKT signaling pathways [27]. Therefore, SP can be an advantageous protection factor in myocardial fibrosis and play its role by way of downstream pathways when bound with SP receptors. But, the specific mechanism between SP and Ang II, TGF-β1, erk as well as smad2/3 protein signaling pathways remains to be elucidated.
Conclusions
SP can inhibit the collagen synthesis of cardiac fibroblasts induced by Ang II after bound with SP receptors in vitro, which was associated with TGF-β1, erk and smad2/3 protein signaling pathways. As a kind of neurotransmitter widely existing in vivo, SP plays a key role in the human bodies’ daily activities. This study indicates that SP can inhibit the collagen synthesis of cardiac fibroblasts and plays a potential protective role in the process of myocardial remodeling and myocardial fibrosis, which are of great significance in the clinical treatment of retroperitoneal fibrosis of myocardial infarction.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Smith SC, Jr, Collins A, Ferrari R, et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke) Circulation. 2012;126:2769–75. doi: 10.1161/CIR.0b013e318267e99f. [DOI] [PubMed] [Google Scholar]
- 2.Dionne RA, Max MB, Gordon SM, et al. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin Pharmacol Ther. 1998;64:562–68. doi: 10.1016/S0009-9236(98)90140-0. [DOI] [PubMed] [Google Scholar]
- 3.Lai JP, Zhan GX, Campbell DE, et al. Detection of substance P and its receptor in human fetal microglia. Neuroscience. 2000;101:1137–44. doi: 10.1016/s0306-4522(00)00398-5. [DOI] [PubMed] [Google Scholar]
- 4.De Giorgio R, Tazzari PL, Barbara G, et al. Detection of substance P immunoreactivity in human peripheral leukocytes. J Neuroimmunol. 1998;82:175–81. doi: 10.1016/s0165-5728(97)00201-4. [DOI] [PubMed] [Google Scholar]
- 5.Blais M, Mottier L, Germain MA, et al. Sensory neurons accelerate skin reepithelialization via substance P in an innervated tissue-engineered wound healing model. Tissue Eng Part A. 2014;20:2180–88. doi: 10.1089/ten.tea.2013.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hongbao M, Yan Y, Shen C. Gender-specific effects of calcitonin gene-related peptide and substance P on coronary blood flow in an experimental model. Angiology. 2009;60:569–75. doi: 10.1177/0003319708325450. [DOI] [PubMed] [Google Scholar]
- 7.Dehlin HM, Levick SP. Substance P in heart failure: the good and the bad. Int J Cardiol. 2014;170:270–77. doi: 10.1016/j.ijcard.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty S, Nepiyushchikh Z, Davis MJ, et al. Substance P activates both contractile and inflammatory pathways in lymphatics through the neurokinin receptors NK1R and NK3R. Microcirculation. 2011;18:24–35. doi: 10.1111/j.1549-8719.2010.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehlin HM, Manteufel EJ, Monroe AL, et al. Substance P acting via the neurokinin-1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. Int J Cardiol. 2013;168:4643–51. doi: 10.1016/j.ijcard.2013.07.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren JY, Song JX, Lu MY, et al. Cardioprotection by ischemic postconditioning is lost in isolated perfused heart from diabetic rats: Involvement of transient receptor potential vanilloid 1, calcitonin gene-related peptide and substance P. Regul Pept. 2011;169:49–57. doi: 10.1016/j.regpep.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Munoz M, Covenas R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids. 2014;46:1727–50. doi: 10.1007/s00726-014-1736-9. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Recio S, Gascon P. Biological and pharmacological aspects of the NK1-receptor. Biomed Res Int. 2015;2015:495704. doi: 10.1155/2015/495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger M, Neth O, Ilmer M, et al. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J Hepatol. 2014;60:985–94. doi: 10.1016/j.jhep.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 14.Mak IT, Kramer JH, Chmielinska JJ, et al. EGFR-TKI, erlotinib, causes hypomagnesemia, oxidative stress, and cardiac dysfunction: Attenuation by NK-1 receptor blockade. J Cardiovasc Pharmacol. 2015;65:54–61. doi: 10.1097/FJC.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruzza C, Rizzi A, Malfacini D, et al. Pharmacological characterization of tachykinin tetrabranched derivatives. Br J Pharmacol. 2014;171:4125–37. doi: 10.1111/bph.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shyu KG, Kuan P, Chang ML, et al. Effects of norepinephrine on apoptosis in rat neonatal cardiomyocytes. J Formos Med Assoc. 2000;99:412–18. [PubMed] [Google Scholar]
- 17.Schwartz K, Mercadier JJ. Molecular and cellular biology of heart failure. Curr Opin Cardiol. 1996;11:227–36. doi: 10.1097/00001573-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Katz AM. Regression of left ventricular hypertrophy: new hope for dying hearts. Circulation. 1998;98:623–24. doi: 10.1161/01.cir.98.7.623. [DOI] [PubMed] [Google Scholar]
- 19.Wang LL, Guo Z, Han Y, et al. Implication of substance P in myocardial contractile function during ischemia in rats. Regul Pept. 2011;167:185–91. doi: 10.1016/j.regpep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza M, Garza MA, Xie M, et al. Substance P is associated with heart enlargement and apoptosis in murine dilated cardiomyopathy induced by Taenia crassiceps infection. J Parasitol. 2007;93:1121–27. doi: 10.1645/GE-596R1.1. [DOI] [PubMed] [Google Scholar]
- 21.Crossman DC, Larkin SW, Dashwood MR, et al. Responses of atherosclerotic human coronary arteries in vivo to the endothelium-dependent vasodilator substance P. Circulation. 1991;84:2001–10. doi: 10.1161/01.cir.84.5.2001. [DOI] [PubMed] [Google Scholar]
- 22.Neves MF, Amiri F, Virdis A, et al. Role of aldosterone in angiotensin II-induced cardiac and aortic inflammation, fibrosis, and hypertrophy. Can J Physiol Pharmacol. 2005;83:999–1006. doi: 10.1139/y05-068. [DOI] [PubMed] [Google Scholar]
- 23.Camm AJ, Al-Khatib SM, Calkins H, et al. A proposal for new clinical concepts in the management of atrial fibrillation. Am Heart J. 2012;164:292–302.e1. doi: 10.1016/j.ahj.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Poczatek MH, Berecek KH, et al. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun. 2006;339:633–41. doi: 10.1016/j.bbrc.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 25.Hough C, Radu M, Dore JJ. Tgf-beta induced Erk phosphorylation of smad linker region regulates smad signaling. PLoS One. 2012;7:e42513. doi: 10.1371/journal.pone.0042513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Fan D, Wang C, et al. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 27.Jubair S, Li J, Dehlin HM, et al. Substance P induces cardioprotection in ischemia-reperfusion via activation of AKT. Am J Physiol Heart Circ Physiol. 2015;309:H676–84. doi: 10.1152/ajpheart.00200.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]