Abstract
Background
Vitamin D deficiency is common in patients with chronic obstructive pulmonary disease (COPD) and has also been linked to comorbidities often present in COPD.
Aim
The aim of this study was to investigate whether vitamin D deficiency was related specifically to airflow limitation or whether vitamin D deficiency was determined by conditions that frequently coexist with COPD: insulin resistance, hypertension, anaemia, obesity and hypercholesterolaemia.
Methods
For this cross-sectional analysis, we included 897 subjects from the Baltimore Longitudinal Study of Aging. Subjects taking vitamin D supplements were excluded. Airflow limitation was defined as FEV1/FVC < lower limit of normal. Logistic regression was used to assess the association between vitamin D deficiency (25-hydroxy vitamin D < 20 ng/mL) and possible determinants.
Results
Vitamin D deficiency was not specific for subjects with airflow limitation. Body mass index (BMI) (OR: 1·05, P < 0·03) and obesity (BMI > 30 kg/m2) (OR: 1·9, P < 0·002) were significantly associated with vitamin D deficiency in the adjusted multivariate regression analysis. Physical activity was associated with a decreased risk of vitamin D deficiency.
Conclusions
Airflow limitation was not an independent determinant of vitamin D deficiency. The effect of weight loss and increased physical activity on vitamin D levels should be investigated further in intervention studies.
Keywords: Airflow limitation, airway obstruction, cardiovascular disease, comorbidities, physical activity, vitamin D
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airflow limitation and the presence of symptoms such as dyspnoea and fatigue [1]. Besides pulmonary symptoms, systemic consequences of the disease are common, for example, impaired lower limb muscle function [2,3], body composition abnormalities [4–6] and various comorbidities [7,8].
Vitamin D deficiency is also common in patients with COPD [9,10] and observational studies have shown a positive association between circulating vitamin D and exercise capacity in patients with COPD [11,12]. In a post hoc subgroup analysis of a larger randomized trial comparing vitamin D supplementation with placebo to reduce time to exacerbations, 50 subjects participated in a rehabilitation programme [13]. In this subgroup, the supplemented group had larger improvement of inspiratory muscle strength and maximal oxygen uptake [13]. Furthermore, vitamin D status has been found to correlate with lung function both in the general population [14] and specifically in patients with COPD [15].
In the general population, vitamin D deficiency has been associated with other COPD-related comorbidities such as cardiovascular disease (CVD), type 2 diabetes, and anaemia [16–18]. As these comorbidities are more prevalent in patients with COPD than in age-matched controls [19], the question arises whether and to what extent vitamin D deficiency is COPD specific or whether it is due to common features of COPD such as smoking, aging, reduced physical activity, and/or the presence of comorbidity?
The aim of this study was to investigate whether and to what extent vitamin D deficiency is related to the degree of airflow limitation in an older population. Furthermore, we wanted to assess whether vitamin D deficiency was determined by conditions that frequently coexist with COPD: insulin resistance, hypertension, anaemia, obesity, and hypercholesterolaemia. We also wanted to examine whether modifiable variables, such as level of physical activity, exercise capacity and smoking, were determinants of vitamin D deficiency.
Materials and methods
The Baltimore Longitudinal Study of Aging (BLSA) is a prospective study of normative aging. All participants were healthy when they entered the study and were followed indefinitely with a serial of evaluations over time (ClinicalTrials.gov identifier: NCT00233272). For this cross-sectional analysis, we included subjects with a serum 25-hydroxy vitamin D (25(OH)D) measurement.
Airflow limitation
In this sample of an older American population, we defined airflow limitation as forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < lower limit of normal. Lower limit of normal was defined as predicted FEV1/FVC minus 1·645 × standard error of the estimate (the lower 5th percentile). The reference values were from the Third National Health and Nutrition Examination Survey (NHANES III) [20]. Calculations were according to sex and ethnicity. For the mixed race, non-Caucasian and non-African American (n = 127), we used the reference values for Caucasians.
Definition of morbidities and vitamin D deficiency
Comorbidities were defined as diseases coexisting with airflow limitation. If the diseases were reported in subjects without airflow limitation, they were referred to as morbidities not including airflow limitation. The following morbidities were objectively defined as follows: hypertension: systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg; insulin resistance: body mass index (BMI) > 28·9 + HOMA > 4·56 or BMI > 27·5 + HOMA > 3·6 [21]; the homeostasis model assessment (HOMA) index is calculated as glucose mg/dL × insulin µU/mL)/405; hypercholesterolaemia: total cholesterol > 200 mg/dL; obesity: BMI > 30 kg/m2; anaemia: haemoglobin < 13 g/dL in men and < 12 g/dL in women. Vitamin D deficiency was defined as serum 25(OH)D < 20 ng/mL (~50 nM).
Season
As plasma vitamin D concentration is dependent on the season, the winter season was defined as the months October–March and the summer season was defined as the months April–September. This variable was used as confounding variable for vitamin D concentration.
Laboratories measures
Total cholesterol in plasma was measured by an enzymatic method (ABA-200 ATC Biochromatic Analyzer; Abbott Laboratories, Irving, TX, USA). Haemoglobin A1c was measured by Automated DiaSTAT analyzer (Bio-Rad Laboratories, Hercules, CA, USA). Fasting plasma glucose was measured with a bichromatic endpoint method (Vista) during the period 30 June 2003–11 July 2009 and spectrophotometry (SPEC) during the period 12 July 2009–present. Plasma fasting insulin was measured by enzyme-linked immunosorbent assay (ELISA); the interassay variability was 2·6–3·6%. Serum 25(OH)D concentrations were measured by liquid chromatography–mass spectrometry at the Mayo Clinic laboratories (Rochester, MN, USA). The lower limit of detection was 4 ng/mL, and the interassay coefficient of variation was 10%.
Modifiable variables
The modifiable variables used in the statistical models were the following: Walking time (seconds to perform 6 m walk), smoking status (never smoker, former smoker and current smoker) and physical activity (BLSA physical activity category 0–3). Information on physical activity was obtained from a questionnaire. The activities were then converted into Metabolic Equivalents [22] (METs)/day (0 = 0–49 METs/day; 1 = 50–249 METs/day; 2 = 250–499 METs/day; and 3 = 500+ METs/day).
Outcomes associated with vitamin D deficiency
A dual-energy X-ray absorptiometry (DXA) scan was performed (using Lunar prodigy 10190 and after 2006 prodigy advance PA + 130024 from GE Healthcare, Madisson, WI, USA) at the same visit as the measurement of the vitamin D status. Osteoporosis and osteopenia were defined based on the DXA scan by the lowest T score of the hip and the lumbar spine, respectively, lower than −2·5 or lower than −1 and higher than −2·5.
Statistics
Patient characteristics are reported as mean (standard deviation) or per cent. Chi-squared test (or Fisher’s exact test when more than one cell had an expected count < 5) was used for comparing binary variables and an independent t-test was used for continuous variables. To compare vitamin D levels between subjects with different numbers of comorbidities, one-way anova test was used. A P-value < 0·05 was considered statistically significant.
All the explanatory variables were entered in a univariate logistic regression analysis with vitamin D deficiency (yes/no) as the outcome variable. The explanatory variables were the measurements that were used to define the morbidities (blood pressure, HOMA index, cholesterol, BMI and haemoglobin), the morbidities as dichotomous variables (morbidity yes/no), the general determinants (age, sex, ethnicity and season) and the modifiable variables (walking time, physical activity and smoking status).
In the first multivariate model (model 1) with vitamin D deficiency as outcome variable, either the measurement variables or the morbidity variables with P < 0·1 in the univariate analysis were entered as explanatory variables adjusted for age, sex, season and ethnicity. In the second multivariate model (model 2), the modifiable explanatory variables with P < 0·1 in the univariate analysis were added to model 1. The variables were entered en bloc.
The analyses were performed using Statistical Package for Social Sciences (spss) version 20 (SPSS Inc., Chicago, IL, USA). A P-value < 0·05 was considered statistically significant.
Results
In this study, 897 subjects from the BLSA were included. None of the 897 subjects were taking vitamin D supplementation. The average age was 66 years (SD ± 13·0) (87% were older than 50 years). Of the 897 subjects, 88 (9·8%) had airflow limitation. Most of these subjects were never smokers or former smokers. Subjects with airflow limitation had lower BMI and worse lung function (FEV1) compared with subjects without airflow limitation (Table 1). Only few subjects had self-reported obstructive lung disease such as asthma and COPD, 8·4% and 1·8%, respectively.
Table 1.
Subject characteristics, mean (SD) or %
| Subject characteristics | All N = 897 |
Subjects with airflow limitation N = 88 |
Subjects without airflow limitation N = 809 |
P* |
|---|---|---|---|---|
| Sex, %male | 50 | 46 | 51 | 0·3 |
| Age, years | 66·1 (13·0) | 66·1 (14·0) | 66·1 (12·9) | 1·0 |
| Caucasian | 61 | 59 | 61 | 0·7 |
| African American | 24 | 22 | 24 | 0·6 |
| Other ethnicity | 15 | 19 | 15 | 0·3 |
| 25(OH)D, ng/mL | 31·8 (11·7) | 31·7 (12·2) | 31·9 (11·6) | 0·9 |
| Vitamin D deficiency | 14·5 | 15·9 | 14·3 | 0·7 |
| Non-smoker | 51·8 | 53·4 | 51·7 | 0·8 |
| Former smoker | 44·5 | 39·8 | 45·0 | 0·8 |
| Smoker | 3·7 | 6·8 | 3·3 | 0·1 |
| BMI, kg/m2 | 27·3 (5·0) | 26·3 (4·6) | 27·4 (5·0) | 0·04 |
| Waist circumference, cm | 91·1 (13·9) | 89·4 (13·5) | 91·2 (13·9) | 0·2 |
| FEV1, L | 2·48 (0·87) | 1·84 (0·82) | 2·55 (0·84) | < 0·001 |
| FEV1, % pred | 93·6 (29·2) | 69·8 (22·1) | 96·3 (20·8) | < 0·001 |
| FVC, L | 3·3 (1·1) | 3·3 (1·2) | 3·3 (1·1) | 0·6 |
| FEV1/FVC | 0·75 (0·09) | 0·56 (0·10) | 0·78 (0·06) | < 0·001 |
| Glucose, mg/dL | 89·8 (16·7) | 87·2 (12·7) | 90·1 (17·0) | 0·2 |
| Insulin, µU/mL | 8·8 (6·5) | 8·4 (5·1) | 8·9 (6·7) | 0·6 |
| HOMA index | 2·2 (1·9) | 2·0 (1·2) | 2·2 (1·9) | 0·2 |
| Cholesterol, mg/dL | 190·4 (37·4) | 191·0 (35·5) | 190·4 (37·6) | 0·9 |
| Haemoglobin, g/dL | 13·7 (1·4) | 13·6 (1·2) | 13·7 (1·4) | 0·4 |
| Systolic blood pressure, mmHg | 115·3 (14·6) | 116·4 (16·7) | 115·1 (14·4) | 0·4 |
| Diastolic blood pressure, mmHg | 65·0 (8·9) | 65·2 (9·2) | 65·0 (8·8) | 0·8 |
| Prevalence of morbidity, % | ||||
| Airflow limitation | 9·8 | – | – | |
| Obesity | 25·8 | 22·7 | 26·1 | 0·5 |
| Hypertension | 4·6 | 5·7 | 4·4 | 0·6 |
| Hypercholesterolaemia | 37·7 | 43·2 | 37·1 | 0·3 |
| Insulin resistance | 8·7 | 2·3 | 9·4 | 0·02 |
| Anaemia | 12·7 | 9·1 | 13·1 | 0·3 |
BMI, body mass index; FVC, forced vital capacity.
Comparing subjects with and without airflow limitation.
The values highlighted in bold are statistically significant (P-value < 0.05).
Morbidity
Sixty-six per cent of the subjects with airflow limitation had one or more comorbidities; 62% of the subjects without airflow limitation had one or more morbidities (NS). The most prevalent morbidity was hypercholesterolaemia both in subjects with and without airflow limitation: 43% and 37%, respectively (P = 0·3) (Table 1). There was no difference between the prevalence of morbidities in subjects with or without airflow limitation, except insulin resistance, which was less prevalent in subjects with airflow limitation (P = 0·02).
Vitamin D levels
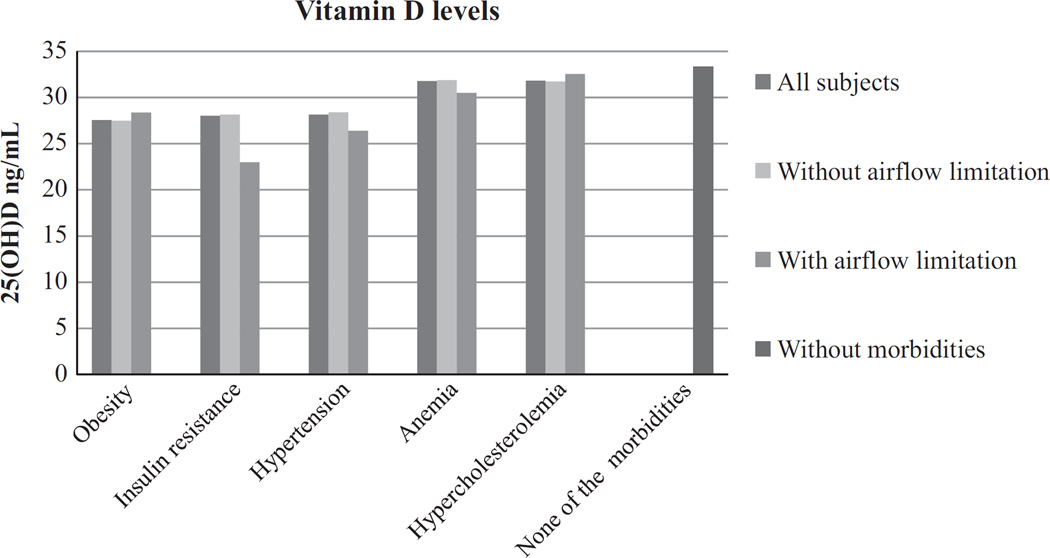
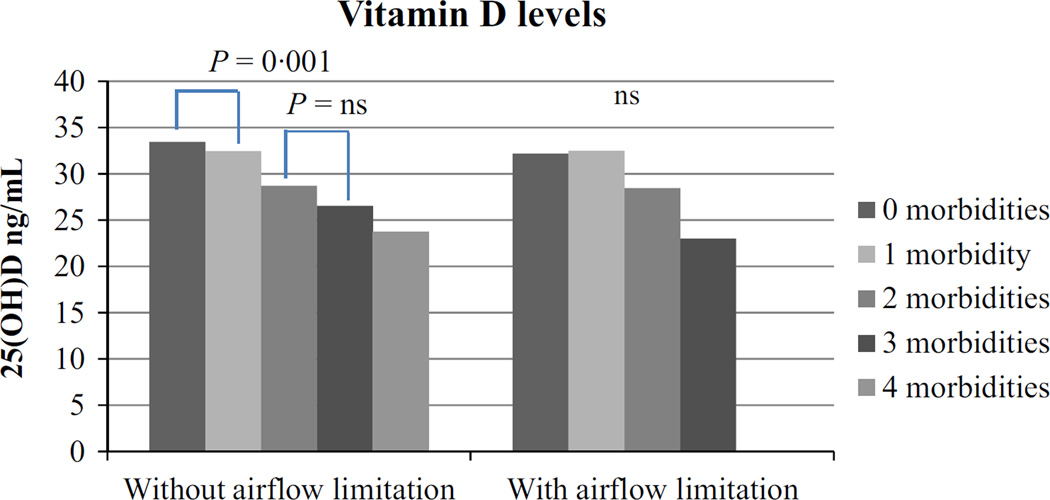
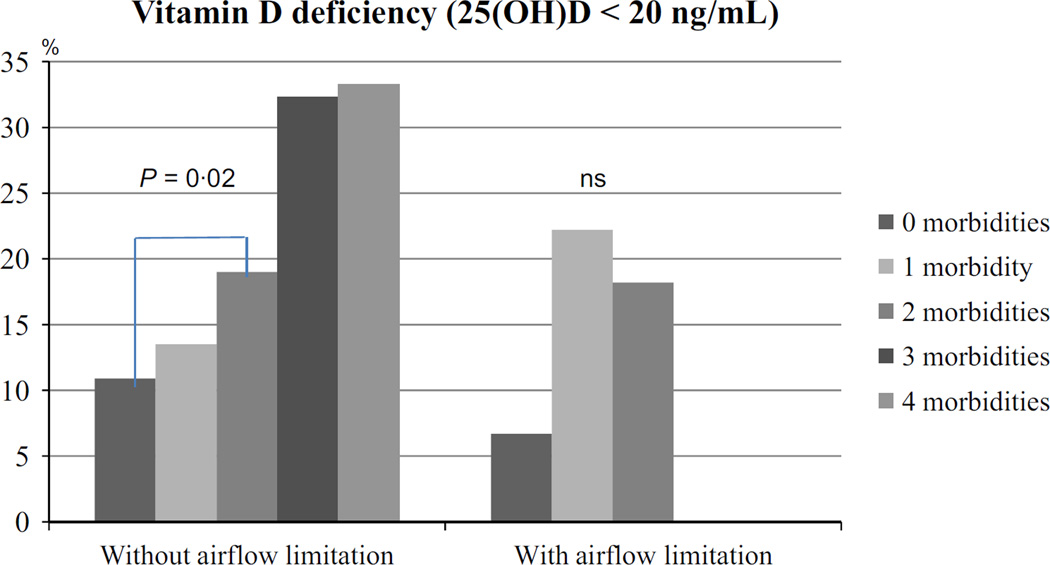
In subjects without airflow limitation, the mean serum 25(OH) D was 32 ng/mL, which was similar in subjects with airflow limitation (Table 1). Vitamin D levels were lower in men (30·3 ng/mL) compared with women (33·4 ng/mL) (P < 0·001). Overall, around 15% of the 897 subjects were vitamin D deficient, 13·9% among women and 15·1% among men (P = 0·6). In subjects without airflow limitation, the mean serum 25(OH)D during winter was 30·8 ng/mL (SD: 11·5 ng/mL) (n = 402) and 32·9 ng/mL (11·7 ng/mL) (n = 407) during summer (P = 0·01). This difference was not seen in subjects with airflow limitation as the mean serum 25(OH)D during winter was 31·5 ng/mL (SD: 14·7 ng/mL) (n = 41) and during summer 31·8 ng/mL (SD: 9·7 ng/mL) (n = 47) (P = 0·9). In subjects with and without airflow obstruction, there was no difference in vitamin D concentration after stratification for comorbidity (Fig. 1). However, plasma vitamin D concentration decreased from 0 to 1 and from 2 to 3 comorbidities in the subjects without airflow obstruction (Fig. 2). Subsequently, vitamin D deficiency was more prevalent in the subjects with two morbidities compared with no morbidities in the subjects without airflow limitation (P = 0·02) (Fig. 3).
Figure 1.
Plasma vitamin D levels by comorbidity. No significant difference between all subjects, without and with airflow limitation.
Figure 2.
Vitamin D levels in subjects without and with airflow limitation by number of comorbidity. One-way anova was used to assess the difference between groups. In subjects with airflow limitation, there was no significant difference between the groups.
Figure 3.
Vitamin D deficiency. Chi-squared test or Fisher’s exact test was used to assess difference between groups. In subjects with airway limitation, there was no significant difference between groups.
Determinants of vitamin D deficiency
In the univariate analysis age, ethnicity (African American vs. Caucasian), BMI, blood pressure and HOMA index as well as the dichotomous variables obesity and physical activity were associated with vitamin D deficiency, as shown in Table 2. In the multivariate logistic regression (model 1) of the measurement variables, age, ethnicity, season and BMI were significant and remained significant after physical activity was added (model 2), as shown in Table 3. In the multivariate logistic regression (model 1) of the morbidity variables, only obesity was significantly associated with vitamin D deficiency after adjustment for age, ethnicity and season, as shown in Table 4. In model 2, the only significant modifiable variable was physical activity.
Table 2.
Possible determinants of vitamin D deficiency. Univariate logistic regression analysis
| Univariate – logistic regression |
||
|---|---|---|
| Explanatory variables | OR | P |
| Age, years | 0·98 | 0·003 |
| Sex (men) | 1·1 | 0·6 |
| Ethnicity (African American vs. Caucasian) | 4·7 | < 0·001 |
| Ethnicity (Other vs. Caucasian) | 1·3 | 0·4 |
| Season (summer) | 0·7 | 0·06 |
| BMI, kg/m2 | 1·1 | < 0·001 |
| Systolic blood pressure, mmHg | 1·01 | 0·01 |
| Diastolic blood pressure, mmHg | 1·03 | 0·003 |
| OMA index | 1·1 | 0·01 |
| FEV1/FVC | 2·5 | 0·4 |
| Cholesterol, mg/dL | 1·001 | 0·6 |
| Haemoglobin, g/dL | 0·96 | 0·5 |
| Obesity | 2·3 | < 0·001 |
| Insulin resistance | 1·6 | 0·1 |
| Hypertension | 1·7 | 0·2 |
| Hypercholesterolaemia | 1·4 | 0·1 |
| Airflow limitation (FEV1/FVC < LLN) | 1·1 | 0·7 |
| Anaemia | 0·8 | 0·5 |
| Seconds to perform 6 m walk | 1·1 | 0·5 |
| Baltimore Longitudinal Study of Aging physical activity category | ||
| 0 vs. 1 | 0·5 | 0·03 |
| 0 vs. 2 | 0·3 | < 0·001 |
| 0 vs. 3 | 0·4 | 0·004 |
| Smoking | ||
| Current smoker vs. never smoker | 1·3 | 0·5 |
| Former smoker vs. never smoker | 0·95 | 0·8 |
The values highlighted in bold are statistically significant (P-value < 0.05).
BMI, body mass index; FVC, forced vital capacity.
Table 3.
Multivariate logistic regression. Determinants of vitamin D deficiency. Measurement variables with P < 0·1 in the univariate analysis entered en bloc with general determinants (model 1) and with modifiable variables (model 2). Only significant variables are shown
| Multivariate (model 1) Measurement variables |
Multivariate (model 2) Measurement variables |
|||
|---|---|---|---|---|
| OR | P | OR | P | |
| Age, years | 0·98 | 0·042 | 0·98 | 0·013 |
| Sex (men) | 1·32 | 0·22 | 1·48 | 0·07 |
| Ethnicity (African American vs. Caucasian) |
4·89 | < 0·001 | 4·52 | < 0·001 |
| Season (summer) | 0·54 | 0·005 | 0·64 | 0·031 |
| BMI, kg/m2 | 1·05 | 0·03 | 1·07 | < 0·001 |
| BLSA physical activity category (2 vs. sedentary) |
– | – | 0·31 | 0·001 |
| BLSA physical activity category (3 vs. sedentary) |
– | – | 0·48 | 0·04 |
BLSA, Baltimore Longitudinal Study of Aging; BMI, body mass index.
Model 1: General determinants + measurement variables with P < 0·1 in the univariate model.
Model 2: Model 1 + modifiable determinants with P < 0·1 in the univariate model.
Table 4.
Multivariate logistic regression. Determinants of vitamin D deficiency [serum 25(OH) < 20 ng/mL]. Morbidity variables with P < 0·1 in the univariate analysis entered en bloc with general determinants (model 1) and with modifiable variables (model 2). Only significant variables are shown
| Multivariate (model 1) Morbidity variables |
Multivariate (model 2) Morbidity variables |
|||
|---|---|---|---|---|
| OR | P | OR | P | |
| Age, years | 0·98 | 0·02 | 0·98 | 0·01 |
| Sex (men) | 1·4 | NS | 1·5 | 0·046 |
| Ethnicity (African American vs. Caucasian) |
4·7 | < 0·001 | 4·5 | < 0·001 |
| Season (summer) | 0·65 | 0·03 | 0·63 | 0·03 |
| Obesity (BMI > 30 kg/m2) | 1·9 | 0·002 | 1·7 | 0·02 |
| BLSA physical activity category (2 vs. sedentary) |
– | – | 0·30 | 0·001 |
| BLSA physical activity category (3 vs. sedentary) |
– | – | 0·47 | 0·04 |
BLSA, Baltimore Longitudinal Study of Aging; BMI, body mass index.
Model 1: General determinants + morbidities with P < 0·1 in the univariate model.
Model 2: Model 1 + modifiable variables with P < 0·1 in the univariate model.
Clinical outcomes associated with vitamin D deficiency
Of the 897 subjects, 415 (46%) subjects had osteopenia and 80 (9%) had osteoporosis. Osteopenia was associated with vitamin D deficiency (P = 0·036), whereas osteoporosis was not (P = 0·63).
Discussion
In this sample of older subjects, airflow limitation was not a determinant of vitamin D deficiency. BMI/obesity and physical activity were the main determinants of vitamin D deficiency. Our data suggest that the frequent presence of vitamin D deficiency in patients with airflow limitation is due to general and modifiable features.
Black and Scragg [14] were the first to show an association between vitamin D and lung function in the NHANES III cohort, also after adjusting for age, sex, ethnicity, height, smoking, and BMI. Since then, this finding was confirmed; among others, COPD has been suggested to be an independent risk factor for vitamin D deficiency (adjusted for BMI, GOLD stage, smoking and season) [10], and vitamin D deficiency was more frequent in patients with airflow limitation compared with controls [9]. In the latter study, the usually seen inverse relationship between BMI and serum 25(OH)D was not found [9]. Thus, it was plausible that airflow limitation could influence vitamin D levels. Our hypothesis was that airflow limitation is an independent determinant of vitamin D deficiency, even after considering common features like aging, comorbidity, reduced physical activity and smoking status, which are risk factors of vitamin D deficiency in the general population [23]. Our results suggest, however, that other determinants of vitamin D deficiency are more influential than airflow limitation by itself. In line with this, it has to be acknowledged that Black and Scragg [14] did not find any difference in the FEV1/FVC ratio between the highest and the lowest quintiles of vitamin D. And in a recent study by Afzal et al. [24], FEV1% predicted and FVC % predicted were lower for lowest vs. highest decile of 25(OH)D in two general population samples; however, the association of 25(OH)D and FEV1/FVC ratio was inconclusive.
Studies assessing the influence of vitamin D in CVD have found an association between elevated BMI and vitamin D deficiency [25–27]. A physiological explanation for this relationship is that vitamin D metabolites are sequestered in fat tissue [28]. In some of the studies that have linked vitamin D deficiency to CVD risk or outcome, adjustment for BMI did not change the result [29–31]. In the present study, obesity was the only morbidity significantly associated with vitamin D deficiency in the multivariate regression analysis. We found that serum 25(OH)D levels were higher in women than in men and increased with age. Several studies have found that lower vitamin D levels are associated with female sex [32,33], but in a study of patients with type 2 diabetes and in another study of morbidly obese patients women had higher levels of 25(OH)D; in both studies, the association was independent of BMI [34,35]. The positive association between age and vitamin D levels have been reported in some studies [36,37], but not in others [32,38]. In the present study of older subjects, only 14% of the women and 15% of the men were vitamin D deficient. A multicentre study from 1995 of an older European population (age: 70–75 years) reported that 36% of the men and 47% of the women had serum 25(OH)D below 30 nM (~12 ng/mL) [39]. A study from Germany in which all the participants were at least 60 years old found that about 26% of the women and 20% of the men had 25(OH)D <50 nM (~20 ng/mL) [40]. In both studies, subjects taking vitamin D supplements were not excluded, while this was the case in the present study. Furthermore, it has to be taken into consideration that Baltimore is located at the 39°N, which is comparable with the south of Italy. This could be another factor explaining the difference in prevalence of vitamin D deficiency between the studies.
We showed that decreased physical activity is associated with increased risk for vitamin D deficiency. As stated in the literature, physical activity has been linked to spending more time outside and thus possibly being exposed to more sunlight [41] and increased physical activity may very well be related to a lower BMI and a more healthy lifestyle in general. Yet, it is less clear whether vitamin D supplementation has a direct effect on muscle strength and physical capacity. In a clinical trial of patients participating in pulmonary rehabilitation and randomized to either 100 000 IU vitamin D3 or placebo, the patients receiving vitamin D had significantly larger improvements in inspiratory muscle strength and maximal oxygen uptake, but improvements in quadriceps strength or 6-min walking distance were not significantly different from the effects in the placebo group [13]. In another clinical trial, patients with COPD received 2000 IU vitamin D3 daily with no effect on physical performance after 6 weeks of treatment [42]. A limitation of these intervention studies was that vitamin D deficiency was not an inclusion criterion; it is thus essential to confirm these findings in a population with vitamin D deficiency. The hypothesis that increased physical activity positively influences the vitamin D concentration has yet to be tested.
Osteopenia was associated with vitamin D deficiency whereas osteoporosis was not. The reason for these results could be the fact that only subjects who did not take vitamin D supplements were included. Subjects already diagnosed with osteoporosis prior to entering the BLSA and at the time of the diagnosis were vitamin D deficient have most likely been supplemented with vitamin D and have thus been excluded from the analyses of the present study.
As populations are aging, multimorbidity is often present and is defined as the coexistence of two or more noncommunicable diseases in the same individual. The terms comorbidity and multimorbidity are often used interchangeable depending on the context. Common risk factors such as smoking, poor nutrition and physical inactivity are leading to simultaneous development of noncommunicable diseases, but there is no standardized definition yet [43]. The implications of the concept of multimorbidity are better understanding of the aging process [44] and the possibility of future treatments targeting common pathways [45]. It could thus be speculated that vitamin D deficiency is just another condition in the multimorbid individual caused by aging.
Our study has several limitations. First, the clinical diagnosis COPD was not established in this population. Information on self-reported asthma and COPD did only in part correspond to the observed airflow limitation. Nevertheless, the prevalence of airflow limitation was lower (9·8%) compared with that of NHANES 2007–2010 in which the prevalence of airflow limitation was 15% using the LLN criterion and prebronchodilator spirometry values on subjects aged 40–79 [46]. Of the subjects with airflow limitation 53·4% were never smokers. These subjects could have asthma or be never smokers with COPD. Never smokers with COPD have been characterized as having milder disease, being older and not having an increased risk of CVD comorbidity compared with current and former smokers [47]. The implications in relation to vitamin D status have not been studied. A second limitation of our study is the cross-sectional design; we cannot infer cause and effect relationship between the found determinants and vitamin D levels. Moreover, we do not know the dietary intake of vitamin D or the estimate of sunlight exposure.
In conclusion, vitamin D deficiency was not specific in subjects with airflow limitation in this sample of older subjects. BMI and physical activity as modifiable factors were associated with vitamin D deficiency. The effect of weight loss and increased physical activity on vitamin D levels should be investigated further in intervention studies.
Acknowledgments
We would like to thank Jørgen Vestbo for valuable advice and insight during the writing of this manuscript. Mia Moberg was funded by TrygFonden.
Footnotes
Conflict of interest
The authors report no conflict of interests.
References
- 1.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36:81–88. doi: 10.1183/09031936.00104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigare R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breyer MK, Spruit MA, Hanson CK, Franssen FM, Vanfleteren LE, Groenen MT, et al. Prevalence of metabolic syndrome in COPD patients and its consequences. PLoS ONE. 2014;9:e98013. doi: 10.1371/journal.pone.0098013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breyer M-K, Spruit MA, Celis APM, Rutten EPA, Janssen PP, Wouters EFM. Highly elevated C-reactive protein levels in obese patients with COPD: a fat chance? Clin Nutr. 2009;28:642–647. doi: 10.1016/j.clnu.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J. 2009;34:209–218. doi: 10.1183/09031936.50130408. [DOI] [PubMed] [Google Scholar]
- 7.Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E, Jr, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 9.Mahlin C, von Sydow H, Osmancevic A, Emtner M, Gronberg AM, Larsson S, et al. Vitamin D status and dietary intake in a Swedish COPD population. Clin Respir J. 2014;8:24–32. doi: 10.1111/crj.12030. [DOI] [PubMed] [Google Scholar]
- 10.Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS ONE. 2012;7:e38934. doi: 10.1371/journal.pone.0038934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringbaek T, Martinez G, Durakovic A, Thogersen J, Midjord AK, Jensen JE, et al. Vitamin d status in patients with chronic obstructive pulmonary disease who participate in pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2011;31:261–267. doi: 10.1097/HCR.0b013e31821c13aa. [DOI] [PubMed] [Google Scholar]
- 12.Romme EA, Rutten EP, Smeenk FW, Spruit MA, Menheere PP, Wouters EF. Vitamin D status is associated with bone mineral density and functional exercise capacity in patients with chronic obstructive pulmonary disease. Ann Med. 2013;45:91–96. doi: 10.3109/07853890.2012.671536. [DOI] [PubMed] [Google Scholar]
- 13.Hornikx M, Van Remoortel H, Lehouck A, Mathieu C, Maes K, Gayan-Ramirez G, et al. Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir Res. 2012;13:84. doi: 10.1186/1465-9921-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 15.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–968. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perlstein TS, Pande R, Berliner N, Vanasse GJ. Prevalence of 25-hydroxyvitamin D deficiency in subgroups of elderly persons with anemia: association with anemia of inflammation. Blood. 2011;117:2800–2806. doi: 10.1182/blood-2010-09-309708. [DOI] [PubMed] [Google Scholar]
- 19.Baty F, Putora PM, Isenring B, Blum T, Brutsche M. Comorbidities and burden of COPD: a population based case-control study. PLoS ONE. 2013;8:e63285. doi: 10.1371/journal.pone.0063285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54:333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 24.Afzal S, Lange P, Bojesen SE, Freiberg JJ, Nordestgaard BG. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax. 2014;69:24–31. doi: 10.1136/thoraxjnl-2013-203682. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Song Y, Manson JE, Pilz S, Marz W, Michaelsson K, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5:819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51:228–233. doi: 10.1016/j.ypmed.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Pilz S, Tomaschitz A, Marz W, Drechsler C, Ritz E, Zittermann A, et al. Vitamin D, cardiovascular disease and mortality. Clin Endocrinol (Oxf) 2011;75:575–584. doi: 10.1111/j.1365-2265.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- 28.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–260. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 32.Omdahl JL, Garry PJ, Hunsaker LA, Hunt WC, Goodwin JS. Nutritional status in a healthy elderly population: vitamin D. Am J Clin Nutr. 1982;36:1225–1233. doi: 10.1093/ajcn/36.6.1225. [DOI] [PubMed] [Google Scholar]
- 33.Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65:67–71. doi: 10.1093/ajcn/65.1.67. [DOI] [PubMed] [Google Scholar]
- 34.Al-Daghri NM, Alkharfy KM, Al-Othman A, Yakout SM, Al-Saleh Y, Fouda MA, et al. Effect of gender, season, and vitamin D status on bone biochemical markers in Saudi diabetes patients. Molecules. 2012;17:8408–8418. doi: 10.3390/molecules17078408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson LK, Hofso D, Aasheim ET, Tanbo T, Holven KB, Andersen LF, et al. Impact of gender on vitamin D deficiency in morbidly obese patients: a cross-sectional study. Eur J Clin Nutr. 2012;66:83–90. doi: 10.1038/ejcn.2011.140. [DOI] [PubMed] [Google Scholar]
- 36.Andersen R, Brot C, Jakobsen J, Mejborn H, Molgaard C, Skovgaard LT, et al. Seasonal changes in vitamin D status among Danish adolescent girls and elderly women: the influence of sun exposure and vitamin D intake. Eur J Clin Nutr. 2013;67:270–274. doi: 10.1038/ejcn.2013.3. [DOI] [PubMed] [Google Scholar]
- 37.Chao YS, Brunel L, Faris P, Veugelers PJ. The importance of dose, frequency and duration of vitamin D supplementation for plasma 25-hydroxyvitamin D. Nutrients. 2013;5:4067–4078. doi: 10.3390/nu5104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoben AB, Kestenbaum B, Levin G, Hoofnagle AN, Psaty BM, Siscovick DS, et al. Seasonal variation in 25-hydroxyvitamin D concentrations in the cardiovascular health study. Am J Epidemiol. 2011;174:1363–1372. doi: 10.1093/aje/kwr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 40.Jungert A, Roth HJ, Neuhauser-Berthold M. Serum 25-hydroxyvitamin D3 and body composition in an elderly cohort from Germany: a cross-sectional study. Nutr Metab (Lond) 2012;9:42. doi: 10.1186/1743-7075-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168:577–586. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjerk SM, Edgington BD, Rector TS, Kunisaki KM. Supplemental vitamin D and physical performance in COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis. 2013;8:97–104. doi: 10.2147/COPD.S40885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
- 44.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: new tasks, priorities, and frontiers for integrated gerontological and clinical research. J Am Med Dir Assoc. 2015 doi: 10.1016/j.jamda.2015.03.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barnes PJ. Mechanisms of development of multimorbidity in the elderly. Eur Respir J. 2015;45:790–806. doi: 10.1183/09031936.00229714. [DOI] [PubMed] [Google Scholar]
- 46.Tilert T, Dillon C, Paulose-Ram R, Hnizdo E, Doney B. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: the National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir Res. 2013;14:103. doi: 10.1186/1465-9921-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomsen M, Nordestgaard BG, Vestbo J, Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med. 2013;1:543–550. doi: 10.1016/S2213-2600(13)70137-1. [DOI] [PubMed] [Google Scholar]