Abstract
Manipulations in cell culture and mouse models have demonstrated that reduction of collagen V results in altered fibril structure and matrix assembly. A tissue-dependent role for collagen V in determining mechanical function was recently established, but its role in determining regional properties has not been addressed. The objective of this study was to define the role(s) of collagen V expression on establishing the site-specific properties in the supraspinatus tendon. The insertion and midsubstance of tendons from wild type, heterozygous and tendon/ligament-specific null mice were assessed for crimp morphology, fibril morphology, cell morphology, as well as total collagen and pyridinoline crosslink (PYD) content. Fibril morphology was altered at the midsubstance of both groups with larger, but fewer, fibrils and no change in cell morphology or collagen compared to the wild type controls. In contrast, a significant disruption of fibril assembly was observed at the insertion site of the null group with the presence of structurally aberrant fibrils. Alterations were also present in cell density and PYD content. Altogether, these results demonstrate that collagen V plays a crucial role in determining region-specific differences in mouse supraspinatus tendon structure.
Keywords: tendon, EDS, supraspinatus, fibril, collagen
1. Introduction
Classic Ehlers-Danlos Syndrome (EDS) is an inheritable disease that affects approximately 1 in 20–40,000 people worldwide. Clinical manifestations of the disease include joint hypermobility, skin hyperextensibility, and poor wound healing.1 These functional deficiencies can often lead to recurrent joint dislocations, particularly at the knee and shoulder,1; 2 as well as to injury. The major disease-causing mechanism of classic EDS is reduced availability of collagen V, with collagen V mutations identified in over 90% of patients3; 4 and approximately half are null-allele mutations resulting in COL5A1 haploinsufficiency.3; 5; 6 Collagen V is a fibril-forming collagen found as heterotypic fibrils with collagens I, II, and III.7; 8 Although it is a minor component of mature tendon composition quantitatively, collagen V plays a critical role in fibrillogenesis, specifically by nucleating fibril formation. Manipulation of these collagens both in cell culture and mouse models have demonstrated that reduction of collagen V results in decreased fibril assembly and increased fibril diameters in a number of tissues, including skin, cornea, anterior cruciate ligament, and flexor digitorum longus tendon.7–11
Due to the strong role of collagen suprastructure in determining mechanical properties, alterations in fibril structure, number and higher order organization resulting from reduced collagen V expression are thought to be responsible for the functional deficiencies in the EDS patient population. Recent mechanical evaluations have confirmed this hypothesis with collagen V deficient mouse tendons exhibiting as much as 80% reduction in tissue modulus.8; 10; 12 However, these changes were tissue-dependent, with the strong changes found in the knee and rotator cuff.12 It’s still unclear whether the role of collagen V in determining tendon structure and composition is also tissue-dependent since some previous tissues, specifically tendons, that have been characterized are highly organized and homogeneous. Characterization of a more complex tendon, such as the supraspinatus tendon, could provide insight into what factors contribute to the differential regulation of fibril assembly by collagen V.
Therefore, the objective of this study was to determine the differential roles of collagen V in determining regional differences, i.e., midsubstance versus insertion, in the mouse supraspinatus tendons, specifically focused on parameters that are thought to contribute to mechanical function. We accomplished this using our established traditional heterozygous collagen V and tendon/ligament specific collagen V-null mouse models. We hypothesized that reduction of collagen V expression would result in altered fibril morphology (increased fibril diameters and decreased fibril density) with no additional alterations in cell morphology, fiber morphology or extracellular matrix (ECM) composition.
2. Experimental Procedures
2.1 Animals and Sample Collection
A total of 144 mice from three genotypes were used in this study: C57BL/6 control (WT), Col5a1+/− (HET), and a tendon/ligament specific collagen V-null model, Col5a1Δten/Δten (NULL).9; 10 Animal use was approved by The University of Pennsylvania and University of South Florida’s Institutional Animal Care and Use Committees. All mice were bred to 120 days of age by the authors at the University of South Florida and shipped to the University of Pennsylvania following sacrifice and tissue harvest.
2.2 Collagen Fiber Crimp Morphology
Tendons designated for assessment of crimp morphology were prepared for mechanical testing as described and preconditioned for 10 cycles between 0.02 and 0.04N.13; 14 Following a 1 minute hold after preconditioning, tendons were immediately flash frozen,14; 15 detached from mechanical testing setup and embedded in tissue freezing medium for frozen histology. Samples were sectioned at 20 µm and stained with Picrosirius Red and Hematoxylin. At least two images at each region (insertion, midsubstance) were taken under polarized light per sample. Images were analyzed using custom quantitative software (Matlab, Natick, MA) to calculate crimp frequency and amplitude as determined by pixel fluctuations along the length of a collagen fiber.15; 16 Values from multiple images were averaged within a specimen.
2.3 Fibril Morphology
Tendons designated for fibril morphology analysis (n=20/group) were prepared as described previously.17 The samples were fixed for 2h at 4°C in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate and 8 mM CaCl2, pH 7.4. Tendons were then rinsed with cacodylate buffer and post-fixed for 1 h with 1% osmium tetroxide. This was followed by dehydration in an ethanol series followed by 100% propylene oxide, infiltrated and embedded in a mixture of Embed 812, nadic methyl anhydride, dodecenylsuccinic anhydride and DMP-30 (EM Sciences, Fort Washington, PA) and polymerized over-night at 60 °C.18; 19 Prior to embedding, tendons were separated into the insertion site and midsubstance for analysis. Ultra-thin cross-sections (~90 nm) were prepared using a Leica UCT ultramicrotome and post-stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. The sections were examined and imaged at 80 kV using a JEOL 1400 transmission electron microscope (JEOL Ltd., Tokyo, Japan) equipped with a Gatan Orius widefield side mount CC Digital camera (Gatan Inc., Pleasanton, CA).
Ten digital images from each tendon were taken from non-overlapping areas at ×60,000. Images were randomized and masked before fibril diameters were measured using a RM Biometrics-Bioquant Image Analysis System (Nashville, TN). A region of interest (ROI) of appropriate size was determined within the image so that a minimum of 80 fibrils were measured from each image. All fibrils in the region of interest were measured and multiple regions of interest were used if necessary to collect at least 80 fibril diameter measurements per image. Fibril diameters were measured along the minor axis of the fibril cross-section. Fibril density was obtained as the fibril number per unit area. A measure of fibril roundness, fibril irregularity factor (FIF), was defined as the ratio of the radius as determined from a circle with the fibril’s perimeter to the radius as determined from a circle with the fibril’s area, where increasing values would define an increasing number of folds along the surface of the fibril.
2.4 Cell Morphology
For assessment of cell morphology, supraspinatus tendons (n=8/group) were grossly harvested from the shoulder, leaving the muscle and bony insertions intact. Specimens were immediately fixed, decalcified, and processed for paraffin-embedding using standard techniques. Coronal sections were cut to 7µm thickness and stained with hematoxylin and eosin (H&E). The insertion site and midsubstance of each sample was then imaged at 20× and evaluated using BioQuant software (Bioquant Image Analysis Corporation, Nashville, TN) for cellularity and cell shape.17; 20 In this study, only tendon proper, and not the surrounding sheaths, was analyzed for changes in cellularity and cell shape. Cellularity was calculated as a cell density per area of region analyzed. Cell shape was measured as the average aspect ratio of nuclei in a region of interest on a scale from 0 to 1, where 1 represented a perfect circular shape.
2.5 Biochemistry
Tendons designated for biochemistry were dissected for location-dependent analysis as described previously.21 All tendons were removed with muscle intact from the insertion site. The muscle was then removed with a scalpel blade to leave only the tendon portion. A consistent piece of the insertion and the midsubstance was taken from each tendon by splitting the tissue into two even 1-mm regions. Biochemical analysis was performed on intact regional tendon samples, including tendon proper and all associated sheaths. Samples were then digested with Proteinase K (5 mg/ml) and ammonium acetate buffer, pH 7.0 overnight at 37°C. The digest was then hydrolyzed with hydrochloric acid, resuspended in assay buffer and used to quantify total collagen (COL) using a hydroxyproline (OHP) assay22; 23 and pyridinoline crosslinks (PYD) using the MicroVue PYD EIA kit (Quidel Corp., San Diego, CA), which has been used previously to measure pyridinoline crosslinks in engineered tissues, annulus fibrosis, and urine.24–26 This procedure is capable of measuring a high range of concentrations (15–750 nmol concentrations) with a very high precision (3–11% error between runs) and is designed to work with the typically low concentrations found in human urine, making it well suited for measurements in mouse tissue. PYD content was normalized to total OHP content.
2.7 Statistical Analysis
All data was analyzed using a one-way ANOVA with post-hoc Bonferroni-corrected t-tests. Comparisons were only made between the genotypes and not between the insertion site and midsubstance in order to test the specific hypotheses of the study. Statistical significance was set at p<0.05 and a trend was noted at p<0.10.
3. Results
3.1 Fiber Morphology
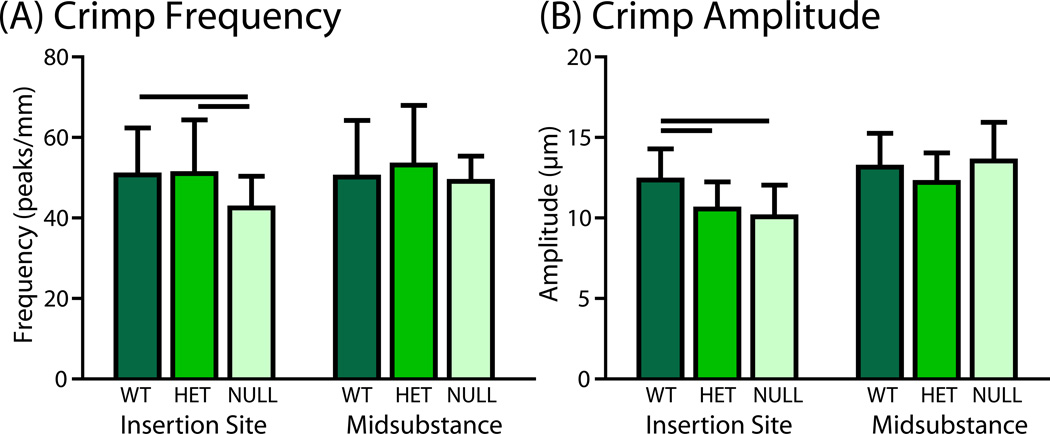
Reduction in collagen V had differential effects on crimp morphology in different regions of the SST. No differences between groups were detected in crimp frequency or amplitude in the midsubstance. However, collagen fibers in null tendons exhibited altered crimp morphology at the insertion site, with decreased crimp frequency and amplitude compared to wild type controls (Fig. 1). The heterozygous group also displayed decreased crimp amplitude at the insertion site, but crimp frequency was unchanged.
Figure 1.
(A) Crimp frequency was decreased in the null tendons compared to both other groups at the insertion site. (B) Crimp amplitude was decreased in the heterozygous and null groups at the insertion site. No differences between groups were detected in crimp frequency or amplitude at the midsubstance.
3.2 Fibril Morphology
Collagen fibril assembly in wild type, heterozygous and null tendons was analyzed using transmission electron microscopy at the insertion site and midsubstance. Wild type, heterozygous and null tendon collagen fibrils at the midsubstance were all exhibited normal circular cross-sectional profiles throughout the tendon extracellular matrix (Fig. 2). Fibril diameters were larger in the heterozygous and null tendons compared to the wild type group. In contrast, null tendons at the insertion site exhibited altered fibril structure and cross-sectional profiles. Increased numbers of large and small diameter fibrils were present in this region with altered fibril arrangement. In addition, null tendons fibrils were structurally abnormal, exhibiting irregular cross-sectional profiles. These irregular fibrils appeared to have a large center with smaller round protrusions surrounding it, rather than the traditionally irregular ‘cauliflower-shaped’ fibrils seen in other studies.8; 27; 28
Figure 2.
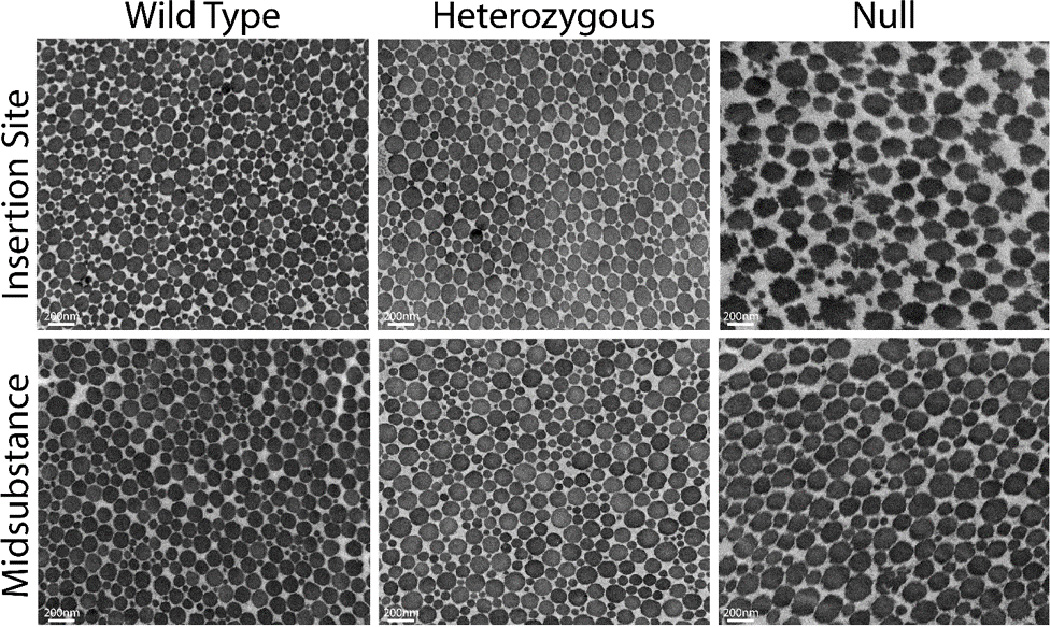
Representative micrographs of fibrils from insertion site (top) and midsubstance (bottom) in wild type (left), heterozygous (middle) and null (right) tendons.
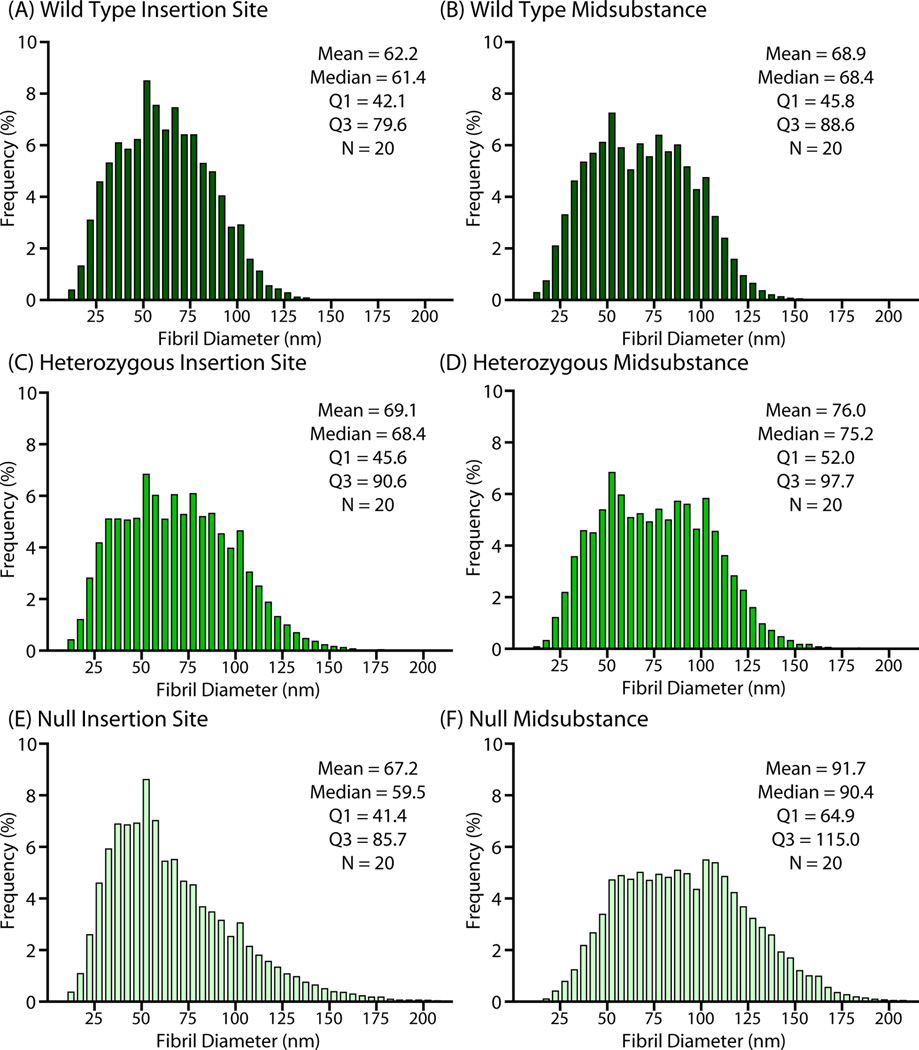
Wild type tendons exhibited a distribution of collagen fibril diameters in the tendon midsubstance with the median diameter at approximately 70nm (Fig. 3). At the insertion site, the wild type fibril distribution was shifted towards smaller diameter fibrils compared to the midsubstance (median 65nm). In the heterozygous tendons, there was an increase in large diameter fibrils at both the insertion (median 66nm) and the midsubstance compared to the wild type group (median 80nm). At the midsubstance of the null group, there was also a shift with increased large diameter fibrils, with diameters greater than both the wild type and heterozygous group (median 88nm). However, the insertion site exhibited an increase in both large and small diameter fibrils and the distribution was visibly more asymmetric than the other groups with the peak shifted to the left, towards smaller diameter fibrils (right-skewed).
Figure 3.
TEM analysis of fibril diameter distribution (n = 20/genotype) for (A/B) wild type, (C/D) heterozygous, and (E/F) null tendons for both insertion site (left column) and midsubstance (right column).
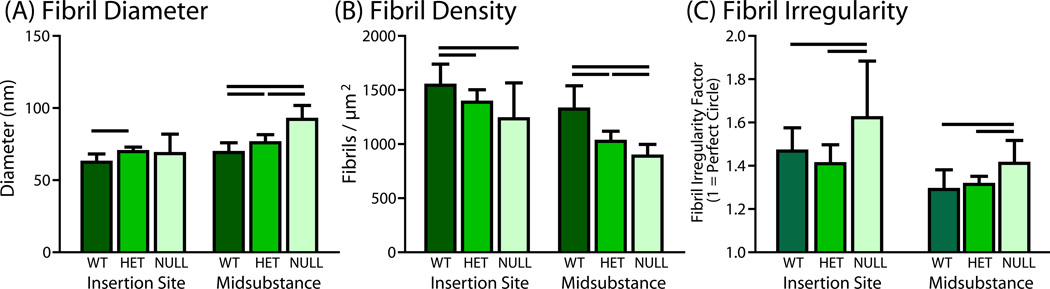
Quantitatively, mean fibril diameters were larger in the heterozygous group than the wild type group at both the insertion site and midsubstance (Fig. 4). The null group had larger fibril diameters than the wild type, but only at the midsubstance. Fibril density was decreased in both experimental groups in both regions. Furthermore, the differences in fibril diameter and fibril density from the wild type to collagen V heterozygous and null groups were not proportional in this study, suggesting an overall change in overall collagen fibril packing. Interestingly, the null tendons also exhibited fibrils with irregular fibril structure (not round), which was not found in any of the other groups or region (Fig. 4C). When fibril irregularity at the insertion site was quantified, the null group had more irregularly shaped fibrils than both the wild type and heterozygous tendons.
Figure 4.
Quantitative analysis confirmed that (A) fibril diameter was larger in the heterozygous groups at both regions but only in the null group at the midsubstance. (B) Fibril density was decreased in both groups at both regions. (C) Fibril irregularity was quantified and was significantly increased in the null group at the insertion site.
3.3 Cell Morphology
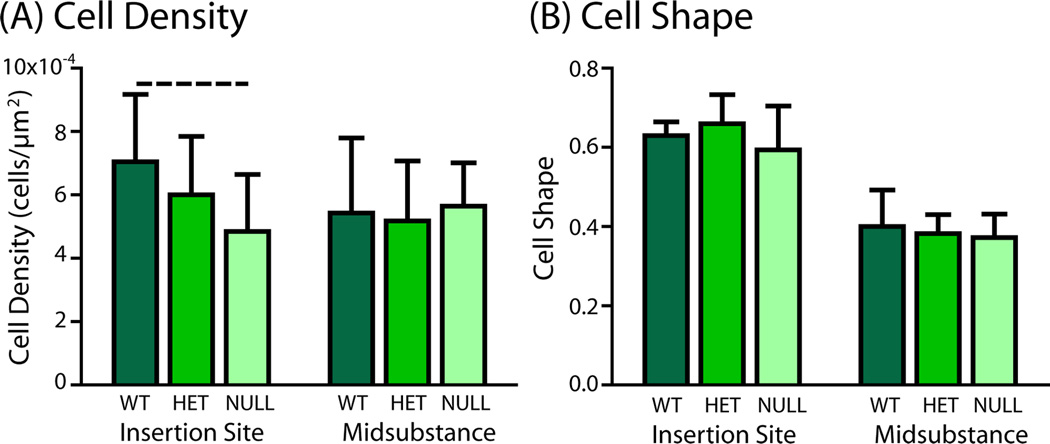
Cell density was slightly decreased (trend) in the tendon proper at the insertion site of the null tendons, but there were no differences in any of the genotypes at the midsubstance (Fig. 5). Similarly, there were no differences in cell shape between groups at either the insertion site or midsubstance, although cell shape was always rounder at the insertion site than in the midsubstance. Examination of histological sections demonstrated small pockets of hypercellular tissue between collagen fibers of the tendon proper in the null groups (not shown). These pockets were not present in either the heterozygous or wild type groups.
Figure 5.
(A) Cell density in tendon proper was slightly decreased (trend) at the insertion site of the null group compared to the wild type group. (B) There were no differences in cell shape between groups in either region.
3.4 Regional Collagen and Crosslink Content
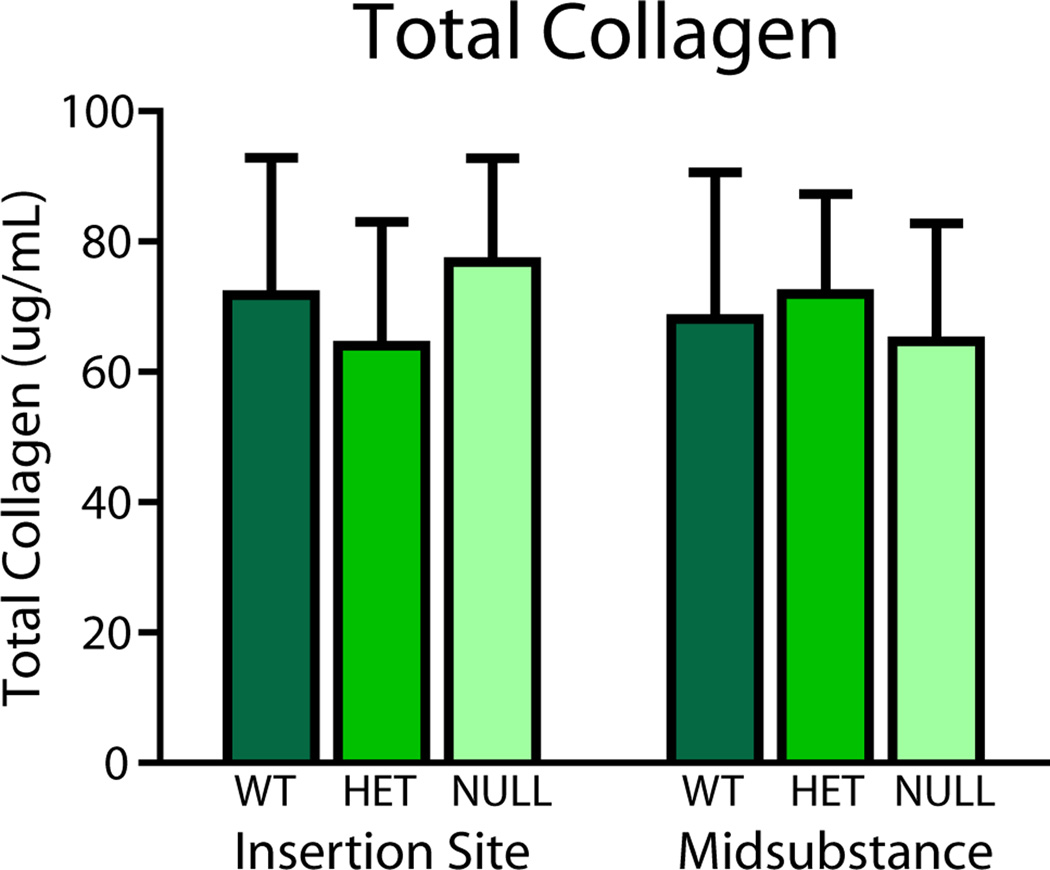
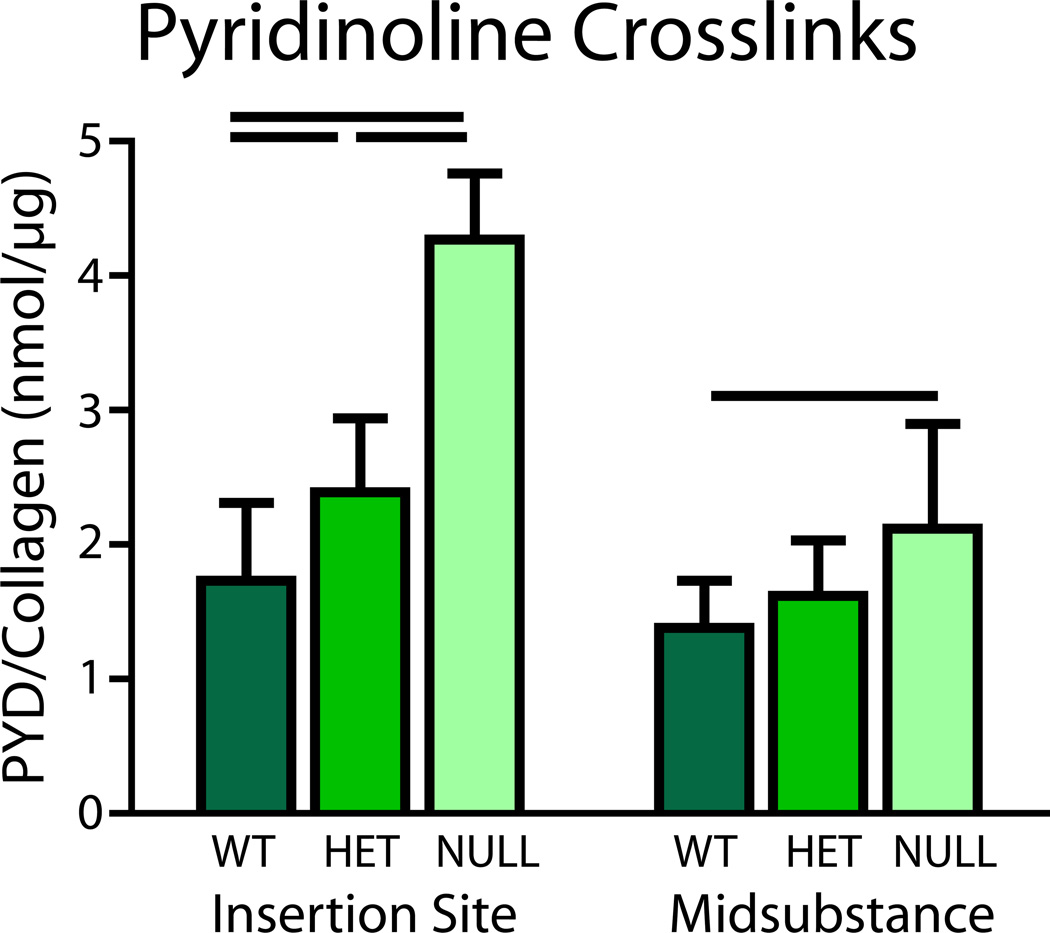
No differences in total collagen content were found between groups at the midsubstance or insertion site of the tissue (Fig. 6). However, pyridinoline, a mature collagen cross-link, was increased in the heterozygous group at the insertion site and in the null group at both regions (Fig. 7).
Figure 6.
Biochemical analysis of tendon proper and associated sheaths showed no differences in collagen content in either region.
Figure 7.
Biochemical analysis of tendon proper and associated sheaths showed increased pyridinoline crosslinks in the heterozygous group at the insertion site and in the null group at both regions when compared to the wild type.
4. Discussion
This study investigated regional collagen structure and extracellular matrix composition at multiple hierarchical scales in mouse supraspinatus tendons as a function of collagen V content. Fiber morphology and structure at the microstructural level was disrupted in both the heterozygous and null tendons. Reduced crimp amplitude and crimp frequency in the experimental groups could be due to alterations in fibril size or distribution.29; 30 Alternatively, alterations in crimp structure have been attributed to changes in ECM content as well as changes in actin cytoskeleton tensioning.31–34 Functionally, decreased crimp morphology indicates that the period of uncrimping is shorter, thus putting strain on the fibers and subsequently the fibrils earlier. This could ultimately lead to earlier damage of the tendon structure.
Reduction in collagen V expression in the supraspinatus tendon caused changes in fibril morphology in both regions. In the wild type tendons, the distribution of fibril diameters was shifted towards smaller diameters at the insertion site compared to the midsubstance. Previous studies have shown that fibril diameter distributions can differ from the bone-tendon junction to the myotendinous junction in superficial digital flexor tendons.35; 36 Small diameter fibrils provide less overall surface area on which frictional forces can act, thus possibly allowing for more dynamic motion (re-alignment, fibril deformation, sliding, etc.), which has been recently reported in these tendons.37 With the reduction of collagen V expression, the midsubstance fibril diameter became larger and fibrils were less dense in both groups, which is consistent with previous literature in tendons and ligaments from other mouse studies as well as with tendons from human studies.7; 10; 38; 39 A larger, but less dense fibril population stems from a decrease in fibril nucleation sites due to the lack of collagen V during fibrillogenesis.8; 11 This is also present at the insertion site of the heterozygous tendons. Interestingly, the fibril distribution at the insertion site of the null tendons includes an increase in large diameter fibrils, but also an increase in small diameter fibrils. An increase in small diameter fibrils has been observed in collagen V heterozygous tendons in the past,8 and could indicate increased dynamic motion during movement as discussed above. However, with repeated or increased magnitude of movement, smaller diameter fibrils will not be able to withstand the same amount of loading and will likely suffer early damage, resulting in lower stiffness tendons as seen in previous mechanical studies.37–39
Fibril irregularity was also present in the groups with reduced collagen V expression, particularly at the insertion site of the null tendons. Fibrils with abnormal shapes were present in many specimens and these fibrils also appeared to be less dense, perhaps due to their irregular shapes. ’Cauliflower-like’ fibrils have been seen previously in approximately 5% of fibrils visualized in classic EDS patient skin27 and tendon biopsies,38; 39 as well as in mouse skin,28 collagen V and collagen XI heterozygous mouse tendons,8 as well as other animal models with genetic manipulation of matrix proteins.8; 40 Regulation of collagen fibrillogenesis involves a sequence of regulatory interactions and therefore disruption of any step would be expected to share some phenotypic endpoints. However, the irregular fibrils in our study appeared to have a large center with smaller round protrusions surrounding it, as if a large fibril had fused with several small diameter fibrils around it. This was distinctively different from previously described fibrils with abnormal morphology and the severity of fibril irregularity was directly related to the amount of collagen V expression in this study. Irregular fibril shapes indicate that fibril assembly is either disrupted or unregulated due to the lack of collagen V expression. The particular phenotype found in this study suggests additionally that there may be fusion of small and large diameter fibrils during maturation in groups without collagen V expression, which has not been observed with the deletion of other regulators of fibril assembly. Irregular fibrils could have an abnormal relationship with the interfibrillar matrix (increased friction, altered ECM production), thus disrupting important dynamic re-organizations during movement.41–43 Additionally, aberrant assembly at the fibril level likely also causes disruption of assembly at higher orders, such as the fiber and fascicle level.
Although fiber and fibril morphology was disrupted in the null group, no large differences in cell morphology were found in the tendon proper, except for a slight decrease in cell density. Since it’s likely that these tendons are experiencing the same magnitude of loading during normal cage activity, this suggests that the number or morphology of cells is not directly related to ECM composition or structure alone. Pockets of hypercellular tissue were observed in histological sections in the null group, particularly in between the tendon fibers (data not shown). An increase in cells, particularly those residing in the tendon sheaths, could be a response to injury or trauma, as a compensatory mechanism to facilitate quick repair due to repeated sub-failure damage that is occurring during everyday activities.44; 45 However, without further analysis of specific cell types present or their origins, it’s still unclear whether the ECM composition was altered prior to or as a result of the recruitment of these cells. Future studies should be aimed at addressing these questions as the presence of these cells could alter not only the active mechanical response, but also future ECM remodeling in response to various mechanical and biological stimuli.
Interestingly, pydrinoline, a mature collagen crosslink, was increased in the heterozygous and null groups in both locations. This suggests that collagen processing could be altered with the removal of collagen V expression. Collagen V at the tenocyte surface has been shown to restrict early stages of fibril assembly to the cell surface domain where efficient processing, including lysyl oxidase mediated crosslink formation, is expected to occur.46 Furthermore, lysyl oxidase activity has been linked recently with other regulators of fibrillogenesis47; 48 and alterations in lysyl oxidase activity in vitro led to structurally abnormal collagen fibrils, similar to those seen in classic EDS phenotypes.49 An alternative explanation for this finding is that crosslink density must be increased to handle stresses on the tendon that cannot be otherwise reduced via dynamic re-organizations during loading, similar to increased crosslinks found in aged tendons.50
This study rigorously evaluated and defined regional changes in mouse supraspinatus composition and structure, however, several limitations exist. Given the small amount of tissue available, several semi-quantitative measures were made in this study which could have benefited from quantitative metrics, such as ELISA or western blotting techniques. Furthermore, a commercially-available kit was used to quantify pyridinoline crosslinks as opposed to the more standard optical quantification methods established previously.51; 52 While there are a number of studies that have shown the versatility and precision of the kit used in this study,24–26 it is originally designed for quantification of crosslinks in urine and has been adapted for mouse tissue in this study. Furthermore, this study was designed as a basic measurement of only one particular collagen crosslink and supports the need for additional work in this area. Additionally, fibril measurements were taken from two-dimensional cross-sections to measure collagen fibril diameter, density and irregularity. However, it would be useful to determine if there were any differences in connectivity between fibrils or significant branching effects using three-dimensional fibril tracking. In addition, this study determined regional dependence of collagen V’s role in establishing regional differences, but it is unclear if these differences are present across other tendons with similar structure, such as the Achilles tendon. Based on previous studies investigating tendon-specific differences in mechanical function, we hypothesize that these structural alterations would be much less exaggerated in those with less severe mechanical deficiencies and that collagen V is likely a regulatory path that influences those differences. Future studies will address these limitations and build upon this work.
Altogether, these results demonstrate that the role of collagen V in determining the structure and composition of mouse supraspinatus tendons is location-dependent. Although there were fibril morphology changes without any other compositional change at the midsubstance, the insertion site displayed alterations in almost every parameter. This could be explained by the increased functional requirements that are present at the insertion site compared to the tendon midsubstance or by the presence of two tissues, bone and tendon, that are both affected by reduced collagen V expression during development.53; 54 However, the cascade of events that leads to the abnormal structure and composition in the null tendons is still unclear as it’s difficult to specifically separate the effects that one might have on the other and the timing of those effects. Future studies also will build upon this work by investigating the effect of reduced collagen V expression on tendon injury in both a normal and abnormal fibril structure, which could elucidate mechanisms for the development of altered structure and composition. Furthermore, understanding the results of these mouse studies combined with the current ongoing studies in human tendons will further aid in developing a therapeutic target for classic EDS patients.
Acknowledgments
This study was supported by NIH/NIAMS (T32-AR007132, AR044745, AR065995) and the Penn Center for Musculoskeletal Disorders (NIH, P30 AR050950). The authors acknowledge Pankti Bhatt, Mei Sun, and Qingmei Yao for their assistance and technical expertise.
Footnotes
Author Contributions Statement: BK Connizzo has contributed to all aspects of this study, including research design, data acquisition, interpretation/analysis of data, and drafting/revision of the manuscript. SM Adams and T Adams were significantly involved in data acquisition and analysis/interpretation of the data. DE Birk and LJ Soslowsky have contributed significantly in research design, interpretation/analysis of data and drafting/revision of the manuscript. All authors have read and approved the final submitted manuscript.
References
- 1.Ainsworth SR, Aulicino PL. A survey of patients with Ehlers-Danlos syndrome. Clin Orthop Relat Res. 1993:250–256. [PubMed] [Google Scholar]
- 2.Stanitski DF, Nadjarian R, Stanitski CL, et al. Orthopaedic manifestations of Ehlers-Danlos syndrome. Clin Orthop Relat Res. 2000:213–221. doi: 10.1097/00003086-200007000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Symoens S, Syx D, Malfait F, et al. Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat. 2012;33:1485–1493. doi: 10.1002/humu.22137. [DOI] [PubMed] [Google Scholar]
- 4.Malfait F, De Paepe A. The Ehlers-Danlos syndrome. Advances in experimental medicine and biology. 2014;802:129–143. doi: 10.1007/978-94-007-7893-1_9. [DOI] [PubMed] [Google Scholar]
- 5.Malfait F, Coucke P, Symoens S, et al. The molecular basis of classic Ehlers-Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat. 2005;25:28–37. doi: 10.1002/humu.20107. [DOI] [PubMed] [Google Scholar]
- 6.Malfait F, De Paepe A. Molecular genetics in classic Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. 2005;139C:17–23. doi: 10.1002/ajmg.c.30070. [DOI] [PubMed] [Google Scholar]
- 7.Wenstrup RJ, Florer JB, Davidson JM, et al. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 8.Wenstrup RJ, Smith SM, Florer JB, et al. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun M, Chen S, Adams SM, et al. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011;124:4096–4105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, Connizzo BK, Adams SM, et al. Targeted deletion of collagen v in tendons and ligaments results in a classic ehlers-danlos syndrome joint phenotype. Am J Pathol. 2015;185:1436–1447. doi: 10.1016/j.ajpath.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wenstrup RJ, Florer JB, Cole WG, et al. Reduced type I collagen utilization: a pathogenic mechanism in COL5A1 haplo-insufficient Ehlers-Danlos syndrome. J Cell Biochem. 2004;92:113–124. doi: 10.1002/jcb.20024. [DOI] [PubMed] [Google Scholar]
- 12.Connizzo BK, Freedman BR, Fried JH, et al. Regulatory role of collagen V in establishing mechanical properties of tendons and ligaments is tissue-dependent. J Orthop Res. 2015 doi: 10.1002/jor.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connizzo BK, Sarver JJ, Birk DE, et al. Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon. J Biomech Eng. 2013;135:021019. doi: 10.1115/1.4023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connizzo BK, Sarver JJ, Han L, et al. In situ fibril stretch and sliding is location-dependent in mouse supraspinatus tendons. J Biomech. 2014;47:3794–3798. doi: 10.1016/j.jbiomech.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller KS, Connizzo BK, Feeney E, et al. Examining differences in local collagen fiber crimp frequency throughout mechanical testing in a developmental mouse supraspinatus tendon model. J Biomech Eng. 2012;134:041004. doi: 10.1115/1.4006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller KS, Connizzo BK, Feeney E, et al. Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J Biomech. 2012;45:2061–2065. doi: 10.1016/j.jbiomech.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birk DE, Southern JF, Zycband EI, et al. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- 19.Birk DE, Zycband EI, Woodruff S, et al. Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Dev Dyn. 1997;208:291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Connizzo BK, Yannascoli SM, Tucker JJ, et al. The detrimental effects of systemic Ibuprofen delivery on tendon healing are time-dependent. Clin Orthop Relat Res. 2014;472:2433–2439. doi: 10.1007/s11999-013-3258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connizzo BK, Bhatt PR, Liechty KW, et al. Diabetes alters mechanical properties and collagen fiber re-alignment in multiple mouse tendons. Ann Biomed Eng. 2014;42:1880–1888. doi: 10.1007/s10439-014-1031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ansorge HL, Adams S, Birk DE, et al. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng. 2011;39:1904–1913. doi: 10.1007/s10439-011-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dourte LM, Pathmanathan L, Jawad AF, et al. Influence of decorin on the mechanical, compositional, and structural properties of the mouse patellar tendon. J Biomech Eng. 2012;134:031005. doi: 10.1115/1.4006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjork JW, Meier LA, Johnson SL, et al. Hypoxic culture and insulin yield improvements to fibrin-based engineered tissue. Tissue Eng Part A. 2012;18:785–795. doi: 10.1089/ten.tea.2011.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes DH, Han WM, Smith LJ, et al. Mechanical properties of the extra-fibrillar matrix of human annulus fibrosus are location and age dependent. J Orthop Res. 2013;31:1725–1732. doi: 10.1002/jor.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson DA, Rudser K, Kunin-Batson A, et al. Biomarkers of bone remodeling in children with mucopolysaccharidosis types I, II, and VI. J Pediatr Rehabil Med. 2014;7:159–165. doi: 10.3233/PRM-140285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausser I, Anton-Lamprecht I. Differential ultrastructural aberrations of collagen fibrils in Ehlers-Danlos syndrome types I-IV as a means of diagnostics and classification. Hum Genet. 1994;93:394–407. doi: 10.1007/BF00201664. [DOI] [PubMed] [Google Scholar]
- 28.Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 29.Morgan M, Kostyuk O, Brown RA, et al. In situ monitoring of tendon structural changes by elastic scattering spectroscopy: correlation with changes in collagen fibril diameter and crimp. Tissue Eng. 2006;12:1821–1831. doi: 10.1089/ten.2006.12.1821. [DOI] [PubMed] [Google Scholar]
- 30.Magnusson SP, Qvortrup K, Larsen JO, et al. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21:369–377. doi: 10.1016/s0945-053x(02)00011-2. [DOI] [PubMed] [Google Scholar]
- 31.Grant TM, Yapp C, Chen Q, et al. The Mechanical, Structural, and Compositional Changes of Tendon Exposed to Elastase. Ann Biomed Eng. 2015 doi: 10.1007/s10439-015-1308-5. [DOI] [PubMed] [Google Scholar]
- 32.Schiele NR, von Flotow F, Tochka ZL, et al. Actin cytoskeleton contributes to the elastic modulus of embryonic tendon during early development. J Orthop Res. 2015 doi: 10.1002/jor.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legerlotz K, Dorn J, Richter J, et al. Age-dependent regulation of tendon crimp structure, cell length and gap width with strain. Acta Biomater. 2014;10:4447–4455. doi: 10.1016/j.actbio.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 34.Buckley MR, Sarver JJ, Freedman BR, et al. The dynamics of collagen uncrimping and lateral contraction in tendon and the effect of ionic concentration. J Biomech. 2013;46:2242–2249. doi: 10.1016/j.jbiomech.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe T, Imamura Y, Hosaka Y, et al. Graded arrangement of collagen fibrils in the equine superficial digital flexor tendon. Connect Tissue Res. 2007;48:332–337. doi: 10.1080/03008200701692800. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T, Imamura Y, Suzuki D, et al. Concerted and adaptive alignment of decorin dermatan sulfate filaments in the graded organization of collagen fibrils in the equine superficial digital flexor tendon. J Anat. 2012;220:156–163. doi: 10.1111/j.1469-7580.2011.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connizzo BK, Han L, Birk DE, et al. Collagen V-heterozygous and -null supraspinatus tendons exhibit altered dynamic mechanical behaviour at multiple hierarchical scales. Interface Focus. 2016;6:20150043. doi: 10.1098/rsfs.2015.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen RH, Couppe C, Jensen JK, et al. Low tendon stiffness and abnormal ultrastructure distinguish classic Ehlers-Danlos syndrome from benign joint hypermobility syndrome in patients. FASEB J. 2014;28:4668–4676. doi: 10.1096/fj.14-249656. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen RH, Holm L, Jensen JK, et al. Tendon protein synthesis rate in classic Ehlers-Danlos patients can be stimulated with insulin-like growth factor-I. J Appl Physiol. 2014;117:694–698. doi: 10.1152/japplphysiol.00157.2014. 1985. [DOI] [PubMed] [Google Scholar]
- 40.Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 41.Szczesny SE, Elliott DM. Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater. 2014;10:2582–2590. doi: 10.1016/j.actbio.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta HS, Seto J, Krauss S, et al. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J Struct Biol. 2010;169:183–191. doi: 10.1016/j.jsb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Screen HR, Shelton JC, Chhaya VH, et al. The influence of noncollagenous matrix components on the micromechanical environment of tendon fascicles. Ann Biomed Eng. 2005;33:1090–1099. doi: 10.1007/s10439-005-5777-9. [DOI] [PubMed] [Google Scholar]
- 44.Dyment NA, Liu CF, Kazemi N, et al. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PLoS One. 2013;8:e59944. doi: 10.1371/journal.pone.0059944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dyment NA, Hagiwara Y, Matthews BG, et al. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One. 2014;9:e96113. doi: 10.1371/journal.pone.0096113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, Zhang G, Birk DE. Collagen V localizes to pericellular sites during tendon collagen fibrillogenesis. Matrix Biol. 2014;33:47–53. doi: 10.1016/j.matbio.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalamajski S, Bihan D, Bonna A, et al. Fibromodulin Interacts with Collagen Cross-linking Sites and Activates Lysyl Oxidase. J Biol Chem. 2016 doi: 10.1074/jbc.M115.693408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalamajski S, Liu C, Tillgren V, et al. Increased C-telopeptide cross-linking of tendon type I collagen in fibromodulin-deficient mice. J Biol Chem. 2014;289:18873–18879. doi: 10.1074/jbc.M114.572941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herchenhan A, Uhlenbrock F, Eliasson P, et al. Lysyl Oxidase Activity Is Required for Ordered Collagen Fibrillogenesis by Tendon Cells. J Biol Chem. 2015;290:16440–16450. doi: 10.1074/jbc.M115.641670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Couppe C, Hansen P, Kongsgaard M, et al. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol. 2009;107:880–886. doi: 10.1152/japplphysiol.00291.2009. 1985. [DOI] [PubMed] [Google Scholar]
- 51.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984;137:380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 52.Walters C, Eyre DR. Collagen crosslinks in human dentin: increasing content of hydroxypyridinium residues with age. Calcif Tissue Int. 1983;35:401–405. doi: 10.1007/BF02405067. [DOI] [PubMed] [Google Scholar]
- 53.Roulet M, Ruggiero F, Karsenty G, et al. A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: a clue for understanding collagen V function in developing connective tissues. Cell Tissue Res. 2007;327:323–332. doi: 10.1007/s00441-006-0294-1. [DOI] [PubMed] [Google Scholar]
- 54.Beighton P, Thomas ML. The radiology of the Ehlers-Danlos syndrome. Clin Radiol. 1969;20:354–361. doi: 10.1016/s0009-9260(69)80085-1. [DOI] [PubMed] [Google Scholar]