Abstract
Purpose
To determine if paraproteinemic keratopathy (PPK) in the setting of monoclonal gammopathy of undetermined significance (MGUS) causes distinct patterns of corneal opacification that can be distinguished from hereditary, immunologic, or inflammatory causes.
Methods
A retrospective, interventional study of patients showed distinct bilateral opacity patterns of the cornea at the eye clinics of Hanau, Mainz, Helsinki, Marburg, and Berlin between 1993 and 2015. Data on patient characteristics and clinical features on ophthalmic examination were collected, and serum protein profiles were evaluated. A literature review and analysis of all published studies of MGUS with PPK is also presented.
Results
The largest group of patients diagnosed with MGUS-induced PPK is analyzed in this study. We studied 22 eyes of 11 patients (6 male, aged 43 to 65, mean age 54; 5 female, aged 49 to 76, mean age 61) with distinct corneal opacities and visual impairment who were first suspected of having hereditary, inflammatory, or immunologic corneal entities. Subsequently, serum protein electrophoresis revealed MGUS to be the cause of the PPK. Literature review revealed 72 patients with bilateral PPK (34 male, mean age 57; 38 female, mean age 58) in 51 studies of MGUS published from 1934 to 2015 and disclosed six additional corneal opacity patterns.
Conclusions
This thesis shows that MGUS is not always an asymptomatic disorder, in contrast to the hematologic definition, which has no hint of PPK. The MGUS-induced PPK can mimic many other diseases of the anterior layer of the eye. A new clinical classification for PPK in MGUS is proposed.
INTRODUCTION
DEFINITION OF MULTIPLE MYELOMA
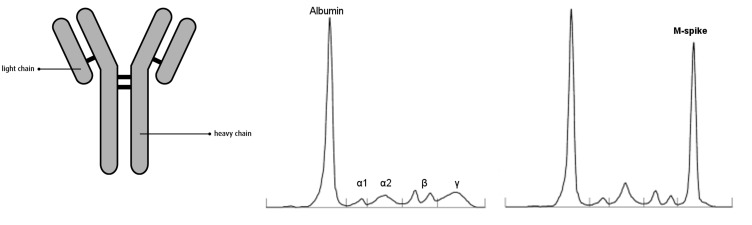
Systemic multiple myeloma is a malignancy of plasma cells. Multiple myeloma is one of the most common hematologic malignancies that show a marked increase in incidence with age.1 It represents one part of the spectrum of monoclonal gammopathies that includes the much larger category of monoclonal gammopathies of undetermined significance (MGUS), which are also age-related. Normal plasma cells, originating in the bone marrow, are white blood cells that help to defend the body against infection by producing antibodies (proteins). There are five kinds of heavy chains, termed immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin D (IgD), and immunoglobulin E (IgE), and two distinct types of light chain, termed kappa (κ) and lambda (λ). IgG typically consists of four polypeptide chains, including two heavy chains and two light chains (Figure 1, left). Serum protein electrophoresis and immunofixation electrophoresis are essential examinations to reveal monoclonal gammopathies (Figure 1, middle and right). A monoclonal protein (M protein) is characterized by the presence of a sharp, well-defined band with a single heavy chain and a similar band with a κ or λ light chain.2 The quantity of M protein can help to differentiate multiple myeloma from MGUS. Heavy chains have molecular weights of approximately 50,000, and light chains 25,000. Allansmith and McClellan3 reported that almost all immunoglobulins can be found in normal corneas and that their concentration correlates with the serum level (Figure 1, middle). They concluded that corneal immunoglobulins are derived mainly from the serum by diffusion from perilimbal vessels.
FIGURE 1.
Left, Structure of an immunoglobulin, composed of four polypeptide chains: two light chains (λ and κ) and two heavy chains. Middle, Normal pattern of serum protein electrophoresis. Right, Abnormal pattern of serum protein electrophoresis in a patient with monoclonal gammopathy presenting as the large M spike in the γ region.
Multiple myeloma is characterized by an increased number of plasma cells that produce an abnormal protein. The disease has numerous consequences, including anemia causing fatigue; bone loss resulting in weakening of the bones, bone fractures, and pain; kidney damage sometimes resulting in the need for dialysis; high calcium levels; altered immunity resulting in infections; and nerve damage that can cause numbness, tingling, or even pain or loss of strength. Bourne and coworkers4 found a corneal opacification in the form of crystals in only one of 100 unselected patients with a confirmed diagnosis of multiple myeloma and concluded that slit-lamp examination was not useful in screening for these conditions. The rarity of such corneal changes in patients with multiple myeloma is due to two factors: a gammopathy of IgGκ light chain must be present, and the disease must be chronic.5 Multiple myeloma is a disorder characterized by the production of a homogeneous M protein, usually one of the immunoglobulins or its subunit.6 Therefore the myeloma can be classified by the type of heavy and light chains produced, such as IgGκ or IgGλ or others. The most common type of heavy chain produced in myeloma is IgG. It is possible that IgG is preferentially deposited, compared with secretory IgA and IgM, because of its lower molecular weight and easier penetration into the tissues. Occasionally, the malignant plasma cells produce only the light-chain component of the antibody. In such cases, the light chains are often excreted into urine and can be identified with a variety of assays.
DEFINITION OF MONOCLONAL GAMMOPATHY OF UNDETERMINED SIGNIFICANCE AND SMOLDERING MULTIPLE MYELOMA
According to a 1984 study by Kyle,7 monoclonal gammopathies can be divided into systemic monoclonal gammopathy, such as multiple myeloma and similar diseases, and MGUS, formerly known as benign monoclonal gammopathy. The diagnosis of MGUS assumes that a complex systemic workup has excluded the presence of multiple myeloma, macroglobulinemia, amyloidosis, and other disorders. The incidence of MGUS is as high as 6% in people from 60 to 80 years of age when immunofixation techniques are used to make the diagnosis.1 The International Myeloma Working Group defined three criteria for the diagnosis of MGUS: (1) M protein concentration in serum of 3 g/dL or less, (2) a proportion of plasma cells in the bone marrow of 10% or less, and (3) absence of lytic bone lesions, anemia, hypercalcemia, and renal insufficiency related to the proliferation of monoclonal plasma cells.8
Smoldering (asymptomatic) multiple myeloma (SMM) requires the presence of a monoclonal protein level of 3 g/dL or more or a proportion of clonal plasma cells in the bone marrow of 10% or more but no end-organ damage.9 It needs to be distinguished from MGUS because of a higher risk of progression to multiple myeloma or a related disorder—10% per year for SMM vs 1% per year for MGUS. Among 21,463 residents of Olmsted County, Minnesota, MGUS was found in 694 (3.2%). The prevalence of MGUS was 5.3% among persons 70 years of age or older and 7.5% among those 85 years of age or older.10 The isotype of the monoclonal immunoglobulin was IgG in 68.9% of the 694 patients with MGUS, IgM in 17.2%, IgA in 10.8%, and biclonal in 3.0%. Regrettably, however, no slit-lamp examination of the cornea was performed.
Another study that showed that the presence of an M protein in 93% of patients with multiple myeloma 7 years before diagnosis of multiple myeloma strongly confirms that a protracted premalignant stage (MGUS) precedes nearly all cases of multiple myeloma.11 However, the precise mechanisms that maintain the MGUS state and the mechanisms that trigger progression from MGUS to multiple myeloma are still not known. Loss of chromosome 13 is a well-documented adverse prognostic indicator in multiple myeloma, but is also seen in MGUS and is thought to be an early event in disease progression.12 The simultaneous occurrence of MGUS and paraproteinemic keratopathy (PPK) could be the first sign of progression from MGUS to SMM. Recently, Balderman and Lichtman13 described forms of ocular injury that may accompany essential monoclonal gammopathy, which include crystalline keratopathy, crystal-storing histiocytosis, hypercupremic keratopathy, and maculopathy.
IMMUNOTACTOID KERATOPATHY OR PARAPROTEINEMIC KERATOPATHY IN MONOCLONAL GAMMOPATHY
The initial description of corneal noncrystalline deposits in association with paraproteinemias was by Meesmann14 in 1934 in the setting of multiple myeloma. Blobner15 was the first to describe crystalline deposits in the whole cornea associated with a paraproteinemia. In 1958 Bürki16 reported a patient with multiple myeloma and bilateral corneal crystals. The definitive evidence that corneal crystals in gammopathies are immunoglobulins was demonstrated by Klintworth and coworkers17 in 1978 using immunofluorescence and immunoperoxidase techniques. The classic appearance of these deposits on electron microscopy is that of parallel, banded crystalloids with an internal periodicity of 10 to 13 nm, although other ultrastructural configurations have been observed, including hexagonal crystals and granular material. The occurrence of corneal opacities in monoclonal gammopathies is rare.18 The classic corneal involvement consists of bilateral crystals in all or different layers of the cornea, often combined with diffuse or patch-like opacities. There are several reports of singular multiple myeloma cases that demonstrate noncrystalline corneal manifestations, such as posterior honeycomb pattern and vortex keratopathy.18,19
Over the last three decades, reports in the renal literature have described immunotactoid deposition associated with glomerulopathy—organized microtubular deposits of IgGκ that measured 32 to 50 nm in diameter in renal biopsies.18 The term immunotactoid keratopathy (ITK) was coined by Garibaldi and coworkers18 to describe the corneal deposition of IgGκ as tubular, electron-dense, crystalloid deposits with a central lucent core on electron microscopy. This aligns the ophthalmic literature with the renal, which has defined immunotactoid glomerulopathy by similar criteria since its description by Schwartz and coworkers in 2002.20 This nomenclature references the resemblance of the elongated immunoglobulin to the linear crystallization of hemoglobulin S as tactoids in sickle cell anemia.
Monoclonal gammopathy of undetermined significance is one of the most common premalignant disorders in Western countries, with a prevalence of 3.2% in the white general population 50 years of age or older.7 Hematologists interpret MGUS as an asymptomatic condition characterized by the presence of a monoclonal immunoglobulin (M protein) in the absence of any clinical signs or symptoms of multiple myeloma or other lymphoproliferative malignancies.9 However, ophthalmologists have an essential role in detecting MGUS because ITK can represent the first evident sign of this disease.18 The different patterns of ITK are relatively unknown and can mimic some forms of hereditary and degenerative corneal disorders. Lisch and coworkers,21 in 2012, applied the ultrastructural term immunotactoid keratopathy to the clinical spectrum of crystalline keratopathy in the setting of MGUS. Despite the low incidence of ocular findings in the setting of paraproteinemias, these systemic diseases must be included in the differential diagnostic considerations in corneal opacification and corneal deposits, as corneal findings may be the initial manifestation of systemic disease.18
Five distinct patterns of corneal protein deposition were described by Lisch and coworkers21 (Table 1). Hall and coworkers22 confirmed this classification with four of their own cases. They concluded that ophthalmologists should familiarize themselves with the Lisch ITK classification to enable the detection of corneal immunoglobulin in its various forms. Today, we have to point out that the term immunotactoid keratopathy is justified to use only in descriptions of the crystalline opacity pattern of the cornea in MGUS that looks the same as immunotactoid nephropathy on electron microscopy. However, we know there are other corneal opacity patterns in MGUS that show no crystalline deposits either clinically or histologically. That is why Milman and coworkers23 propose to use currently the general term paraproteinemic keratopathy to describe the distinct corneal crystalline and noncrystalline deposits in MGUS. IgGκ is the most frequently reported immunoglobulin fraction associated with intracorneal deposition.1
TABLE 1.
CLASSIFICATION OF MGUS-INDUCED ITK IN 2012*
|
ITK, immunotactoid keratopathy; MGUS, monoclonal gammopathy of undetermined significance.
From Lisch et al.21
OBJECTIVE
The International Myeloma Working Group has issued updated criteria for the diagnosis of monoclonal gammopathies. They define MGUS as the absence of subjective symptoms and objective signs in the form of hypercalcemia, renal insufficiency, anemia, and bone lesions. An annual internal and hematologic check of MGUS is recommended because of the occurrence of a systemic monoclonal gammopathy in 30% of cases. We hypothesize that MGUS might not always be asymptomatic but may be a myriad of completely distinct, bilateral corneal opacity patterns that can be differentiated from various forms of corneal dystrophy, metabolic diseases associated with corneal involvement, and others. These paraproteinemia-associated corneal opacities frequently result in significant visual impairment and in such cases cannot be further defined as a condition “of undetermined significance.”
In this study we describe 11 patients with bilateral distinct corneal opacities evaluated between 1993 and 2015. For all patients, family history regarding ocular diseases was examined, and in two patients DNA analyses were performed. None of the patients had a family history of corneal disease. No known disease-causing mutations were detected in the decorin or transforming growth factor beta–induced (TGFBI) genes. One female patient already had a documented follow-up of 10 years of MGUS, which currently has progressed to SMM. One male patient from Helsinki had an M component diagnosed 6 years previously. In two patients a corneal biopsy and in one patient a bilateral penetrating keratoplasty were performed. Histologic and immunohistochemical studies of the corneas revealed the diagnosis of MGUS-induced PPK in these three cases. During the months following penetrating keratoplasty, corneal opacities recurred in the patient’s graft. Consequently, the primary diagnosis of a stromal corneal dystrophy was excluded. Only late serum protein electrophoresis, following the ophthalmologic evaluation, disclosed the diagnosis of MGUS-induced PPK in 9 patients with unknown history of MGUS. The MGUS-induced PPK appeared in most cases in the form of superficial punctiform or comma-shaped crystals, lattice-like units, central or peripheral granular changes, and geographic-like opacity patterns. One male patient showed primarily signs of peripheral corneal inflammation and stem cell insufficiency without corneal neovascularization. Also, this patient’s serum protein electrophoresis confirmed an MGUS-induced PPK. The female patient with SMM presented with additionally a golden-brown opacity at the level of Descemet’s membrane. A hypercupremia with a copper serum level of more than 1000 μg/dL was identified. The association of MGUS, hypercupremia, and golden-brown discoloration of Descemet’s membrane represents a rare syndrome that we propose to call Lewis syndrome. We further hypothesize that an expanded classification system may be required to describe the distinct morphologic forms of PPK in MGUS, based on our data and that from previously published literature.
In conclusion, our study shows the necessity of careful slit-lamp examination in each MGUS patient in order to reveal a potential MGUS-induced corneal involvement. The intensive collaboration between the hematologist and ophthalmologist in the future can disclose whether or not the ocular involvement in MGUS represents a risk factor for progression to SMM and multiple myeloma. In general, systemic treatment of MGUS is not indicated according to the hematologic definition of this disease. However, in case of a severe visual impairment as a result of MGUS-induced PPK, systemic therapy in the form of dietary options, a systemic corticosteroid, and/or chemotherapy must be discussed with the patient and the hematologist. A penetrating keratoplasty cannot be recommended because of the quick recurrence in the graft during the first year postoperatively without any systemic therapy. The etiology of the complex MGUS-induced PPK remains undefined on account of the ill-defined mechanisms of immunoglobulin transport into the cornea. The tear film, diffusion from aqueous fluid from the anterior chamber, influx via the paralimbal vascular arcades, extracellular stromal matrix, glycosaminoglycans, and abnormal synthesis within keratocytes are discussed. The corneal opacity patterns of PPK resemble those observed in other corneal entities. The chameleon-like appearance of corneal opacity patterns has to be differentiated from distinct hereditary corneal entities and inflammatory processes of the anterior eye.
METHODS AND MATERIALS
Ethics approval for this retrospective analysis was obtained from the Ethics Committee of Rhineland-Palatine, Germany. All components of research in this study herein have adhered to the tenets set forth in the Declaration of Helsinki as well as all state and local laws. The institutional review board determined and granted an exemption status for this study due to its retrospective nature and the lack of revelation of any patient identifiers and the maintenance of confidentiality on all privacy information related to all study subjects. Because this was a retrospective study, registration for clinical trials was not needed. This study has complied with all regulations stipulated by HIPAA (the Health Insurance Portability and Accountability Act).
This was a retrospective study involving five centers (City Eye Clinic of Hanau; Department of Ophthalmology, University Medical Center of the Johannes Gutenberg University Mainz; Department of Ophthalmology, University of Helsinki; Department of Ophthalmology, University of Marburg; Department of Ophthalmology, Campus Virchow-Klinikum, University Medical Center Charité Berlin). Between 1993 and 2015, 11 patients (5 female, mean age 60; 6 male, mean age 54) with unclear bilateral corneal opacities were transferred from external ophthalmologists to the eye clinics of Hanau, Mainz, Helsinki, Marburg, and Berlin to elucidate the correct diagnosis. The author (W.L.) was able to evaluate 8 of the 11 patients himself in Hanau, Mainz, and Marburg. Two patients were examined and documented by coauthor Kivelä in Helsinki and one patient by coauthor Pleyer in Berlin. One female patient with a new unclear corneal opacity already had a documented 10-year history of monoclonal gammopathy and a current diagnosis of SMM. In a male patient from Helsinki, an M component was diagnosed 6 years before a bilateral corneal opacity could be revealed. Nine patients did not have a known history of MGUS or other hematologic entities at the time of the ophthalmologic evaluation. In all patients an exact inquiry was made regarding the first occurrence of corneal changes.
Bilateral slit-lamp examination with direct and indirect illumination from the iris and retina by dilated pupil was performed and documented. Confocal microscopy and optical coherence tomography (OCT) provided additional data. For all 11 patients an examination of the family members and in two patients a DNA analysis were performed. In a male patient with diffuse stromal opacity of the cornea, DNA sequencing of polymerase chain reaction products was generated from amplification of exons and adjacent introns of the decorin gene. In the same male patient and in a female patient with lattice-like stromal opacity genomic, DNA was extracted from peripheral blood leukocytes by standard methods. Linkage analysis to the TGFBI gene was evaluated using microsatellite markers. In two patients a corneal biopsy and in one a bilateral penetrating keratoplasty were performed. Light and electron microscopic and immunohistochemical studies revealed the diagnosis of MGUS-induced PPK in these three cases. Immunohistochemical staining was performed with primary antibodies against heavy chains and kappa and lambda light chains. Additional evaluation of the copper level in serum and 24-hour urine collection, as well as coeruloplasmin in serum, was performed in the female SMM patient with golden-brown predescemental opacity. The amount of protein-bound copper in serum was determined at room temperature by membrane filtration on a 10-mL Amicon Diaflo ultrafiltration cell (Amicon Corp) with a membrane ability to retain molecules with molecular weight greater than 10,000. Copper in the filtrate was measured by atomic absorption spectrophotometry and compared to the copper in the serum sample. In nine patients without a known history of MGUS, serum and urine protein electrophoresis tests were performed in distinct intervals from weeks to 3 years after the first diagnosis of corneal involvement. Electrophoresis was analyzed on agarose gel. We show an exemplary presentation and follow-up of two patients from Mainz (a 61-year-old woman with a 17-year follow-up and a 49-year-old man with a 7-year follow-up) with additional intensive internal evaluation.
RESULTS
One female patient already had a documented 10-year history of MGUS and a current diagnosis of progression to SMM. One female patient had an M component diagnosed 6 years before the first corneal involvement occurred. In nine cases without a known history of hematologic disorders, the morphology of the cornea was suggestive of a corneal dystrophy, a systemic hereditary disease with corneal involvement, or an inflammatory process of the anterior eye. The general systemic checkup, examination of the family members, and DNA analyses could not confirm the clinical suspicion. After weeks, months, and years of follow-up, the serum/urine protein electrophoresis, the immunofixation test, and in part the light and electron microscopic and immunohistochemical examination after corneal biopsy or penetrating keratoplasty elucidated the correct hematololgic diagnosis of MGUS. Electrophoresis revealed an IgGκ light chain in six patients, an IgGλ light chain in one patient, an IgGκ and IgGλ light chain in one patient, IgGλ and IgAλ light chains as a biclonal gammopathy in one patient, and an M component without other signs in a female patient. In many cases the corneal opacity patterns of PPK resemble other corneal entities (Figures 2, 3, and 4 ). The types of corneal opacity patterns observed in our patients and their differentiation to distinct hereditary corneal entities and inflammatory processes of the anterior eye are presented in Table 2.
FIGURE 2.
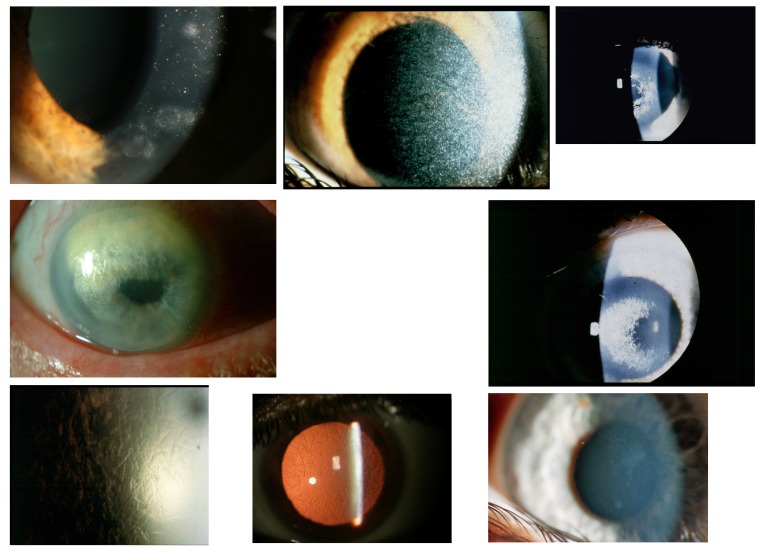
Top row, Case 1 Table 2, MGUS-induced punctiform corneal crystals + patches (left) vs punctiform crystals in cystinosis without patches (middle) and vs crowded comma-shaped crystals of the crystalline type of Schnyder corneal dystrophy + haze (right). Middle row, Case 2 Table 2, MGUS-induced anterior comma-shaped crystals (left) vs crowded comma-shaped crystals of Schnyder corneal dystrophy (right). Bottom row, Case 3 Table 2, MGUS-induced lattice-like lines in retroillumination from the iris (left) vs true lattice lines in retroillumination from the retina of lattice corneal dystrophy type 1 (middle); disappearing of the lattice lines of case 3 and transforming of mild diffuse opacities with focal epithelium elevations after a follow-up of 22 years (right).
FIGURE 3.
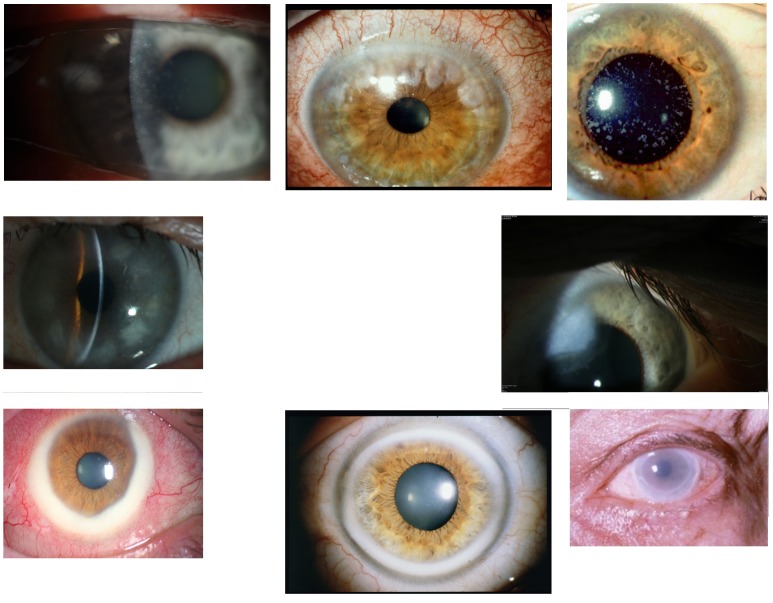
Top row, Case 4 Table 2, MGUS-induced central granules (left) and Case 5 Table 2, MGUS-induced peripheral granules (middle) vs granules of granular corneal dystrophy type 1 (right). Middle row, Case 6 Table 2, MGUS-induced peripheral patch-like opacities (left) vs peripheral hypertrophic degeneration (right). Bottom row, Case 7 Table 2, MGUS-induced peripheral large circular yellowish band with conjunctival injection (left) vs arcus lipoides (middle) and vs peripheral ring of lecithin cholesterol acetyltransferase deficiency (right).
FIGURE 4.
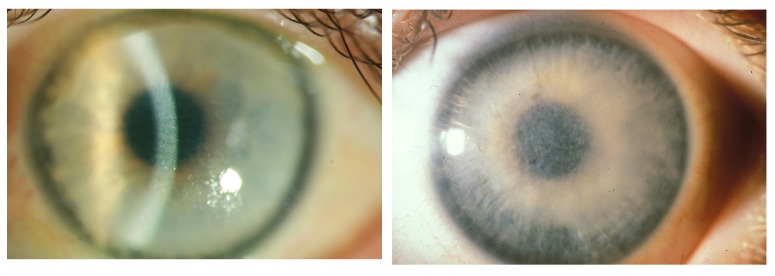
Case 8 Table 2, MGUS-induced superficial geographic-like opacity with transparent limbus zone (left) vs geographic-like opacity pattern of Reis-Bücklers corneal dystrophy (right).
TABLE 2.
CORNEAL OPACITY PATTERNS IN PATIENTS WITH MGUS-INDUCED COMPLEX PPK
| CASE NUMBER, FIGURE | AGE (YR), GENDER | BCVA RIGHT, LEFT | TYPE OF CORNEAL OPACITY PATTERN | FIRST SUSPICIOUS DIAGNOSIS | SERUM PROTEIN ELECTROPHORE SIS AND IMMUNOFIXATIO N TEST | CORRECT DIAGNOSIS PPK | TIME BETWEEN FIRST OPHTHALMOLGIC EVALUATION AND CORRECT DIAGNOSIS | FOLLOW-UP |
|---|---|---|---|---|---|---|---|---|
| Case 1 Figure 2, top row left |
49, F | R/L: 20/60 | Epithelial, punctiform crystals + patches |
|
IgGκ light chain | Punctiform crystalline-like PPK | 1 year | 1 year |
| Case 2 Figure 2, middle row left |
76, F | R/L: Hand movements | Epithelial, comma-shaped crystals + diffuse opacities | Schnyder CD | M component | Comma-shaped crystalline PPK | Few weeks | 4 years with progression of corneal opacities |
| Case 3 Figure 2, bottom left |
49, F | R: 20/60 L: 20/40 |
Lattice-like opacity pattern | Lattice CD | IgGκ light chain | Lattice-like PPK | 2 years | 22 years; disappearing of lattice lines and transforming of diffuse opacities 4 years with slow progression of corneal opacities |
| Case 4 Figure 3, top left |
60, M | R/L: 20/20 | Central multiple granules | Granular CD, type1 | IgGλ light chain | Central granular-like PPK | First M component, 6 years later occurrence of keratopathy | |
| Case 5 Figure 3, top middle |
58, M | R/L: 20/200 (age-related macular degeneration) | Peripheral multiple granules | Granular CD, type1 | IgGκ light chain | Peripheral granular-like PPK | 1 year | 1 year |
| Case 6 Figure 3, middle left |
65, M | R/L: 20/40 | Peripheral multiple patches | Peripheral hypertrophic degeneration | IgGκ light chain | Peripheral multiple patch-like PPK | 1 year | 1 year |
| Case 7 Figure 3, bottom left |
53, M | R/L: 20/20 | Peripheral large circular yellowish band + conjunctival injection | Corneal inflammation of limbal stem cells | IgGκ light chain | Peripheral inflammatory band-like PPK | 2 months | 3 years; progression of limbal band |
| Case 8 Figure 4, left |
73, F | R/L: 20/80 | Subepithelial, geographic opacities |
|
IgGκ and IgGλ light chains | Geographic-like PPK | 3 months | 3 years |
| Case 9 | 47, M | R/L: 20/20 | Diffuse opacities at the posterior cornea | Cornea farinata | Free light chain κ/λ ratio, 2.2 mg/L (normal range, 0.26–1.65 mg/L); precursor of MGUS? | Flake-like PPK at the posterior cornea | 1 month | 1 year |
| Case 10 Figure 5, top left |
59, F | R: 20/25 L: 20/80 (cataract) |
Central disc at Descemet | Posterior corneal pigmentation | IgGλ and IgAλ of SMM | Central Descemet disc + hypercupremia + SMM | 3 years | 17 years |
| Case 11 Figure 6, top left |
43, M | R: 20/40 L: 20/32 |
Flake-like stromal opacification | Diffuse stromal CD | IgGκ light chain | Stromal flake-like PPK | 6 years | 7 years |
BCVA, best-corrected visual acuity; CD, corneal dystrophy; IgA, immunoglobulin A; IgG, immunoglobulin G; L, left; M component, monoclonal component; MGUS, monoclonal gammopathy of undetermined significance; PPK, paraproteinemic keratopathy; R, right; SMM, smoldering multiple myeloma.
THE COMPLEX DIAGNOSTIC AND THERAPEUTIC CHALLENGE OF PPK
The detailed results of two patients with MGUS, presented below, illustrate the complicated differential diagnostic considerations in the long-term hematologic and ophthalmologic follow-up and the unique corneal involvement.
Case 10
In 1998, a 42-year-old female patient (Table 2, case 10) showed a biclonal gammopathy of undetermined significance of IgGλ and IgAλ light chains (Table 3). At that time the kappa-lambda quotient was 0.2 (normal. 1.1–2.7). Results of tests for Bence-Jones proteinuria and electrophoresis of urine for kappa and lambda chains were consistently negative. Bone marrow biopsy revealed 8% plasma cells (normal, >2%) with discrete atypia. Physical examination and bone x-rays revealed no abnormality. Both corneas showed no signs of opacification. The patient appeared to be in healthy condition. Regular checkups every 3 months were recommended, but no therapy. In 2012, after a follow-up of 14 years, the first corneal changes were revealed by an external ophthalmologist. The hematologic workup revealed IgGλ of 11.3 g/L and IgAλ of 25 g/L. There was a bone marrow infiltration of 15% to 20% plasma cells. The bone scintigram showed no osteolytic lesions.
TABLE 3.
| IgG, g/L (normal range,7.0–16.0) | IgA, g/L (normal range, 0.7–4.0) | IgM, g/L (normal range, 0.4–2.3) |
|---|---|---|
| 9.6 | 15.8 | 0.54 |
Hematologic diagnosis was biclonal gammopathy IgGλ and IgAλ of undetermined significance.
Same patient as in Table 4.
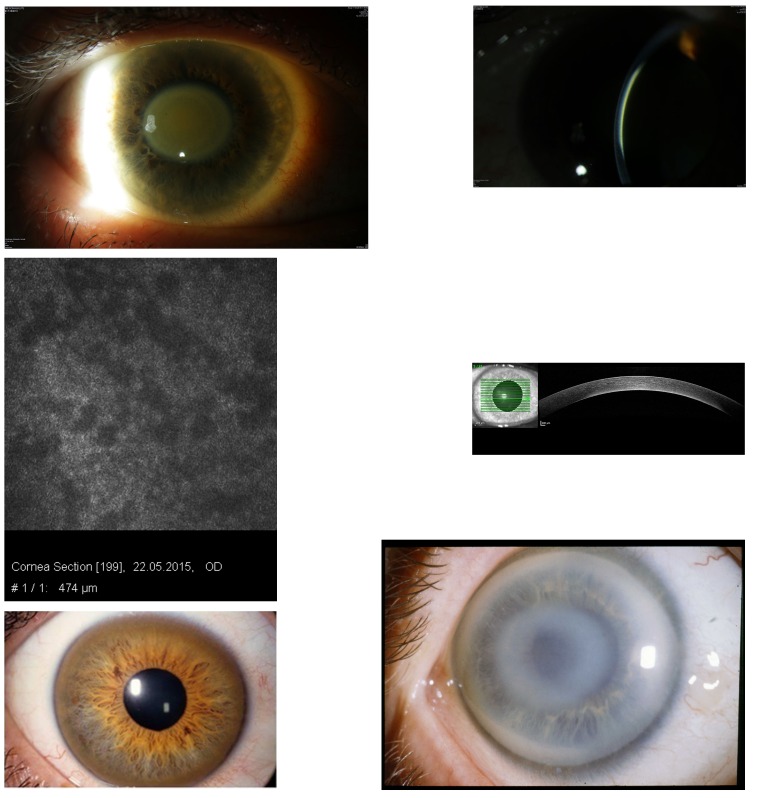
In 2015, the patient was 59 years old and in healthy condition, and after a follow-up of 17 years, best-corrected visual acuity was 20/25 OD and 20/80 OS. Slit-lamp examination revealed a central discoid, golden-brown discoloration of each cornea secondary to pigmentation at the level of Descemet’s membrane with clear overlying and peripheral stroma (Figure 5, top left). The thin slit beam shows the opacity at the level of Descemet’s membrane (Figure 5, top right). No pigment deposition was noted on the anterior or posterior lens capsule or the surface of the iris. There was an age-related anterior and posterior opacity of the lens cortex in the left eye. The confocal microscopy showed dark areas at the corneal level of 474 μm, partly covering the opaque endothelial cells (Figure 5, middle left). The OCT showed a hyperreflectivity of posterior cornea (Figure 5, middle right). The hematologic workup revealed an increase of serum IgG to 45.6 g/L. The kappa-lambda quotient was 0.3. The bone marrow biopsy contained 25% plasma cells. The hematologists interpreted the findings as SMM with no indication for systemic therapy. The patient had a marked elevated serum copper level at 1326 μg/dL (normal, 76–152) (Table 4). Copper in 24-hour urine collection (12 μg/dL, normal 10–60) and the level of ceruloplasmin in serum (0.23 g/L, normal 0.2–0.6) were normal. The association of MGUS + hypercupremia + corneal golden-brown opacification is the first such case reported in Europe and is an indication of a syndrome. The MGUS + hypercupremia-induced disc-like corneal opacity must be differentiated from the Kayser-Fleischer ring of Wilson’s disease (Figure 5, bottom left) and from noncrystalline Schnyder corneal dystrophy (Figure 5, bottom right).
FIGURE 5.
Case 10 Table 2, central discoid, golden-brown discoloration of the cornea in a 59-year-old female patient with biclonal gammopathy of undetermined significance and hypercupremia (top left). The thin slit beam shows that the opacity is at the level of Descemet’s membrane (top right). Confocal microscopy at the level of 474 μm reveals irregular dark areas, which partly cover the endothelial cells (middle left). Optical coherence tomography shows the opacity on the posterior cornea of the patient (middle right). Kayser-Fleischer peripheral brownish ring in Wilson’s disease (bottom left). Noncrystalline Schnyder corneal dystrophy with subepithelial central opacity and arcus lipoides (bottom right).
TABLE 4.
| IgG, g/L | IgA, g/L | IgM, g/L | Copper, μg/dL (nomal range, 76–152) |
|---|---|---|---|
| 57.7 | 0.88 | 0.23 | 1326 |
Hematologic diagnosis was smoldering multiple myeloma IgGλ and IgAλ of undetermined significance + hypercupremia. Ophthalmologic diagnosis was bilateral corneal opacity due to smoldering multiple myeloma + hypercupremia.
Same patient as in Table 3.
Case 11
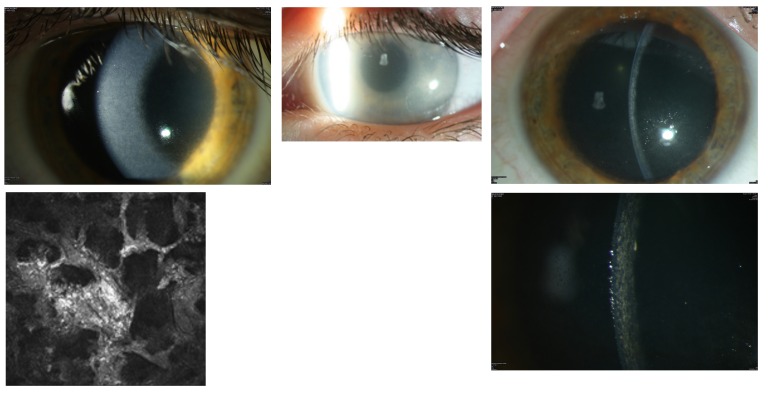
In 2009, a 43-year-old man (Table 2, case 11) sought treatment for bilateral visual impairment. The patient reported visual reduction with photophobia for 2 years. Visual acuity was 20/40 OD and 20/32 OS. Slit-lamp examination revealed a bilateral flake-like opacification of the whole corneal stroma (Figure 6, top left). The thin slit beam showed that the opacity was throughout the whole stroma without epithelial and endothelial involvement (Figure 6, top right). Confocal microscopy revealed large irregular whitish bands masquerading the keratocytes (Figure 6, bottom left). The phenotypical corneal aspect was very similar to that of congenital stromal corneal dystrophy (Figure 6, top middle). However, the late occurrence of the patient’s corneal opacity was not compatible with the diagnosis of this autosomal dominantly inherited corneal dystrophy. Examination of the patient’s parents and three sisters disclosed bilateral completely uninvolved corneas. As expected, no known disease-causing mutations were detected in the TGFBI and decorin genes.
FIGURE 6.
Case 11 Table 2, a 43-year-old male patient with MGUS-induced flake-like corneal opacification in 2009 (top left) vs flake-like phenotype of congenital stromal corneal dystrophy (top middle). Thin slit beam shows that the MGUS opacity involves the whole stroma (top right). Confocal microscopy shows large bands that mask the keratocytes (bottom left) and progression of the stromal opacities on the right eye in 2012 (bottom right).
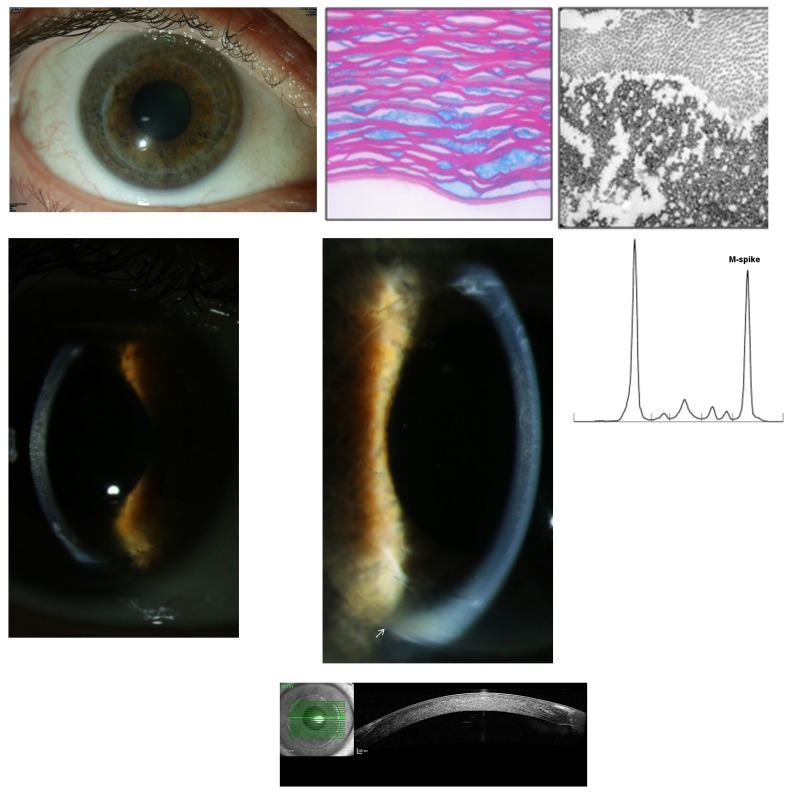
Severe bilateral progression of the patient’s stromal opacities occurred from 2009 to 2012 (Figure 6, bottom right). Visual acuity was 20/160 OU. The patient underwent bilateral penetrating keratoplasty procedures in 2012. The first 10 postoperative months showed no clinical complications (Figure 7, top row left). Light microscopy examination of both corneal discs revealed irregular stromal, predominantly extracellular deposits staining red with Masson trichrome and partly swollen keratocytes (Figure 7, top row middle). Transmission electron microscopy demonstrated almost exclusively extracellular, electron-dense, well-demarcated stromal deposits (Figure 7, top row right). The patient’s left graft showed the first signs of recurrence in the anterior stroma 12 months postoperatively. Best-corrected visual acuity was 20/32 OU. The patient had progressive bilateral visual impairment during the following months in 2014. Best-corrected visual acuity was 20/80 OD and 20/63 OS at the beginning of 2015. Slit-lamp examination revealed a bilateral flake-like opacification of the stromal graft, completely identical to the primary corneal involvement (Figure 7, middle row left and middle). At that time, serum protein electrophoresis was performed. It revealed MGUS of IgGκ light chains (Figure 7, middle row right). The OCT of the right graft showed the diffuse haze throughout the whole stroma (Figure 7, bottom). However, according to hematologic guidelines, MGUS does not require any systemic therapy. We are considering starting chemotherapy in this case owing to the bilateral PPK of the grafts resulting in progressive visual impairment. A further surgical corneal option is contraindicated because of the quick recurrence. The immunohistochemistry of the cornea and the serum is shown in Figure 8.
FIGURE 7.
Case 11 Table 2. Top row, Left eye 12 month after penetrating keratoplasty showing no complications (left); histologic evaluation of penetrating keratoplasty specimens demonstrates irregular corneal deposits exhibiting placoid configuration (Masson’s trichrome, ×136) (middle); and transmission electron microscopy image demonstrating stromal extracellular, electron-dense deposits and on the superior part of the image normal collagen fibrils (×81,000) (right). Middle row, Recurrence of stromal involvement, identical to the primary opacity pattern, in the graft of the right eye (left) and of the left eye (arrow localizing graft’s border) in 2014 (middle); protein electrophoresis of the serum with an elevated M spike (right). Bottom, OCT of the right graft showing dense stromal opacities.
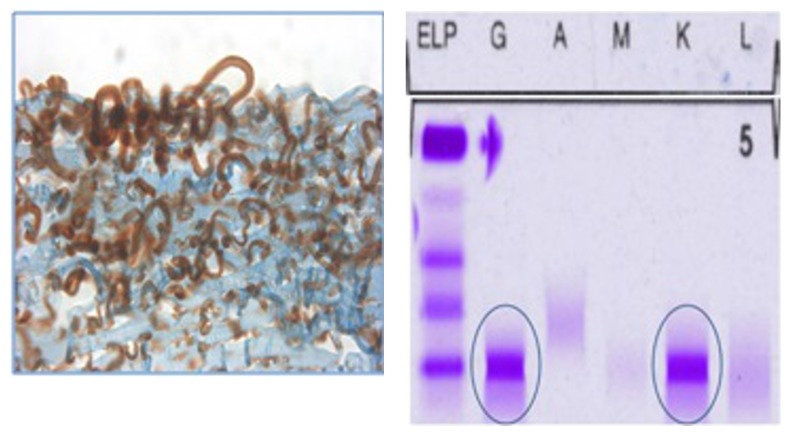
FIGURE 8.
Case 11 Table 2, Immunhistochemistry of the cornea in 2015 (left) showing brownish staining of subepithelial material in an immunoperoxidase reaction for IgG, and with agarose gel electrophoresis of the serum in 2015 (right) that confirms an MGUS-induced paraproteinemic keratopathy of IgGκ light chain type, identified with blue circles; ELP, electrophoresis; G, IgG; A, IgA; M, IgM; K, kappa light chain; L, lambda light chain.
Our study makes it possible to present a new, enlarged classification system of 11 distinct forms of MGUS-induced PPK (Table 5).
TABLE 5.
NEW CLASSIFICATION OF MGUS-INDUCED PPK BASED ON THE PRESENT STUDY
|
MGUS, monoclonal gammopathy of undetermined significance; PPK, paraproteinemic keratopathy.
DISCUSSION
There are many other cases of distinct involvement of MGUS- or SMM-induced PPK that either are identical to the 11 distinct forms in our classification system (Table 5) or showed additional corneal opacity patterns. Forty-one reports dealt with one MGUS patient with distinct corneal involvement. 13–16,19,24,26,28, 32,34,36,38,43,45–48,50–53,55,58–60,64–68, 70–72,76–78 Seven reports each described two MGUS patients with different corneal opacity patterns. 17, 27, 33–35, 44, 49, 54 Hall and coworkers22 described four MGUS patients, Lisch and coworkers21 six MGUS patients, and Milman and coworkers23 seven MGUS patients with distinct forms of PPK. In total, 34 male patients (mean age, 57) and 38 female patients (mean age, 58) with bilateral PPK have been reported in the literature.
SUPERFICIAL PUNCTIFORM CRYSTALLINE-LIKE PPK (WITH AND WITHOUT PERIPHERAL PATCHES)
This corneal opacity pattern has been described in seven publications17, 23–28 and is similar to that shown for the MGUS-induced corneal change with peripheral patches (Case 1, Table 2; Figure 2, top row left).21 From 1947, Palm24 discussed a 46-year-old woman with corneal crystals and rheumatoid arthritis and spondyloarthritis ankylopoietica. In 1942, Holmberg and Grönwall25 from Sweden examined the blood plasma of the same patient and found a previously unknown globulin. Klintworth and coworkers17 reported a 59-year-old man with bilateral punctiform crystals at the level of corneal epithelium after a follow-up of 5 years. An MGUS with IgGκ was diagnosed. The corneal crystals had completely disappeared after 2 years of chemotherapy. Grossniklaus and coworkers26 described a 64-year-old woman with IgAκ light and heavy chains disclosing crystals distributed throughout superficial corneas and conjunctivas bilaterally. They concluded that systemic evaluation, including bone marrow biopsy, will determine the presence of multiple myeloma vs a benign paraproteinemia. Froussart and coworkers27 described therapy with chlorambucil and a regression of corneal crystals in a 68-year-old female patient.
Kleta and coworkers28 reported a 49-year-old woman who was first diagnosed as having a presumed ocular cystinosis presenting as crystals in a whorl-like fashion. The serum IgG concentration was twice that of normal but remained constant for the next 12 years. During the preceding 5 years, the patient had complained of decreased vision under glare conditions. Protein electrophoresis (IgG 8.7 g/dL) and bone marrow biopsy (40% plasma cells) confirmed the diagnosis of multiple myeloma. A 53-year-old patient of Milman and coworkers23 had IgAλ SMM and underwent chemotherapy with cyclophosphamide, etoposide, and dexamethasone, followed by high-dose melphalan-conditioned autologous stem cell transplantation. Six months later, the patient remained in complete biochemical remission with a reduction in corneal opacities and improved vision. Milman and coworkers23 were able to examine in addition three MGUS patients with central crystalline opacities and partly peripheral patches.
SUPERFICIAL COMMA-SHAPED CRYSTALLINE-LIKE PPK (WITH AND WITHOUT HAZE)
This form of PPK has been reported in a few publications27–29 and is similar the MGUS-induced corneal opacity including haze that we describe in a patient (Case 2, Table 2; Figure 2, middle row left). Ann and coworkers (written communication, May 9, 2012) reported a 67-year-old woman with needle-like crystals first diagnosed as a presumed Schnyder corneal dystrophy. However, the Schnyder-related UbiA prenyltransferase domain containing one gene–UBIAD1–did not indicate Schnyder corneal dystrophy, and further systemic evaluation revealed the diagnosis of MGUS. Nik and coworkers29 described a 65-year-old woman with fine, needle-like, refractile, coarse crystals in mid-to-deep stroma of both corneas, with greater density centrally. Serum immunoelectrophoresis showed an MGUS IgAκ light chain. Additionally, there were macular drusen in both eyes. Perry and coworkers28 presented a 52-year-old man who showed distinct polychromatic linear crystals still limited to the epithelium. Histopathologic examination of a superficial trephine biopsy specimen of the cornea showed rectangular to rhomboidal crystalline deposits. Immunohistochemistry of the corneal biopsy and serum electrophoresis disclosed an IgGκ SMM. Six years later, a diagnosis of multiple myeloma was made. Following chemotherapy, the patient went into remission and most of the crystals disappeared.
STROMAL PUNCTIFORM OR COMMA-SHAPED CRYSTALLINE-LIKE PPK
We were not able to see this form of MGUS-induced PPK. There are 13 case reports on this form of PPK in the literature (Table 6). 13, 15–17, 23, 27, 31–37 The first was by Blobner,15 who reported a 45-year-old female patient with dense blue-green crystals throughout the whole stroma and additional patch-like opacities on the left eye. She had elevated serum protein and urine protein levels. In 1958, Bürki16 described crystalline punctiform and colored crystals throughout the whole corneal stroma combined with a severe recurrent uveitis intermedia in a 50-year-old female patient. There was a 1-mm clear limbal zone. The corneal changes had existed for 10 years and were first suggestive of cystinosis. Protein electrophoresis showed an elevation of the gamma globulins with the diagnosis of PPK. Bürki recommended that an electrophoresis of the serum and a bone marrow biopsy should be performed in each case of crystalline corneal degeneration. Klintworth and coworkers17 described a 46-year-old female patient with MGUS-induced stromal crystals. A lamellar keratoplasty on the left eye was performed after a diminution in vision due to a progression of stromal crystals in 1976 (Table 6). An IgGκ light chain was diagnosed. Some time later multiple myeloma was diagnosed on the basis of a 25% proportion of plasma cells in the bone marrow and lytic bone lesions. There was a recurrence of the crystals in the graft’s epithelium. The crystals in both eyes decreased after the patient had received chemotherapy for several months. In 1977, the bone marrow contained less than 1% plasma cells.17 Barr and coworkers33 described two patients with stromal crystals (Table 6) who were first diagnosed as having cystinosis. Ormerod and coworkers34 reported a 50-year-old woman with stromal crystals, from limbus to limbus. De Alba Campomanes and coworkers36 showed a 66-year-old male patient with penetrating keratoplasty and clear graft 1 year postoperatively. Steinberg and coworkers37 presented a 35-year-old man with bilateral white crystalline-like opacities throughout the entire depth of the cornea.
TABLE 6.
STROMAL CRYSTALLINE-LIKE PPK
| AUTHOR (n=11) | GENDER | AGE (YEARS) | MGUS/SMM | BCVA, RIGHT, LEFT | FOLLOW-UP (YEARS) | TREATMENT |
|---|---|---|---|---|---|---|
| Blobner15 | F | 45 | + | R: 6/8 L: 6/8 |
2 | Not reported |
| Bürki16 | M | 50 | + | R: 6/6 L: 6/6 |
9 | Not reported |
| Laibson31 | F | 45 | +, IgGκ light chain | R: 6/7.5 L: 6/6 |
2 | Not reported |
| Pinkerton32 | M | 50 | +, M spike | Not reported | 2 | Not reported |
| Klintworth17 | F | 46 | +, IgGκ light chain | R: 6/12 L: 6/15 |
15 | Lamellar keratoplasty |
| Barr33 | F | 33 | +, IgGκ light chain | R: 6/6 L: 6/7.5 |
2 | Not reported |
| Barr33 | F | 57 | +, IgGκ light chain | R: 6/10 L: 6/7.5 |
1 | Not reported |
| Ormerod34 | F | 50 | +, IgGκ light chain | R: 6/15 L: 6/15 |
13 | Penetrating keratoplasty |
| Froussart27 | F | 55 | +, IgGκ and IgGλ light chain | R: 6/15 L: 6/10 |
3 | Chemotherapy, penetrating keratoplasty 2× |
| Milman23 | M | 53 | +, IgGλ light chain | R: 6/10 L: 6/20 |
5 | Chemotherapy |
| Balderman13 | F | 54 | +, IgGκ light chain | Not reported | 16 | Penetrating keratoplasty |
| Hutchinson35 | M | 53 | +, IgGκ light chain | R: 6/6 L: 6/6 |
Not reported | Not reported |
| De Alba Campomanes36 | M | 66 | +, IgGκ light chain | R: 6/60 L: 6/20 |
1 | Penetrating keratoplasty |
| Steinberg37 | M | 35 | +, IgGκ light chain | R: 6/7.5 L: 6/6 |
Not reported | Not reported |
BCVA, best-corrected visual acuity; IgG, immunoglobulin G; L, left; M spike, monoclonal spike; MGUS, monoclonal gammopathy of undetermined significance; PPK, paraproteinemic keratopathy; R, right; SMM, smoldering multiple myeloma.
DEEP CRYSTALLINE-LIKE PPK
We were not able to see this rare form of PPK. Rodrigues and coworkers38 reported a 74-year-old woman with bilateral deep stromal, patchy crystalline deposits, just anterior to Descemet’s membrane. The diagnosis of Schnyder corneal dystrophy was discussed. Three years later, a penetrating keratoplasty was performed. Serum immunoelectrophoresis demonstrated elevated levels of IgGκ light chains. Immunoelectrophoresis of the urine demonstrated the presence of IgGκ light chains. Nik and coworkers29 showed a 65-year-old white woman with a IgAκ light chain and bilateral fine, needle-like crystals in mid-to-deep stroma. Font and coworkers39 described a 52-year-old man who was first diagnosed as having MGUS IgGκ. More than 1 year later, bilateral corneal crystals could be revealed at the level of Descemet’s membrane involving mostly the central cornea. Subsequently, another hematologic evaluation established a diagnosis of multiple myeloma.
DIFFERENTIAL DIAGNOSIS OF CRYSTALLINE-LIKE PPK
The primary purpose of differential diagnosis of crystalline-like PPK is to differentiate it from the crystalline type of autosomal dominantly inherited Schnyder corneal dystrophy and from the adult and adolescent cystinosis.40,41 The punctiform crystalline-like PPK can show patch-like lesions (Case 1, Table 2; Figure 2, top row left), in contrast to the dense-shaped punctiform crystals of cystinosis, often combined with photophobia (Figure 2, top row middle). Recurrent erosion and retinal crystals and alterations can be observed in cystinosis. Schnyder corneal dystrophy shows crowded comma-shaped crystals (Figure 2, top row right), sometimes with haze (Figure 2, middle row right) that can be very similar to the comma-shaped crystalline-like PPK with additional diffuse opacities (Case 2, Table 2; Figure 2, middle row left). Weiss42 pointed out that nearly all patients with Schnyder corneal dystrophy who are between the ages of 23 and 38 have an arcus lipoides. In none of our MGUS patients was an arcus lipoides visible. The deep crystalline-like PPK must be differentiated from the rare crystalline form of pre-Descemet corneal dystrophy.40
SUPERFICIAL DIFFUSE AND FLECK-LIKE PPK
Beebe and coworkers43 described a 52-year-old man who had bilateral superficial amorphous grey-white deposits causing focal epithelium elevations. In addition, the anterior stroma showed a diffuse haze extending over the entire cornea. The first diagnosis of the healthy patient was IgGκ light chains, and several months later the diagnosis was multiple myeloma. Left penetrating keratoplasty was performed. Systemic therapy was initiated with melphalan and prednisone. The investigators observed that in the later course of the disease, further deep stromal deposits resembling vertical striations developed. However, in spite of the successful lowering of serum immunoglobulin levels, the density of the corneal deposits occurred in a differential manner: the accumulation of protein seemed more prominent in the grafted eye compared to the fellow eye.43 Kocabeyoglu and coworkers44 reported a 62-year-old woman with MGUS and bilateral diffuse gray-white deposits at the level of the anterior stroma. We also observed a transformation of lattice lines into a superficial diffuse and fleck-like PPK with focal epithelium elevations during follow-up of over 20 years (Case 3, Table 2; Figure 2, bottom row right).21
SUPERFICIAL GEOGRAPHIC-LIKE PPK
We were not able to find a case in the literature similar to our 73-year-old female patient (Case 8, Table 2; Figure 4, left) with MGUS IgGκ light chains, examined by protein electrophoresis and immunofixation of the serum with an M (monoclonal) spike of 16.7% (normal, 9%–16%). Immunohistochemistry of the corneal biopsy revealed a diffuse reactivity regarding IgGκ light chains. The corneal involvement could be observed for the first time in 2011. Bilateral cataract surgery was performed without reporting corneal involvement in 2007. This form of PPK shows similarity to Reis-Bücklers corneal dystrophy (Figure 4, right).
CENTRAL GRANULAR-LIKE PPK
We were able to describe a 60-year-old man with MGUS IgGλ light chain. Six years after the first diagnosis of MGUS, a keratopathy was noted in the form of paracentral granular superficial opacities (Case 4, Table 2; Figure 3, top row left). Moeller and coworkers45 described a 52-year-old previously healthy woman with MGUS and a granular corneal opacification. Granular corneal dystrophy type 1 was the initial diagnosis. However, no corneal involvement was found in the other family members. The protein electrophoresis of the serum showed a monoclonal gammopathy of IgGκ light chain. Moeller and coworkers45 pointed out that this form of PPK and granular corneal dystrophy type 1 are similar in appearance. In the PPK there were elements not only close to the center of the cornea but also in the periphery. In patients with granular corneal dystrophy type 1, there is a free peripheral zone of 2 to 3 mm (Figure 3, top row right).
PERIPHERAL GRANULAR-LIKE PPK
We were able to present this MGUS-induced corneal opacity pattern (Case 5, Table 2; Figure 3, top row middle).21 Sekundo and Seifert46 described a 52-year-old white woman in whom Schnyder corneal dystrophy had been diagnosed by a local ophthalmologist 2 years previously. No other family members showed any corneal changes. Slit-lamp examination showed bilateral dense, whitish plaques in the corneal periphery, leaving both the axial and the perilimbal cornea clear. Serum immunofixation electrophoresis demonstrated MGUS with IgGκ light chains. In contrast, granular corneal dystrophy type 1 and 2 are free of corneal deposits in the peripheral cornea.
PERIPHERAL SUPERFICIAL CIRCULAR BAND-LIKE PPK WITH AND WITHOUT CONJUNCTIVAL INFLAMMATION
We were able to present a patient with this PPK with conjunctival inflammation (Case 7, Table 2; Figure 3, bottom row left).21 Eiferman and Rodrigues47 described a 39-year-old woman with severe photophobia. Examination of the cornea showed a dense gray-white band running circumferentially around the cornea without a clear interval. Several finger-like projections extended centrally into the cornea. Immunoelectrophoresis revealed an IgG monoclonal gammopathy. Bone marrow and bone biopsy specimens were normal. Kremer and coworkers48 reported a 60-year-old male patient with raised gelatinous grey-white subepithelial avascular nodules in the corneal periphery but sparing the limbus. These confluent noncrystalline nodular masses extended toward the visual axis by finger-like projections. The conjunctiva was completely normal. There wasan MGUS of IgGκ cryoglobulinemia. The proteins in cryoglobulinemia clump together in the cold. The cause is unknown but may be associated with monoclonal gammopathies. Henderson and coworkers49 presented a 65-year-old man with a peripheral yellow-white opacification encircling both corneas and accompanied by increased limbal vascularity. Singh50 showed a 66-year-old man who had MGUS with IgGκ light chain and peripheral white infiltrate at the limbus in both eyes. Differential diagnosis must include the large arcus lipoides (Figure 3, bottom row middle) and the thin and line-like peripheral ring of LCAT deficiency (Figure 3, bottom row right).
PERIPHERAL INFLAMMATORY-LIKE HAZE OF PPK WITH AND WITHOUT CORNEAL VASCULARIZATION
We did not observe this form of PPK in our patients. Hall and coworkers22 reported a 48-year-old woman with a peripheral large and yellowish pannus with superficial vascularization. The central cornea was clear. Serum protein electrophoresis revealed a monoclonal band IgGκ protein, compatible with MGUS. Schelonka and coworkers51 presented a 43-year-old patient with a 5.3-mm-wide band of multiple, punctuate, fleck-like corneal opacities, mostly at the level of the posterior stroma, extending from the inferotemporal limbus toward the center of the right cornea. An asymmetric distribution of episcleral hyperemia coincided with the corneal opacity. The left eye was unremarkable. Serum immunoelectrophoresis demonstrated an IgM monoclonal gammopathy. Milman and coworkers23 reported a 76-year-old woman with progressive bilateral corneal haze associated with peripheral deep corneal stromal vascularization attributed to inactive interstitial keratitis. The hematologic diagnosis was IgGκ light chains. The same investigators described a 50-year-old woman disclosing bilateral peripheral anterior to mid-stromal patch-like deposits associated with peripheral superficial corneal vascularization.23 A progression of peripheral corneal vascularization was also noted. M protein spikes were present, identified by immunofixation as IgMκ, IgGκ, and IgGλ. The findings were interpreted as compatible with MGUS. This form of PPK masquerades as an inflammatory process in the sense of an interstitial keratitis or limbal stem-cell deficiency. Milman and coworkers23 showed that corneal vascularization, a previously underemphasized finding, can be observed in patients with MGUS.
PERIPHERAL SUPERFICIAL PATCH-LIKE PPK
We previously reported two patients with this form of PPK (Case 6, Table 2; Figure 3, middle left).21 Hall and coworkers22 reported a 66-year-old man with MGUS who had bilateral subepithelial patch-like opacities. The same investigators observed an 81-year-old woman who showed bilateral linear and plaque-like opacities in the local peripheral cornea. Serum protein electrophoresis revealed a monoclonal IgGκ light chain. A presumptive diagnosis of MGUS was established.22 In all patients with so-called peripheral hypertrophic corneal degeneration (Figure 3, middle row right), immunoelectrophoresis of the serum should be performed to include or exclude a PPK.
STROMAL LATTICE-LIKE PPK
We previously reported a 49-year-old woman with stromal lattice lines in 1993 (Case 3, Table 2; Figure 2, bottom row left).21 Our initial diagnosis was lattice corneal dystrophy. However, the patient had no family history of corneal involvement. The histology and immunohistochemical reaction showed no hints of amyloidosis. Protein serum immunoelectrophoresis disclosed MGUS IgGκ light chain (Table 7).21
TABLE 7.
STROMAL LATTICE–LIKE PPK
| AUTHOR (n=8) | GENDER | AGE (YEARS) | MGUS | BCVA, RIGHT, LEFT | FOLLOW-UP (YEARS) | TREATMENT |
|---|---|---|---|---|---|---|
| Yassa52 | M | 34 | +, IgG heavy and light chain | R: 6/6 L: 6/15 |
20 | Bilateral penetrating keratoplasty |
| Spiegel53 | M | 76 | +, IgGκ light chain | R: 6/60 L: 20/400 |
3 | Bilateral penetrating keratoplasty |
| Stirling54 | F | 73 | +, IgGκ and IgMκ | Not reported | Not reported | Bilateral penetrating keratoplasty |
| Stirling54 | M | 42 | +, IgGκ light | Not reported | Not reported | Bilateral penetrating keratoplasty |
| Kamal55 | M | 65 | +, M spike | R: 6/7.5 L: 6/10 |
Not reported | Not reported |
| Lisch21 | F | 49 | +, IgGκ light chain | R: 6/20 L: 6/12 |
Transformation from lattice-like to fleck-like PPK during 20 years | Not reported |
| Hall22 | M | 66 | +, IgGκ | R: 6/6 L: 6/6 |
Several months | Not reported |
BCVA, best-corrected visual acuity; Ig, G immunoglobulin G; L, left; M spike, monoclonal spike; PPK, paraproteinemic keratopathy; R, right.
Ann and coworkers (written communication, May 9, 2012) reported a 60-year-old woman with bilateral stromal lattice lines first diagnosed as a presumed lattice corneal dystrophy. The TGFBI gene did not contain any known disease-causing mutations. Further systemic evaluation revealed the diagnosis of MGUS. There are six published reports of lattice-like opacity patterns and the diagnosis of MGUS (Table 7). 21, 22, 52–55 Yassa and coworkers51 described a 34-year-old man who showed first the phenotype of lattice corneal dystrophy and later those of “deep filiform dystrophy.” However, the protein immunoelectrophoresis was normal. Twenty years later, the immunoperoxidase stains showed that the deposits were positive for MGUS IgGλ light chains in both corneas. Spiegel and coworkers53 reported a 76-year-old man with lattice-like corneal opacities and pointed out that the late onset of the patient’s corneal pathology and a negative family history decreased the likelihood of lattice dystrophy as a diagnosis. The investigators presented an excellent survey about the distinct phenotypes of PPK. Milman and coworkers23 reported about two additional MGUS patients with lattice-like lines of the cornea.
We postulate that patients with lattice-like pathology, without family history and TGFBI gene mutations, may have lattice-like PPK. The autosomal dominantly inherited lattice corneal dystrophy type 1 and variants (Figure 2, bottom row middle) show histologically amyloid deposits in the cornea and are caused by mutations in the TGFBI gene on chromosome 5q31.40 The autosomal dominantly inherited Meretoja syndrome represents a systemic amyloidosis with lattice lines in the cornea and a laxity of the facial skin. The disease is caused by mutation in the gelsolin gene on chromosome 9q34.
STROMAL FLAKE-LIKE PPK
We were able to present a male MGUS patient with this form of PPK (Case 11, Table 2; Figure 6, top left). There are some published reports of similar flake-like opacities of the corneal stroma in patients with monoclonal gammopathy.49,56–59 Henderson and coworkers49 described, in their case 2, a 62-year-old woman with diffuse linear to ground-glass opacity throughout the whole stroma. She was thought to have a corneal dystrophy of uncertain type. Protein immunoelectrophoresis revealed an MGUS IgGκ light chain. Graichen and coworkers56 described a 62-year-old man with bilateral irregular, grayish-white stromal opacities and an MGUS IgGκ light chain. Penetrating keratoplasty was performed on the left eye. The graft remained clear and the patient was healthy, showing no evidence of a systemic disorder. The investigators did not state the length follow-up after keratoplasty. Conway and coworkers59 reported a 49-year-old woman with grayish-white deposits throughout the stroma of both eyes. The hematologic diagnosis was a SMM IgGκ light chain. The phenotype of this form of PPK is similar to those of congenital stromal corneal dystrophy (Figure 6, top middle). However, in an adult patient without a family history of stromal corneal dystrophy, MGUS showing bilateral stromal flake-like changes should be suspected.
STROMAL PUNCTIFORM PPK
We did not observe this form of PPK in our patients. Meesmann14 described a 64-year-old male patient who showed a myriad of fine grayish yellow punctifom deposits throughout the whole corneal stroma in 1934. The outermost corneal periphery was free of deposits. Epithelium and endothelium were uninvolved. Bence Jones protein in the urine was elevated. Meesmann speculated that the corneal deposits had been created from metabolic pathways. Cherry and coworkers60 could also see a myriad of tiny grayish spots throughout the corneal stroma in a 57-year-old man in whom these corneal changes were present for 14 years. The patient did not have ocular complaints, and the general physical examination yielded normal findings. The clinical checkup disclosed a SMM IgGκ, but no freeκ chains were detected in the blood. The investigators concluded that the presence of corneal deposits should alert the ophthalmologist to the possibility of dysproteinemia.
MONOCLONAL GAMMOPATHY + CENTRAL GOLDEN-BROWN DISCOLORATION OF DESCEMET + HYPERCUPREMIA
We present the first patient in Europe with this syndrome (Case 10, Table 2; Figure 5, top left). However, we know of 10 published reports on the coincidence of monoclonal gammopathy + PPK + hypercupremia from the United States, one from Canada, and one from Brazil (Table 8).61–72 Immunoelectrophoresis showed MGUS in four reports, 63,66,68,72 MGUS combined with pulmony carcinoma in one patient,65 SMM in one patient,64 and multiple myeloma in four patients. 61,67,69,71 Goodman and coworkers61 first reported the association of hypercupremia with multiple myeloma and finely granular discoloration of the corneal endothelium and Descemet’s membrane. Lewis and coworkers63 described this association of MGUS + hypercupremia + PPK in 1975. We propose to call this true syndrome Lewis syndrome. In 1976, Lewis and coworkers64 published a second report about this association and presented an extensive clinical and copper study that showed MGUS IgGλ light chain, hypercupremia, corneal opacification, and normal physical findings. Their MGUS patient had no anemia, bone pain, osteoporosis, osteolytic lesion, proteinuria, or cupriuria, in contrast to Goodman and coworkers’ patient with the association of multiple myeloma, hypercupremia, and corneal opacification.61,62 Edward and coworkers71 could show histologically in their case of Lewis syndrome a brownish granular band of copper deposition in the thin central Descemet membrane, with attenuated endothelium. Shah and coworkers72 reported a 46-year-old black woman with Lewis syndrome who underwent Descemet-stripping endothelial keratoplasty. Two months following surgery her acuity corrected to 6/6 in dark and bright room illumination.
TABLE 8.
MONOCLOMNAL GAMMOPATHY + HYPERCUPREMIA + KERATOPATHY
| AUTHORS (n=7) | GENDER | AGE (YEARS) | MGUS | GOLDEN-BROWN DISCOLORATION AT THE DESCEMET LEVEL | COPPER LEVEL (μG/DL) | FOLLOW-UP THERAPY |
|---|---|---|---|---|---|---|
| Goodman61 | F | 69 | MM, IgGκ light chains, anemia | +□, copper lens involvement | 1780–3350 | D-penicillamin |
| Ellis62 | F | 69 | MM | +□, copper lens involvement | 1800–3400 | Cyclophosphamide, D-penicillamin |
| Lewis*63 | F | 41 | +, IgGλ light chain; depression of IgA | +□, copper lens involvement | 1740 | D-penicillamin |
| Lewis*63 | F | 42 | SMM, IgGλ light chain; depression of IgA and IgM | +□, copper lens involvement | 1000–1740 | D-penicillamin |
| Martin65 | M | 60 | +, IgGλ light chain; pulmonary carcinoma | +□, copper lens involvement | 770 | Death due to metastasis of lung cancer |
| Probst66 | M | 65 | +, IgGκ light chain | +□, sunflower cataract | 203 | Oral zinc glucomate, 25 mg 3 × a day |
| Hawkins67 | F | 64 | MM, IgGκ light chain | +□, copper lens involvement | 2380 | Vitamins |
| Tzelikis68 | F | 49 | +, IgGλ light chain | +□, copper lens involvement | 335 | Not reported |
| Garg*69 | F | 70 | MM, IgGκ light chain | +□, copper lens involvement | 2380 | No therapy |
| Aldave70 | F | 65 | IgG elevated, chronic lymphocytic leukemia | +□ | >500 | Not reported |
| Edward*71 | F | 75 | MM, IgGκ light chain | +□, copper lens involvement | Not reported | Right eye penetrating keratoplasty; histologic evaluation |
| Shah72 | F | 46 | +, IgGλ light chain | +□ | 1473 | DMEK |
+, MGUS positive; +□, golden-brown discoloration at the Descemet; DMEK, Descemet membrane endothelial keratoplasty; F, female; Ig, immunoglobulin; M, male; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; SMM, smoldering multiple myeloma.
One and the same patient;
DIFFERENTIAL DIAGNOSIS OF WILSON’S DISEASE VS LEWIS SYNDROME
Wilson’s disease is the most common copper storage disease with autosomal recessive inheritance and is caused by mutations in the Wilson disease protein.73 The gene of this protein is located on chromosome 13. The protein it codes for is an ATPase responsible for providing copper to other proteins, such as coeruloplasmin. The genetic defect causes excessive copper accumulation in the liver, brain, and other body tissues. Young patients can have signs of lenticular degeneration, with major dysfunction of the basal ganglia. Spasticity, dysarthria, dysphasia, tremor, ataxia, and incoordination are the presenting manifestations. Some patients with Wilson’s disease have cirrhosis and kidney dysfunction. The Kayser-Fleischer ring of the cornea is an important sign of Wilson’s disease and is present in 98% of neurologically symptomatic patients (Figure 5, bottom left).74 It is a golden-brown, green, or ruby-red band in the peripheral cornea at the level of Descemet’s membrane beginning at the limbus and gradually extending centrally and circumferentially. In Lewis syndrome, the hypercupremia, normal coeruloplasmin, normal liver morphology, and normal physical findings without neurologic symptoms clearly distinguish this entity from Wilson’s disease.64 There is a strong copper-binding affinity to the IgGκ light chain or to the IgGλ light chain, or to both. The corneal opacity shows a golden-brown discoloration with only a narrow rim of clear cornea peripherally or a central and paracentral discoid golden-brown opacity pattern at the level of Descemet’s membrane (Figure 5, top left).
VERTICILLATA-LIKE APPEARANCE OF PPK
Chong and coworkers19 described a 52-year-old woman with MGUS and a diffuse layer of golden, intraepithelial microcysts in both corneas. There was streaming of the microcysts into a vortex configuration. Hutchinson and coworkers57 reported a 58-year-old woman with MGUS and superficial and deep corneal deposits with a verticillata-like pattern in the overlying epithelium. Kleta and coworkers28 saw a 49-year-old woman with a 17-year follow-up from MGUS to multiple myeloma and fine crystals in the epithelium of both corneas, with a brown pigment deposit in a whorl-like fashion. Finis and Stammen57 reported a 70-year-old woman with multiple myeloma and stromal crystals of the cornea combined with a whorl-like appearance.
MIXED-LIKE APPEARANCE OF PPK
Miller and coworkers75 reported a 63-year-old woman disclosing two types of corneal opacities: fine refractile bodies in the epithelium and large, white, lobulated, discrete opacities within the stroma at all levels. Immunoperoxidase stains were positive for IgG most strongly, and also for IgAκ and λ light chains. Graichen and coworkers56 presented a 62-year-old patient with bilateral irregular, grayish-white stromal opacities with scattered guttae excrescences and moderate stromal edema. Kato and coworkers reported a 55-year-old female patient with MGUS who had bilateral diffuse, amorphous, white deposits throughout the corneal stroma that were more prominent in the anterior stroma.76 Koo and coworkers77 showed a 62-year-old woman with corneal stromal edema and Descemet’s fold in both eyes. Epithelial bullae were found in the center of the right eye. The patient was diagnosed as having MGUS IgGκ light chain.
Table 9 shows our final classification of MGUS-induced PPK, based on the presented study and on the elaboration of all cases in the past literature.
TABLE 9.
NEW CLASSIFICATION OF MGUS-INDUCED PPK BASED ON THE PRESENTED STUDY AND PAST LITERATURE
|
MGUS, monoclonal gammopathy of undetermined significance; PPK, paraproteinemic keratopathy.
HISTOLOGY OF PPK
The pathologic features of PPK are manifold, similar to the distinct clinical phenotype.23 Histologic evaluation of PPK was possible either after corneal biopsy23,26,33,47,77 or after lamellar or penetrating keratoplasty.17,23,34,38,43,48–50,54,56,76,77 Immunoglobulin can be observed in any layer of the cornea as intracellular or extracellular, nonbirefringent, eosinophilic deposits that are acid fuchsinophilic with Masson’s trichrome stain and demonstrate positive results with periodic acid–Schiff but negative results with Congo red.23 Immunohistochemical and immunofluorescent techniques demonstrate reactivity of the deposits for immunoglobulin light or heavy chains, or both.17,18,23 These examinations are very important in the evaluation of PPK because of the multiple unspecific light and electron microscopic features of MGUS-induced corneal opacities. There are distinct ultrastructural patterns of immunoglobulin deposition in conjunctival fibroblasts, limbal vascular endothelium, corneal keratocytes, and stroma.23,26 There is currently no clear understanding as to why certain immunoglobulin molecules crystallize in tissues, whereas the majority do not.49 There are seven distinct ultrastructural patterns of PPK23,43,49,54
Fibrillary crystalloids with a curvilinear filamentous substructure
Lysosome-like granules with amorphous or granular contents
Angulated geometric crystalloids composed of filaments with 9- to 10-nm line-to-line periodicity
Cord-like crystalloids composed of thick-walled hollow tubules approximately 40 nm in diameter (ultrastructural similarity to immunotactoid glomerulopathy–immunotactoid keratopathy)
Large scroll-like tubules
Immune complex-like deposits
Randomly arranged fibrillary deposits 15 to 20 nm in diameter
Light and electron microscopy of our patient with flake-like opacification of the corneal stroma (Case 11, Figure 7) is illustrated in Figure 7, top row, middle and right. The immunohistochemistry of the cornea and the serum is demonstrated in Figure 8.
PPK AND OTHER OCULAR AND EXTRAOCULAR SIGNS IN MGUS
There are few reports about a simultaneous occurrence of MGUS and acute and chronic uveitis anterior. 12,33,34 Maculopathy can be a rare association with MGUS.13 Munteanu78 reported an association between Doyne’s dystrophy and benign monoclonal gammopathy and corneal deposits. Cherry and coworkers60 revealed similar changes of the retina. Nik and coworkers27 saw in their MGUS patient an association with foveolar drusen. Palm24 reported an association of MGUS with rheumatoid arthritis and spondyloarthritis ankylopoietica. Crystal-storing histiocytosis, which involves, for example, marrow, liver, spleen, lymph nodes, and other extramedullar tissues, represents a rare association with MGUS.13
THERAPEUTIC ASPECTS IN MGUS PATIENTS WITH PPK
The corneal opacity pattern of PPK can result in a severe visual impairment. The entity is primarily misdiagnosed as corneal dystrophy, such as in our patient in 2012. A bilateral penetrating keratoplasty was performed in 2012 (Figure 7, top left). Recurrence after 12 months postoperatively hinted at a systemic disease (Figure 7, middle row left and middle). The serum protein electrophoresis and immunohistochemistry showed an MGUS that induces the PPK (Figure 8). The patient and the hematologists are discussing the possible use of chemotherapy to reduce risk of recurrent opacities in the graft.
The possible use of chemotherapy is a new situation in patients with MGUS and PPK with severe visual impairment. There are several reports with a similar follow-up: first misdiagnosis, eg, Schnyder or lattice corneal dystrophy, then penetrating or lamellar keratoplasty with the result of quick corneal recurrence after months. 17,27,34,38,39,43,48,52,53,77
However, Milman and coworkers23 reported a 76-year-old woman with MGUS-induced PPK mimicking an inactive interstitial keratitis. After bilateral keratoplasty no recurrence could be observed during a follow-up of 2 years. Graichen and coworkers56 demonstared a 66-year-old man with crystalline keratopathy and MGUS. A penetrating keratoplasty showed after 1-year follow-up a clear graft. Balderman and Lichtman13 pointed out that in cases with severe corneal involvement penetrating keratoplasty can be performed, but corneal deposition can recur if the monoclonal immunoglobulin remains present. Treatment targeting the underlying monoclonal gammopathy usually improves ocular symptoms.13 There are few reports of patients with multiple myeloma that describe the positive effect of chemotherapy in the disappearing or regression of PPK.17,23,27,30 Beebe and coworkers34 could not observe a regression of corneal opacities in their patient with monoclonal gammopathy in spite of chemotherapy.
CONCLUSION
Monoclonal gammopathy of undetermined significance represents one of the most common premalignant disorders in Western countries. The hematologic definition of MGUS reveals no clinical signsand no indication for therapy. The complex PPK represents a facultative clinical sign of MGUS. The limitation of our study is that we cannot show whether PPK is a precursor of MGUS or not. Only a common hematologic and ophthalmologic prospective, multicenter study could answer this question. However, every patient with MGUS should be examined by the ophthalmologist at the slit lamp to include or exclude a PPK. In case of superficial PPK-induced visual impairment, a laser-assisted phototherapeutic keratectomy could postpone systemic therapy for MGUS. Lamellar or penetrating keratoplasty of PPK in patients with MGUS should be assessed as a contraindication—in contrast to the corneal dystrophies—because of the quick recurrence of the original opacity patterns in the graft. Severe visual impairment due to PPK in patients with MGUS represents a delicate clinical decision regarding therapy. In such a situation, an intensive cooperation between hematologist, ophthalmologist, and patient is necessary to discuss the pros and cons of systemic chemotherapy. There are few controversial reports in the literature on whether chemotherapy can influence and reduce the PPK in patients with monoclonal gammopathy. Again, a common hematologic and ophthalmologic prospective study could elucidate this question.
The ophthalmologist has the important responsibility to diagnose PPK vs distinct heritable and inflammatory corneal entities. A family history that lacks this condition argues against an autosomal dominantly inherited corneal dystrophy, such as Schnyder corneal dystrophy, which can be easily misdiagnosed in cases of crystalline-like PPK. Distinct further forms of PPK can be misdiagnosed as corneal dystrophies: lattice corneal dystrophy, granular corneal dystrophy, Reis-Bücklers corneal dystrophy, stromal corneal dystrophies, and pre-Descemet corneal dystrophy. The autosomal recessive inherited cystinosis is often the wrong first diagnosis in case of crystalline-like PPK. Distinct bilateral peripheral degenerative and inflammatory signs of the cornea with and without neovascularization may be first interpreted as stem cell insufficiency. However, the serum protein electrophoresis can show an MGUS-induced PPK.
We propose that a serum protein electrophoresis be performed in all cases of unclear bilateral corneal opacification without and with corneal neovascularization to include or exclude a PPK. The chameleon-like appearance of PPK, as presented in our new classification system, makes it impossible to draw any generalization with regard to the characteristics, type, and severity of the MGUS-induced corneal opacities. The rare association of MGUS + hypercupremia +central golden-brown discoloration at the level of Descemet’s membrane should be called Lewis syndrome and should be differentiated from Wilson’s disease with the peripheral golden-brown Kayser-Fleischer ring, also at the level of Descemet’s membrane. The pathology of PPK is manifold. There are seven distinct ultrastructural patterns of PPK that can give hints to the diagnosis of monoclonal gammopathy. Immunohistochemical and immunofluorescent techniques of the serum and the cornea can be decisive regarding differential diagnosis, because they demonstrate reactivity of the deposits for immunoglobulin light and heavy chains.
ACKNOWLEDGMENTS
Funding/Support: This study was supported in part by the Research to Prevent Blindness (J.S.W.) and the Lions Eye Foundation (J.S.W.).
Financial Disclosures: None.
Author Contributions: Design and conduct of study (W.L., J.S.W.); Collection and management of data (W.L., J.W.-P., T.K., U.S.-S., J.M.R., W.S., U.P., C.L.); Analysis and interpretation of data (W.L., J. W.-P., U. S.-S.,W.S., A. D., H. R., J.S.W.); Preparation, review, approval of manuscript (W.L., J. W.-P., T.K., U.P., C.L., J.S.W.)
REFERENCES
- 1.Crawford J, Eye MK, Cohen HJ. Evaluation of monoclonal gammopathies in the “well” elderly. Am J Med. 1987;82(1):39–45. doi: 10.1016/0002-9343(87)90375-5. [DOI] [PubMed] [Google Scholar]
- 2.O’Connell TX, Horita TJ, Kasravi B. Understanding and interpreting serum protein electrophoresis. Am Fam Physician. 2005;71(1):105–112. [PubMed] [Google Scholar]
- 3.Allansmith MR, McClellan BH. Immunoglobulins in the human cornea. Am J Ophthalmol. 1975;80(1):123–132. doi: 10.1016/0002-9394(75)90882-x. [DOI] [PubMed] [Google Scholar]
- 4.Bourne WM, Kyle RA, Brubaker RF, Greip PR. Incidence of corneal crystals in the monoclonal gammopathies. Am J Ophthalmol. 1989;107(2):192–193. doi: 10.1016/0002-9394(89)90225-0. [DOI] [PubMed] [Google Scholar]
- 5.Steuhl K-P, Knorr M, Rohrbach JM, Lisch W, Kaiserling E, Thiel H-J. Paraproteinemic corneal deposits in plasma cell myeloma. Am J Ophthalmol. 1991;111(3):312–318. doi: 10.1016/s0002-9394(14)72315-3. [DOI] [PubMed] [Google Scholar]
- 6.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyle RA. “Benign” monoclonal gammopathy: a misnomer?”. JAMA. 1984;251(4):1849–1854. [PubMed] [Google Scholar]
- 8.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121(5):749–757. [PubMed] [Google Scholar]
- 9.Kyle RA, Durie BGM, Rajkumar SV, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24(6):1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 11.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., III Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo clinic series 25 years later. Mayo Clin Proc. 2004;79(7):859–866. doi: 10.4065/79.7.859. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann H, Ackermann J, Baldia C, et al. Both IGH translocations and chromosome 13q deletions are early events in monoclonal gammopathy of undetermined significance and do not evolve during transition to multiple myeloma. Leukemia. 2004;18(9):1879–1882. doi: 10.1038/sj.leu.2403518. [DOI] [PubMed] [Google Scholar]
- 13.Balderman SR, Lichtman MA. Unusual manifestations of monoclonal gammopathy: I. ocular disease. Rambam Maimonides Med J. 2015;6(3):1–15. doi: 10.5041/RMMJ.10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meesmann A. Über eine eigenartige Hornhautdegeneration (?) (Ablagerung des Bence-Jones-schen Eiweisskörpers in der Hornhaut) Ber Dtsch Ophthalmol Ges. 1934;50:311–315. [Google Scholar]
- 15.Blobner F. Kristallinische Degeneration der Bindehaut und Hornhaut. Klin Monatsbl Augenheilkd. 1938;100:588–593. [Google Scholar]
- 16.Bürki E. Über Hornhautveränderungen bei einem Fall von multiplem Myelom (Plasmacytom) Ophthalmologica. 1958;135(5–6):565–572. doi: 10.1159/000303353. [DOI] [PubMed] [Google Scholar]
- 17.Klintworth GK, Bredehoeft SJ, Reed JW. Analysis of corneal crystalline deposits in multiple myeloma. Am J Ophthalmol. 1978;86(3):303–313. doi: 10.1016/0002-9394(78)90230-1. [DOI] [PubMed] [Google Scholar]
- 18.Garibaldi DC, Gottsch J, de la Cruz Z, Haas M, Green WR. Immunotactoid keratopathy: a clinicopathologic case report and a review of reports of corneal involvement in systemic paraproteinemias. Surv Ophthalmol. 2005;50(1):61–80. doi: 10.1016/j.survophthal.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Chong E-M, Campell RJ, Bourne W. Vortex keratopathy in a patient with multiple myeloma. Cornea. 1997;16(5):592–594. [PubMed] [Google Scholar]
- 20.Schwartz MM, Korbet SM, Lewis AJ. Immunotactoid glomerulopathy. J Am Soc Nephrol. 2002;13(5):1390–1397. doi: 10.1097/01.asn.0000013397.06964.19. [DOI] [PubMed] [Google Scholar]
- 21.Lisch W, Saikia P, Pitz S, et al. Chameleon-like appearance of immunotactoid keratopathy. Cornea. 2012;31(1):55–58. doi: 10.1097/ICO.0b013e31821ddd0c. [DOI] [PubMed] [Google Scholar]
- 22.Hall RB, Yeung SN, Yenson PR, Moloney G. A series of 4 cases of distinct keratopathy secondary to dysproteiemia: immunotactoid keratopathy. Can J Ophthalmol. 2014;49(4):388–391. doi: 10.1016/j.jcjo.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Milman T, Kao AA, Chu D, et al. Paraproteinemic keratopathy. The expanding diversity of clinical and pathological manifestations. Ophthalmology. 2015;122(9):1748–1756. doi: 10.1016/j.ophtha.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Palm E. A case of crystal deposits in the cornea. Precipitation of a spontaneously crystallizing plasma globulin. Acta Ophthalmol. 1947;25(2):165–174. [Google Scholar]
- 25.Holmberg CG, Grönwall A. Ein neues kristallinisches Serumglobulin. Hoppe Seylers Z Physiol Chem. 1942;273(4–5):199–205. [Google Scholar]
- 26.Grossniklaus HE, Stulting RD, L’Hernault N. Corneal and conjunctival crystals in paraproteinemia. Hum Pathol. 1990;21(11):1181–1183. doi: 10.1016/0046-8177(90)90156-y. [DOI] [PubMed] [Google Scholar]
- 27.Froussart F, Clay C, Leblond V, Chaouat D, d’Hermies F, Renard G. Kératopathie cristalline des gammapathies monoclonales: à propos de deux cas. J Fr Ophtalmol. 2001;24(7):738–743. [PubMed] [Google Scholar]
- 28.Kleta R, Blair SC, Bernardini I, Kaiser-Kupfer MI, Gahl WA. Keratopathy of multiple myeloma masquerading as corneal crystals of ocular cystinosis. Mayo Clin Proc. 2004;79(3):410–412. doi: 10.4065/79.3.410. [DOI] [PubMed] [Google Scholar]
- 29.Nik NA, Martin NF, Berler DK. Corneal crystalline deposits and drusenosis associated with IgA-kappa chain monoclonal gammopathy. Ann Ophthalmol. 1985;17(5):303–307. [PubMed] [Google Scholar]
- 30.Perry HD, Donnenfeld ED, Font RL. Intraepithelial corneal immunoglobulin crystals in IgG-kappa multiple myeloma. Cornea. 1993;12(5):448–450. doi: 10.1097/00003226-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Laibson P, Damiano V. X-ray and electron diffraction of ocular and bone marrow crystals in paraproteinemia. Science. 1969;163(2):581–583. doi: 10.1126/science.163.3867.581. [DOI] [PubMed] [Google Scholar]
- 32.Pinkerton RMH, Robertson DM. Corneal and conjunctival changes in dysproteiemia. Invest Ophthalmol. 1969;8(4):357–364. [PubMed] [Google Scholar]
- 33.Barr CC, Gelender H, Font RL. Corneal crystalline deposits associated with dysproteinemia. Report of two cases and review of the literature. Arch Ophthalmol. 1980;98(5):884–889. doi: 10.1001/archopht.1980.01020030878015. [DOI] [PubMed] [Google Scholar]
- 34.Ormerod LD, Collin HB, Dohlman CH, Craft JL, Desforges JF, Albert DM. Paraproteinemic crystalline keratopathy. Ophthalmology. 1988;95(2):202–212. doi: 10.1016/s0161-6420(88)33200-8. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson K, Dal Pra M, Apel A. Immunoglobulin G crystalline keratopathy associated with monoclonal gammopathy. Aust N Z J Ophthalmol. 1998;26(2):177–179. doi: 10.1111/j.1442-9071.1998.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 36.De Alba Campomanes AG, Rutar T, Crawford JB, Seiff S, Goodman D, Grenert J. Crystal-storing histiocytosis and crystalline keratopathy caused by monoclonal gammopathy of undetermined significance. Cornea. 2009;28(9):1081–1084. doi: 10.1097/ICO.0b013e318199f73b. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg J, Eddy M-T, Katz T, et al. Bilateral crystalline corneal deposits as a first clinical manifestation of monoclonal gammopathy: a case report. Case Rep Ophthalmol. 2011;2(7):222–227. doi: 10.1159/000330334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues MM, Krachmer JH, Miller SD, Newsone DA. Posterior corneal crystalline deposits in benign monoclonal gammopathy: a clinicopathologic case report. Arch Ophthalmol. 1979;97(1):124–128. doi: 10.1001/archopht.1979.01020010058014. [DOI] [PubMed] [Google Scholar]
- 39.Font RL, Matoba AY, Prabhakaran VC. IgK-k immunoglobulin deposits involving the predescemetic region in a patient with multiple myeloma. Cornea. 2006;25(10):1237–1239. doi: 10.1097/01.ico.0000240094.78095.f5. [DOI] [PubMed] [Google Scholar]
- 40.Weiss JS, Moeller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies —edition 2. Cornea. 2015;34(2):117–159. doi: 10.1097/ICO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 41.Weiss JS, Khemichian AJ. Differential diagnosis of Schnyder corneal dystrophy. In: Lisch W, Seitz B, editors. Corneal Dystrophies. Basel: Karger; 2011. pp. 67–96. [DOI] [PubMed] [Google Scholar]
- 42.Weiss JS. Visual morbidity in thirty-four families with Schnyder crystalline corneal dystrophy (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2007;105:616–648. [PMC free article] [PubMed] [Google Scholar]
- 43.Beebe WE, Webster RG, Jr, Spencer WB. Atypical corneal manifestations of multiple myeloma. A clinical, histopathologic, and immunohistochemical report. Cornea. 1989;8(4):274–280. [PubMed] [Google Scholar]
- 44.Kocabeyoglu S, Mocan MC, Haznedaroglu IC, et al. In vivo confocal microscopic characteristics of crystalline keratopathy in patients with monoclonal gammopathy: report of two cases. Indian J Ophthalmol. 2014;62(9):938–940. doi: 10.4103/0301-4738.143933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moeller HU, Ehlers N, Bojsen-Moeller M, Ridgway AEA. Differential diagnosis between granular corneal dystrophy Groenouw type 1 and paraproteinemic crystalline keratopathy. Acta Ophthalmol. 1993;71(4):552–555. doi: 10.1111/j.1755-3768.1993.tb04635.x. [DOI] [PubMed] [Google Scholar]
- 46.Sekundo W, Seifert P. Monoclonal corneal gammopathy: topographic considerations. Ger J Ophthalmol. 1996;5(5):262–267. [PubMed] [Google Scholar]
- 47.Eiferman RA, Rodrigues MM. Unusual superficial stromal corneal deposits in IgGK monoclonal gammopathy. Arch Ophthalmol. 1980;98(1):78–81. doi: 10.1001/archopht.1980.01020030080003. [DOI] [PubMed] [Google Scholar]
- 48.Kremer I, Wright P, Merin S, Weiss J, Pick AI, Kaufman H. Corneal subepithelial monoclonal kappa IgG deposits in essential cryoglobulinaemia. Br J Ophthalmol. 1989;73(8):669–673. doi: 10.1136/bjo.73.8.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson DW, Stirling JW, Lipsett J, Rozenbilds MA, Roberts-Thomson PJ, Coster DJ. Paraproteinemic crystalloidal keratopathy: an ultrastructural study of two cases, including immunoelectron microscopy. Ultrastruct Pathol. 1993;17(6):643–668. doi: 10.3109/01913129309027800. [DOI] [PubMed] [Google Scholar]
- 50.Singh K. Immunotactoid microtubular corneal deposits in bilateral paraprotein crystalline keratopathy. Cornea. 2009;28(7):829–831. doi: 10.1097/ICO.0b013e318191449a. [DOI] [PubMed] [Google Scholar]
- 51.Schelonka LP, Ogawa GS, O’Brien TP, Green WR. Acute unilateral corneal immunoprotein deposition in IgM monoclonal gammopathy. Arch Ophthalmol. 2000;118(1):125–126. doi: 10.1001/archopht.118.1.125. [DOI] [PubMed] [Google Scholar]
- 52.Yassa NH, Font RL, Fine BS, Koffler BH. Corneal immunoglobulin deposition in the posterior stroma. A case report including immunohistochemical and ultrastructural observations. Arch Ophthalmol. 1987;105(1):99–103. doi: 10.1001/archopht.1987.01060010105040. [DOI] [PubMed] [Google Scholar]
- 53.Spiegel P, Grossniklaus HE, Reinhart WJ, Thomas RH. Unusual presentation of paraproteinemic corneal infiltrates. Cornea. 1990;9(1):81–85. [PubMed] [Google Scholar]
- 54.Stirling JW, Henderson DW, Rozenbilds MA, Skinner JM, Filipic M. Crystalloidal paraprotein deposits in the cornea: an ultrastructural study of two new cases with tubular crystalloids that contain IgG kappa light chains and IgG gamma heavy chains. Ultrastruct Pathol. 1997;21(4):337–344. doi: 10.3109/01913129709021931. [DOI] [PubMed] [Google Scholar]
- 55.Kamal KM, Rayner SA, Chen MC, Aldave AJ. Classic lattice dystrophy associated with monoclonal gammopathy after exclusion of a TGFBI mutation. Cornea. 2009;28(1):97–98. doi: 10.1097/ICO.0b013e31818200f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Graichen DF, Perez E, Jones DB, Font RL. Kappa-immunoglobulin corneal deposits associated with monoclonal gammopathy. Immunohistochemical and electron microscopic findings. Ger J Ophthalmol. 1994;3(1):54–57. [PubMed] [Google Scholar]
- 57.Finis D, Stammen J. Ungewöhnliche stromale Hornhauteinlagerungen. Ophthalmologe. 2010;107(5):471–473. doi: 10.1007/s00347-010-2129-4. [DOI] [PubMed] [Google Scholar]
- 58.Klingenstein A, Mayer WJ, Kampik A. Decrease in bilateral corneal deposits after bortezomib/dexametasone chemotherapy in monoclonal gammopathy: case report. J Anal Oncol. 2012;1(1):103–106. [Google Scholar]
- 59.Conway AB, Kaufman SC, Rudrapatna VK, Linden MA. Crystallizing immunoglobulin presenting as polychromatic crystalline keratopathy: an unusual clinical presentation of monoclonal gammopathy of undetermined significance (MGUS) Lab Med. 2013;44(4):344–347. [Google Scholar]
- 60.Cherry PM, Kraft S, McGowan H, Ghosh M, Shenken E. Corneal and conjunctival deposits in monoclonal gammopathy. Can J Ophthalmol. 1983;18(3):143–149. [PubMed] [Google Scholar]
- 61.Goodman SI, Rodgerson DO, Kaufman J. Hypercupremia in a patient with multiple myeloma. J Lab Clin Med. 1967;70(1):57–62. [PubMed] [Google Scholar]
- 62.Ellis PP. Ocular deposition of copper in hypercupremia. Am J Ophthalmol. 1969;68(3):423–427. doi: 10.1016/0002-9394(69)90706-5. [DOI] [PubMed] [Google Scholar]
- 63.Lewis RA, Falls HF, Troyer DO. Ocular manifestations of hypercupremia associated with multiple myeloma. Arch Ophthalmol. 1975;93(10):1050–1053. doi: 10.1001/archopht.1975.01010020824017. [DOI] [PubMed] [Google Scholar]
- 64.Lewis RA, Hultquist DE, Baker BL, Falls HF, Gershowitz H, Penner JA. Hypercupremia associated with a monoclonal immunoglobulin. J Lab Clin Med. 1976;88(3):375–388. [PubMed] [Google Scholar]
- 65.Martin NF, Kincaid MC, Starl WJ, et al. Ocular copper deposition associated with pulmonary carcinoma, IgG monoclonal gammopathy and hypercupremia. Ophthalmology. 1983;90(1):110–116. doi: 10.1016/s0161-6420(83)34599-1. [DOI] [PubMed] [Google Scholar]
- 66.Probst LE, Hoffman E, Cherian MG, et al. Ocular copper deposition associated with benign monoclonal gammopathy and hypercupremia. Cornea. 1996;15(1):94–98. [PubMed] [Google Scholar]
- 67.Hawkins AS, Stein RM, Gaines BI, Deutsch T. Ocular deposition of copper associated with multiple myeloma. Am J Ophthalmol. 2001;131(2):257–259. doi: 10.1016/s0002-9394(00)00657-7. [DOI] [PubMed] [Google Scholar]
- 68.Tzelikis PF, Laibson PR, Ribeiro MP, Rapuano CJ, Hammersmith KM, Cohen EJ. Ocular deposition associated with monoclonal gammopathy of undetermined significance: case report. Arq Bras Oftalmol. 2005;68(4):539–541. doi: 10.1590/s0004-27492005000400021. [DOI] [PubMed] [Google Scholar]
- 69.Garg S, Jampol LM, Lewis RA, Penner JA. Corneal copper deposition secondary to a variant of multiple myeloma: 30-year catamnesis. Arch Ophthalmol. 2006;124(1):130–132. doi: 10.1001/archopht.124.1.130. [DOI] [PubMed] [Google Scholar]
- 70.Aldave AJ, King JA, Kim BT, Hopp L. Corneal deposition associated with chronic lymphocytic leukemia. Am J Ophthalmol. 2006;142(1):174–176. doi: 10.1016/j.ajo.2006.01.078. [DOI] [PubMed] [Google Scholar]
- 71.Edward DP, Patil AJ, Sugar J, Parikh M. Copper deposition in a variant of multiple myeloma: pathologic changes in the cornea and the lens capsule. Cornea. 2011;30(3):360–363. doi: 10.1097/ICO.0b013e3181ee67fd. [DOI] [PubMed] [Google Scholar]
- 72.Shah S, Espana EM, Margo CE. Ocular manifestations of monoclonal copper-binding immunoglobulin. Surv Ophthalmol. 2014;59(3):115–123. doi: 10.1016/j.survophthal.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Strausak D, Mercer JF, Dieter HH, Stemmel W, Multhaup G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res Bull. 2001;55(2):175–185. doi: 10.1016/s0361-9230(01)00454-3. [DOI] [PubMed] [Google Scholar]
- 74.Lößner A, Lößner J, Bachmann H, Zotter J. The Kayser-Fleischer ring during long-term treatment in Wilson’s disease (hepatolenticular degeneration). A follow-up study. Graefess Arch Clin Exp Ophthalmol. 1986;224(2):152–155. doi: 10.1007/BF02141489. [DOI] [PubMed] [Google Scholar]
- 75.Miller KH, Green WR, Stark WJ, Wells HA, Mendelsohn G, Kanhofer H. Immunoprotein deposition in the cornea. Ophthalmology. 1980;87(9):944–950. doi: 10.1016/s0161-6420(80)35154-3. [DOI] [PubMed] [Google Scholar]
- 76.Kato T, Nakayasu K, Omata Y, Watanabe Y, Kanai A. Corneal deposits as an alerting sign of monoclonal gammopathy: a case report. Cornea. 1999;18(6):734–738. doi: 10.1097/00003226-199911000-00018. [DOI] [PubMed] [Google Scholar]
- 77.Koo H, Oh D-H, Chun YS, Kim JC. A case of crystalline keratopathy in monoclonal gammopathy of undetermined significance (MGUS) Korean J Ophthalmol. 2011;25(3):202–205. doi: 10.3341/kjo.2011.25.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Munteanu G. L’hérédodystrophie maculaire de Doyne et la gammopathie monoclonale benign. Corrélations génétiques et pathogéniques. J Fr Ophtalmol. 1980;3(7):753–758. [PubMed] [Google Scholar]