Abstract
Previous studies have shown inconsistent results regarding the actions of antidepressants on glucocorticoid receptor (GR) signalling. To resolve these inconsistencies, we used a lentiviral-based reporter system to directly monitor rat hippocampal GR activity during stress adaptation. Temporal GR activation was induced significantly by acute stress, as demonstrated by an increase in the intra-individual variability of the acute stress group compared with the variability of the non-stress group. However, the increased intra-individual variability was dampened by exposure to chronic stress, which was partly restored by fluoxetine treatment without affecting glucocorticoid secretion. Immobility in the forced-swim test was negatively correlated with the intra-individual variability, but was not correlated with the quantitative GR activity during fluoxetine therapy; this highlights the temporal variability in the neurobiological links between GR signalling and the therapeutic action of fluoxetine. Furthermore, we demonstrated sequential phosphorylation between GR (S224) and (S232) following fluoxetine treatment, showing a molecular basis for hormone-independent nuclear translocation and transcriptional enhancement. Collectively, these results suggest a neurobiological mechanism by which fluoxetine treatment confers resilience to the chronic stress-mediated attenuation of hypothalamic–pituitary–adrenal axis activity.
Introduction
The glucocorticoid receptor (GR) has a key role in the stress system by functioning to maintain molecular, cellular and systemic homeostasis.1,2 Dysfunctions in the GR have been implicated in the pathogenesis of depression.3,4
Antidepressant medications are known to normalize hypothalamic–pituitary–adrenal axis activity by restoring GR function,5, 6, 7 but the results regarding the effects of antidepressants on in vivo GR activity remain inconsistent and controversial. For example, fluoxetine is reported to increase,8,9 decrease10 or have no effect11,12 on GR expression. The mechanistic action of GR as a transcription factor involves sequential steps: upon binding to glucocorticoids (GCs) released from the adrenal cortex in response to stress, the GR undergoes a conformational change and translocates to the nucleus, where it modulates the expression of diverse target genes via binding to specific GC response elements (GREs) proximal to gene promoters.13,14 Considering this complex process, simple quantification of GR expression may not accurately reflect GR function. An integrative approach, such as measuring in vivo GR transcriptional activity, is necessary to clearly establish the relationship between GRs and the pharmacological effects of antidepressants.
Using live-cell imaging, Hagar's group demonstrated dynamic GRE binding activation coupled to the ultradian mode of hormone stimulation.15 This implies that GR function has been optimized to accurately regulate its target genes during hormonal changes. In line with this, in vivo studies using end-point analyses revealed oscillating target gene transcription synchronous with corticosterone (CORT) pulses in the adrenalectomized (ADX) model.15,16 Although these findings imply that the dynamics of GR signalling are important, most of the current research has focused on the consequent changes of GR signalling. In the end-point analyses, however, experimental variation derived mainly from inter-individual differences contributes significantly to the observed overall variation.17 Thus, it is difficult to assess whether true dynamics exist on the basis of the results of studies examining consequent changes of GR signalling.
The hippocampus reportedly has essential roles in the stress response,18 sleep behaviour,19 major depressive disorder20,21 and cognitive functions.22 Especially, a recent study on dorsal CA1 damage showed memory impairments only when the temporal processing demands were increased,23 suggesting an association with time-dependent brain functions. Thus, we targeted the dorsal CA1 as an anatomically logical region where the GR is highly expressed to study the temporal response of GR signalling in response to stress.
In the present study, we aimed to examine the association between the antidepressant fluoxetine and GR dynamics by analysing intra-individual variability24 of GR activity in the CA1 region of the hippocampus. This is important, as resilience has been conceptualized as a dynamic process.25,26 We used a lentiviral-based reporter to temporally monitor GR activation in live rats following stress±fluoxetine treatment. We further investigated the molecular mechanisms underlying the therapeutic action of GR dynamics by assessing GR nuclear translocation and its corresponding phosphorylation status.
Materials and methods
Construction of lentiviral-based reporters
Four lentiviral-based reporter vectors were constructed in this study (Supplementary Figure 1). For the GRE-luciferase reporter gene (GRE-Luc), a BamHI/EcoRI fragment containing the 5 × GRE-adenovirus intermediate early promoter and luciferase derived from pGRE-Luc14 was replaced with enhanced green fluorescence protein (EGFP) cDNA in the pWPXL transfer vector (a kind gift from Dr Didier Trono, Geneva, Switzerland), and the elongation factor 1α promoter was eliminated by restriction digestion with SalI and XhoI (New England Biolabs, Ipswich, MA, USA). A chicken β-globin insulator (kindly provided by Dr Tisdale)27 was inserted in front of the promoter region at the XbaI site to increase the specificity of the 5 × GRE enhancer. The basal-Luc reporter (ΔGRE-Luc), a negative control for GR signalling, was produced by deleting the 5 × GRE DNA fragment in the GRE-Luc reporter at the BamHI/XbaI sites. The GRE-EGFP reporter was constructed by replacing the luciferase cDNA in the GRE-Luc reporter with the polymerase chain reaction product of EGFP cDNA using a template from pEGFP-C2 (Clontech, Mountain View, CA, USA). The LV-Luc positive control reporter was also produced by replacing EGFP cDNA in the pWPXL with the polymerase chain reaction product of luciferase cDNA. The lentiviral constructs were confirmed by restriction enzyme maps in agarose gels, and by sequencing the ligated regions (Bioneer, Daejeon, Korea).
Animals
In this study, we used male Wistar rats weighing 280–300 g (Japan SLC, Hamamatsu, Japan). The animals were individually housed in transparent plastic cages with wire grid covers under controlled temperatures (22–24 °C) with a 12-h light/dark cycle (lights on from 0800 to 2000 hours). The rats were given at least 1 week to adapt to their environment before the experiments. The Institutional Animal Care and Use Committee at the Korea Basic Science Institute (KBSI-AEC 1109) reviewed and approved this study. All animal procedures were in accordance with the Guide for the Care and Use of Laboratory Animals issued by the Laboratory Animal Resources Commission of KBSI.
Experimental design for hippocampal GR activity analysis
Rats injected with one of the lentiviral reporters (GRE-Luc or ΔGRE-Luc) were tested in three independent experiments. In the first experiment, following 2 weeks of recovery from surgery, the rats were randomly assigned to either a non-stress (NS) or acute stress (AS) group with a 2-h immobilization condition (IMO, typically between 0900 and 1100 hours), which was determined by previous data for GR-mediated transcripts.28, 29, 30 In the second experiment, 7 days after lentiviral injection, rats were adrenalectomized (ADX) bilaterally and after another 7 days were randomly assigned to two groups; one group was subjected to 2-h IMO stress and the second subcutaneously injected with CORT (3.0 mg kg−1 in sesame oil; Sigma, St Louis, MO, USA) at a volume adjusted to 1 ml kg−1 body weight.31 In the third experiment, thirty rats were exposed daily to 2-h IMO stress for 21 consecutive days during which they were randomly assigned to receive daily injection of either fluoxetine (chronic stress plus fluoxetine (CS+F); 10 mg kg−1, intraperitoneal, dissolved in saline solution, Daewoo Pharmaceuticals, Busan, Korea) or 0.9% saline (chronic stress plus vehicle (CS); 1 ml kg−1) 30 min before IMO stress. Following the last chronic injection on day 21, reporter activity was assessed using the IVIS 200 imaging system (Xenogen Corporation, Alameda, CA, USA) before and after the 2-h IMO stress exposure, and then every 2 h after the stress exposure for 6–10 h.
Imaging of in vivo and ex vivo GR activity
We recently reported in vivo visualization of luciferase activity using the bioluminescence imaging (BLI) technique.32, 33, 34 Fifteen minutes before every BLI, all animals received an intraperitoneal injection of 150 mg kg−1 D-luciferin (Biosynth International, Naperville, IL, USA) dissolved in Dulbecco's phosphate-buffered saline, and were then anaesthetized in an induction chamber with 2.5% isoflurane in 100% oxygen at a flow rate of 1.0 l min−1 for 10 min. For the in vivo BLI analyses, three live rats were imaged simultaneously for 5 min using the IVIS system with a 2.0% mixture at 0.5 l min−1, and the regions of interest were quantified with photon flux (p/s) using Living Image software v4.2 (Xenogen Corporation). The data represent BLI signals from an individual rat, combined from at least two independent studies. Quantitative BLI for the GRE-Luc reporter was divided by the averaged BLI value for the ΔGRE-Luc control reporter (Supplementary Figure 1) from at least two independent experiments to normalize the temporal increase of luciferase activity (Supplementary Figure 2). For the ex vivo BLI analyses, after completing the AS, rats were injected with luciferin 15 min before euthanization. The brain was removed and dissected into 1-mm coronal or sagittal segments using a slicer matrix (ASI Instruments, Warren, MI, USA).
Results
Validation of lentiviral-based GR reporters
To visualize GR activity in living rats, we used a lentiviral GRE reporter (Figure 1a). The GRE-Luc reporter comprised the firefly luciferase reporter gene under the control of five repetitive GREs (Supplementary Figure 1), which selectively sensed GR signalling in the hippocampal neuronal cells (Supplementary Figures 3a and b).35 Similar results were observed with COS-1 cells, which exogenously overexpress mineralocorticoid receptor or GRs by transient transfection (Supplementary Figure 3c). We also examined in vivo responsivity to stress for the lentiviral Luc reporters including a positive control for luciferase activity (LV-Luc, Supplementary Figure 4) and found no effect. These results indicate that our lentiviral reporter system can monitor GR activity induced by stress without interference from intrinsic noise signals.
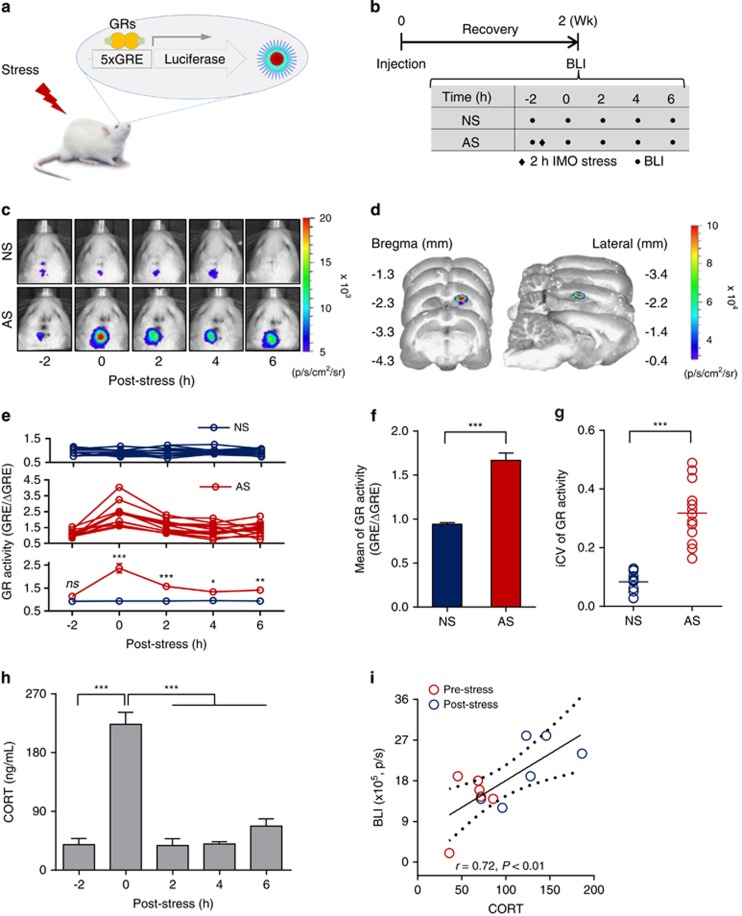
Figure 1.
Temporal response of hippocampal glucocorticoid receptor (GR) activity to acute stress. (a) Schematic illustration of stress-induced GR activation in a living rat. (b) Schedule of the experimental procedure for temporal monitoring of GR activation. (c) Representative in vivo bioluminescent images (BLIs) in the hippocampal CA1 region of non-stressed (NS, upper panel) and acutely stressed (AS, lower panel) rats stereotaxically injected with the glucocorticoid response element-luciferase (GRE-Luc) reporter. (d) Representative ex vivo BLIs in serial sections obtained 25 min following stress and intraperitoneal luciferin injection. BLI signals were detected at the site of the stereotaxic injection. (e) Individual profiles of BLI signals in the NS group (n=11, top panel) and AS group (n=13, middle panel), and the averaged signal for both groups (bottom panel). *P<0.05, **P<0.01 and ***P<0.001 by two-way repeated measures analysis of variance (ANOVA). (f) Comparison of averaged GR activity between the NS and AS groups. Data represent the average value of GR activity for the four time points (0, 2, 4 and 6 h) for each individual and are presented as the mean±s.e.m. (NS, n=44; AS, n=52) ***P<0.001 by Student's t-test. (g) Effect of acute stress on the variability of GR activation. Data represent the intra-individual coefficient of variation (iCV; intra-individual standard deviation divided by the individual mean) over an 8-h imaging period. ***P<0.001 by Student's t-test. (h) Corticosterone (CORT) response to acute stress, presented as the mean±s.e.m. (n=10 animals per time point). ***P<0.001 by one-way repeated measures ANOVA. Wk, week. (i) Correlation analysis between the CORT level and GR activity both pre- (−2 h) and post-stress (0 h). Scatter plots with fitted linear regression lines and a 95% confidence interval (dashed lines) for that line are shown for 12 pairs conducted between the CORT level and GR activity both pre- and post-stress.
Hormone-dependent GR activation induced by AS
To measure the response of GR activation to stress, we monitored hippocampal GR activity after each 2-h exposure to AS for a period of 8 h, beginning before the stress exposure (Figure 1b). GR activity increased immediately after the stress at 0 h post-stress, whereas no change was observed in the NS group (Figures 1c and e). The two-way analysis of variance indicated that GR activity was significantly affected by time (F4,88=21.60, P<0.001) and stress exposure (F1,22=33.58, P<0.001). There was also a significant interaction between time and stress (F4,88=21.58, P<0.001). The AS group exhibited a 77% increase in average GR activity compared with the average GR activity of the NS group (Figure 1f). Furthermore, temporal analyses showed a 60% increase in the intra-individual coefficients of variation (iCV; Figure 1g). The anatomical accuracy of GR activation in hippocampal CA1 neurons was confirmed by ex vivo BLI (Figure 1d) and immunohistochemical staining with the GRE-enhanced green fluorescent protein reporter (Supplementary Figure 1), which was detected in neuronal nuclei (NeuN)-positive CA1 neurons that were colocalized with GRs (Supplementary Figure 5).
The CORT level peaked at 0 h post-stress (Figure 1h; F4,45=47.74, P<0.001) and corresponding GR nuclear translocation was detected immediately after AS (Supplementary Figure 6; F2,9=4.56, P<0.05), consistent with functional coupling between CORT levels and GR activation in the normal stress response. This was supported by a significant positive correlation between CORT and GR activity (Figure 1i). We tested this coupling in ADX rats (Supplementary Figure 7a), and found that AS did not induce GR activation in ADX rats (Supplementary Figure 7b left; t=1.44, P=0.17), but GR activity was significantly increased in CORT-treated ADX rats compared with that in vehicle-treated rats (Supplementary Fig. 7b right; t=6.84, P<0.001). The highest CORT level was measured at 45 min after injection (Supplementary Figure 7c right; t=4.96, P<0.001), whereas there was no change in the CORT level of the ADX-AS group (Supplementary Figure 6c left; t=0.53, P=0.60). There was also a significant positive correlation between CORT and GR activity in ADX rats (Supplementary Figure 7d).
Dynamic GR activation restored by fluoxetine treatment
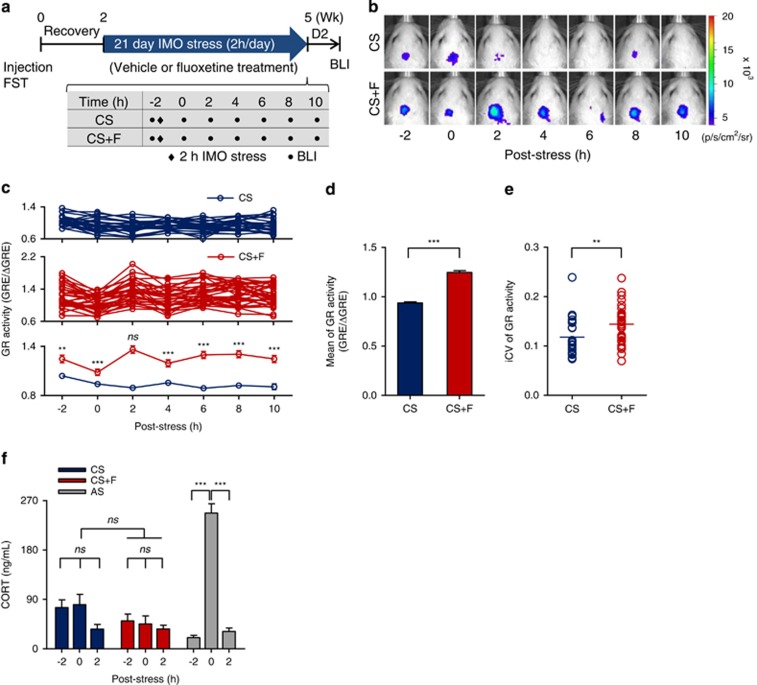
To examine the effect of fluoxetine on GR activation, we monitored GR activity following fluoxetine treatments in the CS group (Figure 2a). There were significant increases in GR activation over time in the CS+F group compared with that in the CS group (Figures 2b and c; time: F6,354=5.49, P<0.001; treatment: F1,59=50.54, P<0.001; time × treatment: F6,354=10.09, P<0.001). Significant average GR activity and iCV differences were also discerned between the two groups, with a 33% increase in average GR activity (Figure 2d) and a 30% increase in iCV (Figure 2e) in the CS+F group compared with that in the CS group. Dynamic GR activation by fluoxetine was corroborated by a widened and right-shifted distribution of GR activity in the CS+F group (Supplementary Figure 8). Although the maximum peak of GR activation was clearly evident at 2 h post-stress in the CS+F group (Figure 2c bottom; t=8.39, P<0.001 vs CS), an induced CORT response was not detected (Figure 2f; time: F2,54=2.78, P=0.07; treatment: F1,54=3.39, P=0.07; time × treatment: F2,54=0.95, P=0.39).
Figure 2.
Effect of fluoxetine treatment on glucocorticoid receptor (GR) activity under chronic stress. (a) Schematic of the experimental design. (b) Representative bioluminescent images (BLIs) after chronic stress (CS) by 21-day immobilization (IMO) with vehicle (CS, upper panel) or fluoxetine treatment (CS+F, lower panel). (c) Individual profiles of BLI signals by CS (n=27, top panel) and CS+F (n=34, middle) from three independent studies, and the averaged signal (bottom panel) of GR activity. **P<0.01, ***P<0.001 by two-way repeated measures analysis of variance (ANOVA) followed by Bonferroni's multiple comparison post hoc test. (d) Comparison of averaged GR activity between CS and CS+F group. Data represent the average value of GR activity for all the time points for each individual and are presented as the mean±s.e.m. (CS, n=189; CS+F, n=238) ***P<0.001 by Student's t-test. (e) Intra-individual coefficients of variation (iCV) over the 12-h imaging period. **P<0.01 by Student's t-test. (f) Comparison of corticosterone (CORT) responses (mean±s.e.m.) to chronic stress in the CS and CS+F groups compared with acutely stressed (AS) rats of the same age. Data are presented as the mean±s.e.m. (CS and CS+F, n=10 per time point; AS, n=5 per time point). ***P<0.001 by one-way repeated measures ANOVA followed by Bonferroni's multiple comparison post hoc test. D2, 2 days; ns, nonsignificant; Wk, week.
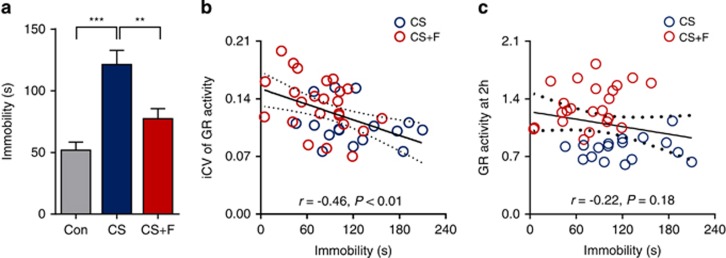
To investigate the molecular links to therapeutic effects, we performed the forced-swim test (FST) 2 days after BLI in the CS and CS+F groups. As expected, chronic stress exposure increased the immobility duration in the FST, which was alleviated by chronic fluoxetine treatment (Figure 3a and Supplementary Figure 9). In the correlation analysis, a significant and negative correlation was found between the iCV and the retention of immobility (Figure 3b). However, for the GR activity at 2 h post-stress, no significant difference was found with regard to the retention of immobility duration (Figure 3c). Similar results were also obtained for the GR activity at the other six time points (Supplementary Figure 10).
Figure 3.
Correlation between glucocorticoid receptor (GR) activity and depression-like behaviour. (a) The effect of fluoxetine on the retention of immobility in the forced-swim test (FST). Two days after imaging, the FST was conducted in the last two of three experiments. Data are presented as the mean±s.e.m. (n=10 for control (Con), n=17 for chronic stress plus vehicle (CS), and n=24 for chronic stress plus fluoxetine (CS+F)). **P<0.01, ***P<0.001 by one-way analysis of variance. (b and c) Pearson correlation between the FST immobility duration and the intra-individual coefficient of variation (iCV; b) or GR activity at 2 h post-stress (c). Scatter plots with fitted linear regression lines and a 95% confidence interval (dashed lines) for that line are shown for 41 pairs.
Hormone-independent GR nuclear translocation in response to fluoxetine treatment
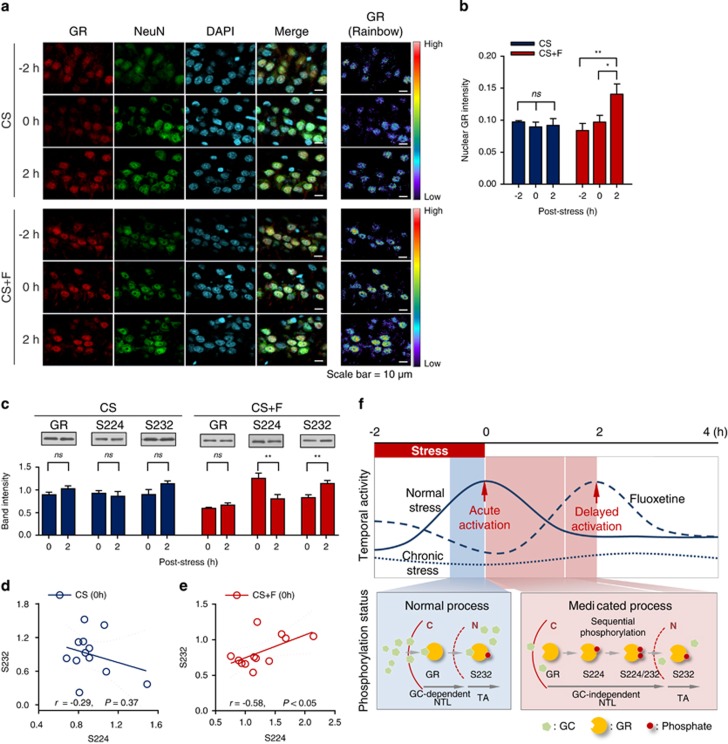
Next, we tested whether treatment with fluoxetine might affect GR nuclear translocation, even without hormonal induction, which is the step preceding GR transactivation. Triple immunohistochemical staining (Figure 4a) showed that nuclear translocation was significantly increased at 2 h post-stress in the CS+F group (Figure 4b right; F2,9=9.27, P<0.01), whereas it was unchanged in the CS group (Figure 4b left; F2,9=0.28, P=0.77). From the western blot analysis, we found that the total amount of GR expression was unchanged within (Figure 4c) and between groups (Supplementary Figure 11). These results indicate that GR nuclear translocation involves GR activation in a hormone-independent manner during fluoxetine treatment.
Figure 4.
Effect of fluoxetine treatment on glucocorticoid receptor (GR) nuclear translocation and phosphorylation. (a) Representative confocal images of the hippocampal CA1 region. Hippocampal sections were stained with GR (red), the neuronal marker NeuN (green), and 4',6-diamidino-2-phenylindole (DAPI; cyan) to detect GR nuclear translocation. In addition, GR intensity images (right panel) are represented linearly on a rainbow scale with red being the maximum signal and black being the lowest signal. Scale bar, 10 μm. (b) Quantification of nuclear GR intensity in neurons at the indicated time points. Data are presented as the mean±s.e.m. (n=4 per time point). *P<0.05, **P<0.01 by one-way repeated measures analysis of variance followed by Bonferroni's multiple comparison post hoc test. (c) Representative images for western blots of hippocampal lysates (insets) and quantification of band intensity in the chronic stress (CS) and chronic stress plus fluoxetine (CS+F) groups (n=12 per group). Expression levels were normalized by β-actin. **P<0.01 by Student's t-test. (e and f) Pearson's correlation between S224 and S232 in the CS (d) and CS+F (e) groups at 0 h post-stress, showing linear regression (solid line) within 95% confidence intervals (dashed lines). (f) Proposed model of the delayed GR activation underlying the molecular action of fluoxetine. Schematic representation of GR activation shows how the different processes of GR nuclear translocation affect peak timing. S224 and S232 in rat GR correspond to S203 and S211 in human GR. S224, phosphorylated GR at Serine 224; S232, phosphorylated GR at Serine 232; GC, glucocorticoid; ns, nonsignificant; NTL, nuclear translocation; TA, transactivation.
Next, we examined GR phosphorylation (pGR), which is known to modulate GR nuclear translocation.36 Western blot analyses showed that there was a 36% decrease in pGR (S224) and a 37% increase in pGR (S232) at 2 h post-stress in the CS+F group (Figure 4c, right), whereas the CS group did not show any significant changes (Figure 4c, left). Furthermore, a significant positive correlation at 0 h post-stress was found in the CS+F group (Figure 4e), showing interdependent phosphorylation before GR activation.37 There was no correlation between the phosphorylated sites at 2 h post-stress (Supplementary Figure 12). Collectively, these findings suggest that the molecular basis of hormone-independent nuclear translocation involves the sequential phosphorylation of pGR (S224) and pGR (S232), which may have a key role in the delayed GR activation in response to fluoxetine (Figure 4f).
Discussion
In this study, using an in vivo bioluminescent reporter system, we monitored hippocampal GR activity in rats subjected to immobilization stress. When rats were exposed to a single 2-h immobilization (AS), hippocampal GRs were activated by stress-induced circulating GCs, indicating a functional coupling between GR activity and GCs. This was confirmed using ADX rats, which exhibited hippocampal GR activation if administered corticosteroids, but not if subjected to AS. In contrast, rats subjected to multiple immobilizations (2 h daily for 21 days, chronic stress) showed both dampened adrenal medullary GC secretion and hippocampal GR activation.30,38,39 However, chronically immobilized animals treated with the antidepressant fluoxetine showed increased hippocampal GR activity and iCV in the absence of altered GC secretion. In the FST, immobility retention was negatively correlated with the iCV, but not with GR activity, suggesting that GR dynamics might be of potential therapeutic significance. On the basis of the combined biochemical results of GR nuclear translocation and phosphorylation status, we propose that hormone-independent GR activation is involved in the therapeutic action of fluoxetine. Collectively, our observations of GR activity during fluoxetine therapy provide clear evidence for the neurobiological links between GR signalling and antidepressant action.
One of the conundrums in current stress research is the individual variability in the adaptation to stress.40 Particularly, the individual variation in in vivo GR activity in response to stress is poorly understood. Our results showed that AS initially induced GR activation with various responses, but it is questionable whether these variations are individual-specific. There are two potential explanations for these observations. The first involves different efficiencies regarding the delivery and cellular uptake of a locally administered lentiviral reporter to targeted cells. In H19-7 hippocampal neuronal cells, however, the correlation between the responses of the GRE-Luc and CORT treatment was strong (r=0.96, P<0.05, data not shown), suggesting that the efficacy of the uptake and delivery for the lentiviral reporter is at least partly homogeneous.41 The second possibility is that individual variations in the GCs' response to stress may be involved with the individual variations in GR activation, as the initial GR activation superimposes on the peak of GCs. Furthermore, strong correlates between GR activity and stress-induced CORT (Figure 1i), as well as exogenous CORT in ADX rats (Supplementary Figure 7d), were detected in independent experiments, supporting the prediction that CORT has a key role in the individual variability of GR activation as a main determinant for translocation and transcription in the normal stress response.
Our prospective finding was significant in terms of showing the neurobiological variability of hippocampal GR activity during fluoxetine therapy, as well as during the normal response to stress. Alterations in physiological variability are thought to be associated with disease. Particularly, decreased heart rate variability was identified as an indicator of emotional stress in severe depression.42,43 In line with this physiological phenomenon, we found attenuated variability in hippocampal GR activity under chronic stress,38,39 which was partly restored by fluoxetine therapy. These findings indicate that temporal variability entails both neurobiological and physiological processes to prevent health disturbances after adversity, which can recover from adversity-related mental illnesses by antidepressant therapy. The pathological significance of GR variability is supported by reports that alterations in the dynamic assembly of the GR-chaperone complex have been implicated as vulnerability factors in posttraumatic stress disorder and mood disorders.44, 45, 46 Moreover, a recent study showed an association between genetic variations in GR function and individual variability in the therapeutic responses to antidepressant-like effects.47 It is also interesting to note that temporal variability was significantly correlated with immobility in the FST, although this correlation was not statistically significant for quantitative GR activity. Therefore, it seems that the simple changes in GR activity are not as strongly related to depressive behaviours as is its variability. Such findings may explain the contrastive results of forebrain GR-knockdown transgenic mouse studies, which show an impaired behavioural response to stress with hypothalamic–pituitary–adrenal axis dysregulation.18,48 Simultaneously, transgenic mice with overexpressed GRs also display a significant increase in anxiety- and depression-like behaviours.49
Another prospective finding was hormone-independent GR activation during fluoxetine therapy. Hormone assays at each time point showed functional coupling between the GC pulses and GR activation in the normal stress response, which was confirmed in an ADX model administrated with a single injection of GC. This coupling corroborates the findings of studies on GR-targeted nascent RNA expression16 and GRE binding activity,15 indicating that the primary secretion of GC has a key role in dynamic GR activation during normal stress adaptation. However, it is unlikely that this functional coupling underlies the molecular actions of fluoxetine, as no GC induction was observed despite activation of the GR reporter during fluoxetine treatment. In agreement with these findings, we showed that fluoxetine treatment had no effect on retention of acquired immobility in the second test session (Supplementary Figure 9), corresponding to the blunted CORT response to fluoxetine.50 These findings indicate that GCs are not necessary for the therapeutic action of fluoxetine.51,52
Nevertheless, significant nuclear translocation corresponding to the GR activation was detected in our study. This hormone-independent translocation was reported by an in vitro study, in which the antidepressant desipramine induced GR nuclear translocation in the absence of corticosteroids.53 Further investigation of the GR phosphorylation status showed that sequential S224 and S232 phosphorylation was involved in GR nuclear translocation. pGR (S224) is known to stimulate S232 phosphorylation to increase GR transcriptional activity,54, 55, 56 suggesting that the phosphorylation of GR (S224) could be an initial target for the therapeutic effect of fluoxetine. Meanwhile, a recent study reported that phospholipase C and protein kinase C signalling have a key role in the modulatory effect of antidepressants on GR-induced gene transcription,57 suggesting the involvement of other signalling mechanisms along with GR phosphorylation. Thus, on-going studies are focused on establishing how these signalling pathways might be linked to the phosphorylation status of GRs during fluoxetine therapy by performing temporal analyses of their associations. Our findings, clearly reflective of the hormone-independent pathway, provide some clues for understanding the molecular mechanisms that are potentially involved in the therapeutic action of fluoxetine on GR dynamics.
The strength of this study is the temporal analysis of GR activity in vivo using a lentiviral reporter system. Additional advantages of the lentiviral reporter include: (i) the integrative readouts of in vivo GR function; (ii) the detection of GR-specific activity without interference from mineralocorticosteroid receptor signalling; (iii) the accurate quantification of GR activity by luminescent enzymatic reporters; (iv) anatomical localization by stereotaxic injection of the reporter; and (v) rapid and easy measurement. A technical limitation of the lentiviral reporter system is the relatively long interval time for monitoring GR activation (2 h), as the in vivo half-life of luciferase activity was estimated at ~102 min (Supplementary Figure 2). Despite this limitation, we were able to detect a significant increase in GR variability by fluoxetine treatment. The second limitation is the unavailability of a true internal control for GRE-Luc signals. We used the average signal obtained from different animals transduced with the ΔGRE-Luc reporter as the reference for normalization of GRE-Luc signals. For an internal control, the ΔGRE-Luc reporter should be injected into the right hippocampal CA1 region (anterior −3.6 mm, lateral +2.0 mm, ventral −2.9 mm) as a counter partner for the GRE-Luc in the left hippocampal CA1 (anterior −3.6 mm, lateral −2.0 mm, ventral −2.9 mm) of the same animal on the basis of coordinates from the bregma and dura mater according to the atlas of Paxinos and Watson.58 However, the resolution of BLI is insufficient to discern the two signals generated by the reporters with a 4-mm lateral distance (data not shown). This low spatial resolution due to light scattering59 has made it very difficult to study strategies for generating a true internal control in the same animal. If the signal outputs from the ΔGRE-Luc reporter result in net changes in the response to stress, it is necessary to assess the signals in a corresponding region in the same animal. These changes are nonsignificantly small and can be neglected in the face of distinct changes in the GRE-Luc (Supplementary Figure 4). The third limitation is the end-point analysis of GR phosphorylation, that is, it was not possible to determine how the phosphorylation changes, especially of pGR (S224), are associated with GR variability and depression-like behaviours. It is tempting to speculate that the dynamic modification of GR phosphorylation underlying the temporal variability of GR activity may be a molecular marker predicting the individual response to antidepressants.60,61
In conclusion, this study identified the neurobiological links between the temporal variability of hippocampal GR activity and the therapeutic action of fluoxetine as demonstrated by an increase in temporal variability and its significant correlation with depressive behaviours. These findings provide new mechanistic insights for the development of biomarkers and novel antidepressants.
Acknowledgments
We thank Dr Didier for the pWPXL transfer vector and Dr Tisdale for the chicken β-globin insulator. This study was supported by Korea Basic Science Institute grants (T32902 and D33400).
AF Schatzberg is a co-founder and has equity in Corcept Therapeutics. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann NY Acad Sci 1998; 840: 33–44. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neurosci Biobehav Rev 1992; 16: 115–130. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Reder AT, Antel JP, Meltzer HY. Glucocorticoid resistance in depression: the dexamethasone suppression test and lymphocyte sensitivity to dexamethasone. Am J Psychiatry 1984; 141: 1365–1370. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry 2002; 7: 924, 985–994. [DOI] [PubMed] [Google Scholar]
- Barden N, Reul JM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system? Trends Neurosci 1995; 18: 6–11. [DOI] [PubMed] [Google Scholar]
- Reul JM, Stec I, Soder M, Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic-pituitary-adrenocortical system. Endocrinology 1993; 133: 312–320. [DOI] [PubMed] [Google Scholar]
- Reul JM, Labeur MS, Grigoriadis DE, De Souza EB, Holsboer F. Hypothalamic-pituitary-adrenocortical axis changes in the rat after long-term treatment with the reversible monoamine oxidase-A inhibitor moclobemide. Neuroendocrinology 1994; 60: 509–519. [DOI] [PubMed] [Google Scholar]
- Brady LS, Gold PW, Herkenham M, Lynn AB, Whitfield HJJr. The antidepressants fluoxetine, idazoxan and phenelzine alter corticotropin-releasing hormone and tyrosine hydroxylase mRNA levels in rat brain: therapeutic implications. Brain Res 1992; 572: 117–125. [DOI] [PubMed] [Google Scholar]
- Lai M, McCormick JA, Chapman KE, Kelly PA, Seckl JR, Yau JL. Differential regulation of corticosteroid receptors by monoamine neurotransmitters and antidepressant drugs in primary hippocampal culture. Neuroscience 2003; 118: 975–984. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Jacobson L. Widespread hypothalamic-pituitary-adrenocortical axis-relevant and mood-relevant effects of chronic fluoxetine treatment on glucocorticoid receptor gene expression in mice. Eur J Neurosci 2010; 31: 892–902. [DOI] [PubMed] [Google Scholar]
- Rossby SP, Nalepa I, Huang M, Perrin C, Burt AM, Schmidt DE et al. Norepinephrine-independent regulation of GRII mRNA in vivo by a tricyclic antidepressant. Brain Res 1995; 687: 79–82. [DOI] [PubMed] [Google Scholar]
- Szymanska M, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Kubera M, Leskiewicz M et al. The effect of antidepressant drugs on the HPA axis activity, glucocorticoid receptor level and FKBP51 concentration in prenatally stressed rats. Psychoneuroendocrinology 2009; 34: 822–832. [DOI] [PubMed] [Google Scholar]
- Kumar R, Thompson EB. Gene regulation by the glucocorticoid receptor: structure:function relationship. J Steroid Biochem Mol Biol 2005; 94: 383–394. [DOI] [PubMed] [Google Scholar]
- Her S, Patel PD, Schatzberg AF, Lyons DM. Mutations in squirrel monkey glucocorticoid receptor impair nuclear translocation. J Steroid Biochem Mol Biol 2005; 94: 319–326. [DOI] [PubMed] [Google Scholar]
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 2009; 11: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC et al. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol 2010; 22: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, McCoy Z, Lambert JM, Andries MJ, Lucier GW. Relationships among benzo(a)pyrene metabolism, benzo(a)pyrene-diol-epoxide:DNA adduct formation, and sister chromatid exchanges in human lymphocytes from smokers and nonsmokers. Cancer Res 1989; 49: 6503–6511. [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC et al. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience 2012; 203: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 2009; 12: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004; 29: 417–426. [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 2004; 56: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 2005; 25: 11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farovik A, Dupont LM, Eichenbaum H. Distinct roles for dorsal CA3 and CA1 in memory for sequential nonspatial events. Learn Mem 2010; 17: 12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Nyberg L, Backman L. Intra-individual variability in behavior: links to brain structure, neurotransmission and neuronal activity. Trends Neurosci 2006; 29: 474–480. [DOI] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev 2000; 71: 543–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D. Resilience under conditions of extreme stress: a multilevel perspective. World Psychiatry 2010; 9: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Washington KN, Lap CJ, Hsieh MM, Tisdale JF. Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol Ther 2011; 19: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsink MC, Steenbergen PJ, Vos JB, Karst H, Joels M, De Kloet ER et al. Acute activation of hippocampal glucocorticoid receptors results in different waves of gene expression throughout time. J Neuroendocrinol 2006; 18: 239–252. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Fuxe K. Time-course of immediate early gene expression in hippocampal subregions of adrenalectomized rats after acute corticosterone challenge. Brain Res 2008; 1215: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N et al. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology 2010; 151: 1177–1186. [DOI] [PubMed] [Google Scholar]
- Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology 2007; 148: 5470–5477. [DOI] [PubMed] [Google Scholar]
- Lee MS, Jung JI, Kwon SH, Lee SM, Morita K, Her S. TIMP-2 fusion protein with human serum albumin potentiates anti-angiogenesis-mediated inhibition of tumor growth by suppressing MMP-2 expression. PLoS One 2012; 7: e35710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kwon EH, Choi HS, Kwon SH, Lee CH, Shim IS et al. Quantification of cellular uptake and in vivo tracking of transduction using real-time monitoring. Biochem Biophys Res Commun 2010; 394: 348–353. [DOI] [PubMed] [Google Scholar]
- Lee MS, Her S. Direct quantification of PTD transduction using real-time monitoring. Methods Mol Biol 2013; 991: 307–314. [DOI] [PubMed] [Google Scholar]
- Iniguez-Lluhi JA, Pearce D. A common motif within the negative regulatory regions of multiple factors inhibits their transcriptional synergy. Mol Cell Biol 2000; 20: 6040–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco DB, Qi M, Borror KC, Garabedian MJ, Brautigan DL. Protein phosphatase types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Mol Endocrinol 1991; 5: 1215–1228. [DOI] [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation ‘code' of the glucocorticoid receptor in vivo. J Biol Chem 2002; 277: 26573–26580. [DOI] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci 2013; 7: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse SK, Nyhuis TJ, Masini CV, Day HE, Campeau S. Central gene expression changes associated with enhanced neuroendocrine and autonomic response habituation to repeated noise stress after voluntary wheel running in rats. Front Physiol 2013; 4: 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev 2010; 34: 853–866. [DOI] [PubMed] [Google Scholar]
- Lee MS, Park WS, Kim YH, Ahn WG, Kwon SH, Her S. Intracellular ATP assay of live cells using PTD-conjugated luciferase. Sensors (Basel) 2012; 12: 15628–15637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol 2000; 37: 121–133. [DOI] [PubMed] [Google Scholar]
- Stein PK, Carney RM, Freedland KE, Skala JA, Jaffe AS, Kleiger RE et al. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res 2000; 48: 493–500. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet 2004; 36: 1319–1325. [DOI] [PubMed] [Google Scholar]
- Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci USA 2008; 105: 8766–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology 2009; 34(Suppl 1): S186–S195. [DOI] [PubMed] [Google Scholar]
- Espallergues J, Teegarden SL, Veerakumar A, Boulden J, Challis C, Jochems J et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. J Neurosci 2012; 32: 4400–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Muglia LJ. Defining brain region-specific glucocorticoid action during stress by conditional gene disruption in mice. Brain Res 2009; 1293: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis HD, De Korte CC, De Kloet ER. Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur J Pharmacol 1985; 115: 211–217. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Carson SW, Breese GR. Differential effects of chronic antidepressant treatment on swim stress- and fluoxetine-induced secretion of corticosterone and progesterone. J Pharmacol Exp Ther 1998; 285: 579–587. [PMC free article] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry 1998; 43: 547–573. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Owens MJ, Miller AH. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol Pharmacol 1997; 52: 571–581. [DOI] [PubMed] [Google Scholar]
- Hu A, Josephson MB, Diener BL, Nino G, Xu S, Paranjape C et al. Pro-asthmatic cytokines regulate unliganded and ligand-dependent glucocorticoid receptor signaling in airway smooth muscle. PLoS One 2013; 8: e60452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min G, Kemper JK, Kemper B. Glucocorticoid receptor-interacting protein 1 mediates ligand-independent nuclear translocation and activation of constitutive androstane receptor in vivo. J Biol Chem 2002; 277: 26356–26363. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem 1999; 274: 1005–1010. [DOI] [PubMed] [Google Scholar]
- Budziszewska B, Jaworska-Feil L, Kajta M, Lason W. Antidepressant drugs inhibit glucocorticoid receptor-mediated gene transcription—a possible mechanism. Br J Pharmacol 2000; 130: 1385–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Jansen ED, Pickett PM, Mackanos MA, Virostko J. Effect of optical tissue clearing on spatial resolution and sensitivity of bioluminescence imaging. J Biomed Opt 2006; 11: 041119. [DOI] [PubMed] [Google Scholar]
- Lee MS, Park WS, Kim YH, Kwon SH, Jang YJ, Han D et al. Antidepressant-like effects of Cortex Mori Radicis extract via bidirectional phosphorylation of glucocorticoid receptors in the hippocampus. Behav Brain Res 2013; 236: 56–61. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim YH, Park WS, Ahn WG, Park OK, Kwon SH et al. Novel antidepressant-like activity of propolis extract mediated by enhanced glucocorticoid receptor function in the hippocampus. Evid Based Complement Alternat Med 2013; 2013: 217853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.