Abstract
ErbB-3 and its ligand NRG-1β are key players in driving oncogenic signaling and resistance to therapy through the activation of the PI3K/Akt pathway. We have recently reported that EV20, a humanized anti-ErbB3 antibody, possesses a marked antitumor activity in a variety of human tumor models, including pancreatic cancer (PC). Here, we report that despite epidermal growth factor receptor overexpression, PC cells are more sensitive to NRG-1β than EGF in terms of Akt activation and cell proliferation. Using stable ErbB-3-knocked down cells and EV20 in combination with trastuzumab, we showed that dual targeting of ErbB-2 and ErbB-3 was necessary to completely abrogate ErbB-3 signaling and to impair cell proliferation. Similarly, enhanced therapeutic efficacy of the antibody combination was seen in xenografts originating from K-Ras-mutated HPAF-II and SW1990 cells, without increasing the toxicity. These results indicate that dual targeting of ErbB-2 and ErbB-3 could represent a new therapeutic approach in PC.
Introduction
Pancreatic cancer (PC) is the fourth deadliest cancer in men and women1 and is one of the most challenging conditions to treat because most of the patients present with locally advanced or metastatic disease and no effective therapy has yet been identified.2 The disease prognosis is extremely poor, with the overall 5-year survival rate of less than 5%, the figure which has largely remained unchanged over the last decades.3 Therefore, the development of novel targeted treatment approaches has recently been a focus of expectation for this malignancy. Overexpression of the epidermal growth factor receptor (EGFR), ErbB-2 and ErbB-3 receptors (ErbB family of receptor tyrosine kinases) has been associated with poor prognosis and disease progression, thereby they have been considered as promising targets for the treatment of PC.4–7
However, the application of anti-EGFR and anti-ErbB-2 agents has not given effective performances in eligible patients. One phase II clinical trial of trastuzumab with gemcitabine in high ErbB-2 expression patients and a phase III clinical trial of cetuximab with gemcitabine in patients with EGFR-expressing tumors have shown no advantage for the combination therapies over gemcitabine alone.8,9 Only one phase III clinical trial with erlotinib plus gemcitabine has shown yet less than a month advantage for combination treatment over gemcitabine alone.10 These data underscore the modest effectiveness of the anti-EGFR and anti-ErbB-2-targeting approaches in PC. This is consistent with the notion that other ErbB receptors, their ligands or other components of the ErbB family network are able to compensate the oncogenic activities of the ‘addicted' receptor once targeted.11–14 Notably, along with the expression of multiple ErbB receptors, most epithelial tumors also express one or more EGF-related ligands, which activate certain types/combinations of the ErbB receptors.15 Recently, ErbB-3, another member of the ErbB receptor family, has emerged as a key player in the development and progression of PC, and its high expression has been correlated with advanced stage of disease and decreased survival.4,16,17 Importantly, high expression of NRG-1β, the ligand promoting ErbB-2/ErbB-3 dimerization, has been correlated with worse clinical prognosis,18 confirming a relevant role of the NRG-1β/ErbB-3/Akt cell signaling axis in PC. In this regard, although ErbB-3 receptor has a very limited innate functioning kinase compared with the other members of the family,19 the ErbB-2/ErbB-3 heterodimer is considered the most potent ErbB pair with respect to strength of interaction, ligand-induced tyrosine phosphorylation and downstream signaling, and functions as a powerful oncogenic unit.20,21
Therefore, we decided to investigate the therapeutic potential of ErbB-2/ErbB-3 dual targeting in a set of PC cells with different ErbB-2/ErbB-3 expression levels, K-Ras status and sensitivity to erlotinib (Table 1). For ErbB-3 inhibition, we utilized EV20, a humanized antibody recently developed in our laboratory that proved to have an antitumor activity in different cancer models, including PC cells, by ligand-dependent and -independent ErbB-3/Akt inhibition.22,23 To target ErbB-2, we used trastuzumab, a monoclonal antibody currently used for treatment of breast and gastric cancer.24,25
Table 1. PC cell lines used in the study and their features.
| Cell line | Metastatic | K-Ras status | EGFR | ErbB-2 | ErbB-3 | Erlotinib sensitivity |
|---|---|---|---|---|---|---|
| BxPC-3 | No | WT | 41.59±7.39 | 4.48±0.95 | 3.66±1.34 | High |
| HPAF-II | Yes | Mutant | 32.34±7.4 | 6.69±0.6 | 4.98±1.2 | Moderate |
| SW1990 | Yes | Mutant | 23.3±6.25 | 7.53±2.26 | 2.13±0.26 | Low |
Abbreviations: EGFR, epithelial growth factor receptor; PC, pancreatic cancer; WT, wild type.
Fluorescence-activated cell sorting data relative to EGFR, ErbB-2 and ErbB-3 surface expression level are expressed as mean florescence intensity (MFI) ratio (MFI of cells stained with primary antibody followed by anti-human secondary antibody/MFI of cells stained with PBS followed by anti-human secondary antibody) and the values indicated are the mean±s.d.
We found that the combination of trastuzumab and EV20 exerts a superior antitumor activity compared with either agent alone both in vitro and in vivo. These results underline the potential efficacy of ErbB-2/ErbB-3 dual targeting, providing a rationale for further investigation in order to obtain a novel therapeutic strategy in PC.
Results
NRG-1β is superior to EGF in activating ErbB-3/PI3K/Akt axis
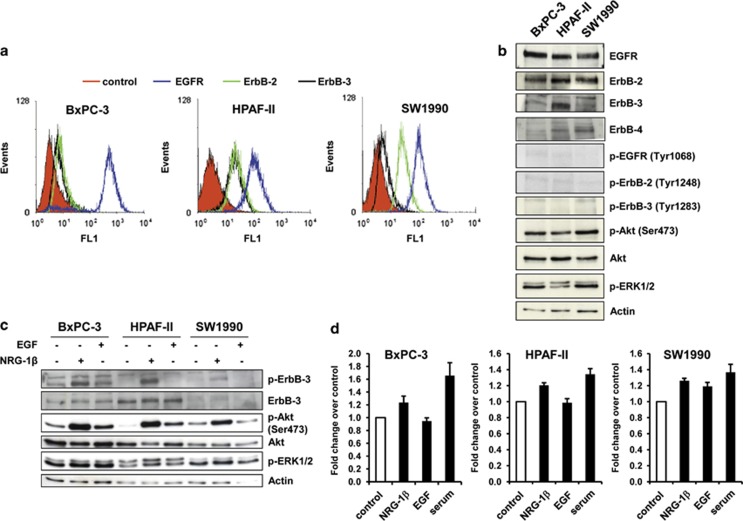
First, we evaluated baseline surface expression of EGFR, ErbB-2 and ErbB-3 in PC cell lines by fluorescence-activated cell sorting. All three cell lines showed overexpression of EGFR. ErbB-2 expression was low in BxPC-3 and moderate in HPAF-II and SW1990. As for ErbB-3, expression scored moderate in HPAF-II cells, and low in BxPC-3 and SW1990 (Figure 1a and Table 1). Consistent results were obtained by western blotting analysis (Figure 1b). ErbB-4 was expressed by HPAF II and SW 1990, whereas it was barely detectable in BxPC-3 cells. Of note, we observed constitutive phosphorylation of Akt in all three cell lines, whereas EGFR, ErbB-2 and ErbB-3 phosphorylation resulted to be almost undetectable (Figure 1b).
Figure 1.
NRG-1β more strongly than EGF activates the ErbB-3/Akt axis. (a) EGFR, ErbB-2 and ErbB-3 surface expression level was analyzed by fluorescence-activated cell sorting analysis. One representative of three independent experiments is shown. (b) Immunoblot analysis of total expression and basal activity of ErbB receptors and downstream signaling pathways. (c) After 24 h of serum starvation, cells were stimulated with either NRG-1β (10 ng/ml) or EGF (20 ng/ml) for 5 min, then cell lysates were blotted as indicated. (d) For cell proliferating assay, 24 h serum-starved cells were grown in the presence or not (control) of NRG-1β (10 ng/ml), EGF (20 ng/ml) or serum (medium containing 10% fetal bovine serum) and stained with MTT after 72 h. Results are expressed as fold change over control.
It has been extensively demonstrated that EGF and NRG-1β, ligands of EGFR and ErbB-3, respectively,26 induce the formation of the EGFR/ErbB-3 and ErbB-2/ErbB-3 heterodimers, which in turn activate the downstream signaling pathways.27 Therefore, we sought to compare the influence of these ligands on ErbB-3 activation and downstream signaling. NRG-1β more efficiently than EGF induced ErbB-3, Akt and ERK phosphorylation (Figure 1c). Of note, NRG-1β markedly activated Akt phosphorylation in BxPC-3 and SW1990 cells despite the low level of ErbB-3 in these cells. The higher stimulatory activity of NRG-1β was confirmed in cell proliferation assays (Figure 1d). These results support the concept that targeting NRG-1β-driven ErbB-3 activation could represent an effective therapeutic strategy in PC.
Dual inhibition of ErbB-2/ErbB-3 is required to completely inhibit Akt activity
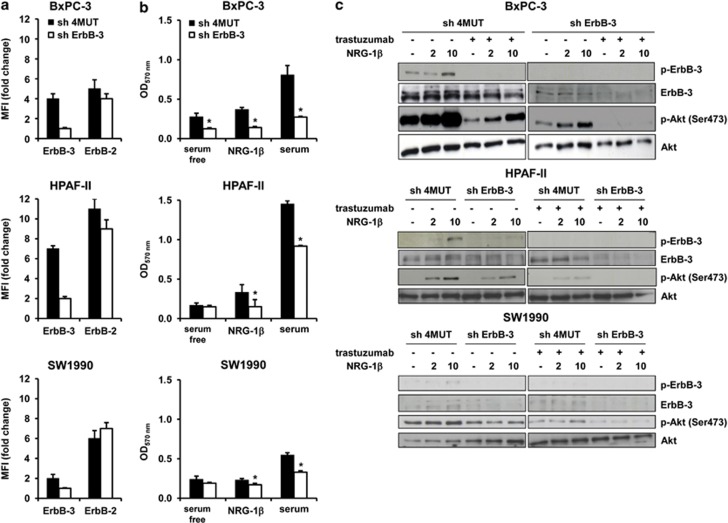
To further elucidate the therapeutic potential of targeting ErbB-3, we stably knocked down its expression in PC cells. Receptor downregulation was specific for ErbB-3, as no difference between shErbB-3 and control sh4Mut cells was observed for ErbB-2 expression (Figure 2a). In keeping with previous data,28 ErbB-3 silencing significantly reduced both NRG-1β- and serum-dependent cell proliferation (Figure 2b). Interestingly, either ErbB-3 knockdown or exposure to trastuzumab produced a marked, although incomplete, inhibition of NRG-1β-driven Akt activation. A complete shutdown was only obtained when ErbB-3-knocked down cells were treated with trastuzumab (Figure 2c). These data underlie the key role of NRG-1β/ErbB-3/Akt axis in PC cell growth and suggest that dual inhibition of ErbB-2/ErbB-3 is required to fully abolish NRG-1β-driven ErbB receptor signaling (Figure 2c).
Figure 2.
Dual inhibition of ErbB-2/ErbB-3 is required to fully inhibit Akt activity. (a) ErbB-3 and ErbB-2 surface expression level was monitored by fluorescence-activated cell sorting in ErbB-3-silenced (shErbB-3) and control (sh4Mut) cells. MFI (mean fluorescence intensity) is expressed as fold change over control (cells stained with secondary antibody alone). (b) Cell proliferation assay by MTT; cells were serum starved for 24 h and then stimulated with NRG-1β or serum for 72 h. The values represents mean±s.d. of quadruplicate measurements from one of two independent experiments. *P<0.05. (c) Cells were serum starved for 24 h, incubated with trastuzumab for 2 h as indicated and then stimulated with 10 ng/ml NRG-1β for 5 min. Lysates were then immunoblotted as indicated.
Trastuzumab and EV20 cooperate to disrupt the NRG-1β/ErbB-3/Akt axis
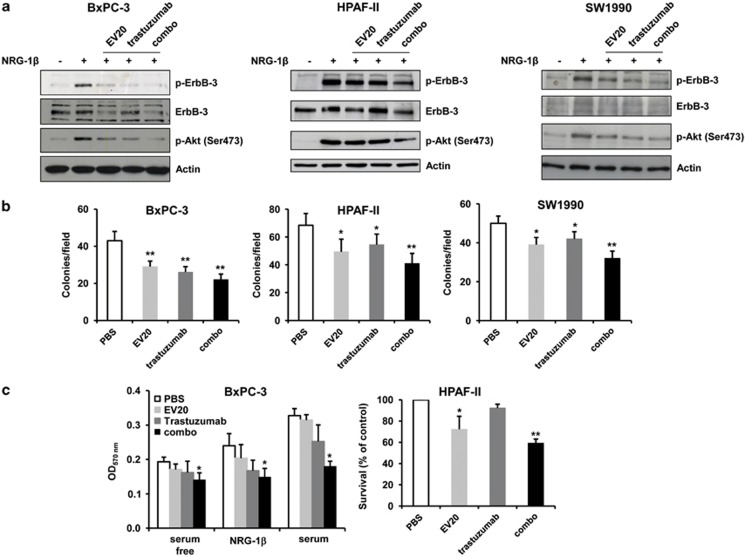
Next, we evaluated whether treatment with EV20 could replace ErbB-3 silencing in the dual inhibition strategy. To this end, PC cells were exposed to EV20, trastuzumab or their combination, before being analyzed for ErbB-3/Akt activation. Both EV20 and trastuzumab, used as a single agent, were able to reduce the level of ErbB-3 and Akt phosphorylation, with EV20 also promoting ErbB-3 receptor downregulation, although with variable efficacy depending on the cell line used (Figure 3a). Importantly, exposure of the cells to trastuzumab along with EV20 (combo) resulted in a greater inhibition than either agent alone (Figure 3a). We then analyzed whether this stronger inhibition associated with a higher antitumor activity. Both EV20 and trastuzumab significantly reduced the colony formation abilities of PC cells; however, treatment with the two agents resulted in a stronger inhibition as compared with either single agent (Figure 3b). Similar results were obtained in the cell proliferation assays (Figure 3c).
Figure 3.
Trastuzumab and EV20 cooperate to disrupt the NRG-1β/ErbB-3/Akt axis and inhibit cell proliferation. (a) Serum-starved (24 h) cells were treated with EV20, trastuzumab or their combination (10 μg/ml of each antibody) for 2 h and then stimulated with NRG-1β (10 ng/ml) for 5 min; cell lysates were blotted as indicated. (b) For anchorage-independent growth assay, cells were treated twice a week with EV20, trastuzumab or their combination for 3 weeks. Data are represented as number of colonies per field. The values are expressed as mean±s.d. of triplicate experiments. *P<0.05; **P<0.01. (c) BxPC-3 cells were grown under the condition indicated and treated with the antibodies or PBS (control) for 72 h before performing MTT staining. Results are expressed as mean of quadruplicate experiments. HPAF-II cells were grown in medium containing 0,5% fetal bovine serum and treated with the antibodies or PBS (control) for 96 h before performing MTT assay. Medium was renewed every 48 h. Results are expressed as mean of triplicate experiments. *P<0.05; **P<0.01.
Dual treatment with EV20 and trastuzumab restrains tumor growth in nude mice
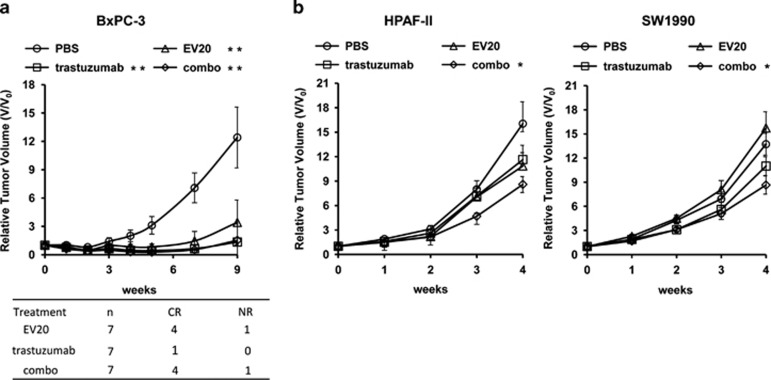
The above cell culture studies revealed that EV20 in combination with trastuzumab is able to fully abolish NRG-1β-driven Akt activation and to inhibit cell growth in vitro. We previously showed that EV20 markedly reduced the growth of several tumor xenografts, including those originating from pancreatic BxPC-3 cells.23 Thus, we wondered whether the dual inhibition of ErbB-2/ErbB-3 by EV20 and trastuzumab combination could result in a potentiation of therapeutic activity in vivo. To this end, animals bearing xenografts originating from BxPC-3, HPAF-II and SW1990 cells were treated either with EV20, trastuzumab or their combination. In the case of BxPC-3 xenografts, single treatment or combo led to a marked suppression of the tumor growth (P<0.001). However, the therapeutic benefit was significantly greater in both the EV20 and combo groups, as compared with the trastuzumab group, with four out of seven mice showing complete remission of the tumors and one out of seven, respectively (Figure 4a). Interestingly, in the case of the HPAF-II or SW1990 xenografts, although no significant restrain of tumor growth was achieved, treating animals with either agent alone, a significant fold change decrease in tumor growth was seen with combination treatment (Figure 4b). No weight loss of toxicity was detected for any treatment.
Figure 4.
Dual targeting of ErbB-2/ErbB-3 delays tumor growth of PC cells in nude mice. (a, b) Tumor growth was assessed as described in Materials and methods. Mice harboring tumors were treated with EV20, trastuzumab or their combination (10 mg/kg of each antibody) twice a week. *P<0.05; **P<0.001. In the table (a) the number of complete remissions (CR) and non-responders (NR) tumors in each treatment arm are indicated. CR indicates no longer palpable tumors. NR indicates tumors with growth curves similar to control.
Discussion
In the present study, we analyzed three PC cell lines with different K-Ras status and ErbB-2/ErbB-3 expression levels (Table 1); we proved that NRG-1β, the ligand promoting ErbB-2/ErbB-3 dimerization, activates the downstream PI3K/Akt pathway more efficiently then EGF, despite EGFR overexpression in all cell lines. This finding is particularly remarkable as the overexpression of NRG-1β has been correlated with worse clinical outcomes in PC. Indeed, cancer-associated fibroblasts have been documented to secrete NRG-1β, which in turn promotes ErbB-3/Akt activation and mediates the growth and survival of pancreatic tumors.15 Indeed, reactivation of PI3K/Akt is a crucial step in the occurrence of therapy resistance.29,30 Here, we show that silencing of ErbB-3 by RNAi resulted in a decrease of cell proliferation and a marked, although incomplete, impairment of NRG-1β-dependent Akt activation. Interestingly, exposure to the anti-ErbB-2 antibody trastuzumab was able to abrogate the residual Akt phosphorylation in these cells. These findings suggest that the dual inhibition of ErbB-2/ErbB-3 receptors might represent an effective strategy to fully disrupt NRG-1β/Akt axis in PC cells. Therefore, we tried to investigate the potential therapeutic application of this approach, studying ErbB-2/ErbB-3 dual inhibition by means of the two monoclonal antibodies trastuzumab and EV20.
In our previous work, we have demonstrated that EV20 exerts a marked antitumor activity in several models, including the K-Ras wild-type PC BxPC-3 cells. Indeed, exposure of these cells to EV20 lead to receptor downregulation and inhibition of ligand-dependent and -independent ErbB-3/Akt phosphorylation.23 Here, we show that EV20 in combination with trastuzumab produces a complete suppression of the NRG-1β-driven Akt induction and a marked inhibition of cell growth in vitro, (Figure 3).
The potentiation of the antitumor activity of the two monoclonal antibodies when given in combination has also been achieved in vivo (Figure 4). The growth of xenografts originating from BxPC-3 cells was similarly restrained when EV20 and trastuzumab were given alone or in combination. Instead, the growth of HPAF-II and SW1990 xenografts was only reduced in animals treated with the combination of the two monoclonal antibodies. The results obtained with BxPC-3 xenograft are in accordance with previous reports, where single treatment with EV2023 or trastuzumab5 caused a significant restrain of tumor growth. Here, we show for the first time that dual targeting of ErbB-2 and ErbB-3 significantly delays tumor growth of xenografts originating from K-Ras-mutated HPAF-II and SW1990 cells. This finding is particularly relevant considering that these cells are poorly sensitive to erlotinib,28 thus suggesting that targeting ErbB-2/ErbB-3 is a preferable therapeutic possibility over targeting EGFR.
In sum, dual targeting of these receptors with the combination of trastuzumab and EV20 in cell culture and mouse xenograft models resulted in enhanced antitumor activity. These data provide a rationale for further investigation of this combination as a potential strategy for treatment of PC.
Materials and methods
Reagents
Antibodies were as follows: phosphorylated EGFR (Tyr1068), phosphorylated ErbB-2 (Tyr1248), phosphorylated ErbB-3 (Tyr1289), phosphorylated Akt (Ser473), phosphorylated ERK1/2, Akt and ERK1/2 from Cell Signaling Technology (Danvers, MA, USA); C-17 ErbB-3 and C-18 ErbB-4 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); ErbB-2 antibody for western blot was kindly provided by Dr O Segatto; anti-Actin was from Sigma-Aldrich Corporation (St. Louis, MO, USA). Herceptin (trastuzumab) was from Roche Pharma AG (Grenzach-Wyhlen, Germany), Erbitux (cetuximab) was from Merck KGaA (Darmstadt, Germany). NRG-1β was purchased from R&D (R&D Systems, Inc., Minneapolis, MN, USA) or Cell Signaling Technology. 3-(4,5-Dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich Corporation.
Cell lines
BxPC-3, HPAF-II and SW1990 PC cells were purchased from American Type Culture Collection (Rockville, MD, USA). The cells were grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich Corporation) and incubated at 37 °C in humidified air with 5% CO2. For stable ErbB-3 silencing, cells were infected with pSuper retro–based vectors as described.22 Control vector pSuper 4Mut contains a four-point mutated sequence unable to target the human ErbB-3 mRNA.
Cell proliferation assay
Cells were seeded at a density of 5 × 103 cells/well in 96-well plates or 2 × 104 cells/well in 24-well plates in 10% fetal bovine serum-containing medium and allowed to attach and propagate overnight before the treatments. The cells were washed once with prewarmed phosphate-buffered saline (PBS), and were treated in the serum-free condition in the presence or absence of the specified experimental conditions as indicated. After treatments, the cells were incubated with MTT (final concentration of 0.5 mg/ml) at 37 °C for 2–3 h. Spectrophotometric absorbance of the samples was determined at 570 nm. Epidermal growth factor (EGF; 20 ng/ml) or NRG-1β (10 ng/ml) was added to the media whenever necessary as indicated. All experiments were performed in quadruplicate. P-values were determined by Student's t-test and considered significant for P<0.05.
Soft agar colony formation assay
For the anchorage-independent growth assay, 2 × 104 cells/well were suspended in RPMI 1640 containing 0.3% agarose and 10% fetal bovine serum and layered onto a 1-ml bed of 0.6% agarose in a 12-well plate dish in triplicate. Dishes were incubated in a humidified atmosphere containing 5% CO2 at 37 °C. A measure of 10 μg/ml of EV20 and/or 2 μg/ml of trastuzumab was added to the cells two times a week and the number of colonies was determined after 3 weeks. P-values were determined by Student's t-test and considered significant for P<0.05.
Immunochemistry
Lysates from cells in culture were prepared by washing cells twice in cold PBS followed by lysis with either HNTG buffer (50 mM HEPES pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 5 mM EGTA) or RIPA lysis buffer supplemented with protease and phosphatase inhibitors (Sigma-Aldrich Corporation). Immunoblotting was performed as described.22
Fluorescence-activated cell sorting analysis
EGFR, ErbB-2 and ErbB-3 cell surface expression was analyzed by flow cytometry using a FACSCalibur cytometer (Becton Dickinson, Buccinasco, MI, Italy). Briefly, around one million of proliferating cells were harvested and labeled with cetuximab for EGFR, trastuzumab for ErbB-2 or EV20 for ErbB-3 and left on ice for 20 min. Cells were then washed with 2 ml PBS, pulled down and stained with an Alexa Fluor 488 Goat anti-Human antibody (Molecular Probes, Life Technologies, Paisley, UK). The data were analyzed using CELLQuest 3.2.1.f1 software (Becton Dickinson).
In vivo tumor growth
Athymic CD-1nu/nu mice (5–7 weeks old) were purchased from Charles River Laboratories (Calco, LC, Italy) and maintained under specific pathogen-free conditions with food and water provided ad libitum and the animals' health status was monitored daily. Procedures involving animal and their care were established according to the institutional guidelines in compliance with national and international policies.
BxPC-3 (5 × 106), HPAF-II (3.5 × 106) and SW1990 (5 × 106) cells were injected subcutaneously into the right flank of mice. When xenografts became palpable, tumor-bearing mice were divided into four groups (n=7 for BxPC-3, n=10 for HPAF II and SW1990 xenografts) in a manner to provide a similar range of tumor size in each group. The test groups received twice a week intraperitoneal injections of EV20 (10 mg/kg), trastuzumab (10 mg/kg) or EV20/trastuzumab combination (10 mg/kg of each mAb), whereas the control groups received PBS only. Tumor volumes were monitored every week by a caliper and volumes were calculated using the following formula: tumor volume=(length × width2)/2. Results are expressed as Relative Tumor Volume, that is, (volume/initial volume). P-values were determined by Student's t-test and considered significant for P<0.05.
Abbreviations
EGF, epidermal growth factor; FACS, fluorescence activated cell sorting; PI3K, phosphoinositide 3-kinase.
Acknowledgments
We thank A Di Risio and R La Sorda for EV20 antibody purification and excellent technical assistance, Dr S Traini for helping in soft agar colony assays, Dr A Sala, Professor V De Laurenzi and Dr L Sibilio for helpful discussion.
The authors declare no conflict of interest.
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- Hidalgo M. Pancreatic cancer. New Engl J Med 2010; 362: 1605–1617. [DOI] [PubMed] [Google Scholar]
- Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol 2009; 6: 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess H, Yamanaka Y, Kobrin MS, Do DA, Buchler MW, Korc M. Enhanced erbB-3 expression in human pancreatic cancer correlates with tumor progression. Clin Cancer Res 1995; 1: 1413–1420. [PubMed] [Google Scholar]
- Larbouret C, Robert B, Navarro-Teulon I, Thezenas S, Ladjemi MZ, Morisseau S et al. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res 2007; 13: 3356–3362. [DOI] [PubMed] [Google Scholar]
- Saxby AJ, Nielsen A, Scarlett CJ, Clarkson A, Morey A, Gill A et al. Assessment of HER-2 status in pancreatic adenocarcinoma: correlation of immunohistochemistry, quantitative real-time RT-PCR, and FISH with aneuploidy and survival. Am J Surg Pathol 2005; 29: 1125–1134. [DOI] [PubMed] [Google Scholar]
- Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y et al. Epidermal growth factor receptor expression in human pancreatic cancer: Significance for liver metastasis. Int J Mol Med 2003; 11: 305–309. [PubMed] [Google Scholar]
- Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest 2004; 22: 706–712. [DOI] [PubMed] [Google Scholar]
- Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010; 28: 3605–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–1966. [DOI] [PubMed] [Google Scholar]
- Kruser TJ, Wheeler DL. Mechanisms of resistance to HER family targeting antibodies. Exp Cell Res 2010; 316: 1083–1100. [DOI] [PubMed] [Google Scholar]
- Smith BL, Chin D, Maltzman W, Crosby K, Hortobagyi GN, Bacus SS. The efficacy of Herceptin therapies is influenced by the expression of other erbB receptors, their ligands and the activation of downstream signalling proteins. Br J Cancer 2004; 91: 1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012; 487: 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012; 12: 553–563. [DOI] [PubMed] [Google Scholar]
- Liles JS, Arnoletti JP, Kossenkov AV, Mikhaylina A, Frost AR, Kulesza P et al. Targeting ErbB3-mediated stromal-epithelial interactions in pancreatic ductal adenocarcinoma. Br J Cancer 2011; 105: 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov A, Schuller K, Tzeng CW, Cannon EE, Ku BC, Howard JH et al. ErbB3 expression and dimerization with EGFR influence pancreatic cancer cell sensitivity to erlotinib. Cancer Biol Ther 2007; 6: 548–554. [DOI] [PubMed] [Google Scholar]
- Liles JS, Arnoletti JP, Tzeng CW, Howard JH, Kossenkov AV, Kulesza P et al. ErbB3 expression promotes tumorigenesis in pancreatic adenocarcinoma. Cancer Biol Ther 2010; 10: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A, Kleeff J, Arnold N, Giese NA, Giese T, Korc M et al. Expression and differential signaling of heregulins in pancreatic cancer cells. Int J Cancer 2007; 120: 514–523. [DOI] [PubMed] [Google Scholar]
- Shi F, Telesco SE, Liu Y, Radhakrishnan R, Lemmon MA. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA 2010; 107: 7692–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995; 10: 1813–1821. [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res 2003; 284: 54–65. [DOI] [PubMed] [Google Scholar]
- Sala G, Traini S, D'Egidio M, Vianale G, Rossi C, Piccolo E et al. An ErbB-3 antibody, MP-RM-1, inhibits tumor growth by blocking ligand-dependent and independent activation of ErbB-3/Akt signaling. Oncogene 2012; 31: 1275–1286. [DOI] [PubMed] [Google Scholar]
- Sala G, Rapposelli IG, Ghasemi R, Piccolo E, Traini S, Capone E et al. EV20, a novel Anti-ErbB-3 humanized antibody, promotes ErbB-3 down-regulation and inhibits tumor growth in vivo. Transl Oncol 2013; 6: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M et al. Adjuvant trastuzumab in HER2-positive breast cancer. New Engl J Med 2011; 365: 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- Carraway KL, Soltoff SP, Diamonti AJ, Cantley LC. Heregulin Stimulates Mitogenesis and Phosphatidylinositol 3-Kinase in Mouse Fibroblasts Transfected with erbB2/neu and erbB3. J Biol Chem 1995; 270: 7111–7116. [DOI] [PubMed] [Google Scholar]
- Lemmon MA. Ligand-induced ErbB receptor dimerization. Exp Cell Res 2009; 315: 638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck E, Eyzaguirre A, Haley JD, Gibson NW, Cagnoni P, Iwata KK. Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther 2006; 5: 2051–2059. [DOI] [PubMed] [Google Scholar]
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer 2009; 9: 463–475. [DOI] [PubMed] [Google Scholar]
- Lee Y, Ma J, Lyu H, Huang J, Kim A, Liu B. Role of erbB3 receptors in cancer therapeutic resistance. Acta Biochim Biophys Sin (Shanghai) 2014; 46: 190–198. [DOI] [PubMed] [Google Scholar]