Abstract
Recent genomic studies have revealed that chromosomal structures are formed by a hierarchy of organizing processes ranging from gene associations, including interactions among enhancers and promoters, to topologically associating domain formations. Gene associations identified by these studies can be characterized by microscopic analyses. Fission yeast is a model organism, in which gene associations have been broadly mapped across the genome, although many of those associations have not been further examined by cell biological approaches. To address the technically challenging process of the visualization of associating gene loci in the fission yeast nuclei, we provide, in detail, an IF–FISH procedure that allows for covisualizing both gene loci and nuclear structural markers such as the nuclear membrane and nucleolus.
1. INTRODUCTION
Next-generation DNA sequencing combined with the molecular biology procedure called chromosome conformation capture (3C), referred to as Hi-C, allows one to investigate a series of genome-organizing events and to map gene associations throughout the genomes of various organisms (Lieberman-Aiden et al., 2009; Tanizawa & Noma, 2012). Hi-C-related approaches have been successfully applied to fission yeast cells to map the yeast global gene association network (Grand et al., 2014; Mizuguchi et al., 2014; Tanizawa et al., 2010). An alternative approach, chromatin interaction analysis by paired-end tag sequencing (ChIA-PET), has also been employed to identify genome-wide associations mediated by particular proteins (Fullwood et al., 2009).
It is becoming clear that the organization of the genome is tightly connected to nuclear activities such as transcriptional regulation, repair, and DNA replication (Misteli, 2007). The nucleus consists of various well-defined nuclear domains including nucleoli, Cajal bodies, and PML bodies, which are also linked to nuclear activities (Spector, 2001). How gene associations and global genome architecture, as determined by genomic approaches, are coupled to nuclear activities and various subnuclear domains remain largely unknown. The application of cell biological approaches to the study of these processes can help fill this significant gap. In this chapter, we describe a practical protocol combining IF (immunofluorescence) and FISH (fluorescence in situ hybridization), which allows investigators to covisualize gene loci and nuclear structural components such as the nuclear membrane and nucleoli. The application of this method will contribute to the understanding of how and where gene associations occur in the fission yeast nucleus.
2. CASE STUDIES IN THE APPLICATION OF THE IF–FISH APPROACH
2.1 Scoring Associations Between Two Gene Loci
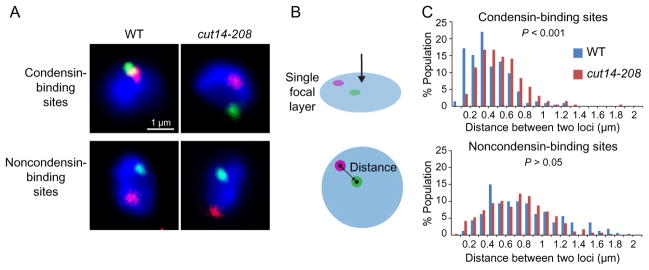
An IF–FISH approach can be used to detect and confirm gene associations predicted by a Hi-C analysis. The IF–FISH images shown in this chapter were captured by a Zeiss Axioimager Z1 fluorescence microscope with an oil immersion objective lens (Plan Apochromat, 100×, NA 1.4, Zeiss). Multicolor FISH can be used to covisualize two different gene loci in a nucleus (Fig. 1A). IF can be used to visualize α-tubulin in the same cell using 1:10-diluted antitubulin TAT1 monoclonal antibody (Woods et al., 1989). Based on tubulin staining patterns, cell cycle phases can be estimated (Funabiki, Hagan, Uzawa, & Yanagida, 1993). Since gene associations are highly transient, these associations are scored as frequencies of nearby localization of two gene loci (Iwasaki et al., 2015; Kim et al., 2013). Centers of FISH foci are defined as positions of the loci, and the distance between two foci present on the same focal layer must be measured in order to minimize errors derived from low resolution along the z-axis (Fig. 1B). The distance between two loci should be measured in more than 100 cells for reliability. It is also important to have a negative control, where two loci separated by the same genomic distance are predicted not to associate with each other (Fig. 1A). In order to examine whether a specific mutation affects a particular gene association in a statistically significant manner, distributions of distances between two loci in wild-type and mutant cells can be subjected to the Mann–Whitney U test (Fig. 1C).
Fig. 1.
Analysis on FISH foci reflecting positions of two gene loci. (A) The two paired gene loci were visualized in wild-type and cut14-208 condensin mutant cells. Because the cut14-208 mutation is temperature sensitive, wild-type and mutant cells were cultured at the restrictive temperature (36°C) for 1 h. The FISH foci (green and red) representing the two loci bound by condensin were positioned nearby in wild-type cells but not in the mutant (top), whereas the two control loci (noncondensin-binding sites) were consistently separated (bottom). DAPI signals are shown in blue. (B) Two FISH foci are present on a single focal layer (top), and the distance between centers of the two FISH foci is defined as a physical distance between the two loci (bottom). (C) Distributions of distances between the two gene loci in wild-type and cut14-208 mutant cells.
2.2 Visualizing Clusters of Centromeres and Telomeres
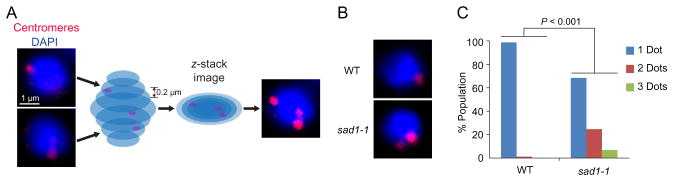
Fission yeast centromeres and telomeres are known to form respective clusters at the nuclear periphery, and this clustering is linked to chromosome dynamics during mitosis (Funabiki et al., 1993). The plasmid pRS140 is used for preparing FISH probes specific to centromeres (Chikashige et al., 1989). FISH foci represent centromeres of all the three chromosomes (Fig. 2A). The cosmid cos212 is used to prepare a telomere-specific FISH probe, which visualizes the left and right subtelomeric regions of the chromosomes 1 and 2 (Sadaie, Naito, & Ishikawa, 2003). To visualize every FISH focus reflecting centromeres or telomeres, images are acquired at 0.2 μm intervals in the z-axis, and z-stack images should be used to count the total number of FISH foci in a nucleus as demonstrated (Fig. 2A).
Fig. 2.
Analysis on centromeric clusters. (A) FISH visualization of centromeric foci (red). Centromeric clusters are captured by a z-stack projection. DAPI signals are shown in blue. (B) Centromeric clusters in the wild-type and sad1-1 mutant. Since the sad1-1 is a temperature-dependent mutation, wild-type and mutant cells were cultured at 36° C for 1 h, and subjected to FISH analysis. (C) Numbers of centromeric clusters in wild-type and mutant cells.
To demonstrate the disruption of the centromeric clustering we used the sad1 gene mutant. Sad1 is a nuclear membrane protein bearing the Sad1- UNC-84 (SUN) domain, and the temperature-sensitive sad1-1 mutation is known to disrupt the clustering of centromeres in fission yeast (Hagan & Yanagida, 1995; Hou et al., 2012). As an example, we visualized centromeres in wild-type and sad1-1 mutant cells (Fig. 2B). The difference in numbers of centromeric FISH foci in wild-type and mutant cell populations (n>100) was evaluated by the chi-square test (Fig. 2C).
2.3 Coordinating Gene Positioning to the Nuclear Architecture
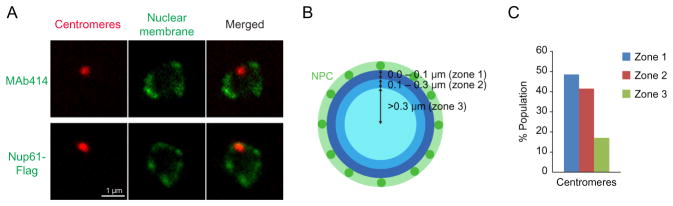
In yeast, gene loci tend to be positioned within subnuclear domains, and gene territories are often coordinated with two nuclear structural markers, such as the nuclear membrane and nucleolus (Berger et al., 2008; Kim et al., 2013). A particular gene locus and nuclear structural components can be covisualized by an IF–FISH approach (Fig. 3A). To visualize the nuclear membrane, a nuclear pore complex (NPC) component (Nup61) tagged with Flag can be visualized using 1:500-diluted mouse monoclonal anti-Flag antibody (F1804, Sigma-Aldrich). Alternatively, 1:100-diluted mouse monoclonal anti-NPC proteins (MAb414, Covance) can be used. The nucleolus can also be visualized using 1:20-diluted mouse monoclonal anti- Nop1 (Encor Biotechnology). Nop1 is known to function in the processing of ribosomal RNA and localizes in the nucleolus (Henriquez, Blobel, & Aris, 1990).
Fig. 3.
Gene positioning relative to the nuclear architecture. (A) Anti-NPC protein antibody (MAb414) was used to visualize the nuclear membrane (top). Alternatively, a NPC subunit (Nup61) fused to a Flag epitope was expressed from the endogenous locus with its own promoter, and Nup61-Flag proteins were visualized by an IF approach (bottom). In the same cells, centromeres were covisualized by FISH (red). (B) NPC signals are used to trace the nuclear membrane, and the nucleus is divided into the three zones based on distance from the membrane. (C) The distance between the centromeric signal and the nuclear membrane was measured in more than 100 cells, and assigned to one of the nuclear zones.
The nucleus is divided into three zones according to distance from the nuclear periphery visualized by IF (Fig. 3B). The distance between the nuclear membrane and a gene locus should be measured in more than 100 cells and binned into one of the assigned zones. As an example, the distance between the centromeric FISH foci and the nuclear periphery was measured and summarized in a graph (Fig. 3C). IF–FISH images carrying both a centromeric focus and clear NPC signals should be employed for this analysis.
3. SUPPLIES
3.1 Equipment
Water bath (37°C and 65°C)
Heat block (75°C and 95°C)
Air incubator (37°C and 40°C)
18°C Water bath shaker in a cold room
37°C Water bath shaker
Rotator
Thermomixer (Eppendorf )
Vacufuge (Eppendorf )
Centrifuges, micropipettes, and other items associated with standard molecular biology experiments.
3.2 Materials
Restriction enzymes (AluI, RsaI, HaeIII, BfuCI, and DdeI; New England BioLabs)
Phenol chloroform isoamyl alcohol (P3803, Sigma-Aldrich)
Amicon Ultra-30K and 10K centrifugal filters (UFC503024 and UFC501024, Millipore)
Random primer DNA labeling kit (TAK6045, Takara)
Cy3-dCTP or Cy5-dCTP (PA53021 and PA55021, GE Healthcare)
30% Paraformaldehyde in YEA medium
-
2.4 M Sorbitol in YEA medium (Alfa, Fantes, Hyams, McLeod, & Warbrick, 1993)
YEA: 5 g yeast extract, 30 g glucose, and 75 mg adenine for 1 L (pH 5.5)
2.5 M Glycine (filtered)
1 mg/mL Zymolyase 100 T (07665-55, Seikagaku) in PEMS (stored at −80°C)
Primary antibodies described earlier
-
Secondary antibodies that successfully worked for our experiments
1:500-Diluted Alexa Flour 488-conjugated anti-mouse IgG (Life Technologies)
1:400-Diluted Alexa Flour 594-conjugated anti-mouse IgG (Life Technologies)
1:400-Diluted Alexa Flour 488-conjugated anti-rabbit IgG (Life Technologies)
1:4000-Diluted Cy3-conjugated anti-rabbit IgG (Jackson ImmunoResearch)
30 μg/μL RNase A solution (R4642, Sigma-Aldrich)
1 μg/mL DAPI in PBS buffer (1 mg/mL DAPI-PBS stock solution at −20°C)
-
Antifade solution (P6001, Sigma-Aldrich)
Prepare 10 mg/mL p-phenylenediamine in 1 MTris–HCl (pH 8.0) and store at −20°C. Mix 5 μL solution with 45 μL glycerol before use
Poly-L-lysine (P8920, Sigma-Aldrich)
Glass slides and coverslips
1×PBS and 1×PBS+0.1% NaN3
Nail polish.
3.3 Buffers
-
PEM
Component Final Conc. (mM) Stock (mM) Amount PIPES (pH 6.9) 100 30.233 g EGTA 1 500 2 mL MgSO4 1 500 2 mL Adjust to pH 6.9 with 10 N NaOH. Add H2O up to 1 L. -
PEMS
1 M Sorbitol in PEM buffer.
-
PEMBAL
Component Final Conc. Stock Amount BSA 1% 1 g L-Lysine 0.1 M 1.46 g NaN3 0.1% 10% 1 mL Add PEM buffer up to 100 mL. -
Hybridization buffer
Component Final Conc. Stock Amount (μL) Formamide 500 SSC 2× 20× 100 Denhardt’s solution 5× 50× 100 Dextran sulfate 10% 50% 200 DW 100
4. PROTOCOL
4.1 Preparation of the FISH Probe Templates
Digest 1 μg of DNA fragments (see Note 1) with 4-base cutters (AluI, RsaI, HaeIII, BfuCI, and DdeI) (see Note 2) in 100 μL total volume at 37°C for 3 h.
Add 130 μL H2O and extract DNA by phenol/chloroform.
Transfer aqueous phase (200 μL) to Amicon Ultra-30K centrifugal filter.
Spin at 13,000 rpm for 5 min and discard the flow-through (avoid completely drying the filter).
Add 200 μL H2O to the filter column.
Spin at 13,000 rpm for 5 min and discard the flow-through.
Repeat four more times (steps 5 and 6).
Invert the filter column and spin at 3000 rpm for 3 min.
Reduce the sample volume to 20 μL using Vacufuge for approximately 12 min.
Store the FISH probe template at 4°C.
4.2 Fluorescent Labeling of the Templates
Prepare 16.5 μL of the FISH probe template in 1.5-mL microcentrifuge tube.
Add 2 μL of random primer mix and mix well.
Denature DNA at 95°C for 3 min.
Immediately transfer to ice and leave for 5 min to prevent renaturation of single-stranded DNA. Flash spin at 13,000 rpm.
Add random labeling mix (total volume 6.5 μL) and mix by pipetting. 2.5 μL 10× buffer, 2.5 μL dNTP mix, 0.5 μL Cy3- or Cy5-dCTP, 1.0 μL Klenow enzyme.
Incubate at 37°C for 1 h with shaking at 500 rpm using Thermomixer. Protect sample from light using aluminum foil from here on.
Inactivate Klenow enzyme at 65°C for 10 min and incubate on ice for 3 min.
Add 150 μL TE buffer and apply to Amicon Ultra-10K centrifugal filter.
Spin at 13,000 rpm at 4°C for 10 min and discard the flow-through.
Apply 200 μL TE buffer to the filter column.
Spin at 13,000 rpm at 4°C for 10 min and discard the flow-through.
Repeat three more times (steps 10 and 11).
Invert the column and spin at 3000 rpm for 3 min to recover the FISH probe.
Adjust to 100 μL with TE buffer.
Store the FISH probe at 4°C in dark (stable for a few years).
4.3 Fixation of the Fission Yeast Cells
Prepare logarithmically growing cells in 20 mL YEA medium at 30°C (OD595 =0.5).
Prepare 30% paraformaldehyde in YEA medium (see Note 3).
Add 1 volume (20 mL) of 2.4 Msorbitol in YEA medium to the culture.
Agitate the culture at 18°C for 5 min using water bath shaker (cold room).
Add 6 mL of 30% paraformaldehyde and shake at 18°C for 10 min.
Add 2.4 mL of 2.5 M glycine and shake at 18°C for 5 min.
Transfer to 50-mL centrifuge tube.
Spin at 3000 rpm at 4°C for 5 min and remove the supernatant.
4.4 Permeabilization of the Cells
Resuspend the cells in 1 mL PEM buffer and transfer the cells to a 1.5-mL tube.
Spin down the cells at 7000 rpm for 1 min and remove the supernatant (see Note 4).
Resuspend the cells in 1 mL PEMS buffer and let stand at room temperature for 5 min.
Spin down the cells at 7000 rpm for 1 min and remove the supernatant.
Resuspend the cells in 1 mL of 1 mg/mL Zymolyase 100 T in PEMS. Cells become fragile after this step. Therefore, cut 2–3 mm from the end of pipette tips or mix by tapping.
Incubate the cells at 37°C (water bath) for 30 min with occasional tapping. Check the cell wall digestion with 2 μL sample mixed with 2 μL of 10% SDS (see Note 5).
Spin at 7000 rpm for 1 min and remove the supernatant.
Resuspend the cells in 1 mL PEMS buffer.
Spin down the cells at 7000 rpm for 1 min and remove the supernatant.
Resuspend the cells in 500 μL PEMS containing 1% Triton X-100 and leave at room temperature for 30 s.
Spin at 7000 rpm for 1 min and remove the supernatant.
Resuspend the cells with 1 mL PEMS.
Spin at 7000 rpm for 1 min and remove the supernatant.
Repeat steps 12 and 13 (namely, wash the cells with 1 mL PEMS twice).
Wash the cells with 1 mL PEM twice.
4.5 Antigen–Antibody Reactions (IF) and Fixation
Resuspend the cells in 500 μL PEMBAL and rotate at room temperature for at least 1 h (see Note 6).
Spin at 7000 rpm for 1 min and remove the supernatant.
Resuspend the cells in 200 μL PEMBAL containing primary antibodies as detailed earlier.
Rotate the tube at room temperature for 14–16 h.
Spin at 7000 rpm for 1 min and remove the supernatant.
Wash the cells with 1 mL PEMBAL three times.
Add 200 μL PEMBAL containing secondary antibodies as detailed earlier.
Wrap the tube in foil and rotate at room temperature for 4 h (see Note 7).
Spin at 7000 rpm for 1 min and remove the supernatant.
Resuspend the cells in 500 μL PEMBAL and rotate at room temperature for 15 min.
Spin at 7000 rpm for 1 min and remove the supernatant.
Repeat steps 10 and 11 for a total of three times.
Wash the cells with 500 μL PEM twice.
Resuspend the cells in 900 μL PEM and add 100 μL of 30% paraformaldehyde in PEM (see Note 8).
Rotate the tube for 20 min at room temperature in the dark.
Add 40 μL 2.5 M glycine.
Wash with 1 mL PEM twice.
Resuspend the cells in 500 μL of 0.1 N HCl and rotate at room temperature for 5 min (see Note 9).
Wash the cells with 1 mL PEM twice.
Resuspend the cells with 500 μL PEMBAL and add 1.6 μL of RNase A solution (30 μg/μL).
Cover the sample with aluminum foil and rotate at 37°C (air incubator) for 1.5 h (see Note 10).
4.6 Hybridization (FISH)
Prepare the hybridization buffer as described earlier.
Mix 10 μL (100–150 ng) FISH probe with 100 μL hybridization buffer.
Incubate at 75°C (heat block) for 15 min.
Spin the RNase-treated cells at 7000 rpm for 1 min and remove the supernatant.
Wash the cells with 500 μL PEM twice.
Resuspend the cells in 110 μL hybridization buffer containing the FISH probe.
Incubate the cells at 75°C (heat block) for 5 min (see Note 11).
Wrap the tube in aluminum foil and rotate at 40°C (air incubator) for 12–14 h.
Add 100 μL 2× SSC and mix well by pipetting.
Spin at 7000 rpm for 2 min and decant. Spin again at 7000 rpm for 2 min and remove the remaining supernatant (see Note 12).
Resuspend the cells with 100 μL 2×SSC and rotate at room temperature for 30 min in the dark.
Spin at 7000 rpm for 30 s and decant. Spin again at 7000 rpm for 30 s and remove the remaining supernatant.
Repeat steps 11 and 12 twice (three times total).
4.7 Preparation of the Cells for Microscopy
Resuspend the cells with 200 μL of 1 μg/mL DAPI in PBS buffer.
Incubate at room temperature for 5 min with rotation.
Wash the cells with 100 μL PBS twice.
Resuspend the cells in 10–100 μL of PBS containing 0.1% NaN3 solution.
Spread 2 μL of the cells onto a poly-L-lysine-coated coverslip (see Note 13).
Leave for 5 min in the dark.
Apply 5 μL of antifade solution to the glass slide.
Place the coverslip on the glass slide as the cells touch the antifade solution.
Leave for 5 min in the dark.
Seal the coverslip with nail polish.
Leave for 5–10 min and investigate the cells under a fluorescent microscope.
Acknowledgments
We would like to thank the Wistar Imaging Facility for microscopic analysis. We also thank Louise Showe for critically reading the manuscript and Sylvie Shaffer for editorial assistance. Research reported in this publication was supported by the G. Harold and Leila Y. Mathers Charitable Foundation and the NIH Director’s New Innovator Award Program of the National Institutes of Health under award number (DP2-OD004348 to K.N.). Support for Shared Resources utilized in this study was provided by Cancer Center Support Grant (CCSG) P30CA010815 to The Wistar Institute.
Footnotes
To generate FISH probes, cosmids, plasmids, and PCR products (~15 kb) can be used. Fission yeast cosmid information is found at the Pombase website (http://www.pombase.org/tools/clone-and-mapping-resources), and clones can be obtained from the Sanger Institute. Three 5 kb DNA fragments adjoined but not overlapping each other are amplified by PCR and can be used for generating locus-specific FISH probes.
DNA fragments should be 50–200 bp. For generating a centromeric probe using pRS140, the combination of AluI, RsaI, HaeIII, BfuCI, DdeI, and Sau3AI are optimal for the digestion.
Prepare a fresh solution for each experiment. Add 3 g of paraformaldehyde and 40 μL of 10 N NaOH to about 7 mL of YEA medium and mix well. Incubate at 65°C and occasionally vortex until the solution becomes clear. It takes approximately 20 min. Cool to room temperature and filter with 0.22 μm Steriflip (SE1M179M6, Millipore). Be cautious, as paraformaldehyde powder and its fume are toxic.
Spin at 7000 rpm for 30 s and decant the supernatant. To remove the buffer completely, spin again at 7000 rpm for 30 s and remove the residual supernatant by pipetting.
Keep the sample on ice and check digestion efficiency under the microscope. If cell walls are not digested completely, incubate again at 37°C for 5 min and check the cells. Repeat this step until digestion is complete.
Blocking time is dependent upon antibody specificity. If a background signal is high, increase the blocking time to 2–4 h.
Protect samples against light from this step on because the secondary antibodies are fluorescence conjugated. Wrap tubes in foil.
30% Paraformaldehyde in PEM is prepared by the same procedure as described in Note 3, except for using PEM instead of YEA.
Acid treatment is recommended, because this step helps deproteinize and facilitates penetration of FISH probes (Syrjanen, 1992).
Excess RNase A or prolonged incubation may affect the quality of FISH images, potentially resulting from a DNase activity contaminated in the RNase A solution. Prepare the hybridization buffer and preheat at 75°C during the RNase A treatment.
Resuspend the cells with the hybridization buffer by pipetting. Cut the end of tips to handle the sticky hybridization buffer. By handling multiple samples at 30 s intervals, you can make sure that every sample is treated at 75°C for exactly 5 min.
Mix well with 100 μL 2 × SSC (step 7) and do not remove the supernatant completely until the second spin.
Apply 300 μL of poly-L-lysine to the coverslip and wait for 20 min. Remove poly-L-lysine by vacuum aspiration and protect from dust.
References
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with fission yeast. New York: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Berger AB, Cabal GG, Fabre E, Duong T, Buc H, Nehrbass U, et al. High-resolution statistical mapping reveals gene territories in live yeast. Nature Methods. 2008;5(12):1031–1037. doi: 10.1038/nmeth.1266. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Kinoshita N, Nakaseko Y, Matsumoto T, Murakami S, Niwa O, et al. Composite motifs and repeat symmetry in S. pombe centromeres: Direct analysis by integration of NotI restriction sites. Cell. 1989;57(5):739–751. doi: 10.1016/0092-8674(89)90789-7. [DOI] [PubMed] [Google Scholar]
- Fullwood MJ, Liu MH, Pan YF, Liu J, Xu H, Mohamed YB, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature. 2009;462(7269):58–64. doi: 10.1038/nature08497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. The Journal of Cell Biology. 1993;121(5):961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand RS, Pichugina T, Gehlen LR, Jones MB, Tsai P, Allison JR, et al. Chromosome conformation maps in fission yeast reveal cell cycle dependent sub nuclear structure. Nucleic Acids Research. 2014;42(20):12585–12599. doi: 10.1093/nar/gku965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. The Journal of Cell Biology. 1995;129(4):1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez R, Blobel G, Aris JP. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. The Journal of Biological Chemistry. 1990;265(4):2209–2215. [PubMed] [Google Scholar]
- Hou H, Zhou Z, Wang Y, Wang J, Kallgren SP, Kurchuk T, et al. Csi1 links centromeres to the nuclear envelope for centromere clustering. The Journal of Cell Biology. 2012;199(5):735–744. doi: 10.1083/jcb.201208001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki O, Tanizawa H, Kim KD, Yokoyama Y, Corcoran CJ, Tanaka A, et al. Interaction between TBP and condensin drives the organization and faithful segregation of mitotic chromosomes. Molecular Cell. 2015;59(5):755–767. doi: 10.1016/j.molcel.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KD, Tanizawa H, Iwasaki O, Corcoran CJ, Capizzi JR, Hayden JE, et al. Centromeric motion facilitates the mobility of interphase genomic regions in fission yeast. Journal of Cell Science. 2013;126(Pt. 22):5271–5283. doi: 10.1242/jcs.133678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T. Beyond the sequence: Cellular organization of genome function. Cell. 2007;128(4):787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Fudenberg G, Mehta S, Belton JM, Taneja N, Folco HD, et al. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe. Nature. 2014;516(7531):432–435. doi: 10.1038/nature13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie M, Naito T, Ishikawa F. Stable inheritance of telomere chromatin structure and function in the absence of telomeric repeats. Genes & Development. 2003;17(18):2271–2282. doi: 10.1101/gad.1112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector DL. Nuclear domains. Journal of Cell Science. 2001;114(Pt. 16):2891–2893. doi: 10.1242/jcs.114.16.2891. [DOI] [PubMed] [Google Scholar]
- Syrjanen S. Viral gene detection by in situ hybridization. Oxford: Oxford University Press; 1992. pp. 103–137. [Google Scholar]
- Tanizawa H, Iwasaki O, Tanaka A, Capizzi JR, Wickramasinghe P, Lee M, et al. Mapping of long-range associations throughout the fission yeast genome reveals global genome organization linked to transcriptional regulation. Nucleic Acids Research. 2010;38(22):8164–8177. doi: 10.1093/nar/gkq955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanizawa H, Noma K. Unravelling global genome organization by 3C-seq. Seminars in Cell & Developmental Biology. 2012;23(2):213–221. doi: 10.1016/j.semcdb.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. Journal of Cell Science. 1989;93(Pt. 3):491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]