Abstract
Objective
It had been previously shown that patients who receive neoadjuvant systemic therapy (NST) are more likely to undergo breast-conserving therapy (BCT) than those who have primary surgery. However, the frequency with which patients who are not BCT-eligible prior to NST convert to BCT-eligible with treatment is unknown. To document this conversion rate in a subset of patients expected to have a high clinical response rate to NST, we studied surgical assessment and management of patients enrolled on a randomized neoadjuvant trial for stage II–III HER2-positive breast cancer (HER2 + BC)(CALGB 40601).
Methods
The treating surgeon assessed BCT candidacy based on clinico-radiographic criteria both before and after NST. Definitive breast surgical management was at surgeon and patient discretion. We sought to determine (1) the conversion rate from BCT-ineligible to BCT-eligible (2) the percentage of BCT-eligible patients who chose breast conservation, and (3) the rate of successful BCT. We also evaluated surgeon-determined factors for BCT-ineligibility and the correlation between BCT eligibility and pathologic complete response (pCR).
Results
Of 292 patients with pre- and post-NST surgical assessments, 59 % were non-BCT candidates at baseline. Of the 43 % of these patients who converted with NST, 67 % opted for BCT, with an 80 % success rate. NST increased the BCT-eligible rate from 41 to 64 %. Common factors cited for BCT-ineligibility prior to NST including tumor size (56 %) and probable poor cosmetic outcome (26 %) were reduced by 67 and 75 %, respectively, with treatment, while multicentricity, the second most common factor (33 %), fell by only 16 %. Since 23 % of the BCT-eligible patients chose mastectomy, BCT was the final surgical procedure in just 40 % of the patients. Patients considered BCT-eligible both at baseline and after NST had a pCR rate of 55 %, while patients who were BCT-ineligible prior to NST had the same pCR rate (44 %) whether they converted to BCT-eligible or not.
Conclusions
Many patients with HER2 + BC deemed ineligible for BCT at baseline can be converted to BCT-eligible with NST; excluding patients with multicentric disease substantially increases that percentage. In converted patients who opt for BCT, the success rate is similar to that of patients considered BCT-eligible at baseline. Whether a BCT-ineligible patient converts to BCT eligibility or not does not appear to affect the likelihood of achieving a pCR. Despite the efficacy of NST in this patient cohort, only 40 % of patients had successful BCT; further research into why BCT-eligible patients often opt for mastectomy is needed.
Keywords: Neoadjuvant therapy, HER2-positive breast cancer, Breast conserving therapy
Introduction
Historically, neoadjuvant systemic therapy (NST) was used to convert patients with inoperable breast cancer to operable. More recently, NST has been utilized to allow some patients with stage II–III breast cancer for whom mastectomy is indicated to undergo breast-conserving therapy (BCT) by down-staging the volume of disease [1–4]. The intent to down-stage the primary tumor is often the rationale given to administer systemic therapy in the neoadjuvant rather than the adjuvant setting, especially in patients with aggressive breast cancer subtypes such as triple-negative breast cancer (TNBC) and human epidermal growth factor receptor-2-positive breast cancer (HER2 + BC). BCT after NST has equivalent locoregional recurrence rates and survival compared to BCT performed prior to systemic adjuvant therapy, and is therefore a safe option [1–4].
While some studies that compared the same regimens used as either neoadjuvant or adjuvant therapy have demonstrated higher BCT rates in the NST arm, these reports were based on retrospective analyses with now outdated treatment regimens. Little data exist from large trials to allow estimation of the success rate for converting BCT-ineligible patients to BCT-eligible, nor do we have prospective data as to how often patients converted to BCT-eligible nonetheless chose mastectomy, the likelihood of achieving negative margins when BCT is attempted and what proportion of converted patients achieved a pathologic complete response (pCR). Most previously published neoadjuvant studies did not examine the reasons for surgical-decision making for BCT or mastectomy. Thus, the impact of NST on surgical options remains unclear and a better understanding of these topics is the purpose of the study described here. We have previously published the prospective surgical companion trial for CALGB 40603, on which patients with triple-negative breast cancer (TNBC) received NST, which utilized identical pre- and post-surgical evaluations [5].
CALGB 40601 was a randomized phase III trial that compared lapatinib (L) or the combination of L and trastuzumab (T) to T given in combination with neoadjuvant wP (weekly paclitaxel) in stage II–III HER2 + BC [6]. The primary clinical endpoint was pCR, defined as the absence of residual invasive disease in the breast. CALGB 40601 incorporated a practice-oriented surgical study whose primary surgical endpoint was to assess the percentage of patients who underwent BCT. In addition, the treating surgeon assessed eligibility for BCT both before and after completion of NST and recorded reasons for non-candidacy of BCT. This is a planned analysis of the trial to assess BCT conversion rates and final surgical outcomes.
Methods
Patient eligibility
Patients with stage II or III HER2 + BC with operable, biopsy confirmed, previously untreated, non-inflammatory disease were eligible. HER2 positivity was defined as IHC 3+ or fluorescent-in situ-hybridization (FISH) amplified (ratio ≥ 2.0).
Study procedures
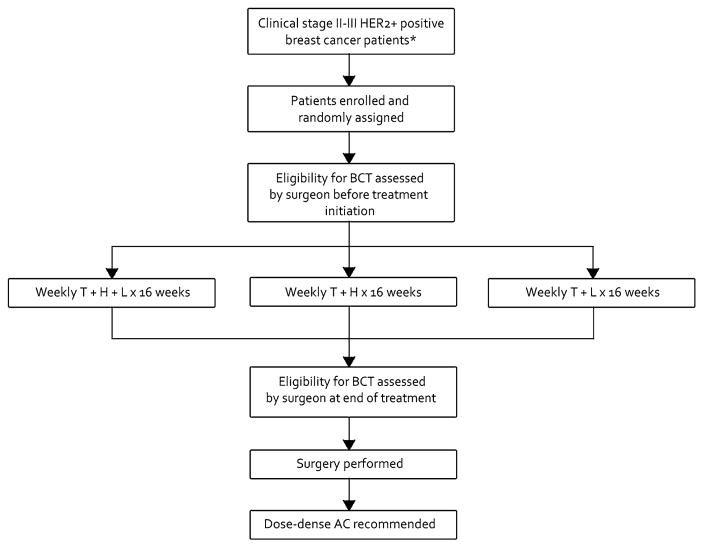
Baseline breast imaging, which included mammography with or without focused breast ultrasound, was required for all patients. Magnetic resonance imaging was recommended but not required. Based upon physical exam, tumor size relative to native breast size, and review of breast imaging studies, the treating breast surgeon assessed eligibility for BCT both before treatment initiation and at the end of NST, and documented all reasons for BCT ineligibility at those time points. Successful BCT was defined as no ink on tumor of a lumpectomy specimen. Mastectomy could be performed either as primary surgery at patient or physician discretion or following unsuccessful attempted BCT. Surgical therapy on CALGB 40601 occurred within 6 weeks of the last dose of wP. Genetic testing for BRCA mutations was not required for the study. The treatment arms described in the introduction are shown in Fig. 1.
Fig. 1.
C40601 Schema T weekly paclitaxel; H trastuzumab; L lapatinib.
*Excluding clinical T4d tumors
Pathologic response was determined locally, with pCR in the breast only defined as the absence of residual invasive disease with or without residual ductal carcinoma in situ (DCIS) (yp T0/is); an additional secondary analysis was done for pCR defined as the absence of both invasive disease and ductal carcinoma in situ (yp T0).
Data collection and analysis
The primary outcome variable of interest was conversion from pre-NST BCT ineligibility to post-NST BCT eligibility. Eligibility for BCT (both pre and post) was scored as yes/no. The conversion rate to BCT candidacy was calculated as the number of patients whose eligibility for BCT changed from ineligible pre-NST to eligible post-NST, divided by the number identified as ineligible pre-NST. Additional items of interest were as follows: (1) proportion of patients who were BCT candidates both before and after NST; (2) proportion of BCT candidates in whom BCT was attempted; (3) BCT success rate; (4) pCR rates by BCT eligibility pre- and post-NST; and (5) final surgical procedure. Proportions and their respective 95 % confidence intervals (CI) were calculated using exact binomial methods. To test the change from pre- to post-NST eligibility, the McNemar’s test was used. Comparisons of proportions across groups were examined with the Chi square test. To model the relation of several baseline clinicopathologic variables to BCT candidacy following NST, logistic regression was used in which post-NST BCT candidacy was the dependent variable. Independent covariables were patient age, tumor grade, and size, whether or not NST was terminated early, and pre-NST BCT candidacy. The observed effect of each independent variable was assessed with its corresponding Wald Chi square. Adjusted odds ratios (OR) and 95 % confidence intervals were taken from the multivariate logistic model. Analyses used an intent-to-treat approach that included all patients who began protocol NST, analyzed according to their randomized assignment.
Data were collected and stored at the CALGB Statistics and Data Center (Durham, NC) with quality ensured through data review performed by the Data Center, the study chairperson (LAC), and surgical co-chairs (MG and DWO). Statistical analyses were performed by CALGB statisticians using SAS 9.2 (Cary, NC). The data cutoff for this report was January 2015.
Results
The trial accrued a total of 305 patients between 2009 and 2012. A total of 292 began protocol therapy, and are included in current analyses. Results for the primary endpoint (pCR) have been previously published [6]. Patient and tumor characteristics are displayed in Table 1. Two-thirds of patients (68 %) were clinical stage II and 32 % were clinical stage III. The median age was 49; 59 % were premenopausal; 66 % had high-grade disease, and 94 % had ductal histology.
Table 1.
Patient and tumor baseline characteristics
| Characteristic | N (%) |
|---|---|
| Total, n (%) | 292 (100) |
| Stratification | |
| Clinical stage, n (%) | |
| II | 200 (68) |
| III | 92 (32) |
| Baseline variables | |
| Patient age, n (%) | |
| 20–29 | 6 (2) |
| 30–39 | 51 (17) |
| 40–49 | 100 (34) |
| 50–59 | 82 (28) |
| 60–69 | 43 (15) |
| 70–79 | 10 (3) |
| Median age (IQR) | 49 (41–56) |
| Minimum age, maximum age | 24–75 |
| Menopausal status, n (%) | |
| Premenopausal | 173 (59) |
| Postmenopausal | 119 (41) |
| Clinical T stage (by physical exam), n (%) | |
| T0 | 3 (1) |
| T1 | 16 (5) |
| T2 | 178 (61) |
| T3 | 75 (26) |
| T4 | 10 (3) |
| Missing/unknown | 10 (3) |
| Tumor grade, n (%) | |
| Low | 10 (3) |
| Intermediate | 84 (29) |
| High | 193 (66) |
| Missing/unknown | 5 (2) |
| Tumor histology n (%) | |
| Ductal | 274 (94) |
| Lobular | 4 (1) |
| Mixed ductal and lobular | 0 (0) |
| Invasive, NOS | 7 (2) |
| Other | 6 (2) |
| Missing/unknown | 1 (<1) |
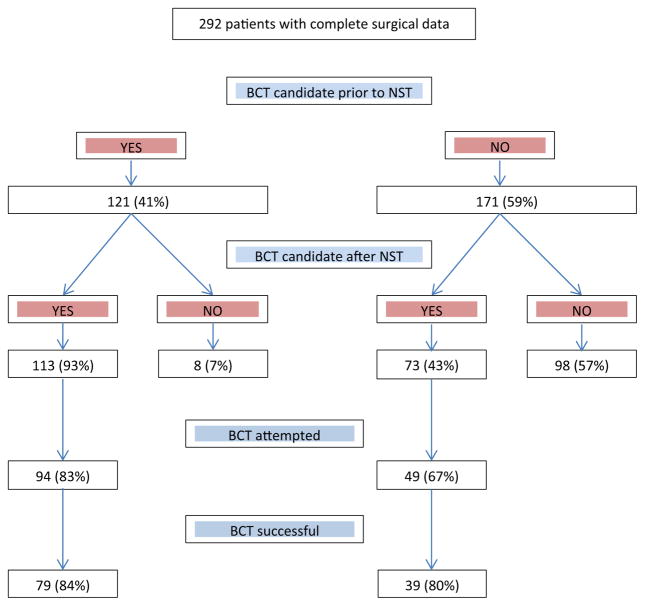
Prior to NST, 121 (41 %) patients were considered eligible for BCT; of these, 113 (93 %) remained eligible after NST and 94 (83 %) underwent BCT, which was successful in 79 (84 %) (Fig. 2). Of the 171 (59 %) patients who were considered ineligible for BCT pre-NST, 73 (43 %) were converted to eligible for BCT after treatment. Of these, BCT was attempted in 49 (67 %) and was successful in 39 (80 %) (Fig. 2). Overall, there was a significant increase in the incidence of surgeon-determined BCT eligibility from 41 % (121/292) prior to NST to 64 % (186/292) after NST (p < 0.001).
Fig. 2.
Surgical outcomes in c40601
Of all patients who were eligible for BCT, 77 % (143/186) attempted BCT with an 83 % (118/143) success rate, while the remaining 23 % (43/186) opted to proceed to mastectomy. Final surgical management was BCT in 118 (40 %) patients and mastectomy in 174 (60 %) patients, including the 25 in whom BCT was unsuccessful.
Of the 171 patients who were judged not to be candidates for BCT prior to NST, the primary reasons identified by the breast surgeon (more than one reason may apply) were as follows: tumor too large [95 (56 %)], multicentric disease [56 (33 %)], probable poor cosmetic outcome [44 (26 %)], and diffuse suspicious microcalcifications [20 (12 %)]. Among the 106 patients deemed not to be candidates for BCT after NST, the primary factors cited were as follows: multicentric disease [47 (44 %)], tumor too large [31(29 %)], diffuse suspicious microcalcifications [17(16 %)], and probable poor cosmetic outcome [11 (10 %)]. (Table 2).
Table 2.
Reasons for BCT non-candidacy
| Characteristic | N (%) |
|---|---|
| Pre-NST (non-candidate) | |
| Total | 171 (100) |
| Tumor too large | 95 (56) |
| Probable poor cosmetic outcome | 44 (26) |
| Contraindication to RT | 1 (1) |
| Diffuse suspicious microcalcifications | 20 (12) |
| Multicentric disease | 56 (33) |
| Post-NST (non-candidate) | 106 (100) |
| Total | 106 (100) |
| Tumor too large | 31 (29) |
| Probable poor cosmetic outcome | 11 (10) |
| Contraindication to RT | 1 (1) |
| Diffuse suspicious microcalcifications | 17 (16) |
| Multicentric disease | 47 (44) |
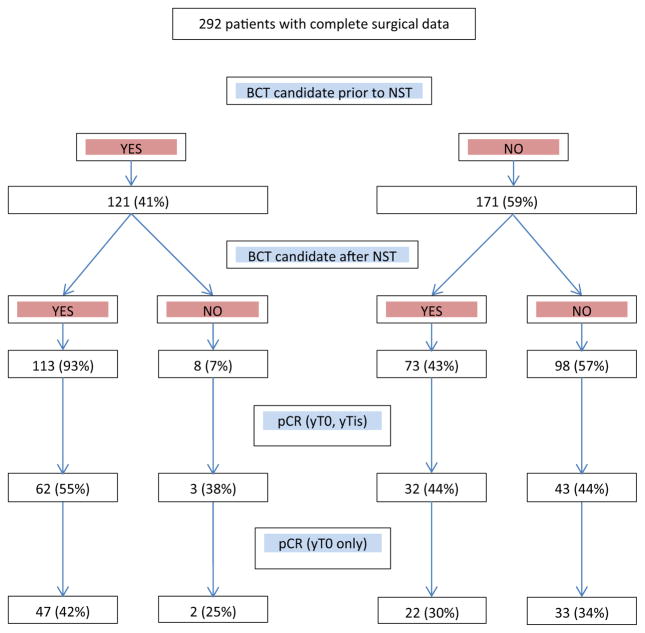
Figure 3 illustrates the incidence of pathologic complete response according to breast conservation candidacy. Of the 113 patients who were candidates for BCT both before and after NST, the pCR rate (ypT0/is) was 55 % (95 % CI 46–64 %). In patients deemed BCT-ineligible before NST, this pCR rate was identical (44 %) among the 73 who converted to BCT-eligible and the 98 who remained BCT-ineligible after NST. (p = 0.13), and was not altered if the more stringent definition of pCR requiring eradication of in situ as well as invasive disease from the breast (ypT0) is used. After adjusting for standard baseline variables of clinicopathologic importance, BCT candidacy prior to NST was the strongest predictor of candidacy post-NST (OR 17.88, 95 % CI 8.03–39.82, p < 0.0001).
Fig. 3.
Surgical outcomes and pCR in c40601
Discussion
In this planned analysis of a prospective surgical sub-study embedded within a large, randomized NST trial, we demonstrate that a substantial fraction (43 %) of patients with HER2 + BC deemed ineligible for BCT at presentation can be successfully converted to BCT-eligible with NST. While these patients were somewhat less likely to an attempt at BCT than patients who were BCT-eligible both at baseline and after NST (67 vs. 83 %), they did their success rate was essentially identical (80 vs. 84 %). In HER2 + BC patients considered BCT-ineligible at baseline due to tumor size or probable poor cosmetic outcome, NST was very likely (67 and 75 % respectively) to eliminate that impediment, whereas in patients with multicentric disease NST was unlikely (16 %) to expand their surgical options. Were we to exclude patients with multicentric disease, currently considered as a contraindication to BCT, from our analysis, the conversion rate would doubtless have been much higher. Similarly, because the extent of diffuse microcalcifications, often a marker of DCIS, is typically not affected by NST, the frequency with which this was cited as a criterion for non-candidacy for BCT remained stable from prior to (n = 20) to after (n = 17) NST.
In addition to the 36 % of patients who remained BCT-ineligible (for whatever reason) after NST, and the 17 % of BCT-eligible patients whose attempted BCT failed to achieve negative margins, leading to subsequent mastectomy, a substantial fraction (23 %) of BCT-eligible patients opted for mastectomy as their primary surgery; as a result, the overall BCT rate was just 40 %. One of the limitations of our study was the failure to collect surgeon and patient reasons for foregoing BCT in eligible patients. Many factors probably weigh into this decision making, including widespread availability of BRCA mutation testing, identifying mutation carriers in whom bilateral mastectomy is considered by many the standard of care, but also the trend towards higher mastectomy and contralateral prophylactic mastectomy rates in BRCA-unknown and even BRCA-negative women seen in the US over the past decade, despite the absence of evidence that mastectomy results in improved survival in non-BRCA carriers.
Whether being able to identify patients who were BCT-ineligible at baseline who were highly likely to have achieved pCR with NST would reduce the mastectomy rate in unknown. However, in our patient population, the inability of conversion from BCT-ineligible to BCT-eligible to predict a greater likelihood of having achieved a pCR limits our ability to reassure the patient about the efficacy of the NST in eradicating disease in the breast, and neither clinical complete response nor resolution of abnormalities on imaging studies with NST has been shown to correlate strongly with achievement of pCR. Nor was BCT-eligibility at baseline or conversion from ineligibility to eligibility with NST substantially more likely to correlate with the more stringent ypTN0 definition of pCR.
Results of multivariable analysis indicated that planned BCT prior to NST was the strongest predictor of BCT eligibility post-NST, mirroring another neoadjuvant trial in HER2 + BC [8].
The results presented here of CALGB 40601 are remarkably similar to those of CALGB 40603, an NST trial for TNBC [5]. The conversion from BCT-ineligible to BCT-eligible was, respectively, 43 vs. 42 %; the patients remaining candidates for BCT was 93 vs. 90 %; the final BCT rate was 40 vs. 47 %; and the overall pCR was 48 vs. 54 %. Surgical practice patterns for these two NST trials run by the same cooperative group which incorporate academic and community centers showed very similar surgical treatment patterns and outcomes for women with TNBC and HER2 + BC.
The incidence of BCT success when attempted was high at 83 %, while the incidence of BCT as the final surgical procedure was 40 %. The latter needs to be viewed in the context of two large, recent neoadjuvant trials that involved women with HER2 + BC. One was Neo-ALLTO, a European phase III neoadjuvant trial of women with HER2 + BC; the other was GeparSixto, a European neoadjuvant trial for HER2 + BC and TNBC conducted by the German Breast Group (GBG) [7, 8]. Neo-ALLTO reported a similar BCT rate of 44 %, but noted that a smaller proportion of patients treated in ‘developing countries’ underwent BCT compared to other countries (32 vs. 53 %). This difference was theorized to be due to a lack of optimal radiotherapy or the absence of a multidisciplinary breast center at participating sites in those countries [7]. In contrast, despite pCR rates similar to those achieved in our trials, in GeparSixto the GBG achieved a BCT rate of nearly 75 %, though the addition of carboplatin to the control neoadjuvant regimen did not raise the BCT rate despite a significant increase in the pCR rate. To what extent these differences in BCT rates are driven by medical versus cultural or economic reasons need to be explored in future NST trials.
When we turn to the neoadjuvant setting to study novel agents, the question remains whether higher pCR rates will be accompanied by a concomitant increase in BCTs. Despite pCR rates of 38–55 %, a sizable increase in BCT eligibility following NST and a high likelihood of success for those who chose BCT, our final BCT rate was only 40 %.
This incongruity between higher clinical and pathologic response rates and the persistence of low overall BCT rates is partially explained by the substantial number of BCT-eligible patients, regardless of whether they were always candidates for BCT or converted to candidates with NST, who choose not to attempt breast conservation. Instead, these patients and their surgeons opted for mastectomy as their definitive oncologic surgery during the first surgical procedure irrespective of the clinico-radiographic response to NST. Patient and surgeon perceptions are complex and layered, with respect to the risks of an in-breast recurrence or development of a secondary primary breast cancer, and likely played a role in many of these decisions [9–11]. These findings underscore the importance of all members of the breast cancer care team to convey to patients an accurate assessment of their risk of loco-regional recurrence and second primary cancers, as well as to discuss surgical risks and likely cosmetic outcomes associated with the various surgical options so that patients can make informed decisions regarding their local management. Surgical oncologists need to incorporate appropriate breast imaging, before and after NST, to improve their assessment of treatment response so that suitable patients can be offered and encouraged to attempt BCT. This incongruity is seen by the over 40 % of patients deemed not candidates for BCT found to have a pCR on final pathology.
Our study has several limitations including the lack of information on genetic propensity to breast cancer that could influence surgical decisions, although it is likely to be of limited impact in HER2 + BC. We did not collect data on factors such as need for additional imaging and biopsy, fear and risk perceptions of HER2 breast cancer subtypes for recurrence, and patient and surgical biases that could play a role in decision-making regarding local therapy [12–14].
In conclusion, we present results from a large modern era NST of HER2 + BC. Despite high rates of pCR and reduction in factors that would preclude BCT, 60 % of patients underwent mastectomy. Our surgical results for HER2 + BC are remarkably similar to our results for TNBC, CALGB 40603. Future trials to address patient- and surgeon-specific factors should be designed in a prospective fashion to tailor recommendations for receipt of BCT [15]. If a major goal of NST is to actually increase BCT rates, while we have demonstrated the ability of NST to increase BCT eligibility in this patient population, the results are disappointing. Our numbers of patients undergoing BCT are lower than decades past when NST regimens had more limited efficacy in terms of reducing in breast tumor burden. These results reflect the need to identify the factors that influence our patients’ decision-making process, and to provide them with information to reduce selection of unnecessarily aggressive treatment options. While mastectomy is not the wrong option for breast cancer local therapy, we believe that it is being used far too frequently in patients who are good candidates for BCT after NST and have no specific indications for (such as a documented BRCA mutation) or contraindications to BCT, given advances in NST, especially in patient populations such as ours (HER2 + BC). Our work highlights the continued trend in the surgical overtreatment of breast cancer, despite ample research demonstrating the equivalence of BCT and mastectomy in clinical outcomes.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), as well as U10CA180888 (to SWOG). Also supported in part by funds from the Breast Cancer Research Foundation, Genentech, and GlaxoSmithKline. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This study presented in part as Oral Presentations at the ASCO Annual Meeting, June 2013 and June 2015.
Conflict of interest All the authors declare no conflict of interest.
Compliance with ethical standards
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Barry PA, Schiavon G. Primary systemic treatment in the management of operable breast cancer: best surgical approach for diagnosis, biological evaluation, and research. J Natl Cancer Inst Monogr. 2015;2015(51):4–8. doi: 10.1093/jncimonographs/lgv008. [DOI] [PubMed] [Google Scholar]
- 2.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 3.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26(5):778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 4.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(22):4224–4237. doi: 10.1200/JCO.2001.19.22.4224. [DOI] [PubMed] [Google Scholar]
- 5.Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant chemotherapy in stage II–III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance) Ann Surg. 2015;262(3):434–439. doi: 10.1097/SLA.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, Ollila DW, Krop IE, Henry NL, Weckstein DJ, Anders CK, Singh B, Hoadley KA, Iglesia M, Cheang MC, Perou CM, Winer EP, Hudis CA. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2016;34(6):542–549. doi: 10.1200/JCO.2015.62.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criscitiello C, Azim HA, de Azambuja E, Rubio IT. Factors affecting surgical management following neoadjuvant therapy in patients with primary HER2-positive breast cancer: results from the NeoALTTO phase III trial. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2014;25(4):910–911. doi: 10.1093/annonc/mdu034. [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–756. doi: 10.1016/S1470-2045(14)70160-3. [DOI] [PubMed] [Google Scholar]
- 9.Debled M, MacGrogan G, Breton-Callu C, et al. Surgery following neoadjuvant chemotherapy for HER2-positive locally advanced breast cancer. Time to reconsider the standard attitude. Eur J Cancer. 2015;51(6):697–704. doi: 10.1016/j.ejca.2015.01.063. [DOI] [PubMed] [Google Scholar]
- 10.Holmes D, Colfry A, Czerniecki B, et al. Performance and practice guideline for the use of neoadjuvant systemic therapy in the management of breast cancer. Ann Surg Oncol. 2015;22(10):3184–3190. doi: 10.1245/s10434-015-4753-3. [DOI] [PubMed] [Google Scholar]
- 11.McGuire KP, Hwang ES, Cantor A, et al. Surgical patterns of care in patients with invasive breast cancer treated with neoadjuvant systemic therapy and breast magnetic resonance imaging: results of a secondary analysis of TBCRC 017. Ann Surg Oncol. 2015;22(1):75–81. doi: 10.1245/s10434-014-3948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caudle AS, Yu TK, Tucker SL, et al. Local-regional control according to surrogate markers of breast cancer subtypes and response to neoadjuvant chemotherapy in breast cancer patients undergoing breast conserving therapy. Breast Cancer Res BCR. 2012;14(3):R83. doi: 10.1186/bcr3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cureton EL, Yau C, Alvarado MD, et al. Local recurrence rates are low in high-risk neoadjuvant breast cancer in the I-SPY 1 Trial (CALGB 150007/150012; ACRIN 6657) Ann Surg Oncol. 2014;21(9):2889–2896. doi: 10.1245/s10434-014-3721-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kummel S, Holtschmidt J, Loibl S. Surgical treatment of primary breast cancer in the neoadjuvant setting. Br J Surg. 2014;101(8):912–924. doi: 10.1002/bjs.9545. [DOI] [PubMed] [Google Scholar]
- 15.Rippy EE, Ainsworth R, Sathananthan D, Kollias J, Bochner M, Whitfield R. Influences on decision for mastectomy in patients eligible for breast conserving surgery. Breast. 2014;23(3):273–278. doi: 10.1016/j.breast.2013.12.009. [DOI] [PubMed] [Google Scholar]