Abstract
Copper-catalyzed functionalization of terminal or 1,1-disubstituted alkenes with bis(pinacolato)diboron and methanol provides formal hydroboration products with exceptional regiocontrol favoring the branched isomer. Through pairing this procedure with photocatalytic cross couplings using iridium and nickel co-catalysis, an effective, highly regiose-lective procedure for the hydroarylation of terminal alkenes is provided.
Graphical Abstract
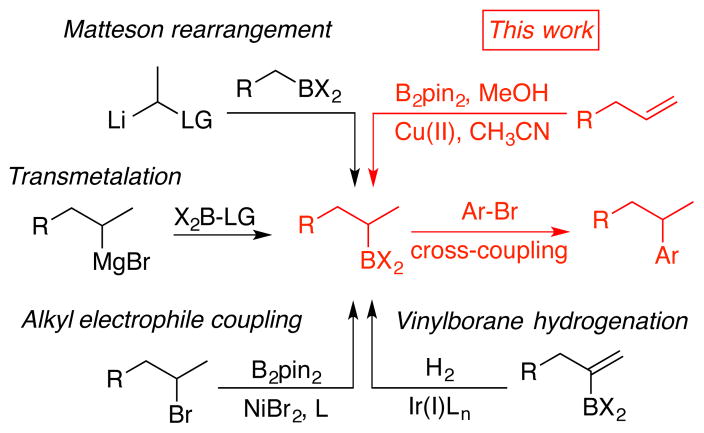
The hydroboration of simple terminal alkenes is a transformation of fundamental importance in synthesis, and the hydroboration/oxidation sequence serves as the introduction to anti-Markovnikov regioselectivity in most introductory organic chemistry courses.1 Despite the long history of this process, a general approach to reversing the regioselectivity of hydrob-orations of simple alkenes bearing aliphatic substituents has remained elusive. Coupled with oxidation of the resulting organoborane, this outcome would provide an equivalent process to the acid-catalyzed hydration of alkenes with accompanying advantages in scope and functional group tolerance. Additionally, we envisioned that other processes such as metal-catalyzed cross-coupling, when paired with a reversal of regiochemistry in hydroborations, would provide a useful and simple method for the synthesis of tertiary carbon frameworks through the two-step branch-selective reductive union of alkenes and sp2-carbon electrophiles. Considering the tremendous recent advances in cross-couplings of secondary organoboranes,2–5 the development of more efficient methods for accessing the requisite branched organoboranes could find immediate utility.
Most catalytic hydroboration methods provide the linear alkylborane product, often with efficiencies that greatly exceed the direct thermal addition of boranes to alkenes.6 The direct access to branched boranes from the hydroboration of terminal olefins is typically accompanied by chain walking7 that often leads to terminal or benzylic boranes. Advances in reversing the regiochemical outcome of hydroborations are often accomplished through the installation of directing groups, which can override normally expected preferences based on the alkene substitution pattern.8 More recent advances illustrated that subtle remote electronic biases can also be used as a handle for regiocontrol.9 Alkenes such as styrenes provide an electronic bias for the development of catalyst-substrate interactions that enable regioselectivity reversals, with notable illustrations in directly accessing benzylic boranes from the rhodium-catalyzed hydroboration of styrenes.10 The copper-catalyzed addition of bis(pinacolato)diboron (B2Pin2) in the presence of methanol has been utilized by Hoveyda as a formal hydroboration of terminal alkynes with regioreversal compared with traditional strategies,11 whereas styrenes afforded the terminal borane product.11b The procedure, which involves borylmetalation followed by protonation of the resulting Cu-C bond, provides considerable utility as a means to access the more hindered alkenylborane products. Secondary alkylboranes, such as those that potentially could be made by a branch-selective hydroboration of terminal alkenes, are typically accessed by methods such as addition of organolithium or organomagnesium reagents to boron electrophiles,12 conversion of alkyl halides to alkylboranes,13 hydrogenations of vinylboranes,14 or variations of the Matteson rearrangement, which has proven to be immensely useful in asymmetric versions (Scheme 1).15 Impressive cascade functionalizations of terminal alkenes have enabled preparation of secondary alkylboranes in tandem with arylation or alkenylation of the terminal alkene carbon,16 and reports of aminoboration and carboboration were recently disclosed.17 Furthermore a recent approach from Ito described the formal hydroboration of terminal alkenes via a process catalyzed by copper phosphine complexes.18 While these methods have enabled many impressive advances in the synthesis and application of secondary alkylboranes, a more general approach to the hydroboration of a variety of terminal and 1,1 disubstituted alkenes to provide a regiochemical outcome opposite that of classical thermal additions of borohydrides to alkenes would provide an important entry to branched alkylboranes for utilization in synthesis. The development of a highly regio- and branch-selective hydroboration process using a copper-NHC catalyst is described herein, and the method utility with photocatalytic cross-couplings3 is demonstrated.
Scheme 1.
Approaches to Secondary Alkyl-boranes.
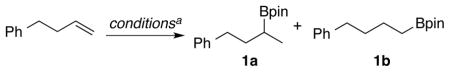
Efforts to access branched alkylboranes from terminal alkenes began with exploration of hydroborations using HBPin, with Ni, Pd, Pt, Co, and Rh catalysts and the N-heterocyclic carbene ligands IMes, SIMes, IPr, and SIPr (Table 1, entries 1–7, selected data). In all cases, the terminal alkylborane product was favored, with Co- and Rh-catalyzed processes most strongly favoring the linear products, and no more than 21% of the desired branched products being observed in any case. We next examined additions using B2Pin2 with methanol using Cu(I) catalysts given the success seen in regiochemistry reversals involving terminal alkynes from Hoveyda with similar catalyst systems.11 The combination of CuCl with IMes provided a nearly 2:1 ratio of regioisomers favoring branched isomer 1a (Table 1, entry 8), whereas the use of SIPr provided excellent regioselectivities favoring 1a, albeit in modest yield (Table 1, entry 9). A solvent screen illustrated that CH3CN provided superior conversions compared with THF and other common solvents, and a variety of Cu(I) and Cu(II) precatalysts were therefore examined in CH3CN for yield optimization (Table 1, entries 10–13). In cases where Cu(II) precatalysts are effective, it is likely that Cu(I) species generated under the reaction conditions are functioning as the active catalyst.19 Among the cases explored, the use of the preformed complex Cu(Cl)IPr with B2Pin2/MeOH (Table 1, entry 11) provided an optimum outcome in terms of conversion, regioselectivity, and reproducibility across various substrate classes, and this protocol was thus selected for further study.
Table 1.
Optimization of Branched Alkylborane Synthesis.
| |||||
|---|---|---|---|---|---|
| entry | catalyst | ligandb | reagent | solvent | 1a : 1b (yield, %)c |
| 1 | Pd2dba3 | IMes | HBPin | THF | 17:83 (62) |
| 2 | Pd2dba3 | SIPr | HBPin | THF | 21:79 (47) |
| 3 | Ni(cod)2 | IMes | HBPin | THF | 21:79 (14) |
| 4 | Ni(cod)2 | SIPr | HBPin | THF | 13:87 (<5) |
| 5 | PtCl2 | SIPr | HBPin | THF | 7:93 (74) |
| 6 | CoBr2 | SIPr | HBPin | THF | <2:98 (62) |
| 7 | Rh(PPh3)3Cl | SIPr | HBPin | THF | <2:98 (33) |
| 8 | CuCl | IMes | B2Pin2 | THF | 67:33 (3) |
| 9 | CuCl | SIPr | B2Pin2 | THF | 79:21 (48) |
| 10 | CuCl | SIPr | B2Pin2 | CH3CN | 95:5 (52) |
| 11 | Cu(Cl)IPr | — | B2Pin2 | CH3CN | 92:8 (81)d |
| 12d | Cu(OAc)2 | SIPr | B2Pin2 | CH3CN | 93:7 (62) |
| 13e | CuBr2 | SIPr | B2Pin2 | CH3CN | 96:4 (78) |
All experiments were conducted at rt for 2 h at 0.2 M under nitrogen atmosphere. Experiments with B2Pin2 also used CH3OH (2 equiv). B2Pin2 = bis(pinacolato)diboron.
Ligand HCl salts were used with KO-t-Bu; IMes•HCl = 1,3-bis(mesityl)-imidazolium chloride; SIPr•HCl = 1,3-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazolium chloride. IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene.
With the exception of entry 11, NMR yields are shown, using 1,3,5-trimethoxybenzene as internal standard. Regioselectivity ratios were determined on crude reaction mixtures.
Isolated yield is given.
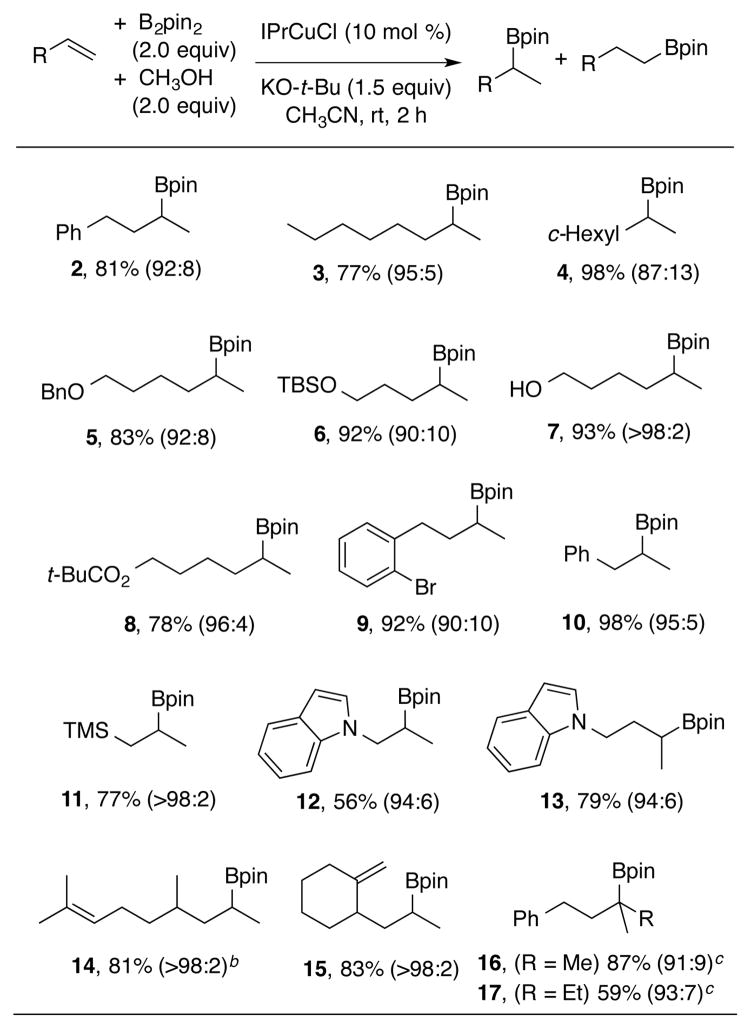
A range of simple terminal olefins were examined in the regioselective formation of branched alkylborane products. Using either 4-phenyl-1-butene or 1-octene, excellent yields and regioselectivities favoring the branched isomer were observed (compounds 2-3, Scheme 2). Branching at the allylic position was tolerated (compound 4, Scheme 2). Other tolerated functional groups included benzyl ethers, silyl ethers, unprotected hydroxyls, pivalate esters, and bromoarenes (compounds 5–9, Scheme 2). Allylbenzene was a highly effective substrate, and no isomerization to the styrene or to the corresponding benzylic borane product was noted (compound 10, Scheme 2). Notably, compound 10 was previously prepared in highly en-antioselective fashion from prop-1-en-1-ylbenzene via Cu-NHC catalyzed hydroboration.20 Examination of similar chiral NHC’s in the current procedure using allylbenzene as substrate led to poor enantioselectivities (see Supporting Information for details). Therefore, the current procedure will be most useful for non-styrenyl substrates in comparison to this alternative method that is highly effective for styrene hydroborations. As additional examples, allylsilanes were tolerated in the procedure, leading to 1,2-bis-metalated products (compound 11, Scheme 2). Indoles were also tolerated to afford products borylated at the β- or γ-positions of the N-alkyl chain (compounds 12–13, Scheme 2). To explore selectivity among different olefin classes, hydroboration of a substrate possessing both a trisubstituted alkene and a monosubstituted alkene afforded complete selectivity for the terminal alkene (compound 14, Scheme 2). Similarly, complete selectivity was seen for hydroboration of the terminal alkene when a 1,1-disubstituted alkene was present (compound 15, Scheme 2). While alkenes that possess two or more substituents were generally unreactive, 1,1-disubstututed alkenes could be converted to tertiary borane products under modified conditions (compounds 16–17, Scheme 2).21
Scheme 2.
Scope of Branched Alkylborane Synthesis.a
aB2Pin2 = bis(pinacolato)diboron; IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene. Regioselectivity ratios were determined on crude reaction mixtures. Isolated yields are given. bDiastereomeric ratio (1:1). cReaction was conducted in CH2Cl2 using NaO-t-Bu at rt.
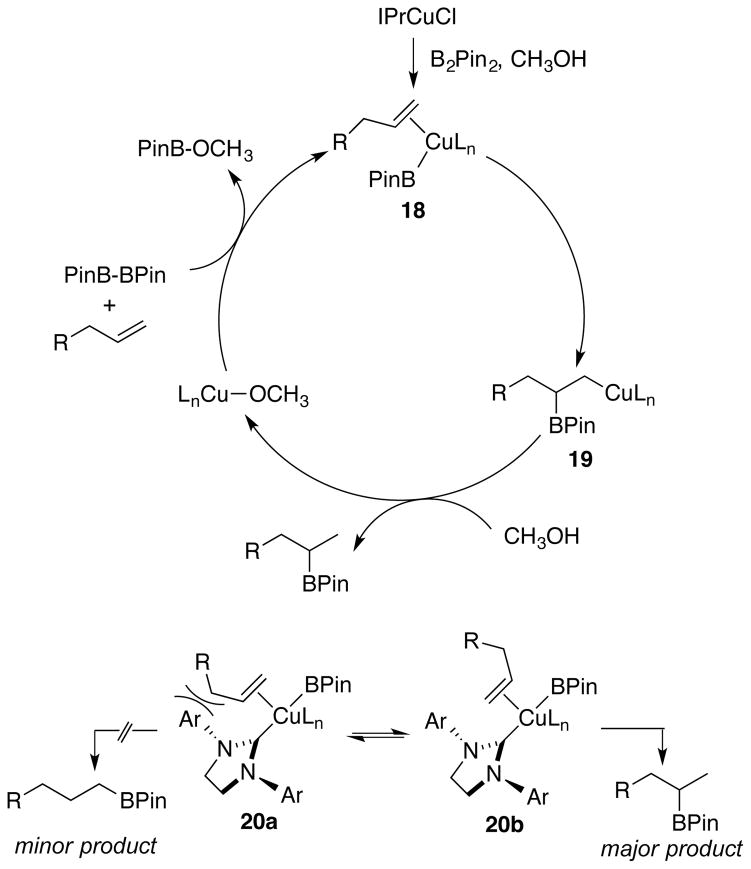
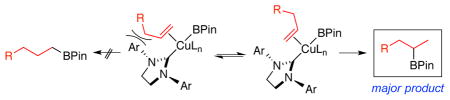
In analogy with other reports of copper-catalyzed additions of B2Pin2 and methanol to alkynes and styrenes,11,17 the mechanism of the process likely involves addition of a copper borane intermediate to the terminal alkene as the regiochemistry-determining step as depicted in the 18 to 19 conversion (Scheme 3). Formation of the linear borane is disfavored as ligand size increases (compare Table 1, entries 8 and 9) due to developing steric interactions experienced in complex 20a.22 Alternatively, complex 20b, which leads to the observed branched product, avoids steric interactions between the ligand and alkene substituent. Following the sterically preferred formation of 19, protonation of the metal-copper bond produces the desired product along with the formation of a copper methoxide species, which is converted to the reactive Cu-BPin complex by the reaction with B2Pin2.
Scheme 3.
Mechanism of Secondary Alkylborane Formation.
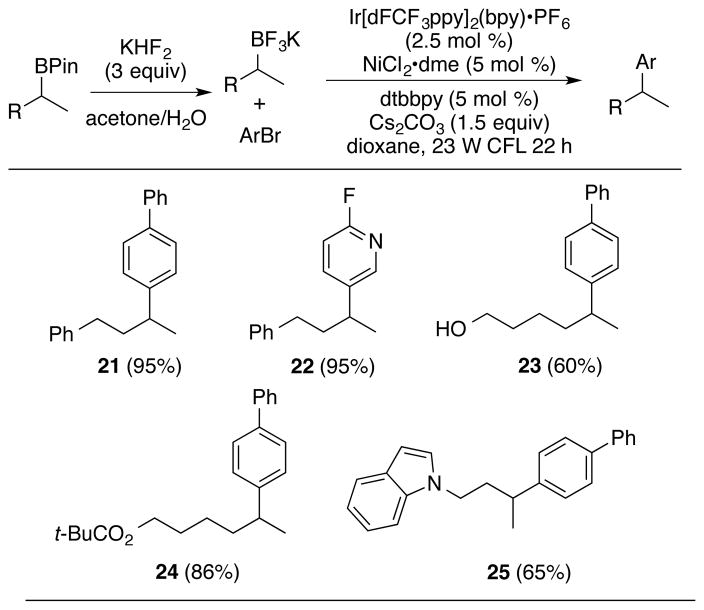
Recent advances in Suzuki couplings can benefit from the facile entry to the secondary alkylboranes provided by this procedure.2,3 For example, the nickel-catalyzed photocatalytic cross-coupling procedure recently developed by Molander,3b when paired with the above developments, provides a convenient and versatile method for the direct conversion of terminal alkenes to tertiary branched motifs. Alkyl(BPin) derivatives accessed by the catalytic, regiocontrolled addition of B2Pin2 with methanol to terminal alkenes (Scheme 4) are readily converted to the corresponding trifluoroborate derivatives,3a,23 while maintaining the versatile functional group tolerance of the hydroboration procedure (Scheme 4). The resulting functionalized trifluoroborate derivatives then directly participate in nickel-catalyzed cross-couplings in the presence of iridium photocatalysts following the Molander protocol. Both electron-rich and electron-deficient bromoarenes participate in the sequence (21–22, Scheme 4). Notably, the functional group tolerance demonstrated in the synthesis of branched alkyl-boranes is carried through the trifluoroborate synthesis/photocatalytic cross-coupling sequence to provide the preservation of sensitive functional groups such as free alcohols, esters, and indoles (23–25, Scheme 4).
Scheme 4.
Photocatalytic Cross-Couplings of Branched Trifluoroalkylboranes.a
aIsolated yields for the photocatalytic cross-coupling step are provided in the Table. Details for the synthesis of the RBF3 salts are provided in the Supporting Information. dFCF3ppy = 2-(2,4-difluorophenyl)-5-(trifluoromethyl) pyridine; dme = dimethoxyethane; dtbbpy = 4,4’-di-tert-butyl-2-2’-bipyridine.
In summary, the regiodivergent hydroboration of a broad range of simple terminal alkenes may now be readily accomplished.24 While a range of thermal or catalyzed additions of boranes provides access to linear alkylboranes according to previous reports, the copper-catalyzed addition of B2Pin2 with methanol reported herein provides highly regioselective access to the isomeric branched alkylboranes. This procedure complements previous methods for accessing secondary alkylborane structures, and the utilization of the products obtained in nickel-catalyzed photocatalytic cross-couplings provides a branch-selective strategy for the reductive cross-coupling of alkenes and aryl bromides. Further exploration of asymmetric versions of the formal hydroboration is in progress, and utilization of the racemic organoboranes will be possible with emerging developments in stereoconvergent cross-couplings.3
Supplementary Material
Acknowledgments
The authors wish to thank the National Institutes of Health (R35GM118133) for support of this research. The Stephenson group (University of Michigan) is thanked for helpful discussions on the use of photocatalysis processes.
Footnotes
Synthetic details and spectral data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Brown HC, Rao BCS. J Org Chem. 1957;22:1136. [Google Scholar]; (b) Brown HC, Zweifel G. J Am Chem Soc. 1961;83:2544. [Google Scholar]
- 2.(a) Li L, Zhao SB, Joshi-Pangu A, Diane M, Biscoe MR. J Am Chem Soc. 2014;136:14027. doi: 10.1021/ja508815w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sandrock DL, Jean-Gérard L, Chen C-y, Dreher SD, Molander GA. J Am Chem Soc. 2010;132:17108. doi: 10.1021/ja108949w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Molander GA, Wisniewski SR. J Am Chem Soc. 2012;134:16856. doi: 10.1021/ja307861n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ding JY, Rybak T, Hall DG. Nat Commun. 2014;5:5474. doi: 10.1038/ncomms6474. [DOI] [PubMed] [Google Scholar]
- 3.(a) Tellis JC, Primer DN, Molander GA. Science. 2014;345:433. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Primer DN, Karakaya I, Tellis JC, Molander GA. J Am Chem Soc. 2015;137:2195. doi: 10.1021/ja512946e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gutierrez O, Tellis JC, Primer DN, Molander GA, Kozlowski MC. J Am Chem Soc. 2015;137:4896. doi: 10.1021/ja513079r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Noble A, McCarver SJ, MacMillan DWC. J Am Chem Soc. 2015;137:624. doi: 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For alternate approaches to couplings of secondary carbon centers: Lundin PM, Fu GC. J Am Chem Soc. 2010;132:11027. doi: 10.1021/ja105148g.Lu Z, Fu GC. Angew Chem Int Ed. 2010;49:6676. doi: 10.1002/anie.201003272.Smith SW, Fu GC. Angew Chem Int Ed. 2008;47:9334. doi: 10.1002/anie.200802784.
- 6.(a) Männig D, Nöth H. Angew Int Ed Engl. 1985;24:878. [Google Scholar]; (b) Evans DA, Fu GC, Hoveyda AH. J Am Chem Soc. 1992;114:6671. [Google Scholar]; (c) Burgess K, Van der Donk WA, Jarstfer MB, Ohlmeyer MJ. J Am Chem Soc. 1991;113:6139. [Google Scholar]; (d) Burgess K, Ohlmeyer MJ. Chem Rev. 1991;91:1179. [Google Scholar]; (e) Tseng KNT, Kampf JW, Szymczak NK. ACS Catal. 2015;5:411. [Google Scholar]; (f) Zhang L, Huang Z. Synlett. 2013;24:1745. [Google Scholar]; (g) Thomas SP, Aggarwal VK. Angew Chem Int Ed. 2009;48:1896. doi: 10.1002/anie.200805604. [DOI] [PubMed] [Google Scholar]
- 7.(a) Scheuermann ML, Johnson EJ, Chirik PJ. Org Lett. 2015;17:2716. doi: 10.1021/acs.orglett.5b01135. [DOI] [PubMed] [Google Scholar]; (b) Prokofjevs A, Boussonniere A, Li LF, Bonin H, Lacote E, Curran DP, Vedejs E. J Am Chem Soc. 2012;134:12281. doi: 10.1021/ja305061c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Morrill TC, D'Souza CA, Yang L, Sampognaro AJ. J Org Chem. 2002;67:2481. doi: 10.1021/jo0109321. [DOI] [PubMed] [Google Scholar]
- 8.Hoveyda AH, Evans DA, Fu GC. Chem Rev. 1993;93:1307. [Google Scholar]
- 9.Xi Y, Hartwig JF. J Am Chem Soc. 2016;138:6703. doi: 10.1021/jacs.6b02478. [DOI] [PubMed] [Google Scholar]
- 10.Evans DA, Fu GC, Anderson BA. J Am Chem Soc. 1992;114:6679.Evans DA, Fu GC. J Org Chem. 1990;55:2280.Crudden CM, Hleba YB, Chen AC. J Am Chem Soc. 2004;126:9200. doi: 10.1021/ja049761i.Lata CJ, Crudden CM. J Am Chem Soc. 2010;132:131. doi: 10.1021/ja904142m.Wen Y, Xie J, Deng C, Li C. J Org Chem. 2015;80:4142. doi: 10.1021/jo502606x.Conjugate additions: Wu H, Radomkit S, O’Brien JM, Hoveyda AH. J Am Chem Soc. 2012;134:8277. doi: 10.1021/ja302929d.Touney EE, Van Hoveln R, Buttke CT, Freidberg MD, Guzei IA, Schomaker JM. Organometallics. 2016 doi: 10.1021/acs.organomet.6b00652.
- 11.(a) Jang H, Zhugralin AR, Lee Y, Hoveyda AH. J Am Chem Soc. 2011;133:7859. doi: 10.1021/ja2007643. [DOI] [PubMed] [Google Scholar]; (b) Corberán R, Mszar NW, Hoveyda AH. Angew Chem Int Ed. 2011;50:7079. doi: 10.1002/anie.201102398. [DOI] [PubMed] [Google Scholar]
- 12.Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257. doi: 10.1021/ja8031423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Dudnik AS, Fu GC. J Am Chem Soc. 2012;134:10693. doi: 10.1021/ja304068t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Atack TC, Lecker RM, Cook SP. J Am Chem Soc. 2014;136:9521. doi: 10.1021/ja505199u. [DOI] [PubMed] [Google Scholar]
- 14.(a) Bull JA. Angew Chem Int Ed. 2012;51:8930. doi: 10.1002/anie.201203876. [DOI] [PubMed] [Google Scholar]; (b) Moran WJ, Morken JP. Org Lett. 2006;8:2413. doi: 10.1021/ol060735u. [DOI] [PubMed] [Google Scholar]; (c) Ganić A, Pfaltz A. Chem Eur J. 2012;18:6724. doi: 10.1002/chem.201200246. [DOI] [PubMed] [Google Scholar]; (d) Paptchikhine A, Cheruku P, Engman M, Andersson PG. Chem Commun. 2009:5996. doi: 10.1039/b912590f. [DOI] [PubMed] [Google Scholar]
- 15.(a) Matteson DS, Majumdar D. Organometallics. 1983;2:230. [Google Scholar]; (b) Sadhu KM, Matteson DS. Organometallics. 1985;4:1687. [Google Scholar]; (c) Leonori D, Aggarwal VK. Acc Chem Res. 2014;47:3174. doi: 10.1021/ar5002473. [DOI] [PubMed] [Google Scholar]; (d) Stymiest JL, Dutheuil G, Mahmood A, Aggarwal VK. Angew Chem Int Ed. 2007;46:7491. doi: 10.1002/anie.200702146. [DOI] [PubMed] [Google Scholar]; (e) Blakemore PR, Marsden SP, Vater HD. Org Lett. 2006;8:773. doi: 10.1021/ol053055k. [DOI] [PubMed] [Google Scholar]; (f) Blakemore PR, Burge MS. J Am Chem Soc. 2007;129:3068. doi: 10.1021/ja068808s. [DOI] [PubMed] [Google Scholar]
- 16.Mlynarski SN, Schuster CH, Morken JP. Nature. 2014;505:386. doi: 10.1038/nature12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Sakae R, Hirano K, Miura M. J Am Chem Soc. 2015;137:6460. doi: 10.1021/jacs.5b02775. [DOI] [PubMed] [Google Scholar]; (b) Kageyuki I, Yoshida H, Takaki K. Synthesis. 2014;46:1924. [Google Scholar]; (c) Semba K, Nakao Y. J Am Chem Soc. 2014;136:7567. doi: 10.1021/ja5029556. [DOI] [PubMed] [Google Scholar]; (d) Moon JH, Jung HY, Lee YJ, Lee SW, Yun J, Lee JY. Organometallics. 2015;34:2151. [Google Scholar]; (e) Su W, Gong TJ, Lu X, Xu MY, Yu CG, Xu ZY, Yu HZ, Xiao B, Fu Y. Angew Chem Int Ed. 2015;54:12957. doi: 10.1002/anie.201506713. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto H, Kubota K, Ito H. Chem Commun. 2016;52:5916. doi: 10.1039/c6cc00782a.For regiochemistry reversals in alkene hydrosilylations: Du X, Zhang Y, Peng D, Huang Z. Angew Chem Ed Engl. 2016;55:6671. doi: 10.1002/anie.201601197.
- 19.(a) Lee K-s, Brown MK, Hird AW, Hoveyda AH. J Am Chem Soc. 2006;128:7182. doi: 10.1021/ja062061o. [DOI] [PubMed] [Google Scholar]; (b) Dabrowski JA, Villaume MT, Hoveyda AH. Angew Chem Int Ed. 2013;52:8156. doi: 10.1002/anie.201304035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhu S, Buchwald SL. J Am Chem Soc. 2014;136:15913. doi: 10.1021/ja509786v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee YM, Hoveyda AH. J Am Chem Soc. 2009;131:3160. doi: 10.1021/ja809382c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.For excellent overviews of the synthesis and utility of tertiary boranes: Joshi-Pangu A, Wang CY, Biscoe MR. J Am Chem Soc. 2011;133:8478. doi: 10.1021/ja202769t.Odachowski M, Bonet A, Essafi S, Conti-Ramsden P, Harvey JN, Leonori D, Aggarwal VK. J Am Chem Soc. 2016;138:9521. doi: 10.1021/jacs.6b03963.
- 22.For other examples of regiochemistry reversals using NHC ligands: (Malik HA, Sormunen GJ, Montgomery J. J Am Chem Soc. 2010;132:6304. doi: 10.1021/ja102262v.Liu P, Montgomery J, Houk KN. J Am Chem Soc. 2011;133:6956. doi: 10.1021/ja202007s.Miller ZD, Li W, Belderrain TR, Montgomery J. J Am Chem Soc. 2013;135:15282. doi: 10.1021/ja407749w.Miller ZD, Montgomery J. Org Lett. 2014;16:5486. doi: 10.1021/ol502766q.Donets PA, Cramer N. Angew Chem Int Ed. 2015;54:633. doi: 10.1002/anie.201409669.Jackson EP, Montgomery J. J Am Chem Soc. 2015;137:958. doi: 10.1021/ja511778a.
- 23.Molander GA, Shin I. Org Lett. 2012;14:4458. doi: 10.1021/ol301955s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.For other regiodivergent couplings, see reference 19 and the following: (Hyster TK, Dalton DM, Rovis T. Chem Sci. 2015;6:254. doi: 10.1039/c4sc02590c.Moragas T, Cornella J, Martin R. J Am Chem Soc. 2014;136:17702. doi: 10.1021/ja509077a.Xu K, Thieme N, Breit B. Angew Chem Int Ed. 2014;53:7268. doi: 10.1002/anie.201403682.Tani Y, Fujihara T, Terao J, Tsuji Y. J Am Chem Soc. 2014;136:17706. doi: 10.1021/ja512040c.Zhao Y, Weix DJ. J Am Chem Soc. 2014;136:48. doi: 10.1021/ja410704d.Bausch CC, Patman RL, Breit B, Krische MJ. Angew Chem Int Ed. 2011;50:5686. doi: 10.1002/anie.201101496.Donets PA, Cramer N. Angew Chem Int Ed. 2015;54:633. doi: 10.1002/anie.201409669.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.