Abstract
Purpose
The purpose of this study was to develop a radiation therapy (RT) contouring atlas and recommendations for women with postoperative and locally advanced vulvar carcinoma.
Methods and Materials
An international committee of 35 expert gynecologic radiation oncologists completed a survey of the treatment of vulvar carcinoma. An initial set of recommendations for contouring was discussed and generated by consensus. Two cases, 1 locally advanced and 1 postoperative, were contoured by 14 physicians. Contours were compared and analyzed using an expectation-maximization algorithm for simultaneous truth and performance level estimation (STAPLE), and a 95% confidence interval contour was developed. The level of agreement among contours was assessed using a kappa statistic. STAPLE contours underwent full committee editing to generate the final atlas consensus contours.
Results
Analysis of the 14 contours showed substantial agreement, with kappa statistics of 0.69 and 0.64 for cases 1 and 2, respectively. There was high specificity for both cases (≥99%) and only moderate sensitivity of 71.3% and 64.9% for cases 1 and 2, respectively. Expert review and discussion generated consensus recommendations for contouring target volumes and treatment for postoperative and locally advanced vulvar cancer.
Conclusions
These consensus recommendations for contouring and treatment of vulvar cancer identified areas of complexity and controversy. Given the lack of clinical research evidence in vulvar cancer radiation therapy, the committee advocates a conservative and consistent approach using standardized recommendations.
Introduction
Vulvar cancer is a relatively uncommon neoplasm responsible for 5% of gynecologic malignancies (1). There has been an incremental rise over the last 2 decades, including in premenopausal women (2). Up to 95% of these cancers are squamous cell carcinoma (SCC) and occur on the labia majora and other primary sites such as labia minora, clitoris, and perineum (3). Known causes include human papillomavirus infection and lichen sclerosis.
Radiation therapy (RT) has a major role in curative treatment of vulvar cancer patients in both postoperative (4) and preoperative (5) settings. Due to the proximity of sensitive organs at risk (OARs) and steep changes in source-to-skin distance, the vulva and groin may be a challenge to treat with 3-dimensional (3D) RT (6). Intensity modulated RT (IMRT) improves the avoidance of critical structures, while maintaining adequate tumor volume coverage (7). This benefit in the treatment of vulvar carcinoma, especially in difficult cases, has been reported previously (8–10). As in anal cancer, IMRT has rapidly become a standard option in vulvar cancer and is now used in NRG Oncology protocol (Gynecologic Oncology Group [GOG] 0279) (11). Given the significant challenges in treating vulvar cancer with RT, an international committee was formed to address modern approaches. In an attempt to standardize treatment for both postoperative and locally advanced vulvar cancer, the committee agreed to create a consensus atlas and generate treatment recommendations. This paper describes the development of summary points based on committee consensus and presents the summary atlas, which will be listed on the NRG website at www.nrgoncology.org/Resources/Contouring-Atlases.
Methods and Materials
To establish details of the current practice of vulvar and nodal contouring to treat carcinoma of the vulva, a survey was conducted among an international consortium of radiation oncologists, including members of the Radiation Therapy Oncology Group (RTOG) Gynecologic Working Group and selected others with a known interest in gynecologic radiation oncology. In total, 35 physicians completed the survey. The survey revealed most radiation oncologists had treated few patients by using IMRT for vulvar carcinoma; 45% of respondents had treated 1 to 5 vulvar cases in the previous 12 months. The most common year IMRT was started was 2006; 11 of the respondents did not use IMRT for vulvar cancer. Of the respondents, 51% were from the United States, 23% from Australia, 17% from Canada, and 9% from Europe. Areas of clinical controversy were identified, and survey results were discussed at multiple meetings. An initial draft of consensus contouring recommendations was generated. These findings were presented at the 2011 American Society for Radiation Oncology conference (12). The panel focused on external beam RT. Brachytherapy is an excellent modality for delivering high dose and restrict dose to OARs for some vulvar lesions; however, brachytherapy planning and dose delivery were felt to be outside the scope of the panel’s deliberations.
Committee members were invited to contour 2 cases in which clinical findings were described and were given explicit instructions and the initial diagnostic positron emission tomography (PET)/computed tomography (CT) images. The locally advanced case, stage IVa (T1bN3M0), had examination findings and a PET/CT that demonstrated a 3.5-cm right-sided mass involving the labia majora, 2.5 cm from midline, with biopsy examination that revealed SCC. A 7-cm fixed nodal conglomerate in the right groin with smaller nodes extending toward the primary was identified both clinically and using PET/CT. There was no palpable disease in the left groin. PET/CT images demonstrated marked avidity in the vulva and right groin.
The second case was that of a 73-year-old patient who underwent a left vulvectomy and bilateral inguinal dissection revealing stage IIIAi (T1bN1aM0) disease. Pathology examination revealed 2.5- × 2.0-cm grade 3 SCC with 0.5-cm depth of invasion, 4-mm margin, and extensive lymphovascular space invasion (LVSI). Inguinal nodal dissection revealed the right groin had no disease and that the left groin contained 2 of 7 total lymph nodes that harbored metastases. The largest nodal deposit was 4 mm, and there was no extracapsular extension.
Contouring instructions for the 2 cases mandated that physicians contour both the vulvar and nodal regions clinical tumor volume (CTV) as a single structure according to initial consensus on lymph node groups and primary as developed from recommendations from the survey. Digital imaging and communication in medicine (DICOM) files were submitted to the Advanced Technology Consortium for analysis.
A 95% confidence interval contour was developed from 14 contours from each case, using Computerized Environment for Radiation Research. Contours were compared and analyzed using an expectation-maximization algorithm for simultaneous truth and performance level estimation (STAPLE) (13). The level of agreement between contours was assessed by a kappa statistic (14). The conformity indices (mean-to-union volume ratio) were calculated. STAPLE sensitivity and specificity values were generated. The outlined contours underwent expert editing using MIM software (MIM Software, Inc, Cleveland, OH). After multiple reviews by the committee, the 95% consensus CTV contour was felt to be too close to skin and was retracted in select locations to prevent excess skin toxicity for areas deemed low risk. The CTV should include the skin if grossly involved. Additionally, tissue posterior and lateral to femoral vessels was felt to be at low risk, and in most slices, this area was removed from the consensus contour. This was then re-presented to the group and approved. Further refining of this recommendation document continued until all contributing authors were satisfied that it was both comprehensive and safe for adoption by radiation oncologists working in a variety of settings.
Results
Survey results indicated the areas of greatest agreement were inclusion of vulvar, inguinal, and pelvic nodes. Areas of initial controversy included delineation and inclusion of the “skin bridge,” the width of the inguinal contour, the inclusion of skin above the inguinal nodes, and the superior border of the pelvic nodes. After contouring, face-to-face meetings, and multiple discussions, consensus was achieved for these recommendations.
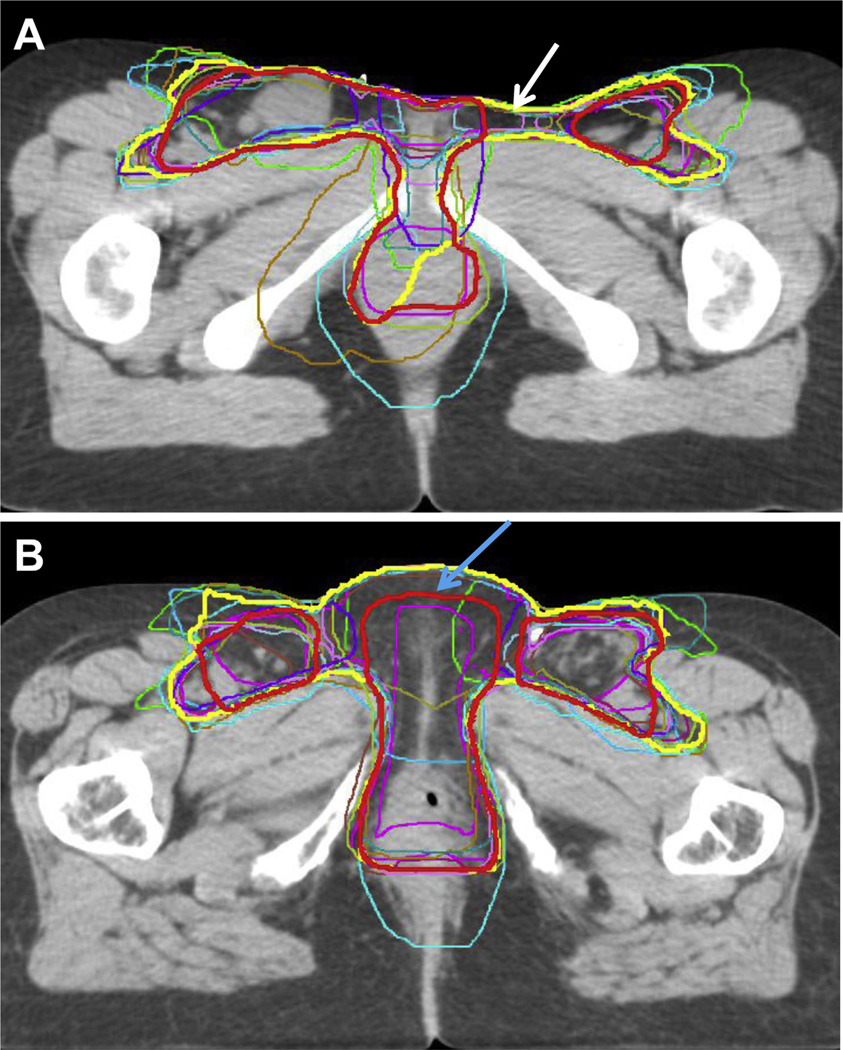
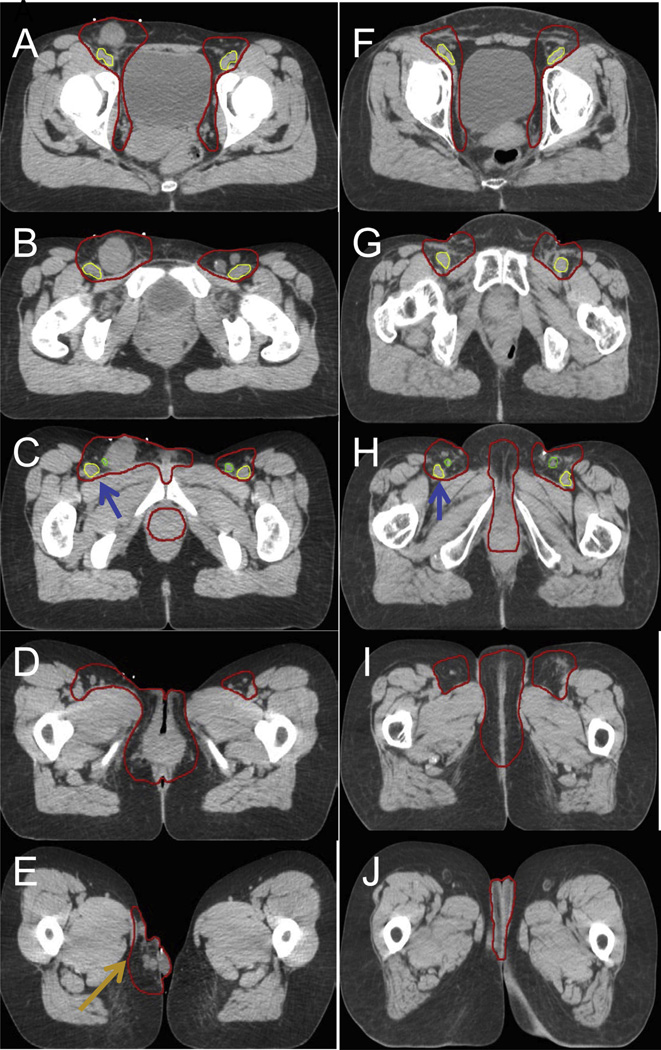
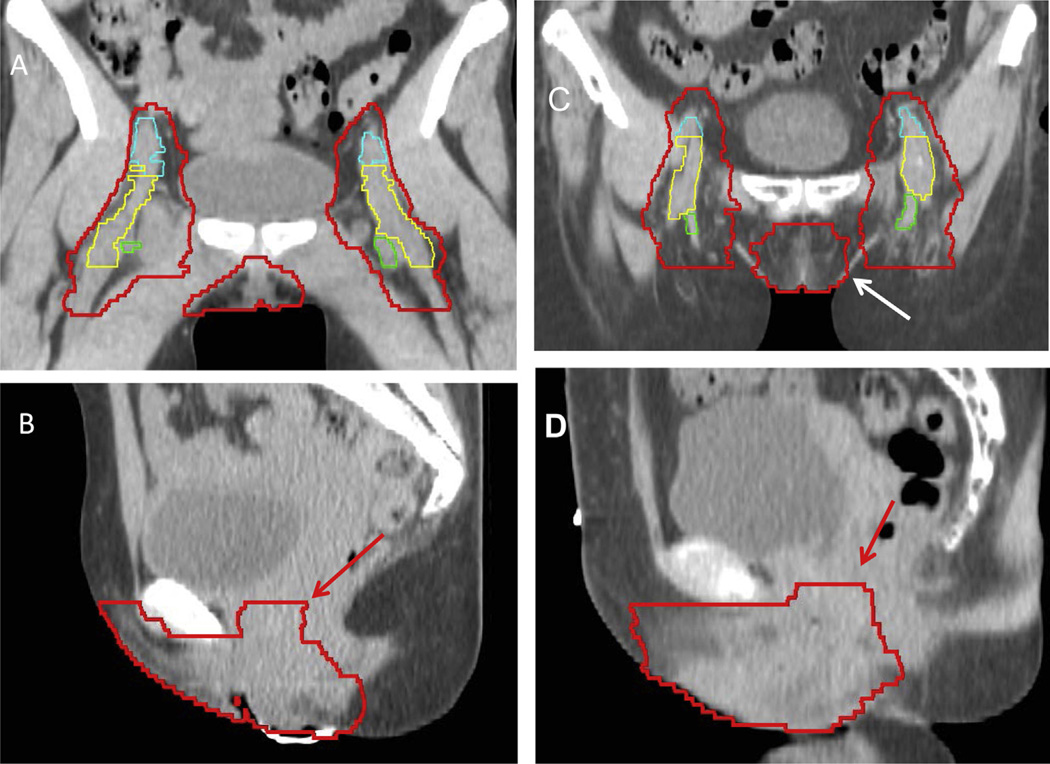
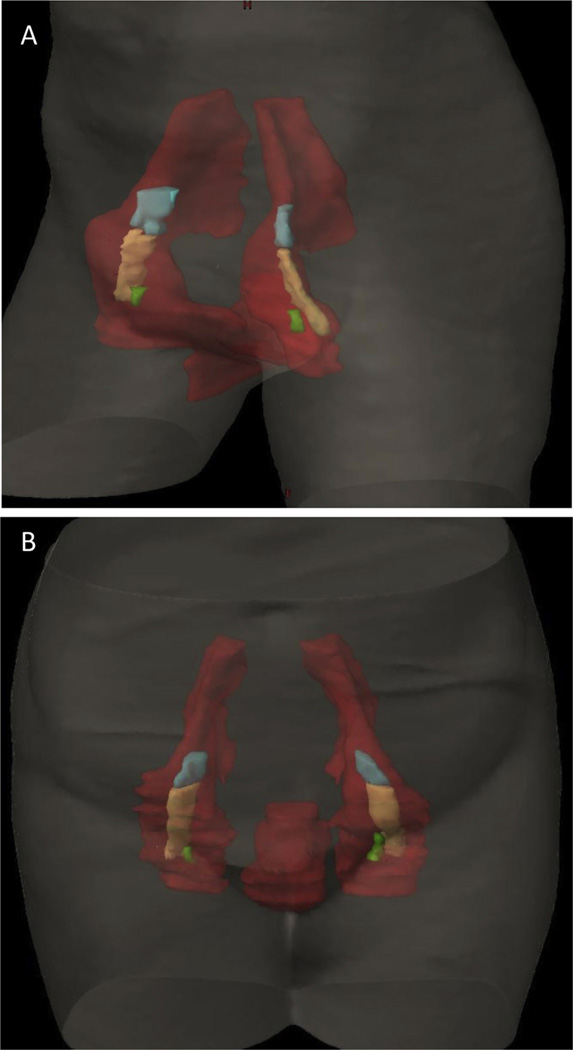
Contouring results of the 14 contours showed a substantial level of agreement, with kappa statistics of 0.69 and 0.64 for cases 1 (locally advanced) and 2 (postoperative), respectively (Table 1, Fig. 1). There was high specificity for both cases (≥99.0%) and only moderate sensitivity of 71.3% and 64.9% for cases 1 and 2, respectively. Thus, the physicians had higher confidence, or agreement, in which structures to exclude compared to which structures to include from the CTV. Specifically, in their contours of cases 1 and 2, some physicians did not include the skin bridge, that is, skin adjacent to an unoperated groin or tissue adjacent to the deep femoral vessels. After expert consensus editing, the 95% confidence intervals and individual contours were re-presented for the locally advanced case and postoperative case (Figs. 2 and 3). The authors appreciated that the 3-dimensional structure of a vulvar CTV is very complex (Fig. 4). Given the rarity of vulvar cancer, the authors urged physicians to exercise great care in contouring this challenging site. Participants agreed upon the following consensus contouring recommendations.
Table 1.
Statistical analysis of contours
| Structure measure | Case 1 (14 contours) | Case 2 (14 contours) |
|---|---|---|
| Sensitivity | 71.3% | 64.8% |
| Specificity | 99.1% | 99.2% |
| Mean volume/minimum/maximum (±SD) (cc) | 688.4/547.1/885.3 (±108.1) | 811.3/327.9/1122.0 (±196.0) |
| STAPLE/intersection/union volume (cc) | 806.8/234.1/1506.0 | 1085.0/158.2/1833.7 |
| Kappa value | 0.69 | 0.64 |
Abbreviation: STAPLE = simultaneous truth and performance level estimation.
Fig. 1.
Consensus contour (yellow), modified consensus contour (red), and individual contours from 14 different physicians for a locally advanced vulvar case (case 1) (A) and postoperative case (case 2) (B). The modified consensus contour was retracted from the space between the vulva and groin (white arrow) and skin surface (blue arrow) when it was believed to be at low risk.
Fig. 2.
(A–E) Axial slices from a locally advanced case, showing modified consensus contour. (F–J) Consensus contour in the postoperative case. Additionally, the space deep to the femoral vessels was not included in the CTV (blue arrows). The locally advanced case had satellite lesions (E orange arrow), an uncommon clinical scenario; hence, the CTV extended inferior to the medial thigh. Abbreviation: CTV = clinical target volume.
Fig. 3.
A coronal (A) and sagittal (B) slice from a locally advanced case and for the postoperative case (C and D) with the modified consensus contour shown. External iliac vessels are shown in cyan (A), femoral vessels in yellow (A), and saphenous vessels in green (A). Evaluation of coronal and sagittal images is essential for accurate delineation of the vulvar and groin CTV. Coronal images can be useful for identifying the lateral extent of the vulva (white arrow), and on the sagittal images, extension into the vagina is specifically included within the CTV (red arrows).
Fig. 4.
Volumetric 3D rendering of the modified consensus contour (red) is shown with external iliac vessels in cyan, femoral vessels in yellow, and saphenous vessels in green, from a locally advanced case (A) and from a postoperative case (B).
Simulation
Patient position for simulation and treatment
Clinical findings should be documented prior to simulation. A “frog leg” position is generally preferred and allows sparing of the skin in the upper inner thigh. In selected patients with difficulty moving into a frog leg position, clinicians may prefer a straight-leg position. Thermoluminescent dosimeters or electronic dosimeters should be used early in treatment to confirm the intended dose is delivered. Thermoluminescent dosimeters should be considered both with and without bolus to fully evaluate skin dose.
Bladder and rectal filling
A previous IMRT atlas discusses contouring of pelvic lymph nodes and recommends using specific bladder and rectal filling protocols (15). In the case of vulvar carcinoma, some clinicians in the group treat patients who have full bladder in an attempt to limit radiation dose to the small bowel, whereas others advocate treating patients who have an empty bladder because of better reproducibility. The committee recommends that integrated target volume (ITV) bladder and rectal contours be generated for locally advanced cases, including those that are deemed inoperable due to vaginal, urethral, and/or anal involvement, or based on tumor size. If the rectum is distended at simulation (eg, >3.5-cm diameter), it is recommended that simulation be repeated after further bowel preparation.
Placement of bolus and wire on scars
Use of bolus should be closely examined to see if it is necessary to cover the entire extent of the primary lesion and to determine whether the entire vulvar surface needs to be covered in the planning target volume (PTV). It is recommended that the patient undergo scanning at the time of simulation with bolus both on and off, with documentation of in vivo dosimetry in the event the bolus needs to be removed during treatment. Alternatively, virtual bolus can also be added and used to guide actual bolus as needed.
Bolus over the groins is not routinely recommended. In postoperative cases, bolus over the groin should be considered if there is extracapsular extension of lymph nodes or skin involvement. If used, bolus should be placed over scars plus a margin of at least 3 cm. In preoperative cases, large or superficially located lymph nodes or skin involvement requires use of bolus. Bolus should be carefully tailored to cover only the specific area of concern. Given uncertainties associated with the delivery of multiple tangential beams, in vivo dosimetry may be used to confirm the intended dose is the dose received and close clinical review during treatment is advised. Some institutions create a false structure in air above the skin to ensure adequate PTV dose coverage. Wires or other radio-opaque material should be used on gross disease and surgical scars.
Locally advanced vulvar cancer
Contouring of the primary vulvar region
Magnetic resonance imaging (MRI) may be useful for delineating the full extent of all disease (gross tumor volume [GTV]) as CT may not document the entirety of gross disease. Clinical examination, including examination under anesthesia, is of paramount importance and anatomic drawings of precise tumor location is encouraged. A GTV should be contoured with careful attention to disease extending beyond the vulva ensuring that the CTV incorporates all areas at risk. The entire vulva should be included in the CTV for all primary lesions; if the GTV extends beyond the vulva, this region plus a 1-cm margin should be encompassed by the CTV, with the understanding that other factors, as discussed below, may impact the specific margins used. Scrutiny of coronal and sagittal views can help identify skin folds that separate the vulva from the skin of the thigh and buttock (Fig. 3). Soft tissues of the thigh can be excluded unless intentionally being treated due to tumor involvement. In some patients with specific features such as satellite lesions, extensive LVSI, or dermal lymphatic invasion, extra margins of skin and/or subcutaneous tissue surrounding the primary lesion may be included in the CTV. If the tumor is seen invading muscle on the MRI or abuts muscle on the CT, a rim of muscle should be included in the CTV. The width of the rim of muscle should depend on the clinical scenario and extent of invasion seen on imaging studies. CTV (or ITV)-to-PTV margins should be 0.7 to 1 cm depending on factors including patient body habitus and stability (16–18). Additionally, margin width may depend on treatment technique (ie, 3D vs IMRT or volumetric arc therapy) and verification method of image guidance.
Lesions invading the vagina
If the primary vulvar lesion involves the vagina (ie, tumor proximal to the hymenal ring), gross disease plus 3 cm should be included in the CTV. If there is any uncertainty as to the proximal extent of the vaginal extension or if there is LVSI, the entire vaginal length should be included in the CTV. MRI can be valuable to assess the extent of the primary tumor and is felt to be the optimal imaging test to evaluate local extent of disease. If the vulvar lesion involves the vagina and if the rectum remains full after 2 attempts at bowel preparation and simulation, then one of a number of strategies must be used to avoid underdosage to targets (eg, a vaginal ITV should be generated by combining vaginal contours on a full-rectum CT and an empty-rectum CT, or fiducial markers should be placed in the vagina to verify that the target is being adequately covered).
Lesions invading the anus or anal canal, bladder, or rectum
If the primary vulvar lesion involves the anus, anal canal, or bladder, gross disease plus at least 2 cm of anorectum (or bladder) is included in the CTV.
Periurethral lesions
If the primary vulvar lesion is periurethral (ie, involving the urethral meatus), gross disease plus at least 2 cm of urethra is included in the CTV. If disease extends into the mid or proximal urethra, the entire urethra and bladder neck should be included in the CTV.
Periclitoral lesions
For clitoral lesions, gross disease plus at least 2 cm is included in the CTV, and in many cases, the CTV should cover the suspensory ligament of the clitoris, which extends to the pubic bone.
Lymph nodes
Definition of lymph node (LN) regions and OAR may be found in Appendix E1 (available online at www.redjournal. org). General recommendations are as follows: include any involved LN regions, including grossly involved LN; and GTV may be defined on either MRI or CT and encompass the entirety of the node(s) involved. Margins for GTV-to-CTV expansion should encompass the entire nodal bed in order to ensure that any extranodal spread (extracapsular extension) is covered; this nodal CTV contouring is described in detail in Appendix E1 (available online at www.redjournal.org). Treat the “echelon above” the highest involved LN; if 1 groin (inguinofemoral region) is involved, treat the other groin as well (due to potential changes in lymphatic flow). Generally, LN coverage for the CTV should include the same LN regions on each side. Admittedly, there are few data for this issue. In highly selected cases, the upper level of the CTV may be at different levels on the left and right sides, but this is not recommended outside of a clinical trial as there is no evidence that it is safe to treat asymmetrically.
LN groups to be included in the CTV for primary vulvar lesions involving the vulva only or vulva and distal vagina, periurethral, or periclitoral
If the primary vulvar lesion involves the distal vagina, the following LN groups should be included in the CTV: bilateral inguinofemoral, bilateral obturator, bilateral internal, and external iliac groups. The distal vagina is defined as that adjacent to the introitus. If the primary tumor involves the proximal half of the posterior vaginal wall, including pre-sacral LNs (from S1–S3) in the CTV should be considered. Admittedly there are only anecdotal data, and the response reflects consensus opinion.
LN groups to be included in the CTV for primary vulvar lesions involving the anus or anal canal
If the primary vulvar lesion involves the anus or anal canal, the following LN groups should be included in the CTV: bilateral inguinofemoral, bilateral obturator, bilateral internal and external iliac nodes, perirectal (including mesorectum), and presacral LNs (from sacral S1–S3).
Postoperative vulvar cancer
Vulvar primary (negative margins)
The CTV should cover the entire operative bed. Selective bolus may be needed. Fiducial markers may be used to identify close or postoperative margin sites for a boost. Adaptive RT or soft tissue image guidance may be needed for cases of leg or vulvar edema.
Vulvar primary (close or positive margins)
Close or positive margins should be well within the CTV, with a margin of approximately 2 cm. Wire placed on scars may be used to identify close or positive margin sites for a boost.
Dose and chemotherapy
Radiation dose and chemotherapy recommendations can be found in Appendix E2 (available online at www.redjournal.org).
Other considerations
Adaptive therapy
Replanning during IMRT treatment should be considered for some patients, especially when tumor shrinkage, vulvar edema, or other developments (eg, lymphocyst formation) during treatment changes the position of either the tumor or an OAR.
Limitations on curative therapy
There is no clear evidence from which to define how advanced vulvar cancer may be where there is still a chance of cure (especially in terms of extent of cephalad lymph nodes). Thaker et al (19) recently documented a 5-year overall survival rate of 43% in patients with gross pelvic nodal involvement, questioning the current utility of the stage IVB designation (19). It is reasonable to offer curative intent RT to patients with involved inguinofemoral LNs, external iliac LNs, internal iliac LNs, or obturator LNs. In selected cases, clinicians may wish to consider curative intent RT in patients with involved common iliac LNs or lower para-aortic LNs; however, there is no evidence to demonstrate that these patients have curable disease, thus it is unknown as to whether the patient would benefit. IMRT may be a valuable technique in curative intent RT.
Discussion
Treatment of vulvar cancer is complex given the rarity of the disease, the sensitivity of the tissues, the irregularities in shape, and the requirements for differential dosing of many different target regions. To date, no consensus statements regarding contouring have been published. The present set of recommendations strives to provide a comprehensive guide for practitioners treating patients who have vulvar carcinoma with radiation. Through a consensus committee, several areas of controversy and complexities were worked through and the final document and atlas generated.
Advances in radiologic imaging have improved the radiation oncologist’s ability to identify disease beyond the clinical examination. The use of diagnostic MRI scanning enables identification of a GTV, whether in the primary vulvar region or in the nodal regions, and the standardized use of CT simulation confirms regions for treating a CTV. Contouring an accurate CTV for patients with vulvar carcinoma is challenging because each case is highly individualized in terms of the site, size, and surgical status of the primary lesion and involved LNs.
We demonstrated a substantial level of interobserver agreement in contours, with high specificity. After the initial set of contours was created, interobserver analysis was performed, and a group consensus of contours was created, a comprehensive process of discussion was held in order to develop this statement further for all to come to an agreement on the exact definitions, regions to include in each region, and margins and doses required. This entailed multiple online conferences (Webex, Milpitas, CA) with all expert participants commenting on the final demonstrated contours, which will be posted on the NRG website (www.nrgoncology.org/Resources/Contouring-Atlases).
There is a paucity of dose-volume data for vulvar RT and no randomized series on radiation dose or volume. Recommendations were made with regard to the dose of radiation recommended for both postoperative and locally advanced cases. In the postoperative setting, the GOG 37 randomized trial compared 45 to 50 Gy to surgical resection of the pelvic and inguinal nodes. This and other trials standardized the use of 45 to 50 Gy as has been recommended (20). For treatment of the vulva in the postoperative setting, 3 series recommend incorporating radiation for close margins (21–23).
For locally advanced cases, GOG trials 101 and 205 continue to escalate dose grossly involved regions, although these trials were conducted in the era before vulvar IMRT, and therefore, further dose escalation is being tested in ongoing trials (GOG 279) (5, 24). In the most recently completed GOG study of 58 patients with T3 or T4 tumors (GOG 205), the clinical complete response rate was 64% after a dose of 57.6 Gy (24). Correspondingly, in the penultimate GOG study (GOG 101), the dose was 10 Gy less, and the clinical complete response rate was 46%, indicating that these doses are on the steep portion of the dose-response curve (5). Therefore, the committee recommended a dose escalation of 60 to 70 Gy, as this was the consensus based on the committee’s current clinical practice. RT may be used to effectively manage the inguinal region. Katz et al (25) reported on 227 patients treated at MD Anderson Cancer Center. In that retrospective review, 29 clinically node-negative inguinal node patients, 60% of whom had a T3 or T4 primary tumor, were treated with RT. Inguinal nodal control was equivalent when RT alone was compared to surgery or combined surgery and RT. The optimal dose to unresected gross nodal disease was felt to be 60 to 70 Gy. Hence, the consensus statements above and those outlined in the appendix are based on expert opinion with attention to extant published reports. In the 2 cases contoured, substantial agreement was found for the vulvar CTV in the locally advanced case and the postoperative case.
These recommendations provide an overview for contouring the most important clinical scenarios. However, other challenges may face the treatment team, and clinical experience and judgement are necessary. Additional reports of patterns of failure following definitive RT for vulvar cancer and detailed anatomic studies should provide information to help refine these target volumes. Given the many uncertainties and lack of evidence in RT, a conservative approach was deliberately taken, such that the guidelines produced may be used by a variety of clinicians. Given the low incidence of vulvar cancer and the complexities involved in treatment, optimal results may be seen in patients treated by physicians with specific experience and a dedicated multidisciplinary team. The guidelines are not intended to be prescriptive for every possible patient scenario. As always, clinicians must tailor treatment to suit the individual needs of their patients.
Conclusions
These consensus recommendations for contouring and treating vulvar cancer identified areas of complexity and controversy. Given the lack of clinical research evidence, the committee advocates a conservative and consistent approach using standardized recommendations. Due to vulvar anatomy and proximity of sensitive OARs, highly conformal techniques such as IMRT are valuable in vulvar cancer.
Supplementary Material
Summary.
Consensus recommendations for contouring and treating vulvar carcinoma were generated. Substantial agreement (kappa statistic: 0.64–0.69) was observed. There was high specificity for both of the cases (≥99%) and only moderate sensitivity of 64% to 71%. Areas of complexity and controversy were identified. The authors recommend separate orthogonal views for contouring a vulvar clinical target volume (axial, sagittal, and coronal) and a conservative and consistent approach using standardized recommendations to achieve optimal clinical results.
Acknowledgments
C.M.Y. has received honoraria from Varian Medical Systems, Inc. W.R.B. has received grant support from National Institutes of Health/National Cancer Institute. S.B. is an Associate Editor with the International Journal of Radiation Oncology Biology Physics.
The authors thank Michelle Denney for editorial assistance with the manuscript.
Footnotes
The findings of this study were presented at the 53rd Annual Meeting of the American Society for Radiation Oncology, Miami Beach, FL, October 2–6, 2011.
Conflict of interest: All other authors report no conflicts of interest.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.National Cancer Institute Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: Vulvar cancer. [Accessed February 27, 2016]; Available at: http://seer.cancer.gov/statfacts/html/vulva.html.
- 2.Hampl M, Deckers-Figiel S, Hampl JA, et al. New aspects of vulvar cancer: Changes in localization and age of onset. Gynecol Oncol. 2008;109:340–345. doi: 10.1016/j.ygyno.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Klemba A, Kukwa W, Semczuk A, et al. Vulvar cancer as a target for molecular medicine. Front Biosci. 2011;3:136–144. doi: 10.2741/s139. [DOI] [PubMed] [Google Scholar]
- 4.Kunos C, Simpkins F, Gibbons H, et al. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: A randomized controlled trial. Obstet Gynecol. 2009;114:537–546. doi: 10.1097/AOG.0b013e3181b12f99. [DOI] [PubMed] [Google Scholar]
- 5.Moore DH, Thomas GM, Montana GS, et al. Preoperative chemoradiation for advanced vulvar cancer: A phase II study of the Gynecologic Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:79–85. doi: 10.1016/s0360-3016(98)00193-x. [DOI] [PubMed] [Google Scholar]
- 6.Carter JS, Downs LS., Jr Vulvar and vaginal cancer. Obstet Gynecol Clin North Am. 2012;39:213–231. doi: 10.1016/j.ogc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 7.D’Souza DP, Rumble RB, Fyles A, et al. for the IMRT Indications Expert Panel. Intensity-modulated radiotherapy in the treatment of gynaecological cancers. Clin Oncol (R Coll Radiol) 2012;24:499–507. doi: 10.1016/j.clon.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Beriwal S, Shukla G, Shinde A, et al. Preoperative intensity modulated radiation therapy and chemotherapy for locally advanced vulvar carcinoma: Analysis of pattern of relapse. Int J Radiat Oncol Biol Phys. 2013;85:1269–1274. doi: 10.1016/j.ijrobp.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Beriwal S, Coon D, Heron DE, et al. Preoperative intensity-modulated radiotherapy and chemotherapy for locally advanced vulvar carcinoma. Gynecol Oncol. 2008;109:291–295. doi: 10.1016/j.ygyno.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Beriwal S, Heron DE, Kim H, et al. Intensity-modulated radiotherapy for the treatment of vulvar carcinoma: A comparative dosimetric study with early clinical outcome. Int J Radiat Oncol Biol Phys. 2006;64:1395–1400. doi: 10.1016/j.ijrobp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2012;84:e2–e28. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King B, Barkati M, Fyles A, et al. Current practice of IMRT to treat carcinoma of the vulva—Results of an international survey. Int J Radiat Oncol Biol Phys. 2011;81:s4–s46. [Google Scholar]
- 13.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): An algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23:903–921. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 15.Small W, Jr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:428–434. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CJ, Chin YY, Leung SW, et al. Topographic distribution of inguinal lymph node metastasis: Significance in determination of treatment margin for elective inguinal lymph nodes irradiation of low pelvic tumors. Int J Radiat Oncol Biol Phys. 1996;35:133–136. doi: 10.1016/s0360-3016(96)85021-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim CH, Olson AC, Kim H, et al. Contouring inguinal and femoral nodes; how much margin is needed around the vessels? Pract Radiat Oncol. 2012;2:274–278. doi: 10.1016/j.prro.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Taylor A, Rockall AG, Reznek RH, et al. Mapping pelvic lymph nodes: Guidelines for delineation in intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1604–1612. doi: 10.1016/j.ijrobp.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 19.Thaker NG, Klopp AH, Jhingran A, et al. Survival outcomes for patients with stage IVB vulvar cancer with grossly positive pelvic lymph nodes: Time to reconsider the FIGO staging system? Gynecol Oncol. 2015;136:269–273. doi: 10.1016/j.ygyno.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homesley H, Bundy B, Sedlis A, et al. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68:733–740. [PubMed] [Google Scholar]
- 21.Viswanathan AN, Pinto AP, Schultz D, et al. Relationship of margin status and radiation dose to recurrence in post-operative vulvar carcinoma. Gynecol Oncol. 2013;130:545–549. doi: 10.1016/j.ygyno.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Heaps JM, Fu YS, Montz FJ, et al. Surgical-pathologic variables predictive of local recurrence in squamous cell carcinoma of the vulva. Gynecol Oncol. 1990;38:309–314. doi: 10.1016/0090-8258(90)90064-r. [DOI] [PubMed] [Google Scholar]
- 23.Faul CM, Mirmow D, Huang Q, et al. Adjuvant radiation for vulvar carcinoma: Improved local control. Int J Radiat Oncol Biol Phys. 1997;38:381–389. doi: 10.1016/s0360-3016(97)82500-x. [DOI] [PubMed] [Google Scholar]
- 24.Moore DH, Ali S, Koh WJ, et al. A phase II trial of radiation therapy and weekly Cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: A gynecologic oncology group study. Gynecol Oncol. 2012;124:529–533. doi: 10.1016/j.ygyno.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Katz A, Eifel PJ, Jhingran A, et al. The role of RT in preventing regional recurrences of SCCA of the vulva. Int J Radiat Oncol Biol Phys. 2003;57:409–418. doi: 10.1016/s0360-3016(03)00591-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.