Figure 3. ΔNp63α promotes MM1 proteasome-dependent degradation.
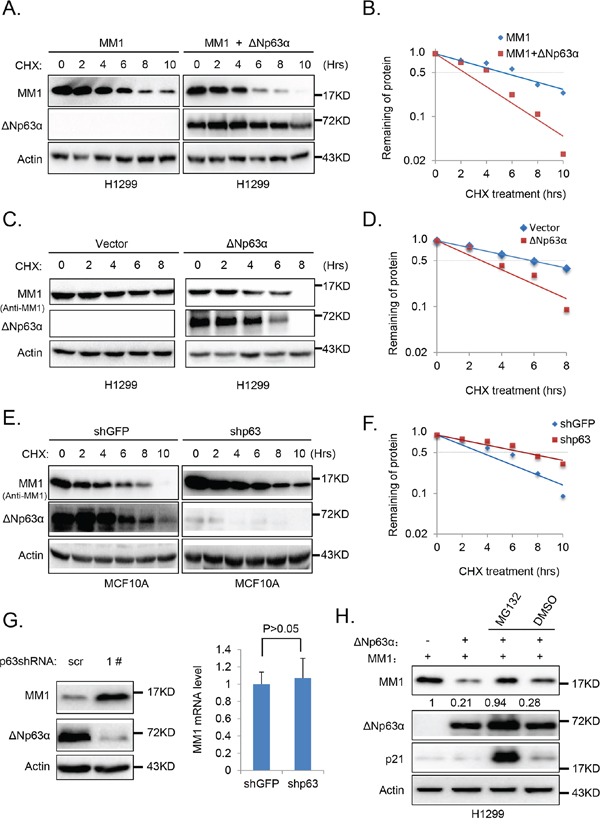
A. Ectopic expression of ΔNp63α shortens half-life of MM1 protein. H1299 cells were co-transfected with FLAG-MM1 together with vector control (FLAG-MM1) or ΔNp63α (FLAG-MM1 + ΔNp63α). 24 hours post transfection, cells were treated with 100 μg/ml cycloheximide (CHX) for indicated time intervals. Cells were harvested and subjected to IB analysis. B. Quantification results of panel A. Percentages of FLAG-MM1 protein were normalized with Actin, and that for the time point of 0 hour was set as 100. C. and D. Ectopic expression of ΔNp63α shortens half-life of endogenous MM1 protein. H1299 cells transfected with vector control or ΔNp63α were subjected to measurement of MM1 protein half-life, using methods abovementioned. E. and F. Depletion of ΔNp63α extends half-life of endogenous MM1 protein. MCF10A cells were infected with lentiviral-based shRNA specific for p63 (1#) to knockdown endogenous ΔNp63α, using lentiviral-based shRNA specific for GFP as a control. 48 hours post infection, cells were lysed and subjected to measurement of MM1 protein half-life. G. Knockdown of endogenous p63 up-regulates endogenous MM1. MCF10A cells were infected with lentiviral-based scrambled or p63 (1#) shRNAs. 72 hours post infection, cells were subjected to IB or Q-PCR analysis. The Q-PCR data were presented as as means±s.e. to measure mRNA levels of MM1, from three independent experiments performed in triplicate. H. Inhibition of proteasome abrogates ΔNp63α-induced down-regulation of MM1. H1299 cells were co-transfected with MM1 plasmid plus vector control or ΔNp63α plasmid. 24 hours post transfection, cells were treated with MG132 or DMSO for additional 6 hours. Cells were then harvested and subjected to immunoblot analysis. Intensities of MM1 bands were normalized with Actin bands.
