Abstract
Fibroblast growth factor (FGF) signaling is essential for normal and cancer biology. Mammalian FGF family members participate in multiple signaling pathways by binding to heparan sulfate and FGF receptors (FGFR) with varying affinities. FGF2 is the prototype member of the FGF family and interacts with its receptor to mediate receptor dimerization, phosphorylation, and activation of signaling pathways, such as Ras-MAPK and PI3K pathways. Excessive mitogenic signaling through the FGF/FGFR axis may induce carcinogenic effects by promoting cancer progression and increasing the angiogenic potential, which can lead to metastatic tumor phenotypes. Dysregulated FGF/FGFR signaling is associated with aggressive cancer phenotypes, enhanced chemotherapy resistance and poor clinical outcomes. In vitro experimental settings have indicated that extracellular FGF2 affects proliferation, drug sensitivity, and apoptosis of cancer cells. Therapeutically targeting FGF2 and FGFR has been extensively assessed in multiple preclinical studies and numerous drugs and treatment options have been tested in clinical trials. Diagnostic assays are used to quantify FGF2, FGFRs, and downstream signaling molecules to better select a target patient population for higher efficacy of cancer therapies. This review focuses on the prognostic significance of FGF2 in cancer with emphasis on therapeutic intervention strategies for solid and hematological malignancies.
Keywords: bFGF, FGF2, diagnosis, prognosis, malignancy
INTRODUCTION
The fibroblast growth factor (FGF) family consists of 23 FGF signaling polypeptides that function as potent mitogens [1–3]. FGFs exert broad mitogenic activity by stimulating the growth of fibroblasts, endothelial, and cancer cells. The family plays important roles in embryonic development, maintenance of adult organ systems, tissue regeneration, wound repair, and hematopoiesis. FGF2, also known as basic FGF (bFGF), is the prototypical and most studied member of the FGF superfamily. FGF2 is an important regulator of cell growth and differentiation under physiological and pathological conditions [1–3]. Previous studies have suggested a role of FGF2 as a prognostic marker for different types of malignancies. This review summarizes the biology of FGF signaling by demonstrating biological roles of FGF2 in regards to pathogenesis and prognosis of solid and hematological tumors with a special focus on clinical development of FGF2 inhibitors in the era of personalized medicine.
FGF2 isoforms, receptors, binding partners, and signaling
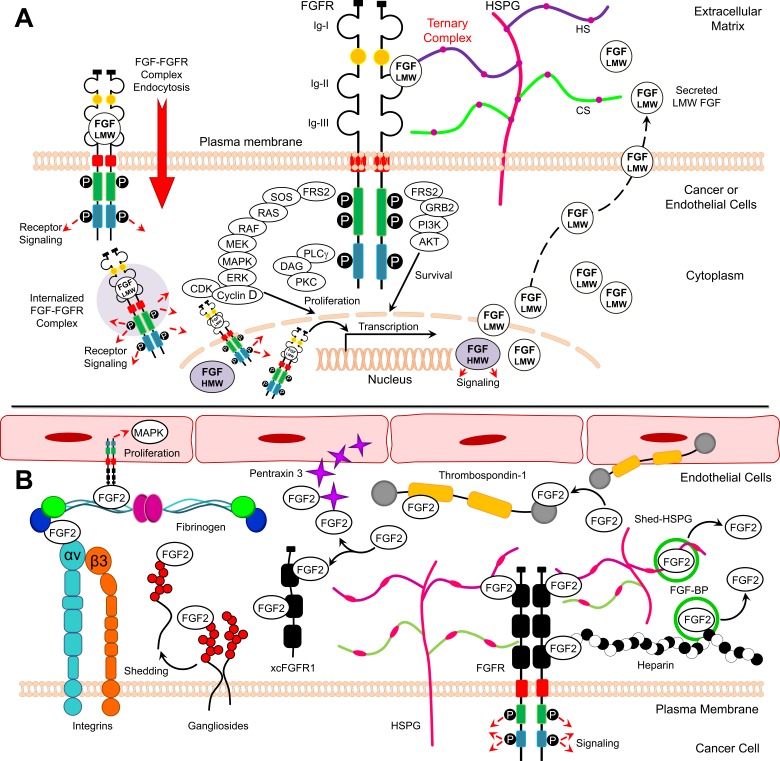
FGF2 exists in low and high molecular weight isoforms, which are translated from a single common mRNA through alternative translation-initiation codons [4]. Low molecular weight (LMW) FGF2 is an 18 kDa protein translated from a conventional AUG start codon [5]. LMW FGF2 is found in the cytoplasm and nucleus and can be also secreted by target cells [6]. In order to initiate signaling, LMW FGF2 interacts with cell surface heparan sulfate proteoglycans (HSPGs) and fibroblast growth factor receptor (FGFR) in a ternary complex consisting of FGF2, FGFR, and HSPG resulting in activation of downstream signaling pathways, including Ras, Raf, MAPK and ERK (Figure 1A) [7]. High molecular weight (HMW) FGF2 isoforms (22-, 22.5-, 24-, and 34-kDa) are produced through translation initiation at CUG sites upstream and in frame of the AUG codon. HMW FGF2 localizes to the nucleus and signals independently of FGFRs [8]. Similar to HMW FGF2, LMW FGF2 can also function in the cytosol and nucleus of cells through endocytosis of activated FGF-FGFR complexes [9]. FGFR1 and FGF2 have been shown to co-localize in the nuclear matrix, where together they may co-activate transcription and thus control cell proliferation (Figure 1A) [10, 11].
Figure 1. Model for FGF2/FGFR signaling and FGF2 binding partners.
A. Structure of activated FGFR and downstream signaling B. FGF2 soluble and membranous binding partners.
Five FGFRs have been identified, four of which (1-4) are highly conserved single-pass transmembrane tyrosine kinase receptors [12]. The extracellular regions of these receptors contain three immunoglobulin (Ig)-like domains (designated as IgI, IgII, and IgIII) linked to the cytoplasmic domain via a transmembrane α-helix (Figure 1A). FGFRs 1-3 can undergo alternative splicing during gene expression, and the IgIII domain is composed of an invariant IgIIIa exon alternatively spliced to IgIIIb or IgIIIc. The expression of IgIIIb and IgIIIc is important in defining FGF signaling specificity. While FGF1 binds to all FGFRs, FGF2 binds to FGFR1 (IIIb), FGFR1 (IIIc), FGFR2 (IIIc), and FGFR4 [2]. It has been reported that LMW FGF2 predominantly binds to FGFR1 (IIIc) and weakly to the other FGFRs [5, 13]. The cytoplasmic domain of FGFRs 1-4 contains a juxtamembrane split kinase domain, which contains tyrosine kinase motifs and a C-terminal tail [12]. Although FGFR5 lacks intracellular tyrosine kinase domain, this receptor can bind to multiple FGF ligands acting as a negative regulator of signaling [14].
FGF2 utilizes HSPGs, such as syndecans (SDC), as binding partners to stabilize the FGF-FGFR interaction and enhance resistance to proteolysis [15, 16]. FGF2 cannot activate FGFRs in cells lacking heparan sulfate [17]. After the binding of FGF and HSPG to FGFR to form a ternary FGF:FGFR:HSPG complex, FGFRs dimerize leading to conformational changes in FGFR structure and subsequent intermolecular transphosphorylation of multiple cytoplasmic tyrosine residues (Figure 1A) [12, 18]. FGFR transmits extracellular signals to two main intracellular substrates, which are phospholipase C-γ1 (PLC-γ1) (also known as FRS1) and FGFR substrate 2 (also known as FRS2) (Figure 1A). The phosphorylation of FGFR1 tyrosine residues creates binding sites for SH2 domain of PLC-γ required for phosphorylation and activation of PLC-γ. Conversely, FRS2 constitutively associates with the juxtamembrane region of the FGFR. The phosphorylation of FRS2 is essential for activation of the Ras-mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase-Akt (PI3K-Akt) signaling pathways in cancer and endothelial cells (Figure 1A) [12, 19].
FGF2 also interacts with immobilized molecules bound to extracellular matrix (ECM), including cell membrane receptors and soluble molecules (Table 1, Figure 1B). The complex interactions between FGF2 and these molecules control bioavailability, stability, and concentration of FGF2 in the microenvironment [20]. FGF2 can tightly bind HSPG in ECM and is only released through the action of FGF-binding protein (FGF-BP), which is a critical controller of FGF bioavailability (Table 1, Figure 1B). In addition, the binding of FGF to heparin, released HSPG, or cell surface-bound HSPG also regulate FGF bioavailability and the interactions with FGFRs (Table 1, Figure 1B). Conversely, thrombospondin-1 (TSP-1) and pentraxin 3 (PTX3) prevent the interaction of FGF2 with cell surface HSPGs and FGFRs. Similarly; xcFGFR1 (a soluble form of the extracellular portion of FGFR1) binds FGF2 and prevents FGF2/FGFR interaction (Table 1, Figure 1B).
Table 1. FGF2 binding partners and associated proteins.
| Associated Protein | Association | Level of Interaction | Cell Type | Pathway | Ref |
|---|---|---|---|---|---|
| FGFR(s) | FGF2 binds to extracellular domain of FGFRs which causes receptor dimerization and autophosphorylation of tyrosine kinase residues on cytoplasmic domain | Cell membrane | Endothelial cells, cancer cells, fibroblasts | FGFR | [12, 101] |
| HSPG | FGF2 binds with low affinity to heparan sulfate chains of HSPG. This interaction can activate intracellular signaling, promote FGF2 internalization, or by presenting FGF2 to FGFR in proper conformation. HSPG also act as reservoirs for FGF2 which protect them from degradation | Cell membrane | Cancer, endothelial cells | FGFR | [102–104] |
| αvβ3 integrin | FGF2 binds to αvβ3 integrin leads to assembly of focal adhesion plaques | Cell membrane | Endothelial cells | FAK | [105] |
| Gangliosides | Gangliosides bind FGF2 via Neu-Ac residues and acts as coreceptors | Cell membrane | Endothelial cells | FGFR | [106] |
| Free gangliosides | Exogenous gangliosides affect the angiogenic activity of FGF2. Exogenous gangliosides act as FGF2 antagonists when added to endothelial cell cultures | Soluble or associated with ECM | Cancer, endothelial cells | FGFR | [107] |
| Heparin | Short heparin chains bind FGF2, thus interfering with mitogenic signaling through activation of FGFR, relatively longer chains are expected to induce the adverse effect of potentiating the mitogenic signaling | Soluble or associated with ECM | Tumor Cells | FGFR | [108] |
| xcFGFR1 | A soluble form of extracellular domain of FGFR1 which binds to FGF2 which leads to suppression of FGF2/FGFR1 interaction | Soluble | Endothelial ECM | FGFR | [107] |
| TSP | TSP-1 and TSP-2 regulate angiogenesis through binding and sequestration of FGF2 | Soluble or associated with ECM | Cancer, endothelial cells | FGFR | [109, 110] |
| PTX3 | PTX3 prevents FGF2 binding to FGFRs on endothelial cells, leading to inhibition cell proliferation and motility. PTX3 suppressed neovascularization triggered by FGF2 in the chick embryo chorioallantoic membrane | Soluble or associated with ECM | Endothelial cells | FGFR | [111] |
| Fibrinogen/ fibrin | Binding of FGF2 to fibrinogen or fibrin provides protection of FGF2 from proteolytic degradation. Fibrinogen potentiates FGF2-stimulated proliferation of endothelial cells. Fibrinogen promotes growth of cancer cells through interaction with FGF2 | Soluble or associated with ECM | Endothelial cells, cancer cells | FGFR | [112, 113] |
| α2M | α2M induces FGF2 expression in embryonic stem cells | Nucleus | Embryonic stem cells | ERK1/2, PI3K, | [114] |
| PDGF | FGF2 stimulates PDGFR-α and β expression in endothelial cells | Nucleus | Endothelial cells | Transcriptional | [115] |
| PF4 | PF4 inhibits FGF2-induced proliferation of endothelial cells. PF-4 binds to FGF2 and inhibits FGF2 dimerization, binding to FGFRs, and internalization | Soluble or associated with ECM | Endothelial cells | ERK | [116, 117] |
| uPA | FGF2 upregulates uPA receptor and stimulates uPA production by endothelial cells | Nucleus | Endothelial cells | Transcriptional | [118] |
| CXCL13 | CXCL13 chemokine displaces FGF2 binding to endothelial cells, inhibits FGF2 homodimerization, and induces the formation of CXCL13-FGF2 heterodimers | Soluble or associated with ECM | Endothelial cells | FGFR | [119] |
| IL-6 | FGF2 induces IL-6 release in human pancreatic periacinar myofibroblasts. Overexpression of FGF2 (24-kDa isoform) upregulates IL-6 transcription in NIH-3T3 cells. FGF2 is downstream effector of IL-6-induced angiogenic activity in cancer cells | Soluble or associated with ECM/Nucleus | Fibroblasts, cancer cells | IL-6, ERK1/2 and p38 MAP kinases | [120–122] |
| E-cadherin | FGF2 downregulates E-cadherin expression through the activation of PI3K/Akt/mTOR and MAPK signaling, and upregulates Slug and ZEB1 in human ovarian cancer cells | Nucleus | Cancer, endothelial cells | PI3K/Akt and ERK | [123] |
| Bcl-2 | FGF2 downregulates Bcl-2 and promotes apoptosis in human breast cancer cells | Nucleus | Cancer cells | Transcriptional | [124] |
| Aquaporin3 | Aquaporin3 is required for FGF2-induced cell migration in human breast cancer cells | Soluble or associated with ECM | Cancer cells | Trans-epithelial fluid transport | [125] |
| Translokin | Translokin interacts specifically with LMW FGF2. Inhibiting Translokin expression by RNA interference reduces the translocation of FGF2 | Soluble or associated with ECM | Fibroblasts | FGF2 trafficking | [126] |
| Thrombin | Thrombin cleaves HMW FGF2 into a LMW FGF2-like form that stimulates endothelial cell migration and proliferation | Soluble or associated with ECM | Endothelial, cancer cells | Endothelial cell migration | [127] |
| FGF-binding protein | FGF-BP 1 binds FGF2 and enhances FGF2-dependent proliferation of NIH-3T3 fibroblasts and FGF2-induced extracellular signal-regulated kinase 2 phosphorylation | Soluble or associated with ECM | Squamous, epithelial cells, fibroblasts | FGFR | [128] |
α2M: α2-macroglobulin; ECM: extracellular matrix; FAK: focal adhesion kinase; HSPG: heparan sulfate proteoglycan; IL-6: interleukin-6; PDGF: platelet-derived growth factor; PF4: platelet factor 4; PTX3: p; TSP: thrombospondin-2; uPA: urokinase-type plasminogen activator
Gangliosides are glycosphingolipids bound to cell membranes regulating growth of a wide variety of normal cells by binding to FGFs. Gangliosides are highly synthesized in metastatic tumors and are known to shed into the ECM. Integrins are transmembrane receptors that regulate the response to soluble FGF2 in endothelial cells. The interaction between αvβ3 integrin and FGF2 promotes endothelial cell proliferation by activating the MAPK pathway [21]. In addition, the binding of fibrinogen to FGF2 requires αvβ3 integrin to promote endothelial cell proliferation (Table 1, Figure 1B). These findings indicate that FGF2 bioactivity and interaction with FGFR is highly regulated by a complex network of interactions with various FGF2 binding partners.
ROLES OF FGF2 IN TUMOR PROGRESSION
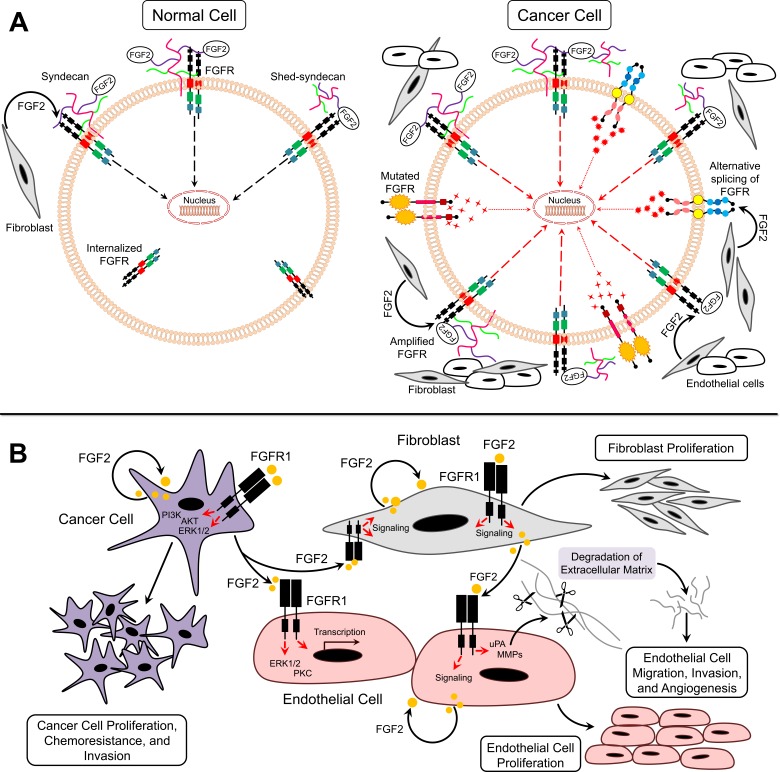
Accumulating evidence suggests that FGF2/FGFR signaling is involved in several biological functions, such as embryonic development, tissue regeneration, wound repair, and normal hematopoiesis [1–3]. Expression of FGF2 and FGFRs in normal cells is highly regulated, and termination of FGF2 signaling is achieved through receptor internalization (Figure 2A) [1–3]. However, FGF2/FGFR signaling in cancer cells is dysregulated, which may contribute to the pathogenesis of many types of cancer (Figure 2A). Several studies have shown that FGF2 is a key tumor-promoting factor in the tumor microenvironment. The following section reviews current knowledge of the molecular pathways associated with FGF2 signaling in cancer, which represents a critical step for the implementation of strategies toward the development of personalized cancer therapy.
Figure 2. FGF2/FGFR signaling in cancer.
A. Model for FGF2/FGFR function under normal and cancer conditions B. Paracrine and autocrine signaling of FGF2 in tumor microenvironment.
Deregulation of FGFR signaling
FGFR amplification and/or upregulation occur in cancer due to deregulated transcription or chromosomal amplification (Figure 2A) [22]. The upregulation of FGFR1 induces cellular transformation of non-transformed cells [23]. FGFR2 upregulation is associated with poor prognosis in patients of multiple cancer types [24]. Furthermore, FGFR2 amplification has been shown to be accompanied with C-terminal exon abrogation, which regulates receptor internalization [25]. Impaired termination of FGFR signaling leads to continuous receptor activation [22]. In addition, mutation of FGFR can also render it insensitive to endocytosis by maintaining its expression at the cell surface [22]. A number of germline activating point mutations of FGFRs have been identified in human cancers and are associated with poor survival and chemoresistance [26, 27]. Mutations in the extracellular domain of FGFRs facilitate ligand binding, while mutations in transmembrane and kinase domains lead to constitutive activation of receptors (Figure 2A) [22]. Furthermore, alternative splicing of the third Ig-like domain could promote tumorigenesis. Paracrine signaling typically occurs through FGFR-IIIb that is expressed on mesenchymal cells and -IIIc expressed on epithelial cells [22]. In cancer models, however, the switch from FGFR IIIb to FGFR IIIc by alternative splicing results in autocrine activation of the receptor (Figure 2A) [28]. For example, FGFR2-IIIb to IIIc switch is related to increased invasiveness in bladder and prostate cancers [29, 30]. In addition, FGFR1-IIIc has been upregulated in pancreatic cancer where it is regarded as a strong oncogene [31].
FGF2 as pro-angiogenic agent
FGF2 is an extremely potent pro-angiogenic growth factor. FGF2 exerts its effects on endothelial cells via a paracrine mode after being released by tumor and stromal cells or through mobilization from ECM (Figure 2B) [32]. In addition, FGF2 plays autocrine roles in endothelial cells [32]. It has been reported that endothelial cells predominantly express FGFR1 and to some extent FGFR2 [33, 34]. Activation of these receptors by FGF2 leads to endothelial cell proliferation, migration, protease production, and angiogenesis. Furthermore, the full mitogenic and chemotactic responses of FGF2 in endothelial cells require activation of ERK1/2 and protein kinase C (PKC) signaling pathways [35]. FGF2 upregulates plasmin-plasminogen activator (uPA) and matrix metalloproteinase (MMP) production in endothelial cells eventually leading to ECM degradation and angiogenesis [36]. In addition, the response of endothelial cells to FGF2 can be regulated by integrins [21]. Immobilized FGF2 binds to αvβ3 integrin causing endothelial cell adhesion, migration, proliferation, and morphogenesis (Figure 2B) [37]. There is also considerable cross-talk between FGF and vascular endothelial growth factor (VEGF) signaling, whereby FGF-induced signaling promotes resistance to VEGF receptor signaling for blocking of the VEGF [38]. Moreover, transient expression of FGF2 in endothelial cells control the expression of genes implicated in cell cycle, differentiation, adhesion, and cell survival [39]. Taken together, these data suggest an important role of FGF2 in promoting endothelial cell angiogenesis (Figure 2B).
FGF2 as mitogen for tumor cells
Although FGF2 levels are elevated in several human cancers, FGF2 levels do not always correlate with microvessel density [40]. For example, in a study conducted by Kuwahara et al, majority of pancreatic ductal carcinomas were positive for VEGF and FGF2 [41]. A significant correlation was observed between VEGF expression and MVD but not between FGF2 and MVD [41]. However, in a study conducted by Garcia de la Torre et al, FGF2 expression was high in primary parathyroid hyperplasia (PPH) and FGF2 scores and MVD were significantly correlated [42]. Therefore, FGF2 may contribute to cancer progression through alternative mechanisms involve acting directly on tumor cells [19]. Mutations in genes encoding FGFs and FGFRs deregulate FGFR signaling [43, 44]. However, no activating mutations have been reported as yet for FGF2 [44]. FGF2-induced activation of FGFR signaling and subsequent activation of PI3K/Akt and ERK1/2 signaling pathways in cancer cells [19, 45]. FGF2 contributes to tumor progression through enhanced expression and/or release from tumor, endothelial, or stromal cells as well as release from local reservoirs in the ECM (Figure 2B) [43]. Secretion of proteases leads to release of the sequestered FGF2 [43]. Therefore, FGF2 functions in an autocrine or paracrine manner in cancer cells (Figure 2B).
FGF2 as mitogen for stromal cells
Tumor progression to metastatic stage is promoted by fibroblasts in tumor stroma through secretion of multiple paracrine factors [46, 47]. FGF2 secreted by stromal fibroblasts induces tumor cell proliferation via FGFR paracrine signaling (Figures 2A, 2B) [48]. In addition, fibroblasts within tumor stroma can be activated by FGF2 secreted from endothelial and tumor cells (Figure 2B) [49]. Activated fibroblasts also produce proteases, such as MMPs that degrade ECM and promote secretion of growth factors including FGF2 in the tumor microenvironment [50].
Dysregulated downstream FGF2/FGFR signaling pathway in hematological tumors
Growing evidence supports a role of FGF2 in hematopoiesis starting at early stages of development through adulthood. In early stages of development, FGF2 has an important role in the proliferation of hemangioblasts, which are common progenitors of hematopoietic and endothelial cells [51] that play a central role in hematopoietic and angiogenic differentiation [52]. In addition, FGF2 plays a role in self-renewal, cell survival, and cell adhesion of human embryonic stem cells [53]. In adult hematopoiesis, FGF2 induces proliferation of stromal cells of bone marrow [54]. FGF2 also induces the production of interleukin-6 (IL-6) [55] and counteracts the suppressive effect of transforming growth factor beta (TGF-β) on myeloid progenitor cells [56]. Myeloid precursor cells can be induced by FGF2 to give rise to erythroid progenitors [57]. In the absence of FGF2, myeloid progenitors generate cells of the neutrophil-granulocyte lineage upon FGF2 induction [58].
Neoplastic cells that define each hematological tumor are descendants of a specific lineage of the hematopoietic process. The involvement of FGF2 in various stages of hematopoiesis suggests that its dysregulation can result in hematological cancers [59]. FGFRs are expressed on all cell types of haematopoietic origin, and deregulation of FGFR gene expression or mutation has been observed in haematologic malignancies [59]. The ability of FGF2 to induce stem cell proliferation and differentiation implies that FGF2 is involved in very early stages of hematopoiesis. It has been reported that lymphoma cell lines express FGF2 and FGFRs and release FGF2 into culture media [45].
Putative mechanisms of FGF2 in Hodgkin Lymphoma
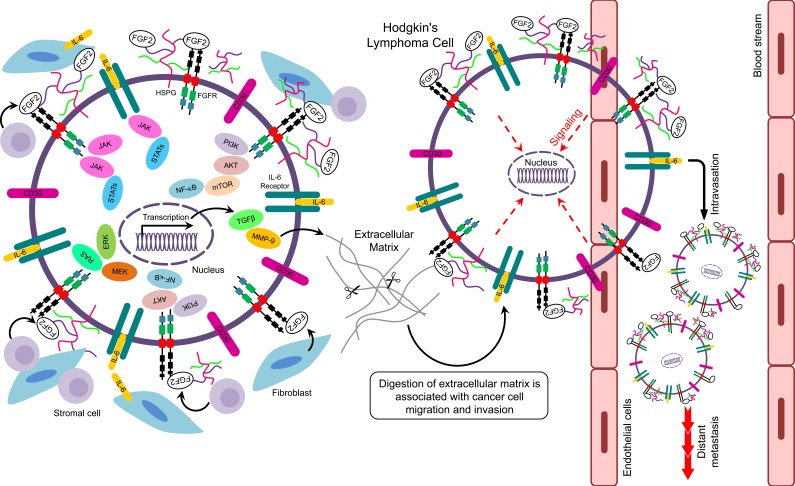
Hodgkin lymphoma (HL) is a rare B-cell malignant neoplasm characterized by a paucity of malignant Hodgkin and Reed-Sternberg cells (HRS cells) embedded within an inflammatory infiltrate [60]. FGF2 causes aberrant signaling activities in HRS cells involving SDC1, NF-κB, IL-6, Ras/ERK, and JAK/STAT as shown below.
SDC1 regulates bioavailability, dimerization, and interaction of FGF2 with FGFRs [20]. Witzig and colleagues reported that SDC1 was expressed in the bone marrow of patients with plasma cell proliferative disorders [61]. In line with this, increased expression of FGF2 and SDC1 was also reported in HL cell lines [68]. Overall, findings suggest that increased expression of FGF2, FGFR, and SCD1 is associated with poor prognosis and chemoresistance [62].
Activation of the NF-κB pathway is a well-established mechanism for protection of tumor cells from apoptosis [63, 64]. In HL, NF-κB is constitutively activated and represents an important step for the proliferation of neoplastic HRS cells (Figure 3) [65]. Epstein-Barr Virus (EBV) is a risk factor for HL [74]. In EBV-positive (EBV+) HL, the EBV oncoprotein, latent membrane protein-1 (LMP1), has been implicated in the activation of NF-κB signaling leading to enhanced B-cell survival [65]. Alternatively, NF-κB may serve as a transcription factor for the FGF2 gene regulating expression and release of FGF2 by LMP1 [66, 67]. This process could be of clinical importance for determining the relationship between EBV status and FGF2 levels in HL patients (Figure 3). IL-1β, which is expressed in subsets of cells in the HL tumor microenvironment, activates PI3K signaling pathway to upregulate FGF2 production through NF-κB [67].
Figure 3. Putative signaling pathways related to FGF2 in Hodgkin's lymphoma.
The interleukin-6 (IL-6) signaling pathway has also been implicated in tumor progression [68, 69]. In multiple myeloma, stromal-derived IL-6 stimulates FGF2 expression in tumor cells, which in turn stimulates the secretion of IL-6 [70]. In addition, IL-6 and FGF2 together can enhance proliferation of myeloma cells [71]. In HL, IL-6 and its receptors are expressed by HRS cells (Figure 3) [72, 73]. Moreover, IL-6 is upregulated in serum of HL patients resulting in poor prognosis [74]. FGF2 upregulates IL-6 gene expression in the fibroblast NIH-3T3 cell line [75]. Moreover, in a basal cell carcinoma cell line, IL-6 mediates upregulation of FGF2 through activation of JAK/STAT3 and PI3K/Akt pathways which are aberrantly activated in HL [65]. Therefore, IL-6 and FGF2 may be involved in paracrine and autocrine interactions to promote chemoresistance in relapsed/refractory HL.
Components of the Ras/ERK pathway are aberrantly expressed in malignancies and associated with chemoresistance [76]. MEK/ERK signaling pathway is essential for proliferation and survival of neoplastic HRS cells (Figure 3) [77]. FGF2 induces MEK signaling to upregulate anti-apoptotic proteins and enhance chemoresistance [78]. In addition, FGF2 mediates chemoresistance to doxorubicin in endothelial cells by inhibiting the pro-apoptotic protein ASK1, which is a member of the MEKK family [79].
The Janus kinase-signal transducer and activator of transcription (JAK/STAT) is a frequently altered pathway in the pathogenesis of HL [80]. The JAK/STAT pathway has been implicated in FGF2-induced chemoresistance in human cancer cells [81]. The JAK2 inhibitor lestaurtinib can overcome JAK/STAT-induced drug resistance in refractory HL cell lines [82]. Therefore, FGF2 may promote chemoresistance by deregulation of JAK/STAT signaling in HRS cells of relapsed and refractory HL patients (Figure 3).
The above studies demonstrated key downstream signaling pathways in HL, including cell growth, survival, and angiogenesis. It seems clear that the main pathways contributing to HL are regulated, at least in part, by FGF2 (Figure 3). Therefore, clinical trials using agents that target FGF pathway may be promising for treatment of HL.
CLINICAL PROGNOSTIC VALUE AND FUNCTIONAL SIGNIFICANCE OF FGF2 IN SOLID AND HEMATOLOGICAL TUMORS
Several studies have compared FGF2 serum levels in cancer patients to those in healthy volunteers (Tables 2 and 3). FGF2 expression in sera quantified by ELISA was strongly increased in cancer patients compared to healthy donors. Significant correlations between serum FGF2 levels and tumor stage, size, and metastasis were reported in endometrial, colorectal, esophageal, head and neck, liver, renal, and testicular cancers. However, no significant correlation was observed between increased serum FGF2 levels and tumor grade in bladder, breast, lung, and prostate cancers. In all leukemia and lymphoma studies, there was no correlation between increased serum levels of FGF2 and microvessel density or stage of the disease. However, high serum FGF2 levels were significantly correlated with tumor bulkiness in Non-Hodgkin's lymphoma (NHL). Therefore, the levels of serum FGF2 may have prognostic significance in these cancers, and quantification of FGF2 may provide an indirect, non-invasive way to monitor patients with high risk of relapse from solid and hematological tumors (Tables 2 and 3).
Table 2. Studies evaluating FGF2 as a prognostic biomarker in cancer patients with solid tumors.
| Cancer Type | Patient Number | Specimen Type | Cancer Subtype | Method | FGF2 Expression Pattern | Prognosis | Associated with | Ref |
|---|---|---|---|---|---|---|---|---|
| Bladder Cancer | 32 | Resection | − | RT-PCR | Elevated in high stage vs. low stage patients (p= 0.001) | − | High stage, local relapse | [129] |
| 82 vs. 20 controls | Untreated serum | Noninvasive, invasive | ELISA | (↑) FGF2 levels vs. controls (p=0.083); (↑) FGF2 in noninvasive vs. invasive (P= 0.013) | No significant difference | No correlation with tumor grade, patient age | [130] | |
| Breast Cancer | 64 | Cytosolic extract | Primary | ELISA | (↑) >2 fold FGF2 levels in tumor vs. controls and non-malignant mastectomy specimens (p < 0.01) | − | − | [131] |
| 79 | Sections | − | IHC | (↑) FGF2 in 38% neoplastic cells, 37% in stromal cells | − | Recurrence, aggressiveness | [132] | |
| 136 vs. 65 controls | Diagnostic biopsy, | ER-(+) and (−); PR (+) and (−) | IHC | In 84% tumors FGF2 staining limited to cytoplasm, 16% tumors limited to both cytoplasm and nuclei vs. positivity limited to the cell nuclei of the basal layer of mammary ducts in normal mammary epithelium | − | Not correlated with clinical, pathological and biological characteristics | [133] | |
| Serum | ELISA | (↑) in tumors vs. healthy controls (p < 0.001) | ||||||
| 111 | Treated resection | ER (+/−)- PR (+/−) | IHC | 70% tumors positive, 30% tumors showed strong staining, (↑) with histological grade (p < 0.05) | (↓) OS in FGF2 (+)vs. (−) group in negative nodal status sub-group | Negatively correlated with histological grading (p < 0.05) | [134] | |
| 149, 14 non-neoplastic, 7 controls | Resection | Primary | ELISA | (↑) ~3 fold FGF2 in tumors vs. non-neoplastic tissues (p< 0.0001); (↑) ~12 fold FGF2 in tumors vs. normal control tissues (p= 0.0003) | No significant difference | No correlation between FGF2 and MVD | [135] | |
| 97 vs. 46 controls | Untreated nipple aspirate | DCIS and invasive | ELISA | (↑) 11 fold FGF2 in cancer patients vs. controls (p < 0.0001) | − | No correlation with tumor stage, size, nodal spread | [136] | |
| 148 | Untreated resection | Triple negative | IHC | 13% tumors positive vs. normal breast tissue | No significant difference | Basal type cancer | [137] | |
| Colorectal Cancer | 124, 26 polyp patients, vs. 55 controls | Plasma | Primary | ELISA | (↑) 1.8 fold in tumor vs. normal controls (p=0.0004), (↓) 0.6 fold in disease-free patients at follow up vs. pre-operative (p= 0.0004) | − | Metastasis | [138] |
| 35 obstructing carcinoma, 34 non-obstructing | Untreated resection | Obstructing and non-obstructing | IHC | (↑) 1.7 fold FGF2+ inflammatory cell in obstructing vs. non-obstructing carcinoma (p=0.018); no difference in FGF2 between obstructing vs. non-obstructing carcinoma | − | Hsp47 and stromal myofibroblast fibrosis | [139] | |
| Endometrial Cancer | 134 (type I=70 and type II=64) vs. 64 controls | Untreated serum | Type I and II | ELISA | (↑) ~10 and 20 fold FGF2 in type I and type II, respectively vs. healthy controls | (↓) OS and DFS: in type I with high vs. low FGF2 levels and type II with high vs. low FGF2 levels | Advanced tumor stages | [140] |
| Esophageal Cancer | 70 vs. 20 controls | Untreated, treated tissues | ESCC | IHC, WB | Positive expression in tumors vs. absent in normal tissues | (↓) 0.3 fold 2 yr RFS FGF2 expression ((P = 0.005) | Local recurrence, reduced RFS | [141] |
| Glioma | 21 | Untreated resection | Astrocytoma, Glioblastoma | IHC | 87% tumors positive for FGF2 expression vs. absent in normal tissues | − | Degree of malignancy | [142] |
| 61 | Resection | Astrocytoma | IHC | 44% tumors had strong FGF2 expression, stronger staining in higher grades than lower grades (p < 0.05) | (↓) survival in tumors with strong vs. weak staining | High grade tumors | [143] | |
| Head and Neck | 66 vs. 18 controls | Resection | SCC | IHC, ELISA | (↑) ~ 12 fold FGF2 expression in tumors vs. control (P < or = 0.05) | − | Early stage disease | [144] |
| Liver Cancer | 88 | Untreated serum | − | IHC and ELISA | (↑) high serum FGF2 levels | (↓) 0.5 fold months DFS in patients with high (>10.8 pg/mL ) vs. low (<10.8 pg/mL) FGF2 level | Tumor size, invasion, advanced stage, platelet count | [145] |
| 16 | Untreated resection | − | IHC, RT-PCR, WB | Positive expression in hepatoma vs. absent in non-cancerous liver cells | − | − | [146] | |
| Lung Cancer | 106 vs. 17 controls | Serum | ACA, SCC, SCLC | ELISA | (↑) 2.5 fold serum FGF2 levels in tumors vs. normal controls (p< 0.05) | (↑) OS in SCLC patients with high (> 5.4 pg/ml) vs. low (< 5.4 pg/ml) FGF2 | − | [147] |
| 103 | Untreated serum | SCLC | ELISA | 25% patients had FGF2 ≥17 pg/ml | (↓) 7.5 and 0.5 fold 1 yr, 2 yr survival in high ( ≥17 pg/ml) vs. low ( < 17 pg/ml) FGF2 (p = 0.0026) | Poor OS | [148] | |
| 184 vs. 100 controls | Untreated serum | SCLC, NSCLC | ELISA | (↑) FGF2 levels NSCLC median 4.2 pg/ml; SCLC median 1.8 pg/ml; | (↓) ~0.5 mo. survival in high (>3.4 pg/ml) vs. low (<3.4 pg/ml) FGF2 (p= 0.023) | No correlation with clinicopathological parameters | [149] | |
| 40 vs. 22 controls | Untreated serum | NSCLC | ELISA | (↑) 1.6 fold FGF2 levels in tumors vs. controls (p=0.01) | (↓) ~0.5 mo. survival in high (>11.21 pg/ml) vs. low (<11.21 pg/ml) FGF2 | No correlation with stage, TSP-2 concentration | [150] | |
| Melanoma | 76, 43 nevi, 10 dysplastic controls | Biopsy | NM, SSM | IHC | Strong cytoplasmic expression in malignant vs. nuclear staining in benign nevi. | − | Stromal localization | [151] |
| Oral Squamous Cell Carcinoma | 61 | Untreated biopsy | SCC | IHC | Positive FGF2 expression in cancer cells | (↓) ~0.5 fold survival in FGF2 (+) vs. (−) expression (p < 0.01) | Poor differentiation, mode of invasion, lymph node metastasis | [152] |
| Osteosarcoma | 80 | Surgical and biopsy | Intramedullary | IHC | 57.5% tumors strong positive with cytoplasmic and epithelium FGF2 expression | (↓) ~0.5 fold mo. OS in (+) vs. (−) FGF2 (p< 0.006) | MVD | [153] |
| Ovarian Cancer | 117 tumors; 54 benign, 42 normal ovaries | Untreated serum | − | Fluorokine MAP multiplex kits | (↑) 1.6 fold FGF2 levels in malignant tumors vs. controls | No significant difference | PDGF-AA (p<0.001) | [154] |
| 39 vs. 11 controls | Untreated surgical, serum | Serous endometrioid clear cell mixed Braner | RT-PCR | FGF2 gene expression strong in malignant vs. weak detection in control | _ | _ | [155] | |
| IHC | Strong positive in tumors vs. weak staining in normal control | |||||||
| ELISA | (↑) in tumors vs. controls (p=0.04) | |||||||
| Pancreatic Cancer | 78 vs. 16 controls | Surgical | − | Northern blot | (↑) 9-fold in tumors vs. normal controls (p<0.01) | (↓) ~ 0.6 fold months OS in positive vs. negative FGF2 (P < 0.001) | Advanced tumor stage | [156] |
| IHC | Intense staining in cytoplasm and nucleus of cancer cells vs. weak cytoplasmic staining in normal controls | |||||||
| 46 | TMA blocks | Ductal ACA | IF | Cytoplasmic FGF2 expression in tumors vs. nuclear expression in myofibroblasts; (↓) nuclear FGF2 staining in tumors vs. positive in stromal cells (35%) (P < 0.0001) | _ | _ | [157] | |
| Prostate Cancer | 55 vs. 32 benign controls | Untreated serum | − | ELISA | (↑) 2-fold FGF2 in tumors vs. control (P < 0.0007) | − | No correlation with clinical stage, Gleason grade | [158] |
| 47 (36 patients + 11 benign) vs. 23 controls | Serum | − | ELISA | (↑) 5 fold FGF2 levels in tumors vs. control (P= 0.0002) | − | High PSA levels (>100 ng/ml) | [159] | |
| Tissue sections | IHC | strong cytoplasmic expression in carcinoma cells vs. negative benign epithelia | ||||||
| 31 vs. 11 controls | Resection | − | ELISA | (↑) 2.5 fold FGF2 levels in tumors vs. control (P < 0.005) | _ | No correlation with Gleason score, pathological stage | [160] | |
| IHC | Strong stromal and endothelial staining (not epithelial) | |||||||
| Renal Cancer | 206 vs. 10 benign controls | Untreated serum | − | ELISA | (↑) >3 fold FGF2 in tumors vs. benign controls (P=0.03) | (↓) OS in high (>3.0 pg/ml) vs. low FGF2 (<3.0pg/ml) | Tumor stage, tumor grade | [161] |
| Testicular Cancer | 21 vs. 22 control | Serum, tumor biopsy | − | ELISA | (↑) ~ 7.3 fold serum FGF2 levels in tumors vs. control (P < 0.001), positive expression in all tumor biopsies | − | Tumor stage | [162] |
| Thyroid Cancer | 35 vs. 26 controls | Untreated serum | Papillary carcinomas | ELISA | (↑) ~ 2 fold serum FGF2 levels in tumors vs. controls (p < 0.05) | − | − | [163] |
Table 3. Studies evaluating FGF2 as a prognostic biomarker in cancer patients with hematological tumors.
| Cancer Type | Patient Sample Number | Specimen Type | Method | FGF2 Expression Pattern | Prognosis/Associated with | Ref |
|---|---|---|---|---|---|---|
| Acute Lymphoblastic Leukemia (ALL) | 28 vs. 11 controls | Untreated, treated plasma | ELISA | (↑) ~1.4 fold plasma FGF2 levels in tumors vs. normal controls | − | [164] |
| 22 pediatric patients vs. 39 controls | Untreated urine | ELISA | (↑) ~8 fold urine FGF2 levels in tumors vs. normal controls (p< 0.0001) | − | [165] | |
| Acute Myeloid Leukemia (AML) | 113 vs. 11 controls | Untreated, treated plasma | ELISA | (↑) 1.2 fold plasma FGF2 levels in tumors vs. normal controls | − | [164] |
| 81 vs. 18 controls | Untreated BM biopsy | IHC | (↑) 1.6 fold FGF2 levels in tumors vs. normal controls (p=0.04) | No significant correlation between FGF2 and MVD | [166] | |
| Chronic Lymphocytic Leukemia (CLL) | 39 vs. 11 controls | Treated, untreated peripheral blood- plasma | ELISA | (↑) FGF2 levels in 54% tumors vs. normal range in healthy controls | − | [167] |
| 155 vs. 11 controls | Untreated, treated plasma | ELISA | (↑) ~9 fold plasma FGF2 levels in tumors vs. normal controls | − | [164] | |
| 14 vs. 58 controls | Urine | ELISA | (↑) 2 fold FGF2 levels in tumors vs. controls ( P= 0.0001) | − | [168] | |
| 36 vs. 15 controls | Peripheral blood (cell lysates and plasma) | ELISA | (↑) ~64 fold FGF2 levels in tumors with high risk vs. normal controls ( P< 0.0001) | No significant correlation between FGF2 and factors other than stage of disease | [169] | |
| Chronic Myelogenous Leukemia (CML) | 16 vs. 11 controls | Treated, untreated peripheral blood- plasma | ELISA | (↑) FGF2 levels in 44% tumors vs. normal range in healthy controls | − | [167] |
| 53 vs. 11 controls | Untreated, treated plasma | ELISA | (↑) 1.6 fold plasma FGF2 levels in tumors vs. normal controls | − | [164] | |
| Hairy Cell Leukemia (HCL) | 7 vs. 7 controls | Treated, untreated serum and BM aspirates | ELISA | Serum- (↑) ~37 fold FGF2 levels in tumors vs. absent in controls (p< 0.05); BM aspirate: (↑) 16 fold FGF2 levels in tumors vs. absent in controls (p< 0.001) | − | [170] |
| Hodgkin's Lymphoma | 39 | Lymph nodes tissue | IHC | 85% tumors positive | − | [73] |
| 67 | TMA (NS) | RT-PCR | (↑) 246 fold FGF2 levels in PO tumors vs. normal lymph node controls (↑) 10 fold FGF2 levels in GO tumors vs. normal lymph node controls | − | [62] | |
| IHC | Strong positive staining in PO patients than GO patients | |||||
| 37 | Untreated, treated serum | ELISA | FGF2 levels in tumors were normal (p= 0.075) | No significant change in FGF2 levels relative to pre-therapy values | [171] | |
| Multiple Myeloma | 18 vs. 4 controls | BM aspirates | RT-PCR | (↑) FGF2 expression in tumors (13.5 pg/mL) vs. absent in controls (P= 0.02) | − | [70] |
| 44 and 12 anemia patients | Plasma cells | ELISA | (↑) 6.7 fold FGF2 levels in active MM patients vs. non-active ones (p < 0.01) | No significant correlation between FGF2 and BM neovascularization | [172] | |
| 56 vs. 20 controls | Untreated, treated serum | ELISA | (↑) FGF2 levels in tumors vs. controls (p < 0.001) (↓) 0.3 fold FGF2 levels in treated patients with CR vs. untreated patients (p<0.001) | Significant correlation between FGF2, VEGF, HGF, and B2M | [173] | |
| Non-Hodgkin's Lymphoma | 58 untreated, 19 treated, 11 controls | Untreated, treated serum | ELISA | (↑)~2 fold serum FGF2 levels in tumors vs. controls (p < 0.001)- No correlation between FGF2 at diagnosis and after treatment | Correlated with bulky disease | [174] |
| 39 | Biopsy | IHC | Positive expression in 23.1% tumors | (↓) 0.5 and 0.4 fold months OS (p=0.033) and PFS (p=0.003), respectively, in FGF2 positive vs. negative tumors; -correlated with bulky disease | [175] | |
| 27 | BM biopsy | IHC | 7 % positive FGF2 in tumors | − | [176] | |
| 65 | Untreated, treated serum | ELISA | (↑) Untreated FGF2 in tumors vs. controls (p< 0.001) - no significant change in FGF2 relative to untreated sample values | − | [171] |
BM: bone marrow; B2M: Beta-2 microglobulin; CR: complete remission; ELISA: the enzyme-linked immunosorbent assay; GO: good outcome; HGF- hepatocyte growth factor; IHC- immunohistochemistry; MM: multiple myeloma; MVD: microvessel density; NHL- Non-Hodgkin Lymphoma; NS: nodular sclerosis; OS: overall survival; PFS: progression free survival; Poor outcome; TMA: tissue microarray; VEGF: vascular endothelial growth factor.
FGF2 expression in cancer surgical sections has been evaluated using immunohistochemical, Western blot, and qRT-PCR techniques (Tables 2 and 3). Up-to-date diagnostics and antibodies that allow detection and precise quantification of FGF2 are listed in Table 4. Immunohistochemical and immunofluorescence studies have shown that FGF2 staining is heterogeneous and significantly increased in malignant tissues compared to benign or normal tissues (Tables 2 and 3). The differential expression and localization of FGF2 was also studied in different cancers. For example, FGF2 expression is generally limited to the cytoplasm of breast cancer tissues, while it is exclusively expressed in the nuclei of normal mammary tissues. Similarly, FGF2 is strongly expressed in the cytoplasm of malignant melanocytes and prostate cancer tissues, while it is almost entirely restricted to the nuclei of benign cells. In pancreatic cancer cells, FGF2 staining is intense in both the cytoplasm and nucleus, while it is weak in normal control tissues. Different expression and localization of FGF2 suggests that FGF2 and different members of FGFR may have different functions and signaling in various cancers. Moreover, FGF2 expression was elevated in tumor stroma, including inflammatory cells, myofibroblasts, and endothelial cells in colorectal, pancreatic, and prostate carcinomas, respectively (Table 2). These findings suggest that FGF2 can modulate tumor progression by activating signaling pathways in cancer-associated fibroblasts, endothelial cells, and cancer cells. In addition, fibroblasts that are abundant in the stroma of carcinomas at advanced stages of disease can mediate resistance to treatment via FGF2 secretion [83]. Additional studies on tissue sections have revealed that high FGF2 intratumoral levels are associated with advanced tumor stages of bladder, glioma, head and neck, liver, and prostate cancers (Table 2).
Table 4. In vitro diagnostics (IVD) and research use only (RUO) detection methods for FGF2.
| Diagnostic Type | Source (host) | Reactivity | Manufacturer | Market Status | Applications | Ref |
|---|---|---|---|---|---|---|
| Antibody (Clone FGF288) | Mouse | Unique synthetic peptide of FGF2 coupled to keyhole limpet hemocyanin | Biogenex (CA, USA) | Class I IVD | IHC | * |
| Antibody (Clone 3D9) |
Mouse | Recombinant fragment corresponding to amino acids 10-155 human FGF2 | Novus Biologicals (CO, USA); Enzo Life Sciences (NY, USA) | RUO | WB, IF, IHC | ** |
| Antibody (Clone 10043) |
Mouse | Biotin conjugated; detects human FGF2 in ELISA | Novus Biologicals (CO, USA); R&D Systems (MN, USA) | RUO | ELISA | [177] |
| Antibody (Clone 10060) |
Mouse | Recognizes human FGF2 | Novus Biologicals (CO, USA) | RUO | ELISA | [177, 178] |
| Antibody (Clone 2H5G2C1) |
Mouse | Purified recombinant fragment of human FGF2 | Thermo Scientific (MA, USA); Sigma-Aldrich (MO, USA) | RUO | WB, IHC | ** |
| Antibody (Clone FB-8) |
Mouse | Recombinant full length human FGF2 | Novus Biologicals (CO, USA); Thermo Scientific (MA, USA) | RUO | WB, ELISA, RIA | [179] |
| Antibody (Clone MC-GF1) |
Mouse | Recombinant full length human FGF2 | Novus Biologicals (CO, USA); LifeSpan Biosciences (WA, USA) | RUO | WB, ELISA, IHC | [180] |
| Antibody (Clone AS24) |
Mouse | Human FGF2 | Santa Cruz (TX, USA); Thermo Scientific (MA, USA) | RUO | IHC, ELISA | ** |
| Antibody (Clone AS25) |
Mouse | Biotin Conjugated; Human FGF2 | Abcam (UK); Santa Cruz (TX, USA) | RUO | ELISA | ** |
| Antibody (Clone F-343) |
Mouse | Full length native human FGF2 | Abcam (UK); Thermo Scientific (MA, USA) | RUO | ELISA, WB | ** |
| Antibody (Clone F-474) |
Mouse | Full length native human FGF2 | Abnova (Taiwan); Abcam (UK) | RUO | ELISA | [181] |
| Antibody (Clone F-74) |
Mouse | Full length native human FGF2 | Abnova (Taiwan); Abcam (UK) | RUO | ELISA | ** |
| Antibody (Clone JKFb-1) |
Mouse | Recombinant human FGF2 | Novus Biologicals (CO, USA); | RUO | ELISA | ** |
| Antibody (Clone bFM-1) |
Mouse | Purified bovine brain FGF2 | EMD Millipore (MA, USA) | RUO | RIA | [182, 183] |
| Antibody (Clone bFM-2) |
Mouse | Purified bovine brain FGF2 | EMD Millipore (MA, USA) | RUO | WB, IHC, RIA | [184, 185] |
| Antibody (Clone 17F4.1) |
Mouse | Recombinant human FGF2 | EMD Millipore (MA, USA) | RUO | Neutralizing | ** |
| Antibody (Clone EP1735) |
Rabbit | Synthetic human FGF2 | Abcam (UK); OriGene (MD, USA) | RUO | WB, IP, FCM, ELISA | ** |
| Antibody (Clone MM0276-6D38) |
Mouse | Recombinant human FGF2 | Novus Biologicals (CO, USA); Abcam (UK) | RUO | WB | ** |
| Antibody (Clone NYR-hFGF-b) |
Mouse | Full length human FGF2 | Abcam (UK) | RUO | WB, IP, ELISA | ** |
| Antibody (Clone 0.T.50) |
Mouse | Full length native bovine brain FGF2 | Abcam (UK) | RUO | Neutralizing | [186] |
information taken by contacting company's technical department
information taken from company's website
ELISA: enzyme-linked immunosorbent assay; FCM: flow cytometry; IF: immunofluorescence; IHC: immunohistochemistry; IP: immunoprecipitation; IVD: in vitro diagnostic; RIA: radioimmunoassay; RUO: research use only; WB: Western blot
There is an urgent need for the identification of novel prognostic biomarkers to improve treatment of poor outcome cancer patients. It is worth noting that in the vast majority of studies, high serum and intratumoral FGF2 levels were associated with reduced cancer patient survival. In addition, high intratumoral and serum levels of FGF2 were associated with relapse and/or recurrence in various cancers such as bladder, breast, esophageal cancers and HL (Tables 2 and 3). In spite of a few contradictory finding, FGF2 is considered a significant tumor biomarker and a potential therapeutic target. Ongoing and future clinical trials are warranted to determine whether FGF2 could be incorporated in cancer prognosis and whether FGF targeting therapies have a favorable effect on cancer recurrence and mortality.
TARGETING THE FGF2/FGFR PATHWAY IN CANCER
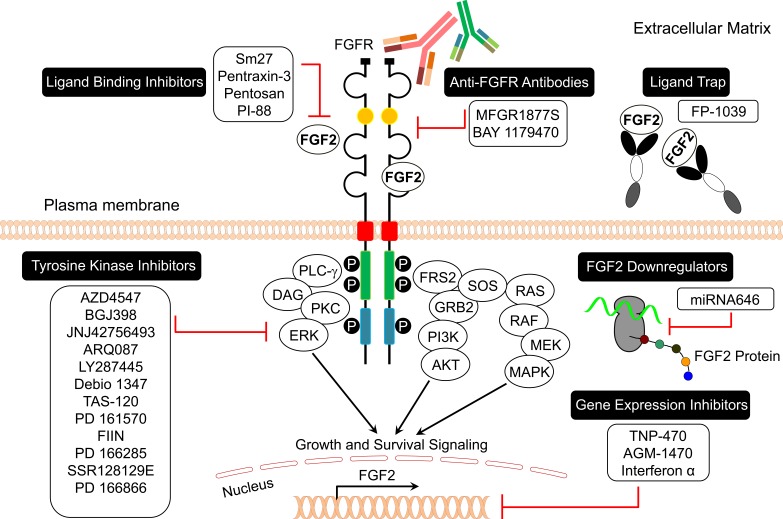
The trend in cancer personalized medicine is to search for biomarkers to predict a patient's response to the targeted therapy and the emergence of secondary resistance [84]. High expression of FGF2 correlates with a worse survival for relapsed/refractory cancer patients (Tables 2 and 3). Therefore, targeting FGF signaling may provide opportunities for personalized therapy in those patients. Several FGF2/FGFR inhibitors have shown promising anticancer and antiangiogenic efficacy in several in vitro assays and in vivo preclinical animal models (Table 5, Figure 4). Clinical studies on these compounds have been conducted in the last decade to evaluate their safety, efficacy, and tolerability (Table 6). Ongoing clinical trials are recruiting patients with metastatic, advanced, or relapsed/refractory cancers to evaluate the importance of blocking the FGF2/FGFR signaling in progressive and poor outcome cancer patients (Table 6).
Table 5. Agents target FGF2/FGFR in cancer.
| Agent | Company | Target | Agent Type | Characteristics | Clinical Trial | Indication/Tested on | Ref. |
|---|---|---|---|---|---|---|---|
| FGF2 inhibitors | |||||||
| FP-1039/GSK3052230 | Five Prime Pharmaceuticals (CA, USA) | FGF2 | Protein | Ligand trap: prevents FGF2 from binding to receptors | Phase I (Ongoing) NCT01868022 | Squamous non-small cell lung cancer, mesothelioma | [86] |
| Interferon-α | − | FGF2 | Protein | Inhibits FGF2 expression and production | Phase II (ongoing) NCT00049530 | Bladder cancer, melanoma | [87, 187] |
| miRNA 646 | − | FGF2 | miRNA | Downregulates FGF2 | − | Osteosarcoma | [188] |
| Sm27 | − | FGF2 | Small molecule | Binds to heparin-binding site on FGF2 and prevents FGF2 interaction with receptors | − | Endothelial cells | [189] |
| Anvirzel | Nerium Biotechnology (Canada) | FGF2 | Glycoside | Inhibits FGF2 export by affecting Na+/K+ pump | − | Prostate cancer | [190] |
| Pentraxin-3 | − | FGF2 | Protein | Inhibits FGF2 binding to FGFR | − | Pancreatic cancer | [191] |
| TNP-470/AGM-1470 | − | FGF2 | Antibiotic | Suppresses expression and production of FGF2 | − | Bladder cancer | [192] |
| Pentosan Polysulfate (Elmiron) | Ortho-McNeil Pharmaceutical (NJ, USA) | FGF2 | Small molecule | Blocks and inhibits activity of FGF2 | − | Various advanced cancers | [193] |
| PI-88 | Progen Pharmaceuticals (Australia) | FGF2 | Small molecule | Binds and inhibits FGF2 associated angiogenesis | − | Liver cancer | [194] |
| Thalidomide | Celgene (NJ, USA) | FGF2 | Small molecule | Inhibits FGF2 induced angiogenesis | − | Multiple cancers | [195] |
| PAMPS, PAS, PSS, PVS | − | FGF2 | Sulfonic acid polymers | FGF2 Antagonists | − | Endothelial cells | [196] |
| Sirolimus (Rapamycin) | Pfizer (NY, USA) | FGF2 | Small molecule | Inhibits FGF2 dependent angiogenesis and proliferation | − | Fibroblasts and endothelial cells | [197] |
| Suramin (Germanin) | Bayer (Germany) | FGF2 | Small Molecule | FGF2 antagonist/reduced FGF2 expression | − | Multiple cancers | [198] |
| Platelet Factor 4 | − | FGF2 | Protein | FGF2 antagonist | − | Endothelial cells | [199] |
| Non-specific Tyrosine Kinase Inhibitors | |||||||
| Lenvatinib (Lenvima) | Eisai (Japan) | FGFR1, PDGFR, VEGFR | Small molecule | Tyrosine kinase and angiogenesis inhibitor | Approved | Progressive, radioactive iodine-refractory thyroid cancer | [200] |
| AP 24534 (Ponatinib, Iclusig) | ARIAD Pharmaceuticals (MA, USA) | BCR-ABL, FGFR1-4 | Small molecule | Tyrosine kinase inhibitor | Approved | CML, ALL | [89] |
| Pazopanib (Votrient) | GlaxoSmithKline (England) | FGFR, PDGFR, VEGFR | Small molecule | Tyrosine kinase and angiogenesis inhibitor | Approved | Renal cell carcinoma, soft tissue sarcoma | [90] |
| Nintedanib (Vargatef, Ofev) | Boehringer Ingelheim (Germany) | FGFR1-3, PDGFR, VEGFR | Small molecule | Tyrosine kinase and angiogenesis inhibitor | Approved (EU) | Non-small-cell lung cancer | [201] |
| BMS582664 (Brivanib) | Bristol-Myers Squibb (NY, USA) | FGFR1, VEGFR1, VEGFR2 | Small molecule | Tyrosine kinase and angiogenesis inhibitor | Phase I/II/III trials NCT00633789 NCT00355238 NCT00435669 | Liver cancer, solid tumors | [202] |
| SU6668, TSU-68 (Orantinib) | SUGEN/Pfizer/Taiho Pharmaceutical (CA, USA/NY, USA/Japan) | FGFR, PDGFR, VEGFR2 | Small molecule | Tyrosine kinase and angiogenesis inhibitor | Phase I/II NCT00024206 NCT00784290 | Advanced solid tumors, liver cancer | [203, 204] |
| TKI-258, CHIR-258 (Dovitinib) | Novartis (Switzerland) | FGFR1-3, PDGFR, VEGFR | Small molecule | Tyrosine kinase and angiogenesis inhibitor | Phase II trials NCT01058434 NCT01831726 NCT01861197 NCT01732107 NCT01719549 | Multiple cancers including relapsed MM, non-small cell lung cancer, bladder cancer, gastric cancer | [205] |
| E3810 (Lucitanib) | EOS/Clovis Oncology (India/CO, USA) | FGFR1-3, VEGFR | Small molecule | Tyrosine kinase inhibitor | PhaseI/II NCT01283945 | Solid tumors | [206] |
| Specific FGFR inhibitors | |||||||
| Debio 1347 | Debiopharm (Switzerland) | FGFR1-3 | Small molecule | Inhibits FGFR autophosphorylation | Phase I NCT01948297 | Advanced solid tumors | [97] |
| AZD 4547 | AstraZeneca (England) | FGFR1-3 | Small molecule | Tyrosine kinase inhibitor | Phase II NCT01795768 | Gastric cancer, esophageal cancer, breast cancer | [98] |
| BGJ 398 | Novartis (Switzerland) | FGFR1-3 | Small molecule | Tyrosine kinase and angiogenesis inhibitor | Phase I NCT01004224 | Advanced solid tumors | [207] |
| JNJ-42756493 | Janssen Oncology (Belgium) | FGFR | Small molecule | Tyrosine kinase inhibitor | Phase I/II NCT01703481 NCT02365597 | Urothelial cancer, glioma | [208] |
| ARQ 087 | Arqule (MA, USA) | FGFR | Small molecule | Tyrosine kinase inhibitor | Phase I NCT01752920 | Solid tumors | [209] |
| LY287445 | LKT Laboratories (MN, USA) | FGFR1-4 | Small molecule | Tyrosine kinase inhibitor | Phase I NCT01212107 | Advanced tumors | [210] |
| TAS-120 | Taiho Pharmaceuticals (Japan) | FGFR | Small molecule | Irreversible FGFR inhibitor | Phase I/II NCT02052778 | Advanced solid tumors, multiple myeloma | [211] |
| MFGR1877S | Genentech/Roche (CA, USA/Switzerland) | FGFR3 | Antibody | Inhibits FGFR3 mediated cell proliferation | Phase I NCT01363024 NCT01122875 | Solid tumors, multiple myeloma | [212, 213] |
| BAY 1179470 | Bayer (NJ, USA) | FGFR2 | Antibody | Inhibits FGFR2 mediated cell proliferation | Phase I NCT01881217 | Advanced, refractory solid tumors | [100] |
| PD 161570 | Parke-Davis/Pfizer (NY, USA) | FGFR | Small molecule | Tyrosine kinase and receptor phosphorylation inhibitor | − | Ovarian cancer | [214] |
| PD 173074 | Parke-Davis/Pfizer (NY, USA) | FGFR | Small molecule | Tyrosine kinase and angiogenesis inhibitor | − | Urothelial carcinoma | [215] |
| PD 166285 dihydrochloride | Parke-Davis/Pfizer (NY, USA) | FGFR | Small molecule | Tyrosine kinase and angiogenesis inhibitor | − | Small cell lung cancer | [215, 216] |
| PD 166866 | Parke-Davis/Pfizer (NY, USA) | FGFR1 | Small molecule | Tyrosine kinase and angiogenesis inhibitor | − | Small cell lung cancer | [217, 218] |
| FIIN hydrochloride | − | FGFR1-4 | Small molecule | Irreversible FGFR inhibitor | − | Cancer cell lines | [219] |
| SU 5402 | − | FGFR, VEGFR | Small molecule | Tyrosine kinase and angiogenesis inhibitor | − | Urothelial carcinoma | [215] |
| SSR128129E | − | FGFR | Small molecule | Binds extracellular domain. Inhibits FGFR signaling | − | Endothelial cells, cancer cell lines | [220, 221] |
Figure 4. FGF2/FGFR signaling inhibitors in cancer.
Table 6. Clinical trials related to FGF2/FGFR pathway.
| Clinical Trial Description ( Trial #) | Participants # | Start Date/Trial Status | Originator | Sponsor | Mechanism of Action | Study Type/Purpose |
|---|---|---|---|---|---|---|
| FGF2 Inhibitors | ||||||
| Phase II study of low dose Pegintron (PEG interferon alfa-2b) in patients with metastatic melanoma over-expressing FGF2 (NCT00049530) | 32 | Sept 2003 / not recruiting - ongoing | Enzon Pharmaceuticals (Piscataway, NJ) | Eastern Cooperative Oncology Group (Boston, MA) | FGF2 inhibitor, interferon alpha stimulant | Interventional, response level of suppression of plasma FGF2 level with low dose Pegintron |
| Phase I, open-label, dose-finding study of FP-1039 in advanced solid tumors (NCT00687505) | 39 | July 2008 / completed | Five Prime Therapeutics, Inc. (San Francisco, CA) | FGFR1 inhibitor | Interventional, assess safety and tolerability | |
| Non-Specific Tyrosine Kinase Inhibitors | ||||||
| Phase I dose escalation study of Lenvima (Lenvatinib) in patients with solid tumors (NCT00280397) | 27 | Jan 2006 – Nov 2008 / completed | Eisai Inc. (Japan) | PDGFR-beta inhibitor; c-kit inhibitor; FGFR inhibitor; VEGFR 1-3 inhibitor | Interventional; adverse events, safety, tolerability | |
| Phase Ib/II, open-label, multicenter study of Lenvima (lenvatinib) alone, and in combination with Everolimus in subjects with unresectable advanced or metastatic renal cell carcinoma following one prior VEGF-targeted treatment (NCT01136733) | 180 | Aug 2010 / not recruiting - ongoing | Eisai Co. Ltd. (Japan) | PDGFR-b antagonist; VEGFR-2 antagonist, FGFR inhibitor | Interventional, assess the dose-limiting and maximally tolerated toxicity, recommended dose, progression-free survival | |
| Phase II, multicenter, randomized, open-label study of Votrient (Pazopanib) in thyroid carcinoma (NCT01813136) | 168 | Mar 2013 / ongoing - recruiting | Centre Leon Berard (France) | GlaxoSmithKline (Philadelphia, PA) | PDGFR antagonist; BRAF inhibitor; c-kit inhibitor; VEGFR 1-3 antagonist | Interventional, efficacy (objective response rate) |
| Phase I/II study of Orantinib for advanced hepatocellular carcinoma (NCT00784290) | 35 | Sept 2003 / completed | Pfizer (New York City, NY) | Taiho Pharmaceutical Co., Ltd. (Japan) | FGF inhibitor; PDGF inhibitor; VEGFR-2 antagonist | Interventional, assess the safety and response rate |
| Phase II study of Dovitinib in patients with gastrointestinal stromal tumors refractory and/or Intolerant to Imatinib (NCT01478373) | 150 | Jan 2012 - July 2014 / completed | Novartis (East Hanover, NJ) | FGF2 inhibitor; PDGFR Δ inhibitor; VEGFR inhibitor | Interventional, measure safety and efficacy | |
| Phase II, open-label study of Lucitanib in patients with FGFR1-driven lung cancer (NCT02109016) | 40 | Apr 2014/ recruiting - ongoing | Advenchen Laboratories (Moorpark, CA) | Clovis Oncology, Inc. (Boulder, CO) | FGFR 1-3 inhibitor; VEGFR1-3 inhibitor | Interventional, efficacy (objective response rate) |
| Phase II study of Vargatef (Nintedanib) in patients with advanced FGFR3 mutated, overexpressed, or wild type urothelial carcinoma of urinary bladder (NCT02278978) | 129 | Oct 2014/not recruiting - ongoing | Boehringer Ingelheim (Germany) | National Taiwan University Hospital (Taiwan) | PDGFR α-Δ inhibitor; FGFR 1-3 inhibitor; VEGFR 1-3 inhibitor | Interventional, safety study with primary purpose of treatment |
| Phase I/II, multicenter, randomized, double-blind study of Vargatef (Nintedanib) in combination with paclitaxel for treatment of patients with BRAF wild-type metastatic melanoma (NCT02308553) | 126 | Jan 2015 / ongoing - recruiting | Boehringer Ingelheim (Ridgefield, CT) | Prof. Dr.Dirk Schadendorf (Germany) | PDGFR-alpha/beta inhibitor; FGFR1-3 inhibitor; VEGF1/2 inhibitor | Interventional, measure of progression-free survival, safety, tolerability |
| Phase III study to compare efficacy and safety of Masitinib in combination with Bortezomib and Dexamethasone to placebo in combination with Bortezomib and Dexamethasone in patients with relapsing multiple myeloma (NCT01470131) | 300 | Apr 2013 | Masitinib: AB Science (France) | Masitinib: AB Science (France) | FGFR modulator; PDGFR antagonist | Interventional, assess overall time to progression and overall survival |
| Bortezomib: Millennium Pharmaceuticals (Cambridge, MA) | Immunomodulator; proteasome inhibitor | |||||
| Dexamethasone: Allergan (Ireland) | Glucocorticoid receptor agonist | |||||
| FGFR Inhibitors | ||||||
| Phase I, multicenter, open label study of oral Debio 1347 (CH5183284) in patients with advanced solid malignancies, whose tumors have an alteration of the FGFR 1, 2 or 3 genes (NCT01948297) | 112 | Aug 2013 / ongoing - recruiting | Chugai Pharmaceutical (Japan) | Debiopharm International SA (Switzerland) | Interventional, measure of safety and tolerability in dose escalation study | |
| Phase I, open-label, multicenter study of AZD4547 in patients with advanced solid tumors (NCT00979134) | 979 | Oct 2009 / not recruiting, ongoing | AstraZeneca (England) | FGFR inhibitor | Investigate the safety, tolerability and maximum tolerated dose | |
| Study of AZD4547 in patients with FGFR1 or FGFR2 amplified tumors (NCT01795768) | 49 | Sept 2012 / ongoing - recruiting | Royal Marsden NHS Foundation Trust (England) | FGFR inhibitor | Interventional, assess efficacy within 8 weeks | |
| Phase I, multi-center, open-label, dose escalation study of oral BGJ398, in adult patients with advanced solid malignancies (NCT01004224) | 190 | Dec 2009 / ongoing - recruiting | Novartis (East Hanover, NJ) | FGFR inhibitor | Interventional, safety, tolerability, pharmacokinetics, pharmacodynamics | |
| Phase I study of JNJ-42756493 in subjects with advanced or refractory solid tumors or lymphoma (NCT01703481) | 260 | Jun 2012 / ongoing - recruiting | Astex Therapeutic (England) | Janssen Research & Development, LLC (Belgium) | FGFR inhibitor | Interventional, safety, tolerability, pharmacokinetics, pharmacodynamics |
| Phase I dose escalation study of ARQ 087 in adult subjects with advanced solid tumors (NCT01752920) | 120 | Dec 2012 / ongoing - recruiting | ArQule (Woburn, MA) | FGFR inhibitor | Interventional, measure of safety and tolerability | |
| Phase I study of LY2874455 in patients with advanced cancer (NCT01212107) | 94 | Dec 2010 – Feb 2015 / completed | Eli Lilly and Company (Indianopolis, IN) | FGFR inhibitor | Interventional, measure of safety and tolerability | |
| Phase I study of TAS-120 in patients with advanced solid tumors with or without FGF/FGFR-Related abnormalities followed by a Phase II study in patients with advanced solid tumors or multiple myeloma with FGF/FGFR-related abnormalities (NCT02052778) | 835 | July 2014 / ongoing - recruiting | Taiho Oncology, Inc. (Japan) | FGFR inhibitor | Interventional, measure of safety and tolerability | |
| Phase I, multicenter, open-label study of MFGR1877S in patients with relapsed or refractory multiple myeloma (NCT01122875) | 14 | Nov 2010 – May 2012 / completed | Genentech, Inc. (South San Francisco, CA) | FGFR3 inhibitor | Interventional, measure of safety and tolerability | |
| Phase I, open-label, dose-escalation study of BAY 1179470 in subjects with advanced, refractory solid tumors (NCT01881217) | 63 | June 2013 / recruiting - ongoing | Bayer (Whippany, NJ) | FGFR2 inhibitor | Interventional, measure of safety, tolerability, pharmacokinetics, and pharmacodynamics | |
It has been reported that various molecules can inhibit FGF2 (ligand) activity, binding, or expression in endothelial and tumor cells (Table 5, Figure 4). FGF ligand traps, such as FP-1039, block FGF2 interaction with FGFR (Tables 5 and 6, Figure 4) [85]. A phase I clinical trial was conducted to investigate the safety and tolerability of FP-1039 in advanced solid tumors (Table 6) [86]. FP-1039 is a soluble FGFR1 Fc fusion protein that was engineered to strongly bind all mitogenic FGF ligands. This compound showed promising results and inhibited FGF-mediated cell proliferation and angiogenesis in lung and endometrial cancer models [86]. AGM-1470, miRNA 646, and interferon alpha downregulate FGF2 expression in cancer cells (Table 5, Figure 4). A phase II clinical study was conducted to evaluate the efficacy of pegintron (peginterferon alpha-2b) in patients with stage IV metastatic melanoma overexpressing FGF2 (Table 6) [87]. The results showed that peginterferon alpha-2b suppressed FGF2 levels in 97% of patients with metastatic melanoma to reference range with a median progression free survival (PFS) and overall survival (OS) of 2.0 and 9.7 months, respectively (Table 6) [87].
Other FGF2 antagonists are under investigation for cell line and animal preclinical cancer models. Small molecules, such as sm27, pentosan, and PI-88 as well as proteins such as pentraxin-3 inhibit FGF2 binding to FGFRs (Table 5, Figure 4) [3]. The capability of FGF2 to bind heparin/heparan sulfate indicates that molecules able to interfere with this interaction may act as angiogenesis inhibitors. On this basis, compounds such as suramin, which mimic heparin, can interfere with FGF2 signaling (Table 5). Thalidomide, PAMPS, sirolimus, suramin, and platelet factor 4 inhibit FGF2-induced angiogenesis, while anvirzel inhibits FGF2 export via Na+/K+ pumps (Table 5).
It has been reported that direct inhibition of FGFRs using small molecule inhibitors may be effective in cancer treatment. Several FGFR tyrosine kinase inhibitors (TKIs) are currently in early clinical development (Tables 5 and 6, Figure 4), and many of them exhibit dual specificity for FGFR and VEGFR due to similarity in the ATP binding pocket structure [85]. The first generation of inhibitors were developed as VEGFR and PDGFR inhibitors, but also can inhibit FGFR [88]. These compounds were successful in clinical trials and some of these drugs and drug combinations were subsequently approved by regulatory administrations worldwide for the treatment of different cancers (Tables 5 and 6).
Recently, the U.S. Food and Drug Administration (FDA) granted approval to Lenvima (lenvatinib, developed by Eisai) based on the results of a study on 392 patients with locally recurrent or metastatic, progressive, radioactive iodine-refractory differentiated thyroid cancer (DTC) who were randomly assigned to receive either Lenvima or a placebo. Study results showed that Lenvima-treated participants lived a median of 18.3 months (PFS) compared to a median of 3.6 months for participants who received placebo. Moreover, approximately two thirds of participants treated with Lenvima showed a reduction in tumor size compared to only two percent of participants who received placebo. Similarly, FDA recently approved Iclusig (ponatinib, developed by ARIAD Pharmaceuticals), which is a multi-tyrosine kinase inhibitor for the treatment of adult patients with chronic myeloid leukemia or acute lymphoblastic leukemia (Table 5) [89]. Moreover, the multi-tyrosine kinase inhibitor Votrient (pazopanib, developed by GlaxoSmithKline) was approved by FDA to treat patients with advanced renal cell cancer and patients with advanced soft tissue sarcoma who have received chemotherapy in the past (Tables 5 and 6) [90].
Dovitinib (TKI-258, CHIR-258, developed by Novartis) is a potent VEGFR, PDGFR and FGFR inhibitor. Dovitinib showed promising anticancer and antiangiogenic activity against multiple myeloma and colon cancer models [91, 92]. A phase II trial was conducted to evaluate the safety and tolerability of Dovitinib in relapsed or refractory multiple myeloma patients, who are with or without t(4;14) chromosomal translocation (Table 6). The t(4;14) translocation is observed in approximately 15-20% of multiple myeloma patients, and it is associated with upregulation of FGFR3 and poor prognosis [93, 94]. Upregulation of FGFR3 occurs in nearly 70% of patients with the t(4;14) translocation. Therefore, development of inhibitors such as Dovitinib may show promise in t(4;14) multiple myeloma patients [94]. Further phase II clinical trials were conducted on Dovitinib for treatment of other types of cancers including lung, bladder and gastric cancers (Table 6).
Other investigational multi-kinase inhibitors, such as Brivanib, Orantinib, and Lucitanib, are currently progressing into phases II and III of clinical trials (Tables 5 and 6). Recent clinical trials have assessed the combination of a tyrosine kinase inhibitor with cytotoxic agents (Table 6). Combination strategies that involve the blockade of FGFR signaling with cytotoxic agents have the best clinical outcome for cancer treatment [85]. Recently, Vargatef (nintedanib, developed by Boehringer Ingelheim) was approved in the EU based on the results of a phase III study comparing Vargatef plus docetaxel to placebo plus docetaxel in patients with locally advanced/metastatic non-small-cell lung carcinoma (NSCLC) after first-line therapy in over 1,300 patients in 27 countries [95]. Vargatef is an oral triple angiokinase inhibitor that simultaneously inhibits the VEGFR, PDGFR, and FGFR signaling pathways. The results indicated that treatment with Vargatef and docetaxel significantly extended the median overall survival from 10.3 to 12.6 months for patients with adenocarcinoma compared to placebo and docetaxel, with a quarter of patients surviving for two years or more with minimum side effects [95]. Several clinical trials are ongoing currently with compounds targeted for FGF2; a phase III study was designed to compare the safety and efficacy of a multi-tyrosine kinase inhibitor, masitinib, in combination with bortezomib and dexamethasone to placebo in combination with bortezomib and dexamethasone in patients with relapsing multiple myeloma. Masitinib is an orally active and bioavailable compound that is a weak inhibitor of FGFR3 [96].
More recently, second-generation inhibitors targeting FGFRs with high selectivity have been developed (Table 5, Figure 4). For example, Debio 1347, developed by Debiopharm Group, a Swiss-based global biopharmaceutical company, is an orally bioavailable inhibitor of FGFRs 1-3 that inhibits FGFR-mediated signal transduction pathways and consequently tumor cell proliferation and angiogenesis [97]. Debio 1347 will be used for personalized cancer treatment through the development of a companion diagnostic. It is currently being evaluated in Europe and the USA in a phase I trial to evaluate its safety and tolerability in patients with advanced solid tumors displaying alterations in FGFRs 1, 2, or 3 genes (Tables 5 and 6) [97]. Similarly, AZD4547, developed by AstraZeneca, is an orally tolerated inhibitor of FGFRs 1-3 [98]. AZD4547 inhibits FGFR kinase activity and tumor growth in vitro and in vivo [98]. This compound is currently being tested for safety and efficacy in different clinical trials against advanced tumors (Table 6). Other small molecule FGFR inhibitors, such as BGJ398, JNJ-42756493, ARQ 087, LY2874455, and TAS-120, are currently under clinical investigation for advanced, relapsed or refractory tumors and most of these trials are still recruiting patients (Tables 5 and 6). Other selective small molecule tyrosine kinase inhibitors, such as PD 161570, PD 173074, PD 166285 dihydrochloride, PD 166866, FIIN hydrochloride, SU 5402, and SSR128129E are currently being tested for their antitumor activity on cell lines and in vivo preclinical models (Table 5). Monoclonal antibodies specifically targeting particular FGFR isoforms are also being developed. MFGR1877S (developed by Genentech) is a human anti-FGFR3 monoclonal antibody that inhibits tumor progression of bladder carcinoma and multiple myeloma xenografts in mice by antagonizing FGFR3 signaling [99]. A phase I clinical trial was conducted to evaluate the response to MFGR1877S in patients with relapsed or refractory multiple myeloma (Tables 5 and 6). Similarly, BAY 1179470 (developed by Bayer) is a human anti-FGFR2 monoclonal antibody. BAY 1179470 showed antitumor activity in gastric cancer xenograft models with high FGFR2 expression [100]. The anti-FGFR2 antibody BAY 1179470 is currently in Phase I testing in subjects with advanced, refractory solid tumors (Tables 5 and 6)
CONCLUSIONS
FGF2 is frequently dysregulated in cancer, especially in advanced stages of disease. The upregulation of FGF2 or FGFRs can promote resistance to chemotherapy. FGF2 is currently being evaluated in clinical studies as a potential predictive biomarker for hematological and solid tumors. In addition, FGF2/FGFR inhibitors are being developed and evaluated as monotherapy or as part of a combination therapy for the treatment of different types of cancer. Identifying patients with advanced, relapsed or refractory cancers that would benefit from FGF2/FGFR signaling inhibition will allow for better treatment options of those patients in the era of personalized medicine.
Acknowledgments
We thank Lisa B. Fishman Foundation and the John Theurer Cancer Center and Hackensack University Medical Center for supporting funds and preparation of this manuscript.
Footnotes
CONFLICTS OF INTEREST
There is no conflict of interest.
REFERENCES
- 1.Powers C, McLeskey S, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 2.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9:639. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florkiewicz RZ, Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989;86:3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahimi OA, Zhang F, Eliseenkova AV, Linhardt RJ, Mohammadi M. Proline to arginine mutations in FGF receptors 1 and 3 result in Pfeiffer and Muenke craniosynostosis syndromes through enhancement of FGF binding affinity. Hum Mol Genet. 2004;13:69–78. doi: 10.1093/hmg/ddh011. [DOI] [PubMed] [Google Scholar]
- 6.Florkiewicz RZ, Majack RA, Buechler RD, Florkiewicz E. Quantitative export of FGF-2 occurs through an alternative, energy-dependent, non-ER/Golgi pathway. J Cell Physiol. 1995;162:388–399. doi: 10.1002/jcp.1041620311. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahimi OA, Zhang F, Hrstka SC, Mohammadi M, Linhardt RJ. Kinetic model for FGF, FGFR, and proteoglycan signal transduction complex assembly. Biochemistry (Mosc) 2004;43:4724–4730. doi: 10.1021/bi0352320. [DOI] [PubMed] [Google Scholar]
- 8.Yu PJ, Ferrari G, Galloway AC, Mignatti P, Pintucci G. Basic fibroblast growth factor (FGF-2): the high molecular weight forms come of age. J Cell Biochem. 2007;100:1100–1108. doi: 10.1002/jcb.21116. [DOI] [PubMed] [Google Scholar]
- 9.Clarke WE, Berry M, Smith C, Kent A, Logan A. Coordination of fibroblast growth factor receptor 1 (FGFR1) and fibroblast growth factor-2 (FGF-2) trafficking to nuclei of reactive astrocytes around cerebral lesions in adult rats. Mol Cell Neurosci. 2001;17:17–30. doi: 10.1006/mcne.2000.0920. [DOI] [PubMed] [Google Scholar]
- 10.Dunham-Ems SM, Lee Y-W, Stachowiak EK, Pudavar H, Claus P, Prasad PN, Stachowiak MK. Fibroblast growth factor receptor-1 (FGFR1) nuclear dynamics reveal a novel mechanism in transcription control. Mol Biol Cell. 2009;20:2401–2412. doi: 10.1091/mbc.E08-06-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng H, Moffett J, Myers J, Fang X, Stachowiak EK, Maher P, Kratz E, Hines J, Fluharty SJ, Mizukoshi E. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Molecular Biol Cell. 2001;12:449–462. doi: 10.1091/mbc.12.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eswarakumar V, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 15.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14:166–180. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akl M, Nagpal P, Ayoub N, Prabhu S, Gliksman M, Tai B, Hatipoglu A, Goy A, Suh K. Molecular and clinical profiles of syndecan-1 in solid and hematological cancer for prognosis and precision medicine. Oncotarget. 2015;6:28693–715. doi: 10.18632/oncotarget.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ornitz D, Yayon A, Flanagan J, Svahn C, Levi E, Leder P. Heparin is required for cell-free binding of basic fibroblast growth factor to a soluble receptor and for mitogenesis in whole cells. Mol Cell Biol. 1992;12:240–247. doi: 10.1128/mcb.12.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lew ED, Furdui CM, Anderson KS, Schlessinger J. The precise sequence of FGF receptor autophosphorylation is kinetically driven and is disrupted by oncogenic mutations. Sci Signal. 2009;2:ra6. doi: 10.1126/scisignal.2000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karajannis MA, Vincent L, Direnzo R, Shmelkov SV, Zhang F, Feldman EJ, Bohlen P, Zhu Z, Sun H, Kussie P, Rafii S. Activation of FGFR1beta signaling pathway promotes survival, migration and resistance to chemotherapy in acute myeloid leukemia cells. Leukemia. 2006;20:979–986. doi: 10.1038/sj.leu.2404203. [DOI] [PubMed] [Google Scholar]
- 20.Presta M, Dell'Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Eliceiri BP. Integrin and growth factor receptor crosstalk. Circ Res. 2001;89:1104–1110. doi: 10.1161/hh2401.101084. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad I, Iwata T, Leung HY. Mechanisms of FGFR-mediated carcinogenesis. Biochim Biophys Acta. 2012;4:850–860. doi: 10.1016/j.bbamcr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Behbod F, Xian W, Shaw CA, Hilsenbeck SG, Tsimelzon A, Rosen JM. Transcriptional profiling of mammary gland side population cells. Stem Cells. 2006;24:1065–1074. doi: 10.1634/stemcells.2005-0375. [DOI] [PubMed] [Google Scholar]
- 24.Jang JH, Shin KH, Park JG. Mutations in fibroblast growth factor receptor 2 and fibroblast growth factor receptor 3 genes associated with human gastric and colorectal cancers. Cancer Res. 2001;61:3541–3543. [PubMed] [Google Scholar]
- 25.Cha JY, Maddileti S, Mitin N, Harden TK, Der CJ. Aberrant receptor internalization and enhanced FRS2-dependent signaling contribute to the transforming activity of the fibroblast growth factor receptor 2 IIIb C3 isoform. J Biol Chem. 2009;284:6227–6240. doi: 10.1074/jbc.M803998200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor JGt, Cheuk AT, Tsang PS, Chung JY, Song YK, Desai K, Yu Y, Chen QR, Shah K, Youngblood V, Fang J, Kim SY, Yeung C, Helman LJ, Mendoza A, Ngo V, et al. Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest. 2009;119:3395–3407. doi: 10.1172/JCI39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thussbas C, Nahrig J, Streit S, Bange J, Kriner M, Kates R, Ulm K, Kiechle M, Hoefler H, Ullrich A, Harbeck N. FGFR4 Arg388 allele is associated with resistance to adjuvant therapy in primary breast cancer. J Clin Oncol. 2006;24:3747–3755. doi: 10.1200/JCO.2005.04.8587. [DOI] [PubMed] [Google Scholar]
- 28.Savagner P, Valles AM, Jouanneau J, Yamada KM, Thiery JP. Alternative splicing in fibroblast growth factor receptor 2 is associated with induced epithelial-mesenchymal transition in rat bladder carcinoma cells. Mol Biol Cell. 1994;5:851–862. doi: 10.1091/mbc.5.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara A, Kan M, Feng S, McKeehan WL. Inhibition of growth of malignant rat prostate tumor cells by restoration of fibroblast growth factor receptor 2. Cancer Res. 1998;58:1509–1514. [PubMed] [Google Scholar]
- 31.Kornmann M, Ishiwata T, Matsuda K, Lopez ME, Fukahi K, Asano G, Beger HG, Korc M. IIIc isoform of fibroblast growth factor receptor 1 is overexpressed in human pancreatic cancer and enhances tumorigenicity of hamster ductal cells. Gastroenterology. 2002;123:301–313. doi: 10.1053/gast.2002.34174. [DOI] [PubMed] [Google Scholar]
- 32.Gualandris A, Rusnati M, Belleri M, Nelli EE, Bastaki M, Molinari-Tosatti MP, Bonardi F, Parolini S, Albini A, Morbidelli L, Ziche M, Corallini A, Possati L, Vacca A, Ribatti D, Presta M. Basic fibroblast growth factor overexpression in endothelial cells: an autocrine mechanism for angiogenesis and angioproliferative diseases. Cell Growth Differ. 1996;7:147–160. [PubMed] [Google Scholar]
- 33.Javerzat S, Auguste P, Bikfalvi A. The role of fibroblast growth factors in vascular development. Trends Mol Med. 2002;8:483–489. doi: 10.1016/s1471-4914(02)02394-8. [DOI] [PubMed] [Google Scholar]
- 34.Dell'Era P, Belleri M, Stabile H, Massardi ML, Ribatti D, Presta M. Paracrine and autocrine effects of fibroblast growth factor-4 in endothelial cells. Oncogene. 2001;20:2655–2663. doi: 10.1038/sj.onc.1204368. [DOI] [PubMed] [Google Scholar]
- 35.Presta M, Tiberio L, Rusnati M, Dell'Era P, Ragnotti G. Basic fibroblast growth factor requires a long-lasting activation of protein kinase C to induce cell proliferation in transformed fetal bovine aortic endothelial cells. Cell Regul. 1991;2:719–726. doi: 10.1091/mbc.2.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dell'Era P, Mohammadi M, Presta M. Different tyrosine autophosphorylation requirements in fibroblast growth factor receptor-1 mediate urokinase-type plasminogen activator induction and mitogenesis. Mol Biol Cell. 1999;10:23–33. doi: 10.1091/mbc.10.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanghetti E, Ria R, Dell'Era P, Urbinati C, Rusnati M, Ennas MG, Presta M. Biological activity of substrate-bound basic fibroblast growth factor (FGF2): recruitment of FGF receptor-1 in endothelial cell adhesion contacts. Oncogene. 2002;21:3889–3897. doi: 10.1038/sj.onc.1205407. [DOI] [PubMed] [Google Scholar]
- 38.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Dell'Era P, Coco L, Ronca R, Sennino B, Presta M. Gene expression profile in fibroblast growth factor 2-transformed endothelial cells. Oncogene. 2002;21:2433–2440. doi: 10.1038/sj.onc.1205301. [DOI] [PubMed] [Google Scholar]
- 40.Hoying JB, Williams SK. Effects of basic fibroblast growth factor on human microvessel endothelial cell migration on collagen I correlates inversely with adhesion and is cell density dependent. J Cell Physiol. 1996;168:294–304. doi: 10.1002/(SICI)1097-4652(199608)168:2<294::AID-JCP8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 41.Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26:344–349. doi: 10.1097/00006676-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Garcia de la Torre N, Buley I, Wass JA, Jackson DG, Turner HE. Angiogenesis and lymphangiogenesis in parathyroid proliferative lesions. J Clin Endocrinol Metab. 2004;89:2890–2896. doi: 10.1210/jc.2003-031651. [DOI] [PubMed] [Google Scholar]
- 43.Haugsten EM, Wiedlocha A, Olsnes S, Wesche J. Roles of fibroblast growth factor receptors in carcinogenesis. Mol Cancer Res. 2010;8:1439–1452. doi: 10.1158/1541-7786.MCR-10-0168. [DOI] [PubMed] [Google Scholar]
- 44.Ribatti D, Vacca A, Rusnati M, Presta M. The discovery of basic fibroblast growth factor/fibroblast growth factor-2 and its role in haematological malignancies. Cytokine Growth Factor Rev. 2007;18(3-4):327–334. doi: 10.1016/j.cytogfr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Krejci P, Faitova J, Laurell H, Hampl A, Dvorak P. FGF-2 expression and its action in human leukemia and lymphoma cell lines: Leukemia. 2003 Apr;17:818–20. doi: 10.1038/sj.leu.2402861. [DOI] [PubMed] [Google Scholar]
- 46.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 47.Su G, Sung KE, Beebe DJ, Friedl A. Functional screen of paracrine signals in breast carcinoma fibroblasts. PLoS One. 2012;7:8. doi: 10.1371/journal.pone.0046685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51:3753–3761. [PubMed] [Google Scholar]
- 49.Choi TH, Tseng SC. In vivo and in vitro demonstration of epithelial cell-induced myofibroblast differentiation of keratocytes and an inhibitory effect by amniotic membrane. Cornea. 2001;20:197–204. doi: 10.1097/00003226-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Rodemann HP, Muller GA. Characterization of human renal fibroblasts in health and disease: II. In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am J Kidney Dis. 1991;17:684–686. doi: 10.1016/s0272-6386(12)80352-0. [DOI] [PubMed] [Google Scholar]
- 51.Kashiwakura I, Takahashi TA. Basic fibroblast growth factorstimulated ex vivo expansion of haematopoietic progenitor cells from human placental and umbilical cord blood. British journal of haematology. 2003;122:479–488. doi: 10.1046/j.1365-2141.2003.04444.x. [DOI] [PubMed] [Google Scholar]
- 52.Pearson S, Sroczynska P, Lacaud G, Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 53.Eiselleova L, Matulka K, Kriz V, Kunova M, Schmidtova Z, Neradil J, Tichy B, Dvorakova D, Pospisilova S, Hampl A. A Complex Role for FGF2 in SelfRenewal, Survival, and Adhesion of Human Embryonic Stem Cells. Stem Cells. 2009;27:1847–1857. doi: 10.1002/stem.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon S-Y, Tefferi A, Li C-Y. Bone marrow stromal cell distribution of basic fibroblast growth factor in chronic myeloid disorders. Haematologica. 2001;86:52–57. [PubMed] [Google Scholar]
- 55.Berardi AC, Wang A, Abraham J, Scadden DT. Basic fibroblast growth factor mediates its effects on committed myeloid progenitors by direct action and has no effect on hematopoietic stem cells. Blood. 1995;86:2123–2129. [PubMed] [Google Scholar]
- 56.Gabrilove J, Wong G, Bollenbacher E, White K, Kojima S, Wilson E. Basic fibroblast growth factor counteracts the suppressive effect of. Blood. 1993;81(4) [PubMed] [Google Scholar]
- 57.Koritschoner NP, Bartunek P, Knespel S, Blendinger G, Zenke M. The fibroblast growth factor receptor FGFR-4 acts as a ligand dependent modulator of erythroid cell proliferation. Oncogene. 1999;18:5904–5914. doi: 10.1038/sj.onc.1202979. [DOI] [PubMed] [Google Scholar]
- 58.Wilson EL, Rifkin DB, Kelly F, Hannocks M-J, Gabrilove JL. Basic fibroblast growth factor stimulates myelopoiesis in long-term human bone marrow cultures. Blood. 1991;77:954–960. [PubMed] [Google Scholar]
- 59.Moroni E, Dell'Era P, Rusnati M, Presta M. Fibroblast growth factors and their receptors in hematopoiesis and hematological tumors. J Hematother Stem Cell Res. 2002;11:19–32. doi: 10.1089/152581602753448513. [DOI] [PubMed] [Google Scholar]
- 60.Fhu CW, Graham AM, Yap CT, Al-Salam S, Castella A, Chong SM, Lim YC. Reed-Sternberg cell-derived lymphotoxin-alpha activates endothelial cells to enhance T-cell recruitment in classical Hodgkin lymphoma. Blood. 2014;124:2973–2982. doi: 10.1182/blood-2014-05-576140. [DOI] [PubMed] [Google Scholar]
- 61.Witzig TE, Kimlinger T, Stenson M, Therneau T. Syndecan-1 expression on malignant cells from the blood and marrow of patients with plasma cell proliferative disorders and B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 1998;31(1-2):167–175. doi: 10.3109/10428199809057596. [DOI] [PubMed] [Google Scholar]
- 62.Gharbaran R, Goy A, Tanaka T, Park J, Kim C, Hasan N, Vemulapalli S, Sarojini S, Tuluc M, Nalley K, Bhattacharyya P, Pecora A, Suh KS. Fibroblast growth factor-2 (FGF2) and syndecan-1 (SDC1) are potential biomarkers for putative circulating CD15+/CD30+ cells in poor outcome Hodgkin lymphoma patients. Journal of hematology & oncology. 2013;6:1756–8722. doi: 10.1186/1756-8722-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Panta GR, Kaur S, Cavin LG, Cortes ML, Mercurio F, Lothstein L, Sweatman TW, Israel M, Arsura M. ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol. 2004;24:1823–1835. doi: 10.1128/MCB.24.5.1823-1835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rocha S, Martin AM, Meek DW, Perkins ND. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol Cell Biol. 2003;23:4713–4727. doi: 10.1128/MCB.23.13.4713-4727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Küppers R. The biology of Hodgkin's lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 66.Wakisaka N, Murono S, Yoshizaki T, Furukawa M, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer research. 2002;62:6337–6344. [PubMed] [Google Scholar]
- 67.Lee JG, Kay EP. NF-B is the transcription factor for FGF-2 that causes endothelial mesenchymal transformation in cornea. Invest Ophthalmol Vis Sci. 2012;53:1530–1538. doi: 10.1167/iovs.11-9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, Epstein J. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- 69.Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995;162:248–255. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- 70.Bisping G, Leo R, Wenning D, Dankbar B, Padró T, Kropff M, Scheffold C, Kröger M, Mesters RM, Berdel WE. Paracrine interactions of basic fibroblast growth factor and interleukin-6 in multiple myeloma. Blood. 2003;101:2775–2783. doi: 10.1182/blood-2002-09-2907. [DOI] [PubMed] [Google Scholar]
- 71.Ishikawa H, Tsuyama N, Liu S, Abroun S, Li F-J, Otsuyama K-i, Zheng X, Ma Z, Maki Y, Iqbal MS. Accelerated proliferation of myeloma cells by interleukin-6 cooperating with fibroblast growth factor receptor 3-mediated signals. Oncogene. 2005;24:6328–6332. doi: 10.1038/sj.onc.1208782. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds GM, Billingham LJ, Gray LJ, Flavell JR, Najafipour S, Crocker J, Nelson P, Young LS, Murray PG. Interleukin 6 expression by Hodgkin/Reed-Sternberg cells is associated with the presence of ‘B'symptoms and failure to achieve complete remission in patients with advanced Hodgkin's disease. Br J Haematol. 2002;118:195–201. doi: 10.1046/j.1365-2141.2002.03575.x. [DOI] [PubMed] [Google Scholar]
- 73.Khnykin D, Troen G, Berner JM, Delabie J. The expression of fibroblast growth factors and their receptors in Hodgkin's lymphoma. J Pathol. 2006;208:431–438. doi: 10.1002/path.1900. [DOI] [PubMed] [Google Scholar]
- 74.Kurzrock R, Redman J, Cabanillas F, Jones D, Rothberg J, Talpaz M. Serum interleukin 6 levels are elevated in lymphoma patients and correlate with survival in advanced Hodgkin's disease and with B symptoms. Cancer research. 1993;53:2118–2122. [PubMed] [Google Scholar]
- 75.Delrieu I, Arnaud E, Ferjoux G, Bayard F, Faye JC. Overexpression of the FGF-2 24-kDa isoform up-regulates IL-6 transcription in NIH-3T3 cells. FEBS letters. 1998;436:17–22. doi: 10.1016/s0014-5793(98)01086-2. [DOI] [PubMed] [Google Scholar]
- 76.McCubrey J, Abrams S, Ligresti G, Misaghian N, Wong E, Steelman L, Bäsecke J, Troppmair J, Libra M, Nicoletti F. Involvement of p53 and Raf/MEK/ERK pathways in hematopoietic drug resistance. Leukemia. 2008;22:2080–2090. doi: 10.1038/leu.2008.207. [DOI] [PubMed] [Google Scholar]
- 77.Zheng B, Fiumara P, Li YV, Georgakis G, Snell V, Younes M, Vauthey JN, Carbone A, Younes A. MEK/ERK pathway is aberrantly active in Hodgkin disease: a signaling pathway shared by CD30, CD40, and RANK that regulates cell proliferation and survival. Blood. 2003;102:1019–1027. doi: 10.1182/blood-2002-11-3507. [DOI] [PubMed] [Google Scholar]
- 78.Pardo OE, Arcaro A, Salerno G, Raguz S, Downward J, Seckl MJ. Fibroblast growth factor-2 induces translational regulation of Bcl-XL and Bcl-2 via a MEK-dependent pathway: correlation with resistance to etoposide-induced apoptosis. J Biol Chem. 2002;277:12040–12046. doi: 10.1074/jbc.M109006200. [DOI] [PubMed] [Google Scholar]
- 79.Alavi AS, Acevedo L, Min W, Cheresh DA. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67:2766–2772. doi: 10.1158/0008-5472.CAN-06-3648. [DOI] [PubMed] [Google Scholar]
- 80.Joos S, Küpper M, Ohl S, von Bonin F, Mechtersheimer G, Bentz M, Marynen P, Möller P, Pfreundschuh M, Trümper L. Genomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cells. Cancer Res. 2000;60:549–552. [PubMed] [Google Scholar]
- 81.Carmo CR, Lyons-Lewis J, Seckl MJ, Costa-Pereira AP. A novel requirement for Janus kinases as mediators of drug resistance induced by fibroblast growth factor-2 in human cancer cells. PloS one. 2011;6:e19861. doi: 10.1371/journal.pone.0019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diaz T, Navarro A, Ferrer G, Gel B, Gaya A, Artells R, Bellosillo B, Garcia-Garcia M, Serrano S, Martínez A. Lestaurtinib inhibition of the Jak/STAT signaling pathway in hodgkin lymphoma inhibits proliferation and induces apoptosis. PloS one. 2011;6:e18856. doi: 10.1371/journal.pone.0018856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med. 2008;5:0050019. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanase C, Codrici E, Popescu ID, Cruceru ML, Enciu A-M, Albulescu R, Ciubotaru V, Arsene D. Angiogenic markers: molecular targets for personalized medicine in pituitary adenoma. Personalized Med. 2013;10:539–548. doi: 10.2217/pme.13.61. [DOI] [PubMed] [Google Scholar]
- 85.Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17:6130–6139. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harding TC, Long L, Palencia S, Zhang H, Sadra A, Hestir K, Patil N, Levin A, Hsu AW, Charych D, Brennan T, Zanghi J, Halenbeck R, Marshall SA, Qin M, Doberstein SK, et al. Blockade of nonhormonal fibroblast growth factors by FP-1039 inhibits growth of multiple types of cancer. Sci Transl Med. 2013;5:3005414. doi: 10.1126/scitranslmed.3005414. [DOI] [PubMed] [Google Scholar]
- 87.Go RS, Lee SJ, Shin D, Callister SM, Jobe DA, Conry RM, Tarhini AA, Kirkwood JM. ECOG phase II trial of graded-dose peginterferon alpha-2b in patients with metastatic melanoma overexpressing basic fibroblast growth factor (E2602) Clin Cancer Res. 2013;19:6597–6604. doi: 10.1158/1078-0432.CCR-13-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jain VK, Turner NC. Challenges and opportunities in the targeting of fibroblast growth factor receptors in breast cancer. Breast Cancer Res. 2012;14:208. doi: 10.1186/bcr3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA, 3rd, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zivi A, Cerbone L, Recine F, Sternberg CN. Safety and tolerability of pazopanib in the treatment of renal cell carcinoma. Expert Opin Drug Saf. 2012;11:851–859. doi: 10.1517/14740338.2012.712108. [DOI] [PubMed] [Google Scholar]
- 91.Kumar SK, Mikhael JR, Buadi FK, Dingli D, Dispenzieri A, Fonseca R, Gertz MA, Greipp PR, Hayman SR, Kyle RA. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc: Elsevier) 2009:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee SH, de Menezes DL, Vora J, Harris A, Ye H, Nordahl L, Garrett E, Samara E, Aukerman SL, Gelb AB. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11:3633–3641. doi: 10.1158/1078-0432.CCR-04-2129. [DOI] [PubMed] [Google Scholar]
- 93.Moreau P, Facon T, Leleu X, Morineau N, Huyghe P, Harousseau J-L, Bataille R, Avet-Loiseau H. Recurrent 14q32 translocations determine the prognosis of multiple myeloma, especially in patients receiving intensive chemotherapy. Blood. 2002;100:1579–1583. doi: 10.1182/blood-2002-03-0749. [DOI] [PubMed] [Google Scholar]
- 94.Kalff A, Spencer A. The t (4; 14) translocation and FGFR3 overexpression in multiple myeloma: prognostic implications and current clinical strategies. Blood cancer journal. 2012;2:e89. doi: 10.1038/bcj.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- 96.Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Casteran N, Borge L, Hajem B, Lermet A, Sippl W, Voisset E, Arock M, Auclair C, Leventhal PS, Mansfield CD, Moussy A, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4:0007258. doi: 10.1371/journal.pone.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakanishi Y, Akiyama N, Tsukaguchi T, Fujii T, Sakata K, Sase H, Isobe T, Morikami K, Shindoh H, Mio T. The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol Cancer Ther. 2014;13:2547–2558. doi: 10.1158/1535-7163.MCT-14-0248. [DOI] [PubMed] [Google Scholar]
- 98.Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S, Rooney C, Coleman T, Baker D, Mellor MJ, Brooks AN, Klinowska T. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–2056. doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- 99.Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K. Antibody-based targeting of FGFR3 in bladder carcinoma and t (4; 14)-positive multiple myeloma in mice. The Journal of clinical investigation. 2009;119:1216. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schatz CA, Kopitz C, Wittemer-Rump S, Sommer A, Lindbom L, Osada M, Yamanouchi H, Huynh H, Krahn T, Asadullah K. Pharmacodynamic and stratification biomarker for the anti-FGFR2 antibody (BAY1179470) and the FGFR2-ADC. Cancer Res. 2014;74(19 Supplement):4766–4766. [Google Scholar]
- 101.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:1–12. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Horowitz A, Tkachenko E, Simons M. Fibroblast growth factor-specific modulation of cellular response by syndecan-4. J Cell Biol. 2002;157:715–725. doi: 10.1083/jcb.200112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rusnati M, Urbinati C, Presta M. Internalization of basic fibroblast growth factor (bFGF) in cultured endothelial cells: Role of the low affinity heparinlike bFGF receptors. J Cell Physiol. 1993;154:152–161. doi: 10.1002/jcp.1041540119. [DOI] [PubMed] [Google Scholar]
- 104.Rusnati M, Presta M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans. Int J Clin Lab Res. 1996;26:15–23. doi: 10.1007/BF02644769. [DOI] [PubMed] [Google Scholar]
- 105.Rusnati M, Tanghetti E, Dell'Era P, Gualandris A, Presta M. v3 integrin mediates the cell-adhesive capacity and biological activity of basic fibroblast growth factor (FGF-2) in cultured endothelial cells. Mol Biol Cell. 1997;8:2449–2461. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rusnati M, Urbinati C, Tanghetti E, Dell'Era P, Lortat-Jacob H, Presta M. Cell membrane GM1 ganglioside is a functional coreceptor for fibroblast growth factor 2. Proc Natl Acad Sci U S A. 2002;99:4367–4372. doi: 10.1073/pnas.072651899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hanneken A, Maher PA, Baird A. High affinity immunoreactive FGF receptors in the extracellular matrix of vascular endothelial cells—implications for the modulation of FGF-2. J Cell Biol. 1995;128:1221–1228. doi: 10.1083/jcb.128.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bergonzoni L, Caccia P, Cletni O, Sarmientos P, Isacchi A. Characterization of a biologically active extracellular domain of fibroblast growth factor receptor 1 expressed in Escherichia coli. Eur J Biochem. 1992;210:823–829. doi: 10.1111/j.1432-1033.1992.tb17485.x. [DOI] [PubMed] [Google Scholar]
- 109.Naumnik W, Ossolinska M, Plonska I, Chyczewska E, Niklinski J. Circulating Thrombospondin-2 and FGF-2 in Patients with Advanced Non-small Cell Lung Cancer: Correlation with Survival. Adv Exp Med Biol. 2015;833:9–14. doi: 10.1007/5584_2014_78. [DOI] [PubMed] [Google Scholar]
- 110.Margosio B, Marchetti D, Vergani V, Giavazzi R, Rusnati M, Presta M, Taraboletti G. Thrombospondin 1 as a scavenger for matrix-associated fibroblast growth factor 2. Blood. 2003;102:4399–4406. doi: 10.1182/blood-2003-03-0893. [DOI] [PubMed] [Google Scholar]
- 111.Rusnati M, Camozzi M, Moroni E, Bottazzi B, Peri G, Indraccolo S, Amadori A, Mantovani A, Presta M. Selective recognition of fibroblast growth factor-2 by the long pentraxin PTX3 inhibits angiogenesis. Blood. 2004;104:92–99. doi: 10.1182/blood-2003-10-3433. [DOI] [PubMed] [Google Scholar]
- 112.Sahni A, Baker CA, Sporn LA, Francis CW. Fibrinogen and fibrin protect fibroblast growth factor-2 from proteolytic degradation. Thromb Haemost. 2000;83:736–741. [PubMed] [Google Scholar]
- 113.Sahni A, Francis CW. Plasmic degradation modulates activity of fibrinogen-bound fibroblast growth factor-2. J Thromb Haemost. 2003;1:1271–1277. doi: 10.1046/j.1538-7836.2003.00228.x. [DOI] [PubMed] [Google Scholar]
- 114.Sauer H, Ravindran F, Beldoch M, Sharifpanah F, Jedelska J, Strehlow B, Wartenberg M. alpha2-Macroglobulin enhances vasculogenesis/angiogenesis of mouse embryonic stem cells by stimulation of nitric oxide generation and induction of fibroblast growth factor-2 expression. Stem Cells Dev. 2013;22:1443–1454. doi: 10.1089/scd.2012.0640. [DOI] [PubMed] [Google Scholar]
- 115.Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Brakenhielm E, Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sulpice E, Bryckaert M, Lacour J, Contreres JO, Tobelem G. Platelet factor 4 inhibits FGF2-induced endothelial cell proliferation via the extracellular signal-regulated kinase pathway but not by the phosphatidylinositol 3-kinase pathway. Blood. 2002;100:3087–3094. doi: 10.1182/blood.V100.9.3087. [DOI] [PubMed] [Google Scholar]
- 117.Perollet C, Han ZC, Savona C, Caen JP, Bikfalvi A. Platelet factor 4 modulates fibroblast growth factor 2 (FGF-2) activity and inhibits FGF-2 dimerization. Blood. 1998;91:3289–3299. [PubMed] [Google Scholar]
- 118.Mignatti P, Mazzieri R, Rifkin DB. Expression of the urokinase receptor in vascular endothelial cells is stimulated by basic fibroblast growth factor. J Cell Biol. 1991;113:1193–1201. doi: 10.1083/jcb.113.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spinetti G, Camarda G, Bernardini G, Romano Di Peppe S, Capogrossi MC, Napolitano M. The chemokine CXCL13 (BCA-1) inhibits FGF-2 effects on endothelial cells. Biochem Biophys Res Commun. 2001;289:19–24. doi: 10.1006/bbrc.2001.5924. [DOI] [PubMed] [Google Scholar]
- 120.Andoh A, Bamba S, Fujino S, Inatomi O, Zhang Z, Kim S, Takayanagi A, Shimizu N, Fujiyama Y. Fibroblast growth factor-2 stimulates interleukin-6 secretion in human pancreatic periacinar myofibroblasts. Pancreas. 2004;29:278–283. doi: 10.1097/00006676-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 121.Delrieu I, Arnaud E, Ferjoux G, Bayard F, Faye JC. Overexpression of the FGF-2 24-kDa isoform up-regulates IL-6 transcription in NIH-3T3 cells. FEBS Lett. 1998;436:17–22. doi: 10.1016/s0014-5793(98)01086-2. [DOI] [PubMed] [Google Scholar]
- 122.Jee SH, Chu CY, Chiu HC, Huang YL, Tsai WL, Liao YH, Kuo ML. Interleukin-6 induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-kinase/Akt pathways. J Invest Dermatol. 2004;123:1169–1175. doi: 10.1111/j.0022-202X.2004.23497.x. [DOI] [PubMed] [Google Scholar]
- 123.Lau M-T, So W-K, Leung PC. Fibroblast growth factor 2 induces E-cadherin down-regulation via PI3K/Akt/mTOR and MAPK/ERK signaling in ovarian cancer cells. PLoS One. 2013;8:e59083. doi: 10.1371/journal.pone.0059083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maloof P, Wang Q, Wang H, Stein D, Denny TN, Yahalom J, Fenig E, Wieder R. Overexpression of basic fibroblast growth factor (FGF-2) downregulates Bcl-2 and promotes apoptosis in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1999;56:151–165. doi: 10.1023/a:1006258510381. [DOI] [PubMed] [Google Scholar]
- 125.Cao X-C, Zhang W-R, Cao W-F, Liu B-W, Zhang F, Zhao H-M, Meng R, Zhang L, Niu R-F, Hao X-S. Aquaporin3 is required for FGF-2-induced migration of human breast cancers. PLoS One. 2013;8:e56735. doi: 10.1371/journal.pone.0056735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bossard C, Laurell H, Van den Berghe L, Meunier S, Zanibellato C, Prats H. Translokin is an intracellular mediator of FGF-2 trafficking. Nat Cell Biol. 2003;5:433–439. doi: 10.1038/ncb979. [DOI] [PubMed] [Google Scholar]
- 127.Yu P-J, Ferrari G, Pirelli L, Galloway AC, Mignatti P, Pintucci G. Thrombin cleaves the high molecular weight forms of basic fibroblast growth factor (FGF-2): a novel mechanism for the control of FGF-2 and thrombin activity. Oncogene. 2008;27:2594–2601. doi: 10.1038/sj.onc.1210899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, Wellstein A. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem. 2001;276:40247–40253. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]
- 129.Gazzaniga P, Gandini O, Gradilone A, Silvestri I, Giuliani L, Magnanti M, Gallucci M, Saccani G, Frati L, Agliano A. Detection of basic fibroblast growth factor mRNA in urinary bladder cancer: correlation with local relapses. Int J Oncol. 1999;14:1123–1130. doi: 10.3892/ijo.14.6.1123. [DOI] [PubMed] [Google Scholar]
- 130.Szatmari T, Mundt F, Heidari-Hamedani G, Zong F, Ferolla E, Alexeyenko A, Hjerpe A, Dobra K. Novel genes and pathways modulated by syndecan-1: implications for the proliferation and cell-cycle regulation of malignant mesothelioma cells. PLoS One. 2012;7:e48091. doi: 10.1371/journal.pone.0048091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, Moghaddam A, Whitehouse R, Bicknell R, Harris AL. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor −1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 132.Visscher D, DeMattia F, Ottosen S, Sarkar F, Crissman J. Biologic and clinical significance of basic fibroblast growth factor immunostaining in breast carcinoma. Mod Pathol. 1995;8:665–670. [PubMed] [Google Scholar]
- 133.Granato AM, Nanni O, Falcini F, Folli S, Mosconi G, De Paola F, Medri L, Amadori D, Volpi A. Basic fibroblast growth factor and vascular endothelial growth factor serum levels in breast cancer patients and healthy women: useful as diagnostic tools. Breast Cancer Res. 2004;6:R38–45. doi: 10.1186/bcr745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Faridi A, Rudlowski C, Biesterfeld S, Schuh S, Rath W, Schröder W. Long-term follow-up and prognostic significance of angiogenic basic fibroblast growth factor (bFGF) expression in patients with breast cancer. Pathol Res Pract. 2002;198:1–5. doi: 10.1078/0344-0338-00176. [DOI] [PubMed] [Google Scholar]
- 135.Smith K, Fox S, Whitehouse R, Taylor M, Greenall M, Clarke J, Harris A. Upregulation of basic fibroblast growth factor in breast carcinoma and its relationship to vascular density, oestrogen receptor, epidermal growth factor receptor and survival. Ann Oncol. 1999;10:707–713. doi: 10.1023/a:1008303614441. [DOI] [PubMed] [Google Scholar]
- 136.Hsiung R, Zhu W, Klein G, Qin W, Rosenberg A, Park P, Rosato E, Sauter E. High basic fibroblast growth factor levels in nipple aspirate fluid are correlated with breast cancer. Cancer J. 2002;8:303–310. doi: 10.1097/00130404-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 137.Lee HJ, Seo AN, Park SY, Kim JY, Park JY, Yu JH, Ahn J-H, Gong G. Low Prognostic Implication of Fibroblast Growth Factor Family Activation in Triple-negative Breast Cancer Subsets. Ann Surg Oncol. 2014;21:1561–1568. doi: 10.1245/s10434-013-3456-x. [DOI] [PubMed] [Google Scholar]
- 138.George M, Tutton M, Abulafi A, Eccles S, Swift R. Plasma basic fibroblast growth factor levels in colorectal cancer: a clinically useful assay? Clin Exp Metastasis. 2002;19:735–738. doi: 10.1023/a:1021322201816. [DOI] [PubMed] [Google Scholar]
- 139.Xu C-J, Mikami T, Nakamura T, Tsuruta T, Nakada N, Yanagisawa N, Jiang S-X, Okayasu I. Tumor budding, myofibroblast proliferation, and fibrosis in obstructing colon carcinoma: The roles of Hsp47 and basic fibroblast growth factor. Pathol Res Pract. 2013;209:69–74. doi: 10.1016/j.prp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 140.Dobrzycka B, Mackowiak-Matejczyk B, Kinalski M, Terlikowski SJ. Pretreatment serum levels of bFGF and VEGF and its clinical significance in endometrial carcinoma. Gynecol Oncol. 2013;128:454–460. doi: 10.1016/j.ygyno.2012.11.035. [DOI] [PubMed] [Google Scholar]
- 141.Chen Y, Li X, Yang H, Xia Y, Guo L, Wu X, He C, Lu Y. Expression of basic fibroblast growth factor, CD31, and -smooth muscle actin and esophageal cancer recurrence after definitive chemoradiation. Tumour Biol. 2014;35:7275–7282. doi: 10.1007/s13277-014-1987-9. [DOI] [PubMed] [Google Scholar]
- 142.Takahashi JA, Fukumoto M, Igarashi K, Oda Y, Kikuchi H, Hatanaka M. Correlation of basic fibroblast growth factor expression levels with the degree of malignancy and vascularity in human gliomas. J Neurosurg. 1992;76:792–798. doi: 10.3171/jns.1992.76.5.0792. [DOI] [PubMed] [Google Scholar]
- 143.Bian X-W, Du L-L, Shi J-Q, Cheng Y-S, Liu F-X. Correlation of bFGF, FGFR-1 and VEGF expression with vascularity and malignancy of human astrocytomas. Anal Quant Cytol Histol. 2000;22:267–274. [PubMed] [Google Scholar]
- 144.Dellacono FR, Spiro J, Eisma R, Kreutzer D. Expression of basic fibroblast growth factor and its receptors by head and neck squamous carcinoma tumor and vascular endothelial cells. Am J Surg. 1997;174:540–544. doi: 10.1016/s0002-9610(97)00169-4. [DOI] [PubMed] [Google Scholar]
- 145.Poon RT-P, Ng IO-L, Lau C, Yu W-C, Fan S-T, Wong J. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182:298–304. doi: 10.1016/s0002-9610(01)00708-5. [DOI] [PubMed] [Google Scholar]
- 146.Kin M, Sata M, Ueno T, Torimura T, Inuzuka S, Tsuji R, Sujaku K, Sakamoto M, Sugawara H, Tamaki S. Basic fibroblast growth factor regulates proliferation and motility of human hepatoma cells by an autocrine mechanism. J Hepatol. 1997;27:677–687. doi: 10.1016/s0168-8278(97)80085-2. [DOI] [PubMed] [Google Scholar]
- 147.Ueno K, Inoue Y, Kawaguchi T, Hosoe S, Kawahara M. Increased serum levels of basic fibroblast growth factor in lung cancer patients: relevance to response of therapy and prognosis. Lung Cancer. 2001;31:213–219. doi: 10.1016/s0169-5002(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 148.Ruotsalainen T, Joensuu H, Mattson K, Salven P. High pretreatment serum concentration of basic fibroblast growth factor is a predictor of poor prognosis in small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1492–1495. [PubMed] [Google Scholar]
- 149.Joensuu H, Anttonen A, Eriksson M, Mäkitaro R, Alfthan H, Kinnula V, Leppä S. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res. 2002;62:5210–5217. [PubMed] [Google Scholar]
- 150.Naumnik W, Ossoliska M, Poska I, Chyczewska E, Nikliski J. Circulating Thrombospondin-2 and FGF-2 in Patients with Advanced Non-small Cell Lung Cancer: Correlation with Survival. Adv Exp Med Biol. 2015;833:9–14. doi: 10.1007/5584_2014_78. [DOI] [PubMed] [Google Scholar]
- 151.Fiuraskova M, Brychtova S, Sedlakova E, Benes P, Zalesak B, Hlobilkova A, Tichy M, Kolar Z. Molecular changes in PDEGF and bFGF in malignant melanomas in relation to the stromal microenvironment. Anticancer Res. 2005;25(6B):4299–4303. [PubMed] [Google Scholar]
- 152.Hase T, Kawashiri S, Tanaka A, Nozaki S, Noguchi N, Kato K, Nakaya H, Nakagawa K. Correlation of basic fibroblast growth factor expression with the invasion and the prognosis of oral squamous cell carcinoma. J Oral Pathol Med. 2006;35:136–139. doi: 10.1111/j.1600-0714.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- 153.Ren T, Qing Y, Dai N, Li M, Qian C, Yang Y, Cheng Y, Li Z, Zhang S, Zhong Z. Apurinic/apyrimidinic endonuclease 1 induced upregulation of fibroblast growth factor 2 and its receptor 3 induces angiogenesis in human osteosarcoma cells. Cancer Sci. 2014;105:186–194. doi: 10.1111/cas.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Madsen CV, Steffensen KD, Olsen DA, Waldstrøm M, SØGAARD CH, Brandslund I, Jakobsen A. Serum platelet-derived growth factor and fibroblast growth factor in patients with benign and malignant ovarian tumors. Anticancer Res. 2012;32:3817–3825. [PubMed] [Google Scholar]
- 155.Le Page C, Ouellet V, Madore J, Hudson TJ, Tonin PN, Provencher DM, MesMasson AM. From gene profiling to diagnostic markers: IL18 and FGF2 complement CA125 as serumbased markers in epithelial ovarian cancer. Int J Cancer. 2006;118:1750–1758. doi: 10.1002/ijc.21521. [DOI] [PubMed] [Google Scholar]
- 156.Yamanaka Y, Friess H, Buchler M, Beger HG, Uchida E, Onda M, Kobrin MS, Korc M. Overexpression of acidic and basic fibroblast growth factors in human pancreatic cancer correlates with advanced tumor stage. Cancer Res. 1993;53:5289–5296. [PubMed] [Google Scholar]
- 157.Coleman SJ, Chioni AM, Ghallab M, Anderson RK, Lemoine NR, Kocher HM, Grose RP. Nuclear translocation of FGFR1 and FGF2 in pancreatic stellate cells facilitates pancreatic cancer cell invasion. EMBO Mol Med. 2014;6:467–481. doi: 10.1002/emmm.201302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meyer GE, Yu E, Siegal JA, Petteway JC, Blumenstein BA, Brawer MK. Serum basic fibroblast growth factor in men with and without prostate carcinoma. Cancer. 1995;76:2304–2311. doi: 10.1002/1097-0142(19951201)76:11<2304::aid-cncr2820761119>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 159.Cronauer MV, Hittmair A, Eder IE, Hobisch A, Culig Z, Ramoner R, Zhang J, Bartsch G, Reissigl A, Radmayr C. Basic fibroblast growth factor levels in cancer cells and in sera of patients suffering from proliferative disorders of the prostate. Prostate. 1997;31:223–233. doi: 10.1002/(sici)1097-0045(19970601)31:4<223::aid-pros3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 160.Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5:1063–1071. [PubMed] [Google Scholar]
- 161.Rasmuson T, Grankvist K, Jacobsen J, Ljungberg B. Impact of serum basic fibroblast growth factor on prognosis in human renal cell carcinoma. Eur J Cancer. 2001;37:2199–2203. doi: 10.1016/s0959-8049(01)00290-8. [DOI] [PubMed] [Google Scholar]
- 162.Aigner A, Brachmann P, Beyer J, Jäger R, Raulais D, Vigny M, Neubauer A, Heidenreich A, Weinknecht S, Czubayko F. Marked increase of the growth factors pleiotrophin and fibroblast growth factor-2 in serum of testicular cancer patients. Ann Oncol. 2003;14:1525–1529. doi: 10.1093/annonc/mdg416. [DOI] [PubMed] [Google Scholar]
- 163.Pasieka Z, Stpie H, Komorowski J, Koomecki K, Kuzdak K. Evaluation of the levels of bFGF, VEGF, sICAM-1, and sVCAM-1 in serum of patients with thyroid cancer. Recent Results Cancer Res. 2003;162:189–194. doi: 10.1007/978-3-642-59349-9_18. [DOI] [PubMed] [Google Scholar]
- 164.Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O'Brien S, Keating M, Freireich E, Albitar M. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]
- 165.Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150:815–821. [PMC free article] [PubMed] [Google Scholar]
- 166.Bieker R, Padro T, Kramer J, Steins M, Kessler T, Retzlaff S, Herrera F, Kienast J, Berdel WE, Mesters RM. Overexpression of basic fibroblast growth factor and autocrine stimulation in acute myeloid leukemia. Cancer Res. 2003;63:7241–7246. [PubMed] [Google Scholar]
- 167.Krejci P, Dvorakova D, Krahulcova E, Pachernik J, Mayer J, Hampl A, Dvorak P. FGF-2 abnormalities in B cell chronic lymphocytic and chronic myeloid leukemias. Leukemia. 2001;15:228–237. doi: 10.1038/sj.leu.2402012. [DOI] [PubMed] [Google Scholar]
- 168.Kini AR, Kay NE, Peterson LC. Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia. 2000;14:1414–1418. doi: 10.1038/sj.leu.2401825. [DOI] [PubMed] [Google Scholar]
- 169.Menzel T, Rahman Z, Calleja E, White K, Wilson EL, Wieder R, Gabrilove J. Elevated intracellular level of basic fibroblast growth factor correlates with stage of chronic lymphocytic leukemia and is associated with resistance to fludarabine. Blood. 1996;87:1056–1063. [PubMed] [Google Scholar]
- 170.Gruber G, Schwarzmeier JD, Shehata M, Hilgarth M, Berger R. Basic fibroblast growth factor is expressed by CD19/CD11c-positive cells in hairy cell leukemia. Blood. 1999;94:1077–1085. [PubMed] [Google Scholar]
- 171.Giles FJ, Vose JM, Do KA, Johnson MM, Manshouri T, Bociek G, Bierman PJ, O'Brien SM, Kantarjian HM, Armitage JO, Albitar M. Clinical relevance of circulating angiogenic factors in patients with non-Hodgkin's lymphoma or Hodgkin's lymphoma. Leuk Res. 2004;28:595–604. doi: 10.1016/j.leukres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 172.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- 173.Sezer O, Jakob C, Eucker J, Niemöller K, Gatz F, Wernecke KD, Possinger K. Serum levels of the angiogenic cytokines basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in multiple myeloma. Eur J Haematol. 2001;66:83–88. doi: 10.1034/j.1600-0609.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- 174.Pazgal I, Zimra Y, Tzabar C, Okon E, Rabizadeh E, Shaklai M, Bairey O. Expression of basic fibroblast growth factor is associated with poor outcome in non-Hodgkin's lymphoma. Br J Cancer. 2002;86:1770–1775. doi: 10.1038/sj.bjc.6600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Salven P, Teerenhovi L, Joensuu H. A high pretreatment serum basic fibroblast growth factor concentration is an independent predictor of poor prognosis in non-Hodgkin's lymphoma. Blood. 1999;94:3334–3339. [PubMed] [Google Scholar]
- 176.Ho CL, Sheu LF, Li CY. Immunohistochemical expression of basic fibroblast growth factor, vascular endothelial growth factor, and their receptors in stage IV non-Hodgkin lymphoma. Appl Immunohistochem Mol Morphol. 2002;10:316–321. doi: 10.1097/00129039-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 177.Gonzalez RM, Seurynck-Servoss SL, Crowley SA, Brown M, Omenn GS, Hayes DF, Zangar RC. Development and validation of sandwich ELISA microarrays with minimal assay interference. J Proteome Res. 2008;7:2406–2414. doi: 10.1021/pr700822t. [DOI] [PubMed] [Google Scholar]
- 178.Rodriguez-Porcel M, Zhu X-Y, Chade AR, Amores-Arriaga B, Caplice NM, Ritman EL, Lerman A, Lerman LO. Functional and structural remodeling of the myocardial microvasculature in early experimental hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H978–H984. doi: 10.1152/ajpheart.00538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang L, Park H, Chhim S, Ding Y, Jiang W, Queen C, Kim KJ. A novel monoclonal antibody to fibroblast growth factor 2 effectively inhibits growth of hepatocellular carcinoma xenografts. Mol Cancer Ther. 2012;11:864–872. doi: 10.1158/1535-7163.MCT-11-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zong F, Fthenou E, Wolmer N, Hollosi P, Kovalszky I, Szilak L, Mogler C, Nilsonne G, Tzanakakis G, Dobra K. Syndecan-1 and FGF-2, but not FGF receptor-1, share a common transport route and co-localize with heparanase in the nuclei of mesenchymal tumor cells. PLoS One. 2009;4:e7346. doi: 10.1371/journal.pone.0007346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Prasse AA, Zauner T, Büttner K, Hoffmann R, Zuchner T. Improvement of an antibody-enzyme coupling yield by enzyme surface supercharging. BMC biotechnology. 2014;14:88. doi: 10.1186/s12896-014-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Candela ME, Geraci F, Turturici G, Taverna S, Albanese I, Sconzo G. Membrane vesicles containing matrix metalloproteinase9 and fibroblast growth factor2 are released into the extracellular space from mouse mesoangioblast stem cells. J Cell Physiol. 2010;224:144–151. doi: 10.1002/jcp.22111. [DOI] [PubMed] [Google Scholar]
- 183.Gutiérrez J, Brandan E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol. 2010;30:1634–1649. doi: 10.1128/MCB.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Waisberg DR, Parra ER, Barbas-Filho JV, Fernezlian S, Capelozzi VL. Increased fibroblast telomerase expression precedes myofibroblast -smooth muscle actin expression in idiopathic pulmonary fibrosis. Clinics. 2012;67:1039–1046. doi: 10.6061/clinics/2012(09)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.DO MKQ, Suzuki T, Gerelt B, Sato Y, Mizunoya W, Nakamura M, Ikeuchi Y, Anderson JE, Tatsumi R. Timecoordinated prevalence of extracellular HGF, FGF2 and TGF3 in crushinjured skeletal muscle. Anim Sci J. 2012;83:712–717. doi: 10.1111/j.1740-0929.2012.01057.x. [DOI] [PubMed] [Google Scholar]
- 186.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Slaton JW, Perrotte P, Inoue K, Dinney CP, Fidler IJ. Interferon-alpha-mediated down-regulation of angiogenesis-related genes and therapy of bladder cancer are dependent on optimization of biological dose and schedule. Clin Cancer Res. 1999;5:2726–2734. [PubMed] [Google Scholar]
- 188.Sun XH, Geng XL, Zhang J, Zhang C. miRNA-646 suppresses osteosarcoma cell metastasis by downregulating fibroblast growth factor 2 (FGF2) Tumour Biol. 2015;36:2127–2134. doi: 10.1007/s13277-014-2822-z. [DOI] [PubMed] [Google Scholar]
- 189.Colombo G, Margosio B, Ragona L, Neves M, Bonifacio S, Annis DS, Stravalaci M, Tomaselli S, Giavazzi R, Rusnati M, Presta M, Zetta L, Mosher DF, Ribatti D, Gobbi M, Taraboletti G. Non-peptidic thrombospondin-1 mimics as fibroblast growth factor-2 inhibitors: an integrated strategy for the development of new antiangiogenic compounds. J Biol Chem. 2010;285:8733–8742. doi: 10.1074/jbc.M109.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Smith JA, Madden T, Vijjeswarapu M, Newman RA. Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem Pharmacol. 2001;62:469–472. doi: 10.1016/s0006-2952(01)00690-6. [DOI] [PubMed] [Google Scholar]
- 191.Alessi P, Leali D, Camozzi M, Cantelmo A, Albini A, Presta M. Anti-FGF2 approaches as a strategy to compensate resistance to anti-VEGF therapy: long-pentraxin 3 as a novel antiangiogenic FGF2-antagonist. Eur Cytokine Netw. 2009;20:225–234. doi: 10.1684/ecn.2009.0175. [DOI] [PubMed] [Google Scholar]
- 192.Inoue K, Chikazawa M, Fukata S, Yoshikawa C, Shuin T. Frequent administration of angiogenesis inhibitor TNP-470 (AGM-1470) at an optimal biological dose inhibits tumor growth and metastasis of metastatic human transitional cell carcinoma in the urinary bladder. Clin Cancer Res. 2002;8:2389–2398. [PubMed] [Google Scholar]
- 193.Marshall JL, Wellstein A, Rae J, DeLap RJ, Phipps K, Hanfelt J, Yunmbam MK, Sun JX, Duchin KL, Hawkins MJ. Phase I trial of orally administered pentosan polysulfate in patients with advanced cancer. Clin Cancer Res. 1997;3(12 Pt 1):2347–2354. [PubMed] [Google Scholar]
- 194.Ferro V, Dredge K, Liu L, Hammond E, Bytheway I, Li C, Johnstone K, Karoli T, Davis K, Copeman E, Gautam A. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin Thromb Hemost. 2007;33:557–568. doi: 10.1055/s-2007-982088. [DOI] [PubMed] [Google Scholar]
- 195.Zhou S, Wang F, Hsieh TC, Wu JM, Wu E. Thalidomide-a notorious sedative to a wonder anticancer drug. Curr Med Chem. 2013;20:4102–4108. doi: 10.2174/09298673113209990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liekens S, Leali D, Neyts J, Esnouf R, Rusnati M, Dell'Era P, Maudgal PC, De Clercq E, Presta M. Modulation of fibroblast growth factor-2 receptor binding, signaling, and mitogenic activity by heparin-mimicking polysulfonated compounds. Mol Pharmacol. 1999;56:204–213. doi: 10.1124/mol.56.1.204. [DOI] [PubMed] [Google Scholar]
- 197.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35(3 Suppl):7S–14S. doi: 10.1016/s0041-1345(03)00211-2. [DOI] [PubMed] [Google Scholar]
- 198.Tayel A, Abd El Galil KH, Ebrahim MA, Ibrahim AS, El-Gayar AM, Al-Gayyar MM. Suramin inhibits hepatic tissue damage in hepatocellular carcinoma through deactivation of heparanase enzyme. Eur J Pharmacol. 2014;728:151–160. doi: 10.1016/j.ejphar.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 199.Hagedorn M, Zilberberg L, Lozano RM, Cuevas P, Canron X, Redondo-Horcajo M, Gimenez-Gallego G, Bikfalvi A. A short peptide domain of platelet factor 4 blocks angiogenic key events induced by FGF-2. FASEB J. 2001;15:550–552. doi: 10.1096/fj.00-0285fje. [DOI] [PubMed] [Google Scholar]
- 200.Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008;14:5459–5465. doi: 10.1158/1078-0432.CCR-07-5270. [DOI] [PubMed] [Google Scholar]
- 201.Popat S, Mellemgaard A, Fahrbach K, Martin A, Rizzo M, Kaiser R, Griebsch I, Reck M. Nintedanib plus docetaxel as second-line therapy in patients with non-small-cell lung cancer: a network meta-analysis. Future Oncol. 2015;11:409–420. doi: 10.2217/fon.14.290. [DOI] [PubMed] [Google Scholar]
- 202.Ayers M, Fargnoli J, Lewin A, Wu Q, Platero JS. Discovery and validation of biomarkers that respond to treatment with brivanib alaninate, a small-molecule VEGFR-2/FGFR-1 antagonist. Cancer Res. 2007;67:6899–6906. doi: 10.1158/0008-5472.CAN-06-4555. [DOI] [PubMed] [Google Scholar]
- 203.Ueda Y, Shimoyama T, Murakami H, Yamamoto N, Yamada Y, Arioka H, Tamura T. Phase I and pharmacokinetic study of TSU-68, a novel multiple receptor tyrosine kinase inhibitor, by twice daily oral administration between meals in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011;67:1101–1109. doi: 10.1007/s00280-010-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Inaba Y, Kanai F, Aramaki T, Yamamoto T, Tanaka T, Yamakado K, Kaneko S, Kudo M, Imanaka K, Kora S, Nishida N, Kawai N, Seki H, Matsui O, Arioka H, Arai Y. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Eur J Cancer. 2013;49:2832–2840. doi: 10.1016/j.ejca.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 205.Angevin E, Lopez-Martin JA, Lin CC, Gschwend JE, Harzstark A, Castellano D, Soria JC, Sen P, Chang J, Shi M, Kay A, Escudier B. Phase I study of dovitinib (TKI258), an oral FGFR, VEGFR, and PDGFR inhibitor, in advanced or metastatic renal cell carcinoma. Clin Cancer Res. 2013;19:1257–1268. doi: 10.1158/1078-0432.CCR-12-2885. [DOI] [PubMed] [Google Scholar]
- 206.Bello E, Colella G, Scarlato V, Oliva P, Berndt A, Valbusa G, Serra SC, D'Incalci M, Cavalletti E, Giavazzi R, Damia G, Camboni G. E-3810 is a potent dual inhibitor of VEGFR and FGFR that exerts antitumor activity in multiple preclinical models. Cancer Res. 2011;71:1396–1405. doi: 10.1158/0008-5472.CAN-10-2700. [DOI] [PubMed] [Google Scholar]
- 207.Sequist LV, Cassier P, Varga A, Tabernero J, Schellens JH, Delord J-P, LoRusso P, Camidge DR, Medina MH, Schuler M. Abstract CT326: Phase I study of BGJ398, a selective pan-FGFR inhibitor in genetically preselected advanced solid tumors. Cancer Res. 2014;74(19 Supplement):CT326–CT326. [Google Scholar]
- 208.Perera T, Jovcheva E, Vialard J, Verhulst T, Esser N, Wroblowski B, Gilissen R, Freyne E, King P, Platero S. JNJ-42756493 is an inhibitor of FGFR-1, 2, 3 and 4 with nanomolar affinity for targeted therapy. Cancer Res. 2014;74(19 Supplement):1738–1738. [Google Scholar]
- 209.Dransfield D, Lee J, Waghorne C, Bull C, Savage RE, Zhao X, Yuan S, Chang E, Nakuci E, Eathiraj S. Abstract A278: ARQ 087, a multi-tyrosine kinase inhibitor with potent in vitro and in vivo activity in FGFR2 driven models. Mol Cancer Ther. 2013;12(11 Supplement):A278–A278. [Google Scholar]
- 210.Zhao G, Li W-y, Chen D, Henry JR, Li H-Y, Chen Z, Zia-Ebrahimi M, Bloem L, Zhai Y, Huss K. A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol Cancer Ther. 2011;10:2200–2210. doi: 10.1158/1535-7163.MCT-11-0306. [DOI] [PubMed] [Google Scholar]
- 211.Ochiiwa H, Fujita H, Itoh K, Sootome H, Hashimoto A, Fujioka Y, Nakatsuru Y, Oda N, Yonekura K, Hirai H. Abstract A270: TAS-120, a highly potent and selective irreversible FGFR inhibitor, is effective in tumors harboring various FGFR gene abnormalities. Mol Cancer Ther. 2013;12(11 Supplement):A270–A270. [Google Scholar]
- 212.Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K. Antibody-based targeting of FGFR3 in bladder carcinoma and t (4; 14)-positive multiple myeloma in mice. J Clin Invest. 2009;119:1216. doi: 10.1172/JCI38017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Trudel S, Bergsagel PL, Singhal S, Niesvizky R, Comenzo RL, Bensinger WI, Lebovic D, Choi Y, Lu D, French D. A phase I study of the safety and pharmacokinetics of escalating doses of MFGR1877S, a fibroblast growth factor receptor 3 (FGFR3) antibody, in patients with relapsed or refractory t (4; 14)-positive multiple myeloma. ASH Annual Meeting Abstracts. 2012:4029. [Google Scholar]
- 214.Batley BL, Doherty AM, Hamby JM, Lu GH, Keller P, Dahring TK, Hwang O, Crickard K, Panek RL. Inhibition of FGF-1 receptor tyrosine kinase activity by PD 161570, a new protein-tyrosine kinase inhibitor. Life Sci. 1997;62:143–150. doi: 10.1016/s0024-3205(97)01060-6. [DOI] [PubMed] [Google Scholar]
- 215.Pardo OE, Latigo J, Jeffery RE, Nye E, Poulsom R, Spencer-Dene B, Lemoine NR, Stamp GW, Aboagye EO, Seckl MJ. The fibroblast growth factor receptor inhibitor PD173074 blocks small cell lung cancer growth in vitro and in vivo. Cancer Res. 2009;69:8645–8651. doi: 10.1158/0008-5472.CAN-09-1576. [DOI] [PubMed] [Google Scholar]
- 216.Panek RL, Lu GH, Klutchko SR, Batley BL, Dahring TK, Hamby JM, Hallak H, Doherty AM, Keiser JA. In vitro pharmacological characterization of PD 166285, a new nanomolar potent and broadly active protein tyrosine kinase inhibitor. J Pharmacol Exp Ther. 1997;283:1433–1444. [PubMed] [Google Scholar]
- 217.Panek RL, Lu GH, Dahring TK, Batley BL, Connolly C, Hamby JM, Brown KJ. In vitro biological characterization and antiangiogenic effects of PD 166866, a selective inhibitor of the FGF-1 receptor tyrosine kinase. J Pharmacol Exp Ther. 1998;286:569–577. [PubMed] [Google Scholar]
- 218.Fischer H, Taylor N, Allerstorfer S, Grusch M, Sonvilla G, Holzmann K, Setinek U, Elbling L, Cantonati H, Grasl-Kraupp B. Fibroblast growth factor receptor-mediated signals contribute to the malignant phenotype of non-small cell lung cancer cells: therapeutic implications and synergism with epidermal growth factor receptor inhibition. Mol Cancer Ther. 2008;7:3408–3419. doi: 10.1158/1535-7163.MCT-08-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhou W, Hur W, McDermott U, Dutt A, Xian W, Ficarro SB, Zhang J, Sharma SV, Brugge J, Meyerson M. A structure-guided approach to creating covalent FGFR inhibitors. Chem Biol. 2010;17:285–295. doi: 10.1016/j.chembiol.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Herbert C, Schieborr U, Saxena K, Juraszek J, De Smet F, Alcouffe C, Bianciotto M, Saladino G, Sibrac D, Kudlinzki D. Molecular mechanism of SSR128129E, an extracellularly acting, small-molecule, allosteric inhibitor of FGF receptor signaling. Cancer Cell. 2013;23:489–501. doi: 10.1016/j.ccr.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 221.Bono F, De Smet F, Herbert C, De Bock K, Georgiadou M, Fons P, Tjwa M, Alcouffe C, Ny A, Bianciotto M. Inhibition of tumor angiogenesis and growth by a small-molecule multi-FGF receptor blocker with allosteric properties. Cancer cell. 2013;23:477–488. doi: 10.1016/j.ccr.2013.02.019. [DOI] [PubMed] [Google Scholar]