Abstract
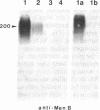
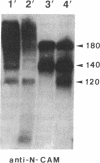
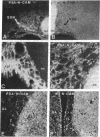
The neural cell adhesion molecule, N-CAM, changes at the cell surface during development, from a highly sialylated form [polysialic acid (PSA)-linked N-CAM, PSA-N-CAM] to several isoforms containing less sialic acid. N-CAM and its polysialic acid may serve to regulate cell apposition, thus affecting a variety of cell interactions. In the nervous system, PSA-N-CAM has until now been localized in developing tissues where it is thought to participate in the structuring of neuronal groups and tissue pattern formation. It has been proposed, however, that PSA-N-CAM may also be expressed in the adult, where it may take part in plasticity and cell reshaping. In the present study, the use of immunoblot and immunocytochemical procedures with a monoclonal antibody that specifically recognizes PSA-N-CAM revealed that the adult rat hypothalamo-neurohypophysial system, which undergoes important neuronal-glial and synaptic rearrangements in response to physiological stimuli, contains high levels of PSA-N-CAM immunoreactivity. The use of a polyclonal serum reacting with all N-CAM isoforms indicated that PSA-N-CAM is expressed together with "adult" forms of N-CAM. Light and electron microscopy demonstrated the presence of PSA-N-CAM immunoreactivity in the supraoptic and paraventricular nuclei of the hypothalamus and in the neurohypophysis; the immunoreactivity was seen in dendrites, axons, and terminals and in associated astrocytes but not in neuronal somata. We propose that the continued expression of PSA-N-CAM confers to magnocellular neurons and their astrocytes the ability to reversibly change their morphology in adulthood. In addition, our observations suggest that evidence for polysialylation may serve to identify other neuronal systems capable of morphological plasticity in the adult central nervous system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaron L. I., Chesselet M. F. Heterogeneous distribution of polysialylated neuronal-cell adhesion molecule during post-natal development and in the adult: an immunohistochemical study in the rat brain. Neuroscience. 1989;28(3):701–710. doi: 10.1016/0306-4522(89)90015-8. [DOI] [PubMed] [Google Scholar]
- Beasley L., Stallcup W. B. The nerve growth factor-inducible large external (NILE) glycoprotein and neural cell adhesion molecule (N-CAM) have distinct patterns of expression in the developing rat central nervous system. J Neurosci. 1987 Mar;7(3):708–715. doi: 10.1523/JNEUROSCI.07-03-00708.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. B., Theodosis D. T., Montagnese C., Poulain D. A., Morris J. F. Osmotic stimulation causes structural plasticity of neurone-glia relationships of the oxytocin but not vasopressin secreting neurones in the hypothalamic supraoptic nucleus. Neuroscience. 1986 Mar;17(3):679–686. doi: 10.1016/0306-4522(86)90039-4. [DOI] [PubMed] [Google Scholar]
- Cole G. J., Glaser L. A heparin-binding domain from N-CAM is involved in neural cell-substratum adhesion. J Cell Biol. 1986 Feb;102(2):403–412. doi: 10.1083/jcb.102.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol. 1986 Mar;102(3):716–730. doi: 10.1083/jcb.102.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossin K. L., Chuong C. M., Edelman G. M. Expression sequences of cell adhesion molecules. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6942–6946. doi: 10.1073/pnas.82.20.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniloff J. K., Chuong C. M., Levi G., Edelman G. M. Differential distribution of cell adhesion molecules during histogenesis of the chick nervous system. J Neurosci. 1986 Mar;6(3):739–758. doi: 10.1523/JNEUROSCI.06-03-00739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M., Marshall P., Covault J., Yamamoto M. Ultrastructural localization of molecular subtypes of immunoreactive neural cell adhesion molecule (NCAM) in the adult rodent striatum. J Neurosci. 1989 Dec;9(12):4158–4168. doi: 10.1523/JNEUROSCI.09-12-04158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenrode T. C., Barr G. A., Battisti W. P., Murray M. Acetylcholine in the interpeduncular nucleus of the rat: normal distribution and effects of deafferentation. Brain Res. 1987 Aug 25;418(2):273–286. doi: 10.1016/0006-8993(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Modulation of cell adhesion during induction, histogenesis, and perinatal development of the nervous system. Annu Rev Neurosci. 1984;7:339–377. doi: 10.1146/annurev.ne.07.030184.002011. [DOI] [PubMed] [Google Scholar]
- Finne J., Finne U., Deagostini-Bazin H., Goridis C. Occurrence of alpha 2-8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983 Apr 29;112(2):482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- Finne J., Mäkelä P. H. Cleavage of the polysialosyl units of brain glycoproteins by a bacteriophage endosialidase. Involvement of a long oligosaccharide segment in molecular interactions of polysialic acid. J Biol Chem. 1985 Jan 25;260(2):1265–1270. [PubMed] [Google Scholar]
- Hatten M. E. Riding the glial monorail: a common mechanism for glial-guided neuronal migration in different regions of the developing mammalian brain. Trends Neurosci. 1990 May;13(5):179–184. doi: 10.1016/0166-2236(90)90044-b. [DOI] [PubMed] [Google Scholar]
- He H. T., Finne J., Goridis C. Biosynthesis, membrane association, and release of N-CAM-120, a phosphatidylinositol-linked form of the neural cell adhesion molecule. J Cell Biol. 1987 Dec;105(6 Pt 1):2489–2500. doi: 10.1083/jcb.105.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M. R., Gaugler L., Deagostini-Bazin H., Bally-Cuif L., Goridis C. Identification of positive and negative regulatory elements governing cell-type-specific expression of the neural cell adhesion molecule gene. Mol Cell Biol. 1990 May;10(5):1959–1968. doi: 10.1128/mcb.10.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Edelman G. M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. M., Agnew W. S. Multiple oligosaccharide chains in the voltage-sensitive Na channel from electrophorus electricus: evidence for alpha-2,8-linked polysialic acid. Biochem Biophys Res Commun. 1987 Oct 29;148(2):817–826. doi: 10.1016/0006-291x(87)90949-1. [DOI] [PubMed] [Google Scholar]
- Landmesser L., Dahm L., Tang J. C., Rutishauser U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic development. Neuron. 1990 May;4(5):655–667. doi: 10.1016/0896-6273(90)90193-j. [DOI] [PubMed] [Google Scholar]
- Langley O. K., Aletsee-Ufrecht M. C., Grant N. J., Gratzl M. Expression of the neural cell adhesion molecule NCAM in endocrine cells. J Histochem Cytochem. 1989 Jun;37(6):781–791. doi: 10.1177/37.6.2723399. [DOI] [PubMed] [Google Scholar]
- Piekut D. T., Casey S. M. Penetration of immunoreagents in Vibratome-sectioned brain: a light and electron microscopic study. J Histochem Cytochem. 1983 May;31(5):669–674. doi: 10.1177/31.5.6341457. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Brackenbury R., Cunningham B. A., Edelman G. M. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982 Sep 25;257(18):11064–11069. [PubMed] [Google Scholar]
- Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Tissue- and developmental stage-specific forms of a neural cell surface antigen linked to differences in glycosylation of a common polypeptide. EMBO J. 1982;1(10):1239–1244. doi: 10.1002/j.1460-2075.1982.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougon G., Dubois C., Buckley N., Magnani J. L., Zollinger W. A monoclonal antibody against meningococcus group B polysaccharides distinguishes embryonic from adult N-CAM. J Cell Biol. 1986 Dec;103(6 Pt 1):2429–2437. doi: 10.1083/jcb.103.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U., Acheson A., Hall A. K., Mann D. M., Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988 Apr 1;240(4848):53–57. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Watanabe M., Silver J., Troy F. A., Vimr E. R. Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J Cell Biol. 1985 Nov;101(5 Pt 1):1842–1849. doi: 10.1083/jcb.101.5.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoul R., Hirn M., Deagostini-Bazin H., Rougon G., Goridis C. Adult and embryonic mouse neural cell adhesion molecules have different binding properties. 1983 Jul 28-Aug 3Nature. 304(5924):347–349. doi: 10.1038/304347a0. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Chapman D. B., Montagnese C., Poulain D. A., Morris J. F. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986 Mar;17(3):661–678. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Poulain D. A. Neuronal-glial and synaptic plasticity in the adult rat paraventricular nucleus. Brain Res. 1989 Apr 10;484(1-2):361–366. doi: 10.1016/0006-8993(89)90382-x. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Morphological adaptability at neurosecretory axonal endings on the neurovascular contact zone of the rat neurohypophysis. Neuroscience. 1987 Jan;20(1):241–246. doi: 10.1016/0306-4522(87)90016-9. [DOI] [PubMed] [Google Scholar]