Abstract
Endogenous brassinosteroids (BRs) in tomato (Lycopersicon esculentum) seedlings are known to be composed of C27- and C28-BRs. The biosynthetic pathways of C27-BRs were examined using a cell-free enzyme solution prepared from tomato seedlings that yielded the biosynthetic sequences cholesterol → cholestanol and 6-deoxo-28-norteasterone ↔ 6-deoxo-28-nor-3-dehydroteasterone ↔ 6-deoxo-28-nortyphasterol → 6-deoxo-28-norcastasterone → 28-norcastasterone (28-norCS). Arabidopsis CYP85A1 that was heterologously expressed in yeast mediated the conversion of 6-deoxo-28-norCS to 28-norCS. The same reaction was catalyzed by an enzyme solution from wild-type tomato but not by an extract derived from a tomato dwarf mutant with a defect in CYP85. Furthermore, exogenously applied 28-norCS restored the abnormal growth of the dwarf mutant. These findings indicate that the C-6 oxidation of 6-deoxo-28-norCS to 28-norCS in tomato seedlings is catalyzed by CYP85, just as in the conversion of 6-deoxoCS to CS. Additionally, the cell-free solution also catalyzed the C-24 methylation of 28-norCS to CS in the presence of NADPH and S-adenosylmethionine (SAM), a reaction that was clearly retarded in the absence of NADPH and SAM. Thus it seems that C27-BRs, in addition to C28-BRs, are important in the production of more active C28-BRs and CS, where a SAM-dependent sterol methyltransferase appears to biosynthetically connect C27-BRs to C28-BRs. Moreover, the tomato cell-free solution converted CS to 26-norCS and [2H6]CS to [2H3]28-norCS, suggesting that C-28 demethylation is an artifact due to an isotope effect. Although previous feeding experiments employing [2H6]CS suggested that 28-norCS was synthesized from CS in certain plant species, this is not supported in planta. Altogether, this study demonstrated for the first time, to our knowledge, that 28-norCS is not synthesized from CS but from cholesterol. In addition, CS and [2H6]CS were not converted into BL and [2H6]BL, respectively, confirming an earlier finding that the active BR in tomato seedlings is not BL but CS. In conclusion, the biosynthesis of 28-norBRs appears to play a physiologically important role in maintaining homeostatic levels of CS in tomato seedlings.
Brassinosteroid (BR)-deficient mutants such as det2 (Li et al., 1996), dwarf4 (Choe et al., 1998), and cpd (Szekeres et al., 1996) in Arabidopsis, dwarf in tomato (Lycopersicon esculentum; Bishop et al., 1999), and lkb in pea (Pisum sativum; Nomura et al., 1997, 1999) showed reduced shoot elongation, reduced fertility, delayed senescence, altered vasculature, and photomorphogenesis. The pleiotropic abnormal developments can be rescued only by application of BRs. Mutants such as bri1 (Li and Chory, 1997), bin2 (Li et al., 2001; Li and Nam, 2002), bak1 (Nam and Li, 2002) in Arabidopsis, lka in pea (Nomura et al., 1997, 2003), and curl-3 in tomato (Koka et al., 2000) also exhibited similar abnormal phenotypes as found in BR-deficient mutants; however, the phenotype of the mutants could not be restored to that of the wild type by application of BRs, indicating that they are BR-insensitive mutants generated by a disruption in BR signaling. Consequently, BRs are now thought of as essential chemical signals, plant hormones, the endogenous levels of which must be properly maintained in plant cells to facilitate the normal growth and developments of plants.
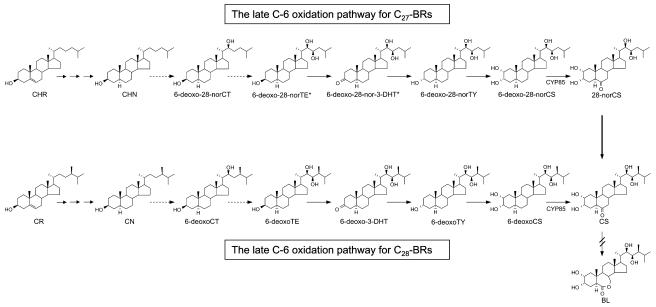
To date, over 50 BRs have been identified from the entire plant kingdom (Fujioka, 1999; Bajguz and Tretyn, 2003). These have been classified as C27-, C28-, and C29-BRs based on the nature of the alkyl groups at the C-24 position in the side chain of a 5α-cholestane carbon skeleton. Among these, C28-BRs possessing a C-24 methyl group such as castasterone (CS) and brassinolide (BL) have been most frequently identified from plant materials. Given their strong biological activities, CS and BL are considered to be the most important BRs in the plant kingdom. The biosynthesis of these BRs in plants has been extensively investigated by employing feeding experiments using isotope-labeled substrates in addition to molecular genetic analyses of BR-deficient mutants (Sakurai, 1999; Bishop and Yokota, 2001; Fujioka and Yokota, 2003). As a result, two parallel pathways, namely the early and late C-6 oxidation pathway for C28-BRs, have been fully established. In the early C-6 oxidation pathway, campestanol (CN) is initially oxidized to 6-oxocampestanol, which then undergoes successive oxidation to cathasterone (CT), teasterone (TE), 3-dehydroteasterone (3-DHT), typhasterol (TY), and CS. In the late C-6 oxidation pathway, CN is initially oxidized at C-22 to yield 6-deoxocathasterone (6-deoxoCT), which then undergoes successive oxidation to 6-deoxoteasterone (6-deoxoTE), 6-deoxo-3-dehydroteasterone (6-deoxo-3-DHT), 6-deoxotyphasterol (6-deoxoTY), 6-deoxocastasterone (6-deoxoCS), and CS (Fig. 1). Finally, CS is converted to BL via 7-oxalactonization. In a recent investigation of endogenous BRs, CT could not be detected in Arabidopsis, pea, tomato (Nomura et al., 2001), and rice (Hong et al., 2002). Consequently, the late C-6 oxidation pathway seems to be predominant in these plants. TE, 3-DHT, and TY frequently detected in plants are likely to be generated via C-6 oxidation of their respective 6-deoxo counterparts. However, even these BRs could not be detected in tomato, indicating that tomato BRs are composed of genuine late C-6 oxidation BRs.
Figure 1.
BR biosynthetic pathways in tomato seedlings. The solid arrows indicate the biosynthetic steps that have been demonstrated. The dotted arrows indicate the biosynthetic steps that have yet to be verified. The presence of BRs with an asterisk has not been demonstrated in plants.
Several genes or enzymes involved in BR biosynthesis have been characterized by molecular analyses of BR biosynthesis mutants. The Arabidopsis DET2 gene possesses sequence identity to mammalian 5α-reductase, which mediates the reduction of testosterone to dihydrotestosterone, indicating that DET2 encodes a steroid 5α-reductase that hydrogenates 24α-methylcholest-4-en-3-one in the conversion of campesterol (CR) to CN (Fujioka et al., 1997; Li et al., 1997; Noguchi et al., 1999). Arabidopsis DWF4 and CPD share high amino acid sequence similarity to mammalian steroid cytochrome P450 monooxygenases (Cyt P450s), and DWF4 and CPD encode Cyt P450s designated as CYP90B1 and 90A1 that catalyze the C-22 and C-23 hydroxylation of BRs, respectively (Szekeres et al., 1996; Choe et al., 1998). The C-6 oxidation of 6-deoxoCS to CS has also been shown to be mediated by a Cyt P450, CYP85, through analysis of the tomato DWARF gene which possesses high homology to the mammalian steroid hydroxylase genes (Bishop et al., 1999). DWARF, its Arabidopsis orthologs AtBR6ox1 (CYP85A1) and AtBR6ox2 (CYP85A2), and its rice ortholog OsDWARF (CYP85A1) have been shown to catalyze the C-6 oxidation of not only 6-deoxoCS but also 6-deoxoTE, 6-deoxo-3-DHT, and 6-deoxoTY (Shimada et al., 2001, 2003; Hong et al., 2002). The pea gene DDWF1 (Dark-induced DWF-like protein 1), which encodes a Cyt P450 (CYP92A6), was claimed to catalyze the C-2 hydroxylation of TY to CS and of 6-deoxoTY to 6-deoxoCS through the action of a small GTP-binding protein, Pra2 (Kang et al., 2001). The in vivo and in vitro C-3 epimerization of 6-deoxoTE and TE to 6-deoxoTY and TY, respectively, is mediated by two or more reversible enzymes. (Choi et al., 1997; Park et al., 1999; Kim et al., 2000c, 2003b; Noguchi et al., 2000). Recently, it was reported that the D2 gene of rice is responsible for the C-3 oxidation involved in C-3 epimerization (Hong et al., 2003). We recently found that in Phaseolus vulgaris, CS 6-oxidase (BL synthase), responsible for the conversion of CS to BL, is located in the endoplasmic reticulum membrane, requires molecular oxygen and NADPH, and is inhibited by Cyt P450 inhibitors including carbon monoxide, the effect of which was reversed by irradiation with blue light (Kim et al., 2004).
C27-BRs such as 28-norcastasterone (28-norCS) occur in various plant species ranging from lower to higher plants (Fujioka, 1999; Bajguz and Tretyn, 2003). However, given their less potent biological activity and thus superficially less frequent occurrence, little attention has been paid to the physiological importance of C27-BRs in plants. C27-BRs possess the same carbon skeleton as that of cholesterol (CHR), suggesting that they are biosynthetically derived from CHR via analogous biosynthetic pathways as C28-BRs. In support of this, the presence of 28-norCS as well as their possible biosynthetic precursors such as CHR, cholestanol (CHN), 6-deoxo-28-norcathasterone (6-deoxo-28-norCT), 6-deoxo-28-nortyphasterol (6-deoxo-28-norTY), and 6-deoxo-28-norcastasterone (6-deoxo-28-norCS) was demonstrated in tomato shoots, where the level of 28-norCS found was comparable to that of CS (Yokota et al., 1997, 2001; Nomura et al., 2001). This finding suggests that the late C-6 oxidation pathway generating C27-BRs is also functional in tomato plants (Yokota et al., 2001). However, metabolic evidence for the presence of a C27-BRs biosynthetic pathway has yet to be obtained.
Some investigations have indicated that 28-norCS frequently cooccurs with CS (Fujioka, 1999; Fujioka et al., 2000). Feeding experiments employing the application of deuterium-labeled CS to seedlings of Arabidopsis, rice, and tomato revealed that [26,28-2H6]CS was converted into [26-2H3]28-norCS (Fujioka et al., 2000). The same demethylation of [26,28-2H6]CN to [26-2H3]28-norCN was also observed in the seedlings of Arabidopsis, rice, and tobacco, as well as in cultured cells of Catharanthus roseus (Nakajima et al., 2002). However, in cell-free enzyme systems derived from P. vulgaris and Marchantia polymorpha, [26,28-2H6]BL and unlabeled BL were metabolized into [26-2H3]28-norBL and unlabeled 26-norBL, respectively, suggesting that natural demethylation of BRs occurs at C-26 but not at C-28, and that C-28 demethylation of [26,28-2H6]BRs is an artifact due to an isotopic effect (Kim et al., 2000a, 2000b). It therefore appears that plant cells recognize [26,28-2H6]BL as a xenobiotic and that deuterium attached to C-28 significantly affected the demethylation reaction due to an isotope effect. Consequently, the biosynthesis of 28-norCS from CS seems unlikely.
In this work, we investigated the biosynthetic pathways of 28-norCS from CHR and related C27 intermediates, in addition to the biosynthetic relationship between 28-norCS and CS, using cell-free preparations derived from tomato seedlings.
RESULTS
28-norCS Is Converted from CHR via C27-BRs in Tomato Enzyme Preparations
Tomato seedlings were homogenized, centrifuged, and precipitated with acetone. The precipitates were dissolved in assay buffer and used as a crude enzyme extract for the in vitro conversion experiments. Since isotope-labeled substrates were not available, unlabeled ([2H0]) CHR, CHN, 6-deoxo-28-norCT, 6-deoxo-28-norteasterone (6-deoxo-28-norTE), 6-deoxo-28-nor-3-dehydroteasterone (6-deoxo-28-nor-3-DHT), 6-deoxo-28-norTY, or 6-deoxo-28-norCS were used as substrates. Enzyme products were purified by HPLC and analyzed by GC-MS or GC-SIM following derivatization. Prior to use in the enzymatic incubations, the absence of expected products in the original enzyme preparations was confirmed by gas chromatography- mass spectrometry (GC-MS) and GC-selected ion monitoring (SIM).
As summarized in Table I, the CHR metabolite, derivatized to the trimethylsilyl ether, yielded an identical mass spectrum and GC retention time to those of authentic CHN trimethylsilyl ether, demonstrating that CHR was converted to CHN in the tomato cell-free system. When 6-deoxo-28-norTE was used, two metabolites consisting of 6-deoxo-28-nor-3-DHT and 6-deoxo-28-norTY were detected, suggesting the conversion of 6-deoxo-28-norTE to 6-deoxo-28-norTY via 6-deoxo-28-nor-3-DHT. 6-Deoxo-28-nor-3-DHT was converted to two metabolites, one of which was identified as 6-deoxo-28-norTE while the other metabolite was identified as 6-deoxo-28-norTY. When 6-deoxo-28-norTY was added to the enzyme mixture, three metabolites were detected. Of these, two metabolites were determined to be 6-deoxo-28-nor-3-DHT and 6-deoxo-28-norTE, indicating that the C-3 epimerization from 6-deoxo-28-norTE to 6-deoxo-28-norTY via 6-deoxo-28-nor-3-DHT is a reversible reaction. The third metabolite was identified as 6-deoxo-28-norCS, demonstrating the C2α-hydroxylation of 6-deoxo-28-norTY to 6-deoxo-28-norCS. 6-Deoxo-28-norCS was converted to 28-norCS, indicating a C-6 oxidation of 6-deoxo-28-norCS to 28-norCS. In contrast to the aforementioned enzyme reactions, the conversion of CHN and 6-deoxo-28-norCT to downstream intermediates could not be demonstrated, even in repeated experiments using higher amounts of substrate. In an effort to overcome this problem, only microsomal enzymes were collected from the solution by ultra-centrifugation and the conversion of CHN to 6-deoxo-28-norTE via 6-deoxo-28-norCT was reexamined. In spite of these efforts, no products were detected, even by GC-SIM analysis. Moreover, the microsomal solution did not mediate the conversion of CN to 6-deoxoTE via 6-deoxoCT, suggesting that the enzyme activity responsible for mediating C-22 and C-23 hydroxylation in the tomato plant is scarce. Overall, the presence of the biosynthetic sequences CHR → CHN and 6-deoxo-28-norTE ↔ 6-deoxo-28-nor-3-DHT ↔ 6-deoxo-28-norTY → 6-deoxo-28-norCS → 28-norCS was clearly demonstrated in the tomato cell-free enzyme system.
Table I.
GC-MS data of metabolites obtained from tomato cell free conversion experiments
| Substrate | Metabolite | RRtd | Prominent Ions |
|---|---|---|---|
| min | m/z, relative intensity % | ||
| CHRa | CHRa | 0.456 | 460(M+, 54), 445(73), 370(28), 355(43), 305(33), 215(100) |
| 6-deoxo-28-norTEb | 6-deoxo-28-nor-3-DHTb | 0.615 | 442(M+, 73), 427(10), 246(12), 231(100), 217(23), 163(20), 141(15) |
| 6-deoxo-28-norTYc | 0.523 | 516(M+, 23), 501(6), 459(4), 426(62), 411(60), 305(11), 230(30), 215(100), 141(17) | |
| 6-deoxo-28-nor-3-DHTc | 6-deoxo-28-norTEc | 0.605 | 516(M+, 73), 501(65), 459(25), 426(23), 411(36), 305(38), 230(26), 215(100), 141(24) |
| 6-deoxo-28-norTYc | 0.523 | 516(M+, 21), 501(5), 459(4), 426(60), 411(59), 305(10), 230(32), 215(100), 141(16) | |
| 6-deoxo-28-norTYc | 6-deoxo-28-nor-3-DHTb | 0.615 | 442(M+, 74), 427(10), 246(12), 231(100), 217(22), 163(20), 141(14) |
| 6-deoxo-28-norTEc | 0.605 | 516(M+, 71), 501(62), 459(25), 426(23), 411(35), 305(36), 230(26), 215(100), 141(23) | |
| 6-deoxo-28-norCSb | 0.619 | 484(M+, 51), 469(16), 288(15), 273(100), 205(24), 141(21) | |
| 6-deoxo-28-norCSb | 28-norCSb | 0.866 | 498(M+, 100), 483(8), 399(3), 358(11), 328(8), 287(35), 141(54) |
| 28-norCSb | CSb | 1.000 | 512(M+, 87), 358(34), 327(11), 287(30), 155(100) |
| CSb | 26-norCSb | 0.900 | 498(M+, 100), 483(8), 399(13), 358(28), 328(10), 287(42), 141(94) |
| [26,28-2H6]CSb | [26-2H3]28-norCSb | 0.863 | 501(M+, 100), 486(8), 399(4), 358(16), 328(11), 287(42), 144(52) |
Analyzed by its trimethylsilyl ether.
Analyzed by its bismethaneboronate.
Analyzed by its methaneboronate-trimethylsilyl ether.
Relative retention time (RRt) with respect to castasterone (26.80 min).
CS Is Converted to 26-norCS But Not 28-norCS in Tomato Enzyme Preparations
The metabolism of CS and [26,28-2H6]CS was investigated separately with the tomato cell-free enzyme solution. HPLC analysis and rice bioassay of the metabolic products derived from CS and [2H6]CS revealed that only fractions 18 and 19, in which both CS and [2H6]CS were eluted, were biologically active. However, in thin-layer chromatography analysis, BR-like purple-bluish spots appeared in fractions 13 and 14. Consequently, these were combined, derivatized, and subsequently analyzed by GC-MS.
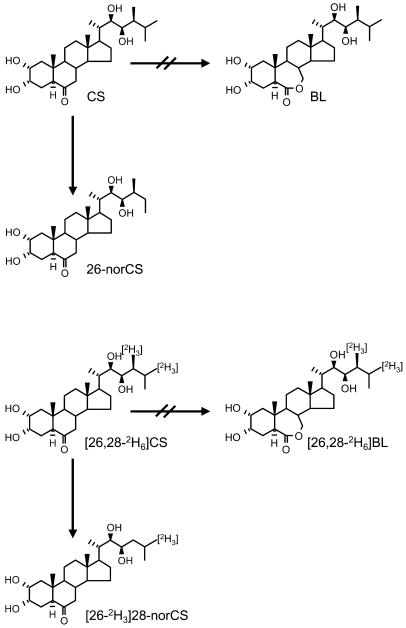
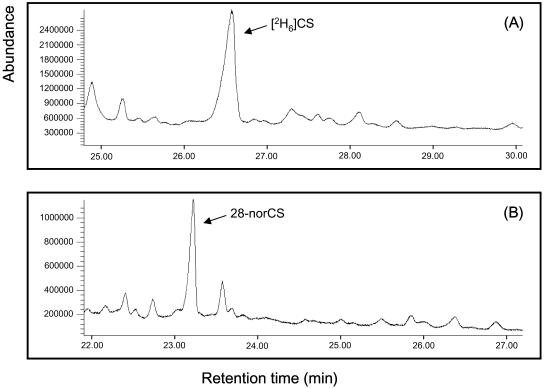
Although BL was assumed to be the most likely product of CS, no trace amounts of BL or [2H6]BL were detected in fractions 13 and 14 pertaining to CS and [2H6]CS feedings, even by GC-SIM analysis (data not shown). Instead, fractions 13 and 14 derived from CS feedings contained a metabolite with mass-to-charge ratio (m/z) 358, 328/327 and 287 ions representative of a bismethaneboronate (BMB), characteristic of the 6-ketone ring structure such as CS BMB. Further peaks were observed corresponding to a molecular ion at m/z 498 and the most abundant ion at m/z 141 due to fission of C-20/C-22. These 2 ions are 14 mass units less than those of CS BMB, suggesting the presence of either 26-norCS or 28-norCS. Under our GC-MS conditions, the 26-norCS and 28-norCS BMBs showed basically the same mass spectrum, although the former displayed a longer retention time than the latter (Table I). Since the CS metabolite BMB had the same retention time as 26-norCS BMB, the metabolite was determined to be 26-norCS. The activity of the enzyme(s) mediating the conversion of CS to 26-norCS was approximately 55.3 ng mg protein−1 min−1.
When [2H6]CS was used, the BMB derivative of the [2H6]CS metabolite obtained yielded characteristic ions for a 6-ketone ring structure at m/z 358, 327/328, and 287, indicating that the metabolite has the same ring structure as [2H6]CS. A molecular ion at m/z 501 and an ion at m/z 144 due to fission of C-20/C-22, both being 17 mass units less than those of [2H6]CS, were also observed, suggesting that the metabolite is either [28-2H3]26-norCS or [26-2H3]28-norCS. The fact that the retention time of the BMB metabolite was 5 s shorter than 28-norCS BMB suggests that the metabolite is [2H3]28-norCS, since under our GC conditions deuterated BRs migrate approximately 5 s faster than the same unlabeled species (Table I). The activity of the enzyme(s) catalyzing the conversion of [2H6]CS to [2H3]28-norCS was approximately 276.6 ng mg protein−1 min−1. Taken together, CS was not converted to BL in the tomato cell enzyme extract, and CS and [26,28-2H6]CS were differentially metabolized to 26-norCS and [26-2H3]28-norCS, respectively (Fig. 2).
Figure 2.
Metabolism of CS and [2H6]CS in young tomato plants. CS and [2H6]CS were not converted to BL and [2H6]BL, respectively, but were catabolized to 26-norCS and [2H3]28-norCS, respectively.
CS Is Also Converted from 28-norCS in Tomato Enzyme Preparations
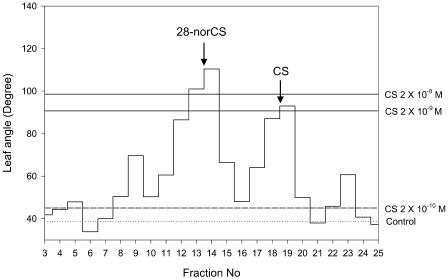
The conversion of 28-norCS to CS represents a reaction in which a methyl group is incorporated at the C-24 position. In plant sterol biosynthesis, alkylation is mediated by a sterol methyltransferase (SMT) with the aid of S-adenosyl-l-Met (SAM; Benveniste, 2002). We therefore examined the conversion of 28-norCS to CS in the presence of SAM and NADPH cofactor in the tomato cell-free solution. The product derived from 28-norCS was purified by a reverse phase HPLC (RP-HPLC), resulting in biological activity in fractions 12 to 14 where 28-norCS eluted, and in fractions 18 and 19 where CS eluted, suggesting that 28-norCS was converted to CS (Fig. 3). As expected, the biologically active BR was identified as CS by GC-MS analysis. The conversion rate of 28-norCS to CS was determined to be approximately 0.6% following addition of an internal standard ([2H6]CS). When NADPH or SAM or both were removed from the assay mixture, the conversion rate was reduced to 36%, 32%, or 19% of the control, respectively (Table II), strongly suggesting that the methylation of 28-norCS to CS is effected by a SAM-dependent SMT.
Figure 3.
Distribution of biological activity following RP-HPLC analysis of the 28-norCS metabolite extant in the young tomato enzyme preparation. Biological activity was based on results of the rice lamina inclination assay. HPLC was carried out using a NovaPak C18 column (8 × 100 mm) at a flow rate of 1 mL min−1 with 40% acetonitrile. Fractions were collected every minute. The arrow indicates the elution points of authentic BRs.
Table II.
Inhibition of enzyme activity for conversion of 28-norCS to CS in young tomato plants by the absence of NADPH and/or SAM
| Treatment | Enzyme Activity |
|---|---|
| % | |
| Control | 100 |
| - NADPH | 36±3 |
| - SAM | 32±2 |
| - NADPH/-SAM | 19±3 |
Biological Activity of C27-BRs
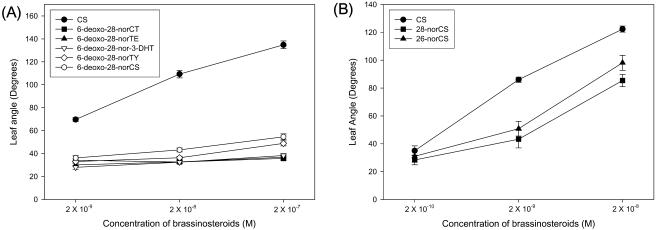
The biological activity of C27-BRs was examined using the rice lamina inclination assay which is based on the specific elongation of adaxial cells of rice lamina joint. As shown in Fig. 4A, 6-deoxo-28-norCT, 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, and 6-deoxo-28-norCS displayed almost no biological activity up to 2 × 10−8 m. At 2 × 10−8 m, 6-deoxo-28-norTY and 6-deoxo-28-norCS exhibited slight activity, but much less activity than CS, suggesting that hydroxylation at C-22 and C-23 of the side chain, C-3 epimerization, and C-2α hydroxylation of 6-deoxo C27-BRs have no significant effects on the increase in the bending angle in the assay. 28-norCS displayed much higher activity than 6-deoxo-28-norCS, indicating that C-6 oxidation of C27-BR is essential for effecting the observed bending activity. It has been reported (Fujioka et al., 2000) that CS displayed approximately 10 times higher activity than 28-norCS. This indicates that the aforementioned C-24 methylation involved in the conversion of 28-norCS (C27-BR) to CS (C28-BR) is an important biosynthetic step that effects an increase in the activity. 26-NorCS possessed approximately one-seventh of the activity compared to that of CS, which is consistent with other reported data (Watanabe et al., 2001; Kim et al., 2000a). This biological activity appears to be inherent to 26-norCS since it was not converted to CS in the tomato cell-free system (data not shown). Consequently, it is concluded that C-26 demethylation represents a degradation step of CS (Fig. 4B).
Figure 4.
Biological activity of C27-BRs in the rice lamina inclination assay. The activity of 6-deoxo-28-norCT, 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, and 6-deoxo-28-norCS was measured in the concentration range from 2 × 10−9 m to 2 × 10−7 m (A), while 26-norCS, 28-norCS, and CS was measured in the concentration range 2 × 10−10 m to 2 × 10−8 m (B).
CYP85 Is Responsible for the C-6 Oxidation of 6-deoxo-28-norCS to 28-norCS in Tomato
Studies based on a heterologous functional assay of enzymes involved in the biosynthesis and catabolism of C28-BRs have been limited only to 5α-reductase, C-6 oxidase, and 26-hydroxylase. 5α-reductases (DET2 and PnDET2) were cloned from Arabidopsis and morning glory and their functions determined (Li et al., 1997; Suzuki et al., 2003), and CYP72B1 from Arabidopsis was found to be a 26-hydroxylase of BL and CS whose action inactivated these BRs (Neff et al., 1999; Turk et al., 2003). Functional analysis of a tomato dwarf mutant revealed that a Cyt P450, CYP85, catalyzed the conversion of 6-deoxoCS to CS (Bishop et al., 1999). In Arabidopsis and rice, CYP85A1, CYP85A2, and OsDWARF that possess high homology to tomato CYP85 were identified, and their heterologous expression in yeast revealed that they have the same function and substrate specificity to those of CYP85 from tomato (Shimada et al., 2001, 2003; Hong et al., 2002). Given that the conversion of 6-deoxo-28-norCS to 28-norCS is basically the same reaction as the conversion of 6-deoxoCS to CS, C-6 oxidation involved in C27-BR biosynthesis might also be catalyzed by CYP85. To confirm this possibility, the cDNA of Arabidopsis CYP85A1 was cloned into a Gal-inducible expression vector, pYeDP60 (V60), and transformed into a yeast strain, WAT21, where the expression of Arabidopsis NADPH-Cyt P450 reductase could be induced by Gal (Pompon et al., 1996; Urban et al., 1997). In an effort to determine whether overexpressed CYP85A1 in the transformed strain CYP85A1/V60/WAT21 was functional, [2H6]6-deoxoCS was fed to the strain and the metabolite was analyzed by GC-MS following methaneboronation. As shown in Figure 5A, the BMB of the metabolite showed a strong peak on the total ion chromatogram which was assigned to [2H6]CS by direct comparison of its mass spectrum and GC retention time to those of authentic [2H6]CS, indicating that the overexpressed CYP85A1 maintained proper functionality. After confirming that the empty vector transformed yeast (V60/WAT21) did not catalyze the C-6 oxidation of 6-deoxo-28-norCS, 6-deoxo-28-norCS was fed to the transformed strain and the metabolite was analyzed by GC-MS. The BMB of the metabolite displayed the same mass spectrum and GC retention time to those of 28-norCS BMB, indicating that the C-6 oxidation of 6-deoxo-28-norCS successfully occurred by means of Arabidopsis CYP85A1 (Fig. 5B).
Figure 5.
Total ion chromatogram of the product of CYP85A1, which was heterologously expressed in the transformed yeast strain, CYP85A1/pYeDP60/WAT21. The arrowed peaks in the upper (A) and lower (B) chromatograms show the products of [2H6]6-deoxoCS and 6-deoxo-28-norCS, respectively.
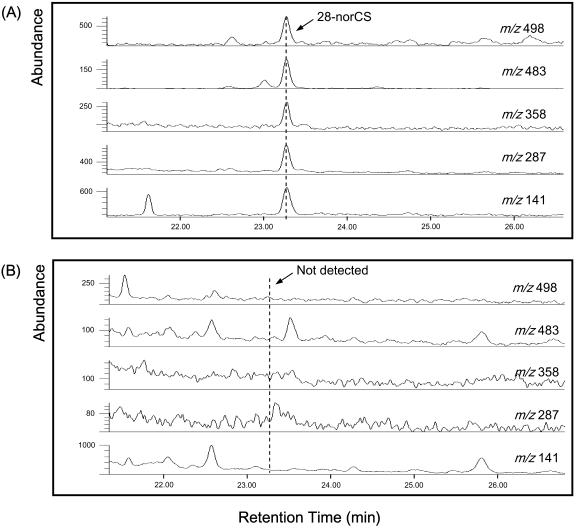
In an effort to determine whether tomato CYP85 possesses the activity, the in vitro conversion of 6-deoxo-28-norCS to 28-norCS was investigated using crude enzyme extracts obtained from wild-type and dwarf mutant tomato plants. Prior to feeding, the absence of 28-norCS in the enzyme preparation of wild-type tomato was confirmed. As expected, enzyme activity for the conversion was detected in the wild-type tomato (Fig. 6A) but not in the dwarf mutant (Fig. 6B). Therefore, it is concluded that tomato CYP85 is responsible for the C-6 oxidation of both 6-deoxoCS and 6-deoxo-28-norCS.
Figure 6.
GC-SIM analysis for the conversion of 6-deoxo-28-norCS to 28-norCS in wild type (A) and dwarf mutant (B) of young tomato plants. Enzyme activity was detected in the wild type but not in the dwarf mutant.
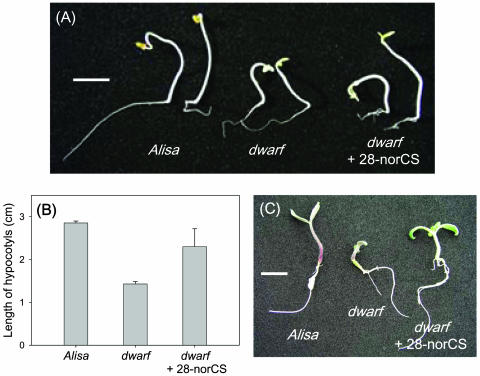
Rescue of the Tomato dwarf Mutant by 28-norCS
The physiological importance of C27-BRs biosynthesis was examined in the tomato dwarf mutant that possesses a defect in the C-6 oxidation of both C27- and C28-BRs. As shown in Figure 7, application of 28-norCS clearly reversed the dwarfism in both dark- and light-grown seedlings of the mutants (Fig. 7, A and C). In particular, it was found that the length of hypocotyls in the dark-grown seedlings recovered by up to 80% compared to that of the wild type (Fig. 7B). Therefore, it may be concluded that C27-BRs biosynthesis plays an important role in the BR physiology of tomato plants.
Figure 7.
Growth recovery of the tomato dwarf mutant following treatment with 28-norCS. A, Dark-grown seedlings. B, Average length (cm) of hypocotyls of dark-grown seedlings. C, Light-grown seedlings. Alisa is the wild-type tomato. Bar represents 1 cm.
DISCUSSION
The conversion of CS to BL has been demonstrated in cultured cells of C. roseus and M. polymorpha and the seedlings of C. roseus and Arabidopsis (Suzuki et al., 1993, 1995; Noguchi et al., 2000; Kim et al., 2003a). Consequently, CS is now considered to be a direct biosynthetic precursor of BL. In certain plants, however, where a large amount of CS is present, no trace amount of BL was detected (Yokota, 1997; Fujioka, 1999). Additionally, the conversion rate of CS to BL is extremely low even in plants where the conversion was confirmed (Suzuki et al., 1995; Sakurai, 1999; Noguchi et al., 2000). These findings suggest the possibility that CS is not only a biosynthetic precursor of BL, but also exerts its own biological activity. Although a higher concentration of CS is needed compared to BL, CS exogenously applied to plants displays the same biological activity as that induced by BL and can rescue abnormal pleiotropic phenotypes in BR-deficient mutants. Moreover, it has recently been demonstrated, albeit to a lesser extent compared to BL, that CS and BL can bind to BRI1, a plasma membrane located receptor of BL, supporting the notion that CS in addition to BL possess biological activity (Wang et al., 2001).
Tomato dwarf and dumpy mutants showing abnormalities in growth and differentiation were rescued only by application of BRs (Bishop et al., 1999; Koka et al., 2000). The tomato curl-3 mutant exhibits the same abnormal phenotype as that found in the dwarf and dumpy mutants; however, this phenotype could not be rescued through the exogenous application of BRs (Koka et al., 2000). Consequently, it is thought that the homeostatic regulation and signaling of BRs are necessary for the normal development of tomato plants. Several independent studies employing wild-type and BR-related mutants of tomato revealed that young tomato plants contain a considerable amount of CS but no BL (Yokota et al., 1997; Bishop et al., 1999; Koka et al., 2000; Nomura et al., 2001). In addition, the present study demonstrated that the enzyme activity mediating the conversion of CS to BL, namely CS 6-oxidase (BL synthase), could not be detected in young tomato plants. Coupled with the fact that CS can restore the unusual phenotype of the dwarf and dumpy mutants (Bishop et al., 1999; Koka et al., 2000), the available evidence indicates that the active BR in tomato seedlings is not BL but CS. If this is indeed the case, the levels of CS must be regulated through degradation into less active catabolite(s) after exerting its activity in the tomato seedlings. Alternatively, a high level of CS may result in feedback regulation of Cyt P450 enzyme activity pertaining to BRs biosynthesis (Mathur et al., 1998).
The present cell-free study revealed that CS is metabolized into a less active C27-BR, namely 26-norCS, indicating that C-26 demethylation represents one of the deactivation steps of CS in tomato. The same C-26 demethylation deactivates BL in P. vulgaris and M. polymorpha (Kim et al., 2000a, 2000b). Thus, it appears that deactivation via C-26 demethylation is a ubiquitous mechanism that operates in plants.
In tomato, it has been suggested that 28-norCS is synthesized from CHR, the most abundant sterol, through late C-6 oxidation as is the case with the biosynthesis of CS from CR (Nomura et al., 2001). This study demonstrated that the tomato seedlings contain enzymes that mediate the conversion of CHR to CHN and 6-deoxo-28-norTE to 28-norCS via 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY and 6-deoxo-28-norCS, although the precise enzymes connecting CHN to 6-deoxo-28-norTE via 6-deoxo-28-norCT could not be verified. On the other hand, 6-deoxo-28-norCT has been identified as an endogenous BR in tomato seedlings (Yokota et al., 2001), strongly suggesting that the aforementioned unverified pathway is present in tomato. Tomato seedlings also contain a complete set of C28-6-deoxoBRs. Taken together, it is likely that the same late C-6 oxidation pathway, i.e. the same enzyme system, operates in generating both C27-BRs and C28-BRs in tomato seedlings. In support of this, it was confirmed that heterologously expressed CYP85A1 catalyzed the C-6 oxidation of both 6-deoxo-28-norCS and 6-deoxoCS.
In tomato shoots, C28 6-oxo-intermediates involved in the early C-6 oxidation pathway are not synthesized (Bishop et al., 1999; Nomura et al., 2001). By analogy, it is most likely that C27 6-oxo-intermediates involved in the early C-6 oxidation pathway are not present in tomato shoots. At present only 28-norTY has been detected as such an intermediate from Arabidopsis seedlings (Fujioka et al., 2000). Interestingly, heterologously expressed tomato CYP85 catalyzed the C-6 oxidation of 6-deoxoTE, 6-deoxo-3-DHT, and 6-deoxoTY although their products are not endogenous (Shimada et al., 2001) and, by analogy, is also supposed to catalyze the C-6 oxidation of their C27 counterparts. The presence of C27 6-oxo-intermediates in tomato and their production from their respective 6-deoxoBRs by tomato CYP85 remain to be determined.
As stated in the introductory remarks, an earlier indication that 28-norCS is synthesized from CS (Fujioka et al., 2000) seems unjustified. The present study using tomato demonstrated that [26,28-2H6]CS, as used by Fujioka et al. (2000), is indeed converted to [26-2H3]28-norCS but that unlabeled CS is only metabolized to 26-norCS. Additionally, we recently confirmed that the identical reactions also occur in cultured cells of P. vulgaris and M. polymorpha (T.-W. Kim, H.-H. Park, and S.-K. Kim, unpublished data). This indicates that plant cells recognized [26,28-2H6]CS as a xenobiotic, resulting in the generation of an artifact. Thus, demethylation of naturally occurring BRs in planta proceeds through loss of C-26 rather than C-28, suggesting that 28-norCS is not biosynthesized from CS in the plants.
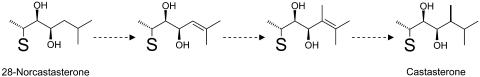
Conversely, this study using a tomato cell-free system resulted in the unexpected finding that CS is synthesized from 28-norCS in the presence of NADPH and SAM which act as proton and methyl donors, respectively. This is the first demonstration, to our knowledge, that CS is biosynthesized from cholesterol via C27 intermediates. To our knowledge, C27 BRs have been identified from as many as 12 species (Fujioka et al., 2000; Bajguz and Tretyn, 2003). Furthermore, it has recently been demonstrated that considerable amounts of CHR are present not only in tomato but also in Arabidopsis and pea (Nomura et al., 2001). Thus CHR and 28-norCS appear to be more widely distributed in plants than expected. Since the conversion of 28-norCS to CS involves an activation reaction, the activity of 28-norCS should be seriously taken into account when attempting to interpret the BR-related growth and development of plants. Higher plants contain C-24 methylsterols such as campesterol and C-24 ethylsterols such as sitosterol as major 4-demethylsterols. Sterol methyltransferase 1 (SMT1) catalyzes the first methylation of cycloartenol to 24-methylenecycloartenol, which is subsequently used as a source for C-24 methylsterols (Diener et al., 2000; Fujioka and Yokota, 2003), while SMT2 and SMT3 mediate the second methylation of 24-methylenelophenol to 24-ethylidenlophenol, which is used as a source for C-24 ethylsterols (Schaeffer et al., 2001; Fujioka and Yokota, 2003). Cycloartenol possesses a Δ24 double bond in the side chain, and this moiety is required by SMT1 to facilitate the introduction of a methyl group to C-24 using SAM. Supposing that SMT1 is involved in the conversion of 28-norCS to CS, the introduction of a Δ24 double bond is necessary for the methylation of 28-norCS. Therefore, we speculate that the conversion of 28-norCS to CS may consist of at least three reactions: (1) desaturation of 28-norCS to form Δ24 28-norCS, (2) methylation of Δ24 28-norCS with SAM to form 24-methylene-28-norCS (dolichosterone), and (3) reduction of dolichosterone by NADPH to form CS (Fig. 8). This hypothesis is consistent with our finding that SAM and NADPH are required for the methylation reaction.
Figure 8.
A hypothetical scheme representing the C-24 methylation of 28-norCS to CS in young tomato plants. S indicates the same ring structure as that of 28-norCS and CS.
MATERIALS AND METHODS
Plant Materials and Chemicals
Tomato (Lycopercicon esculentum) plants (Poong-kwang) were grown in an environmental growth chamber at 28°C under a 16-h light/8-h dark cycle. Young plants grown for 3 to 4 weeks were harvested and stored at −80°C until required. The tomato dwarf mutant and its corresponding wild type (Alisa) were grown under the same conditions and used in enzyme assay to examine CYP85 function. All chemicals used in the biochemical analyses were obtained from Sigma Chemicals (St. Louis). The C27-BRs, 6-deoxo-28-norCT, 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, 6-deoxo-28-norCS, 28-norCS, and 26-norCS were synthesized according to literature procedures (Takatsuto et al., 1984; Watanabe et al., 2001; Yokota et al., 2001). CHR and CHN were obtained from Sigma.
Enzyme Assays
Harvested plants (30 g) were ground in a prechilled mortar and pestle in 80 mL of 0.1 m sodium phosphate (pH 7.4) containing 15 mm 2-mercaptoethanol, 1 mm EDTA, 1 mm dithiothreitol, 0.1 mm phenylmethylsulfonyl fluoride, 40 mm ascorbate, 250 mm Suc, and 10% (v/v) glycerol. The homogenate was filtered and centrifuged at 8,000g for 15 min. The supernatant was recentrifuged at 20,000g for 30 min. The resulting supernatant was precipitated by the addition of cold acetone to 40% (v/v) final concentration. The supernatant-acetone mixture was kept at −25°C for 10 min and then centrifuged at 13,000g for 10 min. The resulting precipitate was dissolved in 10 mL of 0.1 m sodium phosphate (pH 7.4) containing 1.5 mm 2-mercaptoethanol and 20% (v/v) glycerol and was used as the cell-free enzyme solution. The protein concentration of the enzyme solution was estimated with a microassay from Bio-Rad (Cambridge, MA) using bovine serum albumin as a standard.
Enzyme assays for the conversion of CHR to 28-norCS were initiated by the addition of substrates (5 μg each) CHR, CHN, 6-deoxo-28-norCT, 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, and 6-deoxo-28-norCS to the cell-free enzyme solution (3–4 mg protein mL−1) in the presence of NADP/NADPH (for the conversion of CHR to CHN) or NADPH (for the conversion of CHN to 6-deoxo-28-norCT and 6-deoxo-28-nor TY to 28-norCS via 6-deoxo-28-norCS). Following incubation at 37°C for 30 min, enzyme reactions were terminated by the addition of ethyl acetate (1.2 mL × 3). The ethyl acetate-soluble fractions were concentrated in vacuo, dissolved in 50% methanol (MeOH), loaded onto a C18 SepPak cartridge column (SepPak plus C18, Waters, Milford, MA), and eluted with 50% and 100% MeOH (7 mL each). The fraction eluted with 100% MeOH was dried, dissolved in a small amount of MeOH, and then subjected to RP-HPLC (Senshu Pak C18, 10 × 150 mm) eluted at a flow rate of 2.5 mL min−1 with 100% MeOH for the CHR metabolite or acetonitrile (MeCN)-water gradients: 0 to 20 min, 45% MeCN; 20 to 40 min, 45% to 100% MeCN; 40 to 70 min, 100% MeCN for other metabolites. Fractions were collected every minute. Under the same RP-HPLC conditions eluted with 100% MeOH, authentic CHN was detected in fractions 35 to 37. Under the same RP-HPLC conditions eluted with the MeCN-water gradient, authentic 6-deoxo-28-norCT, 6-deoxo-28-norTE, 6-deoxo-28-nor-3-DHT, 6-deoxo-28-norTY, 6-deoxo-28-norCS and 28-norCS were detected in fractions 36 to 38, 49 to 51, 46 to 48, 44 to 46, 59 to 61, and 13 to 15, respectively. Fractions were then analyzed by capillary GC-MS or GC-SIM following suitable derivatization.
The conversion of 28-norCS to CS was carried out with the cell-free enzyme solution containing 0.8 mm NADPH, 0.8 mm SAM, and 5 μg 28-norCS in a total volume of 1.2 mL. Following incubation at 37°C for 30 min, [2H6]-CS was added for quantitative analysis, the enzyme product was extracted with ethyl acetate and then loaded onto a C18 SepPak cartridge column as described above. Further purification was achieved by RP-HPLC (NovaPak C18, 8 × 100 mm, Waters) at a flow rate of 1 mL min−1 with 40% MeCN. Fractions were collected every minute and fractions 18 and 19, which showed biological activity in the rice lamina inclination assay, were analyzed by GC-MS following methaneboronation. The specific enzyme activity was calculated by the ratio of the product/the [2H6]CS added as an internal standard.
Metabolism of CS and [2H6]CS were also examined with the same enzyme solution in the presence of NADPH cofactor. Following the enzyme assays, products were extracted with ethyl acetate (1.2 mL), chromatographed on a SepPak cartridge column, and finally purified by RP-HPLC as described for the conversion of 28-norCS to CS. In the rice lamina inclination assay, only fractions 18 and 19, where CS and [2H6]CS were added as substrates, showed biological activity. However, in the thin-layer chromatography (Merck HPTLC F254) analysis, a blue-purplish spot at Rf 0.30 was detected in fractions 13 to 15 obtained from both assays after treatment with 70% sulfuric acid followed by heating. Fractions 13 to 15 were combined and subsequently analyzed by GC-MS following methaneboronation.
Bioassay
Rice lamina inclination assays using cv Koshihikari were carried out in an effort to examine the biological activity of BRs (Arima et al., 1984).
GC-MS and GC-SIM
The GC-MS and GC-SIM analyses were carried out on a Hewlett-Packard (Palo Alto, CA) 5973 mass spectrometer (electron impact ionization, 70 eVage) coupled to 6890 gas chromatography fitted with a fused silica capillary column (HP-5, 0.25 mm × 30 m, 0.25 μm film thickness). The oven temperature was maintained at 175°C for 2 min, elevated to 280°C at a rate of 40°C min−1 and then maintained at 280°C. Helium was used as the carrier gas at a flow rate of 1 mL min−1, and samples were introduced using an on-column injection mode. Methaneboronation was carried out by heating samples dissolved in pyridine containing methaneboronic acid (2 mg mL−1) at 70°C for 30 min. N-methyl-N-TMS-trifluoroacetamide (MSTFA) was used to effect trimethylsilylation.
Heterologous Expression of CYP85A1
Total RNAs were extracted from Arabidopsis seedlings using the TRI Reagent (Sigma). cDNA was synthesized from 1 ug of total RNA using the MMLV-reverse transcription system (Promega, Madison, WI) according to the manufacturer's instructions. CYP85A1 (At5g38970) cDNA was amplified by PCR using specific primers to delete the 5′- and 3′-noncoding regions of CYP85A1 cDNA. Specific primers were designed to introduce a BamHI restriction site immediately upstream of the initiation codon and a KpnI site following the stop codon: forward primer 5′-ggatccTGGGAGCAATGATGGTGATGAT-3′; reverse primer 5′-ggtaccTTAGT AGGGTGAAATCCTAAGATG-3′. CYP85A1 cDNA was amplified using 36 thermal cycles (94°C 20 s, 61°C 30 s, 72°C 2 min) with Ex Taq polymerase (Takara Shuzo, Shiga, Japan) and cloned into the pGEM-T easy vector (Promega). PCR error in the nucleotide sequence was checked by DNA sequencing using T7 and Sp6 universal primers. CYP85A1 cDNA fragments digested by BamHI and KpnI were subcloned into the BamHI and KpnI sites of a yeast expression vector (pYeDP60) that contained two selection markers (ADE2 and URA3). The CYP85A1/pYeDP60 construct (85A1/V60) was transformed into WAT21 strain according to the method of Gietz et al. (1992) and positively transformed strains (85A1/V60/WAT21) were screened onto synthetic dropout medium without uracil and adenine. The selected 85A1/V60/WAT21 clones were subcloned and the respiration capability was tested by growth on glycerol containing N3 medium (yeast extract, 10 g L−1; bactopeptone, 10 g L−1; glycerol, 2% [v/v] in 50 mm phosphate buffer, pH 6.2) before Gal induction. A N3-positive 85A1/V60/WAT21 single clone was inoculated into 30 mL of YPG medium (Glc, 20 g L−1; yeast extract, 10 g L−1; bactopeptone, 10 g L−1) and grown at 28°C for 48 h. Cells harvested by centrifugation were then reinoculated into 200 mL of YPL medium (Gal, 20 g L−1; yeast extract, 10 g L−1; bactopeptone, 10 g L−1) and grown at 28°C for 12 h. Gal-induced cells were then diluted in 20 mL of YPL to an OD550 of 0.4 to 0.6 and 5 ug each of [2H6]6-deoxoCS and [2H0]6-deoxo-28-norCS was added to the cells. After 6 h, metabolites were extracted using 20 mL of ethyl acetate (three times) and then concentrated in vacuo. The extracts were passed through a silica column (SepPak SiO2 Plus, Waters) and eluted with 8 mL of chloroform (CHCl3), 2% (v/v) MeOH in CHCl3, and 8% (v/v) MeOH in CHCl3. The 8% MeOH fraction was purified by passage through a SepPak C18 cartridge column and eluted with 10 mL of 50% MeOH (v/v) in water, 5 mL of 100% MeOH. The 100% MeOH fraction was concentrated in vacuo and then subjected to RP-HPLC as described above.
Rescue of the dwarf Mutant by 28-norCS
For the dark-grown seedlings, tomato seeds (Alisa and dwarf) were surface sterilized in 10% bleach for 30 min. The seeds were rinsed with sterilized water, incubated at 4°C for 2 d, and then planted on an agar-solidified plate containing 1% Suc-MS medium with or without 1 μm 28-norCS. Following 9 d at 22°C under continuous darkness, the seedlings were photographed using a digital camera. For the light-grown seedlings, sterile tomato seeds were planted on 1% Suc-MS medium for 4 d at 22°C under a 16-h/d light cycle. Following this, 1 μm 28-norCS in a 95% ethanol solution was applied to shoot apexes and the roots of the dwarf mutant using a μ-drop method. Seedlings were photographed after 3 d.
Acknowledgments
The authors are grateful to Dr. D. Pompon and Dr. P. Urban for providing pYeDP60 and the yeast strain WAT21 and to Dr. Gerard B. Bishop for supplying tomato seeds (dwarf and Alisa).
This work was supported by the Korean Science and Engineering Foundation (grant no. R01–2002–000–00367–0 to S.-K.K. and S.C.C.), by the Japan Society for the Promotion of Science (grant no. 1146007 to T.Y.), and by Human Frontier Research Program (grant no. 2000–162 to T.Y.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043588.
References
- Arima M, Yokota T, Takahashi N (1984) Identification and quantification of brassinolide-related steroids in the insect gall and healthy tissues of the chestnut plant. Phytochemistry 23: 1587–1591 [Google Scholar]
- Bajguz A, Tretyn A (2003) The chemical characteristic and distribution of brassinosteroids in plants. Phytochemistry 62: 1027–1046 [DOI] [PubMed] [Google Scholar]
- Benveniste P (2002) Sterol metabolism. In The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD [DOI] [PMC free article] [PubMed]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JD, Kamiya Y (1999) The tomato DWARF enzyme catalyzes C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA 96: 1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Yokota T (2001) Plants steroid hormones, brassinosteroids: current highlights of molecular aspects on their synthesis/metabolism, transport, perception and response. Plant Cell Physiol 42: 114–120 [DOI] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA (1998) The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell 10: 231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-H, Fujioka S, Nomura T, Harada A, Yokota T, Takatsuto S, Sakurai A (1997) An alternative brassinolide biosynthetic pathway via late C-6 oxidation. Phytochemistry 44: 609–613 [Google Scholar]
- Diener AC, Li H, Zhou WX, Whoriskey WJ, Nes WD, Fink GR (2000) Sterol methyltransferase 1 controls the level of cholesterol in plants. Plant Cell 12: 853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S (1999) Natural occurrence of brassinosteroids in the plant kingdom. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids: Steroidal Plant Hormones. Springer-Verlag, Tokyo, pp 21–45
- Fujioka S, Li J, Choi Y-H, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J, et al (1997) The Arabidopsis deetiolated2 mutant is blocked early in Brassinosteroid biosynthesis. Plant Cell 9: 1951–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Noguchi T, Sekimoto M, Takatsuto S, Yoshida S (2000) 28-Norcastasterone is biosynthesized from castasterone. Phytochemistry 55: 97–101 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Yokota T (2003) Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol 54: 137–164 [DOI] [PubMed] [Google Scholar]
- Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved methods for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agematsu M, Yoshida S, Watanabe Y, et al (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32: 495–508 [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka SY, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M (2003) A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Plant Cell 15: 2900–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JG, Yun J, Kim DH, Chung KS, Fujioka S, Kim JI, Dae HW, Yoshida S, Takatsuto S, Song PS, et al (2001) Light and brassinosteroid signals are integrated via a dark-induced small G protein in etiolated seedling growth. Cell 105: 625–636 [DOI] [PubMed] [Google Scholar]
- Kim T-W, Chang SC, Choo J, Watanabe T, Takatsuto S, Yokota T, Lee JS, Kim SY, Kim S-K (2000. a) Brassinolide and [26,28-2H6] brassinolide are differently demethylated by loss of C-26 and C-28, respectively, in Marchantia polymorpha. Plant Cell Physiol 41: 1171–1174 [DOI] [PubMed] [Google Scholar]
- Kim T-W, Chang SC, Lee JS, Hwang B, Takatsuto S, Yokota T, Kim S-K (2004) Cytochrome P450-catalyzed brassinosteroid pathway activation through synthesis of castasterone in Phaseolus vulgaris. Phytochemistry 65: 679–689 [DOI] [PubMed] [Google Scholar]
- Kim T-W, Han K-S, Joo S-H, Kang M-W, Kim S-K (2000. b) Metabolism of brassinolide in suspension cultured cells of Phaseolus vulgaris. B Kor Chem Soc 21: 1044–1046 [Google Scholar]
- Kim Y-S, Kim T-W, Kim S-K (2003. a) Conversion of 6-deoxocastasterone to brassinolide in a Liverwort, Marchantia polymorpha. B Kor Chem Soc 24: 1385–1388 [Google Scholar]
- Kim Y-S, Kim T-W, Kim S-K (2003. b) Biotransformation of 6-Deoxotyphasterol in a liverwort, Marchantia polymorpha. B Kor Chem Soc 24: 1541–1543 [Google Scholar]
- Kim T-W, Park S-H, Han K-S, Choo J, Lee JS, Hwang S, Kim S-K (2000. c) Occurrence of teasterone and typhasterol, and their enzymatic conversion in Phaseolus vulgaris. B Kor Chem Soc 21: 373–374 [Google Scholar]
- Koka CV, Cerny RE, Gardner RG, Noguchi T, Fujioka S, Takatsuto S, Yoshida S, Clouse SD (2000) A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol 122: 85–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Biswas M, Chao A, Russel D, Chory J (1997) Conservation of function between mammalian and plant steroid 5a-reductase. Proc Natl Acad Sci USA 94: 3554–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90: 929–938 [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398–401 [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH (2002) Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295: 1299–1301 [DOI] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J (2001) BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol 127: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur J, Molnár G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C, et al (1998) Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J 14: 593–602 [DOI] [PubMed] [Google Scholar]
- Nakajima N, Fujioka S, Tanaka T, Takatsuto S, Yoshida S (2002) Biosynthesis of cholestanol in higher plants. Phytochemistry 60: 275–279 [DOI] [PubMed] [Google Scholar]
- Nam KH, Li J (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Neff MM, Nguyen SM, Malancharuvil EJ, Fujioka S, Noguchi T, Seto H, Tsubuki M, Honda T, Takatsuto S, Yoshida S, et al (1999) BAS1: a gene regulating brassinosteroid levels and light responsiveness in Arabidopsis. Proc Natl Acad Sci USA 96: 15316–15323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Tax FE, Yoshida S, Feldmann KA (2000) Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol 124: 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Takatsuto S, Sakurai A, Yoshida S, Li J, Chory J (1999) Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-en-3-one to (24R)-24-methyl-5α-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol 120: 833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Bishop GJ, Kaneta T, Reid JB, Chory J, Yokota T (2003) The LKA gene is a brassinosteroids insensitive1 homolog of pea. Plant J 36: 291–300 [DOI] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T (1999) Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiol 119: 1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T (1997) Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol 113: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Sato T, Bishop GJ, Kamiya Y, Takatsuto S, Yokota T (2001) Accumulation of 6-deoxocathasterone and 6-deoxocastasterone in Arabidopsis, pea and tomato is suggestive of common rate-limiting steps in brassinosteroid biosynthesis. Phytochemistry 57: 171–178 [DOI] [PubMed] [Google Scholar]
- Park S-H, Han K-S, Kim T-W, Shim J-K, Takatsuto S, Yokota T, Kim S-K (1999) In vivo and in vitro conversion of teasterone to typhasterol in cultured cells of Marchantia polymorpha. Plant Cell Physiol 40: 955–960 [Google Scholar]
- Pompon D, Louerat B, Bronine A, Urban P (1996) Yeast expression of animal and plant P450s in optimized redox environments. Methods Enzymol 272: 51–64 [DOI] [PubMed] [Google Scholar]
- Sakurai A (1999) Biosynthesis. In A Sakurai, T Yokota, SD Clouse, eds, Brassinosteroids: Steroidal Plant Hormones. Springer-Verlag, Tokyo, pp 137–161
- Schaeffer A, Bronner R, Benveniste P, Schaller H (2001) The ratio of campesterol to sitosterol that modulates growth in Arabidopsis is controlled by STEROL METHYLTRANSFERASE 2:1. Plant J 25: 605–615 [DOI] [PubMed] [Google Scholar]
- Shimada Y, Fujioka S, Miyauchi N, Kushiro M, Takatsuto S, Nomura T, Yokota T, Kamiya Y, Bishop GJ, Yoshida S (2001) Brassinosteroid 6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiol 26: 770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Goda H, Nakamura A, Takatsuto S, Fujioka S, Yoshida S (2003) Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiol 131: 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Fujioka S, Takatsuto S, Yokota T, Murofushi N, Sakurai A (1993) Biosynthesis of brassinolide from castasterone in cultured cells of Catharanthus roseus. J Plant Growth Regul 12: 101–106 [Google Scholar]
- Suzuki H, Fujioka S, Takatsuto S, Yokota T, Murofushi N, Sakurai A (1995) Biosynthesis of brassinosteroids in seedlings of Catharanthus roseus, Nicotiana tabacum, and Oryza sativa. Biosci Biotech Bioch 59: 168–172 [Google Scholar]
- Suzuki Y, Saso K, Fujioka S, Yoshida S, Nitasaka E, Nagata S, Nagasawa H, Takatsuto S, Yamaguchi I (2003) A dwarf mutant strain of Pharbitis nil, Uzukobito (kobito), has defective brassinosteroid biosynthesis. Plant J 36: 401–410 [DOI] [PubMed] [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kausch-mann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C (1996) Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85: 171–182 [DOI] [PubMed] [Google Scholar]
- Takatsuto S, Yazawa N, Ishiguro M, Morisaki M, Ikekawa N (1984) Stereoselective synthesis of plant growth-promoting steroids, brassinolide, castasterone, typhasterol, and their 28-nor analogues. J Chem Soc Perkin Trans I: 139–146 [Google Scholar]
- Turk E, Fujioka S, Seto H, Shimada Y, Takatsuto S, Yoshida S, Denzel M, Torres Q, Neff MM (2003) CYP72B1 inactivates brassinosteroid hormones. An intersection between photomorphogenesis and plant steroid signal transduction. Plant Physiol 133: 1643–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P, Mignotte C, Kazmaier M, Delorme F, Pompon D (1997) Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J Biol Chem 272: 19176–19186 [DOI] [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J (2001) BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410: 380–383 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noguchi T, Yokota T, Shibata K, Koshino H, Seto H, Kim S-K, Takatsuto S (2001) Synthesis and biological activity of 26-norbrassinolide, 26-norcastasterone and 26-nor-6-deoxocastasterone. Phytochemistry 58: 343–349 [DOI] [PubMed] [Google Scholar]
- Yokota T (1997) The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci 2: 137–143 [Google Scholar]
- Yokota T, Nomura T, Nakayama M (1997) Identification of brassinosteroids that appear to be derived from campesterol and cholesterol in tomato shoots. Plant Cell Physiol 38: 1291–1294 [Google Scholar]
- Yokota T, Sato T, Takeuchi Y, Nomura T, Uno K, Watanabe T, Takatsuto S (2001) Roots and shoots of tomato produce 6-deoxo-28-norcathasterone, 6-deoxo-28-nortyphasterol and 6-deoxo-28-norcastasterone, possible precursors of 28-norcastasterone. Phytochemistry 58: 233–238 [DOI] [PubMed] [Google Scholar]