Abstract
Tocopherols (vitamin E) are lipophilic antioxidants presumed to play a key role in protecting chloroplast membranes and the photosynthetic apparatus from photooxidative damage. Additional nonantioxidant functions of tocopherols have been proposed after the recent finding that the Suc export defective1 maize (Zea mays) mutant (sxd1) carries a defect in tocopherol cyclase (TC) and thus is devoid of tocopherols. However, the corresponding vitamin E deficient1 Arabidopsis mutant (vte1) lacks a phenotype analogous to sxd1, suggesting differences in tocopherol function between C4 and C3 plants. Therefore, in this study, the potato (Solanum tuberosum) ortholog of SXD1 was isolated and functionally characterized. StSXD1 encoded a protein with high TC activity in vitro, and chloroplastic localization was demonstrated by transient expression of green fluorescent protein-tagged fusion constructs. RNAi-mediated silencing of StSXD1 in transgenic potato plants resulted in the disruption of TC activity and severe tocopherol deficiency similar to the orthologous sxd1 and vte1 mutants. The nearly complete absence of tocopherols caused a characteristic photoassimilate export-defective phenotype comparable to sxd1, which appeared to be a consequence of vascular-specific callose deposition observed in source leaves. CO2 assimilation rates and photosynthetic gene expression were decreased in source leaves in close correlation with excess sugar accumulation, suggesting a carbohydrate-mediated feedback inhibition rather than a direct impact of tocopherol deficiency on photosynthetic capacity. This conclusion is further supported by an increased photosynthetic capacity of young leaves regardless of decreased tocopherol levels. Our data provide evidence that tocopherol deficiency leads to impaired photoassimilate export from source leaves in both monocot and dicot plant species and suggest significant differences among C3 plants in response to tocopherol reduction.
The Suc export defective1 (sxd1) mutant from maize (Zea mays) has long been thought to represent the only known mutant showing altered plasmodesmata (PD) structure and function during leaf development. This assumption was based on a typical photosynthate export-deficient phenotype characterized by an overall growth reduction and source leaf-specific accumulation of anthocyanins and starch. In addition, minor veins of maturating sxd1 leaf blades exhibited ultrastructural alterations and callose occlusion of a specific class of PD between bundle sheath (BS) and vascular parenchyma (VP) cells (Russin et al., 1996; Botha et al., 2000). This suggested an inhibition of symplastic continuity at the BS-VP boundary, leading to a block of Suc transport into the phloem. Indeed, symplastic transport of the fluorescent dye Lucifer Yellow into minor veins was specifically blocked in anthocyanin-accumulating source regions of sxd1 leaf blades (Botha et al., 2000). Therefore, it was tempting to speculate that the respective mutation would reside in a gene that is essential for PD function. However, after identification of the sxd1 locus, SXD1 turned out to be a novel chloroplast-targeted protein of unknown function. Thus, a model was proposed that SXD1 is involved in a chloroplast-to-nucleus signaling pathway in maize BS cells essential for PD formation during sink-source transition (Provencher et al., 2001).
Recently, evidence for the function of SXD1 has been provided by genetic analysis of the Arabidopsis vte1 mutant, which was discovered during a mutant screen for altered tocopherol (vitamin E) content and composition (Porfirova et al., 2002). In general, tocopherols are amphipathic molecules that are composed of a polar chromanol head and a hydrophobic isoprenoid (prenyl) tail. Therefore, substrates for tocopherol biosynthesis are drawn from two different metabolic pathways, the shikimate pathway and the plastid-localized nonmevalonate pathway. The first committed step in tocopherol biosynthesis is the condensation of homogentisate and phytyl pyrophosphate by homogentisate phytyl transferase to yield 2-methyl-6-phytyl-1,4-hydroquinone (Collakova and DellaPenna, 2001; Savidge et al., 2002). Subsequent ring methylation and ring cyclation reactions lead to the formation of the four major tocopherol derivates (α-, β-, γ-, and δ-tocopherol) that differ only in number and position of methyl substituents on the aromatic ring. The vte1 mutant lacked all four derivatives of tocopherol and was devoid of tocopherol cyclase (TC) activity (Porfirova et al., 2002). Genetic mapping of vte1 combined with a genomics-based approach identified VTE1 as a gene encoding TC. Surprisingly, the sequence of VTE1 shared a high degree of similarity to SXD1 and, based on Arabidopsis genome and maize expressed sequence tag (EST) database analysis, it was suggested that VTE1 and SXD1 represent single-copy orthologs, both encoding an enzyme with TC activity (Porfirova et al., 2002). Lately, the functional equivalency of SXD1 and VTE1 could be verified unequivocally by different approaches (Sattler et al., 2003). First, sxd1 mutant leaves showed tocopherol deficiency and accumulated 2,3-dimethyl-5-phytyl-1,4-hydroquinone (DMPQ), a substrate of TC; second, recombinant SXD1 protein exhibited TC activity in vitro; and third, expression of maize SXD1 in the Synechocystis sp. strain PCC 6803 protein slr1737 knockout mutant complemented the lack of tocopherol cyclase activity and restored vitamin E synthesis (Sattler et al., 2003).
Despite the molecular and biochemical similarities of VTE1 and SXD1 in vte1 and sxd1 mutant leaves, the Suc export phenotype was absent in vte1 Arabidopsis plants (Sattler et al., 2003). Thus, unlike the sxd1 mutant, vte1 did not accumulate carbohydrates and anthocyanins and did not exhibit stunted growth. In addition, chlorophyll content and photosynthetic efficiency of vte1 plants were very similar to wild type under optimal growth conditions (Porfirova et al., 2002). However, during photooxidative stress at high light conditions, chlorophyll content and photosynthetic quantum yield decreased slightly as compared to wild type (Porfirova et al., 2002). These data appeared to be in good agreement with the well-defined in vitro ability of tocopherols to scavenge and quench reactive oxygen species and lipid peroxy radicals by physical and chemical means (for review, see Fryer, 1992; Munné-Bosch and Alegre, 2002).
The mechanistic link between tocopherol deficiency and the formation of aberrant PD at the BS-VP interface leading to the Suc transport defect in the sxd1 maize mutant is completely unknown. Moreover, the reasons for phenotypic differences between Arabidopsis and maize remain elusive. Sattler et al. (2003) postulated that additional biological activities of tocopherols, which are not related to their antioxidant function, might be involved in the different responses of Arabidopsis and maize to tocopherol deficiency. There is accumulating evidence from studies in mammalian systems that specific tocopherols are able to modulate signal transduction pathways or act as signal molecules themselves (for review, see Azzi et al., 2002; Rimbach et al., 2002). Although direct evidence for analogous nonantioxidant roles of tocopherols in plants is lacking, it was speculated that tocopherol deficiency in sxd1 interfered with signal transduction events that are required specifically for the formation of BS-VP PD (Sattler et al., 2003). This tocopherol-dependent signaling pathway might be different or absent in Arabidopsis due to anatomical and physiological differences between C3 and C4 and/or between monocot and dicot species.
To address the principal question of whether the secondary effects of tocopherol deficiency on PD function and photoassimilate transport are restricted to the sxd1 mutant from maize and thus could be related to special features of C4 plants, comparative experiments on different plant systems are required. Therefore, we have chosen potato (Solanum tuberosum L. var. Solara) as an alternative model plant. Potato represents a C3 crop plant and is one of the best-characterized plants with respect to sink-source relations and carbohydrate metabolism (Stitt and Sonnewald, 1995; Herbers and Sonnewald, 1998). In this study, the SXD1 ortholog from potato was isolated and functionally analyzed in vitro as well as in planta using an RNAi-silencing approach. We could demonstrate that the reduction of tocopherol cyclase enzyme activity in transgenic potato lines resulted in tocopherol deficiency and caused a characteristic photoassimilate export-deficient phenotype, most likely due to vascular-specific callose deposition in source leaves. These data provide evidence that the impact of tocopherol deficiency on PD function and carbohydrate metabolism is similar in both monocot and dicot species and thus cannot be assigned to specific anatomical or biochemical features of C4 metabolism. Furthermore, the absence of comparable phenotypic changes in the Arabidopsis vte1 mutant suggests significant differences in the C3 plant family in response to tocopherol deficiency.
RESULTS
Isolation of SXD1 cDNA from Potato and Enzymatic Activity of Recombinant Protein
To identify a potato ortholog of maize SXD1 (ZmSXD1) and Arabidopsis VTE1 (AtSXD1) proteins, EST databases were searched for homologous sequences using tBLASTn (BLAST; Altschul et al., 1990). A partial EST sequence derived from mature potato tubers (accession no. BF460070) was amplified by reverse transcription (RT)-PCR from potato leaf material. After obtaining 5′ and 3′ sequences by RACE techniques, the full-length cDNA, designated StSXD1 (accession no. AY536918) was isolated and fully sequenced. StSXD1 encoded a 56.2-kD protein with 501 amino acids that shared approximately 62% amino acid identity with the TCs from maize and Arabidopsis, respectively, and also showed substantial similarity to predicted TCs from different photosynthetic bacteria, including the Synechocystis sp. protein encoded by open reading frame slr1727 (34% amino acid identity), that was previously shown to be a functional TC (Sattler et al., 2003).
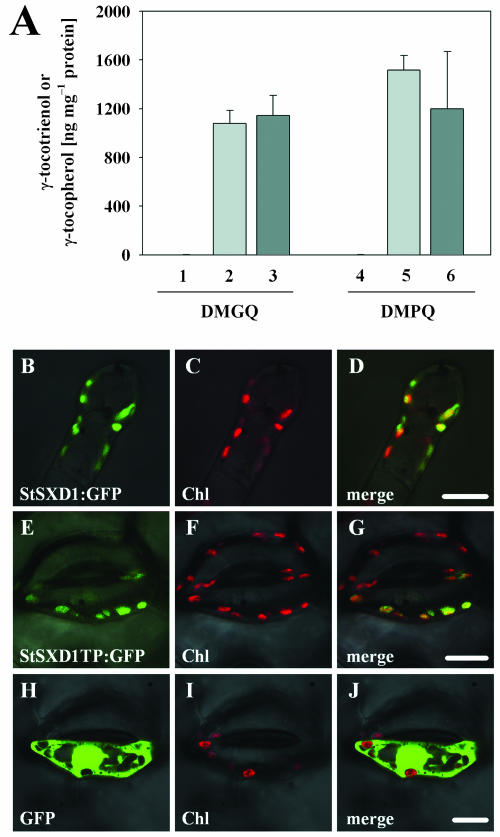
To provide evidence that the potato SXD1 ortholog codes for a functional protein with TC activity, the StSXD1 cDNA was heterologously expressed in Escherichia coli and used for enzyme assays. Since StSXD1 contained in accordance with ZmSXD1 and AtSXD1/VTE1 (Provencher et al., 2001) an apparent N-terminal transit peptide (TP) for chloroplast targeting, the precursor protein (StSXD1), as well the predicted mature part lacking the N-terminal 89 amino acid (StSXD1ΔTP), was analyzed. As demonstrated in Figure 1A, recombinant StSXD1 and StSXD1ΔTP proteins showed high TC activity with the substrates DMPQ and 2,3-dimethyl-5-geranlygeranyl-1,4-hydroquinone (DMGQ), leading to the synthesis of γ-tocopherol and γ-tocotrienol, respectively. This clearly demonstrated that StSXD1 is a functional TC whose activity is not influenced by the N-terminal targeting signal in vitro.
Figure 1.
TC activity of recombinant StSXD1 protein in E. coli and intracellular targeting of StSXD1 in leaves. A, The premature (columns 3 and 6) and mature part (columns 2 and 5) of StSXD1 cDNA was expressed in E. coli cells and used for TC assay with DMGQ and DMPQ as substrates. The products, γ-tocotrienol and γ-tocopherol, were quantified by fluorescence HPLC. The values represent the mean of three independent experiments and sd. E. coli cells transformed with an empty vector pQE11 served as control (columns 1 and 4) and accumulated less than 2 ng γ-tocotrienol or 1 ng γ-tocopherol/mg of protein. B–J, GFP fusion proteins of full-length StSXD1 (StSXD1:GFP) and N-terminal TP (StSXD1TP:GFP) as well as GFP protein alone were transiently expressed in tobacco epidermal cells by microprojectile bombardment and analyzed by confocal microscopy after 20 h. Green color indicates GFP fluorescence and red color reveals chlorophyll (Chl) fluorescence. B, Intracellular localization of StSXD1:GFP in terminal trichome cell. C, Chl fluorescence indicates chloroplast distribution. D, Merged image. E, Intracellular localization of StSXD1TP:GFP in stomata cell. F, Chl fluorescence of chloroplasts. G, Merged image. H, Intracellular localization of free GFP in stomata cell. I, Chl fluorescence of chloroplasts. J, Merged image. Bars represent 10 μm.
StSXD1 Is Targeted into Chloroplasts
To analyze the functionality of the predicted N-terminal TP in planta, intracellular localization of full-length StSXD1, as well as the N-terminal portion (89 amino acid) fused to green fluorescent protein (GFP; Chalfie et al., 1994), respectively, was determined after transient expression in epidermal cells of tobacco (Nicotiana tabacum) source leaves. As depicted in Figure 1, B to J, StSXD1-GFP appeared to be targeted into chloroplasts since it colocalized in the merged picture with the red fluorescence of chlorophyll. Transient expression of the StSXD1 TP-GFP fusion resulted in a similar chloroplastic targeting, whereas expression of GFP alone revealed only cytosolic localization. These data indicate that StSXD1 contains a functional TP and is targeted into chloroplasts.
RNAi-Mediated Silencing of StSXD1 Results in Tocopherol Deficiency in Potato Plants
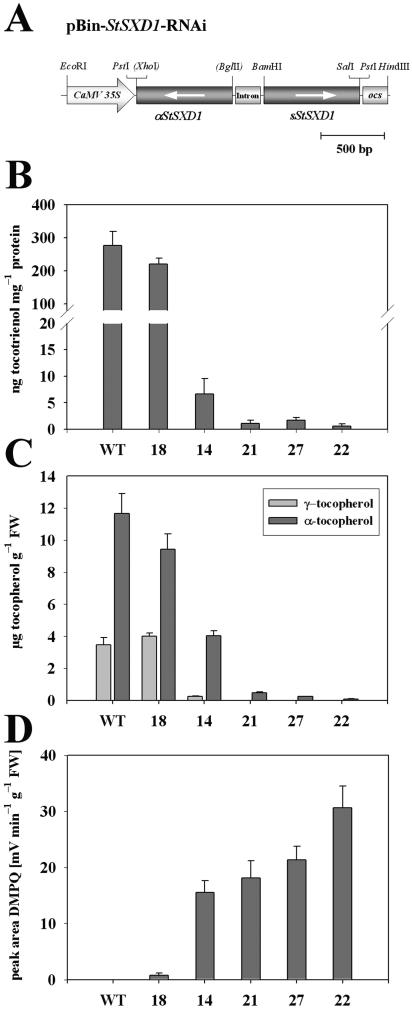
To functionally analyze the in planta role of StSXD1, transgenic potato plants constitutively expressing an intron-spliced hairpin RNA (RNAi) construct targeted at StSXD1 were generated (pBin-StSXD1-RNAi; Fig. 2A). Twenty-eight kanamycin-resistant transformants were screened for reduction in StSXD1 transcript level by northern-blot analysis (data not shown). Four transgenic plants showing strong silencing, designated StSXD1-RNAi-14, -21, -22, and -27, respectively, and, in addition, StSXD1-RNAi-18 with similar transcript levels as nontransformed wild-type plants, were selected and vegetatively multiplied in tissue culture for biochemical analysis. Five weeks after transfer into the greenhouse, TC activity and tocopherol contents were determined in source leaves of transgenic lines. In correlation with decreased StSXD1 transcript levels, TC activity was suppressed in lines 14, 21, 22, and 27 compared to wild type, respectively, leading to dramatically reduced vitamin E levels as indicated by determination of the 2 predominant tocopherol derivatives in leaves, α- and γ-tocopherol (Fig. 2, B and C). Among the selected lines, StSXD1-RNAi-22 showed the strongest repression of TC activity, resulting in a tocopherol-deficient phenotype (0.7% of α-tocopherol wild-type levels), comparable to the Arabidopsis vte1 null mutant. StSXD1-RNAi-27 and -21 were also strongly affected (2.1% and 4.1% of α-tocopherol wild-type levels, respectively), whereas line 14 showed an intermediate phenotype (34.5% of α-tocopherol wild-type levels). In contrast to these significantly silenced lines, StSXD1-RNAi-18 plants exhibited only a minor decrease in TC enzyme activity and slightly lowered α-tocopherol content (80.9% of α-tocopherol wild-type levels) and thus was subsequently included as a weakly affected control line. Apparently in close correlation with the inhibition of TC activity and the reduction of tocopherol content, transgenic lines showed an accumulation of the TC substrate DMPQ (Fig. 2D), which was absent in wild-type plants and thus indicated a specific disruption of TC by silencing of StSXD1. Collectively, these results provide evidence that the SXD1 ortholog from potato codes for a functional TC in planta involved in the biosynthesis of tocopherols.
Figure 2.
RNAi-mediated silencing of StSXD1 leads to suppression of TC activity and results in tocopherol deficiency as well as DMPQ accumulation in transgenic potato plants. A, Schematic structure of the binary intron-spliced hairpin RNA (RNAi) expression construct used for transformation of potato plants. StSXD1 fragments (765 bp, nts 503–1,268 of StSXD1 cDNA; accession no. AY536918) in sense and antisense orientation separated by intron 1 of potato GA20 oxidase (200 bp) were placed between the cauliflower mosaic virus 35S promoter and the ocs terminator of the Bin19-derived vector using the indicated restriction sites. B, TC activity. C, Tocopherol contents (α- and γ-tocopherol). D, DMPQ levels in source leaves of StSXD1-silenced transgenic lines (StSXD1-RNAi-18, -14, -21, -27, and -22) compared to wild type. TC activity was determined using DMGQ as substrate and tocopherols, as well as the prenyl quinone precursor DMPQ, were quantified by fluorescence HPLC. Samples were taken from source leaves 5 weeks after transfer form tissue culture and data represent the means ± se of 5 individual plants.
Tocopherol Deficiency Leads to Impaired Photoassimilate Export
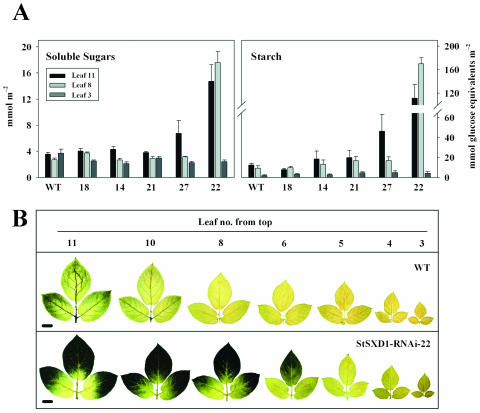
To evaluate whether tocopherol deficiency in StSXD1-silenced potato lines resulted in a Suc export defect similar to the maize sxd1 mutant phenotype (Russin et al., 1996; Provencher et al., 2001) or did not alter carbohydrate metabolism as observed in Arabidopsis vte1 plants (Sattler et al., 2003), steady-state levels of soluble sugars and starch were determined. To this end, the set of StSXD1-RNAi transgenic lines characterized for inhibition of TC activity and tocopherol reduction before (compare Fig. 2) was analyzed in 3 different leaf stages (leaf 11, 8, and 3 from the top) after an additional 4-week growth period. As indicated in Figure 3A, 59-d-old plants of line StSXD1-RNAi-22, showing the strongest reduction in TC activity and tocopherol content, accumulated massive amounts of carbohydrates in lower source leaves (leaf 11) at the end of the dark period. Starch and soluble sugar levels increased 9-fold and 4.1-fold, respectively, compared to wild-type levels. A considerable accumulation in starch and soluble sugar content was also detectable for StSXD1-RNAi-27 plants (3.7-fold and 1.8-fold, respectively), whereas starch levels in lines 21 and 14 were slightly, but not significantly, enhanced relative to wild-type plants. In upper source leaves (leaf 8), carbohydrate status remained dramatically increased in line 22, but was not significantly altered in line 27, as well as in lines 21 and 14, respectively, whereas in young developing leaves (leaf 3) overall carbohydrate levels did not accumulate in any of the transgenic lines. These data clearly suggested a leaf age-dependent occurrence of the phenotype potentially correlating with the sink-source gradient in the plant. To analyze the spatial distribution of phenotype development in more detail, leaves of different developmental stages from line 22 were assessed for starch accumulation by iodine staining at the end of the dark period. As depicted in Figure 3B, the starch-excess phenotype appeared to be source leaf-specific and restricted to the nonvascular regions of the leaf, indicating a defect in photoassimilate export.
Figure 3.
Carbohydrate steady-state levels and spatial distribution of starch accumulation in leaves of tocopherol-deficient potato plants. A, Soluble sugar and starch contents in three different leaf stages (11th, 8th, and 3rd leaf from the top) of 59-d-old plants at the end of the dark period. Two samples were taken from each leaf and averaged; values are given as means ± se of 5 independent plants, and soluble sugars represent the sum of Glc, Fru, and Suc. B, Qualitative determination of starch accumulation in leaves of StSXD1-RNAi-22 and wild type after an extended dark period (16 h) by iodine (KI) staining. Bars represent 2 cm.
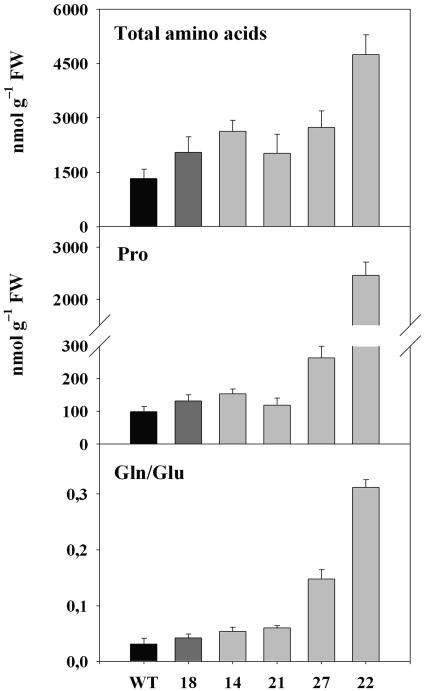
To further elucidate whether the observed export impairment of source leaves would be limited to carbon or would also affect nitrogen allocation, amino acid contents and composition were determined at the end of the photoperiod. Remarkably, total amino acid levels in lower source leaves (leaf 11, see above) increased 3.7-fold in StSXD1-RNAi-22 plants and were also enhanced (between 1.5- and 2.1-fold) in the other transgenic lines (Fig. 4). The strong increase in amino acid levels of StSXD1-RNAi-22 was mainly due to dramatically elevated levels of the osmoprotectant Pro, which accumulated 24.8-fold compared to wild type (Fig. 4). Gln to Glu ratios were also strikingly enhanced in lines 22 and 27, indicating a shift toward the transport forms of amino acids. Although Asn to Asp ratios were not comparably altered (data not shown), the increase in the Gln to Glu ratio correlated well with starch accumulation in the respective transgenic lines (compare Fig. 3A), suggesting an impaired export capacity of source leaves both for carbohydrates and amino acids.
Figure 4.
Amino acid concentrations in source leaves of StSXD1-silenced potato plants. Leaf samples were taken at the end of the light period from lower source leaves (leaf 11 from the top) of 59-d-old plants. Total amino acid levels represent the sum of single amino acid concentrations determined by HPLC. Values are given as means (n = 4) ± se.
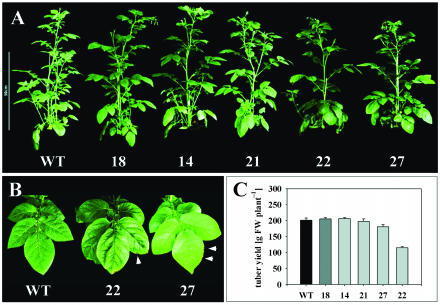
Despite the inhibition of photosynthate transport from source leaves, overall stature and growth of transgenic plants was not substantially changed compared to the control (Fig. 5A). However, carbohydrate-accumulating source leaves of line 27, and especially line 22, developed phenotypic alterations characterized by chlorotic interveinal regions, leaf curling, and necrotic spots at the leaf margins (Fig. 5B). Additionally, tuber yield was significantly reduced in line 22 and slightly lowered in line 27, compared to wild-type plants, respectively, which provided circumstantial evidence that leaves of the strongest affected lines fail to effectively export photoassimilates for storage sink development (Fig. 5C).
Figure 5.
Growth response, leaf morphology, and tuber yield of tocopherol-deficient potato plants. A, Visible phenotype of transgenic lines and wild type after 9 weeks in the greenhouse. Bar represents 50 cm. B, Phenoytpic alterations of lower source leaves from StSXD1-RNAi-22 and -27 compared to wild type. Arrowheads indicate necrotic spots at the leaf margins. C, Tuber yield of StSXD1-RNAi and wild-type plants grown for 13 weeks in the greenhouse. Data represent means ± se of 5 plants.
Tocopherol Deficiency Causes Enhanced Callose Deposition in the Vascular Tissue
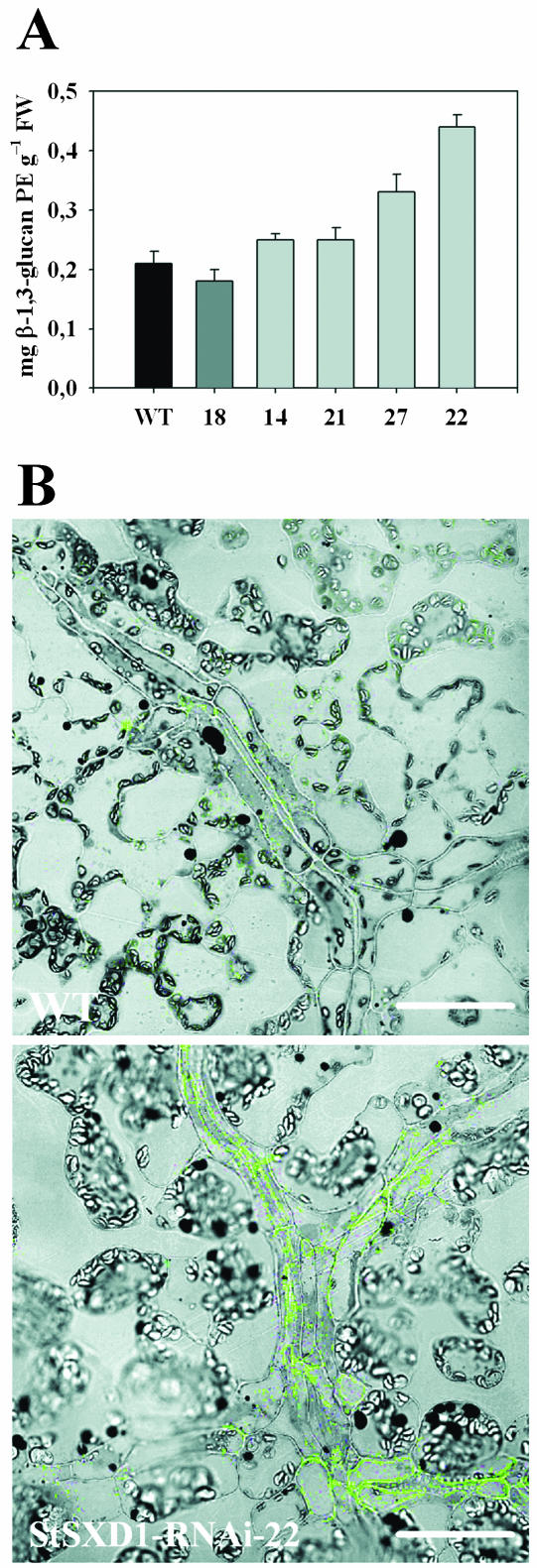
The assimilate export-deficient phenotype in the maize sxd1 mutant has previously been attributed to callose occlusion of PD at the BS-VP interface of maturating leaf blades, thereby preventing the symplastic transport of Suc into the phloem tissue (Botha et al., 2000; Provencher et al., 2001). Therefore, callose concentration and distribution were analyzed in the same lower source leaves of tocopherol-deficient potato lines in comparison to wild type as used for photoassimilate determination before (leaf 11; compare Figs. 3 and 4). Consistent with the development of the starch-excess phenotype, callose accumulated in source leaves of StSXD1-silenced plants and reached highest levels in lines 22 and 27 (2.1-fold and 1.6-fold, respectively) relative to wild type. Using a fluorescein isothiocyanate-labeled β-1,3-glucan antibody and confocal laser scanning microscopy, the callose localization pattern was visualized in leaf sections of the most strongly affected line, StSXD1-RNAi-22, in comparison to the control. As shown in Figure 6B, enhanced callose deposition in tocopherol-deficient plants was obvious as fluorescing aggregates decorating the cell wall of phloem-associated cells, whereas comparable vascular cell types of control plants showed only very little callose-derived fluorescence. In contrast, nonvascular mesophyll cells of both StSXD1-silenced plants and wild-type controls did not exhibit significant callose labeling. These results demonstrated that, in agreement with the sxd1 maize mutant, enhanced callose synthesis and deposition were restricted to the vascular tissue in StSXD1-suppressed potato plants and, therefore, might similarly be the reason for the disruption of assimilate export.
Figure 6.
Callose concentrations and distribution in source leaves of tocopherol-deficient potato plants. A, Callose contents in lower source leaves (leaf 11 from the top) of 59-d-old potato plants at the end of the photoperiod. Data represent the means (n = 5) ± se and are given as β-1,3-glucan pachyman equivalents. B, Immunofluorescence analysis of vascular-specific callose accumulation in StSXD1-RNAi-22 compared to wild type. Samples were taken from lower source leaves of 59-d-old potato plants and callose was detected in longitudinal section using a monoclonal anti-β-1,3-glucan antibody and Alexa Fluor 488 antimouse IgG as fluorescence marker. Callose-derived fluorescence signals in vein class III-associated cells were detected by confocal laser scanning microscopy and superimposed with bright field images. Bars represent 50 μm.
The Assimilate Export-Deficient Phenotype Is Accompanied by Changes in Photosynthetic Capacity and Altered Gene Expression
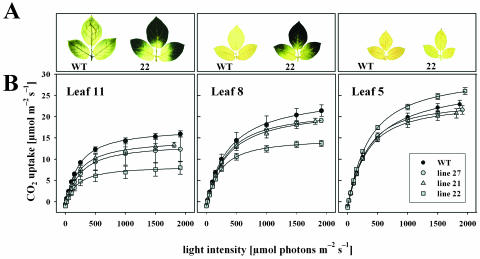
The absence of tocopherol in the vte1 mutant has previously been shown to have no major impact on photosynthetic performance under optimal growth conditions (Porfirova et al., 2002). On the other hand, it is well established that carbohydrate accumulation in leaves results in reduced rates of photosynthesis by direct or indirect feedback inhibition mechanisms (Krapp and Stitt, 1995; Herbers et al., 1997; Paul and Pellny, 2003). Therefore, we investigated whether tocopherol deficiency and/or photosynthate accumulation affected CO2 assimilation in lines StSXD1-RNAi-21, -27, and -22 compared to wild type. Gas-exchange rates were determined in 3 different leaves (corresponding to leaf stages 11, 8, and 5 in Fig. 3), which were chosen according to the occurrence of the starch-excess phenotype in line 22 and included younger leaves that did not exhibit starch accumulation (Fig. 7A). As depicted in Figure 7B, maximum photosynthetic rates of StSXD1-RNAi-22 plants were significantly reduced by 50.3% in lower and 33.7% in upper source leaves as compared to equivalent leaves of wild-type plants under approximately saturating light conditions (1,500 μm photons m−2 s−1). In contrast, CO2 assimilation was significantly enhanced by 11.7% in young leaves of line 22 in comparison to wild-type leaves, suggesting compensatory effects in developing leaves that lack the assimilate export-deficient phenotype. Apparently consistent with the smaller degree of carbohydrate accumulation in lines 27 and 21, respectively, reduction of CO2 exchange rates in lower and upper source leaves of the respective lines relative to wild type was less pronounced and not as significant as observed for line 22. Accordingly, an increase in photosynthesis in young leaves compared to the control was not obvious in StSXD1-RNAi-27 and StSXD1-RNAi-21 plants, respectively. In contrast to maximal photosynthetic rates at saturating light conditions, the initial slope of the light response curves appeared to be less affected in tocopherol-deficient lines relative to wild type. A significant reduction of CO2 exchange rates at low light intensities was only detectable in lower source leaves of phenotypic lines, whereas upper source leaves as well as young leaves were largely unaltered in this respect. Hence, the system of light reactions, including light harvesting and electron transport rate, was only compromised in the oldest starch-accumulating leaf stage. Taken together, these results indicated that carbohydrate accumulation rather than tocopherol deficiency per se influenced photosynthetic performance of StSXD1-silenced potato plants.
Figure 7.
Photosynthetic capacity in tocopherol-deficient potato lines. A, Starch accumulation in three different leaf stages (11th, 8th, and 5th leaf from the top) of wild type and StSXD1-RNAi-22 plants selected for CO2 gas-exchange measurements. B, Light response curves of StSXD1-RNAi-21, -27, and -22 plants in comparison to wild type. Photosynthetic rates were determined at 400 μm m−1 CO2 concentration using a LICOR LI6400 instrument. Plants were analyzed 8 weeks after transfer to the greenhouse and data represent means ± se of 4 plants.
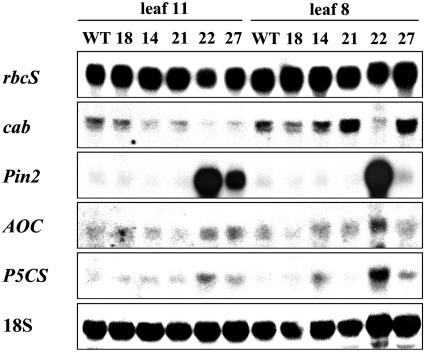
Previously, it was observed that sugar accumulation in leaves, whether as a result of impaired assimilate export or of sugar feeding, negatively regulated the expression of photosynthetic genes and induced defense-related gene expression (Herbers et al., 1996a, 1996b; Rolland et al., 2002). However, the sxd1 maize mutant failed to down-regulate the accumulation of various photosynthetic proteins (Provencher et al., 2001). Therefore, photosynthetic (rbcS and cab) gene expression was studied by northern blotting in the same lower and upper source leaves of StSXD1-silenced plants as used for carbohydrate analysis before (see Fig. 3). Figure 8 shows that rbcS and cab transcript accumulation was reduced in lower source leaves compared to wild type. In upper source leaves, suppression of photosynthetic gene expression occurred in StSXD1-RNAi-22 plants in correlation with excess sugar accumulation (see Fig. 3), whereas the other lines showed no effect on rbcS expression or even exhibited a slight increase of cab transcripts (lines 21 and 27). In addition, transcripts of the defense-related proteinase inhibitor II (Pin2), previously shown to be up-regulated by sugars and jasmonic acid (JA; Johnson and Ryan, 1990; Wasternack and Parthier, 1997), were strongly induced in phenotypic source leaves of StSXD1-RNAi-22 and -27. Possible changes of JA-related gene expression in response to tocopherol deficiency were further supported by the slight transcriptional up-regulation of the JA biosynthetic gene allene oxide cyclase (AOC) in these lines. Consistent with the observed Pro accumulation (Fig. 4), an increase of transcript levels was also detectable for the key enzyme in Pro biosynthesis, Δ1-pyrroline-5-carboxylate synthase (P5CS), which was demonstrated to be inducible by Suc and abscisic acid (Hellmann et al., 2000; Abraham et al., 2003). Together, these indicated carbohydrate- and potentially stress-induced alteration of gene expression in tocopherol-deficient potato plants.
Figure 8.
Gene expression analysis of StSXD1-silenced plants. Total RNA was isolated from lower and upper source leaves (11th and 8th leaves from the top of 59-d-old StSXD1-RNAi lines and control plants (wild type), and transcript levels were analyzed by northern blotting. Thirty micrograms of RNA were loaded per lane and hybridized with cDNA probes of photosynthetic (rbcS, cab), defense-related (Pin2), as well as JA (AOC) and Pro (P5CS) biosynthetic genes. Additional probing with an 18S cDNA served as loading control.
DISCUSSION
Tocopherols are believed to represent key components of the antioxidant network in the chloroplast and to play a protective role against toxic free radicals generated during photosynthesis and various abiotic stresses (Fryer, 1992; Munné-Bosch and Alegre, 2002). However, most of our understanding of tocopherol functions has been derived from nonplant systems, since tocopherols (vitamin E) are essential dietary components for humans and other mammals. Recent efforts to identify the enzymatic steps involved in the tocopherol biosynthetic pathway and to improve vitamin E contents in crop plants by metabolic engineering have provided a number of different mutant and transgenic plants for the analysis of tocopherol function in planta (Shintani and DellaPenna, 1998; Collakova and DellaPenna, 2001, 2003; Porfirova et al., 2002; Savidge et al., 2002; Bergmüller et al., 2003; Cheng et al., 2003; Van Eenennaam et al., 2003). In this respect, it was discovered that the maize sxd1 mutant, originally isolated based on a block in Suc transport from leaves (Russin et al., 1996; Provencher et al., 2001), and the Arabidopsis vte1 mutant were both defective in the TC gene, resulting in a complete lack of tocopherols (Porfirova et al., 2002; Sattler et al., 2003). However, the photoassimilate export-defective phenotype caused by aberrant PD formation and callose deposition in BS cells of sxd1 maize plants was absent in the vte1 Arabidopsis mutant, which suggested additional nonantioxidant and signal transduction-related functions of tocopherols in C4 plants (Sattler et al., 2003). Here, we show that RNAi-mediated disruption of tocopherol cyclase activity in the C3 crop plant potato results in a severe tocopherol-deficient phenotype comparable to the orthologous Arabidopsis and maize mutants. As a consequence, transgenic potato plants exhibited a characteristic photoassimilate export defect as seen in the sxd1 mutant, which appeared to be likewise caused by vascular-specific callose deposition in source leaves. Therefore, the impact of tocopherol reduction on callose accumulation, assimilate transport, and carbohydrate metabolism occurs both in C4 and C3 plants. Additionally, the lack of an sxd phenotype in Arabidopsis suggests significant and potentially nonantioxidant-related differences between C3 species in their pleiotropic response to tocopherol deficiency.
StSXD1 Encodes a Functional Chloroplast-Targeted TC Enzyme
The isolated potato ortholog of the SXD1 gene was characterized in vitro and in planta, providing several lines of evidence that StSXD1 is functionally equivalent to the recently identified TCs from Arabidopsis (AtVTE1/AtSXD1) and maize (ZmSXD1). First, heterologous expression of StSXD1 in E. coli resulted in the synthesis of a protein with high TC activity as revealed by conversion of DMPQ and DMGQ into γ-tocopherol and γ-tocotrienol, respectively (Fig. 1A). A comparable activity has previously been detected for recombinant AtSXD1/AtVTE1 and ZmSXD1 proteins, respectively (Porfirova et al., 2002; Sattler et al., 2003). Second, the N-terminal domain of StSXD1, which shared only a few conserved amino acid residues with other SXD1 plant homologs, targets StSXD1 to the chloroplast as revealed by transient expression of GFP-tagged fusions in tobacco leaves (Fig. 1B). This chloroplastic localization of StSXD1 is consistent with the results obtained by chloroplast import assays for ZmSXD1 (Provencher et al., 2001), as well as with the reported TC activity and tocopherol biosynthesis in plastids (Soll et al., 1985; Arango and Heise, 1998). Third, silencing of StSXD1 resulted in a loss of TC activity and caused the accumulation of the TC substrate DMPQ as well as tocopherol deficiency in leaves of transgenic potato plants, indicating that StSXD1 is required for tocopherol biosynthesis (Fig. 2, A–D).
Severe Tocopherol Deficiency Is Required to Induce a Blockage of Photoassimilate Export
Efficient silencing of StSXD1 was obtained by expression of an intron-spliced hairpin RNA (RNAi) construct, which led to the generation of three strongly affected lines with less than 5% of wild-type α-tocopherol level (Fig. 2C). However, the induction of a severe Suc export-deficient phenotype characterized by a severalfold increase of carbohydrate contents and morphological alterations in source leaves, as well as a strong reduction in tuber yield, was only detectable in line StSXD1-RNAi-22 (Figs. 3 and 5), which nearly showed a complete lack of tocopherols. Slightly higher tocopherol contents resulted in a considerably smaller extent of phenotype development in plants of line 27 or affected assimilate export only marginally as observed for line 21. This indicated a low threshold level and the requirement of a comparably strong impairment of tocopherol biosynthesis as obtained by the sxd1 null mutation to induce the assimilate transport block. The phenotype of StSXD1-RNAi-22 plants was reminiscent of transgenic potato plants inhibited in apoplastic phloem loading of Suc by antisense repression of the Suc transporter SUT1 (Riesmeier et al., 1994; Kühn et al., 1996). However, it is unlikely that carbohydrate accumulation in tocopherol-deficient plants resulted from Suc transporter malfunction, because the block in carbon export was accompanied by impaired amino acid transport as revealed by the accumulation of total amino acids and severalfold increase of Gln to Glu ratios in source leaves (Fig. 4). These effects concur well with the assumption that the blockage of the symplastic transport route into minor veins, as demonstrated previously by dye-coupling microinjection studies for sxd1 mutant leaves (Botha et al., 2000), would likewise affect carbon and nitrogen allocation. The increase of Pro contents in source leaves of phenotypic line StSXD1-RNAi-22, and less pronounced of line StSXD1-RNAi-27, further indicated an enhanced stress response as a consequence of tocopherol reduction and/or excess carbohydrate accumulation. Pro has been shown to function as a protective compatible osmolyte and to accumulate in response to osmotic stress conditions stimulated by salt, cold, drought, and abscisic acid (Hare et al., 1999; Hasegawa et al., 2000), as well as by elevated hexose contents (Bussis et al., 1997). Consistently, the pathway for Pro biosynthesis appeared to be up-regulated as evidenced by increased gene expression of P5CS in phenotypic lines (Fig. 8).
Tocopherol Deficiency Indirectly Affects Photosynthetic Capacity by Carbohydrate-Mediated Feedback Mechanisms
The assimilate export defect in StSXD1-silenced plants was paralleled by a significant decrease in photosynthetic capacity (Fig. 7). This effect appeared to be leaf age-dependent and directly linked to the source leaf-specific carbohydrate accumulation, since young leaves that lacked a starch-excess phenotype either were not affected (StSXD1-RNAi-21, 27) or even showed an increase in CO2 exchange rates for StSXD1-RNAi-22. Additionally, CO2 assimilation at lower light intensities indicated that the light reaction was only affected in lower carbohydrate-accumulating source leaves and, thus, was not generally inactivated by tocopherol deficiency. These results provide clear evidence that tocopherol per se is not essential for photosynthetic performance at optimal growth conditions, which concurs well with previous results obtained for the Arabidopsis vte1 mutant (Porfirova et al., 2002) and for partially tocopherol-deficient tobacco plants repressed in the expression of geranylgeranyl reductase (Grasses et al., 2001). A decrease in photosynthetic rate has frequently been observed in response to soluble sugar and starch accumulation indicative of feedback regulation of photosynthesis (Riesmeier et al., 1994; Kühn et al., 1996; Herbers et al., 1997; Paul and Pellny, 2003, and references therein). Aside from the possibility of impaired chloroplast function as a consequence of excessive starch accumulation, carbohydrate-mediated feedback inhibition of photosynthesis has been attributed to hexose-induced repression of photosynthetic genes via hexokinase-dependent and -independent signals (Herbers et al., 1996a, 1996b; Rolland et al., 2002). Accordingly, rbcS- and cab-specific transcripts appeared to be down-regulated in source leaves of StSXD1-RNAi-22 plants that accumulated large amounts of soluble sugars in addition to starch (Fig. 3A). Consistently, in the absence of sugar accumulation as evident for upper source leaves of lines 21 and 27 (compare Fig. 3), photosynthetic transcripts were either not affected (rbcS) or even slightly up-regulated (cab). The occurrence of carbohydrate-mediated changes of gene expression was further supported by the induction of Pin2 transcripts in sugar-accumulating source leaves, which is in good agreement with the former hypothesis that suppression of photosynthetic genes and activation of defense-related genes might be initiated by common sugar-sensing mechanisms (Herbers et al., 1996a; Rolland et al., 2002). However, despite a similar accumulation of soluble sugars, mature leaves of the sxd1 maize mutant failed to alter photosynthetic gene expression (Provencher et al., 2001) as observed in StSXD1-silenced potato plants. Although the exact reason for this discrepancy is unknown, it might be due to either different threshold levels of soluble sugars for repression of photosynthetic genes in maize and potato plants or the general differences of CO2 assimilation in C4 and C3 plants.
Callose Deposition in the Vascular Tissue Correlates with Tocopherol Deficiency and the Defect in Photoassimilate Export
The assimilate export phenotype in source leaves of StSXD1-repressed transgenic lines correlated well with the enhanced formation and vascular-specific deposition of callose (Fig. 6). This observation appeared to be in close agreement with previous data from sxd1 mutant leaves demonstrating that callose occlusion of PD at the BS-VP interface prevented symplastic transport into the phloem (Botha et al., 2000), and thus resulted in the inhibition of photosynthate export. Although plasmodesmal permeability of callose-accumulating vascular cells in StSXD1-silenced potato plants was not analyzed in this study, it is likely that the observed cell wall deposition of callose went along with PD closure, leading to the interruption of symplastic Suc transport and finally excess assimilate accumulation.
There is compelling evidence in the literature that PD represent highly regulated channels in the cell wall that dynamically respond to external and internal stimuli by altering their size exclusion limits (Haywood et al., 2002; Roberts and Oparka, 2003). In this respect, the formation of callose deposits at the neck region of PD leading to reduced size exclusion limits or PD closure has frequently been described as a specific response of plants to different abiotic and biotic stresses, including wounding, metal toxicity, and microbial infection (Enkerli et al., 1997; Iglesias and Meins, 2000; Sivaguru et al., 2000; Roberts and Oparka, 2003). For instance, enhanced callose accumulation was observed in membranes and PD of pea roots in response to aluminum treatment, leading to the blockage of symplastic transport and inhibition of root elongation (Sivaguru et al., 2000). Interestingly, callose formation was strongly correlated with aluminum-induced oxidative damage to membrane lipids and changes in intracellular calcium homeostasis, suggesting a mechanistic link between lipid peroxidation and callose synthesis (Jones et al., 1998; Yamamoto et al., 2001). Because tocopherols are proposed to function effectively in the scavenging of lipid peroxy radicals responsible for the propagation of polyunsaturated fatty acid oxidation (Wefers and Sies, 1988; McKersie et al., 1990; Fryer, 1992; Munné-Bosch and Alegre, 2002), it is tempting to speculate that tocopherol deficiency may indirectly affect callose synthesis by increasing the extent of lipid peroxidation in the chloroplast membranes. Apparently consistent with this view, it has recently been postulated that tocopherols play a role in intracellular signaling by controlling accumulation of lipid hydroperoxides, which are derived from lipid peroxidation and used for JA biosynthesis (Schaller, 2001; Munné-Bosch and Alegre, 2002; Munné-Bosch and Falk, 2004). Thus, tocopherol levels may indirectly regulate the amount of JA in the leaves and influence JA-dependent gene expression involved, for instance, in the wound stress response. The induction of JA-responsive Pin2 transcripts, as well as the slight up-regulation of JA biosynthetic gene AOC in severely tocopherol-deficient potato plants, might support this hypothesis. Interestingly, a callose synthase gene (GSL5) has recently been isolated from Arabidopsis, which is required for wound- and pathogen-induced callose formation (Jacobs et al., 2003; Nishimura et al., 2003). Further research is needed to determine whether callose-synthesizing activities are indeed responsive to lipid peroxidation-derived or JA-dependent signals and whether other hormones (e.g. salicylic acid) involved in stress signaling, and potentially in the regulation of callose synthesis (Østergaard et al., 2002), are also affected by tocopherols, as suggested recently (Munné-Bosch and Peñuelas, 2003). The absence of lipid peroxidation in vte1 Arabidopsis plants at optimal growth conditions (Bergmüller et al., 2003) might lead to the tempting hypothesis that the crop plant potato and the mustard weed Arabidopsis differ in their basal stress tolerance and sensitivity of membrane lipids to oxidative damage under tocopherol deficiency. However, a detailed analysis of lipid composition and malondialdehyde levels in StSXD1-silenced potato plants, indicative of the extent of lipid peroxidation, is required to draw any further conclusions in this respect. Similarly, based on the various antioxidant-independent functions of tocopherols known from mammalian systems (Brigelius-Flohe and Traber, 1999; Rimbach et al., 2002), it is possible that tocopherol or the prenyl quinone precursor DMPQ acts as signaling molecules and modulators of other stress-unrelated signal transduction pathways in plants, as discussed in detail by Sattler et al. (2003). These functions might differ significantly between the C3 species Arabidopsis and potato and thus have to be equally considered for future attempts to unravel the molecular basis of callose deposition and impaired assimilate export in response to tocopherol deficiency.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Solanum tuberosum L. var. Solara (potato) was obtained from Bioplant (Ebstorf, Germany) and maintained in tissue culture under a 16-h-light/8-h-dark period (150 μm m−2 s−1 light, 21°C) at 50% relative humidity on Murashige and Skoog medium (Sigma, St. Louis) containing 2% (w/v) Suc. Plants used for biochemical and physiological analysis were multiplied vegetatively in tissue culture and then grown in soil either under controlled conditions (70% humidity, 16-h day/8-h night regime, 19°C/15°C, 500 μm m−2 s−1 light) in a growth chamber or in the greenhouse with supplementary light (250 μm m−2 s−1 light).
Cloning of StSXD1 and Heterologous Expression in Escherichia coli
A partial 765-bp EST clone (accession no. BF460070) homologous to Arabidopsis VTE1 and maize (Zea mays) SXD1 cDNAs was amplified by RT-PCR from potato leaf material using M-MLV [H-] reverse transcriptase (Promega, Madison, WI) and oligo(dT)[30] V primer for reverse transcription as well as rTaq-Polymerase (TaKaRa Shouzo, Japan) and gene-specific primers (D184, 5′-GGATCCGGGAGCTCAAATTCTTGGTGCAGATG-3′; D185, 5′-GTCGACAATGTGGTTCCATGATCTTTTGAAG-3′) for PCR amplification. The resulting PCR fragment was subcloned into pCR2.1 vector (Promega) and sequenced to verify the identity of the StSXD1 clone. 5′ and 3′ fragments were obtained by RACE using the SMART RACE cDNA amplification kit (CLONTECH, Palo Alto, CA) and StSXD1-specific primers (StSXD1-5′RACE, 5′-CTCTGTGCGCGGCGATTGAACTCCTGAGG-3′; StSXD1-3′RACE, 5′-GGATTAAGGCGACTTCCGGGATTGAATGAG-3′). Finally, the full-length StSXD1 cDNA clone was amplified by RT-PCR from potato leaves using RACE fragment-deduced oligonucleotides (D278, 5′-AGTTGCCGCTCCTCAAAAACTCTACAWTCC-3′; D279, 5′-TTCTGCTATACTAGCAATAAGATTTTCCAT-3′), and the entire sequence was determined (accession no. AY536918).
For heterologous expression in Escherichia coli, the StSXD1 precursor (nucleotides [nts] 53 to 1,555) as well as the mature part without the apparent signal peptide (StSXD1ΔTP, nts 320–1,555) was PCR amplified from the full-length cDNA clone using the oligonucleotides D292 (5′-GGGATCCCCATGGAGAGCTTTTATAGTGTTTCCGC-3′) and D291 (5′-GTCGACTCAAAGGCCAGGAGGTTTGAGAAGTGAAG-3′) for StSXD1, and D290 (5′-GGGATCCGGACTCCTCATAGCGGGTATCATTTTG-3′) and D291 for StSXD1ΔTP, respectively. The PCR fragments were ligated into the BamHI and SalI sites of pQE11 and transferred into E. coli M15(pREP4) cells for protein expression (Qiagen, Hilden, Germany). Recombinant E. coli cells were harvested 3 to 4 h after induction with 1 mm isopropylthio-β-galactoside, resuspended in 0.1 m potassium phosphate buffer (pH 8.0), and disrupted by sonification. After additional washing of the cell suspension (30 min at 4°C) with potassium phosphate buffer containing 2% (v/v) CHAPS, the insoluble cell debris was removed by centrifugation and the soluble protein fraction was used for enzymatic measurements.
Construction of GFP Fusion Proteins and Microprojectile Bombardment
To obtain GFP fusion constructs of StSXD1 for transient expression in leaf tissue, the complete coding region of StSXD1 (nts 53–1,555), as well as the predicted TP (StSXD1TP) including the 5′UTR (nts 1–334) were PCR amplified from the full-length cDNA clone using oligonucleotides D292 (5′-GGGATCCCCATGGAGAGCTTTTATAGTGTTTCCGC-3′) and D296 (5′-GTCGACAAGGCCAGGAGGTTTGAGAAGTGAAG-3′) for StSXD1, and D311 (5′-GGATCCAGTTGCCGCTCCTCAAAAACTCTA-3′) and D312 (5′-GTCGACCCCGCTATGAGGAGTTCGAAGAGG-3′) for StSXD1TP, respectively. After subcloning into pCR-blunt, the BamHI/SalI-digested PCR fragments were inserted into the BamHI/SalI sites of a pFF19-based vector (Timmermans et al., 1990) containing mGFP5 instead of the GUS gene (pFF19-GFP kindly provided by A. Wachter, Heidelberg) to yield plasmids pFF19-StSXD1:GFP or pFF19-StSXD1TP:GFP. Plasmid DNA was coated onto 1-μm gold particles, and the abaxial epidermis of tobacco (Nicotiana tabacum) source leaves was bombarded using a Helios Gene Gun following the manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA). Images were obtained with a confocal microscope LSM 510 META (Zeiss, Göttingen, Germany). Excitation light of 488 nm produced by krypton/argon laser and emission filters of 510 to 525 and 645 to 700 nm allowed detection of GFP or chlorophyll-derived red fluorescence, respectively, and images were superimposed by means of the Zeiss LSM Version 3.0 software. Transient expression of the empty pFF19-GFP plasmid served as a cytosolic control.
RNAi Plasmid Construction and Potato Transformation
The aforementioned 765-bp PCR fragment of the StSXD1 gene comprising nts 503 to 1,268 and flanked by BamHI/SalI restriction sites (underlined in primers D184 and D185, respectively) was inserted in sense orientation downstream of the GA20 oxidase intron in the pUC-RNAi vector as described by Chen et al. (2003). The same fragment was subsequently placed in antisense orientation into the XhoI/BglII sites of pUC-RNAi already carrying the StSXD1 sense fragment. Finally, the entire RNAi cassette comprising sense and antisense fragments of StSXD1 interspersed by the GA20 oxidase intron was excised from pUC-RNAi using the flanking PstI sites and inserted into the SbfI site of pBinAR (Höfgen and Willmitzer, 1990) between the cauliflower mosaic virus 35S promoter and ocs terminator yielding the construct pBin-StSXD1-RNAi.
Transformation of potato plants by Agrobacterium-mediated gene transfer using Agrobacterium tumefaciens strain C58C1:pGV2260 was carried out as described previously (Rocha-Sosa et al., 1989).
RNA Analysis
Extraction of total RNA from leaf material and northern-blot analysis was performed as described by Chen et al. (2003). Thirty micrograms of RNA per sample were separated on a 1.5% (w/v) formaldehyde-agarose gel, transferred to a nitrocellulose membrane (GeneScreen, NEN Life Science Products, Boston), and hybridized with random-primed [α-32P]-labeled cDNA fragments. Photosynthetic gene-specific (rbcS, cab) cDNAs used for hybridization were described previously (Herbers et al., 1996a, 1996b). Pin2 (accession no. AY129402) and AOC (accession no. AJ272026) cDNAs from tomato (Lycopersicon esculentum) were kindly provided by C. Wasternack (Halle/Saale, Germany). A partial tobacco EST clone showing 97% identity to the tomato P5CS gene (accession no. U60267) was additionally used as cDNA probe. Gel blots were hybridized with 18S rRNA for loading control.
TC Enzyme Assay and Quantification of Tocopherol
TC activity was determined in protein extracts of E. coli cells and leaves using DMPQ and DMGQ as substrates following the method as described by Porfirova et al. (2002). Tocopherol contents were determined after methanol extraction by fluorescence HPLC as described before (Porfirova et al., 2002). The immediate tocopherol precursor DMPQ was determined in the same leaf extracts as used for tocopherol analysis and quantified based on peak area (mV min−1 g−1 fresh weight) using an authentic standard.
Carbohydrate and Amino Acid Determination
Soluble sugars and starch levels were determined in leaf samples extracted with 80% (v/v) ethanol/20 mm HEPES-KOH, pH 7.5 as described (Sonnewald, 1992). Starch accumulation was visualized by iodine staining (0.3% [w/v] I2, 0.7% [w/v] KI) after an extended dark period (16 h) and removal of chlorophyll by 80% (v/v) ethanol at 55°C.
Total amino acids, Pro, Gln, and Glu were analyzed in 25-mg leaf samples extracted with 80% (v/v) ethanol, 20 mm HEPES-KOH, pH 7.5. Extracts were evaporated to dryness, redissolved in purest H2O, and separated on a reversed-phase HPLC system (Waters Associates, Milford, MA) after derivatization using the AccQ-Tag method as described (Rolletschek et al., 2002). Amino acids were identified by cochromatography with authentic standards and quantified by comparison with internal standards.
Callose Determination and Immunofluorescence Analysis
Extraction and measurement of callose from leaf material was done as described (Köhle et al., 1985). Callose quantification was based on comparison with the fluorescence of known amounts of the commercial β-1,3-glucan pachyman (ICN Biomedicals, Irvine, CA), and measurements were performed using a SpectraMax Gemini XS spectrofluorometer (Molecular Devices, Ismaning, Germany). Callose contents were expressed as β-1,3-glucan pachyman equivalents.
For immunofluorescence detection of callose, potato leaf samples were fixated, substituted, and embedded as described previously in Börnke et al. (2002). Semithin sections with a thickness of approximately 1 μm were mounted on slides and immunolabeling was performed by using antimouse monoclonal (1,3)-β-d-glucan antibody (Biosupplies, Parkville, Australia) as primary antibody and Alexa Fluor 488 goat antimouse IgG (Molecular Probes, Eugene, OR) as fluorescence marker. Images were taken with the confocal microscope Zeiss LSM 510 META and fluorescence signals were detected after excitation with a 488-nm krypton/argon laser using an emission filter of 505 to 530 nm.
CO2 Gas-Exchange Measurements
CO2 uptake rates were measured with a portable photosynthesis system LI-6400 (LI-COR, Lincoln, NE). CO2 concentration of the air entering the leaf chamber was adjusted to 400 μm m−1 and leaf temperature was maintained at 20°C. For determination of light response curves of CO2 gas exchange, photosynthetic photon flux density was varied between 0 and 2,000 μm photons m−2 s−1.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AY536918.
Acknowledgments
We especially thank Anita Winger and Ulrike Schlereth for excellent technical assistance, Bernhard Claus for skillful help with confocal laser scanning microscopy, and Andrea Knospe for plant transformation and tissue culture work. We are also grateful to Prof. Dr. Claus Wasternack (Martin-Luther-University, Halle/Saale, Germany) for providing the Pin2 and AOC cDNA fragments, and to Dr. Andreas Wachter (Heidelberg) for the pFF19-GFP vector.
This work was funded by a grant from the Deutsche Forschungsgemeinschaft (DFG; SO 300/6–1).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043927.
References
- Abraham E, Rigo G, Szekely G, Nagy R, Koncz C, Szabados L (2003) Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol 51: 363–372 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Arango Y, Heise KP (1998) Tocopherol synthesis from homogentisate in Capsicum anuum L. (yellow pepper) chromoplast membranes: evidence for tocopherol cyclase. Biochem J 336: 531–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A, Ricciarelli R, Zingg JM (2002) Non-antioxidant molecular functions of α-tocopherol (vitamin E). FEBS Lett 519: 8–10 [DOI] [PubMed] [Google Scholar]
- Bergmüller E, Porfirova S, Dörmann P (2003) Characterization of an Arabidopsis mutant deficient in γ-tocopherol methyltransferase. Plant Mol Biol 52: 1181–1190 [DOI] [PubMed] [Google Scholar]
- Börnke F, Hajirezaei M, Heineke D, Melzer M, Herbers K, Sonnewald U (2002) High-level production of the non-cariogenic sucrose isomer palatinose in transgenic tobacco plants strongly impairs development. Planta 214: 356–364 [DOI] [PubMed] [Google Scholar]
- Botha CEJ, Cross RHM, van Bel AJE, Peter CI (2000) Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath-vascular parenchyma interface. Protoplasma 214: 65–72 [Google Scholar]
- Brigelius-Flohe R, Traber MG (1999) Vitamin E: function and metabolism. FASEB J 13: 1145–1155 [PubMed] [Google Scholar]
- Bussis D, Heineke D, Sonnewald U, Willmitzer L, Raschke K, Heldt HW (1997) Solute accumulation and decreased photosynthesis in leaves of potato plants expressing yeast-derived invertase either in the apoplast, vacuole or cytosol. Planta 202: 126–136 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263: 802–805 [DOI] [PubMed] [Google Scholar]
- Chen S, Hofius D, Sonnewald U, Börnke F (2003) Temporal and spatial control of gene silencing in transgenic plants by inducible expression of double-stranded RNA. Plant J 36: 731–740 [DOI] [PubMed] [Google Scholar]
- Cheng Z, Sattler S, Maeda H, Sakuragi Y, Bryant DA, DellaPenna D (2003) Highly divergent methyltransferases catalyze a conserved reaction in tocopherol and plastoquinone synthesis in cyanobacteria and photosynthetic eukaryotes. Plant Cell 15: 2343–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2001) Isolation and functional analysis of homogentisate phytyltransferase from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 127: 1113–1124 [PMC free article] [PubMed] [Google Scholar]
- Collakova E, DellaPenna D (2003) Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol 131: 632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkerli K, Hahn MG, Mims CW (1997) Immunogold localisation of callose and other plant cell wall components in soybean roots infected with the oomycete Phytophthora sojae. Can J Bot 75: 1509–1517 [Google Scholar]
- Fryer MV (1992) The antioxidant effects of thylakoid vitamin E (α-tocopherol). Plant Cell Environ 15: 381–392 [Google Scholar]
- Grasses T, Grimm B, Koroleva O, Jahns P (2001) Loss of alpha-tocopherol in tobacco plants with decreased geranylgeranyl reductase activity does not modify photosynthesis in optimal growth conditions but increases sensitivity to high-light stress. Planta 213: 620–628 [DOI] [PubMed] [Google Scholar]
- Hare PD, Cress WA, van Staden J (1999) Proline synthesis and degradation: a model system for elucidating stress-related signal transduction. J Exp Bot 50: 413–434 [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51: 463–499 [DOI] [PubMed] [Google Scholar]
- Haywood V, Kragler F, Lucas WJ (2002) Plasmodesmata: pathways for protein and ribonucleoprotein signalling. Plant Cell 14 (Suppl): S303–S325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann H, Funck D, Rentsch D, Frommer WB (2000) Hypersensitivity of an Arabidopsis sugar signaling mutant toward exogenous proline application. Plant Physiol 123: 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Frommer WB, Métraux JP, Sonnewald U (1996. a) Systemic acquired resistance mediated by the ectopic expression of invertase: possible hexose sensing in the secretory pathway. Plant Cell 8: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers K, Meuwly P, Metraux JP, Sonnewald U (1996. b) Salicylic acid-independent induction of pathogenesis-related protein transcripts by sugars is dependent on leaf developmental stage. FEBS Lett 397: 239–244 [DOI] [PubMed] [Google Scholar]
- Herbers K, Tacke E, Hajirezaei M, Krause KP, Melzer M, Rohde W, Sonnewald U (1997) Expression of a luteoviral movement protein in transgenic plants leads to carbohydrate accumulation and reduced photosynthetic capacity in source leaves. Plant J 12: 1045–1056 [DOI] [PubMed] [Google Scholar]
- Herbers K, Sonnewald U (1998) Molecular determinants of sink strength. Curr Opin Plant Biol 1: 207–216 [DOI] [PubMed] [Google Scholar]
- Höfgen R, Willmitzer L (1990) Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum L.). Plant Sci 66: 221–230 [Google Scholar]
- Iglesias VA, Meins F Jr (2000) Movement of plant viruses is delayed in a beta-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J 21: 157–166 [DOI] [PubMed] [Google Scholar]
- Jacobs AK, Lipka V, Burton RA, Panstruga R, Strizhov N, Schulze-Lefert P, Fincher GB (2003) An Arabidopsis callose synthase, GSL5, is required for wound and papillary callose formation. Plant Cell 15: 2503–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Ryan CA (1990) Wound-inducible potato inhibitor II genes: enhancement of expression by sucrose. Plant Mol Biol 14: 527–536 [DOI] [PubMed] [Google Scholar]
- Jones DL, Gilroy S, Larsen PB, Howell SH, Kochian LV (1998) Effect of aluminum on cytoplasmic Ca2+ homeostasis in root hairs of Arabidopsis thaliana (L.). Planta 206: 378–387 [DOI] [PubMed] [Google Scholar]
- Köhle H, Jeblick W, Poten F, Blaschek W, Kauss H (1985) Chitosan-elicited callose synthesis in soybean cells as a Ca2+-dependent process. Plant Physiol 77: 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn C, Quick WP, Schulz A, Riesmeier JW, Sonnewald U, Frommer WB (1996) Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ 19: 1115–1123 [Google Scholar]
- Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the sink-regulation of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195: 313–323 [Google Scholar]
- McKersie BD, Hoekstra FA, Krieg LC (1990) Differences in the susceptibility of plant membrane lipids to peroxidation. Biochim Biophys Acta 1030: 119–126 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21: 31–57 [Google Scholar]
- Munné-Bosch S, Falk J (2004) New insights into the function of tocopherols in plants. Planta 218: 323–326 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Peñuelas J (2003) Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 217: 758–766 [DOI] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC (2003) Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 301: 969–972 [DOI] [PubMed] [Google Scholar]
- Østergaard L, Petersen M, Mattsson O, Mundy J (2002) An Arabidopsis callose synthase. Plant Mol Biol 49: 559–566 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54: 539–547 [DOI] [PubMed] [Google Scholar]
- Porfirova S, Bergmüller E, Tropf S, Lemke R, Dörmann P (2002) Isolation of an Arabidopsis mutant lacking vitamin E and identification of a cyclase essential for all tocopherol biosynthesis. Proc Natl Acad Sci USA 99: 12495–12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher LM, Miao L, Sinha N, Lucas WJ (2001) Sucrose export defective1 encodes a novel protein implicated in chloroplast-to-nucleus signalling. Plant Cell 13: 1127–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbach G, Minihane AM, Majewicz J, Fischer A, Pallauf J, Virgli F, Weinberg PD (2002) Regulation of cell signalling by vitamin E. Proc Nutr Soc 61: 415–425 [DOI] [PubMed] [Google Scholar]
- Roberts AG, Oparka KJ (2003) Plasmodesmata and the control of symplastic transport. Plant Cell Environ 26: 103–124 [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L (1989) Both developmental and metabolic signals activate the promoter of a class I patatin gene. EMBO J 8: 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14 (Suppl): S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolletschek H, Hajirezaei MR, Wobus U, Weber H (2002) Antisense-inhibition of ADP-glucose pyrophosphorylase in Vicia narbonensis seeds increases soluble sugars and leads to higher water and nitrogen uptake. Planta 214: 954–964 [DOI] [PubMed] [Google Scholar]
- Russin WA, Evert RE, Vanderveer PJ, Sharkey TD, Briggs SP (1996) Modification of a specific class of plasmodesmata and loss of sucrose export ability in a sucrose export defective1 maize mutant. Plant Cell 8: 645–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler SE, Cahoon EB, Coughlan SJ, DellaPenna D (2003) Characterization of tocopherol cyclases from higher plants and cyanobacteria. Evolutionary implications for tocopherol synthesis and function. Plant Physiol 132: 2184–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge B, Weiss JD, Wong YH, Lassner MW, Mitsky TA, Shewmaker CK, Post-Beittenmiller D, Valentin HE (2002) Isolation and characterization of homogentisate phytyltransferase genes from Synechocystis sp. PCC 6803 and Arabidopsis. Plant Physiol 129: 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F (2001) Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J Exp Bot 52: 11–23 [PubMed] [Google Scholar]
- Shintani D, DellaPenna D (1998) Elevating the vitamin E content of plants through metabolic engineering. Science 282: 2098–2100 [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Fujiwara T, Samaj J, Baluška F, Yang Z, Osawa H, Maeda T, Mori T, Volkmann D, Matsumoto H (2000) Aluminum-induced 1→3-β-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol 124: 991–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J, Schultz G, Joyard J, Douce R, Block MA (1985) Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts. Arch Biochem Biophys 238: 290–299 [DOI] [PubMed] [Google Scholar]
- Sonnewald U (1992) Expression of E. coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning. Plant J 2: 571–581 [PubMed] [Google Scholar]
- Stitt M, Sonnewald U (1995) Regulation of metabolism in transgenic plants. Annu Rev Plant Physiol Plant Mol Biol 46: 341–368 [Google Scholar]
- Timmermans MC, Maliga P, Vieira J, Messing J (1990) The pFF plasmids: cassettes utilising CaMV sequences for expression of foreign genes in plants. J Biotechnol 14: 333–344 [DOI] [PubMed] [Google Scholar]
- Van Eenennaam AL, Lincoln K, Durrett TP, Valentin HE, Shewmaker CK, Thorne GM, Jiang J, Baszis SR, Levering CK, Aasen ED, et al (2003) Engineering vitamin E content: from Arabidopsis mutant to soy oil. Plant Cell 15: 3007–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Parthier B (1997) Jasmonate-signalled plant gene expression. Trends Plant Sci 2: 302–308 [Google Scholar]
- Wefers H, Sies H (1988) The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem 174: 353–357 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125: 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]