Abstract
Disruption of the FATB gene in Arabidopsis results in a two-thirds reduction in saturated fatty acids, largely palmitate, in the leaf extra-plastidic phospholipids and a reduction in the growth rate of the mutant compared to wild type (Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB [2003] Plant Cell 15: 1020–1033). In this study, we report that although fatb-ko seedlings grow more slowly than wild type, the rate of fatty acid synthesis in leaves of the mutant increases by 40%. This results in approximately the same amount of palmitate exported from the plastid as in wild type but an increase in oleate export of about 55%. To maintain constant amounts of fatty acids in leaves, thereby counterbalancing their higher rate of production, the mutant also increases its rate of fatty acid degradation. Although fatb-ko leaves have higher rates of fatty acid synthesis and turnover, the relative proportions of membrane lipids are similar to wild type. Thus, homeostatic mechanisms to preserve membrane compositions compensate for substantial changes in rates of fatty acid and glycerolipid metabolism in the mutant. Pulse-chase labeling studies show that in fatb-ko leaves there is a net increase in the synthesis of both prokaryotic and eukaryotic lipids and consequently of their turnover. The net loss of palmitate from phosphatidylcholine plus phosphatidylethanolamine is similar for wild type and mutant, suggesting that mechanisms are not present that can preferentially preserve the saturated fatty acids. In summary, the leaf cell responds to the loss of saturated fatty acid production in the fatb-ko mutant by increasing both fatty acid synthesis and degradation, but in doing so the mechanisms for increased fatty acid turnover contribute to the lowering of the percentage of saturated fatty acids found in eukaryotic lipids.
In plants, the major site for de novo fatty acid synthesis (FAS) occurs in the plastid (Ohlrogge et al., 1979). Fatty acids are either utilized in this organelle or exported to supply diverse cytoplasmic biosynthetic pathways and cellular processes. Production of fatty acids for export depends on the activity of acyl-ACP thioesterases (FATs) that hydrolyze acyl-acyl carrier protein (acyl-ACP) to release free fatty acids and ACP (for review, see Voelker et al., 1997). After export, the free fatty acids are re-esterified to CoA to form the cytosolic acyl-CoA pool (Pollard and Ohlrogge, 1999). In mesophyll cells, acyl-CoAs are primarily used for the biosynthesis of membrane glycerolipids in the endoplasmic reticulum (Browse and Somerville, 1991). However, in other cell types exported fatty acids have different fates. For example, in embryo cells of oilseeds, the major fraction is incorporated into triacylglycerols, while in epidermal cells a large fraction is utilized for the synthesis of waxes and cutin (Post-Beittenmiller, 1996; Kolattukudy, 2003). Furthermore, all cells synthesize sphingolipids (Lynch, 1993), and we recently estimated that as much as 30% to 40% of exported palmitate is needed for sphingoid base synthesis in leaves (Bonaventure et al., 2003). Finally, exported 14:0 and 16:0 participate in other acylation reactions, such as protein acylation (for review, see Yalovsky et al., 1999).
Because FATs terminate FAS and allow for the export of fatty acids from plastids, these enzymes may be important determinants of cellular metabolism. Two classes of FAT enzymes have been described in most plants, namely FATA and FATB (Voelker et al., 1997). The FATA class has highest in vitro activity for 18:1-ACP with much lower activity for saturated acyl-ACP substrates. By contrast, the FATB class prefers saturated acyl-ACP substrates but also shows activity for oleoyl-ACPs (Doermann et al., 1995; Voelker et al., 1997; Salas and Ohlrogge, 2002). The Arabidopsis genome encodes two FATA genes and a single FATB gene (Mekhedov et al., 2000; Beisson et al., 2003). We previously described the isolation and analysis of an Arabidopsis mutant disrupted in the FATB gene (fatb-ko; Bonaventure et al., 2003). In this mutant, the total amount of saturated fatty acids in various tissues was reduced by 40% to 50% compared to wild type. This reduction occurred only in the cytosolic pool of saturated fatty acids, affecting the fatty acid composition of extraplastidial phospholipids, waxes in leaves and stems, and triacylglycerols in seeds. However, although sphingolipid synthesis is initiated from 16:0-CoA, no reductions were observed in total sphingoid base content, suggesting that plants may prioritize the synthesis of these essential lipids (Wells and Lester, 1983). Disruption of the FATB gene slowed growth of the mutant, resulting in seedlings approximately half the size of wild-type seedlings by week 4 (Bonaventure et al., 2003). Based on these results, it was concluded that the reduction in the export of saturated fatty acids from plastids affects cellular processes that are critical for plant growth.
Complete suppression or disruption of any of the enzymes of FAS would be expected to reduce FAS and affect plant performance. In support of this, analysis of tobacco (Nicotiana tabacum) plants engineered to constitutively express an antisense transcript of the tobacco biotin carboxylase showed reductions of leaf fatty acid content together with a stunted phenotype (Shintani et al., 1997). Growth phenotypes together with reduction in leaf lipids were also obtained with antisense constructs directed against stearoyl-ACP desaturase and ACP4 and with induced mutations in the fab2 and enoyl-ACP reductase genes (Lightner et al., 1994; Mou et al., 2000; Branen et al., 2003). It is apparent from these studies that disruptions in leaf FAS can have pleiotropic effects on plant growth and development. Some of these effects come from changes in chloroplast membrane structure and, therefore, loss of photosynthetic capability. By contrast, a wide range of mutations in plant fatty acid desaturases, acyltransferases, and condensing enzymes demonstrate that the fatty acid composition of plant membranes can be altered considerably with no apparent phenotype under normal growth conditions (Kunst et al., 1989; Wu et al., 1994; Wallis and Browse, 2002).
Based on these observations, one possible mechanism responsible for the slower growth of fatb-ko plants could be a reduced synthesis of fatty acids and therefore a decline in the rate of membrane glycerolipid biosynthesis. Alternatively, slower growth of the mutant may be the result of reduced synthesis of other critical components such as sphingolipids, cutin, and waxes, or lower rates of acylation reactions. In addition, lipid-derived signaling molecules that affect growth could be affected in the mutant (Nandi et al., 2003). When fatb-ko plants were grown at a range of different temperatures, no suppression of the fatb-ko growth phenotype was observed, suggesting that changes in the bulk properties of cellular membranes were not the main factor affecting plant performance (Bonaventure et al., 2003). Likewise, when the mutant was grown at different relative humidity or in liquid culture, no reversion of the growth phenotype occurred, suggesting that increased water loss through reduced wax content was not a major factor affecting growth. Similar results were obtained when fatb-ko was supplemented with exogenous sugar, and in this case its photosynthetic capability appeared not to limit its growth (Bonaventure et al., 2003). The latter conclusion is substantiated by similar chlorophyll content and chloroplast ultrastructure for wild type and fatb-ko plants (this study; G. Bonaventure and J. Ohlrogge, unpublished data).
To further elucidate the role of saturated fatty acids in plant growth and to understand fatty acid and lipid metabolism in fatb-ko, a series of isotope labeling experiments were conducted. Unexpectedly, the rates of both FAS and lipid turnover were higher in fatb-ko than wild-type leaves. Thus, fatb-ko plants appear to induce a futile cycle of fatty acid production and degradation, perhaps as an attempt to increase saturated FAS.
RESULTS
Rate of Fatty Acid Synthesis in Leaves of Wild-Type Arabidopsis and fatb-ko
The rate of FAS in leaves correlates with the expansion rate of this organ, being higher in younger leaves and reflecting the demand for new membranes to sustain cell division and chloroplast biogenesis (Browse et al., 1981; Bao et al., 2000). It was previously reported that fatb-ko plants showed a 17% decline per week in fresh weight growth rate compared to wild type (Bonaventure et al., 2003). To evaluate if the disruption of the FATB gene alters the rate of FAS in leaves, incorporation of labeled precursors was used to assess the in vivo rate of FAS in Arabidopsis wild type and fatb-ko. Because isotope incorporation rates can be influenced by internal pools of metabolites (e.g. acetate) that might differ between mutant and wild type (Cronan et al., 1975; Nunn et al., 1977), three different labeled substrates were tested, and the results are shown in Table I.
Table I.
Initial rates of FAS in leaves of Arabidopsis wild type and fatb-ko measured by different radiolabeled substrates
| Substrate | Wild Typea | fatb-koa | Increaseb |
|---|---|---|---|
| 3H2O | 2,800 ± 130 | 3,880 ± 140 | 39% |
| 14CO2 | 42 ± 2 | 64 ± 3 | 52% |
| 14C-Acetate | 78 ± 2 | 102 ± 2 | 31% |
Units are in nmol of 3H or 14C h−1 mg−1 chlorophyll incorporated into fatty acids.
Percent increase in the rate of FAS of fatb-ko versus wild type.
The rate of 3H incorporation into fatty acids from 3H2O by leaf strips from fatb-ko plants was 39% higher than for wild type. In this assay system after a lag phase of 10 to 15 min, the incorporation of 3H was linear for 2 h (Browse et al., 1981). An advantage of using 3H2O as the labeled substrate is that water rapidly equilibrates with all tissue water pools and, therefore, total FAS activity can be measured. Because deuterium or tritium water labeling of fatty acids by illuminated leaf tissues shows no kinetic isotope effect and can give complete substitution of hydrogen atoms, the measured rate of FAS in wild type of 2.8 μmol H-atom h−1 mg−1 chlorophyll is a total rate and converts to 1.6 μmol C-atom h−1 mg−1 chlorophyll for the leaf strips. The rate previously measured for Arabidopsis aboveground tissues in intact seedlings was 2.3 μmol C-atom h−1 mg−1 chlorophyll (Bao et al., 2000). Using [14C]acetate labeling of leaf strips at tracer concentrations of acetate (0.09 mm; Pollard and Ohlrogge, 1999), fatb-ko mutant leaves incorporated 14C into fatty acids at a rate 31% higher than wild type. Finally, intact wild-type and mutant seedlings were labeled with tracer concentrations of 14CO2. As shown in Table I, fatb-ko leaves incorporated 14C from CO2 into fatty acids at a 52% higher rate than wild type. In all the experiments, the chlorophyll content per gram fresh weight (gfw) in leaves of the two plant classes was not significantly different (1.05 ± 0.01 mg chlorophyll/gfw for wild type and 1.07 ± 0.03 mg chlorophyll/gfw for fatb-ko). In summary, by using three different radiolabeled substrates with intact seedlings or in leaf strip assays, it was demonstrated that fatb-ko leaves synthesized fatty acids at an average rate 40% higher than wild type.
Rate of Fatty Acid Degradation in Wild-Type and fatb-ko Leaves
We previously reported that leaves from fatb-ko plants contained the same amount of fatty acids per gram of fresh weight as wild type (Bonaventure et al., 2003). Therefore, if the FAS rate in fatb-ko leaves is 40% higher than wild type, the rate of fatty acid degradation must increase concomitantly to maintain fatty acid concentration constant in this tissue.
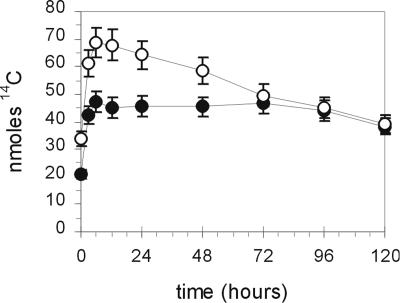
To test this conclusion, fatty acid turnover was evaluated by labeling intact seedlings with a 30-min pulse of 14CO2 and then determining the concentration of 14C in leaf fatty acids at different times up to 120 h (Fig. 1). In this experiment, at the end of the 30-min labeling period, the specific activity of labeled fatty acids was 62% higher in the mutant. During the first 6 h of the chase period, there was continued net accumulation of 14C into fatty acids of both wild type and mutant (Fig. 1). This net accumulation of label may reflect the use of newly labeled 14C carbohydrates as substrates for FAS (Bao et al., 2000). After 6 h of the chase the amount of total label in fatty acids in fatb-ko leaves began to decline, such that by 120 h the radioactivity in fatty acids dropped by 44%. By comparison, the decay in labeled fatty acids in wild type over the entire period was small, and at the most was 10% to 15%. In the first 3 d, wild-type and mutant leaves had an average disappearance rate of 0% and 10% per day, respectively. By day 4, the amounts of wild-type and mutant 14C fatty acids were similar.
Figure 1.
Fatty acid turnover in wild-type and fatb-ko leaves. Intact plants were pulsed for 30 min with 2 mCi of 14CO2 and the label chased for a period of 5 d in an open atmosphere. At the times indicated leaf samples were harvested, the fatty acids purified, and their specific radioactivity measured. Product amount (nmol) was calculated by correcting for the dilution in specific activity caused by growth over the labeling period. Black circles, wild type; white circles, fatb-ko. Bars denote ses.
Analysis of Labeled Polar Lipid Classes in Wild-Type and fatb-ko Leaves
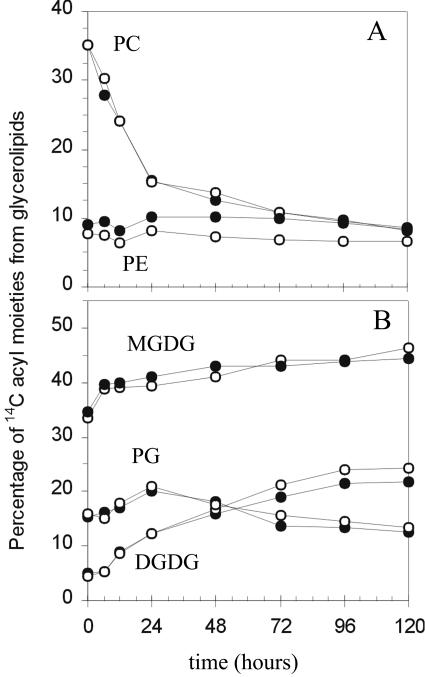
To investigate whether higher rates of fatty acid turnover in fatb-ko leaves were specific for particular membrane glycerolipids, leaf glycerolipid classes were analyzed after pulsing wild-type and fatb-ko seedlings with 14CO2. Because labeled carbon dioxide incorporates not only into the acyl chains of glycerolipids but also into the glycerol and head group moieties (Roughan, 1970), the 14C recovered in the acyl moieties of each 14C glycerolipid class was determined and subtracted from the total 14C in that glycerolipid class. The results shown in Figure 2 indicate that the distribution of radiolabeled acyl moieties of plastidial and extraplastidial glycerolipids was similar between wild-type and fatb-ko leaves.
Figure 2.
Redistribution of 14C acyl moieties in glycerolipids after 14CO2 labeling of wild-type and fatb-ko leaves. A, Extraplastidial glycerolipids. B, Plastidial glycerolipids. Seedlings were pulsed with 14CO2 and radiolabeled glycerolipids separated by TLC. 14C glycerolipids were quantified before and after saponification to determine 14C in the total molecule and acyl moieties, respectively. Black circles, wild type; white circles, fatb-ko. Values represent the average of replicates from two samples. ses are not shown and correspond to less than 5% of the average in all cases.
The fraction of label in phosphatidylcholine (PC) from wild type and fatb-ko declined by approximately 75% over the 120-h pulse-chase period, and most dramatically over the first 24 h (Fig. 2A). This high rate of loss of labeled PC is explained in large part by the donation of diacylglycerol moieties from PC to galactolipids and sulfolipids in chloroplasts (Roughan, 1970). Phosphatidylethanolamine (PE) was the only glycerolipid analyzed with consistently lower label in fatb-ko than wild-type leaves throughout the chase period (Fig. 2A). In wild-type tissue, PE accounted for approximately 9% to 10% of labeled lipids throughout the 5-d period, whereas in the mutant PE represented only 6% to 8%. This observation agreed with the 20% lower steady state amounts of PE observed in fatb-ko leaves compared to wild type (Bonaventure et al., 2003). Lower incorporation of radioactivity in PE of the mutant was also observed at early time points during continuous labeling of leaves with [14C]acetate (data not shown). Although there is a relative decline in label in PE, when the increased rate of FAS is taken into account there is either no change or a slight increase in the absolute amount of PE labeling in the mutant (see below). The relative label associated with phosphatidylserine plus phosphatidylinositol (PS + PI) was similar for wild type and fatb-ko during the entire 0 to 120-h period and fluctuated around 3%. Because 14C PS + PI comprised a small fraction of total label in polar glycerolipids, it was not further evaluated. Likewise, diacylglycerol and triacylglycerol represented less than 2% of 14C glycerolipids, and they were excluded from the analysis.
In the case of plastidial lipids, the percentage of label in monogalactosyl-diacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) increased by 28% and more than 400% respectively in wild type and fatb-ko during the chase period (Fig. 2B). These increments in labeled MGDG and DGDG at longer times represented the redistribution of acyl groups from cytosolic phospholipids to plastid galactolipids (Browse et al., 1989). These data, together with the decline of labeled PC, are consistent with the parallel operation of the prokaryotic and eukaryotic pathways of lipid biosynthesis in wild-type and fatb-ko leaves. The kinetics of 14C labeling in the different lipid classes of wild-type Arabidopsis leaves were in accordance with previous studies in which the same tissue was labeled with 14C-acetate (Browse et al., 1989; Kunst et al., 1989; Wu et al., 1994). Furthermore, the distribution of radioactivity between the lipid classes at the end of the 5-d chase period is in good agreement with the molar distribution of endogenous membrane lipids we reported previously (Bonaventure et al., 2003). In conclusion, with the exception of PE, the similarity in the distribution of label among lipid classes in wild-type and fatb-ko leaves indicated that despite higher rates of FAS and degradation, cells maintained a constant composition of membrane lipids.
Analysis of Radiolabeled C16 and C18 Fatty Acids in Membrane Lipids of Wild-Type and fatb-ko Leaves
The fatty acid composition of leaf membrane glycerolipids differs between wild type and fatb-ko. In this regard PE, PS, and PI in the mutant have reductions of approximately 50% in their 16:0 content compared to wild type, whereas in PC the reduction is 80% (Bonaventure et al., 2003). By contrast, the fatty acid composition of the plastid lipids is barely altered.
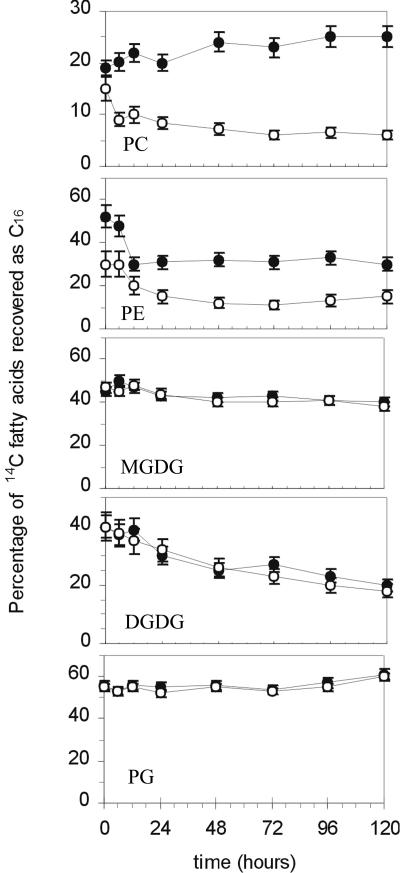
To understand the metabolism leading to these changes in composition, lipids were isolated from wild-type and mutant seedlings pulsed with 14CO2 and chased for different times up to 5 d. Isolated lipid classes were transmethylated and the fatty acid methyl esters (FAMEs) hydrogenated. The resulting labeled 16:0 and 18:0 were separated by reverse phase thin-layer chromatography (TLC) and the label quantified. The results of this analysis are shown in Figure 3 and reveal that, in the mutant, the distribution of labeled C18 and C16 fatty acids was similar to wild type in plastidial lipids but differed substantially in extraplastidial lipids.
Figure 3.
Redistribution of 14C fatty acids in membrane glycerolipids from 14CO2 labeling of wild-type and fatb-ko leaves. Seedlings were pulsed with 14CO2, lipid classes separated by TLC, and their corresponding fatty acids transmethylated, hydrogenated, and separated by reverse-phase TLC for subsequent quantification. Only the percentage of C16 fatty acids is shown. C18 fatty acids can be calculated as (100%–%C16 fatty acids). Black circles, wild type; white circles, fatb-ko. Bars denote ses.
After the labeling period of 30 min, 16:0 in PC is not greatly different, being 19% in wild type and 15% in the mutant (Fig. 3). However, during the chase period, 16:0 increased to 25% in the wild-type PC but decreased to 6% in the mutant. Thus, it is the differential removal of palmitate from PC that is the primary cause of the reduced palmitate level in PC in the fatb-ko mutant. Moreover, much of this change in PC in the mutant occurs during the first 6 h of the chase period, even as the total label in PC increases 1.75-fold (Fig. 4). At the end of the pulse-chase period, the amount of labeled 16:0 in PC was similar to the mass composition of PC in wild-type and fatb-ko leaves (21% and 4.5%, respectively; Bonaventure et al., 2003).
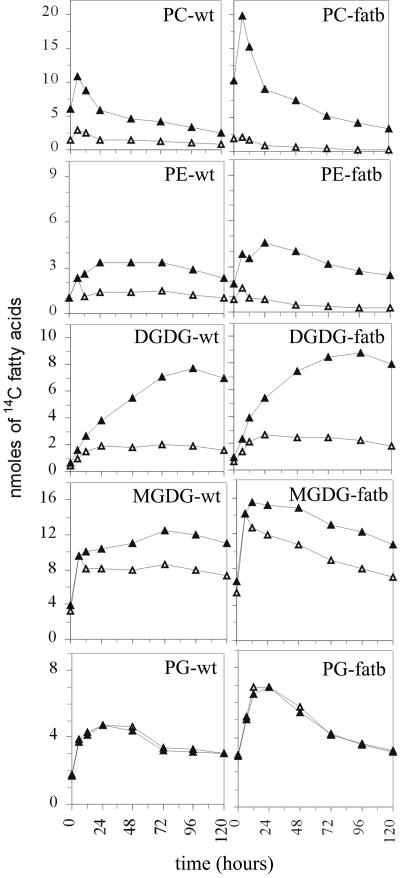
Figure 4.
Redistribution of 14C C18 and C16 fatty acids of polar lipids from 14CO2 labeled wild-type (wt) and fatb-ko (fatb) leaves. Absolute masses of 14C C18 and C16 fatty acids (FA) were calculated using the data from Figures 1 to 3. Black triangles, C18 FA; white triangles, C16 FA.
Initial labeling of PE in the wild type yields 52% labeled 16:0, whereas in the mutant there is only 30% labeled 16:0. During the chase period, both wild-type and fatb-ko leaves show decreases in 16:0 levels in PE (Fig. 3). Thus, in contrast to PC, the steady state difference in the C16 content of PE between wild type and fatb-ko appeared to be primarily the result of lower initial incorporation instead of increased removal of this fatty acid. At the end of the pulse-chase period, the amount of labeled 16:0 in PE was 30% in wild type and 14% in the mutant, values which are similar to the mass composition of PE in leaves (29% and 11.5%, respectively; Bonaventure et al., 2003).
An important observation from Figure 3, when combined with data in Figures 1 and 2, is an estimation of the amount of labeled 16:0 in eukaryotic lipids (PC + PE) after the initial labeling period of 30 min. Labeled 16:0 found in PC + PE, expressed as a percentage of total labeled fatty acids, fell from 11.4% in wild type to 7.6% in the mutant, a reduction of 33%. However, because the overall rate of FAS increased, the total amount of labeled 16:0 in these phospholipids actually increased slightly, from 2.4 nmol to 2.6 nmol. Thus, despite the inactivation of the FATB encoded FAT, the chloroplast actually maintains its flux of 16:0 for export.
In contrast to extraplastidial glycerolipids, the incorporation and redistribution of label in C18 and C16 fatty acids of chloroplast glycerolipids was similar for wild type and fatb-ko (Fig. 3). These observations agree with the absence of significant changes in the fatty acid composition of MGDG, DGDG, and phosphatidylglycerol (PG) found between wild type and the mutant (Bonaventure et al., 2003). In MGDG there was a small decline in C16 fatty acid levels over the chase period as the initially labeled prokaryotic C18C16 species are diluted with eukaryotic C18C18 species. The more pronounced decline of labeled C16 fatty acids in DGDG reflected a small prokaryotic pool initially that was extensively diluted by the synthesis of eukaryotic C18C18 species from PC (Browse et al., 1986). PG synthesis is primarily by the prokaryotic pathway (Browse and Somerville, 1991), and the high level of labeled C16 fatty acids incorporated into this phospholipid initially (55%) reflect the occurrence of 16:0 and 16:1(3-trans) in this lipid at the sn-2 position.
Fluxes of C18C18 and C16C18 Molecular Species of Membrane Lipids in Wild-Type and fatb-ko Leaves
As the labeling method uses carbon dioxide and intact plants, the tracer will accurately mirror endogenous fluxes. In order to better understand these fluxes, the data are now presented as nmol of radiolabeled C16 or C18 acyl groups in each glycerolipid class, as shown in Figure 4. The first noteworthy feature is that both eukaryotic (PC) and prokaryotic (MGDG, PG) lipid synthesis are enhanced in the fatb-ko mutant. Second, there are much greater rates of disappearance of labeled fatty acids in these same lipid classes. To fully understand the data, we must take account of both the disappearance of labeled fatty acids (that is, complete degradation or conversion to nonglycerolipid products) and the relative acyl fluxes between the lipid classes, and particularly the movement from PC via MGDG to DGDG. This flux preferentially utilizes C18C18 species (Kunst et al., 1989). There follows a more detailed analysis, described as nmol of 14C acyl groups in glycerolipids. This accounting shows that the loss of C16 fatty acids from PE and PC that cannot be ascribed to flux through to chloroplast lipids is essentially the same in wild type and the fatb-ko line, but the net loss C18 fatty acids is much increased in the mutant compared to wild type.
In setting up this analysis, we discuss the movement of label between the 6-h time point when the labeling is at a maximum, and the 72-h time point, when the nmol of labeled fatty acids in fatb-ko and wild type become indistinguishable (Fig. 1). Arabidopsis membrane glycerolipids are composed of C16C18, C18C16, or C18C18 molecular species (sn-1:sn-2 acyl groups). Whereas lipids derived from the prokaryotic pathway are predominantly C18C16, those derived from the eukaryotic pathway are either C18C18 or C16C18 (Browse and Somerville, 1991). In order to approximate the flux of acyl groups from cytosolic to plastid lipids, we predict the compositions of labeled chloroplast lipids from the labeled fatty acid distribution by assuming that both positions are equally labeled, an observation based on equal labeling of the sn-1 and sn-2 positions of PC, MGDG, and PG in spinach (Spinacia oleracea) leaves (Roughan et al., 1980). We also use the finding that Arabidopsis DGDG contains about 8% C18C16 species and 25% C16C18 species (Kunst et al., 1989).
In wild type during this period, the label in acyl moieties of PC decreases from 13.8 nmol to 5.2 nmol (Fig. 4), contributed by reductions of 1.7 nmol of C16 and 6.8 nmol of C18 fatty acids (Table II). The net appearance of label in C18C18 species in MGDG is 3.8 nmol and in DGDG is 4.4 nmol, with about 1.1 nmol in new C16C18 eukaryotic species in DGDG. Assuming negligible rates of DGDG degradation, the 8.6 nmol of label that are removed from the acyl moieties of PC provide much of the 9.3 nmol that are destined to be imported into the chloroplast for galactolipid synthesis. In PE there is a loss of 0.9 nmol of C16 fatty acids and a gain of 1.0 nmol of C18 fatty acids and, therefore, the net loss of label is essentially nil. If we combine the changes in PC plus PE, these lipids can donate 5.8 nmol of C18 and 2.6 nmol of C16 acyl groups, while eukaryotic galactolipid synthesis is estimated to require 8.8 nmol of C18 and 0.5 nmol of C16 acyl groups. Thus our estimates suggest that another 3 nmol of C18 fatty acids must be coming from other minor labeled pools, while there is a net disappearance of 2.1 nmol of palmitic acid.
Table II.
Redistribution of 14C acyl groups from 14C glycerolipids during the 6 to 72 h chase period after 14CO2 radiolabeling wild-type and fatb-ko leaves
| nmol 14C Acyl Groups
|
||||
|---|---|---|---|---|
| Wild Type | fatb | |||
| C16 | C18 | C16 | C18 | |
| PC | −1.7 | −6.8 | −1.6 | −14.6 |
| PE | −0.9 | +1.0 | −1.2 | −0.7 |
| MGDG | −0.9 | +4.7 | −5.3 | +9.2 |
| DGDG | +1.0 | +5.4 | +1.0 | +6.2 |
| PG | −0.5 | −1.5 | ||
−, Loss; +, gain.
In the fatb-ko mutant during this period, the label in acyl moieties of PC decreases from 21.7 nmol to 5.5 nmol (Fig. 4), contributed by reductions of 1.6 nmol of C16 and 14.6 nmol of C18 fatty acids (Table II). The net appearance of label in C18C18 species in MGDG is 3.9 nmol and in DGDG is 5.2 nmol, with about 1.2 nmol in new C16C18 eukaryotic species in DGDG. Assuming negligible rates of DGDG degradation, the 16.2 nmol of label that are removed from the acyl moieties of PC readily provides the 10.3 nmol that are destined to be imported into the chloroplast for net galactolipid synthesis. In PE there is a loss of 1.2 nmol of C16 and 0.7 nmol of C18 fatty acids, for a net loss of 1.9 nmol of labeled acyl groups in this phospholipid (Table II). In summary, PC + PE can donate 15.3 nmol of C18 and 2.8 nmol of C16 acyl groups, while eukaryotic galactolipid synthesis is estimated to require 9.7 nmol of C18 and 0.6 nmol of C16 acyl groups. Thus, our estimates suggest that there is a net disappearance of 5.6 nmol of C18 and 2.2 nmol of C16 acyl groups from the two major extraplastidic phospholipids.
In our accounting of nmol of labeled acyl groups, there may be some small inconsistencies. The amounts are based on combining data from several analyses. However, for any time point, when we combine the nmol of C16 and C18 fatty acids from each of the major lipid classes (Fig. 4), we get a sum of total fatty acids that is within 5% of the amount of total fatty acids measured directly from total lipids (Fig. 1). Thus, it is a reasonable first approximation not to have taken into account of the minor glycerolipid classes (e.g. sulfoquinovosyldiacylglycerol, PI, triacylglycerol). There may also be small nonextractable labeled pools that contribute to acyl fluxes. Thus, although the absolute values we provide may be open to some minor corrections, it is the comparison of values between the wild-type and fatb-ko lines under the same set of assumptions that is meaningful. In the fatb-ko line, the loss of palmitate from PE and PC that cannot be ascribed to flux through to chloroplast lipids is essentially the same as wild type (2.2 nmol versus 2.1 nmol, respectively). Likewise, there is a net loss (5.6 nmol) of C18 fatty acids from cytosolic phospholipids in the mutant that cannot be ascribed to flux through to chloroplast lipids, whereas in the wild type the accounting requires a net gain of 3 nmol. The difference of 8.6 nmol between mutant and wild type represents the additional removal of C18 fatty acids from phospholipids in the mutant.
Immunoblot Analysis of Wild-Type and fatb-ko Leaves
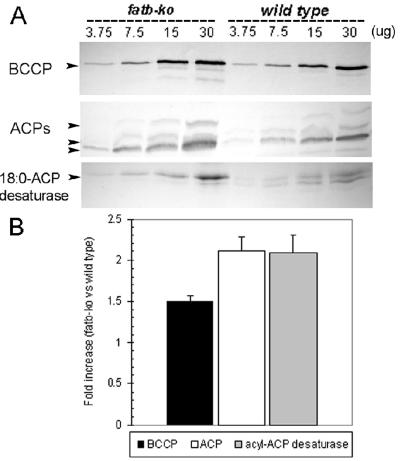
To determine if the increase in the rate of fatty acid biosynthesis in fatb-ko leaves was correlated with an up-regulation of FAS protein expression, specific antibodies against biotin carboxylase carrier protein (BCCP), ACPs, and stearoyl-ACP desaturase were used for immunoblot analysis of protein extracts from wild-type and fatb-ko leaves. As shown in Figure 5, protein levels of the BCCP subunit of plastidic acetyl-CoA carboxylase were increased by 1.5-fold in fatb-ko leaves compared to wild type. Similarly to BCCP, the protein levels of ACPs and stearoyl-ACP desaturase were approximately 2-fold higher in the mutant (Fig. 5). Arabidopsis leaves express several isoforms of plastidic ACPs with ACP-4 as the most abundant in this tissue, followed by ACP-2 and -3 (Hlousek-Radojcic et al., 1992). The immunoblot results indicated that most of ACP isoforms were up-regulated in leaves of the mutant. By contrast, a single band in the blot probed with anti-stearoyl-ACP desaturase antibody appeared up-regulated in fatb-ko leaves, suggesting that one major leaf isoform of this enzyme was induced.
Figure 5.
Immunoblot analysis of BCCP, ACP, and 18:0-ACP desaturase in leaf tissue of wild-type and fatb-ko Arabidopsis. A, Total Arabidopsis protein was extracted from leaves of wild type and mutant and increasing amounts loaded on the gel (3.75, 7.5, 15, and 30 μg). BCCP was detected with anti-biotin antibodies, ACPs, and 18:0-ACP desaturases with specific antibodies against spinach ACP-I and avocado stearoyl-ACP desaturase, respectively. Relative molecular mass for BCCP-1 is 35 kD, for ACP 10 kD, and for 18:0-ACP desaturase 35 kD. B, The amount of each polypeptide was quantitated with ImageQuant software and is expressed as relative fold increase of mutant versus wild type. The fold values shown are the average of the fold values for each protein concentration, and the bars denote the ses. Fold increase values correspond to BCCP-1, all ACPs detected, and the major 18:0-ACP desaturase band. The intensity of Coomassie Blue-stained bands was used to normalize western-blot signals.
Changes in the expression of some FAS proteins occur during leaf development, being higher in young leaves and declining after this tissue completes its expansion (J. Ohlrogge, unpublished data). Thus, to determine whether the increased levels of BCCP, ACPs, and 18:0-ACP desaturase in fatb-ko were the result of differences in the developmental stage of wild-type and mutant leaves, immunoblot analysis was also performed on leaf extracts at different stages of development (2-, 3-, and 4-week-old seedlings). The results showed a consistent increase (1.3–2-fold) in the levels of the three FAS proteins in fatb-ko leaves compared to wild type at the different stages of development (data not shown).
DISCUSSION
FATs initiate the export from the plastid of fatty acids produced by de novo FAS. In this study, pulse-chase labeling experiments were performed to investigate changes in lipid metabolism brought about by disruption of the FATB gene in Arabidopsis (Bonaventure et al., 2003). The results presented in this study give us a qualitatively and quantitatively different view than expected of the effects of disruption of this gene in Arabidopsis. Previously, it was determined that there was a 66% reduction in palmitate in the cytosolic phospholipids of Arabidopsis leaves, with a larger reduction in PC and smaller reductions in other phospholipids (Bonaventure et al., 2003). The mutation did not affect the sphingosine base content of the tissue (sphingosine bases are derived from palmitate). In wild-type leaves, there are about 1.9 μmol palmitate/gfw in glycerolipids, of which about 1.2 μmol/gfw are in eukaryotic phospholipids. In addition, there are at least 0.5 μmol/gfw palmitate used for sphingolipid synthesis, so we estimated that the reduction in palmitate export caused by the mutation was 40% to 50%. No consideration was made of the possibility that a major consequence of the mutation would be increases in both de novo FAS and fatty acid degradation.
Increased Rates of Fatty Acid Biosynthesis and Turnover in fatb-ko
The observation that three different labeling assays, with cut or intact tissue and with tracer substrates (acetate or carbon dioxide) or water labeling to give total FAS rate, gave consistently higher rates of FAS in fatb-ko compared to wild type demonstrates that this mutant increases the rate of fatty acid production in leaves. An important conclusion of these results is that fatty acid production appears not to limit the amount of total membrane lipid biosynthesis and consequently growth of fatb-ko plants. For the appearance of labeled 16:0 in PC + PE, the initial rate of labeling suggests that the reduction of 16:0 export for glycerolipid synthesis is from 11.4% to 7.6% (about a 33% reduction), and, therefore, on an absolute basis (considering the 40% increase in FAS) there is no reduction in the rate of palmitate export. This might imply a mechanism whereby the cell can sense the amount of palmitate exported and attempts to correct its biosynthetic machinery accordingly. However, without FATB, this mechanism is unable to maintain a wild-type balance of C16 to C18 acyl chains. Thus the shortage of palmitate synthesis per se may not be the reason for the slow growth and other phenotypes observed for the fatb-ko mutant. In many membranes of both prokaryotic and eukaryotic organisms, the balance of C16 to C18 acyl chains in lipids is critical to membrane function, and so the changes in the C16 to C18 acyl chain ratio seen in the eukaryotic phospholipids of the mutant could be important determinants of the phenotypic changes.
Because the steady state amount of fatty acids in leaves is not affected by the mutation, an increase in the rate of FAS must be matched by an increase of a similar magnitude in the rate of fatty acid degradation in membrane lipids. This is observed experimentally. Wild-type and fatb-ko leaves presented an average rate of fatty acid degradation of 2% to 3% and 8% to 9% per day, respectively, over the 5-d chase period (Fig. 1). Bao et al. (2000) previously reported a turnover rate of 4% to 5% per day in wild-type Arabidopsis plants based on an isotope dilution method. In the latter study, the plants were grown in a 13-h-light/11-h-dark cycle as opposed to an 18-h-light/6-h-dark cycle in this study. Thus, one explanation for the higher rates of fatty acid degradation observed by Bao et al. (2000) may be the prolonged dark period. The increase in both FAS and degradation can be considered another example of the induction of a futile cycle in the plant cell. Such a cycle was observed when the California bay 12:0-ACP thioesterase was overexpressed in developing Brassica napus seeds (Eccleston and Ohlrogge, 1998). In this system, the redirection of FAS toward medium-chain fatty acids (particularly 12:0) reprograms fatty acid metabolism in a way that both the catabolic and biosynthetic pathways are increased to remove the surplus of 12:0 and to maintain normal levels of C16 and C18 fatty acids.
The response to the disruption of the FATB gene in Arabidopsis was not specifically to increase de novo FAS to provide more fatty acids for export to the eukaryotic pathway. Instead, the acyl product distribution between the prokaryotic and eukaryotic pathways remained unchanged by the increase in de novo FAS. The limited protein expression data we have to date, that is an approximately proportional up-regulation of BCCP, ACP, and 18:0-ACP desaturase protein levels in fatb-ko leaves, is suggestive of a general up-regulation of the FAS system. We do not yet know if other proteins of FAS are likewise up-regulated, nor whether the mechanism of up-regulation will be at the level of gene expression or protein turnover. What does seem likely, however, is that in the mutant, the membrane lipid to protein ratio is kept in balance by an increase in lipid degradation, both within the chloroplast and probably also in the cytosol. In the wild type, there is very little need for acyl degradation. Perhaps the membrane lipid to protein ratios are kept constant by different mechanisms in wild type, or perhaps the protein and lipid synthesis machinery is such a finely balanced metabolic network that under normal circumstances the active acyl editing mechanisms are barely needed and are only exposed by the fatb-ko mutation.
Turnover of Membrane Lipids in Wild Type and fatb-ko
Despite the increases in the rates of FAS and degradation in fatb-ko, the ratio of membrane lipids is preserved. In wild type there is little degradation of acyl groups during the pulse-chase period. However, in the mutant the disappearance of label in the prokaryotic lipids, namely C18C16 species of MGDG and PG, is quite apparent (Fig. 4). MGDG is predominantly 18:3/16:3. Since 16:3(Δ7,10,13) is a fatty acid unique to the chloroplast, its disappearance implies total degradation. This being the case, since C18C16 species of MGDG are lost, 18:3 must also be removed. The molecular mechanism to initiate the removal of MGDG is not revealed by our experiments, that is, whether the molecule is removed intact or is hydrolytically degraded. In wild type, there is about 15% removal of C18C16 species of MGDG between 6 and 24 h in the pulse-chase period. Some of this reduction may arise from conversion of prokaryotic MGDG species to prokaryotic DGDG species, since there are about 10% of such species in DGDG (Kunst et al., 1989). Perhaps the remainder of the reduction is from lipid editing. In the mutant, the decline in labeled MGDG during the pulse-chase period is 50%, an increase of 3.3-fold over wild type (Fig. 4). In PG, there is also a chloroplast unique fatty acid, 16:1(Δ3-trans). We can therefore make a similar argument to MGDG that the disappearance of labeled PG is essentially through complete degradation. Over half of the PG (about 60%) is removed during the pulse-chase period.
In eukaryotic membrane lipids, the increase in the export of 18:1 from the chloroplast by the mutant is manifested most strikingly in the increase in C18 fatty acids in PC (Fig. 4). In wild type, the disappearance of C18 labeled fatty acids in PC can be completely attributed to flux through to DGDG. Palmitate is clearly removed from phospholipids but its fate is unknown. It may be used for protein acylation and supply much or all of the palmitate required for sphingoid base synthesis. However, this hypothesis needs further investigation. The removal of palmitate (measured in nmol) from phospholipids is very similar in the mutant, suggesting that this process may be under tight control in the cell. However, in the mutant, the excess PC synthesis is associated with additional degradation and removal of C18 fatty acids. These C18 fatty acids will be removed by turnover in the phospholipid pool, but we cannot define whether their degradation arises directly from this process or also includes a contribution from increased eukaryotic DGDG synthesis and turnover that leaves the net pool of DGDG largely unchanged.
PE labeling has some distinct differences from PC labeling. First, there is not a significant increase in the total amount of PE synthesis in the mutant. Second, during the pulse-chase period there is only a small decline in the amount of PE in either mutant or wild type, whereas there is a large decline in PC (Fig. 4). And third, in the 24-h period immediately after labeling, the significant change in PE composition in wild type, namely the reduction in palmitate, is retained in the mutant (Figs. 3 and 4) despite the initially lower amount of 16:0 in PE. This is a critical period of phospholipid remodeling that helps to cause the larger drop in 16:0 in PC relative to PE in the mutant. Over this period, phospholipid remodeling will result in a net flux of 16:0 from PC to PE to compensate for the initial loss of 16:0 in PE. Saturated fatty acids, and in particular palmitic acid, play an important role in the control of lipid composition in animal membranes (Seegmiller et al., 2002). Moreover, the incorporation of 16:0 into PE appears to trigger regulatory mechanisms for membrane homeostasis. This effect may be brought about indirectly by changing the biophysical properties of membranes and therefore their structure and function (Dobrosotskaya et al., 2002). Our observations on PE composition changes in wild type and in fatb-ko mutant relative to PC are consistent with a regulatory role for PE in membrane homeostasis in plants. Thus, similar to animal cells, changes in the structure and function of membranes as a result of changes in the composition of PE could trigger signals to activate membrane homeostatic mechanisms. These mechanisms may be different between plants and animals; however, an ancient role for saturated fatty acid could be conserved between these two organisms.
CONCLUSIONS AND FUTURE PERSPECTIVES
Disruption of the fatb-ko gene causes a 33% reduction in the proportion of 16:0 product of FAS exported from the chloroplast. The plant cell responds by (1) increasing the rate of FAS by 40% to compensate, regaining the rate of palmitate export, and by (2) a commensurate increase in rate of lipid turnover and fatty acid degradation. What is not clear is the causal relationship between the increase in FAS and the increase in fatty acid degradation.
One possibility is that the cell responds to the fatb mutation by sensing the lack of palmitate, either as palmitate per se or a metabolite or as an altered C16 to C18 ratio in the cytosolic phospholipids, and increasing FAS to compensate. However, this causes an increase in fatty acids supplied to both the prokaryotic and eukaryotic membrane lipid synthesis machinery and results in higher rates of membrane lipid synthesis. This in turn causes commensurate increases of lipid turnover and fatty acid degradation in both prokaryotic and eukaryotic lipid pools. The alternative is that the cell recognizes the altered C16 to C18 ratio in the cytosolic phospholipids and attempts to correct for this by increased degradation of C18 fatty acids. This in turn alters the membrane lipid to protein ratio and as a consequence FAS is up-regulated. By whatever mechanism, the cell maintains a constant composition of polar lipids, demonstrating the dominance of lipid homeostasis in plant cell membranes biogenesis. A similar conclusion was noted with the Arabidopsis act1 mutant, for which a major disruption of the prokaryotic pathway for lipid biosynthesis did not affect significantly the overall lipid composition of the plant (Kunst et al., 1989).
The molecular mechanisms underlying the changes in lipid metabolism in the fatb-ko mutant remain to be determined. These are important because they clearly reduce the rate of growth of the plant. The increase in FAS and up-regulation of BCCP, ACPs, and 18:0-ACP desaturase protein levels suggest that plant cells have mechanisms capable of sensing subnormal levels of saturated fatty acids and signaling the activation of the FAS machinery and protein expression in order to increase their production. Finding the initial signal, whether it be an acyl-CoA, palmitoyl-phospholipid, sphingolipid, acylated protein, membrane lipid to protein ratio, or other, will be important. In addition, we do not know the mechanisms by which the excess of both prokaryotic and eukaryotic lipid species are recognized and removed, and whether these represent constitutive or induced metabolic systems.
MATERIALS AND METHODS
Plant Material and Growth Conditions
In all the experiments, wild-type Arabidopsis and fatb-ko mutant plants (ecotype Wassilewskija-2; Bonaventure et al., 2003) were grown in growth chambers on a mixture of soil:vermiculite:perlite (1:1:1) for 3 weeks under white fluorescent light (100 μmol m−2 s−1) in a 18-h-light/6–h-dark photoperiod at 22°C. Sowed seeds were always stratified for 4 d at 4°C.
Rate of Fatty Acid Biosynthesis by Arabidopsis Leaves
Rapidly expanding leaves from wild-type and fatb-ko plants (3 weeks old) were cut in strips (0.5 cm wide) and transferred to preweighed glass flasks containing 4.75 mL of incubation buffer (2.5 mm sodium MES, pH 5.7, 0.0075% [w/v] Tween 20, and 2.15 mg/mL of Murashige and Skoog salts). Leaf strips from the same plant were randomly distributed between flasks, which were reweighed after approximately 0.2 g of tissue had been added to obtain gfw values. The assay was started by the addition of either 0.25 mL of 3H20 (100 mCi/mL, 3.7 GBq/mL) or 0.025 mCi of [1-14C]sodium acetate (56 mCi/mmol; American Radiolabeled Chemicals, St. Louis) to each flask and the flasks incubated for different times at 22°C in a temperature-controlled water bath with gentle agitation and continuous illumination. All data points were performed in duplicate and the values presented in Table I represent initial rates of FAS (calculated using data from 0, 10, 20, 40, and 60 min of continuous labeling). At the end of the assay period, the incubation medium was removed and the tissue quickly washed twice with deionized water and quenched by heating in 10 mL of isopropanol for 10 min at 80°C. Lipids were extracted with hexane-isopropanol method (Hara and Radin, 1978). An aliquot of the lipid extract was suspended in acetone:water (4:1, v/v) to determine chlorophyll content (Arnon, 1949). The methodology used to determine the rate of fatty acid biosynthesis using 14CO2 was identical to the methodology used to determine the rate of fatty acid turnover (see below) with the only difference that plants were removed at different time points from the sealed bag using an air trap sealed at both ends.
Rate of Fatty Acid Turnover by Arabidopsis Leaves
Wild-type and fatb-ko plants were grown for 3 weeks as indicated above. One day prior to the experiment, a total of 12 pots (6 pots with wild-type and 6 with fatb-ko plants [15 plants per pot]) were randomly placed inside a transparent glove bag (40 L of gas space, I2R Instruments for Research and Industry, Cheltenham, PA) with circulating air and the same lighting and temperature conditions as indicated above. A 30-min pulse of 14CO2 was given to the plants by mixing 2 mCi of 14C-NaHCO3 (56 mCi/mmol; American Radiolabeled Chemicals) with concentrated sulfuric acid inside the sealed bag and air circulated by using a small battery-driven fan. For the chase period, the radioactive atmosphere was rapidly vented and the plants were placed in a normal (nonradioactive) atmosphere for different times in the same growth conditions as described above. At each time point, 15 wild-type and 15 fatb-ko plants were randomly removed from different pots and separated in two individual samples (7–8 plants/sample for wild type and mutant). Leaf tissue was immediately weighed, frozen in liquid nitrogen, and stored at −80°C for subsequent lipid extraction with hexane-isopropanol method (Hara and Radin, 1978) and for chlorophyll content determination (Arnon, 1949).
Total amounts of fatty acids in the different samples were determined by gas chromatography as described in Bonaventure et al. (2003). These values were used to calculate specific activities of 14C fatty acids during growth of both wild-type and mutant plants in Figure 1. The amount of label at the end of the labeling period is expressed as nmol per mg chlorophyll. Subsequent time points included a correction for the dilution in specific activity caused by growth. This correction is based on the increasing accumulation of fatty acids in the tissue, which by the end of the 5-d chase period has doubled.
Fatty Acid Analysis of Lipid Extracts from Leaf Tissue
For the transmethylation of total lipids, an aliquot of lipid was heated at 90°C for 1 h in 1 mL of 10% (v/v) boron-trifluoride/methanol. After acidification with aqueous acetic acid, FAMEs were extracted two times with hexane and radioactivity in the sample (either 3H or 14C) analyzed by scintillation counting (Beckman Instruments, Fullerton, CA). A second aliquot of total lipid extract from each sample was transmethylated, and FAMES were separated by TLC on K6 silica plates (Whatman, Clifton, PA) using 90:10 (v/v) hexane:diethyl-ether. Radioactive bands corresponding to FAMEs were localized by scanning in an Instant Imager system (Packard, Meriden, CT). The bands corresponding to FAMEs were recovered from the plates and radioactivity measured by scintillation counting (Beckman Instruments).
Individual Glycerolipid Analysis
Glycerolipid classes from total lipid extracts were separated by TLC on K6 silica plates impregnated with 0.15 m ammonium sulfate and activated for 3 h at 110°C (Kahn and Williams, 1977). The TLC plates were developed three times with 91:30:8 (v/v/v) acetone:toluene:water and scanned for radioactivity using an Instant Imager (Packard), both to quantitate radioactivity and to locate the appropriate bands for recovery. Lipids were also detected after spraying with 0.2% (w/v) 2′-7′-dichlorofluorescein/methanol and viewing under UV light. Standards were used to identify the different glycerolipid classes. Lipids were eluted from the silica with chloroform:methanol:water (5:5:1) and FAMEs prepared as described above. FAMEs from the different lipid classes were hydrogenated using hydrogen at slightly greater than atmospheric pressure with a platinum (IV) oxide catalyst in methanol. The reaction was run for at least 2 h to give complete reduction of unsaturated to saturated FAMEs as determined by gas chromatography analysis. Analysis and isolation of 18- and 16-carbon FAMEs were by reverse-phase TLC using KC18 plates (Whatman), developed half and then fully with acetonitrile:methanol:water (130:70:1, v/v/v). Plates were scanned for radioactivity using an Instant Imager to quantitate radioactivity and to locate the appropriate bands for recovery. Scintillation counting was also used to measure radioactivity after scraping off the bands corresponding to 16- and 18-carbon fatty acids from the plates.
To analyze the radioactivity in the acyl moieties of 14C glycerolipids from 14CO2 labeled leaves, lipid classes were isolated as indicated above and divided in two fractions. One fraction was used to measure total radioactivity by scintillation counting. The second fraction was subjected to base transmethylation (Ichihara et al., 1996). Lipids were vortexed with 2 m KOH in methanol (0.6 mL) plus hexane (4 mL) for 3 min at room temperature (Ichihara et al., 1996). The reaction was quenched by adding 4 mL of 1 m aqueous acetic acid. The hexane phase was removed, and the acidified aqueous phase re-extracted with hexane. The hexane phases were combined and extracted with water. The hexane phase was placed in a scintillation vial, evaporated to dryness under nitrogen, scintillation liquid added, and the radioactivity measured by scintillation counting (Beckman Instruments).
SDS-PAGE and Immunoblotting
Extraction of proteins from leaves was performed as follows. Up to 0.1 g of leaf tissue was harvested and placed into a 1.5-mL plastic microcentrifuge tube. Plant material was pulverized with a microcentrifuge pestle in the presence of liquid nitrogen. Powder was immediately reconstituted in 0.2 mL of extraction buffer (2% [v/v] 2-mercaptoethanol, 50 mm HEPES [pH 7.8], 100 mm NaCl, and 0.05% [w/v] SDS) by vortexing. Insoluble debris was collected by centrifugation for 15 min at 12,000g. The supernatant was removed and placed into a fresh microcentrifuge tube. Protein concentration was determined by dye-binding protein assay using bovine serum albumin as the standard (Bradford, 1976).
SDS-PAGE and protein transfer to nitrocellulose was performed using standard conditions. Nitrocellulose membranes were blocked for at least 1 h with 10 mm Tris-HCl, pH 8.0, 0.15 m sodium chloride, 0.3% (v/v) Tween 20 (Tris-buffered saline plus Tween 20 [TBS-T]), and 2% (w/v) nonfat dry milk. Anti-biotin antibodies conjugated to alkaline phosphatase (Kirkegaard and Perry Laboratories, Gaithersburg, MD) were directly added at a 1:5,000 dilution to detect BCCP. Antisera raised against avocado stearoyl-ACP desaturase (Shanklin and Somerville, 1991) and spinach ACP-I (Post-Beittenmiller et al., 1991) were added at a 1:1,000 dilution. Probing proceeded for 16 h at 4°C followed by 1 h at 25°C, after which membranes were briefly rinsed with TBS-T. Antibody-bound proteins were detected by incubating blots for 1 h at 25°C with alkaline-phosphatase-conjugated anti-rabbit secondary antibodies (Kirkegaard and Perry Laboratories). The membranes were then washed six times for 5 min each with TBS-T. Blots were then washed 5 min with developing solution (0.1 m Tris-HCl, pH 9.5, 0.1 m sodium chloride, and 5 mm magnesium chloride) before colorimetric detection in developing solution containing 0.33 mg/mL p-nitroblue tetrazolium chloride and 0.17 mg/mL 5-bromo-4-chloro-3-indoyl phosphate p-toluidine salt. Immunoblot signals were quantitated using ImageQuant software (Molecular Dynamics, Sunnyvale, CA), which accounted for band area plus intensity. Blot signals were corrected for differences in protein loading (as determined by Coomassie Brilliant Blue R-250 staining) and normalized against values of the control plants. Increasing amounts of total protein were resolved by SDS-PAGE for immunoblot analyses to ensure that both major and minor bands were within the linear sensitivity range of the detection system.
This work was supported by the National Science Foundation (grant no. MCB–9817882), by the Department of Energy (DE–FG02–87ER13729), and by the Michigan Experimental Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.043372.
References
- Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Focke M, Pollard M, Ohlrogge J (2000) Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J 22: 39–50 [DOI] [PubMed] [Google Scholar]
- Beisson F, Koo AJ, Ruuska S, Schwender J, Pollard M, Thelen JJ, Paddock T, Salas JJ, Savage L, Milcamps A, et al (2003) Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol 132: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure G, Salas JJ, Pollard MR, Ohlrogge JB (2003) Disruption of the FATB gene in Arabidopsis demonstrates an essential role of saturated fatty acids in plant growth. Plant Cell 15: 1020–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Branen JK, Shintani DK, Engeseth NJ (2003) Expression of antisense acyl carrier protein-4 reduces lipid content in Arabidopsis leaf tissue. Plant Physiol 132: 748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Kunst L, Anderson S, Hugly S, Somerville C (1989) A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol 90: 522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Roughan G, Slack R (1981) Light control of fatty acid synthesis and diurnal fluctuations of fatty acid composition in leaves. Biochem J 196: 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, Somerville C (1991) Glycerolipid synthesis: biochemistry and regulation. Annu Rev Plant Physiol Plant Mol Biol 42: 467–506 [Google Scholar]
- Browse J, Warwick N, Somerville CR, Slack CR (1986) Fluxes through the prokaryotic and eukaryotic pathways of lipid synthesis in the ‘16:3’ plant Arabidopsis thaliana. Biochem J 235: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan JE, Weisberg LJ, Allen RG (1975) Regulation of membrane lipid synthesis in Escherichia coli: accumulation of free fatty acids of abnormal chain length during inhibition of phospholipid synthesis. J Biol Chem 250: 5835–5840 [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB (2002) Regulation of SREBP processing and membrane lipid production in phospholipids in Drosophila. Science 296: 879–883 [DOI] [PubMed] [Google Scholar]
- Doermann P, Voelker T, Ohlrogge JB (1995) Cloning and expression in Escherichia coli of a novel thioesterase from Arabidopsis thaliana specific for long chain acyl-acyl carrier proteins. Arch Biochem Biophys 316: 612–618 [DOI] [PubMed] [Google Scholar]
- Eccleston VS, Ohlrogge JB (1998) Expression of lauroyl-acyl carrier protein thioesterase in Brassica napus seeds induces pathways for both fatty acid oxidation and biosynthesis and implies a set point for triacylglycerol accumulation. Plant Cell 10: 613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A, Radin NS (1978) Lipid extraction of tissues with a low toxicity solvent. Anal Biochem 90: 420–426 [DOI] [PubMed] [Google Scholar]
- Hlousek-Radojcic A, Post-Beittenmiller D, Ohlrogge JB (1992) Expression of constitutive and tissue specific acyl carrier protein isoforms in Arabidopsis. Plant Physiol 98: 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K, Shibahara A, Yamamoto Y, Nakayama T (1996) An improved method for rapid analysis of the fatty acids of glycerolipids. Lipids 31: 535–539 [DOI] [PubMed] [Google Scholar]
- Kahn MU, Williams JP (1977) Improved thin layer chromatographic method for the separation of major phospholipids and glycolipids from plant lipid extracts and phosphatidylglycerol and bis(monoacylglycerol) phosphate from animal lipid extracts. J Chromatogr 140: 179–185 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE (2003) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71: 1–49 [DOI] [PubMed] [Google Scholar]
- Kunst L, Browse J, Somerville C (1989) A mutant of Arabidopsis deficient in desaturation of palmitic acid in leaf lipids. Plant Physiol 90: 943–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner J, Wu JR, Browse J (1994) A mutant of Arabidopsis with increased levels of stearic-acid. Plant Physiol 106: 1443–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DV (1993) Sphingolipids. In TS Moore, ed, Lipid Metabolism in Plants. CRC Press, Boca Raton, FL, pp 285–303
- Mekhedov S, de Ilarduya OM, Ohlrogge J (2000) Toward a functional catalog of the plant genome. A survey of genes for lipid biosynthesis. Plant Physiol 122: 389–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Z, He Y, Dai Y, Liu X, Li J (2000) Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12: 405–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Krothapalli K, Buseman CM, Li M, Welti R, Enyedi A, Shah J (2003) Arabidopsis sfd mutants affect plastidic lipid composition and suppress dwarfing, cell death, and the enhanced disease resistance phenotypes resulting from the deficiency of a fatty acid desaturase. Plant Cell 15: 2383–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn WD, Kelly DL, Stumfall MY (1977) Regulation of fatty acid synthesis during the cessation of phospholipids biosynthesis in Escherichia coli. J Bacteriol 132: 526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Kuhn DN, Stumpf PK (1979) Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci USA 76: 1194–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard MR, Ohlrogge JB (1999) Testing models of fatty acid transfer and lipid synthesis in spinach leaf using in vivo oxygen-18 labeling. Plant Physiol 121: 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post-Beittenmiller D (1996) Biochemistry and molecular biology of wax production in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 405–430 [DOI] [PubMed] [Google Scholar]
- Post-Beittenmiller D, Jaworski JG, Ohlrogge JB (1991) In vivo pools of free and acylated acyl carrier proteins in spinach: evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem 266: 1858–1865 [PubMed] [Google Scholar]
- Roughan PG (1970) Turnover of the glycerolipids of pumpkin leaves, the importance of phosphatidylcholine. Biochem J 117: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan PG, Holland R, Slack CR (1980) The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C] acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J 188: 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas JJ, Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch Biochem Biophys 403: 25–34 [DOI] [PubMed] [Google Scholar]
- Seegmiller AC, Dobrosotskaya I, Goldstein JL, Ho YK, Brown MS, Rawson RB (2002) The SREBP pathway in Drosophila: regulation by palmitate, not sterols. Dev Cell 2: 229–238 [DOI] [PubMed] [Google Scholar]
- Shanklin JS, Somerville C (1991) Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs. Proc Natl Acad Sci USA 88: 2510–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani DK, Roesler K, Shorrosh B, Savage L, Ohlrogge JB (1997) Antisense expression and overexpression of biotin carboxylase in tobacco leaves. Plant Physiol 114: 881–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker TA, Jones A, Cranmer AM, Davies HM, Knutzon DS (1997) Broad-range and binary-range acyl-acyl-carrier protein thioesterases suggest an alternative mechanism for medium-chain production in seeds. Plant Physiol 114: 669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JG, Browse J (2002) Mutants of Arabidopsis reveal many roles for membrane lipids. Prog Lipid Res 41: 254–278 [DOI] [PubMed] [Google Scholar]
- Wells GB, Lester RL (1983) The isolation and characterization of a mutant strain of Saccharomyces cerevisiae that requires a long chain base for growth and for synthesis of phosphosphingolipids. J Biol Chem 258: 10200–10203 [PubMed] [Google Scholar]
- Wu JR, James DW, Dooner HK, Browse J (1994) A mutant of Arabidopsis deficient in the elongation of palmitic acid. Plant Physiol 106: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalovsky S, Rodriguez-Concepcion M, Gruissem W (1999) Lipid modification of proteins slipping in and out of membranes. Trends Plant Sci 4: 439–445 [DOI] [PubMed] [Google Scholar]