Abstract
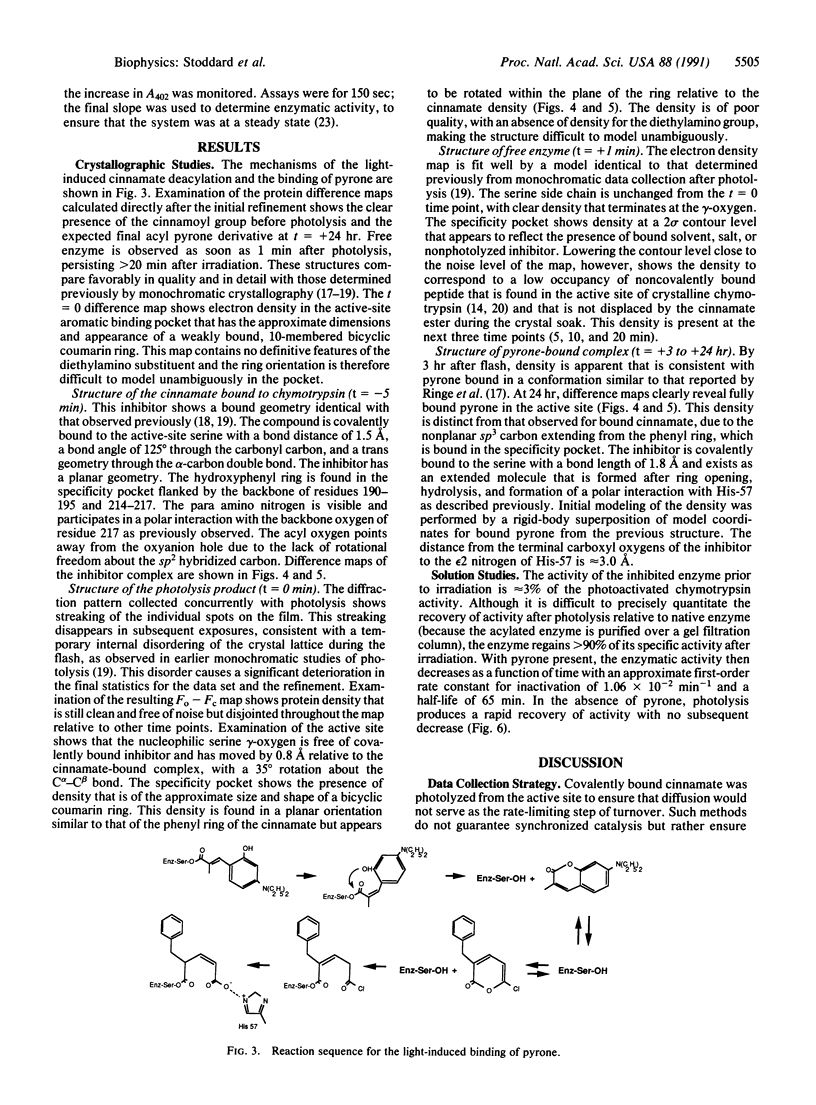
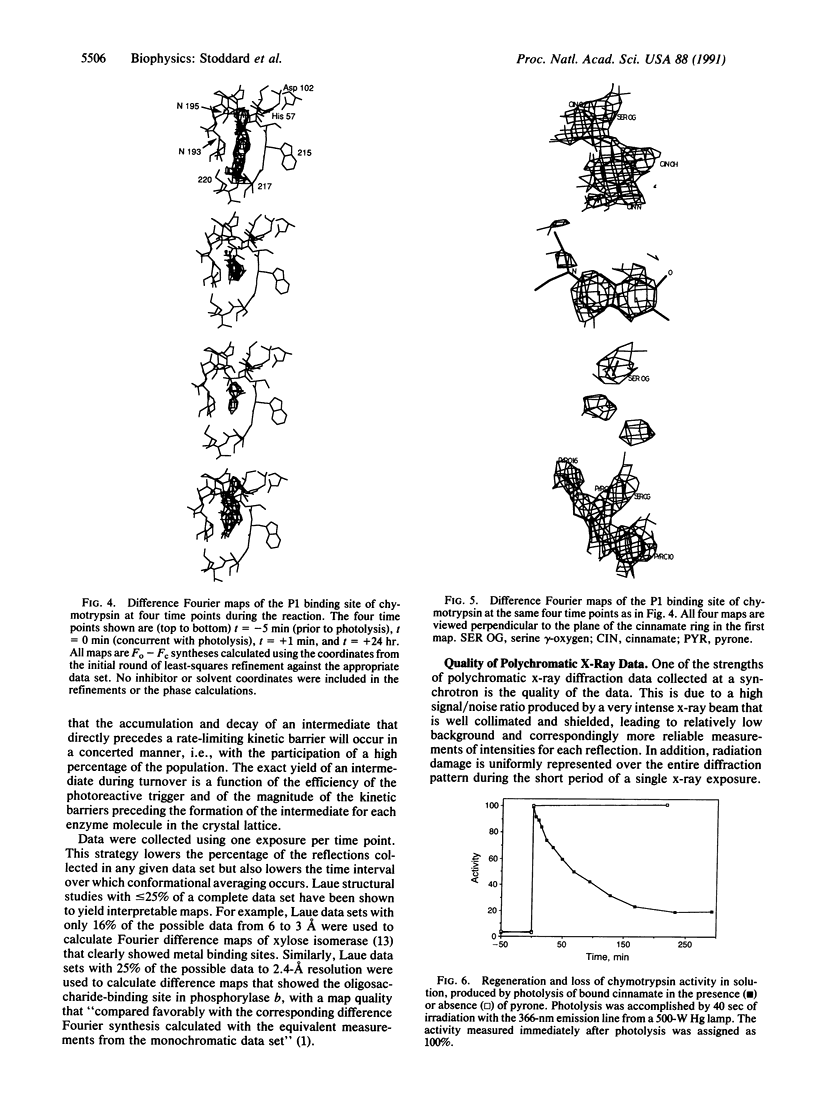
Crystals of gamma-chymotrypsin inhibited with the photodissociable group trans-p-diethylamino-o-hydroxy-alpha-methylcinnamate were irradiated with a 1-msec flash from a high-energy xenon flashlamp in the presence of the mechanism-based inhibitor 3-benzyl-6-chloro-2-pyrone. The ensuing reaction was monitored by collection of sequential, single-exposure Laue x-ray diffraction patterns. The experiment was also performed in solution to verify the regeneration of catalytic activity and the subsequent inhibition of the enzyme by pyrone after photolysis. The resulting crystallographic structures show the presence of covalently bound cinnamate prior to photolysis, the generation of "free" enzyme after irradiation of the crystal, and the slow formation of a pyrone-inhibited complex several hours after photolysis. The structure of the free enzyme shows a significant proportion of the active sites in the crystal to contain a naturally occurring, noncovalently bound tetrapeptide inhibitor [Dixon, M.M. & Matthews, B.W. (1989) Biochemistry 28, 7033-7038], even after cinnamate acylation and photolysis. Data collected simultaneously with irradiation show the crystal to be slightly disordered during photolysis, leading to streaked x-ray photos. The resulting maps are suggestive of a bicyclic coumarin species produced by photolysis and deacylation; however, the electron density is difficult to model unambiguously by one unique chemical state. Nevertheless, Laue crystallography is shown to be capable of visualizing time-dependent chemical changes in the active site of an enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell J. W., Clifton I. J., Greenhough T. J., Hajdu J., Harrison S. C., Liddington R. C., Shrive A. K. Calcium binding sites in tomato bushy stunt virus visualized by Laue crystallography. J Mol Biol. 1990 Aug 5;214(3):627–632. doi: 10.1016/0022-2836(90)90278-T. [DOI] [PubMed] [Google Scholar]
- Dixon M. M., Matthews B. W. Is gamma-chymotrypsin a tetrapeptide acyl-enzyme adduct of alpha-chymotrypsin? Biochemistry. 1989 Aug 22;28(17):7033–7038. doi: 10.1021/bi00443a038. [DOI] [PubMed] [Google Scholar]
- Farber G. K., Machin P., Almo S. C., Petsko G. A., Hajdu J. X-ray Laue diffraction from crystals of xylose isomerase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):112–115. doi: 10.1073/pnas.85.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu J., Acharya K. R., Stuart D. I., Barford D., Johnson L. N. Catalysis in enzyme crystals. Trends Biochem Sci. 1988 Mar;13(3):104–109. doi: 10.1016/0968-0004(88)90051-5. [DOI] [PubMed] [Google Scholar]
- Hajdu J., Acharya K. R., Stuart D. I., McLaughlin P. J., Barford D., Oikonomakos N. G., Klein H., Johnson L. N. Catalysis in the crystal: synchrotron radiation studies with glycogen phosphorylase b. EMBO J. 1987 Feb;6(2):539–546. doi: 10.1002/j.1460-2075.1987.tb04786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu J., Johnson L. N. Progress with Laue diffraction studies on protein and virus crystals. Biochemistry. 1990 Feb 20;29(7):1669–1678. doi: 10.1021/bi00459a001. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Fink A. L. Reactivity and cryoenzymology of enzymes in the crystalline state. Annu Rev Biophys Bioeng. 1977;6:301–343. doi: 10.1146/annurev.bb.06.060177.001505. [DOI] [PubMed] [Google Scholar]
- Moffat K., Szebenyi D., Bilderback D. X-ray Laue Diffraction from Protein Crystals. Science. 1984 Mar 30;223(4643):1423–1425. doi: 10.1126/science.223.4643.1423. [DOI] [PubMed] [Google Scholar]
- Rapp G., Güth K. A low cost high intensity flash device for photolysis experiments. Pflugers Arch. 1988 Feb;411(2):200–203. doi: 10.1007/BF00582315. [DOI] [PubMed] [Google Scholar]
- Ringe D., Mottonen J. M., Gelb M. H., Abeles R. H. X-ray diffraction analysis of the inactivation of chymotrypsin by 3-benzyl-6-chloro-2-pyrone. Biochemistry. 1986 Sep 23;25(19):5633–5638. doi: 10.1021/bi00367a043. [DOI] [PubMed] [Google Scholar]
- Schlichting I., Almo S. C., Rapp G., Wilson K., Petratos K., Lentfer A., Wittinghofer A., Kabsch W., Pai E. F., Petsko G. A. Time-resolved X-ray crystallographic study of the conformational change in Ha-Ras p21 protein on GTP hydrolysis. Nature. 1990 May 24;345(6273):309–315. doi: 10.1038/345309a0. [DOI] [PubMed] [Google Scholar]
- Stoddard B. L., Bruhnke J., Koenigs P., Porter N., Ringe D., Petsko G. A. Photolysis and deacylation of inhibited chymotrypsin. Biochemistry. 1990 Sep 4;29(35):8042–8051. doi: 10.1021/bi00487a008. [DOI] [PubMed] [Google Scholar]
- Stoddard B. L., Bruhnke J., Porter N., Ringe D., Petsko G. A. Structure and activity of two photoreversible cinnamates bound to chymotrypsin. Biochemistry. 1990 May 22;29(20):4871–4879. doi: 10.1021/bi00472a017. [DOI] [PubMed] [Google Scholar]
- Westkaemper R. B., Abeles R. H. Novel inactivators of serine proteases based on 6-chloro-2-pyrone. Biochemistry. 1983 Jun 21;22(13):3256–3264. doi: 10.1021/bi00282a034. [DOI] [PubMed] [Google Scholar]


