Abstract
Current methods for studying oligodendrocyte myelination using primary neurons are limited by the time, cost and reproducibility of myelination in vitro. Nanofibers with diameters of >0.4 μm fabricated from electrospinning of liquid polystyrene are suitable scaffolds for concentric membrane wrapping by oligodendrocytes. With the advent of aligned electrospinning technology, nanofibers can be rapidly fabricated, standardized, and configured into various densities and patterns as desired. Notably, the minimally permissive culture environment of fibers provides investigators with an opportunity to explore the autonomous oligodendrocyte cellular processes underlying differentiation and myelination. The simplicity of the system is conducive to monitoring oligodendrocyte proliferation, migration, differentiation and membrane wrapping in the absence of neuronal signals. Here we describe protocols for the fabrication and preparation of nanofibers aligned on glass coverslips for the study of membrane wrapping by rodent oligodendrocytes. The entire protocol can be completed within 2 weeks.
INTRODUCTION
One of the most intimate and elaborate cellular interactions found in nature occurs in the CNS, when oligodendrocytes deposit multiple concentric layers of compact membrane around axons, with the amount of membrane deposited depending on the size of the axon. The formation of this myelin sheath is crucial to the efficient and rapid transmission of action potentials throughout the vertebrate nervous system. Axonal signaling to oligodendrocytes is generally accepted as controlling myelination1. Therefore, identifying the nature of these signals is paramount for promoting remyelination after injury or after onset of demyelinating diseases, such as multiple sclerosis, and this is indeed the goal of most myelin research.
Much of our current understanding of oligodendrocyte myelination is derived from in vitro culture studies using purified neurons and oligodendrocytes, dispersed mixed glial cultures or organotypic slice cultures. In general, each of these culture methods has its own unique set of advantages for biological experiments. However, these methods also have limitations in terms of time length, cost and reproducibility of experiments. For example, although slice cultures generally produce robust myelination and maintain tissue architecture and neuronal connections2,3, these cultures contain cell types other than neuronal substrates for myelination and oligodendrocytes, such as astrocytes and microglia, which may confound data interpretation. Similarly, dispersed mixed glial cultures established with neurons and oligodendrocytes from cortical tissues4 or spinal cords5,6 also contain similar contaminating cell types that are seen with slice cultures and that yield inconsistent amounts of myelin from culture to culture. In contrast, purified neuron-oligodendroglial coculture systems can be established using dorsal root ganglion neurons7, retinal ganglion neurons8 or hippocampal neurons9,10. However, preparing these coculture systems involves tedious purification procedures and the maintenance of primary neurons. For example, dorsal root ganglion neurons need to mature for ~3 weeks in vitro before oligodendrocyte precursor cells (OPCs) can be seeded7.
Here we describe a rapid and reproducible culture system that is suited for analyzing oligodendrocyte autonomous mechanisms that are important in myelination. Recently, we explored the longstanding notion that the initiation of myelin wrapping is somehow intimately related to axonal diameter, with the larger axons being preferentially myelinated over the smaller ones. For example, an increase in axonal target size correlates with both an increase in axonal diameter and the development of myelin11,6. By using electrospun polystyrene nanofibers that act as pseudo-axonal scaffolds in forming myelin-like segments, we demonstrated that fiber diameter is a permissive axonal cue that is sufficient for initiating membrane wrapping by oligodendrocytes. Fibers with diameters ≥0.4 μm are ensheathed and wrapped by OPCs and oligodendrocytes, respectively. Our findings demonstrate that nanofibers of sufficiently large diameter represent a minimally permissive environment that is ideal for investigating extrinsic factors that may contribute to membrane wrapping12.
Design of nanofibers and development of OPC-fiber culture
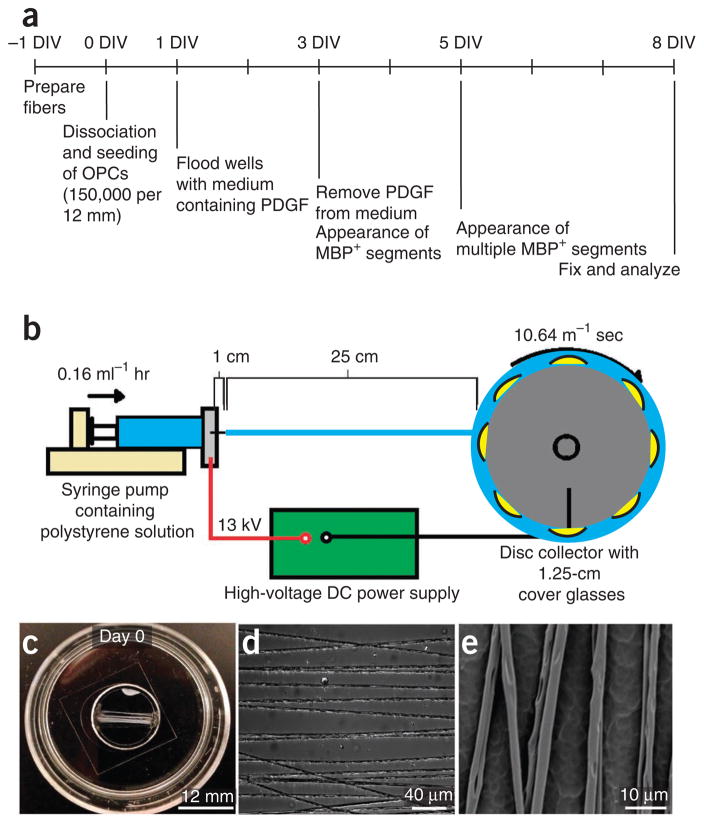
This protocol consists of three major sections: design and fabrication of nanofibers, culture of oligodendrocytes on fibers, and analysis of myelination. First, nanofibers are designed to have a range of diameters that are physiologically relevant and optimal for wrapping (2–4 μm). Fibers are fabricated by electrospinning liquid polystyrene, a material commonly used for tissue culture. Nanofibers can be configured into various diameters, orientations and densities, as desired. In the present protocol, we align the fibers horizontally on 12-mm glass coverslips during the electrospinning process. In order to prevent the fibers from detaching from the glass coverslips when immersed in cell culture medium, both edges of the fibers are attached to the coverslips with silicone adhesive sealant (Fig. 1). Fibers are then sterilized with 70% (vol/vol) ethanol and coated with substrates, such as poly-L-lysine. Next, by using a protocol adapted from previously reported methods8,13, OPCs are dissociated from cortices of postnatal day (P) 7 rat pups or P9 mouse pups and purified through immunopanning. Immediately after purification, a high density of OPCs is seeded onto the fibers in a defined culture medium containing exogenous platelet-derived growth factor (PDGF). OPC-fiber cultures can be examined by light microscopy, fixed for fluorescence microscopy or processed for electron microscopy.
Figure 1.
Fabrication and preparation of the fibers for assaying oligodendroglial membrane wrapping. (a) Timeline for fiber fabrication and OPC culture for analysis of myelin-like segment formation using immunocytochemistry. (b) Diagram of an electrospinning setup consisting of a syringe pump (pale yellow), a high-voltage DC power supply (green) and a rapidly rotating wheel collector (dark gray). A polystyrene solution (cyan) is dispensed from the syringe pump to the collector that is positioned 25 cm from the tip of the syringe (in the figure, the 1-cm length refers to the distance from the tip of the syringe to the tip of the needle, before ejection of the polystyrene liquid starts). A high-voltage DC power supply (green) grounds a rapidly rotating wheel collector containing taped 12-mm coverslips onto which aligned nanofibers are collected. (c) Phase image of the fibers aligned on a coverslip at low magnification. (d) Phase image of the fibers aligned on a coverslip at high magnification. (e) SEM image of 2–4-μm diameter fibers spaced 10–15 μm apart.
Applications and advantages
Electrospun nanofibers provide reproducible and robust scaffolds for concentric membrane wrapping, a process that is indicative of the generation of myelin-like segments by oligodendrocytes. These fibers can be most useful in the investigation of autonomous oligodendrocyte mechanisms that are important for myelination. For example, OPC-fiber cultures can be used to analyze changes in oligodendrocyte-specific gene expression or signaling mechanisms associated with the formation of myelin-like segments. One of the long-standing questions with respect to myelination is the molecular mechanism underlying membrane assembly and wrapping that leads to the characteristic architecture of myelin. Sufficiency of fiber diameter for generating multiple concentric wraps of membrane provides an opportunity to study the molecular pathways regulating this process. Another application of the fibers would be in identifying molecules that promote membrane wrapping around the fibers and in oligodendrocyte proliferation, migration and differentiation. OPCs grown on regular glass coverslips may cluster as mounds of cells upon proliferation, whereas OPCs cultured on the fibers space themselves evenly along the fibers, thereby facilitating analyses that require accurate quantification of cell numbers, such as proliferation or cell death assays. Nanofibers also provide scaffolds on which OPCs can migrate in an orderly and predictable fashion rather than dispersing at random.
As our findings suggest, oligodendrocyte differentiation and myelination may be a continuous process, which cannot be uncoupled, as membrane ensheathment initiates at the level of OPCs before expression of myelin basic protein (MBP)12. Therefore, fibers provide a minimally permissive environment that enables the analysis of extrinsic signals, both molecular and biophysical, that control oligodendrocyte differentiation and membrane wrapping. For example, mechanical properties of extracellular matrix, such as stiffness, can influence oligodendrocyte differentiation and myelination14. Therefore, one could fabricate fibers with varying stiffness and examine effects on oligodendrocyte differentiation and fiber wrapping. In addition, integrin molecules, such as α6β1, have been reported to interact with either axonal or extracellular matrix substrates, such as laminin-2, to modulate oligodendrocyte survival15, axon myelination16 and enhanced membrane formation in vitro17. Coating the fibers with various substrates enables the investigation of the effect that integrin-interacting substrates have on oligodendrocyte survival and differentiation and on membrane wrapping. Furthermore, the OPC-fiber culture can be used to systematically recapitulate the in vivo cellular microenvironments by adding other cellular components, such as astrocytes and/or microglia, to examine the roles these cell types have in oligodendrocyte survival and maintenance, as well as in axon (re)myelination and wrapping. Finally, one can examine oligodendrocyte-specific responses to demyelinating insults by exposing mature oligodendrocyte-fiber cultures to either chemical or physical insult. After the demyelinating insults, signaling mechanism(s) underlying remyelination of previously damaged myelin-like segments deposited on the fibers or recovery of OPCs and oligodendrocytes in culture can be analyzed.
Limitations of the approach and additional considerations
One of the hallmark features of the myelin internode generated on axons is the clustering of axonal sodium channels within the nodes of Ranvier that in turn enable the action potential to jump from one node to the other. In the fiber culture system, myelin-like segments generated along the fibers lack nodal structures. Despite important potential applications of this system, there are limitations for certain uses because of the absence of axonal components on fibers. Therefore, it is not possible to examine axon-mediated myelin changes. These changes include the extent of myelination, such as the thickness of myelin proportional to fiber diameter and the degree of compaction. Conversely, fibers provide investigators an opportunity to dissect axonal signals important for these processes. For example, it has been reported that neuregulin 1 type III regulates myelin thickness in both the CNS and the peripheral nervous system (PNS)18–20. One can identify potential axonal membrane-bound proteins important for completion of later stages of myelination by coating the fibers with axonal membrane or other candidate substrates known to modulate intercellular interactions.
MATERIALS
REAGENTS
Polystyrene solution
Polystyrene 250,000 MW (g per mol) (Acros Organics, cat. no. 178895000)
Dichloromethane (DCM; Acros Organics, cat. no. 40692-0040)
Dimethylformamide (DMF; Fisher Scientific, cat. no. AC11622-0010)
Tetrahydrofuran, 1 liter (THF; Sigma-Aldrich, cat. no. 34865)
Nanofiber preparation
Poly-L-lysine (Sigma-Aldrich, cat. no. P-2636)
Silicone sealant (Grainger, cat. no. 2CTE9)
Ethanol anhydrous (VWR, cat. no. EMEX02853)
OPC purification
-
Female rat with two P7–P8 pups (Sprague-Dawley; Charles River Laboratories) or female mouse with two P9–P10 pups (C57/BL6J, Jackson Laboratories) for one preparation
! CAUTION Necessary approvals must be obtained; all animal procedures must be approved by an institutional animal care and use committee, and they must adhere to all relevant governmental regulations.
Tris (Sigma-Aldrich, cat. no. T1503)
HCl (Fisher Scientific, cat. no. SA49)
NaOH pellets (Sigma-Aldrich, cat. no. 221465)
DMSO (Sigma-Aldrich, cat. no. D2438)
Sodium tetraborate decahydrate (Sigma-Aldrich, cat. no. B9876)
Goat anti-mouse IgG/M (Jackson Immunoresearch Laboratories, cat. no. 115-005-044)
BSA (Sigma-Aldrich, cat. no. A-9647)
NaCl (J.T. Baker, cat. no. 3624-19)
KCl (J.T. Baker, cat. no. 3040-01)
Na2HPO4 (J.T. Baker, cat. no. 3828-01)
KH2PO4 (J.T. Baker, cat. no. 3246-01)
Dulbecco’s PBS with Ca2 + and Mg2 + (D-PBS; Life Technologies, cat. no. 14040-182)
FBS (Life Technologies, cat. no. 10437-028)
Earle’s balanced salt solution (EBSS; Life Technologies, cat no. 14155063)
MgSO4 (Sigma-Aldrich, cat. no. M8226)
Glucose (Sigma-Aldrich, cat. no. G7528)
EGTA (Sigma-Aldrich, cat. no. E3889)
Papain (Worthington Biochemical Corporation, cat. no. 3119)
L-cysteine (Sigma-Aldrich, cat. no. C7352)
DNase I (Worthington Biochemical Corporation, cat. no. 2139)
Ovomucoid protease inhibitor (Worthington Biochemical Corporation, cat. no. 3086)
Trypsin-EDTA, 0.05% (wt/vol; Life Technologies, cat. no. 25300-054)
DMEM (Life Technologies, cat. no. 11995-065)
B27 (Life Technologies, cat. no. 17504-044)
N2, 100× (Life Technologies, cat. no. 17502-048)
Penicillin-streptomycin (Life Technologies, cat. no. 15140-122)
N-acetylcysteine (NAC; Sigma-Aldrich, cat. no. A8199)
Forskolin (EMD, cat. no. 344270)
Human recombinant PDGF-AA (Peprotech, cat. no. 100-13A)
Glacial acetic acid (Sigma-Aldrich, cat. no. A6283)
Rat neural antigen-2 (Ran-2) hybridoma (ATCC, cat. no. TIB-119)
Galactocerebroside (GalC) hybridoma (Ranscht et al.21)
O4 hybridoma (Sommer and Schachner22)
dH2O
Immunostaining
Goat serum (Sigma-Aldrich, cat. no. G9023)
Triton X-100 (Sigma-Aldrich, cat. no. T8787)
Rabbit anti-PDGFRα antibodies (gift of B. Stallcup’s Laboratory, Sanford-Burnham Medical Research Institute; 1:1,000 dilution)
Rat anti-MBP antibodies, 1:200 dilution (Millipore, cat. no. MAB386)
Mouse anti-glial fibrillary acid protein (GFAP) antibodies, 1:500 dilution (Millipore, cat no. AB5804)
Alexa Fluor 488 goat anti-rabbit IgG (H + L), highly cross-adsorbed, 1:1,000 dilution (Life Technologies, cat. no. A11034)
Alexa Fluor 594 goat anti-rat IgG (H + L), 1:1,000 dilution (Life Technologies, cat. no. A11007)
Alexa Fluor 647 goat anti-mouse IgG (H + L), 1:1,000 dilution, highly cross-adsorbed (Life Technologies, cat. no. A21236)
Vectashield mounting medium with DAPI (Vector Laboratories, cat. no. H-1200)
Paraformaldehyde (PFA), 16% (wt/vol) aqueous solution (Electron Microscopy Sciences, cat. no. 15710, 1:4 dilution)
Electron microscopy
OsO4, 4% (wt/vol) (Electron Microscopy Sciences, cat. no. 19140)
UO2(OCOCH3)2·2H2O, 1% (wt/vol) (Electron Microscopy Sciences, cat. no. 22400-1)
EMbed-812 kit (includes EMbed-812, dodecenyl succinic anhydride (DDSA); specially distilled, methylnadic anhydride (NMA) and 2,4,6-tris(dimethylaminomethyl)phenol (DMP)-30) (Electron Microscopy Sciences, cat. no. 14120)
2-Hydroxylpropyl methacrylate (HPMA; Electron Microscopy Sciences, cat. no. 14220)
Silicone embedding mold (Ted Pella, cat. no. 10504)
Glutaraldehyde, 2–5% (vol/vol; optional, see Step 51. Electron Microscopy Sciences, 50% aqueous solution, cat. no. 16320)
EQUIPMENT
Electrospinning equipment
Rotating disc collector (The University of Michigan (Ann Arbor, Michigan) modified by MogulTech (Saline, Michigan))
High-voltage DC power supply (Gamma High Voltage, cat. no. ES40P-5W)
Coverslips with a diameter of 12 mm (Daigger, cat. no. EF15973A)
Carbon tape (Ted Pella, cat. no. 13073-1)
Aluminum foil (VWR, cat. no. 89107-724)
High-torque industrial stirring motor (Caframo)
Blunt metal needles, 23-gauge (Fisher Scientific, cat. no. 13-850-102)
Polypropylene syringe, 3-ml (BD Biosciences, cat. no. 309585)
KDS syringe pump set (KD Scientific, cat. no. KDS 100)
Wire brush (Cerrowire 50 ft., 12-gauge stranded THHN white cable. Home Depot, cat. no 202206538).
12-mm glass coverslips (Fisher Scientific, cat. no. 12-545-80)
25-mm glass coverslips (Fisher Scientific, cat. no. 12-542C)
High-voltage DC power supply (Hipotronics)
OPC purification
Dissection tools
Cell culture dish, 150 × 25 mm (VWR, cat. no. 25383103)
Cell culture dish, 100 × 20 mm (VWR, cat. no. 25382166)
Cell culture dish, 60 × 15 mm (VWR, cat. no. 25382100)
Affinity pure goat anti-mouse IgG + IgM as a secondary antibody for immunopanning (Jackson ImmunoResearch, cat. no. 115-005-044)
Syringe, 35 ml (VWR, cat. no. 82002310)
Syringe filter: Millex-GP, 0.22 μm, polyethersulfone, 33 mm, gamma-sterilized syringe filter (Millipore, cat. no. SLGP033RS)
Tissue culture flask with vented cap, 225 cm2 (VWR, cat. no. 29560-959)
Six-well plate (BD Falcon, cat. no. 353046)
Cell strainer (nylon mesh filter), 40 μm, blue (BD Falcon, cat. no. 352340)
Bright-Line hemocytometer (Sigma, cat. no. Z359629)
Microcentrifuge (e.g., Eppendorf 5702)
REAGENT SETUP
Polystyrene solution for the fibers
Dissolve 1.5 g of 250,000 MW (g per mol) MW polystyrene in 7 ml of DCM and mix until the polystyrene is completely dissolved. Add 2 ml of DMF and 1 ml of THF to the solution. The solution can be stored for a couple of days at most at room temperature (20–25 °C).
Tris-HCl solution (50 mM)
In order to prepare 20× stock, dissolve 121.14 g of Tris in 950 ml of dH2O, and adjust the pH to 9.5 with 10 N HCl; adjust the volume to 1 l with dH2O. Autoclave at 121 °C for 15 min and let it cool to room temperature. To prepare 1× stock, dilute 20-fold with autoclaved, dH2O before use. Both 20× and 1× stocks can be stored for years at room temperature.
Immunopanning buffer (0.2% (wt/vol) BSA in D-PBS)
In order to prepare 25× immunopanning buffer stock, dissolve 5 g of BSA in 80 ml of D-PBS. Adjust the final volume to 100 ml with D-PBS and filter sterilize with a Millex-GP 0.22-μm, polyethersulfone, 33-mm, gamma-sterilized syringe filter. Store 1-ml aliquots at −20 °C. These aliquots can be stored safely for several months at −20 °C. In order to prepare 1× stock, dilute the 25× stock 25-fold with D-PBS before use. The maximum recommended shelf life for 1× stock is 1 month at 4 °C.
PBS (without Ca2+, Mg2+) stock (10×)
To prepare 10× PBS stock, dissolve 80 g of NaCl, 2.0 g of KCl, 11.5 g of Na2HPO4 and 2 g of KH2PO4 in 950 ml of dH2O. Adjust the pH to 7.4 with HCl. Adjust the volume to 1 l with dH2O. Autoclave at 121 °C for 15 min and let it cool to room temperature (20–25 °C). In order to prepare 1× stock, dilute the 10× stock tenfold with autoclaved dH2O before use. Both 10× and 1× stocks can be stored for years at room temperature.
Glucose solution (1.2 M)
Dissolve 10.8 g of glucose in 40 ml of dH2O. Adjust the final volume to 50 ml with dH2O and filter sterilize with a Millex-GP 0.22-μm, polyethersulfone, 33-mm, gamma-sterilized syringe filter. This solution can be stored at 4 °C for several months.
EGTA solution (0.25 M)
Dissolve 4.75 g of EGTA in 40 ml of dH2O and adjust the pH to 8.0 with ~1 g of NaOH pellets. It will not dissolve until a pH of 8.0 is reached. Adjust the final volume to 50 ml with dH2O and filter sterilize with a Millex-GP 0.22-μm, polyethersulfone, 33-mm, gamma-sterilized syringe filter. This solution can be stored for years at room temperature.
MgSO4 solution (100 mM)
Dissolve 476.05 mg of MgSO4 in 40 ml of dH2O. Adjust the final volume to 50 ml with dH2O and filter sterilize with a Millex-GP 0.22-μm, polyethersulfone, 33-mm, gamma-sterilized syringe filter. This solution can be stored for years at room temperature.
DNase I stock (12,500 U ml −1)
Dissolve 125,000 U of DNase I in 10 ml EBSS. Filter sterilize with a Millex-GP 0.22-μm, polyethersulfone, 33-mm, gamma-sterilized syringe filter, and store 1-ml aliquots at −20 °C. These aliquots can be stored for several months at −20 °C.
Papain buffer
A volume of 50 ml of papain buffer consists of 48 ml of EBSS containing 1 mM MgSO4, 2 mM EGTA and 19.8 mM glucose. To prepare 50 ml, mix 500 μl of 100 mM MgSO4, 400 μl of 0.25 M EGTA and 825 μl of 1.2 M glucose. Freshly prepare this buffer before use.
High-ovomucoid (Hi-ovo) protease inhibitor stock (6×)
Dissolve 2 g of ovomucoid protease inhibitor and 2 g of BSA in 33.3 ml D-PBS. Filter sterilize with a Millex-GP, 0.22 μm, polyethersulfone, 33 mm, gamma-sterilized syringe filter, and store 1-ml aliquots at −20 °C. These aliquots can be stored for several months at −20 °C. To prepare 1× stock, dilute sixfold with D-PBS before use.
Low-ovomucoid (Lo-ovo) protease inhibitor stock (10×)
Dissolve 2 g of ovomucoid protease inhibitor and 2 g of BSA in 133.3 ml of D-PBS. Filter sterilize with a Millex-GP 0.22-μm, polyethersulfone, 33-mm, gamma-sterilized syringe filter, and store 1-ml aliquots at − 20 °C. These aliquots can be stored for several months at −20 °C. In order to prepare 1× stock, dilute tenfold with D-PBS before use.
FBS buffer, 30% (vol/vol)
To prepare 50 ml, mix 15 ml of FBS with 35 ml of D-PBS. The maximum recommended shelf life of the resulting solution is 1 month at 4 °C.
NAC stock (5 mg ml−1)
Dissolve 50 mg of NAC in 10 ml of dH2O. Filter sterilize with a Millex-GP 0.22-μm, polyethersulfone, 33-mm, gamma-sterilized syringe filter. Store 500-μl aliquots for several months at −20 °C.
Forskolin stock (10 mM)
Dissolve 10 mg of forskolin in 2.4 ml of sterile DMSO. Store 250-μl aliquots for several months at −20 °C.
Myelin culture medium
OPC culture medium consists of DMEM containing 1× B27, 1× N2, 0.5× penicillin-streptomycin, 5 μg ml −1 of NAC and 5 μM of forskolin. The medium is prepared by mixing 48 ml of DMEM with 1 ml of B27, 500 μl of N2, 250 μl of penicillin-streptomycin, 50 μl of NAC and 25 μl of forskolin. The maximum recommended shelf life of the resulting solution is 1 month at 4 °C.
PDGF-AA stock (100 μg ml −1)
Dissolve 10 μg of PDGF-AA in 100 μl of 10 mM glacial acetic acid. Store 10-μl aliquots for several months at −20 °C. Working aliquots can be stored for 1 week at 4 °C. ▲ CRITICAL Avoid freezing and thawing aliquots.
Poly-L-lysine solution
By using 0.15 M sodium borate buffer (boric acid, target pH achieved with NaOH pellets) to achieve a pH of 8.4, make a 1 mg ml −1 stock of Poly-L-lysine. Do not attempt to filter this solution. In order to prepare the stock for use, dilute it to 0.1 mg ml −1 with sterile water, and then filter it through a 0.22-μm sterile filter. The maximum recommended shelf life of the resulting solution is 3 months at 4 °C.
Hybridoma culture medium
Hybridoma culture medium consists of DMEM containing 10% FBS and 1× penicillin-streptomycin. This medium is prepared by mixing 500 ml of DMEM with 56 ml of FBS and 5 ml of penicillin-streptomycin. Maximum recommended shelf life is 1 month at 4 °C.
Production of hybridoma supernatants containing monoclonal antibodies for immunopanning
Transfer two ~90% confluent cell culture dishes (100 mm × 20 mm) of Ran-2, GalC and O4 hybridomas into separate 225-cm2-vented flasks; culture them in 50 ml of hybridoma culture medium. Add 25 ml of hybridoma culture medium every other day until volume reaches 125 ml or when hybridoma cells begin to be wrinkled in appearance. When ~75% of the cells detach from the bottom of the flask, collect the hybridoma supernatant (containing monoclonal antibodies) by centrifuging at 2,500g using a tabletop microcentrifuge for 20 min at room temperature. Store hybridoma supernatants in 10-ml aliquots at −20 °C. These aliquots can be stored for several months at −20 °C.
Paraformaldehyde solution, 4% (wt/vol)
In order to prepare 40 ml of solution, mix 10 ml of 16% (wt/vol) paraformaldehyde aqueous solution with 30 ml of D-PBS. The maximum recommended shelf life for the resulting solution is 1 month at 4 °C for immunostaining. Freshly prepare before use for electron microscopy samples.
Triton X-100 solution, 10% (vol/vol)
In order to prepare 10 ml of solution, mix 1 ml of 100% (vol/vol) Triton X-100 with 9 ml of D-PBS. This solution can be stored for several months at room temperature.
Immunostaining blocking and permeabilization buffer
In order to prepare 10 ml of buffer, mix 2 ml of goat serum with 8 ml of D-PBS and 200 μl of 10% (vol/vol) Triton X-100 solution. The maximum recommended shelf life for the resulting solution is 1 month at 4 °C.
Immunostaining antibody buffer
To prepare 10 ml of buffer, mix 2 ml of goat serum with 8 ml of D-PBS. The maximum recommended shelf life for the resulting solution is 1 month at 4 °C.
Epon stock
To prepare 22.6 ml of stock, mix 10 ml of EMbed-812, 8 ml of DDSA, 4 ml of NSA and 0.6 ml of BDMA. Store 2-ml aliquots at −20 °C and thaw for >2 h at room temperature before use. These aliquots can be stored for several months at −20 °C.
PROCEDURE
Preparation of polystyrene solution ● TIMING ~1 h
-
1|
Dissolve 1.5 g of 250,000 MW (g per mol) polystyrene in 7 ml of DCM and mix until the polystyrene is completely dissolved.
-
2|
Add 2 ml of DMF and 1 ml of THF to the solution, resulting in a final concentration of 15% (wt/vol) polystyrene solution..
▲ CRITICAL STEP Polystyrene must be completely dissolved by DCM before the addition of DMF and THF. Adding THF and DMF to the solution before the polystyrene has been completely dissolved will markedly increase the amount of time it will take to completely dissolve all of the polystyrene.
-
3|
Load the solution into a 3-ml polypropylene syringe and cap it with a 23-gauge blunt-tip needle.
! CAUTION Perform all of the steps that follow in a fume hood. Please note that ideally, the process of electrospinning should take place inside a glove box to minimize air-flow disturbances that may interfere with the stability of the stream.
Electrospinning of nanofibers for myelination ● TIMING ~1 d
-
4|
Load the syringe containing the polystyrene solution onto the KDS 100 syringe pump set at a dispensing rate of 0.16 ml h−1.
-
5|
Poke the tip of the blunt-tip needle through a 10 cm × 10 cm sheet of aluminum foil and position the sheet 1 cm away from the tip.
-
6|
Attach an alligator clip from a high-voltage DC power supply to the base of the aluminum sheet.
-
7|
Position the syringe pump containing the syringe 25 cm away from the edge of the rotating collector.
-
8|
Tape the 12-mm round-glass coverslips to the edge of the disc collector using a double-sided conductive carbon tape (typically used for mounting samples for scanning electron microscopy (SEM)). Spacing between the coverslips should be at least roughly their length or diameter.
-
9|
Place a wire brush from a second power supply in contact with the side of the disc collector.
-
10|
Attach the axle of the disc collector to a high-torque stirring motor and set the rotating velocity of the disc collector to 10.64 m s−1 (800 r.p.m. for our disc collector).
-
11|
Set the syringe pump to 0.16 ml h−1.
-
12|
Set the high-voltage DC power supply attached to the syringe to 13 kV and set the second power supply contacting the disc collector to - 2 kV. The distance between the fibers and the density of fibers accumulating on the coverslips is dependent on the length of electrospinning; for fiber spacing of 10–15 μm, electrospin for 2 min under 30–40% relative humidity. After the desired density of fibers has covered the surface of the coverslips, turn off both power supplies, the wheel motor and the syringe pump.
! CAUTION This procedure involves the use of high voltage. Precautions should be taken to avoid electrocution.
▲ CRITICAL STEP Check the uniformity of fiber diameters by light microscopy after each electrospinning procedure. Adjustments may be made to the polymer solution or the electrospinning apparatus to ensure the uniformity of fiber diameters. If small beads are observed along the length of the fibers, increase the polymer concentration, as this approach will reduce the appearance of such beads. The polystyrene concentration should, however, not be increased above 17% (wt/vol) in order to maintain the desired fiber diameters of 2–4 μm. These diameters largely depend on the concentration of the polymer and the distance from the tip of the syringe to the edge of the disc. In our hands, the optimal polystyrene concentration and distance for generating fiber diameter of 2–4 μm are 15% and 25 cm, respectively. Fiber diameter, spacing and uniformity should be analyzed using SEM before OPC culture experiments. Please note that we made all adjustments to the procedure to manufacture the fibers on the basis of SEM images of the fibers we obtained.
▲ CRITICAL STEP Solution will begin to collect at the tip of the syringe needle and dry during the electrospinning process. Use a paper towel (or another nonconductive material) held at a safe distance as a swab to unclog the polymer from the needle tip.
! CAUTION This procedure involves the use of high voltage. Precautions should be taken to avoid electrocution..
? TROUBLESHOOTING
Preparation of the fiber-coverslip assembly, immunopanning plates and solutions ● TIMING ~1 d
-
13|
Place the 12-mm glass coverslips containing fibers onto 25-mm coverslips under a dissecting microscope.
-
14|
Apply silicone sealant on both edges of the 12-mm coverslips, including both ends of the fibers, and air-dry the assembly for at least 5 h.
! CAUTION It is important to maintain asepsis of fiber-OPC cultures. Therefore, perform the following steps in a laminar flow tissue culture hood to prevent any bacterial or fungal contamination during fiber preparation, brain dissection, OPC dissociation and immunopanning.
-
15|
Sterilize the fibers by placing the fiber-containing glass coverslips in 2 ml of 75% (vol/vol) ethanol for 2 min in the wells of a six-well plate.
-
16|
Wash the ethanol-treated coverslips with sterile water three times, and then air-dry them in the wells of a six-well plate until no visible liquid remains.
-
17|
To coat the fibers, drop 100 μl of poly(L-lysine) only onto an area of the 12-mm coverslips covered with fibers and incubate the coverslips for 1 h at room temperature; wash the coverslips by immersing them in 2 ml of water for 10 min within the six-well plate. Repeat this procedure three times for a total of 30 min. Air-dry the coverslips. Leave the fiber-containing coverslips in the wells of the six-well plate under the hood until the next day, making sure that the UV light is not on.
▲ CRITICAL STEP During preparation, make sure not to destroy or disrupt fiber alignments with the pipette tip.
? TROUBLESHOOTING
-
18|
Prepare two 150 mm × 25 mm culture dishes and one 100 mm × 20 mm culture dish by incubating them with goat anti-mouse IgG/M secondary antibodies (10 μg ml−1) diluted in 15 ml (per each plate) of 50 mM Tris-HCl (pH 9.5) overnight at room temperature. Use 150 mm × 25 mm dishes for Ran-2 and GalC hybridoma supernatant and 100 mm × 20 mm dishes for the OPC cell-surface antigen O4 hybridoma supernatant.
■ PAUSE POINT Culture dishes containing secondary antibodies are prepared the day before OPC dissociation and immunopanning and are stored in room temperature overnight in a tissue culture hood without UV light or laminar flow.
-
19|
Prepare 100 ml of 30% (vol/vol) FBS and 100 ml of 0.2% (wt/vol) BSA in 1× PBS.
-
20|
Prepare fresh papain buffer by adding 1 mM MgSO4, 0.36% (wt/vol) glucose and 2 mM EGTA in 10 ml of 1× EBSS (Reagent Setup).
Dissociating, immunopanning and seeding rat cortical OPCs on fibers ● TIMING ~8 d
-
21|
On the day of OPC dissociation and immunopanning, gently rinse the culture dishes with 1× D-PBS three times (5 min for each wash), for a total of 15 min, in order to remove the secondary antibody. Thaw out previously prepared frozen aliquots of each hybridoma supernatant for Ran-2 (1:5 dilution in 0.2% (wt/vol) BSA), GalC (1:5 dilution in 0.2% (wt/vol) BSA and O4 (1:5 dilution in 0.2% (wt/vol) BSA), and gently pour them onto the panning plates; as mentioned, the 150 mm × 25 mm culture dishes are used for Ran-2 and GalC hybridoma supernatant, whereas the 100 mm × 20 mm dish is used for the O4 hybridoma supernatant. Culture dishes containing hybridoma supernatants are kept in the hood at room temperature until they are ready to be used for panning.
▲ CRITICAL STEP O4 hybridoma supernatant can be used to isolate both rat and mouse OPCs. Although we are able to purify OPCs from mouse brain with high purity using the same Ran-2 hybridoma supernatant as negative selection and the O4 hybridoma supernatant as a positive selection, we do not have definitive proof that Ran-2 is effective for isolating mouse cells.
■ PAUSE POINT The culture dishes can be stored in this manner for 4–5 h.
-
22|
Prewarm the papain buffer in a 37 °C water bath.
-
23|
Under sterile conditions, remove the brains of two P7 rat pups or two P9 mouse pups and place them into a 60 mm × 15 mm cell culture dish containing 10 ml of 1× PBS (without Mg2+/Ca2+) at room temperature for further dissection.
! CAUTION Necessary approvals must be obtained; all animal procedures must be approved by an institutional animal care and use committee, and they must adhere to all relevant regulations.
-
24|
Under the dissection microscope, separate brains into two halves by cutting along the midline and remove the meninges and all noncortical tissues, including the hippocampus, brainstem and cerebellum, leaving only cerebral cortices. Transfer the cortices into a new 60 mm × 15 mm cell culture dish containing 10 ml of 1× PBS (without Mg2+/Ca2+) at room temperature, ensuring the removal of all cellular debris.
-
25|
Once dissection is complete, measure 200 units of papain and dissolve them alongside 2 mg of L-cysteine into the 10 ml of papain buffer for each preparation.
-
26|
Filter sterilize the solution from Step 25 through a 0.2-μm filter using a 35-ml syringe, and then add 2,500 U of DNase I to it.
-
27|
Remove all the 1× PBS (without Mg2+/Ca2+) and mince the cortices very finely using a sterilized surgical scalpel. Transfer the minced cortical tissues into a 50-ml conical tube containing 10 ml of papain buffer from Step 26 prewarmed to 37 °C; incubate the resulting mixture for 75 min in the 37 °C water bath, gently shaking it every 15 min.
▲ CRITICAL STEP During incubation, minced tissues tend to clump and sink to the bottom of the tube, rendering most of the tissues unexposed to the enzymatic activity of the papain. Gentle shaking minimizes tissue clumping and improves dissociation of tissue by papain. Poorly dissociated tissues require more forceful pipetting during the Steps 31 and 32. Forceful pipetting increases the incidence of cell death and lowers the yield of viable OPCs.
? TROUBLESHOOTING
-
28|
While tissue dissociation is proceeding, prepare 10 ml of Lo-ovo solution containing 1.5 mg ml−1 of ovomucoid protease inhibitor and 1.5 mg ml−1 of BSA by diluting 10× stock solution tenfold in 1× PBS and adding 2,500 U of DNase I. Also prepare 1× Hi-ovo solution containing 6 mg ml−1 ovomucoid protease inhibitor and 6 mg ml−1 BSA by diluting the 6× stock solution sixfold in 1× PBS.
-
29|
Allow the digested tissue to settle to the bottom of the tube, and then remove as much of the papain buffer as possible from the mixture (prepared in Step 27) without losing the brain tissue.
-
30|
Gently add to the container 2 ml of Lo-ovo from Step 29, mix thoroughly, let the tissue settle and then discard as much of the supernatant as possible.
-
31|
Add another 2 ml of Lo-ovo to the minced tissue, triturate with a 5-ml pipette gently (five or six times), allow the cells to settle, remove 1 ml of supernatant from the top of the suspension and transfer it to a 15-ml tube. Repeat this process one more time.
-
32|
Add 1 ml of Lo-ovo and triturate with a P-1000 pipette until a single-cell suspension is obtained. Add back the 2 ml of supernatant set aside in Step 31 to the original tube and spin at 300g for 15 min using a tabletop microcentrifuge.
-
33|
Remove the supernatant and resuspend the cell pellet in 6 ml of Hi-ovo, and then spin at 300g for 15 min using a tabletop microcentrifuge.
-
34|
Remove the supernatant and resuspend the cell pellet in 5 ml of 0.2% (wt/vol) BSA solution. Prewet a nylon mesh filter (40 μm) with 2 ml of 0.2% (wt/vol) BSA solution, and then use it to filter the cell suspension from Step 33 into a 50-ml conical tube. Rinse the filter with another 5 ml of 0.2% (wt/vol) BSA solution.
? TROUBLESHOOTING
-
35|
Rinse the panning plates gently three times with 10 ml of D-PBS (with Mg2+/Ca2+) for 15 min (5 min per wash) at room temperature and apply the cell suspensions from Step 34 to the washed panning plates.
-
36|
Sequentially incubate the cell suspensions in the Ran-2 (30 min), GalC (30 min) and O4 (45 min) culture dishes, gently shaking the mixtures every 15 min.
-
37|
To dissociate OPCs from the final O4 culture dishes, add 3 ml of prewarmed (37 °C) 0.05% (vol/vol) trypsin-EDTA and incubate the dishes for 1 min while monitoring cell dissociation from the plate under a light microscope; next, add 3 ml of pre-warmed (37 °C) 30% (vol/vol) FBS solution onto the dish to stop the enzymatic activity of trypsin. Transfer the supernatant from the dish to a 50-ml conical tube. Add another 5 ml of 30% (vol/vol) FBS to detach the OPCs from the dish by repeated but gentle pipetting with a 5-ml pipette. Repeat this process until all the cells have been detached from the plate.
? TROUBLESHOOTING
-
38|
Spin the supernatant at 300g for 20 min at room temperature using a tabletop microcentrifuge.
-
39|
Gently decant the supernatant without losing the cell pellet and resuspend the cells in myelin culture medium in the appropriate volume (normally 500 μl or 1 ml) to achieve a cell concentration of no less than 1.5 million cells per ml. Normally, the dissociation of four rat brain hemispheres yields 1.5–3 million OPCs. By adding a small volume of medium to initially dissociate the pellet, such as 500 μl, concentration of OPCs can be maintained at no less than 1–2 million cells per ml. Desired concentration can be achieved by further diluting the cells after having determined the concentration using a hemocytometer (see Step 40).
▲ CRITICAL STEP Do not dilute OPCs in a large volume of myelin culture medium, as OPCs should be seeded in a small volume (100 μl) onto the fibers to promote fiber contact.
? TROUBLESHOOTING
-
40|
Calculate OPC yield using a hemocytometer. Normally, two rat brains yield a total of 1.5–3 million OPCs and two mice brains yield 1–1.5 million OPCs.
-
41|
Wet the fibers by adding and removing 100 μl of myelin culture medium containing PDGF before seeding 150,000 OPCs in 100 μl of myelin culture medium. The coverslip assemblies containing the fibers and seeded OPCs in small volume of myelin culture medium are kept in the side wells of a six-well plate in the 37 °C incubator overnight.
▲ CRITICAL STEP Seeding density of OPCs largely depends on the type of experiments desired. The higher the initial seeding density, the sooner OPCs will differentiate into MBP-positive oligodendrocytes. Therefore, a lower seeding density should be used for experiments designed to study OPC proliferation or migration. In addition, homogeneity and purity of OPCs will greatly influence the timing of OPC proliferation and maturation in the culture.
-
42|
After having kept the coverslips containing fibers and OPCs in small volumes overnight to promote cell attachment to fibers (Step 17), flood the wells with 1.5 ml of myelin culture medium containing PDGF and change the medium every 3 d for the remainder of the culture period. PDGF is removed from the medium on the third day. The length of the culture period required for optimal fiber wrapping is 8 d.
? TROUBLESHOOTING
Fixing and immunostaining nanofiber cultures for fluorescence microscope imaging ● TIMING ~1 d
-
43|
Remove the myelin culture medium, rinse the nanofiber cultures gently three times with 1× D-PBS (with Mg2+/Ca2+) for 15 min (5 min per wash) by immersing and incubating cultures with 2 ml of 1× D-PBS for 5 min per wash, and then incubate the cultures with 4% (vol/vol) PFA for 15 min.
-
44|
Remove and rinse away any residual PFA by rinsing three times with 1× D-PBS, as detailed in Step 43.
-
45|
Incubate the fibers with 1.5 ml of a blocking solution containing 20% (vol/vol) goat serum and 0.2% (vol/vol) Triton X-100 in 1× D-PBS for 2 h at room temperature.
-
46|
Incubate the fibers for 2 h at room temperature or overnight at 4 °C with a solution containing the following three primary antibodies diluted in 20% goat serum in D-PBS: rabbit anti-PDGFRα antibody (1:1,000 dilution), rat anti-MBP antibody (1:200 dilution) and mouse anti-GFAP antibody (1:500 dilution).
-
47|
Wash the fibers three times with 1× D-PBS for 15 min by immersing and incubating cultures with 2 ml of 1× D-PBS for 5 min per wash, and then incubate the fibers for 1 h at room temperature with a solution containing the following secondary antibodies (1:1,000 dilution) in 20% goat serum: Alexa Fluor 488 goat anti-rabbit, Alexa Fluor 594 goat anti-rat IgG (H + L) and Alexa Fluor 647 goat anti-mouse IgG (H + L). Please note that a goat serum solution containing normal IgG antibodies from mouse, rat or rabbit can be used as negative controls, ensuring the specificity of the secondary antibodies being used.
-
48|
Rinse the fibers with D-PBS three times for 10 min by immersing and incubating cultures with 2 ml of 1× D-PBS and rinse once with water for 5 min. Blot dry to remove any excess liquid, then carefully cut the edges of the fibers containing sealant using a razor blade to remove the silicone sealant from the fibers.
▲ CRITICAL STEP Perform this operation under a dissecting microscope; take care not to disrupt the integrity of the fibers or break the coverslip.
▲ CRITICAL STEP Complete removal of the sealant ensures an even field of view for optimal imaging.
? TROUBLESHOOTING
-
49|
Mount the coverslip on a glass microscope slide using a small volume of mounting medium.
Preparation of nanofiber cultures for electron microscopy ● TIMING ~3 d
-
50|
Remove the cell culture medium and rinse nanofiber cultures gently with 1× D-PBS (with Mg2+/Ca2+) three times.
-
51|
Fix the fibers with 4% (wt/vol) PFA for 20 min. Please note that 2–5% (vol/vol) glutaraldehyde could be an alternative to PFA and may improve fiber fixation for electron microscopy analysis.
-
52|
Stain the fibers with 1% (wt/vol) OsO4 for 1 h at 4 °C and counterstain them with 1% (wt/vol) UO2(OCOCH3)2·2H2O overnight at 4 °C.
-
53|
Rinse the fibers with dH2O and then dehydrate them via a series of ethanol dehydration treatments (with 50, 70, 95 and 100% ethanol—vol/vol in water).
-
54|
Embed the fibers in a 1:1 mixture of EMBed-812 resin and HPMA for 1 h at room temperature.
▲ CRITICAL STEP The use of HPMA, a water-soluble resin, is important to prevent polystyrene fibers from dissolving during the embedding process.
-
55|
Continue embedding the fibers in a 2:1 EMBed-812 resin:HPMA mix overnight at room temperature, followed by 100% EMBed-812 resin for 3 h at room temperature.
-
56|
Place the fibers in fresh EMBed-812 resin, remove the fibers from the coverslip, carefully transfer the fibers into the silicone embedding mold and cure overnight at 65 °C.
-
57|
Obtain ultrathin sections (70 nm) of the fibers for visualization via electron microscopy.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 1.
TABLE 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 12 | The electrospinning polymer stream ceases | Polymer is collecting and drying at the tip of the needle | Use a paper towel or swab to wipe the collecting polymer away from the tip |
| The stream begins to show unusual behavior, i.e., spitting and waving uncontrollably | There is either too much or too little static charge at the tip of the needle | Adjust the voltage knob on the power supply, contacting the needle until a consistent stream is visible. Also, make sure that the aluminum sheet is making contact with the needle and that the wire brush is making contact with the rotating collector. If the problem persists, adjust the tip-to-collector distance from 25 cm | |
| The tip of the syringe dispenses the polymer in ‘pulses’ or ‘globs’ of large droplets at the collector, instead of in a steady, smooth stream | The solution is too viscous, the feed rate is too low, the setting for the rate of the syringe pump is too high, or there is a bad connection between the power supply and the syringe | Set the syringe pump at a lower feed rate or adjust the diameter settings on the syringe pump to match that of the syringe | |
| 17 | Fibers are too hydrophobic and are difficult to coat with poly-L-lysine | There are too many fibers on the coverslip | Ensure that during fiber deposition onto the glass coverslips, an interfiber distance of 10–15 μm is maintained |
| 27 | Tissue appears sticky and does not disperse when shaken | Dissection took too long | Dissection should take <1.5 h |
| No DNase I was added | Make sure to add DNase I at Step 26 of the PROCEDURE | ||
| 34 | Cell pellet is very small | Cells were accidentally removed during the trituration and treatment with Lo-ovo and Hi-ovo solution (Steps 31–33) | Watch the pipette tip during supernatant removal, and ensure that cellular matter is not removed as well |
| Too much cellular debris is generated during trituration | Pipette up and down gently during trituration | ||
| 37 | Very few OPCs are attached to the O4 plate | Cells are lost during transfer between immunopanning plates | Ensure that all liquid is transferred between immunopanning plates |
| 39 | Low OPC yield | Cell pellet may have been lost during supernatant removal at Steps 34 and 39 | Carefully decant supernatant next time |
| 42 | Slow OPC differentiation | The number of viable OPCs in the culture might be low resulting in OPC proliferation rather than differentiation | Gentle manipulation of culture dishes and cell dissociation techniques need to be employed to ensure viability of purified OPCs (one has to perform another OPC preparation applying gentle techniques, as no parts of the steps can be repeated within the same preparation) |
| 48 | OPCs are found in the space between fibers rather than interacting with fibers | Evaporation after fiber fabrication occurs too quickly, creating fibers that are nonporous | Ensure a higher ambient humidity during electrospinning |
| There are too few fibers on the coverslip | Ensure that fibers fill >50% of the coverslip surface area with a distance of 10–15 μm between fibers | ||
| It is difficult to visualize distinct myelin-like segments because of fiber density | There are too many fibers on the glass coverslip | Ensure 10–15 μm of distance between fibers during fiber deposition onto the coverslip |
● TIMING
Steps 1–3, preparation of polystyrene solution: ~1 h
Steps 4–12, electrospinning of fibers: ~1 d
Steps 13–20, preparation of coverslips with fibers, immunopanning plates and solutions: ~1 d
Steps 21–42, dissociating, immunopanning, and seeding and culturing of OPCs onto fibers: ~8 d
Steps 43–49, fixing and immunostaining nanofibers: ~1 d
Steps 50–57, preparation of nanofibers for electron microscopy: ~3 d
ANTICIPATED RESULTS
The time required to complete the entire protocol (Fig. 1a), from fiber fabrication (Fig. 1b) and validation (Fig. 1b–e) to the analysis of myelin-like segments on the fibers, is reported in Figure 1. Whether an OPC-fiber culture experiment is successful depends largely on the quality of the fibers, and that correct dimensions required for oligodendrocyte membrane wrapping are attained. The key parameters include optimum fiber diameter, spacing and uniformity, which need to be verified by both light microscopy and SEM (Fig. 1c–e).
When initially plated, OPCs appear as small spheres or bipolar cells with short processes scattered along the fibers or in between the fibers (Fig. 2). As time progresses, OPCs associate with more fibers by extending their membrane processes to ensheathe the fibers, while continuing to proliferate and migrate7. OPCs proliferate and migrate in close association with the fibers (in the presence of exogenous PDGF in the medium for 2 d), which can be observed by time-lapse imaging12 and immunostaining (Fig. 2c). Under optimal culture conditions, most of the OPCs prefer associating with fibers rather than with non-fiber-covered areas, and they maintain their contact with fibers. At 3 d in vitro (DIV), PDGFRα-positive OPCs proliferate, migrate and occupy space evenly along the fibers while making thin or tube-like ensheathments. Mature oligodendrocytes then start to produce tube-like segments that are positive for MBP (Fig. 2c).
Figure 2.
Temporal and spatial distribution of OPCs cultured on fibers: proliferation, migration, differentiation and formation of myelin-like segments. (a) Phase image of the fibers seeded with rat cortical OPCs in 100 μl of culture medium containing PDGF, promoting association with the fibers. (b) Immunostaining of OPC-fiber culture reveals that at day 1, OPCs (green, positive for PDGFRα) are spatially organized in culture either ensheathing or contacting several fibers with their processes. They maintain close association with the fibers during proliferation and migration. At this early stage of the culture, very few contaminating GFAP-positive astrocytes are present. (c) At day 3, OPCs start to differentiate into oligodendrocytes and can be identified by MBP. A small number of GFAP-positive astrocytes can be identified at this stage (white arrows). (d) At day 5, more mature MBP-positive oligodendrocytes start to appear among OPCs that are spatially organized around the fibers. (e,f) At day 8, the majority of cells are MBP-positive oligodendrocytes that form longer and mature myelin-like segments shown at low magnification (e) and at high magnification (f). (g) Electron micrographs of the fibers ensheathed by OPCs. (h) A high-magnification image of a cross-section illustrating an oligodendrocyte wrapping multiple layers of membrane around a large-diameter fiber. Arrows indicate dark electron-dense lines.
Fibers ensheathed by OPC membranes can also be seen by electron micrographs of the cultures at this time (Fig. 2g). At later stages, more cells become MBP-positive and produce multiple myelin-like segments (Fig. 2f) that generate multiple, concentric wraps of membrane, which appear as dark, electron-dense lines indicative of partial compaction, as shown in Figure 2h. Full compaction is rarely observed with nanofibers and the membranes are poorly organized.
We have also analyzed the presence of contaminating cells, such as GFAP-positive astrocytes, in OPC-fiber cultures by im-munostaining (Fig. 2b–f). Upon purification, our OPC cultures are >95% pure. The only cell contamination can be attributed to astrocytes that arise from our OPCs as they are maintained in the culture. At 1 DIV, it is difficult to find any contaminating astrocyte present in a particular field under microscopy (Fig. 2b). As OPCs mature in culture starting at 3 DIV, a small number of GFAP-positive astrocytes with long fibrous membrane processes (indicated by white arrows in Fig. 2c–e) can be identified. Cultures can be maintained for 8–10 DIV.
Acknowledgments
We thank all the members of the Chan laboratory and the Multiple Sclerosis Research Group at the University of California, San Francisco, for encouragement, advice and insightful discussion. This work was supported by the US National Multiple Sclerosis Society Career Transition Award (TA 3008A2/T), the Harry Weaver Neuroscience Scholar Award (JF 2142-A2/T) and a grant from the US National Institute of Health/National Institute of Neurological Disorders and Stroke (NS062796-02) to J.R.C.
Footnotes
AUTHOR CONTRIBUTIONS S.L. performed the experiments and analyzed the data. S.J.T. fabricated the fibers. S.L., S.Y.C.C., J.R.C. and J.M.C. provided intellectual contributions and wrote the paper.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Colello RJ, Pott U. Signals that initiate myelination in the developing mammalian nervous system. Mol Neurobiol. 1997;15:83–100. doi: 10.1007/BF02740617. [DOI] [PubMed] [Google Scholar]
- 2.Ravikumar M, Jain S, Miller RH, Capadona JR, Selkirk SM. An organotypic spinal cord slice culture model to quantify neurodegeneration. J Neurosci Methods. 2012;211:280–288. doi: 10.1016/j.jneumeth.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Lewis R, Miller RH. Interactions between oligodendrocyte precursors control the onset of CNS myelination. Dev Biol. 2011;350:127–138. doi: 10.1016/j.ydbio.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubetzki C, et al. Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci USA. 1993;90:6820–6824. doi: 10.1073/pnas.90.14.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash B, et al. Functional duality of astrocytes in myelination. J Neurosci. 2011;31:13028–13038. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson CE, et al. Myelinated, synapsing cultures of murine spinal cord—validation as an in vitro model of the central nervous system. Eur J Neurosci. 2008;28:1518–1535. doi: 10.1111/j.1460-9568.2008.06415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JR, et al. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by γ-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J Biol Chem. 2011;286:25835–25847. doi: 10.1074/jbc.M111.219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner A, Jukkola P, Gu C. Myelination of rodent hippocampal neurons in culture. Nat Protoc. 2012;7:1774–1782. doi: 10.1038/nprot.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989;342:430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci USA. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jagielska A, et al. Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cells Dev. 2012;21:2905–2914. doi: 10.1089/scd.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colognato H, et al. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- 16.Camara J, et al. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J Cell Biol. 2009;185:699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- 18.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 20.Velanac V, et al. Bace1 processing of NRG1 type III produces a myelin-inducing signal but is not essential for the stimulation of myelination. Glia. 2012;60:203–217. doi: 10.1002/glia.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer I, Schachner M. Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]