Abstract
Purpose
The epidermal growth factor receptor inhibitor gefitinib (Iressa®) is currently being studied in patients with bladder cancer and it has significant anti-angiogenic activity. We investigated the relationship between the modulation of vascular endothelial growth factor (Santa Cruz Biotechnology, Santa Cruz, California) expression and the biological efficacy of gefitinib for bladder cancer.
Materials and Methods
In vitro the 4 bladder cancer cell lines 253JB-V, UMUC-3, KU-7 and UMUC-13 were treated with gefitinib and vascular endothelial growth factor secretion was measured. The effects of gefitinib on vascular endothelial growth factor promoter, proliferation, cell cycle and downstream signals were evaluated. In vivo 253JB-V and UMUC-13 were injected into nude mice and tumors were treated with 2 mg gefitinib per day. Tumor kinetics were determined and the levels of phospho-epidermal growth factor receptor (Biosource™), vascular endothelial growth factor, phospho-vascular endothelial growth factor (Cell Signaling Technology®), angiogenesis and apoptosis were measured.
Results
Epidermal growth factor receptor (Neomarkers, Fremont, California) phosphorylation was blocked efficiently in all cell lines at concentrations of 0.5 µM or greater. Gefitinib (1 µM) induced an accumulation of cells in G0/G1 without apoptosis in 253J B-V cells, whereas it had no effect in other cell lines. Gefitinib inhibited vascular endothelial growth factor secretion in 253JB-V and UMUC-13 (concentration inhibiting a 50% response 0.5 and 0.1 µM, respectively) but not in UMUC-3 or KU-7. Gefitinib decreased vascular endothelial growth factor promoter activity in 253JB-V and UMUC-13 by 40% to 60%. In vivo the growth of 253JB-V tumors was significantly inhibited by gefitinib, whereas no effect was demonstrated in UMUC-13 tumors. Vascular endothelial growth factor expression and vascular endothelial growth factor receptor activation were significantly decreased in 253JB-V tumors and to a greater extent in resistant UMUC-13 tumors. Gefitinib inhibited angiogenesis and induced apoptosis in sensitive 253JB-V tumors only.
Conclusions
Epidermal growth factor receptor blockade exerts an anti-angiogenic effect on bladder cancer cells, in part by modulating vascular endothelial growth factor expression. However, down-regulation of vascular endothelial growth factor expression is not sufficient to inhibit bladder cancer growth and it should not be used as a predictor of the therapeutic efficacy of gefitinib.
Keywords: urinary bladder, carcinoma, transitional cell, gefitinib, vascular endothelial growth factor, angiogenesis inhibitors
Vascular endothelial growth factor is one of the most potent inducers of angiogenesis and it has been extensively studied. Higher levels of VEGF in tumor tissue predict an increased risk of bladder transitional cell carcinoma progression and increased urinary levels are a marker of an increased risk of recurrence in patients with superficial lesions.1 In patients with locally advanced urothelial cancer undergoing cystectomy and chemotherapy VEGF over expression in prechemotherapy samples was found to be a strong predictor of recurrence and death from bladder cancer.2 These observations suggest that VEGF expression is mechanistically relevant to clinically aggressive bladder cancer, providing a rationale for the clinical investigation of interventions capable of blocking VEGF signaling.
Concurrently numerous studies have suggested that high levels of EGFR expression in tumors is associated with advanced bladder cancer, the development of a metastatic phenotype and poor prognosis.3 For these reasons the inhibition of EGFR function is attractive among the wide array of biological targets implicated in bladder cancer progression. Although the mechanism by which EGFR regulates tumor biology in bladder cancer is not clearly defined, it has been demonstrated that EGFR signaling regulates cell survival, proliferation, differentiation and invasion.4 Moreover, in in vivo bladder cancer models EGFR is implicated in the induction of tumor induced angiogenesis and metastasis.5 EGFR inhibitors have demonstrated significant antitumor activity for bladder cancer, in part due to their anti-angiogenic effect.6,7
However, clinical trials of EGFR inhibitors in head and neck, lung and colon cancer failed to live up to the expectations generated by laboratory results, in that only a minority of patients seemed to benefit from this approach. In the context of nonsmall cell lung cancer studies have shown that the clinical responses were linked to activating mutations in the EGFR tyrosine kinase domain, suggesting that a better understanding of the biological effects of EGFR inhibitors on tumor cells would help identify tumors that would respond to therapy.8,9 Identifying molecular markers of response to help select patients for such therapy is crucial.
Since VEGF is tightly coupled with bladder cancer progression and implicated in the EGFR signaling axis,6 we evaluated whether VEGF modulation could predict the therapeutic efficacy of EGFR inhibitors and help select patients for such therapy. We hypothesized that, although VEGF has a key role in tumor angiogenesis and growth, its modulation alone may not be a reliable predictor of the biological efficacy of gefitinib for bladder cancer. In this study we used the orally active EGFR-tyrosine kinase inhibitor gefitinib and noted that in in vitro and in vivo models VEGF modulation is indeed a poor predictor of the biological efficacy of EGFR inhibitors for bladder cancer.
MATERIALS AND METHODS
Tumor Cell Lines
The metastatic human transitional cell carcinoma cell lines 253JB-V, UMUC-3, UMUC-13 and KU-7 were maintained as a monolayer in modified Eagle’s minimum essential medium supplemented with 10% fetal bovine serum, vitamins, sodium pyruvate, L-glutamine, penicillin, streptomycin and nonessential amino acids.
Reagents
For in vitro studies gefitinib was reconstituted in dimethyl sulfoxide at a stock concentration of 10 mM and stored at −20C until use. The stock was diluted in medium just before use so that the concentration of dimethyl sulfoxide never exceeded 0.1%. For in vivo studies the powder was dissolved in PBS (pH 5). The antibodies used for immunoblotting were EGFR, p-EGFR, Akt, p-Akt, (Cell Signaling Technology) and VEGF.
Western Blot Analysis
EGF stimulated cells were treated with gefitinib for 1 hour and harvested at approximately 75% to 80% confluence. Western blot analysis was performed using standard methodology, as previously described.10
Cell Proliferation Assay
Cells (5 × 103) were plated in 96-well plates for 24 to 48 hours and then treated with increasing concentrations of gefitinib for 48 hours. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed, as previously described.10 Each experimental data point represents the average of 4 replicates and each experiment was performed at least twice.
Quantification of DNA Fragmentation and Flow Cytometry
The assay was performed as previously described.10 Each experiment was performed at least twice.
Quantification of VEGF Protein
Cells were plated in 96-well plates and 5% modified Eagle’s medium. After 48 hours the cells were treated with increasing dose of gefitinib or inhibitors of the ERK, Jnk, Akt/PI3 kinase, P38 and src pathways for 48 hours. Quantification of secreted VEGF was done using an ELISA kit (Assay Designs, Ann Arbor, Michigan) according to manufacturer instructions. VEGF concentrations were indexed to the total protein concentration of the cell lysate.
Luciferase Assay
All cells lines (253JB-V, KU-7, UMUC-3 and UMUC-13) were grown in 24-well plates to 60% confluence. Cells were transfected transiently using FuGene® reagent with 0.6 µg of a luciferase expression construct containing the VEGF promoter as well as 20 ng β-actin Renilla to assess transfection efficiency. At 24 hours after transfection cells were treated with 2 µM gefitinib overnight. Luciferase activity in cell extracts was measured 36 hours after transfection by chemiluminescence using a dual luciferase reporter assay system (Promega®) in a Luminoskan Ascent® microplate luminometer, as outlined in the manufacturer protocols. Luciferase units were adjusted to the protein concentration of the cell lysates.
Animal Experiments
Male athymic BALB/c nude mice (Animal Production Area, National Cancer Institute, Frederick Cancer Research Facility, Frederick, Maryland) 4 to 6 weeks old were housed in laminar flow cabinets under specific pathogen-free conditions. Animals were maintained in facilities approved by the American Association for Accreditation of Laboratory Animal Care, and in accordance with the current regulations and standards of the United States Department of Agriculture, Department of Health and Human Services, and National Institutes of Health. Mice were acclimatized for 2 weeks and their use in these experiments was approved by the institutional animal care and use committee. Mice were injected in the subcutis (106 cancer cells per injection) with the 4 bladder carcinoma cell lines 253JB-V, UMUC-3, KU-7 and UMUC-13, which were suspended in 200 µl Matrigel™. Two groups of animals were assigned to each cell line, including a treatment arm and a placebo arm. After 1 to 2 weeks when tumors had reached 4 to 5 mm in diameter 6 mice (total of 12 tumors) per group were treated intraperitoneally on days 1 to 5 of each week with 2 mg gefitinib per dose per mouse in the treatment arm and an equal number of mice were treated with placebo (Hanks solution) in the control arm for a total of 3 weeks. Tumor volume was measured and tumor kinetics were established in the various groups. VEGF expression was then evaluated in tumor sections using immunohistochemical techniques.
Immunohistochemistry
Frozen tissue sections (8 µm) were fixed with cold acetone, chloroform/acetone and acetone. Tissue sections (5 µm) of formalin fixed, paraffin embedded specimens were deparaffinized and antigen retrieval was performed with pepsin. Endogenous peroxidase was blocked with 3% hydrogen peroxide. Samples were washed with PBS and incubated with a protein blocking solution containing 5% normal horse serum and 1% normal goat serum. Samples were then incubated with a 1:200 dilution of mouse monoclonal anti-VEGF antibody (Santa Cruz Biotechnology), a 1:100 dilution of mouse monoclonal anti-CD31 antibody (BD Pharmingen™), 1:100 dilution of p-EGFR antibody or a 1:400 dilution of p-VEGFR-1/VEGFR-2 (Oncogene Science, Cambridge, Massachusetts). Samples were rinsed with PBS and incubated with the appropriate dilution of secondary antimouse IgG (Jackson ImmunoResearch Laboratory, West Grove, Pennsylvania). Slides were rinsed with PBS and incubated with diaminobenzidine (Research Genetics, Huntsville, Alabama). Sections were then washed with PBS and counterstained with Gill’s hematoxylin (BioGenex Laboratories, San Ramon, California).
TUNEL Assay
Frozen tissue sections fixed and treated as described were washed with PBS containing 0.1% Brij (volume per volume). TUNEL was performed using a commercial kit (Promega) according to manufacturer instructions. Immunofluorescence microscopy was performed using a Plan-Neofluar® lens on an epifluorescence microscope equipped with narrow band pass excitation filters mounted in a filter wheel (Ludl Electronic Products, Hawthorne, New York) to individually select for green, red and blue fluorescence. Images were captured using a cooled charge coupled device camera (Photometrics™). DNA fragmentation was detected by localized green fluorescence in the nucleus of apoptotic cells.
Quantification of VEGF, Angiogenesis and Apoptosis in In Vivo Tissue Samples
Microvessel density and VEGF expression were determined by immunohistochemistry of tissue sections with anti-CD31 and anti-VEGF antibodies, whereas apoptosis was determined by TUNEL assay. Tissue was photographed using a cooled, charged coupled device Tec 470 camera (Optotronics Engineering, Goleta, California) linked to a computer and digital printer. The number of blood vessels and apoptotic cells were quantified in 5 areas per sample by an image analyzer using Optimas image analysis software (Media Cybernetics®) to obtain an average measurement. The density of blood vessels and apoptotic cells is expressed as the average of the 5 highest areas identified in a single 200× field. Immunostaining intensity was quantified in 5 areas per sample and the quantity of VEGF protein is expressed as the average of the 5 highest areas identified in a single 200× field.
Statistical Analysis
Statistical analysis was performed using SPSS® and Instat ® 3 software. For in vitro data ANOVA was performed and the t test with the Bonferroni correction were used to evaluate significant differences between treated cells at each drug concentration and untreated control cells. Tumor weight and the expression intensity of VEGF, CD31 and TUNEL counts were compared by the unpaired Student t test. For the primary end point of tumor size a sample size of 10 mice per treatment group was expected to have greater than 90% power to detect a minimum difference of 24 mm3 in tumor size at a statistical significance level of 0.05%. Statistical significance for this study was considered at 2-sided p <0.05.
RESULTS
In Vitro
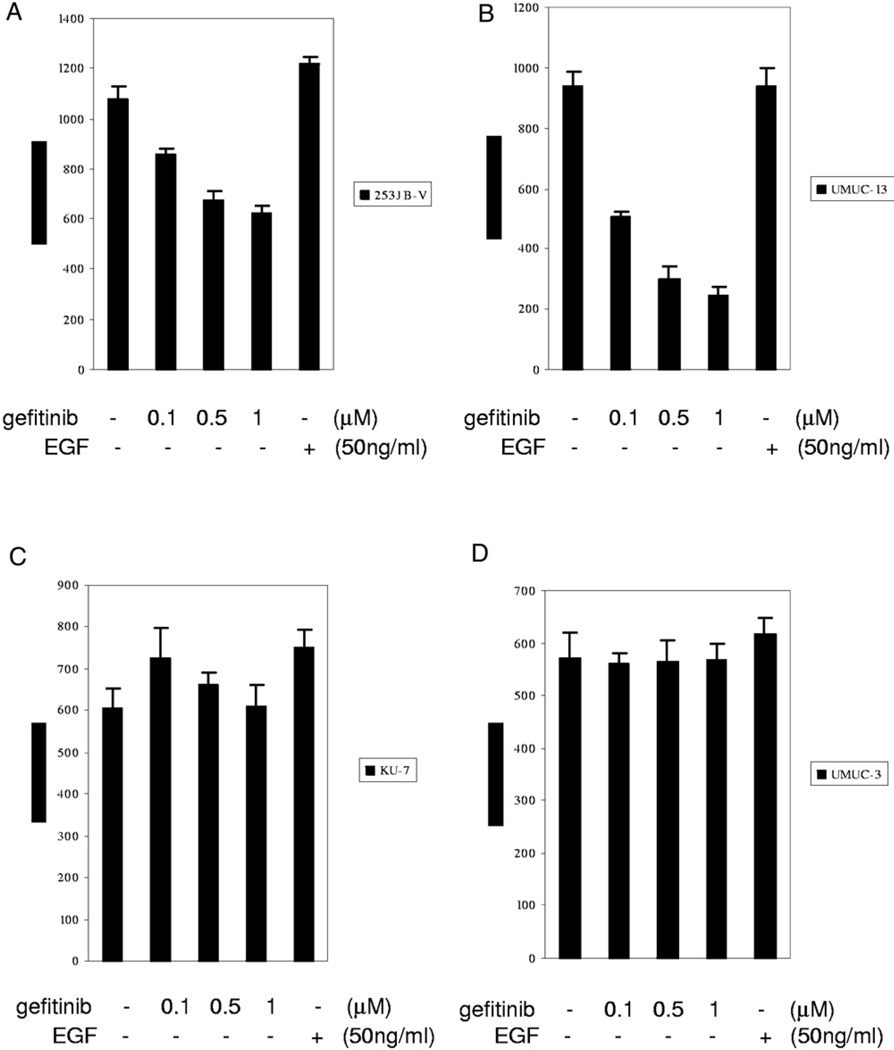
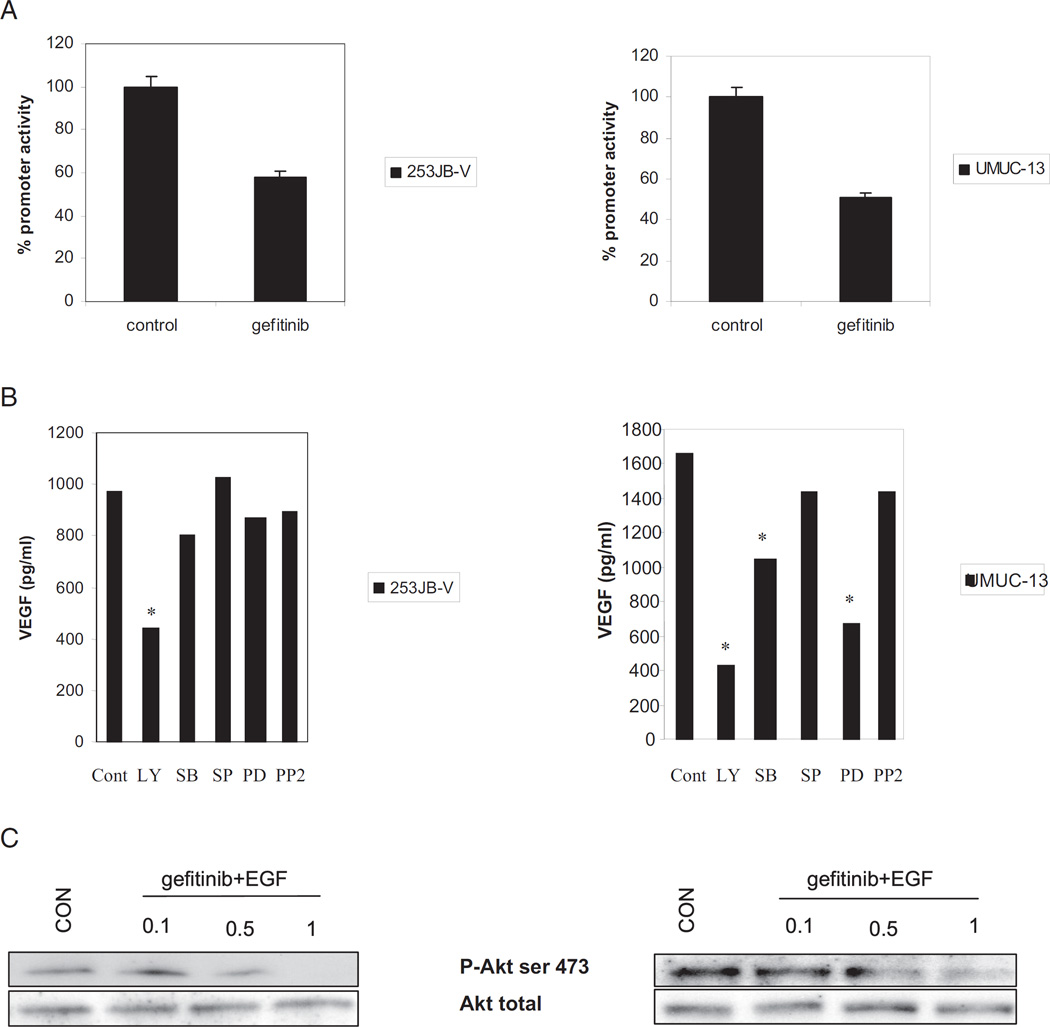
Since VEGF has been shown to be a potent angiogenic factor and it correlates with aggressive tumor biology, we investigated whether VEGF modulation could predict the biological efficacy of gefitinib for human bladder cancer. VEGF quantification by ELISA was performed in the 4 highly malignant bladder cancer cell lines 253JB-V, UMUC-3, KU-7 and UMUC-13, which were treated with gefitinib. Gefitinib significantly decreased VEGF production in 253JB-V and UMUC-13 cells but not in KU-7 or UMUC-3 cells (fig. 1).
Fig. 1.
ELISA for VEGF in duplicate after gefitinib treatment for 48 hours. A, VEGF dose dependent inhibition by gefitinib in 253JB-V cells. B, VEGF dose dependent inhibition by gefitinib in UMUC-13 cells. C, no dose dependent VEGF inhibition by gefitinib in KU-7 cells. D, no dose dependent VEGF inhibition by gefitinib in UMUC-3 cells.
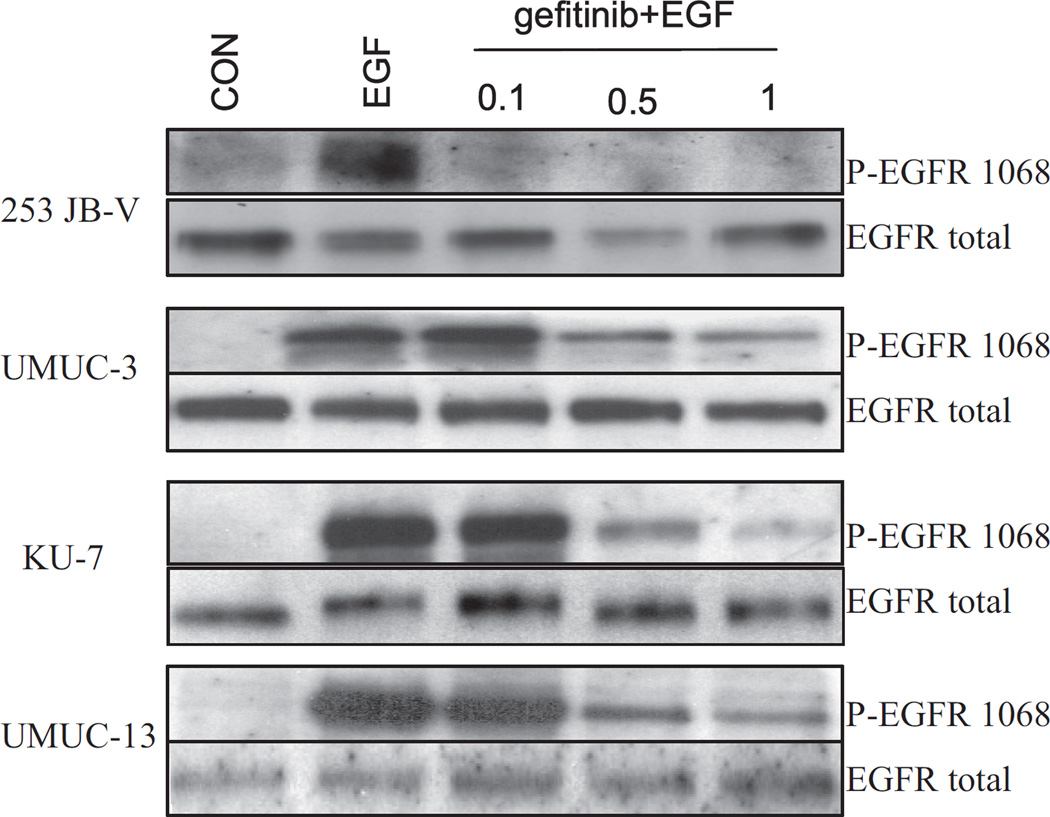
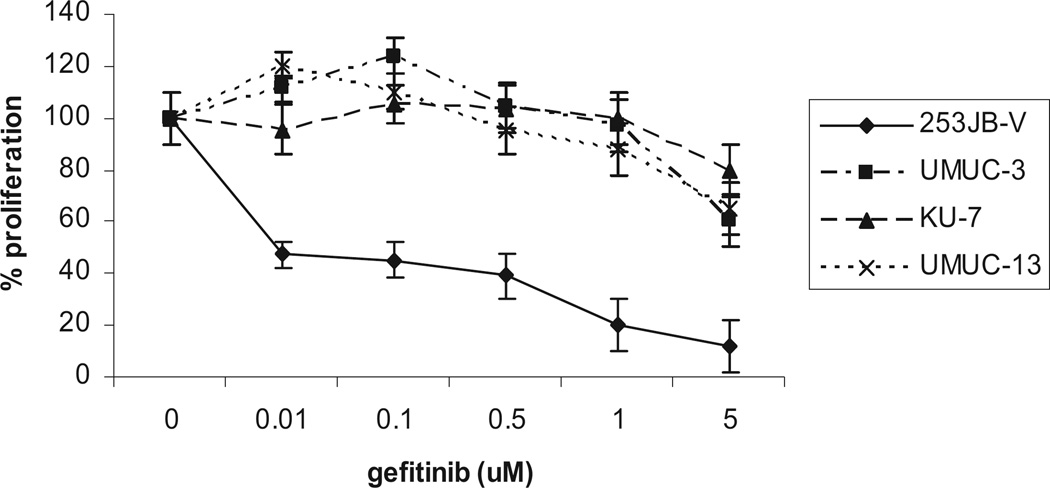
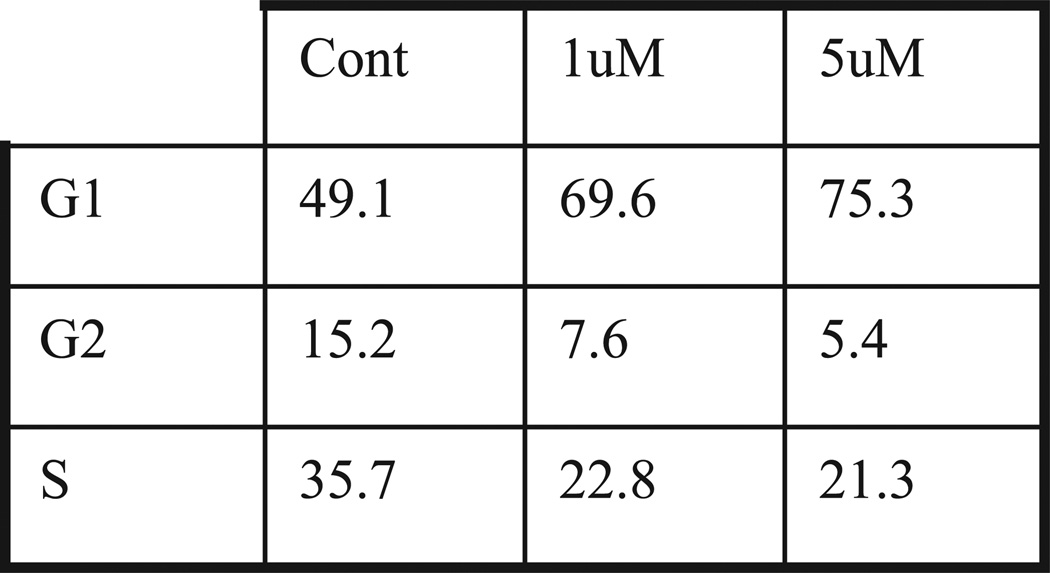
To establish sensitivity to the cytostatic effect of gefitinib we first noted that gefitinib markedly inhibited its target, EGFR phosphorylation, in a dose dependent manner in all 4 cell lines (fig. 2). We then tested the antiproliferative effects of gefitinib in bladder cancer cells. Cells were plated and treated with gefitinib at concentrations of 0.1 to 5 µM for 24 hours. Of the 4 cell lines 253JB-V was the only cell line that was sensitive to the antiproliferative effects of gefitinib (concentration inhibiting a 50% response 0.5 µM), whereas KU-7, UMUC-3 and UMUC-13 were resistant to gefitinib (up to 5 µM) (fig. 3). Gefitinib (1 µM) induced a 31% increase in cell cycle arrest in the G1 phase in 253 JB-V cells (fig. 4), while there was no increase in G1 arrest in the other cell lines. Notably gefitinib did not induce apoptosis (an absence of subG0/G1 cells) in any of the 4 cell lines.
Fig. 2.
Western blot analysis of EGF stimulated human bladder cancer cells treated with various doses of gefitinib for 1 hour.
Fig. 3.
In vitro cell proliferation assay using 3H-thymidine in human bladder cancer cell lines treated with gefitinib for 24 hours
Fig. 4.
Fluorescence based cell cycle sort analysis using propidium iodine staining of bladder cancer cell lines treated with gefitinib for 24 hours shows that 1 µM gefitinib induced 42% increase in cell cycle arrest in G1 phase in 253 JB-V cells but no increase in other cell lines. There was absent subG0/G1 cells in presence of gefitinib in all cell lines (data not shown). Results represent 3 experiments performed in duplicate dishes.
Interestingly VEGF production was markedly decreased by gefitinib in sensitive 253JB-V cells and in resistant UMUC-13 cells. The effect of gefitinib on VEGF production in these cells was regulated through mRNA transcriptional activity. Gefitinib significantly decreased VEGF promoter activity by 40% to 60% in 253JB-V and UMUC-13 cells (fig. 5, A). To investigate the mechanism of VEGF regulation by gefitinib we treated the cells with various inhibitors of the ERK, Jnk, Akt/PI3 kinase, P38 and src pathways, and performed ELISA to quantitate VEGF secretion. We found that inhibition of the Akt/PI3Kinase pathways resulted in the most potent inhibition of VEGF expression in 253JB-V and UMUC-13 cells (fig. 5, B). Inhibition of the ERK pathway inhibited VEGF synthesis to a lesser extent in UMUC-13 cells but not in 253JB-V cells. We noted that gefitinib markedly inhibited Akt phosphorylation in 253JB-V and UMUC-13 cells (fig. 5, C), which is known to regulate VEGF.
Fig. 5.
VEGF regulation by gefitinib. A, after gefitinib treatment for 24 hours promoter assay using luciferase expression construct containing VEGF promoter was performed. B, ELISA for VEGF after cell treatment with inhibitors of ERK, P38-mitogen-activated protein kinase, Jnk, GSK, src and Akt/PI3 kinase pathways for 48 hours. C, Western blot analysis shows significant inhibition of Akt phosphorylation by gefitinib in each cell line. Asterisk indicates p <0.05.
In Vivo
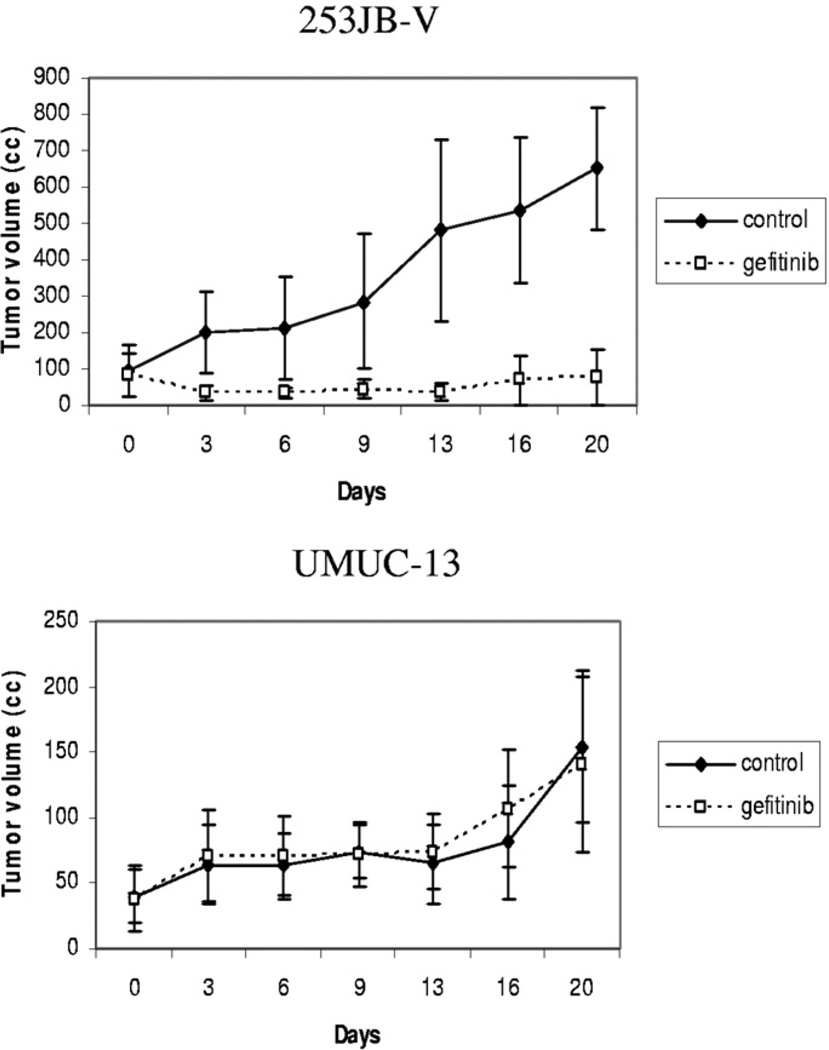
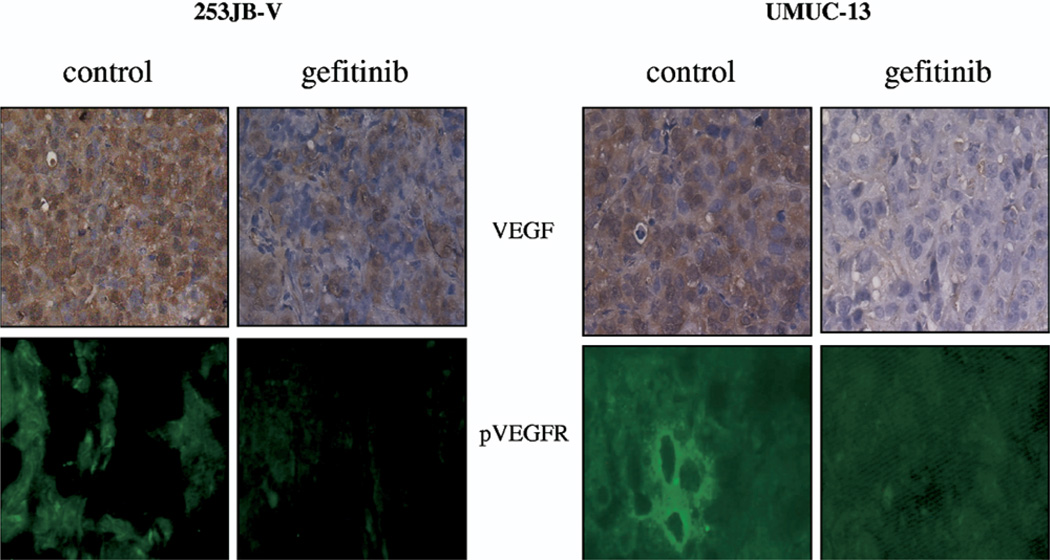
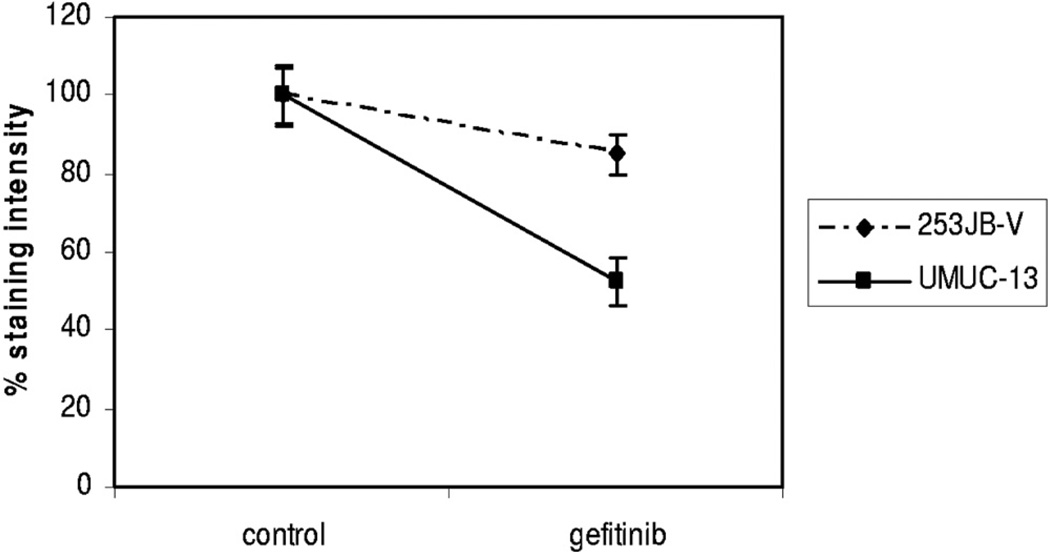
Each cell line was implanted in the subcutis of male nude mice and treated with gefitinib for 3 weeks to determine whether the in vivo effect of gefitinib correlated with its effect on VEGF down-regulation or whether it was related to the antiproliferative effects of this agent. Tumor kinetics were significantly inhibited by gefitinib in 253JB-V tumors but not in UMUC-13 tumors despite significant inhibition of its target, as measured by EGFR phosphorylation in tumor samples (figs. 6, A and 7, A). However, VEGF expression was inhibited in the 2 tumors and to a greater extent in resistant UMUC-13 tumors compared to sensitive 253JB-V preparations (figs. 8 and 9). The decreased VEGF production translated into inhibition of the activation of VEGFR-1/VEGFR-2, as measured by receptor phosphorylation (figs. 8 and 9).
Fig. 6.
Growth kinetics of subcutaneous tumors injected in athymic male nude mice and treated with intraperitoneal injection of 4 mg gefitinib 5 times weekly. Gefitinib induced marked growth inhibition in 253JB-V tumors but not in UMUC-13 tumors.
Fig. 7.
EGFR phosphorylation reveals that gefitinib inhibited its target. A, 253JB-V cell line. B, UMUC-13 cell line. Reduced from ×400
Fig. 8.
Immunohistochemical analysis of VEGF expression and VEGF receptor phosphorylation in subcutaneous 253JB-V and UMUC-13 tumors treated with gefitinib. Reduced from ×400.
Fig. 9.
Inhibition measured by VEGF staining intensity in 253JB-V and UMUC-13 tumors treated with gefitinib
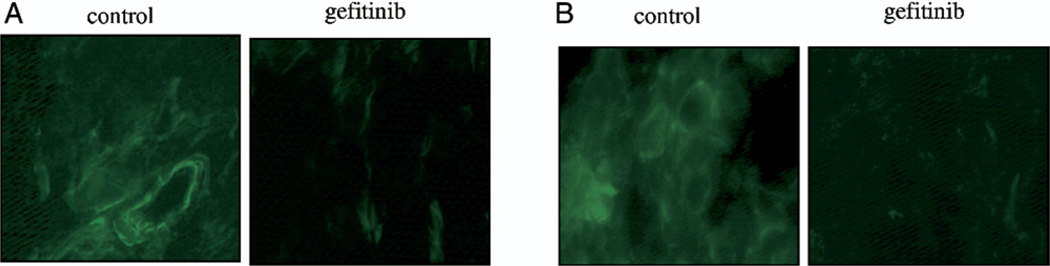
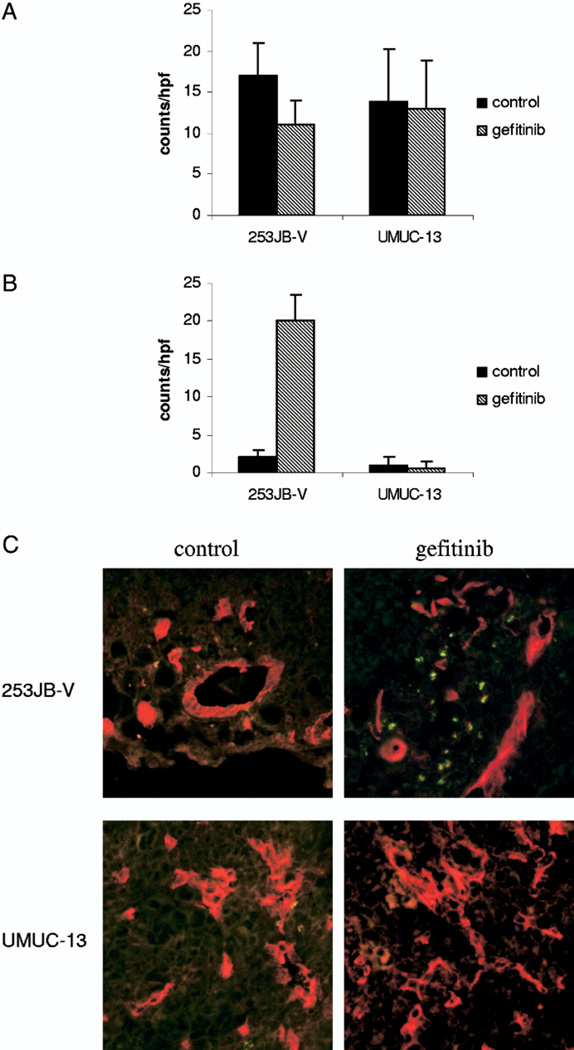
To investigate whether the decrease in VEGF expression was related to tumor angiogenesis and apoptosis the tumors were co-stained with anti-CD31 antibody to identify blood vessels and a TUNEL assay was done to identify apoptotic cells (fig. 10). Gefitinib inhibited angiogenesis by 35% in 253JB-V tumors but this did not attain statistical significance (p = 0.09). Furthermore, gefitinib significantly enhanced apoptosis (greater than 10-fold) in tumor and endothelial cells of 253JB-V lesions (p <0.0001). However, gefitinib did not have any effect on tumor angiogenesis or apoptosis in UMUC-13 tumors. All of our results suggest that VEGF modulation alone is not a reliable predictor of the biological efficacy of gefitinib for tumors that are resistant to the antiproliferative effects of EGFR inhibition.
Fig. 10.
Immunohistochemical analysis of subcutaneous tumors treated with gefitinib. A, angiogenesis. B, apoptotic cells. C, colocalization of apoptotic cells and blood vessels. Reduced from ×400.
DISCUSSION
VEGF is a potent inducer of angiogenesis and it has been extensively studied. VEGF promotes endothelial cell growth, migration, invasion and survival from the preexisting vasculature.11,12 It also has a key role in the early stages of establishing new metastatic foci.13 Higher levels of VEGF in tumor tissue predict an increased risk of disease progression in bladder cancer and increased urinary levels are a marker of an increased risk of recurrence in patients with superficial lesions.1 In patients with locally advanced urothelial cancer undergoing cystectomy and chemotherapy VEGF over expression was found to be a strong predictor of recurrence and death from bladder cancer.2 Regulators of VEGF expression include hypoxia, oncogenes/tumor suppressor genes such as src, p53 and Rb, and activating signaling pathways such as the Akt/PI3Kinase pathway.14–17 In addition, growth factor receptors such as EGFR have also been shown to regulate VEGF expression and angiogenesis.7,16
We investigated whether VEGF modulation could be used as a marker of the response to gefitinib. We already knew that only a minority of patients respond to gefitinib. Thus, if we could identify this group of patients, we would avoid overtreating many who would not benefit from gefitinib and could consider other alternative management options. Of the 4 cell lines examined we selected a sensitive cell line to gefitinib (253JB-V) and a resistant one (UMUC-13) for further investigation. Because of recent data showing that patients with nonsmall cell lung cancer that responds to gefitinib often have a gain of function mutation in EGFR,8,9 the receptor in the sensitive 253JB-V cell line was sequenced and no mutation was found.18 Since gefitinib has been shown to decrease VEGF expression,6 the question arose of whether the biological efficacy of gefitinib could be predicted by VEGF modulation in the tumor. We observed that gefitinib was able to inhibit VEGF synthesis in sensitive and resistant bladder cancer cells (253JB-V and UMUC-13, respectively) in vitro. Similarly we observed that cetuximab (C225, a monoclonal antibody against the extracellular domain of EGFR) was also able to significantly inhibit VEGF synthesis in UMUC-13 (data not shown).
To further elucidate the mechanism by which gefitinib regulated VEGF expression we treated the 2 cell lines with inhibitors of ERK, Jnk, Akt/PI3 kinase, P38, GSK and src, and VEGF protein was then quantified. In each cell line the key pathway for VEGF regulation was the Akt/PI3 kinase pathway. Hence, it is not surprising that gefitinib inhibited VEGF expression in 253JB-V and UMUC-13 cells since it significantly inhibited Akt activation in each cell line. When the cells were implanted in athymic nude mice, in vivo data were consistent with in vitro results and only 253JB-V tumors responded to gefitinib with an 8-fold decrease in the tumor growth rate. Interestingly VEGF production inhibition and VEGF receptor activation were most dramatic in resistant UMUC-13 tumors compared to those in 253JB-V tumors. We noted that the significant decrease in VEGF expression in UMUC-13 was not associated with the inhibition of tumor angiogenesis or enhancement of cell apoptosis, suggesting that VEGF may not be a dominant pro-angiogenic factor in these cell lines. UM-UC13 cells express basic fibroblast growth factor and interleukin-8 but neither was inhibited by gefitinib in vitro (data not shown). Thus, although VEGF has a major role in tumorigenicity and angiogenesis, its modulation by gefitinib is not sufficient to inhibit the growth of a tumor resistant to the antiproliferative effects of this agent.
These findings were not unexpected since there are many endogenous angiogenic inhibitors and inducers that may contribute to tumor angiogenesis. Hence, modulation of a single factor alone, such as VEGF, may not reliably predict the response. Furthermore, other pathways may be involved in driving cellular proliferation despite the down-regulation of angiogenic factors. For example, we recently reported that gefitinib induced activation of GSK-3β has an important role in inhibiting proliferation.10 As long as stimulatory signals maintain GSK-3β in a inactive state, as was the case in UMUC-13 cells, the cytostatic effect of the EGFR inhibitor is minimal.10 In conclusion, we report that in vitro and in vivo VEGF modulation alone is a poor predictor of the biological efficacy of gefitinib for bladder cancer and it should not be considered a primary marker of the response to gefitinib.
Acknowledgments
Cell lines were provided by the Specimens Core of the Genitourinary SPORE in Bladder Cancer. Gefitinib was provided by AstraZeneca, London, United Kingdom.
Study received institutional animal care and use committee approval.
Supported by National Cancer Institute Cancer Center Core Grant CA16672, GU Bladder SPORE CA91846 and a T32 training grant.
Abbreviations and Acronyms
- EGFR
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinase
- GSK
glycogen synthase kinase
- Jnk
c-Jun N-terminal kinase
- p
phospho
- PBS
phosphate buffered saline
- PI3
phosphatidylinositol 3
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
Financial interest and/or other relationship with AstraZeneca, GlaxoSmithKline, National Cancer Institute, Canji/Schering-Plough and Abbott/Vysis.
REFERENCES
- 1.Crew JP. Vascular endothelial growth factor: an important angiogenic mediator in bladder cancer. Eur Urol. 1999;35:2. doi: 10.1159/000019811. [DOI] [PubMed] [Google Scholar]
- 2.Slaton JW, Millikan R, Inoue K, Karashima T, Czerniak B, Shen Y, et al. Correlation of metastasis related gene expression and relapse-free survival in patients with locally advanced bladder cancer treated with cystectomy and chemotherapy. J Urol. 2004;171:570. doi: 10.1097/01.ju.0000108845.91485.20. [DOI] [PubMed] [Google Scholar]
- 3.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 4.Ciardiello F. Epidermal growth factor receptor tyrosine kinase inhibitors as anticancer agents. Drugs, suppl. 2000;60:25. doi: 10.2165/00003495-200060001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn J, Dinney CP. The Willet F. Whitmore, Jr., Lectureship: blockade of epidermal growth factor receptors as anticancer therapy. J Urol. 2001;165:1152. [PubMed] [Google Scholar]
- 6.Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, et al. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7:1459. [PubMed] [Google Scholar]
- 7.Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve B, Hicklin D, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257. [PubMed] [Google Scholar]
- 8.Paez JG, Janne PA, Lee JC, Tracey S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 9.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 10.Kassouf W, Dinney CP, Brown G, McConkey D, Diehl A, Bar-Eli M, et al. Uncoupling between epidermal growth factor receptor and downstream signals defines resistance to the antiproliferative effect of gefitinib in bladder cancer cells. Cancer Res. 2005;65:10524. doi: 10.1158/0008-5472.CAN-05-1536. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 12.Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 13.Li CY, Shan S, Huang Q, Braun R, Lanzen J, Hu K, et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 15.Ellis LM, Staley CA, Liu W, Fleming RY, Parikh NU, Bucana CD, et al. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem. 1998;273:1052. doi: 10.1074/jbc.273.2.1052. [DOI] [PubMed] [Google Scholar]
- 16.Maity A, Pore N, Lee J, Solomon D, O’Rourke DM. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that induced by hypoxia. Cancer Res. 2000;60:5879. [PubMed] [Google Scholar]
- 17.Bouvet M, Ellis LM, Nishizaki M, Fujiwara T, Liu W, Bucana CD, et al. Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 1998;58:2288. [PubMed] [Google Scholar]
- 18.Blehm KN, Spiess PE, Bondaruk JE, Dujka ME, Villares GJ, Zhao WJ, et al. Mutations within the kinase domain and truncations of the epidermal growth factor receptor are rare events in bladder cancer: implications for therapy. Clin Cancer Res. 2006;12:4671. doi: 10.1158/1078-0432.CCR-06-0407. [DOI] [PubMed] [Google Scholar]