Abstract
Pollen tube growth requires a Ca2+ gradient, with elevated levels of cytosolic Ca2+ at the growing tip. This gradient's magnitude oscillates with growth oscillation but is always maintained. Ca2+ influx into the growing tip is necessary, and its magnitude also oscillates with growth. It has been widely assumed that stretch-activated Ca2+ channels underlie this influx, but such channels have never been reported in either pollen grains or pollen tubes. We have identified and characterized stretch-activated Ca2+ channels from Lilium longiflorum pollen grain and tube tip protoplasts. The channels were localized to a small region of the grain protoplasts associated with the site of tube germination. In addition, we find a stretch-activated K+ channel as well as a spontaneous K+ channel distributed over the entire grain surface, but neither was present at the germination site or at the tip. Neither stretch-activated channel was detected in the grain protoplasts unless the grains were left in germination medium for at least 1 h before protoplast preparation. The stretch-activated channels were inhibited by a spider venom that is known to block stretch-activated channels in animal cells, but the spontaneous channel was unaffected by the venom. The venom also stopped pollen tube germination and elongation and blocked Ca2+ entry into the growing tip, suggesting that channel function is necessary for growth.
The growth of lily pollen tubes is a dynamic affair, with the tubes extending at the average rate of about 10 μm min−1. Furthermore, the growth rate is not steady but oscillatory, with a period of 40 to 60 s (Feijo et al., 2001; Robinson and Messerli, 2002). Tip-high gradients of cytosolic Ca2+ are an absolute requirement for pollen tube extension (Rathore et al., 1991; Miller et al., 1992), and it is well established that the Ca2+ gradient oscillates at the same frequency as growth (Holdaway-Clarke et al., 1997; Messerli and Robinson, 1997), although not in the same phase (Messerli et al., 2000). The medium in which lily pollen tubes germinate and grow is quite simple and requires only K+, Ca2+, a trace of boron, and somewhat acidic pH. No inorganic anions are required (Weisenseel and Jaffe, 1976). Ion-specific self-referencing electrodes have detected the oscillatory uptake of three cations at the tip, Ca2+, K+, and H+ (Holdaway-Clarke et al., 1997; Feijó et al., 1999; Messerli et al., 1999), as well as an oscillatory efflux of Cl− of a magnitude that exceeds the magnitude of the other fluxes by a factor of 10 or more (Zonia et al., 2002).
The role of Ca2+ influx at the tip has been brought into question by the fact that the rise in cytosolic Ca2+ occurs 7 to 12 s before the measured influx of Ca2+ (for review, see Robinson and Messerli, 2002). This result gave rise to the suggestion that the Ca2+ influx did not enter the cytosol directly but entered intracellular stores or was bound up in the wall (Holdaway-Clarke et al., 1997). Nevertheless, all recent models of the organization of the machinery to maintain the focused elongation at the pollen tube tip assume the existence of mechanically sensitive, stretch-activated (SA) calcium channels (Derksen, 1996; Feijó et al., 2001; Holdaway-Clarke and Hepler, 2003). No such channels have been identified in pollen grains or tubes, while a number of potassium currents and channels have been identified in pollen by electrophysiological and molecular techniques (Obermeyer and Kolb, 1993; Obermeyer and Blatt, 1995; Fan et al., 2001, 2003; Mouline et al., 2002). SA channels have been reported in other tip-growing organisms, including oomycete hyphae (Garrill et al., 1992) and algal rhizoids (Taylor et al., 1996).
Using the whole-cell patch clamp configuration, Griessner and Obermeyer (2003) recently detected both inward and outward potassium currents in the plasma membranes of protoplasts made from Lilium longiflorum pollen grains and pollen tubes. In contrast to previous studies on lily pollen grains, they found significant activation of inward potassium currents at holding potentials that were in the range of the measured membrane potentials of lily pollen tubes (−102 mV when bathed in 3 mm K+; Messerli et al., 1999). The measured influx of K+ at the lily pollen tube tip is strongly pulsatile, varying in each growth cycle from a basal level of less than 25 pmol cm−2 s−1 to average peaks of 688 pmol cm−2 s−1 or more (Messerli et al., 1999). However, significant variations in membrane potential have not been measured despite efforts to detect them (K. R. Robinson, unpublished data), making it unlikely that changes in K+ flux are regulated by voltage.
Thus, the factor(s) that regulates oscillatory K+ influx as well the other ion fluxes is not known. It is possible that K+ channel activity at the tip is influenced by cytosolic Ca2+; however, the maximum influx of K+ is delay by nearly 7 s from the maximum of cytosolic Ca2+. Another obvious factor to consider is the tension in the membrane. That tension is likely to oscillate as the rate of elongation oscillates, so mechanical sensitivity of the channels is an attractive possibility. SA Ca2+, K+, and Cl− channels, three channels that would seem to be required at the pollen tube tip, have been found in the plasma membranes of Vicia faba guard cells (Cosgrove and Hedrich, 1991). We have carried out patch clamp studies of outside-out patches from lily pollen protoplasts designed to detect SA channels, and we have characterized both K+ and Ca2+ SA channels in the grain, but found no Cl− channel under any of the conditions that were used. In addition, we found a K+ channel that was not activated by stretch or voltage. In patches pulled from wall-free tips of tubes, only SA Ca2+ channels were detected.
RESULTS
The formation of seals between the patch pipettes and the plasma membrane usually required the application of suction (5–10 kPa) for 1 to 2 min. As a result of the suction, the membrane deformed into the pipette, and occasionally SA channels were noted in the form of currents that varied with suction. Occasionally, seals formed almost immediately after contact and with little or no suction; the properties of channels isolated in these patches did not differ from those from patches that required more prolonged suction. After the formation of high resistance seals (3–6 GΩ), the patches were analyzed in order to characterize channels with regard to their mechanical sensitivity and conductance. Three distinct channels were detected in the grains, two of which were stretch activated. In order to detect SA channels, a period of incubation in the standard solution was required. The pollen grains were suspended in the standard solution for 10 min, 30 min, or 1 h before the enzymatic digestion of cell wall. SA channels were only found when the pollen grains were suspended in the standard solution for at least 1 h. Membrane at the growing pollen tube tip was exposed as described in “Methods and Materials,” below, and only an SA Ca2+ channel was detected with similar, but not identical, properties to the grain SA Ca2+ channel.
Spontaneous K+ Channels in Grain Protoplasts
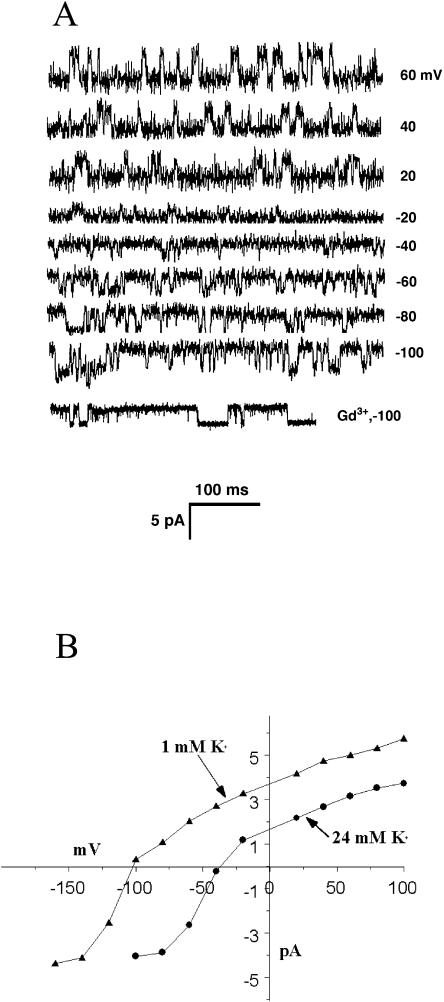
The most abundant channel that we observed, and the only channel seen in the absence of mechanical deformation, was a K+ channel that was detected when the internal solution contained 150 mm K-gluconate and 30 mm sorbitol, buffered to pH 7.2 with 5 mm Tris/HEPES, and the external solution was 24 mm K-gluconate or 1 mm K-gluconate, and 300 mm sorbitol, buffered to pH 5.6 with HEPES/MES. The channel was present in >90% of about 80 patches that were successfully pulled, regardless of the incubation time of the grain before enzymatic removal of the cell wall. Examples of channel activity are shown in Figure 1A, and these data from 75 successful patches are summarized in the I-V curves in Figure 1B. This channel had a cord conductance of 67 pS when the external solution contained 24 mm K+, and 72 pS conductance in 1 mm external K+. The channel open probability changed little with changes in the holding potential (0.18 at −60 mV and 0.21 at +60 mV). The reversal potential was about −40 mV when the external solution contained 24 mm K+ and shifted to −102 mV if the external K+ was 1 mm. The calculated equilibrium potential for K+ in the two external K+ concentration solutions was −40 mV and −120 mV, assuming that the activity coefficients for K+ were 0.75, 0.97, and 0.96 for 150 mm, 24 mm, and 1 mm K+, respectively.
Figure 1.
The spontaneous K+ channel. A, Representative sweeps from an outside-out patch containing the spontaneous K+ channel. In this case, the patch pipette contained 150 mm K+ and bathing solution contained 24 mm K+. The last sweep shows the lack of effect of 50 μm Gd3+ on channel properties. B, The I-V curves for the channel at two different concentrations of K+ in the bathing solution. In both cases, the curves cross the voltage axis near the equilibrium potential for K+. Each point includes data from 75 patches.
The effect of pressure application up to10 kPa and the SA channel blocker Gd3+ was tested on the spontaneous K+ channel. Neither treatment affected any aspect of the channel's activity (Fig. 1A). Even 50 μm Gd3+ had no effect on this channel's properties.
Stretch-Activated K+ Channels in Grain Protoplast
If 10 kPa of suction was applied to patches that were pulled from protoplasts made from grains that were incubated for at least 1 h or more, a different K+ channel appeared in about 8% of the patches. Representative sweeps are shown in Figure 2A, and the I-V curve for many such channels is shown in Figure 2B. The average conductance of the channel in 24 mm K+ was 33 pS. The SA K+ channel did not show fatigue after 30 min of pressure application; that is, its activity in patches did not decline as is common with mechanically sensitive channels (Gustin et al., 1988; Ding and Pickard, 1993).
Figure 2.
The SA K+ channel. A, Representative sweeps from an outside-out patch containing the SA K+ channel. These sweeps are all from the same patch. The first four sweeps were done with −10 kPa pressure applied to the pipette. As the bottom two sweeps show, 10 μm Gd3+ or lack of pressure abolished all channel activity. Gd3+ took about 10 min to be fully effective, and the trace shown was after recorded slightly more than 10 min after Gd3+ addition. B, I-V curve for the channel, compiled from 30 patches.
As shown in Figure 2A, no channel activity was found in patches lacking the spontaneous K+ channels unless suction was applied. When 10 μm Gd3+ was added to the bath, channel activity ceased (Fig. 2A) after about 10 min. If 50 μm Gd3+ was added, channel activity ceased immediately. In addition to its lower conductance and sensitivity to Gd3+, the SA K+ channel differed from the spontaneous K+ channel in its average open probability, which was 0.45 at −80 mV versus 0.20 at −80 mV for the spontaneous K+ channel.
Stretch-Activated Ca2+ Channels in Grain Protoplasts
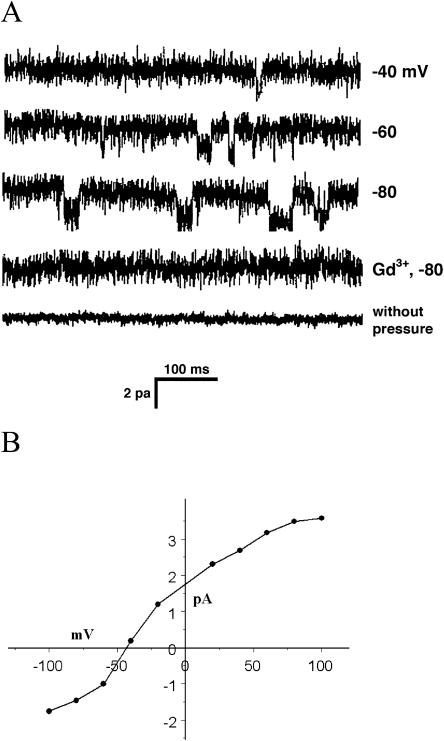
Under conditions where K+ currents are suppressed (no K+ and 30 mm Ca-gluconate in the bath), no channel activity was detected in the absence of suction applied to the patch. However, if the patch was pulled from protoplasts made from grains that were hydrated for 1 h or more before enzyme treatment, and if suction was applied to the pipette, SA Ca2+ channels were occasionally seen (Fig. 3). These channels had an instantaneous conductance of about 15 pS, and the currents were rapidly blocked by 10 μm Gd3+. The average open probability of the channels was 0.28 at −80 mV and 10 kPa pressure. Unlike the SA K+ channel, this channel exhibited fatigue; that is, the activity of a channel in a patch declined noticeably after 30 min of pressure application. Data from the first 10 min after pressure application were used for analysis.
Figure 3.
The SA Ca2+ channel. A, Sweeps from an outside-out patch containing an SA Ca2+ channel. As with the SA K+ channel (Fig. 2), no channel activity was detected in the absence of pressure or the presence of 10 μm Gd3+. In some sweeps, two channels could be seen, as well as channel substates. B, The I-V curve for the SA Ca2+ channels from 25 patches. The curves are approximately linear for negative holding potentials. Also included here are the data from patches pulled from tip protoplasts (see text and Fig. 4).
The overall chance of detecting an SA Ca2+ in a patch was about 2%. It was noticed that the SA Ca2+ channels appeared to be localized on the protoplasts. Occasionally, it was possible to return to the same place on a protoplast from which a patch containing an SA Ca2+ channel was pulled. In those cases, a second patch from the same place inevitably also contained an SA Ca2+ channel. Shortly after the protoplasts are released from the pollen wall, a groove can be seen on the protoplast that is left from the groove in the wall from which the pollen tube will later emerge (see Fig. 4). This groove in the protoplasts quickly disappeared. We found that keeping the protoplasts cold extended the time during which the groove could be seen and that SA channels could be detected in about 97% of the successful patches pulled from this region. SA K+ channels were never found in this region.
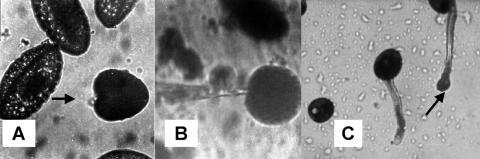
Figure 4.
Photographs of protoplasts. Shown in A is a recently isolated protoplast with the groove (arrow) still visible. This groove was the locus of the SA Ca2+ channels on the grain protoplasts. Shown in B is the same protoplast as the patch electrode makes contact with the groove region. The protoplast was displaced by the electrode from its position shown in A. The patch pulled from this protoplast contained an SA Ca2+ channel. C shows the swelling of a pectinase-treated tube (arrow) from which a patch was successfully pulled.
The internal (pipette) solution contained 150 mm K+ and the external solution contained 30 mm Ca2+. These conditions allow the use of a modified form of the Goldman-Hodgkin-Katz equation to evaluate the relative permeability of the channel to Ca2+ compared to K+ using the equation
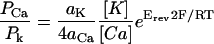 |
where aK is the activity coefficient of K+ (0.75), aCa is the activity coefficient of Ca2+ (0.52), and Erev is the estimated reversal potential from Figure 3, which we estimate to be at least +50 mV (Fatt and Ginsborg, 1958; Ding and Pickard, 1993). This equation gives a value for PCa/PK of 98 under these conditions, indicating that the channel has a high preference for Ca2+ over K+.
Stretch-Activated Ca2+ Channels in Protoplasts from Growing Tips
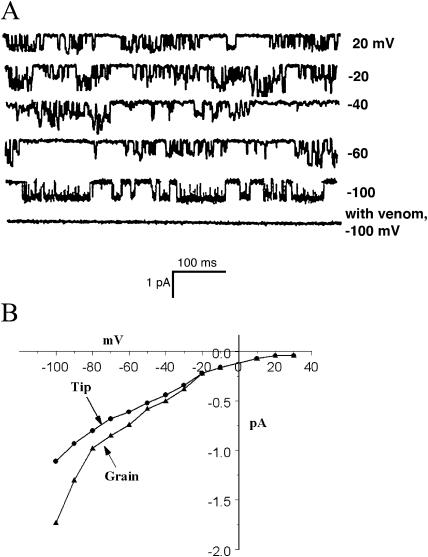
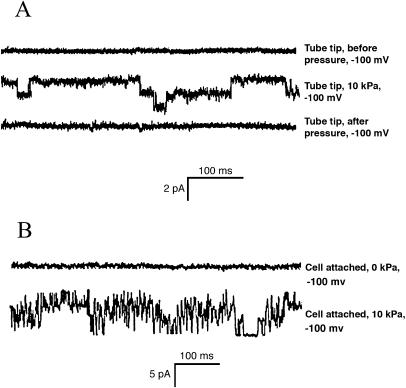
If the osmolarity of the growth medium is raised, extension of pollen tubes can be temporarily halted and the plasma membrane retreats from the cell wall at the tip due to water loss (plasmolysis). Because the cell wall at the tip is composed of pectin, it can be digested with pectinase, leaving intact the more complex cell wall distal to the tip. The pollen tube recovers turgor pressure and extrudes a protoplast in the presence of pectinase that is suitable for patch clamp analysis (Fig. 4C). We exploited this mechanism to characterize the ion channels at the tip of the growing pollen tube. When outside-out patches were drawn from tip protoplasts, only one type of channel could be detected. It was an SA Ca2+ channel with similar properties to the Ca2+ channel found in grain protoplasts. The density of the channels in the tip protoplasts was greater than in the grain, and multiple channels were often detected in patches pulled with our standard 3-μm tip, as shown in Figure 5. Also shown in Figure 5 is a record made in the cell-attached mode. No currents were detected in the absence of pressure, but complex pattern of inward current was seen when negative pressure of 10 kPa was applied.
Figure 5.
SA Ca2+ channels from tip protoplasts. The top three traces show the activity of at least two SA channels in an outside-out patch pulled from a tip protoplast. No channel activity is detected in the patch before or after the application of pressure. The lower two curves show the currents that were recorded before and during the application of pressure to the electrode in the cell-attached mode.
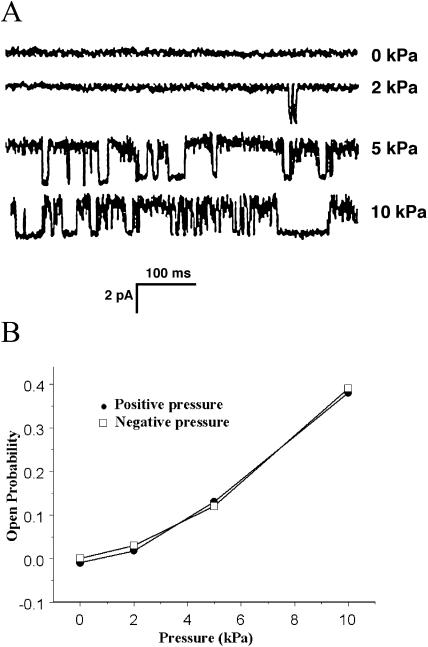
Single channels were further analyzed in outside-out patches made using smaller electrode tips. Figure 6A shows the effects of increasing negative pressure on a channel, and the effects of both negative and positive pressure on the channel open probability are shown in Figure 6B. The channels responded symmetrically to negative and positive pressures in these isolated patch preparations.
Figure 6.
Pressure sensitivity of the SA Ca2+ channels. A, A single channel from a tip protoplast was subjected to positive pressures of different magnitude. All sweeps were at a holding potential of −100 mV. B. The points show the open probability of tip-derived channels in patches subjected to varying positive and negative pressures. The error bars indicate the se of the mean for five different patches.
The tip SA Ca2+ channel differed from the one found in the grain in its conductivity. Figure 4B shows the I-V curves for the two channels, and the tip channel's conductance at −100 mV is about 50% greater than the grain channel.
Effects of Spider Venom on Channels and Growth
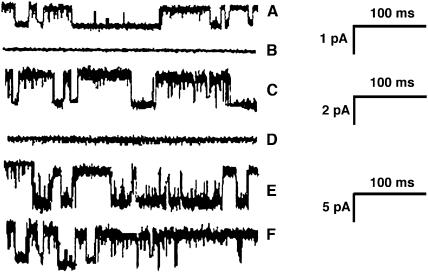
One problem in the study of SA channels has been the lack of specific pharmacological blockers for these channels. It has been reported that the crude venom of the spider Grammostola spatulata, at a dilution of about 1,000, could block SA channels in outside-out patches from pituitary cells (Chen et al., 1996). More recently, a 35-amino acid peptide from the venom has been identified as the active component (Suchyna et al., 2000). We have tested the crude venom (obtained from Spider Pharm, Yarnell, AZ) on the three channel types from pollen protoplasts and also on the growing tubes themselves. We find that the venom, at a final dilution of 3,000, rapidly blocks both the SA K+ channel and the SA Ca2+ channel but had no effect on the spontaneous K+ channel (Figs. 3A and 7).
Figure 7.
The effect of spider venom at a dilution of 3,000 on pollen protoplast channels. All sweeps at −100 mV. A, C, and E show channel opening of the SA Ca2+ channel, the SA K+ channel, and the spontaneous K+ channel, respectively. B, D, and F show the effects of venom on these channels. The venom abolishes activity of two SA channels (B and D) but has no effect on the spontaneous channel (F). The current scale bar represents 1 pA for A and B, 2 pA for C and D, and 5 pA for E and F.
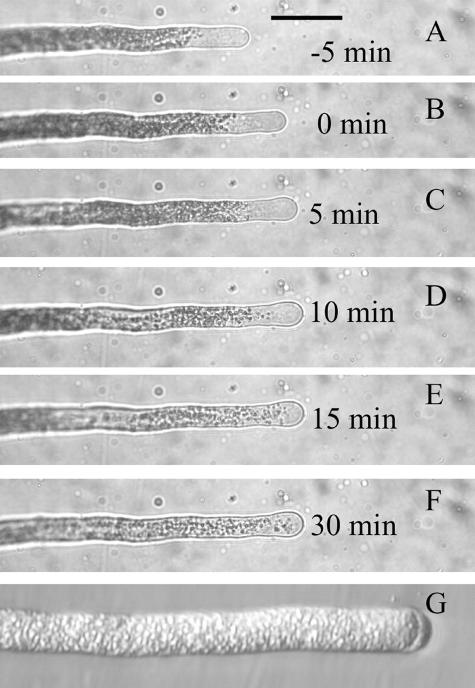
The venom, at a final dilution of 3,000, also blocked pollen tube growth. When added directly to the medium in which the tubes were grown, growth stopped as soon as the tubes could be examined (1–2 min). In order to observe the effects on single pollen tubes, the pollen grains were germinated in a thin layer of low temperature gelling agarose covered with medium, and the venom was added to the covering medium. After a delay of a few minutes due to diffusion through the agarose (Messerli and Robinson, 1997), growth stopped (Fig. 8, A–F). Following cessation of growth, the clear zone disappeared. Streaming at the tip was halted but continued in regions of the tube closer to the grain. Many tubes exposed to venom exhibited plasmolysis at the tip (Fig. 8G). The addition of venom at a final dilution of 4,000 did not affect growth within 30 min.
Figure 8.
Effect of spider venom at a dilution of 3,000 on pollen tube growth. A to F, Successive photographs of a pollen tube taken at the indicated time with respect to the time of addition of venom, which was just after the photograph in B was taken. Pollen tubes were embedded in a thin layer of agarose, which is covered with medium, so small molecules take a few minutes to diffuse to the tubes after they are added to the medium. Growth slows within 5 min and ceases by 10 min after venom addition, and some swelling of the tip is evident. Scale bar for A to F = 50 μm. G, A different tube at higher magnification, 30 min after venom addition. The plasmolysis at the tip and the complete loss of the clear zone are typical results of treatment with venom.
Effects of Spider Venom on Ca2+ Currents
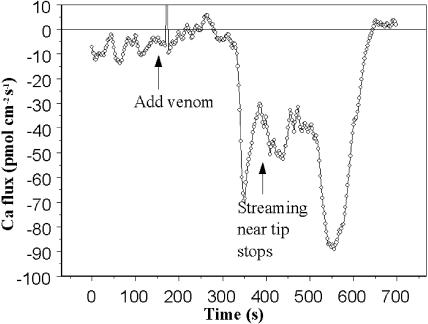
We suspect that the SA Ca2+ channels that we detect at the tips of growing pollen tubes are the means by which Ca2+ enters the tubes. If so, inhibiting the channels with venom should reduce or abolish the known influx of Ca2+ into the tips. Using the self-referencing probe, we tested this inference. We found that the venom rapidly stopped the usual oscillatory Ca2+ influx (Fig. 9). However, within about 1 min, a period of substantial Ca2+ efflux occurred, followed by a large burst of influx. At that point, streaming in the pollen tube stopped and the flux again reversed direction. Variations on this complex pattern were seen in all six tubes that were treated with venom. In all cases, an initial elimination of normal influx occurred, followed by much larger alternations of influx and efflux. Cessation of streaming near the tip always coincided with the onset of large Ca2+ influx.
Figure 9.
The effect of spider venom on Ca2+ fluxes. The Seris Ca2+ probe was used to measure Ca2+ fluxes at the growing pollen tube tip. Initially, the usual oscillatory influx was detected. Upon adding venom, the flux rapidly declined and then reversed direction, becoming outward briefly. About 3 min after venom addition, massive Ca2+ influx occurred and with it, the cessation of streaming near the tip. Note that the pollen tube was not embedded in agarose, as in Figure 8, so the venom reached to tube tip immediately as a result of gentle mixing.
Lack of Cl− Channels
Using protocols that others have used to detect Cl− channels in guard cells (Cosgrove and Hedrich, 1991), we attempted to identify Cl− channels in pollen protoplasts. In no case were such channels detected, either in the presence or absence of suction or at any holding potential. We conclude that such channels must be exceedingly rare or nonexistent.
DISCUSSION
We have identified three, and possibly four, ion channels in the membrane of pollen protoplasts. The channels differ in their frequency of occurrence, conductivity, sensitivity to pressure, selectivity, and distribution. While we have not carried out detailed analysis of the ion specificity of the channels, it should be recognized that lily pollen tubes germinate and grow in a simple medium, with Ca2+ and K+ the only cations (except for protons) in the extracellular medium. The membrane potential of pollen tubes is more negative than the equilibrium potential of any of the cations, so currents through open channels will be inward, carried by one of these three ions. Thus, we characterize these channels as K+ channels or Ca2+ channels. We did evaluate the permeability ratio of the Ca2+ channel for Ca2+ versus K+, and found that the channel was about two orders of magnitude more permeable to Ca2+ than K+.
While the SA Ca2+ channels and the SA K+ channels differed in a number of regards, the most compelling reason for assuming that they are distinct entities rather than different states of the same channel is their distribution. SA K+ channels were never detected in the groove of the pollen grain protoplasts, and SA Ca2+ channels were never detected except in the groove. Likewise, SA K+ channel activity was never detected in the protoplasts made from growing tips, but that region was densely populated with SA Ca2+ channels.
The identification of an SA Ca2+ channel in the grain and at the tip is of considerable significance. Such a channel has long been hypothesized to exist in pollen tube tips to explain the localized, oscillating entry of Ca2+ there. Extracellular Ca2+ and Ca2+ gradients are essential for lily pollen tube growth (Brewbaker and Kwack, 1963; Rathore et al., 1991; Miller et al., 1992), and blocking the entry of Ca2+ abolishes cytoplasmic Ca2+ oscillations (Messerli and Robinson, 1997). Geitmann and Cresti (1998) have shown that lanthanides at low concentrations abolish the growth pulses of Petunia pollen tubes, suggesting that SA channels are a common feature of pollen tube extension. The SA Ca2+ channel is not present in functional form in the recently hydrated pollen grain; it only appears after an hour or more of incubation in the germination medium, shortly before germination would occur. It will be interesting to learn if the SA Ca2+ channel is synthesized and inserted into the membrane or modified to make it functional during that 1-h period. It is unclear if the tip SA Ca2+ channel is a slightly modified form of the grain channel or a completely different entity. The only difference that we noted between the channels from the two locations is in somewhat greater conductivity of the tip channel at more negative holding potentials.
The SA Ca2+ channels are highly localized on the protoplasts. The faint indentation that runs along one part of the newly isolated protoplast persists long enough to permit sampling it for the presence of channels. That region proved to be a rich source of SA Ca2+ channels, while the remainder of the protoplast was devoid of the channels. We think that the indentation marks the region from which the pollen tube would emerge, and, thus, it is likely that the SA Ca2+ channels there would also be found in the tube tip. The SA K+ channels were never found in this region.
There is an important difference in the physical situation in which we have characterized the channel, compared to the situation in the intact pollen grain or tube. We have disrupted any putative links to the cytoskeleton, the extracellular matrix (the cell wall), and the endoplasmic reticulum (Reuzeau et al., 1997). The tethering of both extracellular and intracellular domains of SA channels has been shown to affect the mechanical gating of some channels, while others are gated by membrane deformation (for review, see Hamill and Martinac, 2001). We do not know how the behavior of the SA Ca2+ channel (or the SA K+ channel) would be modulated in the normal membrane environment. The whole cell recordings done at the tip do not address this issue, as the cell wall was eliminated and the cytoskeleton within the tip may have been greatly modified from its normal state in an untreated pollen tube.
A surprising feature of our results is the lack of any K+ channels in the tip protoplasts. It is known that there is a large, oscillatory influx of K+ into the growing tip (Messerli et al., 1999), and the K+ flux is an order of magnitude larger than the Ca2+ flux. The electrochemical driving force for K+ entry is vastly smaller than that for Ca2+, so K+ channels would be expected to be abundant in order to support the large measured influx. K+ is a required component of the growth medium; however, K+ must be rigorously removed from the medium in order to stop growth, and K+ concentrations as low as 10 μm are sufficient to support normal growth rates (Messerli and Robinson, 2003). It has been suggested previously that perhaps the measured K+ influx pulses involved cotransport with H+ rather than movement through an ion channel (Messerli et al., 1999). This suggestion was based on the observation that K+ pulses and H+ pulses were spatially and temporally coincident and had similar magnitudes. Our present failure to detect K+ channels at the growing tip lends support to the notion of H+/K+ cotransport.
The spontaneous K+ channel was ubiquitously distributed on the protoplasts. It had the largest conductance of the three channels that we detected. Its I-V curve was symmetrical around the zero current point, and it was unaffected by membrane tension, Gd3+, or spider venom. The distribution of K+ influx over the surface of the pollen tube has not been carefully studied; attention has been focused on the tip. If this channel were widely distributed on the pollen tube, it would lead to steady K+ uptake. The membrane potential of the pollen tube is somewhat more negative than the K+ equilibrium potential (Messerli et al., 1999) and does not vary with time. It would be surprising, however, if this channel were completely unregulated, so its behavior in the intact membrane may be quite different.
The finding that spider venom blocked both SA channels gives us confidence that the channels are in fact stretch activated and are distinct from the spontaneous K+ channel. The effect of the venom on germination and growth emphasizes the importance of the SA channels to pollen tube physiology. The same dilution of the venom that blocks the channels rapidly stops elongation of the tubes and disrupts the normal Ca2+ influx. The effects of venom on Ca2+ influx were complicated. As expected from the effect on isolated channels, the immediate effect of venom was to reduce the amplitude of the normal Ca2+ influx oscillations. However, within a few minutes, Ca2+ efflux occurred, followed by massive influx that stopped cytoplasmic streaming. We do not understand the mechanism that underlies these later events, which presumably are a pathological response to the initial disruption of normal Ca2+ influx.
Finally, we failed to detect any evidence of anion channels, stretch activated or otherwise. One possibility that we did not explore is Ca2+ regulation of an anion channel. Zonia et al. (2002) have reported massive oscillatory Cl− efflux at the pollen tube tip. Their measured fluxes were an order of magnitude larger than any other measured fluxes, thus the channel underlying the fluxes would be expected to be both abundant and high conductance. The Cl− fluxes reported by Zonia et al. (2002) were disrupted by the injection of Ins(3,4,5,6) P4, so perhaps our patch preparation lacked some critical aspect of inositol phosphate control. It should be noted that others have found that lily pollen germination and growth are independent of extracellular Cl− (Weisenseel and Jaffe, 1976).
MATERIALS AND METHODS
Protoplast Isolation
Anthers from Lilium longiflorum flowers were dried at room temperature for 2 d, and pollen grains were then separated from the anthers by shaking them in a sieve. The grains were then stored in small vials at −20oC. Protoplasts were prepared following the method described by Fan et al. (1999). In order to prepare protoplasts, the pollen grains were hydrated in a standard solution containing 1 mm KNO3, 0.2 mm KH2PO4, 1 mm MgSO4, 1 μm KI, 0.1 μm CuSO4, 5 mm CaCl2, 5 mm MES (pH 5.8 adjusted with Tris), 500 mm Glc, and 500 mm sorbitol (osmolarity = 1.5 Osmol kg−1). The hydrated grains were then filtered through 80-μm nylon mesh and centrifuged at 160g for 5 min. An enzyme solution was prepared by adding to the standard solution 2% (w/v) cellulase, 1% (w/v) macerozyme R-10 (Yokult Honsha, Tokyo), and 0.2% potassium dextran sulfate, and the pelleted pollen grains were suspended in it. The protoplasts were again centrifuged at 160g for 5 min, and the pellet was resuspended with 2 mL of the standard solution. This centrifugation/resuspension cycle was repeated three times, and the washed protoplasts were stored on ice until use.
Preparation of Tip Protoplasts
Pollen grains were incubated in modified Dickinson's medium (Messerli et al., 2000) and allowed to germinate. Pollen tubes were plasmolyzed by increasing the osmolarity of the bathing medium and then were treated with an enzyme solution containing only 2% pectinase (Sigma, St. Louis). As the tubes recovered turgor, a protoplast was extruded from the tip from which patches could be pulled. Before patching, the enzyme solution was replaced with the desired patch clamp solution.
Patch Clamp Methods
Patch pipettes were fabricated from Kimax 51 capillaries 1.5 to 1.8 mm o.d. and 100 mm long using two-step pulling with Narishige vertical puller, and the pipettes were fire polished. The pipettes were backfilled with desired solution, and seals were formed between the pollen plasma membrane and the patch pipettes, and outside-out patches pulled as described by Hamill et al. (1981). The Axopatch-1D amplifier (Axon Instruments, Union City, CA), Digidata 1200, and programs Clampex 8.0 and Clampfit 8.0 were used for recording and analysis of the patch clamp data. After successful patches were pulled, single channel recordings were carried out at membrane potentials from 100 mV to −100 mV (−160 mV in some cases) at the intervals of every 20 mV. The sweep lengths were either 2,000 or 2,500 ms. The records were filtered at 3 kHz.
The experiments were designed to characterize three different channels: Cl−, K+, and Ca2+. The outside-out patches were analyzed with three different sets of solutions. For Cl− channels, the internal solution contained 150 mm KCl, 2 mm MgCl2, 2 mm Mg-ATP, 1 mm EGTA buffered to pH 7.2 with 10 mm Tris/HEPES, and external solution consisted of 40 mm CaCl2, 2 mm MgCl2, and 220 mm sorbitol buffered to pH 5.5 with 10 mm MES/Tris. For K+ channels, internal solution contained 150 mm K-gluconate and 30 mm sorbitol, buffered to pH 7.2 with 4 mm Tris/HEPES, and external solution was 24 mm K-gluconate and 300 mm sorbitol, buffered to pH 5.6 with 5 mm HEPES/MES. For Ca2+ channels, the internal solution contained 150 mm K-gluconate, 30 mm sorbitol, buffered to pH 7.2 with HEPES/MES, and external solution contained 30 mm Ca-gluconate and 300 mm sorbitol buffered to pH 5.6 with 2 mm HEPES/MES (Cosgrove and Hedrich, 1991). Pressure, both positive and negative, was applied to patches by activating a solenoid valve that connected the patch electrode to a gas-tight syringe equipped with a screw drive attached to the plunger. The pressure generated in the system was measured continuously by a digital manometer and was stable for the duration of each recording.
Solutions containing Gd3+ were prepared shortly before use by dissolving GdCl3 directly in the appropriate media. Spider venom was reconstituted from lyophilized solid and stored in small aliquots at −70oC. The venom was used within 2 weeks of reconstitution.
Ca2+ Flux Measurements
Ca2+ fluxes were measured using the self-referencing ion selective (Seris) probe (Smith et al., 1994) as modified for pollen tip measurements (Messerli et al., 1999). Pollen tubes were germinated in modified Dickinson's medium and then transferred to plastic petri dishes that were treated with poly-l-Lys (500 μg/mL) solution for 30 min and then rinsed. This treatment caused the pollen tubes to stick slightly to the dish bottom, which facilitated flux measurements.
This work was supported by the National Science Foundation (grant no. 0087517 IBN).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041483.
References
- Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50: 859–865 [Google Scholar]
- Chen Y, Simasko SM, Niggel J, Sigurdson WJ, Sachs F (1996) Ca2+ uptake in GH3 cells during hypotonic swelling: the sensory role of stretch-activated channels. Am J Physiol 270: C1790–C1798 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ, Hedrich R (1991) Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta 186: 143–153 [DOI] [PubMed] [Google Scholar]
- Derksen J (1996) Pollen tubes: a model system for plant cell growth. Bot Acta 109: 341–345 [Google Scholar]
- Ding J, Pickard B (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3: 83–110 [DOI] [PubMed] [Google Scholar]
- Fan LM, Wang YF, Wang H, Wu WH (2001) In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot 52: 1603–1614 [PubMed] [Google Scholar]
- Fan LM, Wang YF, Wu WH (2003) Outward K+ channels in Brassica chinensis pollen protoplasts are regulated by external and internal pH. Protoplasma 220: 143–152 [DOI] [PubMed] [Google Scholar]
- Fan LM, Wu WH, Yang HY (1999) Identification and characterization of the inward K+ channel in the plasma membranes of Brassica pollen protoplasts. Plant Cell Physiol 40: 859–865 [DOI] [PubMed] [Google Scholar]
- Fatt P, Ginsborg BL (1958) The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol (Lond) 142: 516–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK (1999) Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J Cell Biol 144: 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó JA, Sainhas J, Holdaway-Clarke T, Cordeiro MS, Kunkel JG, Hepler PK (2001) Cellular oscillations and the regulation of growth: the pollen tube paradigm. Bioessays 23: 86–94 [DOI] [PubMed] [Google Scholar]
- Garrill A, Lew RR, Heath IB (1992) Stretch-activated Ca2+ and Ca2+-activated K+ channels in the hyphal tip plasma membrane of the oomycete Saprolegnia ferax. J Cell Sci 101: 721–730 [Google Scholar]
- Geitmann A, Cresti M (1998) Ca2+ channels control the rapid expansions in pulsating growth of Petunia hybrida pollen tubes. J Plant Physiol 152: 439–447 [Google Scholar]
- Griessner M, Obermeyer G (2003) Characterization of whole-cell K+ currents across the plasma membrane of pollen grain and tube protoplasts of Lilium longiflorum. J Membr Biol 193: 99–108 [DOI] [PubMed] [Google Scholar]
- Gustin MC, Zhou XL, Martinac B, Kung C (1988) A mechanosensitive ion channel in the yeast plasma-membrane. Science 242: 762–765 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Martinac B (2001) Molecular basis of mechanotransduction in living cells. Physiol Rev 81: 685–740 [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution recording from cells and cell-free membrane patches. Pflugers Arch 391: 85–100 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke T, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159: 539–563 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijó JA, Hackett GR, Kunkel JG, Hepler PK (1997) Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli M, Robinson KR (1997) Tip localized Ca2+ pulses are coincident with peak pulsatile growth rates in pollen tubes of Lilium longiflorum. J Cell Sci 110: 1269–1278 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Creton R, Jaffe LF, Robinson KR (2000) Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol 222: 84–98 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Danuser G, Robinson KR (1999) Pulsatile fluxes of H+, K+, and Ca2+ lag growth pulses of Lilium longiflorum pollen tubes. J Cell Sci 112: 1497–1509 [DOI] [PubMed] [Google Scholar]
- Messerli MA, Robinson KR (2003) Ionic and osmotic disruptions of the lily pollen tube oscillator: testing proposed models. Planta 217: 147–157 [DOI] [PubMed] [Google Scholar]
- Miller DD, Callaham DA, Gross DJ, Hepler PK (1992) Free Ca2+ gradient in growing pollen tubes of Lilium. J Cell Sci 101: 7–12 [Google Scholar]
- Mouline K, Very AA, Gaymard F, Boucherez J, Pilot G, Devic M, Bouchez D, Thibaud JB, Sentenac H (2002) Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev 16: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer G, Blatt MR (1995) Electrical properties of intact pollen grains of Lilium longiflorum: Characteristics of the non-germinating pollen grain. J Exp Bot 46: 803–813 [Google Scholar]
- Obermeyer G, Kolb H-A (1993) K+ channels in the plasma membrane of lily pollen protoplasts. Bot Acta 106: 26–31 [Google Scholar]
- Rathore KS, Cork RJ, Robinson KR (1991) A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev Biol 148: 612–619 [DOI] [PubMed] [Google Scholar]
- Reuzeau C, Doolittle KW, McNally JG, Pickard BG (1997) Covisualization in living onion cells of putative integrin, putative spectrin, actin, putative intermediate filaments, and other proteins at the cell membrane and in an endomembrane sheath. Protoplasma 199: 173–197 [DOI] [PubMed] [Google Scholar]
- Robinson KR, Messerli M (2002) Pulsating ion fluxes and growth at the pollen tube tip. Science's STKE, http://stke.sciencemag.org/cgi/content/full/sigtrans;2002162/pe51 [DOI] [PubMed]
- Smith PJ, Sanger RH, Jaffe LF (1994) The vibrating Ca2+ electrode: a new technique for detecting plasma membrane regions of Ca2+ influx and efflux. Methods Cell Biol 40: 115–134 [DOI] [PubMed] [Google Scholar]
- Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F (2000) Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol 115: 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Manison NFH, Fernandez C, Wood J, Brownlee C (1996) Spatial organization of calcium signaling involved in cell volume control in the Fucus rhizoid. Plant Cell 8: 2015–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenseel MH, Jaffe LF (1976) The major growth current through lily pollen tubes enters as K+ and leaves as H+. Planta 133: 1–7 [DOI] [PubMed] [Google Scholar]
- Zonia L, Cordeiro S, Tupy J, Feijó JA (2002) Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetrakisphosphate. Plant Cell 14: 2233–2249 [PubMed] [Google Scholar]