Abstract
Many environmental stresses result in increased generation of active oxygen species in plant cells. This leads to the induction of protective mechanisms, including changes in gene expression, which lead to antioxidant activity, the recovery of redox balance, and recovery from damage/toxicity. Relatively little is known about the signaling events that link perception of increased active oxygen species levels to gene expression in plants. We have investigated the role of calcium signaling in H2O2-induced expression of the GLUTATHIONE-S-TRANSFERASE1 (GST1) gene. Challenge with H2O2 triggered a biphasic Ca2+ elevation in Arabidopsis seedlings. The early Ca2+ peak localized to the cotyledons, whereas the late Ca2+ rise was restricted to the root. The two phases of the Ca2+ response were independent of each other, as shown by severing shoot from root tissues before H2O2 challenge. Modulation of the height of Ca2+ rises had a corresponding effect upon H2O2-induced GST1 expression. Application of the calcium channel blocker lanthanum reduced the height of the first Ca2+ peak and concomitantly inhibited GST1 expression. Conversely, enhancing the height of the H2O2-triggered Ca2+ signature by treatment with l-buthionine-[S,R]-sulfoximine (an inhibitor of glutathione synthesis) lead to enhancement of GST1 induction. This finding also indicates that changes in the cellular redox balance constitute an early event in H2O2 signal transduction as reduction of the cellular redox buffer and thus the cell's ability to maintain a high GSH/GSSG ratio potentiated the plant's antioxidant response.
In plants, oxidative stress may be caused either directly through ozone pollution or indirectly, as a result of abiotic stresses (e.g. cold, drought, or high light intensity) leading to perturbations in cellular O2 processing (Vranová et al., 2002). Even during normal metabolism, active oxygen species (AOS) are generated as a side product in electron transport processes, such as photosynthesis and respiration. The plant experiences oxidative stress only once a serious imbalance between the production of AOS and antioxidant defences has occurred (Moller, 2001). Oxidative stress can therefore be defined as a disruption of the cellular redox balance. AOS have traditionally been considered to be toxic by-products of aerobic metabolism. However, in recent years, it has become apparent that plant cells generate low levels of AOS endogenously in response to a number of biotic and abiotic stresses, such as pathogenic elicitors and even ozone (Lamb and Dixon, 1997; Wohlgemut et al., 2002). The paradox of self-imposed stress points to a role of AOS in signaling, where increased accumulation of H2O2 and changes in redox status alerts the plant cell to environmental change (Noctor and Foyer, 1998; Foyer and Noctor, 2003).
Plants have evolved a number of mechanisms to protect themselves from AOS-mediated damage. The term antioxidant can be considered to describe any compound capable of quenching AOS without itself undergoing conversion to a destructive radical (Noctor and Foyer, 1998; Dröge, 2002). Antioxidant enzymes (e.g. ascorbate peroxidases or superoxide dismutases) catalyze such reactions, or are involved in the direct processing of AOS. Considerable amounts of nonprotein thiols are present in plants that function as antioxidants by keeping other redox-active molecules in a reduced state (Noctor and Foyer, 1998). Glutathione, a Gln – Cys – Gly tripeptide, is the major thiol compound, present at concentrations of up to 10 mm in the chloroplast. Two molecules of the reduced form, GSH, are oxidized to a dimer, and connected by a disulfide bond, GSSG. Under most conditions, glutathione is found largely in the reduced form (90%–99%; Foyer et al., 1997). By providing a store for reducing power within the cell, glutathione acts as a redox buffer. Concomitantly, oxidative conditions increase GSH synthesis in Arabidopsis cell suspension cultures, whereas depletion of glutathione by treatment with l-buthionine-[S,R]-sulfoximine (BSO), an inhibitor of glutathione synthesis (Griffith and Meister, 1979), renders them susceptible to oxidative damage (May and Leaver, 1993).
It has been suggested that the GSH/GSSG ratio, indicative of the cellular redox balance, may be involved in AOS perception (Dröge, 2002; Foyer and Noctor, 2003). For example, the bacterial OxyR protein can be activated either directly by hydrogen peroxide or alternatively by changes in the intracellular GSH/GSSG balance, suggesting that the increase in H2O2 is sensed in this manner (Dröge, 2002). In plants, treatments that affected the GSH/GSSG ratios in opposite ways had the opposite effects on the H2O2-triggered rise in the cytosolic Ca2+ concentration ([Ca2+]cyt), even though accumulation of H2O2 was enhanced in both cases (Price et al., 1994). Pretreatment with BSO heightened the rise in [Ca2+]cyt, whereas inhibition of ascorbate peroxidase1 (APX1) activity by treatment with hydroxyurea or hydroxylamine, which should increase the GSH/GSSG ratio by blocking the ascorbate-glutathione cycle, lowered the [Ca2+]cyt elevation. These observations suggested that it may not be H2O2 accumulation per se that is sensed by the plant but its effect on the GSH/GSSG ratio.
Another class of detoxifiying enzymes is constituted by the glutathione-S-transferases (GSTs). GSTs catalyze the conjugation of glutathione to a variety of hydrophobic, electrophilic, and usually cytotoxic substrates and their role in detoxifying electrophilic herbicides is well known (Marrs, 1996). However, the endogenous role of GSTs is less clear, despite numerous reports of increases in GST levels during plant development and after exposure to pathogens and abiotic stresses (Edwards et al., 2000). Their role in protection against oxidative damage may lie in conjugation (and thus detoxification) of metabolites arising from oxidative damage, e.g. lipid peroxidation or oxidative DNA degradation products (Marrs, 1996).
Relatively little is known about the signaling events triggered by oxidative stress that lead to deployment of defenses against it. As a very fast response, Ca2+ elevations upon AOS treatment have been reported in a number of different systems. In Commelina communis guard cells, low concentrations of superoxide and H2O2 triggered rapid increases in [Ca2+]cyt, as measured by changes in Fura-2 fluorescence (McAinsh et al., 1996). The rise in [Ca2+]cyt as well as ensuing stomatal closure were inhibited by the Ca2+ ion chelator EGTA, indicating that external Ca2+ is required. H2O2 challenge of whole tobacco (Nicotiana tabacum) seedlings expressing recombinant aequorin resulted in a rapid, transient elevation of [Ca2+]cyt that was inhibited by lanthanum chloride (LaCl3), a Ca2+ channel blocker, and ruthenium red, an inhibitor of Ca2+ release from internal stores (Price et al., 1994). Activation of Ca2+-permeable channels in response to AOS treatment was demonstrated in Arabidopsis guard cells by patch-clamping (Pei et al., 2000). The study showed that application of H2O2 stimulated Ca2+ influx through a hyperpolarization-activated Ca2+-permeable channel, followed by partial stomatal closing. Thus, increases in [Ca2+]cyt in response to H2O2 have been observed and shown to be necessary for stomatal closure. However, a link between H2O2-triggered rises in [Ca2+]cyt and H2O2-induced gene expression has not been demonstrated.
In this paper, we examine the characteristics of the Ca2+ elevations in Arabidopsis seedlings upon H2O2 treatment and demonstrate that inhibiting or enhancing the height of the [Ca2+]cyt rises leads to inhibition or enhancement of GST1 gene expression, respectively. In addition, the observation that BSO treatment potentiates H2O2-triggered Ca2+ signaling and antioxidant gene induction suggests that the plant may sense increases in H2O2 through changes in the cellular redox balance.
RESULTS
H2O2 Triggers a Biphasic [Ca2+]cyt Response in Arabidopsis Seedlings
Changes in cytosolic calcium concentrations were investigated using Arabidopsis RLD1 seedlings stably transformed with the apoaequorin gene (Knight et al., 1996). Once reconstituted with its luminophore coelenterazine to produce aequorin, this protein emits blue light upon binding of calcium ions. The light can be measured by luminometry, yielding information on timing and magnitude of the response, or with a photon-counting camera, supplying additional spatial information (Knight et al., 1991, 1996). Because the imaging data obtained with the photon-counting camera cannot be calibrated to reflect actual [Ca2+]cyt values, both types of measurements are presented here.
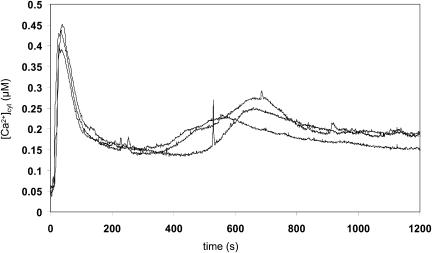
In order to determine whether H2O2 triggers increases in [Ca2+]cyt in Arabidopsis, reconstituted seedlings were treated with 10 mm H2O2 and emitted light was measured using a luminometer (Fig. 1). A biphasic Ca2+ response was detected, with a first rise in [Ca2+]cyt starting 15 s after stress application and reaching a maximum after 35 to 45 s, resulting in [Ca2+]cyt peak values of 0.45 μm (±0.02, n = 3). The second increase in [Ca2+]cyt showed more variability in height and timing (peak at 7–20 min) than the first [Ca2+]cyt peak. Due to this variability, Figure 1 depicts individual traces of three representative seedlings rather than an average of these responses (see also Figs. 3B and 4B). Seedlings treated in these experiments did not manifest any signs of damage and were viable when placed back into water or onto agar plates (data not shown), indicating that the increase in [Ca2+]cyt was not part of a cytotoxic response. Moreover, the height of the Ca2+ response was dependent on the H2O2 concentration administered (data not shown).
Figure 1.
Increase in [Ca2+]cyt in response to H2O2 [Ca2+]cyt was measured by luminometry as described in “Materials and Methods.” For each measurement, an individual 7-d-old reconstituted RLD1.1 seedling was placed into a plastic cuvette containing 0.5 mL water. H2O2 (final concentration 10 mm) was added. At 1,200 s, remaining aequorin was discharged. Traces represent the responses of three individual seedlings per treatment.
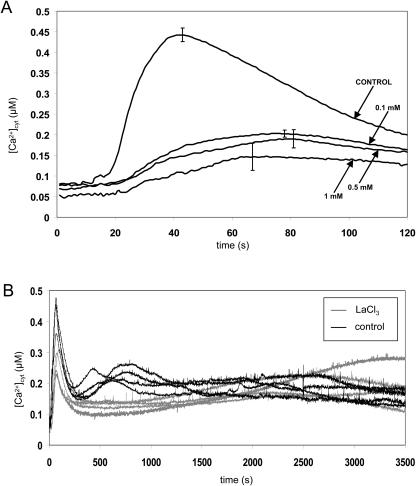
Figure 3.
Effect of the Ca2+ channel blocker LaCl3 on the H2O2-induced [Ca2+]cyt signature [Ca2+]cyt was measured by luminometry as described in “Materials and Methods.” A, Inhibition of the early [Ca2+]cyt peak by LaCl3. Seven-day-old reconstituted RLD1.1 seedlings were incubated for 1 h in LaCl3 solution (concentrations indicated in the legend) or water (control) prior to transfer into plastic cuvettes. H2O2 (final concentration 10 mm) was added at 5 s. At 120 s, remaining aequorin was discharged. Traces represent the mean of 4 seedlings with error bars indicating se at that time point. B, Effect of LaCl3 on the late H2O2-induced [Ca2+]cyt peak. Seven-day-old reconstituted RLD1.1 seedlings were incubated individually in 0.1 mm LaCl3 solution or water (control) for 30 min inside a plastic cuvette. H2O2 (final concentration 10 mm) was added at 5 s. At 3,600 s, remaining aequorin was discharged. Traces represent the responses of 3 individual seedlings per treatment.
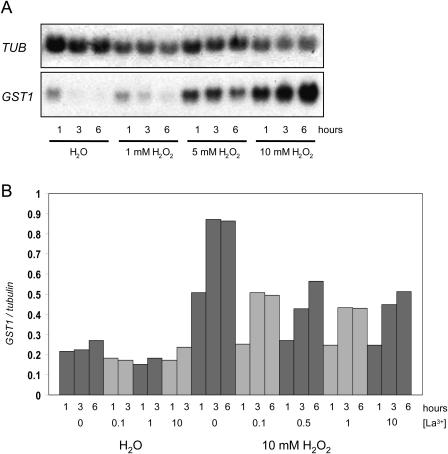
Figure 4.
Inhibition of H2O2-triggered GST1 gene induction by LaCl3. Northern analysis and normalization of gene induction data was carried out as described in “Materials and Methods.” A, Six-day-old RLD1 seedlings were incubated in water or different concentrations of H2O2 for 1, 3, or 6 h as indicated. B, Seven-day-old RLD1 seedlings were transferred into LaCl3 solution (final concentration as indicated). Control samples remained in water. After 1 h incubation, samples were placed into fresh LaCl3 and H2O2 was added where indicated (final concentrations LaCl3 as before; H2O2, 10 mm). Samples were removed and frozen after 1 h, 3 h, or 6 h. Total RNA was extracted, electrophoresed, and transferred to nylon membrane. GST1 and β-TUBULIN probes were labeled and hybridized to the RNA on the membrane. In B, bars represent GST1 gene induction relative to β-TUBULIN mRNA levels.
The Two Phases of H2O2-Triggered [Ca2+]cyt Increase Are Located in Different Tissues of the Seedling
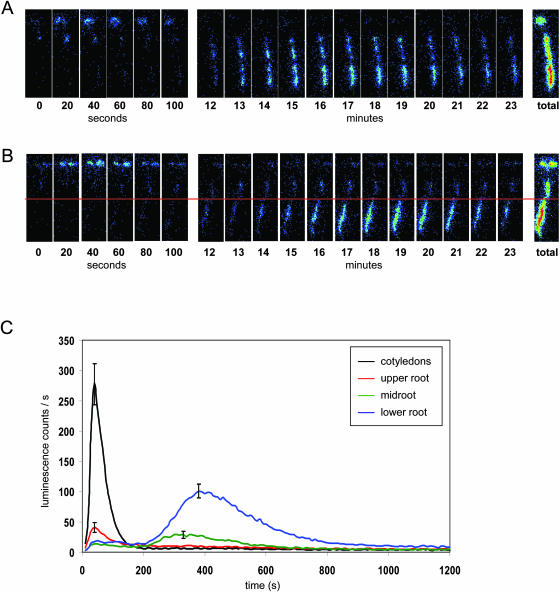
Spatial characteristics of the Ca2+ signal were examined by imaging reconstituted seedlings under the photon-counting camera following transfer onto agar plates containing 10 mm H2O2. Consistent with the luminometry data, seedlings responded to H2O2 with two [Ca2+]cyt elevations (Fig. 2A). Transfer of seedlings to agar plates without H2O2 did not trigger any increases in [Ca2+]cyt (data not shown). On H2O2 challenge, [Ca2+]cyt began to rise after 25 to 30 s and reached a peak at 45 to 60 s. The small delay compared to the timing detected by luminometry stems from the different stress application; under the camera, seedlings are not directly immersed in solution but the oxidative stress agent has to diffuse from the agar into the filter paper to reach the plant. This latter approach is necessary technically to be able to capture all of the first peak during imaging; it is however possible that the concentration of H2O2 reaching the seedling by diffusion is lower than 10 mm. The first increase in [Ca2+]cyt was always located in the cotyledons/leaves and the hypocotyl. The second peak was located exclusively in the root, particularly the lower root. This latter response was variable in timing, with peak times measured between 5 and 20 min after stress application. The photon counting camera experiments were repeated for at least 100 individual wild-type seedlings, and for more than 5,000 seedlings of an EMS-mutagenized population during a mutant screen. The biphasic calcium signature was very reproducible. This is also clear in Figure 2C, which represents the mean calcium signal of 25 imaged seedlings. The height of the second luminescence peak of the lower roots was thus shown to reach only one-third of the early peak measured from the cotyledons. However, as the lower root signal was of much longer duration, 40.5% of the total luminescence was detected in this tissue as opposed to 22.5% in the cotyledons (Table I). In order to eliminate the possibility that luminescence differences between tissues were due to differences in aequorin content, 10 seedlings were cooled repeatedly to 2°C under the camera and subsequently discharged of remaining aequorin by CaCl2 addition in the luminometer (data not shown). Cooling triggers [Ca2+]cyt release in all plant tissues (Knight et al., 1996). Cotyledons, upper root half and lower root half contained 37% (±3.8), 38% (±2.8) and 25% (±2.6) of total luminescence, respectively. Thus, the higher luminescence detected in the lower root as compared to the cotyledons was not due to a higher content in aequorin. To confirm that the observed localizations of the two [Ca2+]cyt peaks also occur under luminometry conditions, seedlings were imaged floating in liquid (data not shown). Again, the early peak was observed in the cotyledons and upper root followed by a later but more prolonged [Ca2+]cyt elevation in the lower root sections. Thus, localization of the Ca2+ elevations is unaffected by the method of H2O2 challenge, allowing direct comparison of data obtained by luminometry and imaging.
Figure 2.
Localization of increases in [Ca2+]cyt in response to H2O2 [Ca2+]cyt was measured under a photon-counting camera as described in “Materials and Methods.” Seven-day-old reconstituted RLD1.1 seedlings were transferred to moist filter paper and left to rest for 45 min. A, After transfer of the filter paper onto agar plates containing 10 mm H2O2, luminescence was counted for 23 min. Images represent the total photon counts for an individual seedling over consecutive 20 s or 1 min intervals, or the entire 23 min period (total). B, Seedlings were treated as in A, but cotyledons and root were severed (as indicated by the red line) with a sharp razor blade before transfer to 10 mm H2O2 agar plates. C, Luminescence was counted for 20 min. Traces represent mean luminescence counts of 25 seedlings imaged on the same stress plate. Error bars indicate se at that time point.
Table I.
Comparison of the Ca2+ peaks triggered by H2O2 treatment
| Plant Tissue | Percentage of Cotyledon Peak Height | Percentage of Total Luminescence |
|---|---|---|
| Cotyledons | (100) | 23 |
| Upper root | 11 | 14 |
| Mid root | 22 | 23 |
| Lower root | 33 | 40 |
The maximum count value of calcium-dependent luminescence (peak height) emitted by the cotyledons was taken as 100% and compared to the peak heights of different root sections. The percentage of total luminescence for each part of the seedling was calculated ([sum of counts for section/sum of all parts] × 100).
The Two H2O2-Triggered [Ca2+]cyt Peaks Are Independent of Each Other
In order to determine whether different tissues within the plant communicate the [Ca2+]cyt signal to unchallenged tissues, 7-d-old seedlings were severed at the hypocotyl and imaged under the photon-counting camera (Fig. 2B). Upon H2O2 challenge, a biphasic Ca2+ signal was observed comparable to that seen in nonsevered (control) seedlings (Fig. 2A). Thus, the two [Ca2+]cyt peaks are independent of each of other.
Inhibition of the Ca2+ Response to H2O2 Inhibits GST1 Gene Induction
In order to establish a link between the Ca2+ signal and downstream effects, we attempted to modulate the Ca2+ response to H2O2 by treatment with the Ca2+ channel blocker LaCl3. LaCl3 was able to inhibit the first [Ca2+]cyt peak of the H2O2 response at a concentration of 0.1 mm (from 0.44 μm ± 0.02 to 0.20 μm ± 0.01 for 0.1 mm LaCl3; n = 4; Fig. 3A). The effect on the second [Ca2+]cyt elevation was variable but generally caused this peak to be delayed and decreased in magnitude (Fig. 3B; first peak is reduced from 0.44 μm ± 0.03 to 0.27 μm ± 0.02; n = 4).
The GST1 gene was induced in a concentration dependent manner in response to H2O2, with strong induction at 10 mm for all time points (1, 3, and 6 h; Fig. 4A).
Incubation of seedlings with LaCl3 before challenge with 10 mm H2O2 reproducibly reduced GST1 induction, at all LaCl3 concentrations that had been shown to inhibit the first [Ca2+]cyt peak (Fig. 4B). This calcium peak is thus necessary for full gene induction in response to H2O2 challenge.
The Ca2+-Signal Is Affected by the Redox State of the Cell
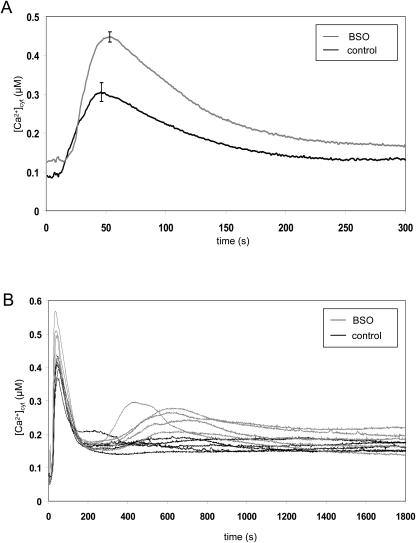
Seedlings were treated with 10 mm BSO, an inhibitor of glutathione synthesis (Griffith and Meister, 1979), prior to challenge with H2O2. BSO treatment significantly increased the first [Ca2+]cyt elevation, from 0.31 μm ± 0.02 to 0.45 μm ± 0.01 (n = 5; Fig. 5A). The treatment also appears to have a stabilizing effect on the second [Ca2+]cyt peak in the H2O2 response; in batches of seedlings that did not exhibit a second peak under control conditions, BSO incubation increased the occurrence and height of the second peak (Fig. 5B; here first peak enhanced from 0.40 μm ± 0.01 to 0.48 μm ± 0.03; n = 5). Hence, the height of the Ca2+-signal appears to be affected by the redox state of the cell.
Figure 5.
Effect of reduced glutathione levels on the H2O2-induced [Ca2+]cyt elevations [Ca2+]cyt was measured by luminometry as described in “Materials and Methods.” Seven-day-old reconstituted seedlings were incubated for 4 to 6 h in 10 mm BSO or water (control) and transferred individually into plastic cuvettes. A, H2O2 (final concentration 10 mm) was added at 5 s. At 300 s, remaining aequorin was discharged. Traces represent the mean of 5 seedlings with error bars indicating se at that time point. B, H2O2 (final concentration 10 mm) was added at 5 s. At 1,800 s, remaining aequorin was discharged. Traces represent responses of 5 individual seedlings per treatment.
An Increase in the Magnitude of the Ca2+ Response Enhances GST1 Gene Expression
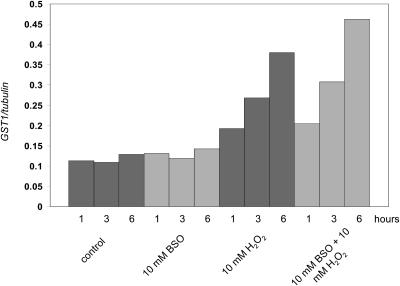
Treatment with BSO was shown to enhance the H2O2-triggered Ca2+ response (increase in the magnitude of the first peak [Fig. 5A] and stabilization of the second peak [Fig. 5B]). In order to determine if this enhancement in the Ca2+ response affects GST1 gene induction, seedlings were incubated in 10 mm BSO before addition of H2O2 (final concentration, 10 mm; Fig. 6). The increase in GST1 levels was enhanced in BSO-treated samples as compared to control samples. A slight, but reproducible increase was seen after 3 h, and a more pronounced increase was seen after 6 h.
Figure 6.
Enhancement of GST1 gene induction by BSO treatment. Northern analysis and normalization of gene induction data was carried out as described in “Materials and Methods.” Seven-day-old RLD1 seedlings were incubated in 5 mL BSO (10 mm) or water (control) for 16 h (overnight, in the dark). H2O2 (final concentration 10 mm) or water was added the following day and samples removed and frozen after 1 h, 3 h, or 6 h as indicated. Total RNA was extracted, electrophoresed, and transferred to nylon membrane. GST1 and β-TUBULIN probes were labeled and hybridized to the RNA on the membrane. Bars represent GST1 gene induction relative to β-TUBULIN mRNA levels.
DISCUSSION
Challenge of whole seedlings with H2O2 triggers a biphasic Ca2+ signature with the first peak located in the cotyledons and the second peak in the root (Figs. 1 and 2, A and C). The relatively short delay between H2O2 application and the first Ca2+ rise indicates that this Ca2+ influx may be one of the earliest responses to H2O2 perception. However, the delay between stress treatment and onset of the second, root-localized Ca2+ peak (5–20 min) indicates that shoot and root tissues employ different H2O2 signal transduction/perception mechanisms. Challenging seedlings expressing aequorin in specific root cell types (Kiegle et al., 2000) with H2O2 demonstrated that externally applied H2O2 had access to internal tissues and that the late second peak in the root is therefore not the result of delayed stimulation (data not shown). Further characterization of the second Ca2+ elevation revealed that it was independent of signals emanating from the shoot (Fig. 2B) and that the roots we responding to local concentrations of H2O2.
Interestingly, a recent study demonstrated that aequorin-expressing tobacco cell cultures also display a biphasic [Ca2+]cyt signature in response to H2O2 challenge (Lecourieux et al., 2002), with similar timing to the two [Ca2+]cyt peaks observed in whole Arabidopsis seedlings. It is surprising that both [Ca2+]cyt peaks can be triggered in cell suspension culture cells despite the differential location in shoot and root in Arabidopsis. Therefore, either tobacco and Arabidopsis differ in their Ca2+ signatures with respect to location of the Ca2+ rise or some of the cells have differentiated within the cell suspension culture. It is also possible that H2O2 or other active oxygen species are generated during regeneration of suspension cells.
The [Ca2+]cyt elevations seen in response to H2O2 treatment may arise through activation of hyperpolarization-activated Ca2+-permeable channels. These channels play a critical role in abscisic acid signal transduction and mediate Ca2+ influx across the plasma membrane in response to H2O2 treatment in Arabidopsis guard cells (Pei et al., 2000). However, the delay between H2O2 application and the [Ca2+]cyt elevation (2.4 ± 0.8 min) and the duration of the [Ca2+]cyt transient (2.8 ± 0.5 min) were longer compared to data presented here. Treatment with 0.1 mm and 1 mm lanthanum strongly inhibited the H2O2-triggered [Ca2+]cyt elevation (Pei et al., 2000), similar to the effect seen on the first cotyledon-localized Ca2+ peak (Fig. 3A).
H2O2 challenge caused a second Ca2+ peak particularly in the lower one-half of the root. This [Ca2+]cyt elevation coincides with the localization of hyperpolarization-activated channels which were detected in the root elongation zone (Kiegle et al., 2000) and the tips of actively growing root hairs (Very and Davies, 2000). Again, lanthanum was shown to block Ca2+ influx in both these studies and correspondingly reduced the root-localized [Ca2+]cyt elevation in the data presented here (Fig. 3B). In contrast to our results, Demidchik et al. (2003) and Foreman et al. (2003) report Ca2+ influx across the plasma membrane in response to OH˙ but not H2O2 application. However, these measurements were performed on protoplasts. It is possible that in our system, H2O2 reacts with cell wall components to yield OH˙ that ultimately triggers the observed rise in Ca2+; alternatively, a different Ca2+ channel could be responsible that requires the presence of the cell wall for activation.
In order to establish a link between early AOS signaling events and protective gene induction, the necessity of the H2O2-triggered Ca2+ response for full induction of GST1 was investigated. GST1 had previously been shown to be induced by AOS and oxidative stress treatment (Levine et al., 1994). In this study, GST1 transcript levels increased in response to H2O2 treatment (Fig. 4A). By modulating the Ca2+ signal, we demonstrated that the magnitude of the Ca2+ response is correlated with the level of GST1 gene induction. Incubation with the Ca2+ channel blocker lanthanum strongly inhibited the first rise in Ca2+ in response to H2O2 treatment (Fig. 3A), whereas reduction of the cellular glutathione content by incubation with BSO enhanced this peak (Fig. 5A). Accordingly, lanthanum treatment decreased GST1 induction in response to H2O2 treatment (Fig. 4B), whereas incubation with BSO enhanced expression (Fig. 6). In a previous study, the role of the ozone-triggered biphasic Ca2+ signature in GST1 induction was investigated (Clayton et al., 1999). The authors speculated that only the second, but not the first, Ca2+ peak was inhibited by lanthanum treatment and consequently that this peak was involved in GST1 induction. However, upon closer inspection of the data, the height of the first Ca2+ peak had also decreased. Therefore, data presented here are consistent with these previous results (if not with the interpretation). Clayton et al. (1999) also demonstrated that treatment of seedlings with the Ca2+-agonist BAYK-8644 is sufficient for induction of GST1 expression, confirming our hypothesis that the Ca2+-signal plays a role in H2O2-triggered GST1 gene induction. However, our observation that GST1 induction is only partially inhibited after LaCl3 treatment (Fig. 4B) may indicate that Ca2+-independent signaling pathways also play a role in H2O2-triggered gene regulation.
Reducing the cellular redox buffer by inhibition of glutathione synthesis, and thus reducing the ability of the cell to maintain a high GSH/GSSG ratio, led to an increase in the magnitude of the early Ca2+ peak in response to H2O2 challenge (Fig. 5A). This result is consistent with a previous study in tobacco, demonstrating that treatments which increase or reduce the cellular GSH/GSSG ratio have opposite effects on the rise in [Ca2+]cyt (Price et al., 1994). BSO treatment was also shown to have a stabilizing effect on the root-localized second Ca2+ peak in response to H2O2 challenge (Fig. 5B), indicating that the redox balance may also play a role in this late [Ca2+]cyt elevation. Hence, the signal transduction pathway in response to AOS may be mediated by redox signaling through changes in the GSH to GSSG ratio upstream of changes in [Ca2+]cyt, leading to activation of downstream protective mechansims such as GST1 gene induction (Fig. 6). Consistent with these findings, the Arabidopsis cad2-1 mutant, which is deficient in glutathione (Cobbett et al., 1998) shows enhanced first and second calcium peaks in response to oxidative stress (N.H. Evans, M.R. McAinsh, A.M. Hetherington, and M.R. Knight, unpublished data).
In conclusion, the data presented here indicate the necessity for Ca2+ responses in linking H2O2 perception with induction of GST1 gene expression. The Ca2+ signals may be triggered by changes in the cellular redox balance, the level of which informs the plant cell of the oxidative stress severity and hence the appropriate level of antioxidant gene induction. Interestingly, H2O2 causes Ca2+ peaks by different mechanisms in shoot and root tissues; the basis for these differences remains to be determined.
MATERIALS AND METHODS
All chemicals were obtained from Sigma (St. Louis) unless stated otherwise.
Plant Material and Growth Conditions
All gene expression studies were carried out with Arabidopsis ecotype RLD1 (Lehle Seeds, Roundrock, TX). For Ca2+ measurements, the transgenic line RLD1.1 (Polisensky and Braam, 1996) constitutively expressing aequorin (Knight et al., 1991) was used. Seedlings were grown on Murashige and Skoog medium (0.8% [w/v] agar) at 21°C, 60 μm m−2 s−1 light intensity with a 16-h photoperiod as described previously (Knight et al., 1999). Seeds were sterilized in 70% (v/v) ethanol for 5 min, dried on filter paper, and vernalized on Murashige and Skoog plates at 4°C for 2 d before transfer to the growth chamber.
In Vivo Reconstitution of Aequorin and Ca2+ Measurements
Aequorin expressing seedlings or plants (RLD1.1) grown on Murashige and Skoog agar were transferred into water and coelenterazine solution (ProLume, Woburn, MA; final concentration 10 μm, 1% [v/v] methanol) was added. Reconstitution was allowed to proceed for a minimum of 16 h at 21°C in the dark (Knight et al., 1991). Six-day-old seedlings (approdimately 100/well in 6-well plates) were floated on 2 mL solution.
For Ca2+ measurements by luminometry, a digital chemiluminometer with discriminator was used (Electron Tubes, Ruislip, UK), fitted with a cooling unit for the photomultiplier tube (to approximately −25°C) in order to reduce background noise (Knight et al., 1991, 1996). After aequorin reconstitution, RLD1.1 seedlings were placed individually into plastic cuvettes (Sarstedt, Nümbrecht, Germany) containing 0.5 mL water. Following a resting period of 25 to 45 min to recover from the touch response, the cuvettes were individually inserted into the luminometer chamber and luminescence counts were recorded at 1 s intervals. After 5 s of counting, 0.5 mL of 20 mm H2O2 solution was added from a syringe through a luminometer port and measurements continued over the indicated time periods. Remaining aequorin was discharged by addition of 1 mL CaCl2 solution (final concentration 1 m, 10% ethanol), and counts recorded for a further 5 min until values were within 1% of the highest discharge value. The luminescence counts obtained were calibrated by applying the equation below, which takes into account the double logarithmic relationship between the concentration of free calcium present in cells and the remaining aequorin discharged at any point in time (Fricker et al., 1999).
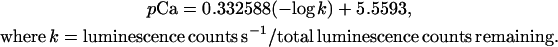 |
This calibration equation was determined empirically and works on the assumption that all aequorin is discharged from all cells and all emitted light is detected. For Ca2+ imaging in the CCD camera, a three microchannel plate intensified CCD camera (Photek 216, Photek, Hastings, UK) was used. Photon emissions were stored by the camera as an array of pixels and the x-y coordinates of the images were established by the software (IFS216 Software, Photek). This data was used to analyze changes in [Ca2+]cyt over time as well as in different parts of the plant.
Following aequorin reconstitution, 7-d-old plants were laid onto moist filter paper (Whatman, Clifton, NJ, Grade 1) placed on top of 0.8% (v/v) agar. Special care was taken that the entire length of the root was in contact with the paper. After a rest period of 30 to 45 min, the filter paper with the plants was transferred onto a 0.8% (v/v) agar plate containing 10 mm H2O2 (prepared on the same day). This stress plate was immediately placed into a specially constructed dark box underneath the CCD camera.
Northern Analysis
Seven-day-old seedlings were transferred from Murashige and Skoog agar plates into H2O and left for 2 h to 3 h (as indicated) to recover from damage caused by the transfer before further treatment. LaCl3 or BSO were added 1 h or 5 h, respectively, prior to H2O2 challenge. Fifty to 100 7-d-old seedlings were harvested into microcentrifuge tubes. Total RNA was extracted using the RNeasy Plant Total RNA kit (Qiagen, Dorking, UK) according to the manufacturer's protocol. For RNA gel-blot hybridizations, total RNA samples (10 μg/lane) were electrophoresed through 1.0% (w/v) agarose formaldehyde gels (Sambrook and Russell, 2001). RNA was transferred to nylon membranes (Roche, Mannheim, Germany) by capillary action. Blots were prehybridized for 4 h and hybridized overnight in 50% (v/v) formamide at 42°C. Probes were labeled using Ready To Go DNA labeling beads (Amersham Pharmacia, Buckinghamshire, UK) and 25 μCi of [α-32P]dCTP (Amersham Pharmacia). Blots were washed twice in each of the following successively: 2× SSC (1× SSC is 0.15 m NaCl and 0.015 m sodium citrate, pH 7.0) and 0.1% (w/v) SDS, followed by 1× SSC and 0.1% (w/v) SDS, and finally 0.1× SSC and 0.1% (w/v) SDS at 42°C. Probes for β-tubulin were prepared from the products of PCR using specific primers as described previously (Knight et al., 1999). Probe for GST1 (At2g29450) was prepared in the same way, using the primers 5′-TTGCTTCTTGCTCTTAACCC-3′ and 5′-CTCAACCTTCTCCAAATTCC-3′. The hybridization signal was quantified using a phosphorimaging system (GS-525 Molecular Imager System, Bio-Rad, Hercules, CA). Counts obtained for each band were normalized by calculating the GST1/β-tubulin ratio. All experiments involving northern analysis were carried out at least twice.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
This work was supported by the Gatsby Charitable Foundation and the BBSRC.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.042663.
References
- Clayton H, Knight MR, Knight H, McAinsh MR, Hetherington AM (1999) Dissection of the ozone-induced calcium signature. Plant J 17: 575–579 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B (1998) The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. Plant J 16: 73–78 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116: 81–88 [DOI] [PubMed] [Google Scholar]
- Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95 [DOI] [PubMed] [Google Scholar]
- Edwards R, Dixon DP, Walbot V (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Foyer CH, LopezDelgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant 100: 241–254 [Google Scholar]
- Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119: 355–364 [Google Scholar]
- Fricker MD, Plieth C, Knight H, Blancaflor E, Knight MR, White NS, Gilroy S (1999) Fluorescence and luminescence techniques to probe ion activities in living plant cells. In W Mason, ed, Fluorescent and Luminescent Probes for Biological Activity. Academic Press, London, pp 569–594
- Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n butyl homocysteine sulfoximine). J Biol Chem 254: 7558–7560 [PubMed] [Google Scholar]
- Kiegle E, Gilliham M, Haselhoff J, Tester M (2000) Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J 21: 225–229 [DOI] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Veale E, Warren GJ, Knight MR (1999) The sfr6 mutation in Arabidopsis suppresses low-temperature induction of genes dependent on the DRT/DRE sequence motif. Plant Cell 11: 875–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytosolic calcium. Nature 352: 524–526 [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251–275 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Marzars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increase in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Marrs K (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- May M, Leaver CJ (1993) Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension-cultures. Plant Physiol 103: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Clayton H, Mansfield TA, Hetherington AM (1996) Changes in stomatal behaviour and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol 111: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561–591 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49: 249–279 [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Polisensky D, Braam J (1996) Cold-shock regulation of the Arabidopsis Tch genes and the effects of modulating intracellular calcium levels. Plant Physiol 111: 1271–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AH, Taylor A, Ripley SJ, Griffiths A, Trewavas AJ, Knight MR (1994) Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6: 1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW (2001) Molecular Cloning, A Laboratory Manual, Ed 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Very A-A, Davies JM (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranová E, Inzé D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53: 1227–1236 [PubMed] [Google Scholar]
- Wohlgemut H, Mittlestrass K, Kschieschan S, Bender J, Weigel H-J, Overmyer K, Kangasjärvi J, Sandermann HJ, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ 25: 717–726 [Google Scholar]