Abstract
Abscisic acid (ABA) is a major regulator in the adaptation of plants to environmental stresses, plant growth, and development. In higher plants, the ABA biosynthesis pathway involves the oxidative cleavage of 9-cis-epoxycarotenoids, which may be the key regulatory step in the pathway catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED). We developed a new inhibitor of ABA biosynthesis targeting NCED and named it abamine (ABA biosynthesis inhibitor with an amine moiety). Abamine is a competitive inhibitor of NCED, with a Ki of 38.8 μm. In 0.4 m mannitol solution, which mimics the effects of osmotic stress, abamine both inhibited stomatal closure in spinach (Spinacia oleracea) leaves, which was restored by coapplication of ABA, and increased luminescence intensity in transgenic Arabidopsis containing the RD29B promoter-luciferase fusion. The ABA content of plants in 0.4 m mannitol was increased approximately 16-fold as compared with that of controls, whereas 50 to 100 μm abamine inhibited about 50% of this ABA accumulation in both spinach leaves and Arabidopsis. Abamine-treated Arabidopsis was more sensitive to drought stress and showed a significant decrease in drought tolerance than untreated Arabidopsis. These results suggest that abamine is a novel ABA biosynthesis inhibitor that targets the enzyme catalyzing oxidative cleavage of 9-cis-epoxycarotenoids. To test the effect of abamine on plants other than Arabidopsis, it was applied to cress (Lepidium sativum) plants. Abamine enhanced radicle elongation in cress seeds, which could be due to a decrease in the ABA content of abamine-treated plants. Thus, it is possible to think that abamine should enable us to elucidate the functions of ABA in cells or plants and to find new mutants involved in ABA signaling.
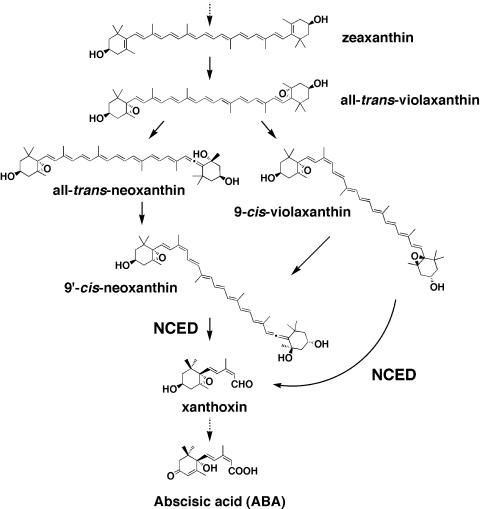
Plants can respond to environmental stresses, such as drought, cold, and high salt, and can control aspects of their growth and development. One important regulator of these responses is abscisic acid (ABA; Shinozaki and Yamaguchi-Shinozaki, 1999; Zhu, 2001; Finkelstein et al., 2002). The phytohormone ABA plays a major role in adaptation to environmental stresses and regulation of growth and development. Unlike other plant hormones, the endogenous concentration of ABA increases more than 10-fold within a few hours of drought stress and decreases dramatically to normal levels following rehydration (Zeevaart, 1980). Much evidence indicates that in higher plants ABA is synthesized via oxidative cleavage of epoxycarotenoids (Zeevaart and Creelman, 1988; Li and Walton, 1990; Parry et al., 1990). A number of steps may be regulated in the ABA biosynthesis pathway in higher plants, but special interest has focused on the enzyme involved in the oxidative cleavage of 9-cis-epoxycarotenoids, which is the key regulatory step in ABA biosynthesis in response to environmental stresses (Fig. 1; Schwartz et al., 1997; Tan et al., 1997; Qin and Zeevaart, 1999). This enzyme, 9-cis-epoxycarotenoid dioxygenase (NCED), catalyzes the cleavage of 9-cis-epoxycarotenoids to apocarotenoid (C25) and xanthoxin (C15; Schwartz et al., 1997) and is up-regulated by water-deficit stress (Bray, 2002). Moreover, NCED genes are not regulated by ABA, which indicates that ABA does not have a positive feedback effect on NCED gene expression (Iuchi et al., 2000; Thompson et al., 2000). NCED genes encoding NCED-like enzymes have been isolated from bean, cowpea, tomato, Arabidopsis, and avocado (Burbidge et al., 1997; Neill et al., 1998; Qin and Zeevaart, 1999; Chernys and Zeevaart, 2000; Iuchi et al., 2000).
Figure 1.
ABA biosynthesis pathway in higher plants. ABA is derived from C40-carotenoids, such as 9-cis-violaxanthin and 9′-cis-neoxanthin, via the oxidative cleavage catalyzed by NCED. This step is the key regulatory step in the ABA biosynthesis pathway.
Several compounds, such as fluridone and norflurazon, have been used to identify ABA functions in plants (Grappin et al., 2000; Thompson et al., 2000; Moreno-Fonseca and Covarrubias, 2001; Ullah et al., 2002). Fluridone and norflurazon inhibit phytoene desaturase, which converts phytoene to phytofluene in the carotenoid biosynthesis pathway. Since carotenoids are the main precursors of ABA in plants, carotenoid biosynthesis inhibitors should also prevent the biosynthesis of ABA (Gamble and Mullet, 1986; Yoshioka et al., 1998; Grappin et al., 2000). However, the upstream inhibition of carotenoid biosynthesis using fluridone and norflurazon causes lethal damage during plant growth because carotenoids play an important role in protecting photosynthetic organisms against photooxidation damage and absorb light energy in plants (Britton et al., 1998). Therefore, the use of these phytoene desaturase inhibitors in the investigation of ABA functions is limited to narrow physiological aspects.
In view of the indispensable nature of carotenoids and the importance of ABA functions in plants, it is worthwhile synthesizing and evaluating specific inhibitors of ABA biosynthesis that would be useful tools for functional studies of ABA biosynthesis and the effects of ABA in higher plants. In such studies, one advantage of ABA biosynthesis inhibitors over ABA-deficient mutants is that inhibitors can be applied to almost every plant. Moreover, ABA biosynthesis inhibitors could provide a useful way to find mutants in which genes involved in ABA signal transduction have been altered, as was seen in mutants of brassinosteroid signal transduction (Wang et al., 2002). In this context, we started designing and synthesizing ABA biosynthesis inhibitors. In developing novel specific ABA biosynthesis inhibitors, NCED is an attractive target because it is the key regulatory enzyme in the ABA biosynthesis pathway (Burbidge et al., 1997). We previously synthesized inhibitors of lignostilbene-α,β-dioxygenase, which has a similar reaction mechanism to NCED (Han et al., 2002, 2003). On the basis of the result of a structure-activity relationship study of LSD inhibitors, we next tried chemical modification of lipoxygenase inhibitor nordihydroguaiaretic acid (NDGA) in order to find NCED inhibitors because NDGA was reported to inhibit ABA biosynthesis (Creelman et al., 1992) and was proved to be an NCED inhibitor in vitro in this report. Eventually, we found a novel NCED inhibitor. This report describes a characteristic of a new inhibitor of the oxidative cleavage of 9-cis-epoxycarotenoids in ABA biosynthesis.
RESULTS
Synthesis of Abamine
LSD catalyzes the oxidative cleavage of the central double bond of stilbene in a manner similar to the cleavage of 9-cis-epoxycarotenoids catalyzed by NCED. As an initial step to develop specific ABA biosynthesis inhibitors, we investigated the structure-activity relationships of LSD inhibitors (Han et al., 2002, 2003). (Z)-1-Fluoro-1-(4-hydroxyphenyl)-2-phenylethene and N-(4-hydroxybenzyl)-3-methoxyaniline were found to be potent competitive inhibitors of this enzyme with IC50 values of 3 and 10 μm, respectively. The polarization induced by fluorine bonded to an sp2-carbon might increase the affinity of this inhibitor to LSD. Moreover, the replacement of a C=C bond with a C-N bond played an important role in the inhibition of LSD. By contrast, these compounds did not inhibit NCED, suggesting that they are specific for LSD.
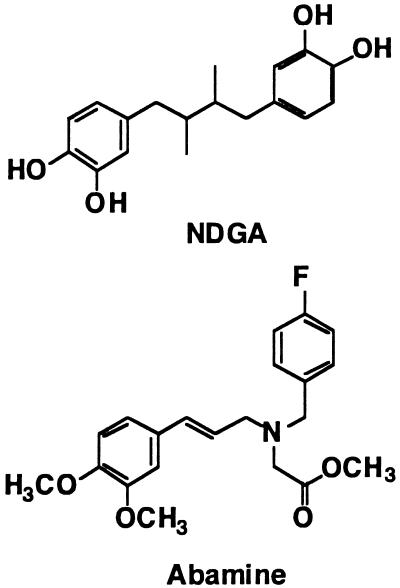
NDGA was reported to inhibit osmotic stress-induced ABA accumulation in vitro (Creelman et al., 1992); however, the target site of NDGA in the inhibition of ABA biosynthesis was unknown. In this study, NDGA was identified as an inhibitor of NCED activity in vitro (Fig. 3) and used as the lead compound in designing ABA biosynthesis inhibitors. NDGA, a catecholic antioxidant, is one of the most effective inhibitors of the lipoxygenase-catalyzed dioxygenation of polyunsaturated fatty acids (Creelman et al., 1992). In addition, NDGA decreases the frequency of mitosis in plant growth, which suggests that the effects of NDGA are linked to a partial blockage of dividing cells in G1 phase. NDGA also strongly inhibits lipid synthesis and affects the morphology of the endoplasmic reticulum in plant cells (Kemal et al., 1987; Merigout et al., 2002). That is, NDGA is not a specific ABA biosynthesis inhibitor and is not useful for understanding the specific functions of ABA in plants. Whitman et al. (2002) reported that alkyl substituted NDGA derivatives did not inhibit any lipoxygenases, demonstrating that a phenol group is required for the inhibition of lipoxygenases. In this context, we assumed that the total shape of NDGA should fit into the NCED binding site instead of its substrate and shows the inhibitory activity of ABA biosynthesis. Therefore, the reduction of the efficacy of NDGA for the lipoxygenase inhibition by alkylation of phenol group could strengthen the specificity of NDGA to ABA biosynthesis inhibition. In order to develop specific ABA biosynthesis inhibitors targeting NCED, a number of compounds were designed and synthesized based on the structures of NDGA and LSD inhibitors. Eventually, a novel inhibitor of ABA biosynthesis was developed and named abamine (ABA biosynthesis inhibitor with an amine moiety); it was the most potent and specific ABA biosynthesis inhibitor targeting NCED (Fig. 2) in our study.
Figure 3.
HPLC analysis of NCED inhibition activities. NCED activity was estimated in the presence of 23 μm 9′-cis-neoxanthin and 3.24 μg/mL enzyme at pH 7.0 at 20°C. The product, C25-apocarotenoid, was analyzed by HPLC at 440 nm wavelength. Error bars indicate the sd.
Figure 2.
The chemical structures of NDGA and abamine. A novel ABA biosynthesis inhibitor was named abamine (ABA biosynthesis inhibitor with an amine moiety).
NCED Assay and Kinetic Analysis
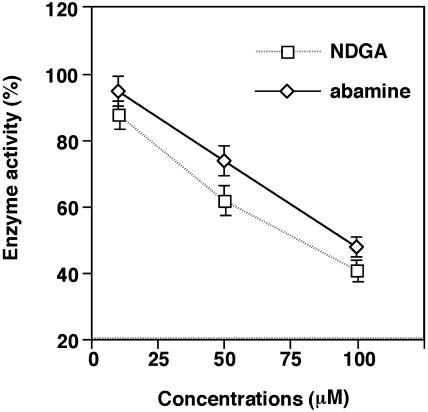
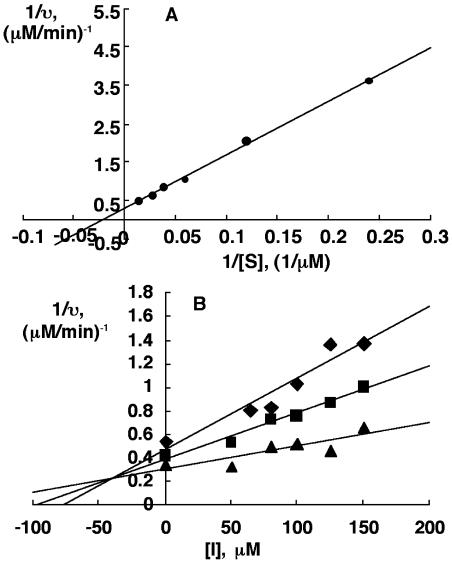
First, we demonstrated the in vitro inhibition of NCED expressed in Escherichia coli. When NCED was incubated with 9′-cis-neoxanthin, the main products of this reaction were C25-apocarotenoid and xanthoxin. To quantify the molar amounts of the products, the C25-products were analyzed by HPLC using all-trans-violaxanthin as an internal standard. As shown in Figure 3, NCED activity was inhibited more than 50% by 100 μm NDGA or abamine in the presence of 23 μm 9′-cis-neoxanthin. This indicates that NDGA and abamine are NCED inhibitors. Using Lineweaver-Burk plots, the Km of NCED for 9′-cis-neoxanthin was determined to be 49.0 μm (Fig. 4A). This Km value was similar to that recently reported by Schwartz et al. (2003). Subsequently, we performed the inhibition kinetic analysis only for abamine because NDGA was phytotoxic in vivo, as described below. The data in Figure 4B show that abamine is a potent competitive inhibitor of NCED, with a Ki of 38.8 μm determined from a Dixon plot.
Figure 4.
Kinetic analysis of the inhibition of NCED by abamine. A, NCED activity was measured in the presence of 4 to 100 μm 9′-cis-neoxanthin and 3.24 μg/mL enzyme at pH 7.0 at 20°C. The Km of NCED for 9′-cis-neoxanthin was determined to be 49.0 μm using Lineweaver-Burk plots. B, NCED activity was measured in the presence of 42 μm (♦), 54 μm (▪), and 67 μm (▴) 9′-cis-neoxanthin and 3.24 μg/mL enzyme with the indicated concentrations of abamine at pH 7.0 at 20°C. Abamine is a potent competitive inhibitor of NCED, with a Ki of 38.8 μm determined from a Dixon plot.
The Effect of Abamine and NDGA on the Regulation of Stomatal Closure in Vitro
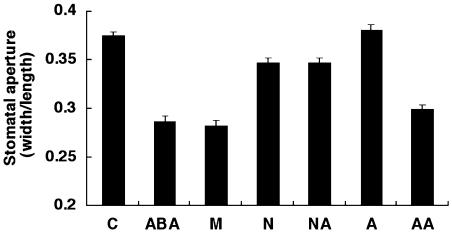
In guard cells, ABA regulates stomatal apertures by inhibiting stomatal opening and inducing stomatal closure in response to drought stress (Uno et al., 2000). The ABA levels increased 10-fold more in water-deficit stressed tissues than in nonstressed tissues (Creelman and Zeevaart, 1985). Since water-deficit stress using mannitol induces ABA accumulation, we tested whether NDGA and abamine inhibit guard cell closure under these conditions. A high concentration of mannitol is expected to mimic the effect of water-deficit stress and increase the ABA concentration. That is, incubation in 0.4 m mannitol imposes osmotic stress on guard cells. As illustrated in Figure 5, we treated epidermal cells from spinach (Spinacia oleracea) with 100 μm abamine, NDGA, or 10 μm ABA to assess the effect of these inhibitors on stomatal closure. Treatment with 0.4 m mannitol caused stomatal closure similar to that seen with 10 μm ABA. Under these conditions, both abamine and NDGA inhibited stomatal closure. The coapplication of 10 μm ABA plus 100 μm abamine produced a response similar to that with ABA alone. By contrast, NDGA abolished the promotion of stomatal closure even when coapplied with ABA, although it reduced stomatal closure in 0.4 m mannitol. In the lipoxygenase assay, abamine showed no inhibitory activity (data not shown). These results also suggest that abamine should be a specific inhibitor of ABA biosynthesis.
Figure 5.
The effect of abamine or NDGA treatment on stomatal aperture. Epidermal strips were immersed in 0.4 m mannitol with or without 100 μm inhibitors or 10 μm ABA and incubated for 3 h: 10 mm HEPES (C), 10 μm ABA (ABA), 0.4 m mannitol (M), 100 μm NDGA + 0.4 m mannitol (N), 100 μm NDGA + 10 μm ABA + 0.4 m mannitol (NA), 100 μm abamine + 0.4 m mannitol (A), 100 μm abamine + 10 μm ABA + 0.4 m mannitol (AA). The data show the mean ± se of at least three independent experiments measuring at least 100 stomata. All solutions included DMSO at approximately 0.02%.
The Accumulation of ABA under Osmotic Stress
To examine whether abamine inhibits osmotic stress-induced ABA accumulation, we determined the ABA content of spinach leaves after incubation in 0.4 m mannitol. The ABA content in 10 mm HEPES (pH 6.5) was 11.3 ng/g fresh weight (FW). After treatment with 0.4 m mannitol, the ABA content in the leaves increased 16-fold to 178.4 ng/g FW. At 100 μm, abamine inhibited the ABA accumulation in response to osmotic stress by 54% (Table I). At 50 μm, NDGA had a weak inhibitory effect (<10%) on ABA accumulation inhibition (data not shown), while abamine caused more than 30% inhibition. These results demonstrate that abamine is a stronger ABA biosynthesis inhibitor than NDGA. The test was repeated in triplicate under the same experimental conditions and similar results were obtained. In a previous study, NDGA inhibited ABA accumulation by more than 90% under conditions similar to those used here (Creelman et al., 1992), while it did not show clear inhibitory activity in our experiments.
Table I.
The effect of abamine on ABA accumulation in spinach leaf slices in the presence of 0.4 m mannitol
| ABA | |
|---|---|
| ng/g FW | |
| Control | 11.3 |
| 0.4 m Man | 178.4 |
| 100A | 82.8 |
| 50A | 120.6 |
Leaf slices were immersed in 10 mm HEPES (pH 6.5 with KOH) plus 0.4 m mannitol with or without abamine and incubated for 4 h. All experiments were performed in triplicate under the same experimental conditions and similar results were obtained: 10 mm HEPES (Control), 0.4 m mannitol (0.4 m Man), 100 μm abamine + 0.4 m mannitol (100A), 50 μm abamine + 0.4 m mannitol (50A).
The Effect of Abamine on RD29B::LUC Expression
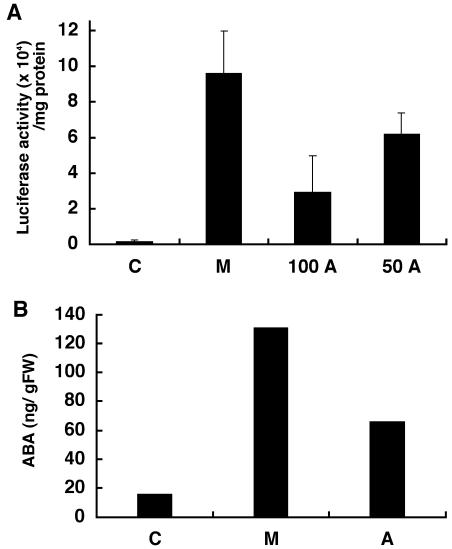
In Arabidopsis, the expression of the endogenous RD29B gene containing ABA-responsive elements in the promoter region is increased by drought stress and exogenous ABA treatment (Yamaguchi-Shinozaki and Shinozaki, 1993; Uno et al., 2000). If abamine inhibits ABA biosynthesis and decreases ABA accumulation, RD29B expression should be down-regulated. In this context, we used RD29B::LUC transgenic Arabidopsis to determine the effect of abamine on ABA biosynthesis. Figure 6A shows the luminescence of transgenic Arabidopsis after treatment with or without 0.4 m mannitol to impose osmotic stress. With 0.4 m mannitol, more RD29B::LUC was expressed than in untreated plants. Treatment with 100 or 50 μm abamine significantly reduced the luminescence as compared with 0.4 m mannitol treatment without the inhibitor, suggesting that abamine inhibits ABA biosynthesis and reduces the expression of this gene.
Figure 6.
The effect of abamine in Arabidopsis. A, RD29B::LUC expression in RD29B::LUC transgenic Arabidopsis. B, The accumulation of ABA in the presence of 0.4 m mannitol: 10 mm HEPES (C), 0.4 m mannitol (M), 100 μm abamine + 0.4 m mannitol (100A), 50 μm abamine + 0.4 m mannitol (50A). All experiments were performed in triplicate under the same experimental conditions and similar results were obtained. The error bars indicate the sd of the means.
The Accumulation of ABA in Arabidopsis
Abamine inhibited stomatal closure and ABA accumulation in spinach leaves incubated in 0.4 m mannitol. To confirm that inhibition of RD29B::LUC expression in Arabidopsis was accompanied by the suppression of ABA accumulation, the amounts of endogenous ABA in 10-d-old RD29B::LUC transgenic Arabidopsis grown in the light were analyzed using the same method as used to analyze ABA accumulation in spinach leaves (Fig. 6B). The ABA content was increased 8-fold in the presence of mannitol as compared with untreated Arabidopsis, but the accumulation of ABA in Arabidopsis treated with 100 μm abamine was about 50% lower than that without abamine. This result was similar to that for spinach and demonstrates that abamine inhibits ABA biosynthesis under osmotic stress in Arabidopsis.
The Effect of Abamine under Dehydration Treatment
To estimate the survival rate of Arabidopsis after drought treatment, 3-week-old Arabidopsis were treated with dimethyl sulfoxide (DMSO; 1 mg/plant), abamine (0.1 mg/plant), or abamine (0.1 mg/plant) plus ABA (0.001 mg/plant), exposed to drought stress for 10 d, and then rehydrated to allow recovery. The survival rate was defined as the number of healthy plants after drought treatment and rehydration, divided by the total number of plants (Table II). Arabidopsis not treated with abamine tolerated the drought treatment, and 83% of the plants survived, whereas virtually all the leaves of abamine-treated Arabidopsis became wilted and curled. Although some plants recovered from drought, up to 72% of abamine-treated Arabidopsis died, and the plants showed reduced growth as compared with controls. The survival rate was altered with exogenous ABA treatment.
Table II.
Survival rates of chemical-treated plants
| Survival Rate | |
|---|---|
| % | |
| Control | 83 ± 17 |
| Abamine | 28 ± 14 |
| Abamine + ABA | 63 ± 23 |
Arabidopsis was grown in a growth chamber at 22°C under 16-h-light/8-h-dark conditions (150 μE m−2 s−1). The 3-week-old plants were treated with DMSO (1 mg/plant), abamine (0.1 mg/plant), or abamine (0.1 mg/plant) + ABA (0.001 mg/plant) and then exposed to drought stress. The experiments were performed in triplicate.
Radicle Elongation in Cress Seeds
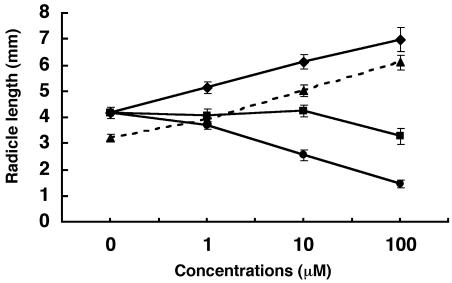
One of the advantages of biosynthesis inhibitors over mutants is that they can allow rapid, conditional, reversible, selective, and dose-dependent control of biological functions: they act like conditional mutations. More importantly, we can test them in every plant and know the function of the targets. Cress (Lepidium sativum) was thus selected to test the efficacy of abamine because no ABA deficient mutant of it has ever been reported. To estimate the effect of abamine on radicle growth, cress seeds were placed in various concentrations of abamine, fluridone, or NDGA with or without 0.1 μm ABA. Abamine had a significant effect on the radicle length measured after a 24-h incubation (Fig. 7). The mean radicle length of controls was 4.2 mm. In abamine-treated seeds, the radicles emerged from the seed coats within 15 h, which was faster than in untreated controls (data not shown). At 100 μm abamine, the radicle length was about 150% that of the controls. NDGA had almost no effect on radicle elongation, possibly due to its phytotoxicity, whereas fluridone had a negative effect on radicle growth (Fig. 7).
Figure 7.
Radicle elongation in cress seeds at various inhibitor concentrations. The length of the radicle was measured after 24 h at 25°C in the dark. Abamine (♦), abamine + 0.1 μm ABA (▴), NDGA (▪), and fluridone (•). Each experiment was performed in triplicate under the same conditions. The error bars indicate the se of the means.
DISCUSSION
Abamine inhibits NCED activity in vitro and stomatal closure, ABA-induced gene expression of RD29B, and the ABA accumulation induced by osmotic stress. These results suggest that abamine should be an ABA biosynthesis inhibitor in planta. The dehydration test also supports the notion that abamine inhibits ABA biosynthesis in mature plants and makes them less tolerant to dehydration. This result is in good agreement with those for ABA-deficient mutants.
Recently, AtNCED3, an Arabidopsis NCED gene, antisense transgenic plants, and T-DNA-tagged knockout mutants have been reported (Iuchi et al., 2001). AtNCED3 antisense plants and T-DNA-tagged mutants are more sensitive to drought, and water loss via transpiration is faster than in wild-type plants. This also demonstrates that abamine inhibits ABA biosynthesis under drought stress, resulting in inhibition of ABA-induced stomatal closure and decreased drought tolerance.
The first visible sign of seed germination is the emergence of the radicle from the testa. Radicle emergence is believed to depend on both cell wall weakening and sufficient growth of the embryo to overcome the resistance of the endosperm. In tobacco seed germination, endosperm rupture is related to the induction of class I β-1,3-glucanase. ABA treatment delays endosperm rupture and inhibits class I β-1,3-glucanase induction (Leubner-Metzger and Meins, 2000). A study of the role of ABA in the weakening of the endosperm cap in tomato seeds suggested that ABA inhibits the second step in the endosperm cap weakening process (Toorop et al., 2000). Moreover, expansins, which are plant proteins expressed in germinating seeds, induce cell wall extension (Chen et al., 2001). It has been suggested that ABA does not prevent the expression of expansins but plays roles in down-regulating them. We found that abamine promoted radicle elongation in cress seeds, probably via the inhibition of ABA biosynthesis, and this effect was inhibited by 0.1 μm ABA (Fig. 7). However, fluridone and NDGA had a negative effect on radicle elongation, probably because of their side effects. The effect of abamine on the promotion of radicle elongation may be applied to screening mutants that are insensitive to ABA deficiency caused by abamine treatment.
Other than ABA, carotenoid cleavage products (apocarotenoids) are widespread in plants and play roles as pigments, flavors, aromas, and defense compounds. The first step in their biosynthesis is also the oxidative cleavage of a carotenoid catalyzed by nonheme iron oxygenase called carotenoid cleavage dioxygenase (CCD). These enzymes have conserved regions present in carotenoid cleavage enzymes (Giuliano et al., 2003). On the basis of the similarities of the sequence and the reaction mechanisms between CCD and NCED, it can be postulated that abamine may target CCD. Further investigation on the kinetic study of abamine against CCD will reveal the specificity of abamine to NCED.
In conclusion, we found that abamine should be an ABA biosynthesis inhibitor that inhibits NCED. The characteristic that distinguishes abamine from phytoene desaturase inhibitors, which have been used to reduce the ABA content in plants, is that abamine does not cause an albino phenomenon in treated plants, which makes it possible to use abamine as a plant growth regulator. In fact, abamine accelerates radicle elongation and stimulates germination under stress conditions. In addition, abamine should be useful in studying ABA function and the mechanism of ABA biosynthesis or catabolism in plants as was demonstrated by acetylenic ABA derivative (Cutler et al., 2001). More importantly, by use of chemical genetic approaches to plant biology (Asami et al., 2003; Blackwell and Zhao, 2003), abamine should prove useful for finding mutants in genes involved in ABA signal transduction, as seen with other plant hormone-related chemicals (Gallardo et al., 2002; Wang et al., 2002; Zhao et al., 2003).
MATERIALS AND METHODS
Chemicals
The chemicals and reagents used in this study were purchased from Wako Pure Chemical, Tokyo, or Kanto Chemical, Tokyo. NDGA was purchased from Tokyo Chemical Industry, Tokyo. 9′-cis-Neoxanthin and all-trans-violaxanthin for the NCED assay were purified from spinach (Spinacia oleracea) leaves (Iuchi et al., 2000). Standard samples of carotenoids were purchased from Wako Pure Chemical.
Synthesis of Abamine, [[3-(3,4-dimethoxyphenyl)allyl]-(4-fluorobenzyl)amino]acetic acid methyl ester
An ABA biosynthesis inhibitor, which is described later in this report, was synthesized from 3,4-dimethoxycinnamic acid using previously reported reactions (Soai et al., 1987; Shishido et al., 1990; Cushman et al., 1993; Tanaka et al., 2001). Liquid, 1H-NMR (300 MHz, CDCl3) δ: 7.34 (2H, m), 7.03-6.87 (4H, m), 6.79 (1H, d, J = 8.2 Hz), 6.47 (1H, d, J = 15.8 Hz), 6.11 (1H, dt, J = 15.8, 6.8 Hz), 3.89 (3H, s), 3.86 (3H, s), 3.77 (2H, s), 3.66 (3H, s), 3.38 (2H, d, J = 6.8 Hz), 3.34 (2H, s). 13C-NMR (125 MHz, CDCl3) δ: 171.7, 162.0 (J = 245.7 Hz), 149.0, 148.8, 134.2, 132.9, 130.5 (d, J = 7.7 Hz), 129.9, 124.8, 119.5, 115.1 (d, J = 21.0 Hz), 111.0, 108.6, 57.4, 56.4, 55.9, 55.8, 53.6, 51.4. Anal. Calcd for C21H24FNO4· 1/3H2O: C, 66.47; H, 6.56; N, 3.69. Found: C, 66.57; H, 6.44; N, 3.62.
Plant Material
Spinach was purchased from a local market and epidermal cells were isolated. Arabidopsis ecotype Columbia was purchased from Lehle Seeds (Round Rock, TX) and used in all the experiments described in this paper. Cress seeds (Lepidium sativum) were purchased locally.
Protein Extraction and Purification
NCED from cowpea (Iuchi et al., 2000) was expressed in Escherichia coli and purified. Its enzymatic activity was assayed as described (Iuchi et al., 2001). The protein concentration was determined using a Bio-Rad kit (Bio-Rad Laboratories, Hercules, CA) based on the Lowry method.
In Vitro NCED Assay
The reaction mixture consisted of 100 mm Tris (pH 7.0), 0.05% (v/v) Triton X-100, 10 mm ascorbate, 0.5 mm FeSO4, and 50 μL of enzyme (0.01 unit = 3.24 μg/mL) solution in a total volume of 200 μL. Appropriate amounts of substrate and inhibitors were added in 5 μL of ethanol. The entire enzyme reaction assay was performed under dim light. The reaction mixture was incubated at room temperature (20°C) for 10 min and was stopped by the addition of 800 μL of water. All-trans-violaxanthin was used as an internal standard. All values were corrected for the recovery of added all-trans-violaxanthin. The products were extracted three times with ethyl acetate (1 mL). The extracts were evaporated to dryness and redissolved in 50 μL of methanol. We identified the predicted C25 compound by HPLC on an ODS H 3151 column (150 mm length, 8 mm i.d.; Senshu Scientific, Tokyo). The column was eluted with a linear gradient between solvents A (85:15, v/v, methanol:water) and B (1:1, v/v, chloroform:methanol) at a flow rate of 1.5 mL/min. The concentration of solvent B was increased from 10% to 50% over 20 min and then kept at 50% for 5 min. The substrate carotenoid and C25 product were monitored with aUV/visible detector at 440 nm. Kinetic parameters, such as Km, were evaluated using Lineweaver-Burk plots. The enzyme-inhibitor inhibition constant Ki and the mechanism of inhibition were determined from Dixon plots.
Stomatal Aperture Measurement
Fully expanded young leaves of spinach were used in all experiments, as reported previously (Creelman and Zeevaart, 1985). Stomatal apertures were measured with a microscope (IX70; Olympus, Tokyo) fitted with a camera (DP50; Olympus) linked to a personal computer. The width of the stomatal aperture and the inner height of stomata were measured. The data show the mean ± se of three independent experiments measuring at least 100 stomata.
Stimulation of ABA Biosynthesis in Spinach Leaf Slices and Arabidopsis Seedlings
Spinach leaf slices were prepared as reported (Creelman and Zeevaart, 1985). Fully expanded leaves were detached, the midribs were removed, and the leaves were sliced into 3-mm-wide strips with a sharp razor blade. The leaf slices were incubated in 10 mm HEPES (pH 6.5 with KOH) containing 2.5 g/L PVP-40 for 10 min and then placed in 10 mm HEPES (pH 6.5 with KOH) for 2 h at each final concentration of inhibitors. To ensure that the slices did not experience anaerobic conditions, air was bubbled into the solution throughout the incubation. Subsequently, the leaf slices were added to10 mm HEPES consisting of 0.4 m mannitol with or without inhibitors and incubated for 4 h. All experiments were performed at least three times. Arabidopsis seeds were surface-sterilized in 1% (w/v) solution of NaOCl for 15 min, washed with sterile distilled water five times, and sown on 0.8% (w/v) agar-solidified medium containing one-half Murashige and Skoog salts and 1.5% (w/v) Suc. The plates were incubated for 3 d at 4°C and then transferred to 22°C under continuous light. The sample for estimating the ABA level of the 10-d-old Arabidopsis was prepared using the same method described above.
ABA Extraction and Quantification
After incubation, the spinach slices and incubation solution were separated and the slices were washed with distilled water (10 mL). Then, the wash water was combined with the incubation solution. The slices were homogenized and extracted in 80% methanol including 2,6-di-tert-butyl-4-methylphenol (200 mg/L). The ABA content was measured using a minor modification of a reported method (Gawronska et al., 1995). Gas chromatography-mass spectrometry analysis was carried out on a JEOL Automass JMS-AM 150 mass spectrometer connected to a Hewlett-Packard 5890-A-II gas chromatograph with a capillary DB-1 (J&W Scientific, Folsom, CA) column (0.25 mm × 15 m, 0.25 mm film thickness). [13C2]ABA was used as an internal standard (Asami et al., 1999). The gas chromatography-selected ion monitoring responses at m/z 190 (base peak of ABA) and m/z 192 (base peak of 13C2-ABA) were monitored. Extraction and quantification of ABA in Arabidopsis was estimated using the same method. This experiment was done in triplicate and gave similar results.
In Vitro Luciferase Assay
RD29B::LUC Arabidopsis (K. Nakashima and K. Yamaguchi-Shinozaki, unpublished data) seeds were surface-sterilized in 1% NaOCl (w/v) for 15 min, washed with sterile distilled water five times, and sown on 0.8% (w/v) agar-solidified medium containing one-half Murashige and Skoog salts and 1.5% (w/v) Suc. The plates were incubated for 3 d at 4°C and then transferred to 22°C under continuous light. Ten-day-old seedlings were pretreated with or without abamine at various concentrations for 2 h, and then each sample was immersed in 0.4 m mannitol (10 mm HEPES, pH 6.5) with or without abamine. After a 4-h incubation, the seedlings were homogenized with a pestle. Luciferase assays were carried out using luciferin as the substrate (Promega, Madison, WI), as described (Kimura et al., 2001).
Dehydration Treatment
Plants were grown in 7.5-cm pots filled with a 1:1 perlite:vermiculite. They were grown under a 16-h-light (150 μE m−2 s−1) and 8-h-dark cycle in a growth chamber at 22°C, with three plants in each pot. The pots were separated into three groups (20 pots per group), and the 3-week-old plants were treated with DMSO (1 mg/plant), abamine (0.1 mg/plant), or abamine (0.1 mg/plant) plus ABA (0.001 mg/plant) and then exposed to drought stress. The plants were retreated with the chemicals at the same dose after 4 d. Drought stress was induced by withholding water for 10 d. The plants were then supplied with water. The numbers of plants that survived and continued to grow were counted. The experiments were repeated three times and gave similar results.
Radicle Elongation
Cress seeds were surface-sterilized as described previously and plated on 0.8% (w/v) agar-solidified medium containing one-half Murashige and Skoog salts and 1.5% (w/v) Suc with or without the indicated concentration of inhibitor. To test the effect of ABA on radicle length, 0.1 μm ABA was used. Plastic plates were wrapped in aluminum foil and incubated for 24 h at 25°C. After 24 h, the seeds were photographed with a digital camera and the radicle length was measured for 30 seeds per treatment. Each experiment was performed in triplicate under the same conditions.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AB030293.
Acknowledgments
We thank Mrs. M. Kobayashi and A. Hanada (RIKEN, Plant Science Center) for helpful technical assistance in the ABA measurements. We thank Dr. J. A. D. Zeevaart (Michigan University) for his helpful comments on this paper.
This work was supported in part by the Bioarchitect Research Program at RIKEN, funded by the Science and Technology Agency of Japan.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.039511.
References
- Asami T, Nakano T, Nakashita H, Sekimata K, Shimada Y, Yoshida S (2003) Influence of chemical genetics on plant sciences: shedding light on functions and action mechanisms of brassinosteroids with brassinosteroid biosynthesis inhibitors. J Plant Growth Regul 22: 251–258 [DOI] [PubMed] [Google Scholar]
- Asami T, Sekimata K, Wang JM, Yoneyama K, Takeuchi Y, Yoshida S (1999) Preparation of (±)-[1,2-13C2] abscisic acid for use as a stable and pure internal standard. J Chem Res Synop 11: 658–659 [Google Scholar]
- Blackwell HE, Zhao Y (2003) Chemical genetic approaches to plant biology. Plant Physiol 133: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA (2002) Abscisic acid regulation of gene expression during water-deficit stress in the era of the Arabidopsis genome. Plant Cell Environ 25: 153–161 [DOI] [PubMed] [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H (1998) Carotenoids, Vol 3: Biosynthesis and Metabolism. Birkhauser Verlag, Basel, pp 117–121
- Burbidge A, Grieve T, Jackson A, Thompson A, Taylor I (1997) Structure and expression of a cDNA encoding a putative neoxanthin cleavage enzyme (NCE), isolated from a wilt-related tomato (Lycopersicon esculentum Mill.) library. J Exp Bot 48: 2111–2112 [Google Scholar]
- Chen F, Dahal P, Bradford K (2001) Two tomato expansin genes show divergent expression and localization in embryos during seed development and germination. Plant Physiol 127: 928–936 [PMC free article] [PubMed] [Google Scholar]
- Chernys JT, Zeevaart JAD (2000) Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant Physiol 124: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Bell E, Mullet JE (1992) Involvement of a lipoxygenase-like enzyme in abscisic acid biosynthesis. Plant Physiol 99: 1258–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Zeevaart JAD (1985) Abscisic acid accumulation in spinach leaf slices in the presence of penetrating and nonpenetrating solutes. Plant Physiol 77: 25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M, He H-M, Lin CM, Hamel E (1993) Synthesis and evaluation of a series of benzylaniline hydrochlorides as potential cytotoxic and antimitotic agents acting by inhibition of tubulin polymerization. J Med Chem 36: 2817–2821 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Rose PA, Squires TM, Loewen MK, Shaw AC, Quail JW, Krochko JE, Abrams SR (2001) Inhibitors of abscisic acid 8′-hydroxylase. Biochemistry 39: 13614–13624 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J, Job D (2002) Proteomics of Arabidopsis seed germination. A comparative study of wild-type and gibberellin-deficient seeds. Plant Physiol 129: 823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble PE, Mullet J (1986) Inhibition of carotenoid accumulation and abscisic acid biosynthesis in fluridone-treated dark-grown barley. Eur J Biochem 160: 117–121 [DOI] [PubMed] [Google Scholar]
- Gawronska H, Yang Y-Y, Furukawa K, Kendrick RE, Takahashi N, Kamiya Y (1995) Effects of low irradiance stress on gibberellin levels in pea seedlings. Plant Cell Physiol 36: 1361–1367 [Google Scholar]
- Giuliano G, Al-Babili S, von Lintig J (2003) Carotenoid oxygenases: cleave it or leave it. Trends Plant Sci 8: 145–149 [DOI] [PubMed] [Google Scholar]
- Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M (2000) Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 210: 279–285 [DOI] [PubMed] [Google Scholar]
- Han S-Y, Inoue H, Terada T, Kamoda S, Saburi Y, Sekimata K, Saito T, Kobayashi M, Shinozaki K, Yoshida S, et al (2002) Design and synthesis of lignostilbene-α,β-dioxygenase inhibitors. Bioorg Med Chem Lett 12: 1139–1142 [DOI] [PubMed] [Google Scholar]
- Han S-Y, Inoue H, Terada T, Kamoda S, Saburi Y, Sekimata K, Saito T, Kobayashi M, Shinozaki K, Yoshida S, et al (2003) N-benzylideneaniline and N-benzylamine are potent inhibitors of lignostilbene-α,β-dioxygenase, a key enzyme in oxidative cleavage of the central double bond of lignostilbene. J Enzyme Inhib Med Chem 18: 279–283 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2000) A stress-inducible gene for 9-cis-epoxycarotenoid dioxygenase involved in abscisic acid biosynthesis under water stress in drought-tolerant cowpea. Plant Physiol 123: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemal C, Louis-Flamberg P, Krupinski-Olsen R, Shorter AL (1987) Reductive inactivation of soybean lipoxygenase 1 by catechols: a possible mechanism for regulation of lipoxygenase activity. Biochemistry 26: 7064–7072 [DOI] [PubMed] [Google Scholar]
- Kimura M, Yoshizumi T, Manabe K, Yamamoto YY, Matsui M (2001) Arabidopsis transcriptional regulation by light stress via hydrogen peroxide-dependent and -independent pathways. Genes Cells 6: 607–617 [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G, Meins F (2000) Sense transformation reveals a novel role for class I-1,3-glucanase in tobacco seed germination. Plant J 23: 215–221 [DOI] [PubMed] [Google Scholar]
- Li Y, Walton DC (1990) Violaxantin is an abscisic acid precursor in water-stressed dark-grown bean leaves. Plant Physiol 92: 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigout P, Kepes F, Perret A-M, Satiat-Jeunemaitre B, Moreau P (2002) Effects of brefeldin A and nordihydroguaiaretic acid on endomembrane dynamics and lipid synthesis in plant cells. FEBS Lett 518: 88–92 [DOI] [PubMed] [Google Scholar]
- Moreno-Fonseca LP, Covarrubias AA (2001) Downstream DNA sequences are required to modulate Pvlea-18 gene expression in response to dehydration. Plant Mol Biol 45: 501–515 [DOI] [PubMed] [Google Scholar]
- Neill SJ, Burnett EC, Desikan R, Hancock JT (1998) Cloning of a wilt-responsive cDNA from an Arabidopsis thaliana suspension culture cDNA library that encodes a putative 9-cis-epoxy-carotenoid dioxygenase. J Exp Bot 49: 1893–1894 [Google Scholar]
- Parry AD, Babiano MJ, Horgan R (1990) The role of cis-carotenoids in abscisic acid biosynthesis. Planta 182: 118–128 [DOI] [PubMed] [Google Scholar]
- Qin X, Zeevaart JAD (1999) The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA 96: 15354–15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JAD, McCarty DR (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276: 1872–1874 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, McCarty DR, Welch W, Zeevaart JAD (2003) Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxygenase in the ABA biosynthetic pathway. Biochim Biophys Acta 1619: 9–14 [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K (1999) Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants. R.G. Landes Company, Austin, TX
- Shishido K, Yamashita A, Hiroya K, Fukumoto K (1990) Synthesis of 2,6-disubstituted dihydronaphthalenes and naphthalenes by electrocyclic reaction of o-quinodimethane. A synthesis of (±)-naproxen. J Chem Soc Perkin Trans 1: 469–475 [Google Scholar]
- Soai K, Yokoyama S, Mochida K (1987) Reduction of symmetric and mixed anhydrides of carboxylic acids by sodium borohydride with dropwise addition of methanol. Synthesis: 647–648
- Tan BC, Schwartz SH, Zeevaart JAD, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci USA 94: 12235–12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Tamai T, Mukaiyama H, Hirabayashi A, Muranaka H, Akahane S, Miyata H, Akahane M (2001) Discovery of novel N-phenylglycine derivatives as potent and selective β3-adrenoceptor agonists for the treatment of frequent urination and urinary incontinence. J Med Chem 44: 1436–1445 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Jackson AC, Parker RA, Morpeth DR, Burbidge A, Taylor IB (2000) Abscisic acid biosynthesis in tomato: regulation of zeaxanthin epoxidase and 9-cis-epoxycarotenoid dioxygenase mRNAs by light/dark cycles, water stress and abscisic acid. Plant Mol Biol 42: 833–845 [DOI] [PubMed] [Google Scholar]
- Toorop PE, van Aelst AC, Hilhorst WM (2000) The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J Exp Bot 51: 1371–1379 [PubMed] [Google Scholar]
- Ullah H, Chen J-G, Wang S, Jones AM (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Nakano T, Gendron J, He J, Vafeados D, Chen M, Yang Y, Fujioka S, Yoshida S, Asami T, et al (2002) BZR1 is a nuclear component of the brassinosteroid signaling pathway. Dev Cell 2: 505–513 [DOI] [PubMed] [Google Scholar]
- Whitman S, Gezginci M, Timmermann BN, Holman TR (2002) Structure-activity relationship studies of nordihydroguaiaretic acid inhibitors toward soybean, 12-human, and 15-human lipoxygenase. J Med Chem 45: 2659–2661 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (1993) Characterization of the expression of a desiccation-responsive RD29 gene of Arabidopsis-thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236: 331–340 [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Endo T, Satoh S (1998) Restoration of seed germination supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol 39: 307–312 [Google Scholar]
- Zeevaart JAD (1980) Changes in the levels of abscisic acid and its metabolites in excised leaf blades of Xanthium strumarium during and after water stress. Plant Physiol 66: 672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39: 439–473 [Google Scholar]
- Zhao Y, Dai X, Blackwell HE, Schreiber SI, Chory J (2003) SIR1, an upstream component in auxin signaling identified by chemical genetics. Science 301: 1107–1110 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6: 66–71 [DOI] [PubMed] [Google Scholar]