Abstract
This study tested the hypothesis that a controlled water deficit during grain filling of wheat (Triticum aestivum) could accelerate grain-filling rate through regulating the key enzymes involved in Suc-to-starch pathway in the grains. Two high lodging-resistant wheat cultivars were field grown. Well-watered and water-deficit (WD) treatments were imposed from 9 DPA until maturity. The WD promoted the reallocation of prefixed 14C from the stems to grains, shortened the grain-filling period, and increased grain-filling rate or starch accumulation rate (SAR) in the grains. Activities of Suc synthase (SuSase), soluble starch synthase (SSS), and starch branching enzyme (SBE) in the grains were substantially enhanced by WD and positively correlated with the SAR. ADP Glc pyrophosphorylase activity was also enhanced in WD grains initially and correlated with SAR with a smaller coefficient. Activities of granule-bound starch synthase and soluble and insoluble acid invertase in the grains were less affected by WD. Abscisic acid (ABA) content in the grains was remarkably enhanced by WD and very significantly correlated with activities of SuSase, SSS, and SBE. Application of ABA on well-watered plants showed similar results as those by WD. Spraying with fluridone, an ABA synthesis inhibitor, had the opposite effect. The results suggest that increased grain-filling rate is mainly attributed to the enhanced sink activity by regulating key enzymes involved in Suc-to-starch conversion, especially SuSase, SSS, and SBE, in wheat grains when subjected to a mild water deficit during grain filling, and ABA plays a vital role in the regulation of this process.
Starch in the endosperm of wheat (Triticum aestivum) is the major form of carbon reserves and comprises 65% to 75% of the final dry weight of the grain (Housley et al., 1981; Dale and Housley, 1986; Hurkman et al., 2003). Grain filling is therefore mainly a process of starch biosynthesis and accumulation. It is generally accepted that four enzymes may play a key role in this process: Suc synthase (SuSase; EC 2.4.1.13), ADP Glc pyrophosphorylase (AGPase; EC 2.7.7.27), starch synthase (StSase; EC 2.4.1.21), and starch branching enzyme (SBE; EC 2.4.1.18; Hawker and Jenner, 1993; Ahmadi and Baker, 2001; Hurkman et al., 2003). SuSase catalyses the cleavage of Suc, the main transported form of assimilates in wheat plants (Fisher and Gifford, 1986), to form UDP-Glc and Fru, which is thought to be the first step in the Suc-to-starch conversion. Its activity is considered to be linked to sink strength in the developing rice (Oryza sativa) grain and tomato (Lycopersicon esculentum) fruit (Sun et al., 1992; Wang et al., 1993; Kato, 1995). AGPase produces ADP-Glc, the primer of the starch chain (Smith and Denyer, 1992), and is regarded as the rate-limiting enzyme in starch biosynthesis (Preiss, 1988). StSase, composed of both soluble and granule-bound isoforms, elongates the amylose and amylopectin chains (Déjardin et al., 1997). Soluble StSase (SSS) activity is reported to be positively correlated with the rate of starch synthesis in wheat grains (Keeling et al., 1993). SBE forms branches on the polymers. It cleaves α-1,4 bonds on both amylose and amylopectin molecules and reattaches the released glucan segments to the same or another glucan chain through the formation of α-1,6 linkages (Hurkman et al., 2003). Its activity is closely associated with the increase in starch content during the development of rice endosperm (Nakamura et al., 1989; Nakamura and Yuki, 1992).
Extensive studies have been done on the effects of heat stress on the activities of enzymes involved in Suc-to-starch metabolism in cereals (Caley et al., 1990; Hawker and Jenner, 1993; Keeling et al., 1993; Jenner, 1994; Cheih and Jones, 1995; Duke and Doehlert, 1996; Wilhelm et al., 1999; Hurkman et al., 2003). A correlation of reduction in starch content with declines in SuSase and SSS activities in heat-treated grains has been reported in these studies. With the exception of the work done by Ahmadi and Baker (2001), who reported that reduction in grain growth rate of water-stressed wheat plants resulted from a reduced SSS activity, whereas growth cessation was due mainly to the inactivation of AGPase, information is scarce on changes in activities of key enzymes in Suc-to-starch catalytic pathway in water-stressed wheat grains during the filling period. Our early work (Yang et al., 2000, 2001b) has shown that a controlled water stress imposed during the grain-filling period of wheat and rice can enhance remobilization of prestored carbon reserves to grains and accelerate grain filling. However, little is known about whether and how the sink strength is involved in these processes. The objective of this study was to test the hypothesis that a controlled water deficit during the grain filling may enhance sink strength by regulating the key enzymes involved. The changes in activities of SuSase, AGPase, StSase, SBE, and acid invertase (AI; EC 3.2.1.26), a possible Suc-cleaving enzyme, in wheat grains and their relationship with grain filling were investigated.
Abscisic acid (ABA) is generally regarded as a very sensitive signal produced during water stress (Zhang and Davies, 1990a, 1990b; Davies and Zhang, 1991). Our earlier work has shown that an increased grain-filling rate is closely associated with an enhanced ABA level in water-stressed grains (Yang et al., 2001a, 2001c). Here in this experiment, the change in ABA content in the grains and its possible role in the regulation of the enzymatic activities were also investigated.
RESULTS
Leaf Water Potential
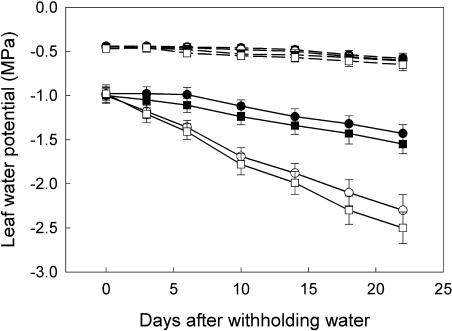
Figure 1 illustrates the progression of leaf water potentials during the first 22 d after withholding water. When plants were well-watered (WW), mid-day (11:30 am) leaf water potentials decreased gradually during grain filling, from −0.96 million Pa (MPa) at the beginning (9 DPA) to −1.43 to −1.55 MPa on 22 d after withholding water (31 DPA). The water-deficit (WD) treatment substantially reduced mid-day leaf water potentials from −0.98 MPa at 9 DPA to −2.31 to −2.50 MPa at 31 DPA. However, the differences in predawn (6 am) leaf water potentials between WW and WD plants were insignificant (Fig. 1), indicating that plants subjected to water deficit could rehydrate overnight.
Figure 1.
Changes of leaf water potentials of wheat during the first 22 d after withholding water. The treatments are NN + WW (•), NN + WD (○), HN + WW (▪), and HN + WD (□). Measurements were made on the flag leaves at predawn (6 am, dashed lines) and at midday (11:30 am, solid lines). Vertical bars represent ±se of the mean (n = 12) where these exceed the size of the symbol.
Carbon Remobilization and Grain-Filling Rate
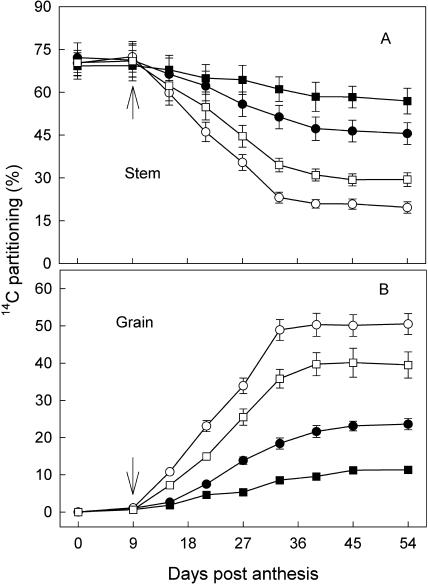
At early grain-filling stage (0–12 DPA), the main form of nonstructural carbohydrate in the stem of wheat was fructan, Suc, Glc, and Fru (56%, 25%, 10%, and 9%, respectively), and the activities of fructan exohydrolase and Suc phosphate synthase in the stem were substantially enhanced by WD (data not shown). The WD facilitated the reallocation of preanthesis assimilates from the stems to grains. Figure 2 shows the disappearance of preanthesis-assimilated 14C in the stems and its appearance in the grains during grain filling. At the start of water withholding (9 DPA), about 70% of 14C fed to the flag leaves at the booting stage was partitioned in the stems and about 5% in the grains. After 24 d (33 DPA), 14C in the stem was reduced by 23% to 34% under WD and 50% to 61% under WW treatments. Opposite to that observed in the stem, the 14C in the grains increased by 39% to 50% for WD plants and only 9% to 18% for WW ones at 33 DPA. In comparison, application of a high amount of N (HN) reduced the 14C reallocation into the grains (Fig. 2).
Figure 2.
Changes of 14C partitioning in the stems (A) and grains (B) of wheat. The 14C was fed to the flag leaves at the booting stage. The treatments are NN + WW (•), NN + WD (○), HN + WW (▪), and HN + WD (□). Arrows in the figure indicate the start of withholding water. Vertical bars represent ±se of the mean (n = 12) where these exceed the size of the symbol.
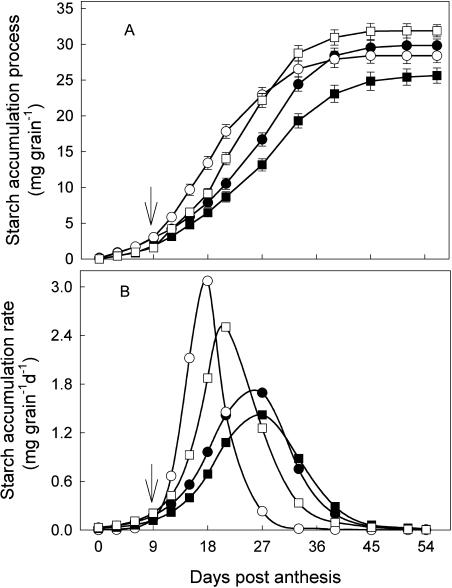
The WD greatly accelerated starch accumulation in grains from 9 to 27 DPA at normal amount of N (NN) and from 12 to 33 DPA at HN (Fig. 3). Eighty-one percent to 92% of the final starch weight in WD grains was accumulated during this period, and only 44% to 52% for WW grains in the same period. HN slowed the starch accumulation either for WW or WD plants. A similar observation was made on grain-filling rate (Table I). The WD substantially increased the grain-filling rate and shortened the grain-filling period at both NN and NH. The active grain-filling period was shortened by 8 d at NN and 10 d at HN, and grain-filling rate increased by 0.54 mg per day per kernel at NN and 0.74 mg per day per kernel at HN, respectively, compared with their respective WW treatments.
Figure 3.
Starch accumulation process (A) and SAR (B) of wheat grains. The treatments are NN + WW (•), NN + WD (○), HN + WW (▪), and HN + WD (□). SAR was calculated according to Richards' (1959) equation. Arrows in the figure indicate the start of withholding water. Vertical bars in A represent ±se of the mean (n = 6) where these exceed the size of the symbol.
Table I.
Grain-filling rate and grain yield of wheat subjected to various N and soil moisture treatments
| Treatment | Active Grain-Filling Period | Grain-Filling Rate | Spike Number | Grain Number | Kernel Weight | Grain Yield |
|---|---|---|---|---|---|---|
| d | mg grain−1 d−1 | spike m−2 | grain spike−1 | mg grain−1 | g m−2 | |
| NN + WW | 27 ba | 1.40 c | 419 a | 41 a | 42 b | 701 b |
| NN + WD | 19 d | 1.94 a | 417 a | 40 a | 41 b | 679 b |
| HN + WW | 34 a | 0.95 d | 424 a | 42 a | 36 c | 628 c |
| HN + WD | 24 c | 1.69 b | 421 a | 43 a | 45 a | 785 a |
The treatments are NN + WW, well watered with normal amount of nitrogen; NN + WD, water deficit with normal amount of nitrogen; HN + WW, well watered with high amount of nitrogen; and HN +WD, water deficit with high amount of nitrogen. Active grain-filling period and grain-filling rate were calculated according to Richards' (1959) equation. Values of spike number per square meter, grain number per spike, and grain weight were means of plants harvested from 2 m2 of each treatment. The grain yield was the means of plants harvested from 18 m2 of each treatment. Statistical comparison was within the same column.
Different letters indicate statistical significance at the P = 0.05 level of probability.
The final grain weight was not significantly different between the WW and WD treatments when NN was applied (Table I). However, it was significantly increased under HN plus WD treatments. A similar result was obtained with grain yield because only the kernel weight, rather than the spike number per m2 or grain number per spike, was influenced by the WD during grain filling in this experiment (Table I).
Changes in Activities of Enzymes
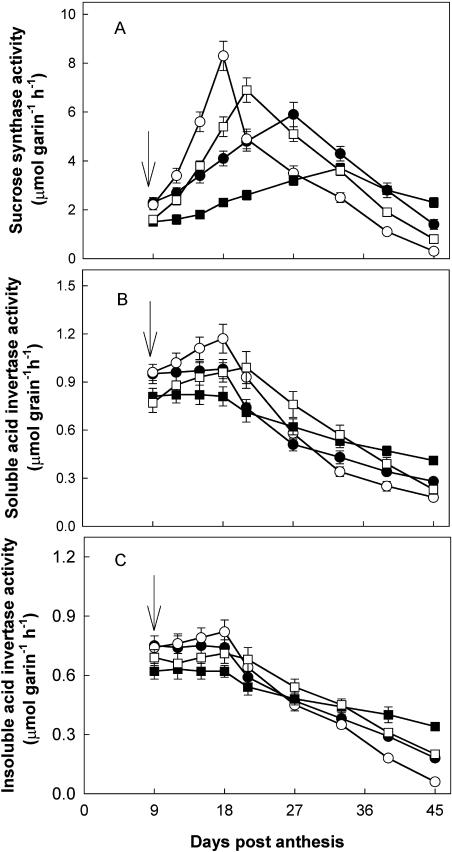
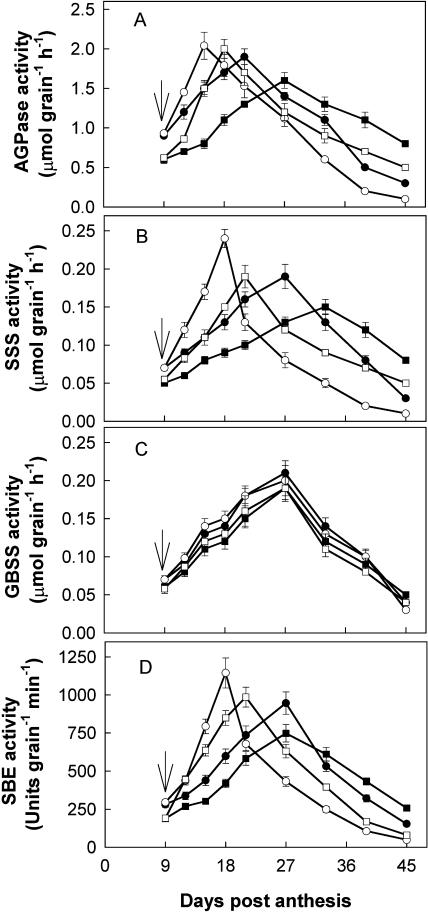
The activities of the five enzymes examined in relation to Suc-to-starch conversion in wheat grains exhibited variable responses with time, soil moisture, and N levels. SuSase activity (assayed in cleavage direction) in WW grains was increased from 9 to 27 DPA and then decreased with grain development (Fig. 4A). It was substantially enhanced by WD during the first 9 to12 d after withholding water, reached its peak 18 and 21 DPA at NN and HN, respectively, and sharply declined thereafter, in good agreement with the starch accumulation rate (SAR; refer to Fig. 3B).
Figure 4.
Changes in activities of SuSase (A), soluble AI (B), and insoluble AI (C) in wheat grains. The treatments are NN + WW (•), NN + WD (○), HN + WW (▪), and HN + WD (□). Arrows in the figure indicate the start of withholding water. Vertical bars represent ±se of the mean (n = 6) where these exceed the size of the symbol.
Irrespective of NN and HN treatments, activities of both soluble and insoluble AI in WW grains were little changed from 9 to 18 DPA and decreased afterward (Fig. 4, B and C). They were enhanced by WD, with soluble AI enhanced more than insoluble AI. SuSase activity, on a per grain basis, was much higher than those of AI during the rapid accumulation period of starch in the grain (9–27 DPA at NN and 12–33 DPA at HN, respectively, for WD plants and 12–39 DPA for WW plants; refer to Fig. 3), indicating that SuSase is a predominant enzyme responsible for Suc cleavage in wheat grains.
Very similar to SuSase activities, SSS and SBE activities were markedly increased in WD grains during the first 9 to 12 d after withholding water (Fig. 5, B and D). The changes in activities of both SSS and SBE were consistent with SAR (refer to Fig. 3). By contrast, granule-bound starch synthase (GBSS) activity was little affected by either the WD or N treatments (Fig. 5C).
Figure 5.
Changes in activities of AGPase (A), SSS (B), GBSS (C), and SBE (D) in wheat grains. The treatments are NN + WW (•), NN + WD (○), HN + WW (▪), and HN + WD (□). Arrows in the figure indicate the start of withholding water. Vertical bars represent ±se of the mean (n = 6) where these exceed the size of the symbol.
AGPase activity exhibited a peak at 15 to18 DPA in WD grains and 21 to 27 DPA in WW grains (Fig. 5A). The peak appeared just before or corresponded to the maximum SAR. The activity in WD grains was greater initially and declined faster after reaching a maximum when compared with that in WW grains. With the exception of GBSS activity, HN reduced activities of all the enzymes at early and mid-grain-filling stages but slowed down their declines at the late filling stage when ψsoil was the same.
Changes in ABA Content
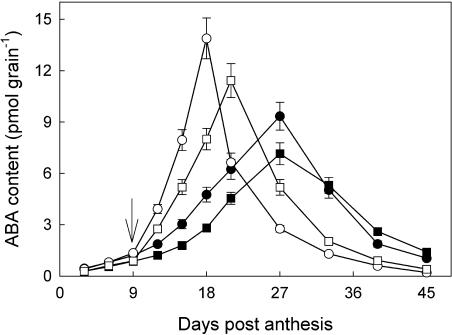
As shown in Figure 6, ABA content in the grains was very low at early grain-filling stage and remarkably enhanced by WD at both NN and HN. At early and mid-grain-filling stages, the grain with NN contained more ABA than those with HN treatments when soil water potential was the same. The change in ABA content in the grains was paralleled with SAR (refer to Fig. 3), and they were very significantly correlated (r = 0.99**, P = 0.01).
Figure 6.
Changes in ABA content in wheat grains. The treatments are NN + WW (•), NN + WD (○), HN + WW (▪), and HN + WD (□). Arrow in the figure indicates the start of withholding water. Vertical bars represent ±se of the mean (n = 6) where these exceed the size of the symbol.
Correlations of Enzyme Activities with ABA Content and Starch Accumulation Rate
During the rapid accumulation period of starch in the grain, the correlations of activities of the five enzymes examined with ABA content and SAR were analyzed (Table II). Activities of SuSase, SSS, and SBE were positively and very significantly correlated with SAR (r = 0.92** to 0.97 **, P = 0.01). AGPase activity was also correlated with SAR with r = 0.69* (P = 0.05), whereas neither of the soluble or insoluble AI nor GBSS was significantly correlated (r = 0.35–0.48, P > 0.05). Very similar correlations were observed between the activities of enzymes and ABA content in the grains (Table II), suggesting that ABA may play an important regulative role in enzyme activities.
Table II.
Relationships of ABA content and (SAR) in wheat grains with the activities of the enzymes involved in Suc-to-starch metabolism during the rapid SAR period (9–27 DPA for NN + WD, 12–33 DPA for HN + WD, and 12–39 DPA for NN + WW and HN + WW treatments)
| Correlation With | ABA Content | SAR |
|---|---|---|
| SuSase | 0.94**a | 0.93** |
| Soluble AI | 0.45 | 0.46 |
| Insoluble AI | 0.36 | 0.35 |
| AGPase | 0.73** | 0.69* |
| SSS | 0.95** | 0.92** |
| GBSS | 0.47 | 0.48 |
| SBE | 0.98** | 0.97** |
Data used for the calculation are from Figures 3 to 6.
The * and ** indicate correlation significance at the P = 0.05 and P = 0.01 levels of probability, respectively.
Effects of Exogenous ABA and Fluridone
When ABA was applied to HN plus WW plants at early grain-filling stage (9–13 DPA), the activities of SuSase, SSS, and SBE and starch accumulation in the grains were very significantly increased (Table III). Effects of ABA application on AGPase and soluble AI activities varied with the determination date. Supplemental ABA had no significant effects on the activities of insoluble AI and GBSS. Opposite to ABA, spraying with fluridone, an inhibitor of ABA synthesis, significantly reduced activities of SuSase, SSS, SBE, and AGPase and starch content in the grains (Table III). The final grain weight was 46.1 g and 26.6 g, respectively, for ABA- and fluridone-treated plants, which was 129% and 75% of that (35.6 g) for the control, respectively.
Table III.
Effects of applied ABA and fluridone on ABA content (pmol grain−1), activities of the enzymes involved in Suc-to-starch metabolism (μmol grain−1 h−1), and starch accumulation (mg grain−1) in the grains of wheat
| ABA Content/Enzyme Activities/Starch Accumulation | 18 DPA
|
21 DPA
|
27 DPA
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| CK | T1 | T2 | CK | T1 | T2 | CK | T1 | T2 | |
| ABA | 4.86 | 8.93**a | 2.37** | 6.42 | 12.4** | 4.76** | 8.73 | 11.5** | 4.21** |
| SuSase | 2.32 | 4.78** | 0.89** | 2.68 | 6.39** | 1.32** | 3.01 | 4.19* | 0.82** |
| Soluble AI | 0.84 | 1.15** | 0.65* | 0.75 | 1.11** | 0.56* | 0.69 | 0.77b | 0.41* |
| Insoluble AI | 0.78 | 0.89b | 0.70b | 0.62 | 0.74b | 0.53b | 0.31 | 0.23b | 0.24b |
| AGPase | 1.11 | 1.78** | 0.86** | 1.56 | 1.58b | 1.27** | 1.44 | 1.35b | 1.23* |
| SSS | 0.10 | 0.21** | 0.06** | 0.14 | 0.32** | 0.07** | 0.15 | 0.25** | 0.05** |
| GBSS | 0.08 | 0.09b | 0.04* | 0.11 | 0.14b | 0.06** | 0.14 | 0.16b | 0.11b |
| SBE | 319 | 647** | 208** | 446 | 751** | 314** | 552 | 636* | 270** |
| Starch | 6.57 | 9.15** | 4.13** | 8.59 | 14.1** | 6.72** | 13.4 | 27.2** | 9.35** |
The plants were tank grown with a HN + WW treatment. The spikes were sprayed either with 20 × 10−6 m ABA (T1) or with 20 × 10−6 m fluridone (T2) daily for 5 d starting at 9 DPA. Control plants (CK) were sprayed with deionized water. Statistical comparison was within the same row and the same determination date.
The * and ** indicate significantly different at the P = 0.05 and 0.01 levels of probability, respectively.
Not significantly different.
DISCUSSION
Water stress imposed during the grain-filling period of wheat, especially at the early filling stage, usually results in a reduction in grain weight and leads to reduced grain yield (Aggarwal and Sinha, 1984; Nicolas et al., 1985b; Kobata et al., 1992; Zhang et al., 1998). We found, however, that if WD during grain filling was controlled properly, plants were able to rehydrate overnight (Fig. 1). A benefit from such a WD was that it could enhance remobilization of carbon reserves from vegetative tissues to the grains (Fig. 2) and accelerate SAR and the grain-filling rate (Fig. 3; Table I). The grain weight and grain yield would not necessarily be reduced when NN was applied (Table I). Moreover, the grain weight and grain yield could be increased when excessive N fertilizer was applied (Table I).
The discrepancies between our results and previous reports (Aggarwal and Sinha, 1984; Nicolas et al., 1985b; Kobata et al., 1992; Zhang et al., 1998) are probably attributable to several reasons. First, the plant materials we used in this experiment are high yielding and strong lodging-resistant wheat cultivars, which have shown slow grain filling and low harvest index because they stay green for too long and remobilize prestored assimilates poorly to the grains (Peng et al., 1992; Yang et al., 2000). A mild WD during grain filling would be beneficial to the grain filling by enhancing remobilization of stored reserves from the stems to grains for these cultivars. Second, the reduction in gain weight in response to water stress during grain-filling period is mainly attributed to the lowered number of endosperm cells, thereby decreasing sink size per kernel (Singh and Jenner, 1982; Nicolas et al., 1985a; Michihiro et al., 1994). In our experiment, WD was initiated at 9 DPA, at which time the active division period of endosperm cells is almost complete (Feng et al., 2000; Wang et al., 2003), and therefore the sink size may not be seriously affected. Third, the WD imposed in our experiments was rather mild and controlled properly such that plants could rehydrate overnight. We have observed that such a mild WD imposed during grain filling would not seriously reduce photosynthesis (Yang et al., 2000, 2001a). Under such conditions, the gain from the accelerated grain-filling rate may outweigh the possible loss of photosynthesis as a result of a shortened grain-filling period, leading to an increase in grain yield in the cases where plant senescence is unfavorably delayed by excessive application of N fertilizer.
It is generally accepted that grain-filling rate in cereals is mainly determined by sink strength (Venkateswarlu and Visperas, 1987; Liang et al., 2001). During grain-filling period of wheat, kernels are very strong sinks for carbohydrate (Ho, 1988; Riffkin et al., 1995). The sink strength can be described as the product of sink size and sink activity (Venkateswarlu and Visperas, 1987). Sink size is a physical restraint that includes cell number and cell size. Sink activity is a physiological restraint that includes multiple factors and key enzymes involved in carbohydrate utilization and storage (Wang et al., 1993). As the sink size was not seriously affected by the WD in this experiment, we speculate that increased carbon remobilization from the stems to grains and accelerated SAR or grain-filling rate may be mainly attributed to an enhanced sink activity under the WD.
It was hypothesized that high levels of enzymes involved in the breakdown of Suc in the sink would increase sink capacity by lowering the local concentration of Suc, thereby generating a gradient that allows further unloading of Suc from phloem (Wardlaw, 1968; Liang et al., 2001). Since both SuSase and invertase are involved in Suc cleavage in sink tissue, their activities are regarded as biochemical markers of sink strength (Wang et al., 1993; Ranwala and Miller, 1998). Our results showed that the activity of SuSase in wheat grains was much higher than those of both soluble and insoluble AI, and the former was enhanced more than the latter by WD (Fig. 4). SuSase activity was positively and very significantly correlated with SAR during the rapid accumulation period, whereas neither soluble nor insoluble AI activities was positively correlated (Table II). The results back the notion that SuSase is predominant in the sink accumulating carbohydrate reserves and catalyzes the first step in the conversion of Suc to starch in the endosperm of cereals (Chourey and Nelson, 1976; Dale and Housley, 1986; Kato, 1995), whereas invertase is mainly present in tissues in which active cell elongation is occurring (Sung et al., 1988; Ranwala and Miller, 1998). The results suggest that the enhanced carbon remobilization and grain-filling rate by a WD imposed during grain-filling period is attributed to, or associated with, an increase in sink strength by regulating SuSase activity in wheat grains.
WD enhanced activities of AGPase, SSS, and SBE at initial or at early and mid-grain-filling stages (Fig. 5). The activities of the three enzymes were positively correlated with SAR (Table II), suggesting that all of the three enzymes in wheat grains play an important role in starch synthesis. We observed that GBSS activity was neither enhanced by the WD nor significantly correlated with SAR (Table II). A probable explanation is that SSS is thought to be responsible for generating polymers that are the substrates for amylopectin synthesis via SBE (Smith and Denyer, 1992). By contrast, GBSS may be involved only in amylose synthesis. Since starch in wheat grains is composed mostly of amylopectin, SSS is therefore considered to play a more important role than GBSS.
It is worth noting that SBE was highly correlated with SAR with the greatest coefficient (r = 0.97) among all the enzymes examined in this experiment (Table II). The result may probably be explained by the fact that SBE is the sole enzyme capable of forming α-1,6-linked branches on already synthesized and/or elongating amylose molecules (Preiss et al., 1991) and thereby plays a key role in starch production in wheat endosperm. A similar observation was made in rice grains (Nakamura et al., 1989; Nakamura and Yuki, 1992). The close correlation of SBE activity with SAR in this study suggests that accelerated grain-filling rate under WD is mainly associated with the enhanced SBE activity in wheat grains.
The results that a mild WD imposed during grain-filling period could enhance activities of key enzymes in Suc-to-starch pathway were also observed on other wheat cultivars and rice (Yang et al., 2001a; Wang, 2003). However, the regulatory mechanisms involved in the enzyme activities are unrevealed. We observed from this experiment that changes in activities of SuSase, SSS, SBE, and AGPase were closely associated with that of ABA content (Figs. 4–6). A positive and very significant correlation between ABA content and activities of the enzymes was found (Table II). Furthermore, the activities of the enzymes were significantly increased when supplemental ABA was applied to the HN plus WW plants, whereas application of fluridone, an inhibitor of ABA synthesis, had the opposite effects (Table III). The results suggest that an elevated ABA level may play an important role in the enhancement of enzymatic activities under the WD. Little is known about how ABA activates the enzymes. It is reported that ABA improves the energization of phloem cells in sink organs by stimulating ATPase activity and thereby regulates the metabolism of assimilates in cells (Peng et al., 2003). A multiplicity of evidence shows that stress-induced ABA plays a role in regulation of gene expression (e.g. Rock and Quatrano, 1995). It is possible that ABA-induced gene expression under WD may contribute to the regulative role over the activities of enzymes involved in Suc-to-starch conversion. Further investigation is needed to elucidate the mechanism at which ABA regulates activities of the enzymes.
CONCLUSIONS
Our results demonstrate that if a WD stress during the grain filling of wheat is controlled properly so that plants can rehydrate overnight, remobilization of prestored carbon from vegetative tissues to the grains can be substantially enhanced and SAR or grain-filling rate be accelerated. The increased remobilization and grain-filling rate are mainly attributed to, or at least linked to, the enhanced sink activity by regulating key enzymes involved in Suc-to-starch pathway, especially SuSase, SSS, and SBE, in the grains when subjected to the WD. ABA plays a vital role in the regulation of this process.
MATERIALS AND METHODS
Plant Materials and Cultivation
The experiment was conducted at Yangzhou University farm, Yangzhou, China (32°30′N, 119°25′E) from November 2002 to June 2003. Two highly lodging-resistant cultivars of semi-winter wheat (Triticum asetivum), cv Yangmai 158 and cv Yangmai 11, currently used in local production, were grown in the field. The sowing date was November 2, and the plant density was adjusted to 150 plants m−2 at three-leaf age. The soil of the field was sandy loam soil (Typic fluvaquents, Entisols; U.S. taxonomy) that contained organic matter at 2.42% and available N-phosphorus-potassium at 110, 36.5, and 70.4 mg kg−1, respectively. On the day of sowing, 14 g N m−2 as urea and 4 g phosphorus m−2 as single superphosphate were applied to the soil. On 30 d after sowing (DAS) and 112 DAS, 6 g and 5 g N m−2 as urea were top dressed, respectively. The soil water content was maintained close to field capacity (soil water potential, ψsoil, at −0.02 to −0.025 MPa) by manual watering until 9 DPA when WD treatments were initiated. Yangmai 158 and Yangmai 11 headed (50% plants) 157 DAS and 158 DAS, respectively, and flowered during 164 to 170 DAS. Air temperatures during grain filling (164–221 DAS), averaged 10 d, were 15.4°C, 16.9°C, 18.1°C, 19.2°C, 21.3°C, and 23.9°C, respectively.
Nitrogen and Water-Deficit Treatments
The experiment was a 2 × 2 × 2 (two cultivars, two levels of N, and two levels of soil moisture) factorial design with eight treatments. Each of the treatments had three plots as repetitions in a complete randomized block design. Plot dimension was 3 m × 4 m, and plots were separated by a ridge (20 cm in width) wrapped with plastic film. Two levels of N treatments were applied at heading (50% of plants headed). One-half of plots were top dresses with either 3 g N m−2 (NN) or 8 g N m−2 (HN) as urea. From 9 DPA (173 DAS for both cultivars) until maturity, two levels of ψsoil were imposed on the plants of both NN and HN treatments by controlling water application. The WW treatment was maintained at −0.02MPa, and the WD treatment was maintained at −0.08 MPa. Soil water potential was monitored in the 15- to 20-cm soil depth. Four tension meters (Soil Science Research Institute, China Academy of Sciences, Nanjing, China) were installed in each plot to monitor. Tension meter readings were recorded at 12 pm each day. When the reading dropped to the designed value, 0.08 and 0.02 m3 of tap water per plot was added to the WW and WD plants, respectively. A rain shelter, consisting of a steel frame covered with plastic sheet, was used to protect the plot during rains.
Radioactive Labeling
At the boot stage (144 DAS for Yangmai 158 and 146 DAS for Yangmai 11), 60 plants from each treatment were labeled with 14CO2. Flag leaves of main stems were used for the labeling between 9 am to 11 am on a clear day with photosynthetically active radiation at the top of the canopy ranging between 1,000 and 1,100 μmol m−2 s−1. The whole flag leaf was placed into a polyethylene chamber (25-cm length and 4-cm diameter) and sealed with tape and plasticine to maintain a gas-tight seal. Ten milliliters of air in the chamber was drawn out, and the same volume of gas was injected into the chamber, which contained 0.015 mol L−1 CO2 at a specific radioactivity of 14C of 2.21 MBq L−1. The chamber was removed after 1 h.
Labeled plants were harvested at 0 DPA (50% anthesis) and from 9 (the initiation of water withholding) to 54 DPA at 6-d intervals, respectively. Each plant was divided into leaf blades, culms plus sheaths, kernels, and other parts on a spike (rachis + palea + lemma + glume). Samples were dried at 80°C to constant weight, ground into powder, and then extracted by shaking for 30 min in 80% (v/v) boiling ethanol. The residue was extracted in 2:1 of 60% (v/v) HClO4 to 30% (v/v) H2O2 for 4 h at 60°C. The radioactivity of 14C in both the extracted aliquots was counted using a liquid scintillation counter (Beckman Instruments, Fullerton, CA), and the data were presented in composite averages. Radioactivity distribution in each part of the plant was expressed as a percentage of total radioactivity remaining in the aboveground portion of the plant.
Sampling
A total of 150 spikes that headed on the same day were chosen and tagged for each plot. Ten tagged spikes from each plot were sampled at 3-d intervals from anthesis to 21 DPA and 6-d intervals from 27 DPA to maturity. All grains from each spikelet were removed. Half of the sampled grains was frozen in liquid nitrogen for 2 min and then stored at −80°C for enzymatic and ABA measurements. The other half of the grains was dried at 70°C to constant weight and weighed. The starch content in the grains was analyzed by the method of Yoshida et al. (1976). The processes of grain filling and starch accumulation in the grain were fitted by Richards' (1959) growth equation as described by Zhu et al. (1988):
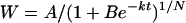 |
(1) |
Both grain-filling rate and SAR (G) were calculated as the derivative of Equation 1:
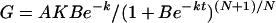 |
(2) |
where W is the grain/starch weight (mg), A is the final grain/starch weight (mg), t is the time after anthesis (d), and B, k, and N are coefficients determined by regression. The active grain-filling period was defined as the days when W was from 5% (t1) to 95% (t2) of A. An average grain filling rate during this period was therefore calculated from t1 to t2.
Plants (except border) from a 4-m2 site from each plot were harvested at maturity for the determination of grain yield. Yield components, i.e. the spikes per square meter, grain number per spike, and grain weight, were determined from plants harvested from a 1-m2 site (excluding the border plants) randomly sampled from each plot.
Measurement of Leaf Water Potential
Leaf water potentials of flag leaves were measured on clear days at predawn (6 am) and midday (11:30 am) on 0, 3, 6, 10, 14, 18, and 22 d after withholding water. Well-illuminated flag leaves were chosen randomly for such measurements. A pressure chamber (model 3000; Soil Moisture Equipment, Santa Barbara, CA) was used for leaf water potential measurement with six leaves for each treatment.
Enzyme Extraction and Assays
All chemicals and enzymes used for enzymatic measurement were from Sigma (St. Louis). All enzyme assays were optimized for pH and substrate concentration and were within the linear phase with respect to incubation time and protein concentration. Protein content was determined according to Bradford (1976), using bovine serum albumin as standard. Enzyme activities were expressed on a per grain basis.
The method for SuSase and AI extraction was modified from Ranwala and Miller (1998). For AI extraction, 30 to 40 grains were homogenized in 4 to 8 mL of chilled 100 mm Tris buffer (pH 7.2) containing 5 mm β-mercaptoethanaol, 10 mm isoascorbate, and 1 mm phenylmethyl-sulfonyl fluoride. The homogenate was centrifuged at 15,000g for 30 min, the supernatant was dialyzed in 15 mm Tris (pH 7.2) containing 5 mm β-mercaptoethanaol overnight, and the dialyzate was used for AI assay.
For SuSase extraction, grains were homogenized with a mortar and pestle in 100 mm HEPES (pH 7.5) containing 10 mm isoascorbate, 3 mm MgCl2, 5 mL dithiothreitol (DTT), 2 mL EDTA, 5% (v/v) glycerol, 3%(w/v) polyvinylpyrrolidone, and 0.01% Triton X-100. After centrifugation at 15,000g for 30 min, the supernatant was desalted on a Sephadex G-25 column, and the proteins were eluted by the reaction buffer that contained 50 mm HEPES (pH 7.5), 10 mm MgCl2, 2 mm EDTA, and 3 mm DTT.
The extraction procedure for AGPase, StSase, and SBE was according to Nakamura et al. (1989). Briefly, 30 to 40 grains were homogenized with a pestle in a precooled mortar that contained 4 to 8 mL frozen extraction medium: 100 mm HEPES-NaOH (pH 7.6), 8 mm MgCl2, 5 mm DTT, 2 mm EDTA, 12.5% (v/v) glycerol, and 5% (w/v) insoluble polyvinylpyrrolidone 40. After being filtered through four layers of cheesecloth, the homogenate was centrifuged at 12,000g for 10 min, and the supernatant was used for the enzyme assay.
The enzyme activities were determined as described previously: SuSase (in cleavage direction; Ranwala and Miller, 1998), soluble and insoluble AI (Zinselmeier et al., 1995), SSS and GBSS (Schaffer and Petreikov, 1997), and AGPase and SBE (Nakamura et al., 1989).
ABA Extraction, Purification, and Quantification
The methods for extraction and purification of ABA [(±)-ABA] were modified from those described by Bollmark et al. (1988) and He (1993). Samples of 20 to 30 grains were ground in a mortar (at 0°C) in 10 mL of 80% (v/v) methanol extraction medium containing 1 mm butylated hydroxytoluene as an antioxidant. The extract was incubated at 4°C for 4 h and centrifuged at 4,800g for 15 min at the same temperature. The supernatants were passed through Chromosep C18 columns (C18 Sep-Park cartridge; Waters, Millford, MA) and prewashed with 10 mL of 100% and 5 mL of 80% methanol, respectively. Impurity in the extraction was checked through dilution test and addition test as described by He (1993). The hormone fractions were dried under N2 and dissolved in 2 mL of phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5) for analysis by an ELISA.
The mouse monoclonal antigen and antibody against ABA, and IgG-horse radish peroxidase (IgG-HRP) used in ELISA were produced at the Phytohormones Research Institute, China Agricultural University, China (see He, 1993). The method for quantification of ABA by ELISA was described previously (Yang et al., 2001a). The recovery percentage of ABA in grains was 81.6 ± 4.6. The specificity of the monoclonal antibody and the other possible nonspecific immunoreactive interference were checked previously and proved reliable (Wu et al., 1988; Zhang et al., 1991; Yang et al., 2001a, 2001c).
Exogenous ABA and Fluridone Applications
Plants were grown in eight cement tanks in open field conditions. Each tank (0.3-cm height, 1.6-m width, and 8.8-m length) was filled with sandy loam soil with the same nutrient contents as the field soil. The sowing date and cultivation were the same as the field experiment. A HN plus WW treatment was conducted as described above.
Starting 9 DPA, either 20 × 10−6 m (±)-ABA (Sigma) or 20 × 10−6 m fluridone (Fluka, NJ; Riedel-de Haën, Seelze, Germany), an inhibitor of ABA synthesis, were sprayed at the rate of 800 mL m−2 on the top of plants (spikes) for 5 d. Both ABA and fluridone were applied between 4 pm and 5 pm on each day. All the solutions contained ethanol and Tween 20 at final concentrations of 0.1% (v/v) and 0.01 (v/v), respectively. Control plants were sprayed with the same volume of deionized water containing same concentrations of ethanol and Tween 20. Each treatment was an area of 2.4 m2 with four replications.
ABA content, enzymatic activities, and starch content in the grains were determined 3, 6, and 9 d after the chemical treatments (15, 18, and 27 DPA). Measurement methods were the same as described above. Fifty plants from each treatment were harvested at maturity for the determination of final grain weight.
Statistical Analysis
The results were analyzed for variance using SAS statistical analysis package (version 6.12; SAS Institute, Cary, NC). Data from each sampling date were analyzed separately. Means were tested by lsd at P0.05 level (lsd0.05). Linear regression was used to evaluate the relationships of enzymatic activities with ABA content and SAR in the grains. Since the two cultivars behaved the same, the data are presented as an average between the two cultivars.
This experiment was also conducted under pot-grown conditions at the same time as the field-grown, and results from the pot experiment were very similar. Only the field experiment was reported in this paper because of limited space.
This work was supported by the Research Grant Council of Hong Kong (RGC 2052/00M), by the Area of Excellence for Plant and Fungal Biotechnology in the Chinese University of Hong Kong, and by the State Key Basic Research and Development Plan (grant no. G1999011704).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041038.
References
- Aggarwal PK, Sinha SK (1984) Effect of water stress on grain growth and assimilate partitioning in two cultivars of wheat contrasting in their yield in a drought environment. Ann Bot (Lond) 53: 329–340 [Google Scholar]
- Ahmadi A, Baker DA (2001) The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat. Plant Growth Regul 35: 81–91 [Google Scholar]
- Bollmark M, Kubat B, Eliasson L (1988) Variations in endogenous cytokinin content during adventitious root formation in pea cuttings. J Plant Physiol 132: 262–265 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Caley CY, Duffus CM, Jeffcoat B (1990) Effects of elevated temperature in developing wheat grains. Aust J Plant Physiol 17: 431–439 [Google Scholar]
- Cheih N, Jones RJ (1995) Heat stress effects on sink activity of developing maize kernels grown in vitro. Physiol Plant 95: 59–66 [Google Scholar]
- Chourey PS, Nelson OE (1976) The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochem Genet 14: 1041–1055 [DOI] [PubMed] [Google Scholar]
- Dale EM, Housley TL (1986) Sucrose synthase activity in developing wheat endosperms differing in maximum weight. Plant Physiol 82: 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Zhang J (1991) Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol 42: 55–76 [Google Scholar]
- Déjardin A, Rochat C, Wuilléme S, Boutin JP (1997) Contribution of sucrose synthase, ADP-glucose pyrophosphorylase and starch synthase to starch synthesis in developing pea seeds. Plant Cell Environ 20: 1421–1430 [Google Scholar]
- Duke ER, Doehlert DC (1996) Effects of heat stress on enzyme activities and transcript levels in developing maize kernels grown in culture. Environ Exp Bot 36: 199–208 [Google Scholar]
- Feng C, Guo W, Shi J, Peng Y (2000) Effect of high temperature after anthesis on endosperm cell development and grain weight in wheat. Acta Agron Sin 26: 399–405 [Google Scholar]
- Fisher DB, Gifford RM (1986) Accumulation and conversion of sugars by developing wheat grains. VI. Gradients along the transport pathway from the peduncle to the endosperm cavity during grain filling. Plant Physiol 82: 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker JS, Jenner CJ (1993) High temperature affects the activity of enzymes in the committed pathway of starch synthesis in developing wheat endosperm. Aust J Plant Physiol 20: 197–209 [Google Scholar]
- He Z (1993) Enzyme linked immunosorbent assay for endogenous plant hormones. In ZP He, ed, Guidance to Experiment on Chemical Control in Crop Plants. Beijing Agricultural University Publishers, Beijing, pp 60–68
- Ho LC (1988) Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Annu Rev Plant Physiol Plant Mol Biol 39: 355–378 [Google Scholar]
- Housley TL, Kirkeis AW, Ohm HW, Patterson FL (1981) An evaluation of seed growth in soft red winter wheat. Can J Plant Sci 61: 525–534 [Google Scholar]
- Hurkman WJ, McCue KF, Altenbach SB, Korn A, Tanaka CK, Kothari KM, Johnson EL, Bechtel DB, Wilson JD, Anderson OD, et al (2003) Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci 164: 873–881 [Google Scholar]
- Jenner CF (1994) Starch synthesis in the kernel of wheat under high temperature conditions. Aust J Plant Physiol 21: 791–806 [Google Scholar]
- Kato T (1995) Change of sucrose synthase activity in developing endosperm of rice cultivars. Crop Sci 35: 827–831 [Google Scholar]
- Keeling PL, Bacon PJ, Holt DC (1993) Elevated temperature reduces starch deposition in wheat endosperm by reducing the activity of soluble starch synthase. Planta 191: 342–348 [Google Scholar]
- Kobata T, Palta JA, Turner TC (1992) Rate of development of postanthesis water deficits and grain filling of spring wheat. Crop Sci 32: 1238–1242 [Google Scholar]
- Liang J, Zhang J, Cao X (2001) Grain sink strength may be related to the poor grain filling of indica-japonica rice (Oryza sativa) hybrids. Physiol Plant 112: 470–477 [DOI] [PubMed] [Google Scholar]
- Michihiro W, Lui JCB, Garvalho GC (1994) Cultivar difference in leaf photosynthesis and grain yield of wheat under soil water deficit conditions. Jpn J Crop Sci 63: 339–344 [Google Scholar]
- Nakamura Y, Yuki K (1992) Changes in enzyme activities associated with carbohydrate metabolism during development of rice endosperm. Plant Sci 82: 15–20 [Google Scholar]
- Nakamura Y, Yuki K, Park SY (1989) Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol 30: 833–839 [Google Scholar]
- Nicolas ME, Gleadow RM, Dalling MJ (1985. a) Effect of post-anthesis drought on cell division and starch accumulation in developing wheat grains. Ann Bot (Lond) 55: 1433–1444 [Google Scholar]
- Nicolas ME, Lambers H, Simpson RJ, Dalling MJ (1985. b) Effect of drought on metabolism and partitioning of carbon in two wheat varieties differing in drought-tolerance. Ann Bot (Lond) 55: 727–747 [Google Scholar]
- Peng Y, Guo W, Yan L (1992) Source-sink relationship in wheat and its regulation. In Y Peng, ed, Wheat Production and Physiology. Dongnan University Publishers, Nanjing, China pp 20–62
- Peng YB, Lu YF, Zhang DP (2003) Abscisic acid activates ATPase in developing apple fruit especially in fruit phloem cells. Plant Cell Environ 26: 1329–1342 [Google Scholar]
- Preiss J (1988) Biosynthesis of starch and its regulation. In J Preiss, ed, The Biochemistry of Plants, Vol 14. Academic Press, New York, pp 181–254
- Preiss J, Ball K, Smith-White B, Iglesias A, Kakefuda G, Li L (1991) Starch biosynthesis and its regulation. Biochem Soc Trans 19: 539–547 [DOI] [PubMed] [Google Scholar]
- Ranwala AP, Miller WB (1998) Sucrose-cleaving enzymes and carbohydrate pools in Lilium longiflorum floral organs. Physiol Plant 103: 541–550 [Google Scholar]
- Richards FJ (1959) A flexible growth function for empirical use. J Exp Bot 10: 290–300 [Google Scholar]
- Riffkin HL, Duffus CM, Bridges IC (1995) Sucrose metabolism during development in wheat (Triticum aestivum). Physiol Plant 93: 123–131 [Google Scholar]
- Rock CD, Quatrano RS (1995) The role of hormones during seed development. In PJ Davies, ed, Plant Hormones, Physiology, Biochemistry and Molecular Biology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 671–697
- Schaffer AA, Petreikov M (1997) Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol 113: 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BM, Jenner CF (1982) A modified method for the determination of cell number in wheat endosperm. Plant Sci Lett 26: 273–278 [Google Scholar]
- Smith AM, Denyer K (1992) Starch synthesis in developing pea embryos. New Phytol 122: 21–33 [DOI] [PubMed] [Google Scholar]
- Sun J, Loboda T, Sung S-JS, Black CC (1992) Sucrose synthase in wild tomato, Lycopersicon chemielewskii, and tomato fruit sink strength. Plant Physiol 98: 1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SJ, Xu DP, Black CC (1988) A reassessment of glycolysis and gluconeogenesis in high plants. Physiol Plant 72: 650–654 [Google Scholar]
- Venkateswarlu B, Visperas RM (1987) Source-sink relationships in crop plants. International Rice Research Paper Series 125: 1–19 [Google Scholar]
- Wang F, Sanz A, Brenner ML, Smith A (1993) Sucrose synthase, starch accumulation, and tomato fruit sink strength. Plant Physiol 101: 321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W (2003) Soil drying in regulation to the remobilization of carbon and nitrogen reserves during grain filling period of wheat and rice and its physiological mechanism. PhD thesis. Yangzhou University, Yangzhou, China
- Wang W, Guo W, Fang M, Zhu X, Peng Y (2003) Endosperm cell proliferation and grain filling dynamics in wheat. Acta Agron Sin 29: 779–784 [Google Scholar]
- Wardlaw IF (1968) The control and pattern of movement of carbohydrates in plants. Bot Rev 34: 79–105 [Google Scholar]
- Wilhelm EP, Mullen RE, Keeling PL, Singletary GW (1999) Heat stress during grain filling in maize: effects on kernel growth and metabolism. Crop Sci 39: 1733–1741 [Google Scholar]
- Wu S, Chen W, Zhou X (1988) Enzyme linked immunosorbent assay for endogenous plant hormones. Plant Physiol Commun 5: 53–57 [Google Scholar]
- Yang J, Zhang J, Huang Z, Zhu Q, Wang L (2000) Remobilization of carbon reserves is improved by controlled soil-drying during grain filling of wheat. Crop Sci 40: 1645–1655 [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2001. a) Water deficit-induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron J 93: 196–206 [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001. b) Remobilization of carbon reserves in response to water deficit during grain filling of rice. Field Crops Res 71: 47–55 [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001. c) Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol 127: 315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Forno D, Cock J, Gomez K (1976) Determination of sugar and starch in plant tissue. In S Yoshida, ed, Laboratory Manual for Physiological Studies of Rice. The International Rice Research Institute, Los Baños, The Philippines, pp 46–49
- Zhang J, Davies WJ (1990. a) Changes in the concentration of ABA in xylem sap as a function of changing soil water status will account for changes in leaf conductance. Plant Cell Environ 13: 277–285 [Google Scholar]
- Zhang J, Davies WJ (1990. b) Dose ABA in the xylem control the rate of leaf growth in soil-dried maize and sunflower plants? J Exp Bot 41: 1125–1132 [Google Scholar]
- Zhang J, He Z, Wu Y (1991) Establishment of an indirect enzyme-linked immunosorbent assay for zeatin and zeatin riboside. J Beijing Agric Univ (China) 17: 145–151 [Google Scholar]
- Zhang J, Sui X, Li B, Su B, Li J, Zhou D (1998) An improved water-use efficiency for winter wheat grown under reduced irrigation. Field Crops Res 59: 91–98 [Google Scholar]
- Zinselmeier C, Westgate ME, Schussler JR, Jones RJ (1995) Low water potential disrupts carbohydrate metabolism in maize (Zea Mays L.) ovaries. Plant Physiol 107: 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Cao X, Luo Y (1988) Growth analysis in the process of grain filling in rice (in Chinese with English abstract). Acta Agron Sin 14: 182–192 [Google Scholar]