Abstract
Infrared video thermography was used to observe ice nucleation temperatures, patterns of ice formation, and freezing rates in nonacclimated and cold acclimated leaves of a spring (cv Quest) and a winter (cv Express) canola (Brassica napus). Distinctly different freezing patterns were observed, and the effect of water content, sugars, and soluble proteins on the freezing process was characterized. When freezing was initiated at a warm subzero temperature, ice growth rapidly spread throughout nonacclimated leaves. In contrast, acclimated leaves initiated freezing in a horseshoe pattern beginning at the uppermost edge followed by a slow progression of ice formation across the leaf. However, when acclimated leaves, either previously killed by a slow freeze (2°C h−1) or by direct submersion in liquid nitrogen, were refrozen their freezing pattern was similar to nonacclimated leaves. A novel technique was developed using filter paper strips to determine the effects of both sugars and proteins on the rate of freezing of cell extracts. Cell sap from nonacclimated leaves froze 3-fold faster than extracts from acclimated leaves. The rate of freezing in leaves was strongly dependent upon the osmotic potential of the leaves. Simple sugars had a much greater effect on freezing rate than proteins. Nonacclimated leaves containing high water content did not supercool as much as acclimated leaves. Additionally, wetted leaves did not supercool as much as nonwetted leaves. As expected, cell solutes depressed the nucleation temperature of leaves. The use of infrared thermography has revealed that the freezing process in plants is a complex process, reminding us that many aspects of freezing tolerance occur at a whole plant level involving aspects of plant structure and metabolites rather than just the expression of specific genes alone.
The initiation of freezing in plants is quite complex (Wisniewski and Fuller, 1999) and involves both extrinsic and intrinsic ice nucleating agents (Lindow, 1983; Ashworth and Kieft, 1995), antifreeze proteins (Griffith et al., 1992; Huang et al., 2002), the permeability and thickness of the cuticle (Wisniewski and Fuller, 1999; Workmaster et al., 1999), and anatomical or morphological physical barriers (Workmaster et al., 1999; Carter et al., 2001). High-resolution infrared video thermography (IRVT) has been used in numerous studies to characterize factors involved in ice nucleation and propagation in specific plant species (Wisniewski and Fuller, 1999; Wisniewski et al., 2002). Collectively, these studies have demonstrated that freezing in herbaceous plants is primarily initiated by extrinsic ice, which must physically penetrate into the interior of the cell. In the absence of extrinsic nucleation, significant supercooling can occur to as low as −12°C (Huang et al., 2002). This is supported by the demonstration that a hydrophobic particle film blocks the growth of extrinsic ice and enhances supercooling in tomato (Lycopersicon esculentum) plants (Wisniewski et al., 2002). In contrast to herbaceous plants, woody plants possess intrinsic ice nucleators that are active at warm, subzero temperatures (Ashworth, 1992). This has been confirmed by studies utilizing IRVT (Wisniewski et al., 1997; Carter et al., 2001). However, current year growth such as recently expanded buds, flowers, and shoots can supercool despite the presence of ice in older stem tissues (Carter et al., 2001).
Pearce and Fuller (2001) presented a comprehensive study of freezing in barley using IRVT in which two distinct freezing events and pathways of ice propagation were observed. Huang et al. (2002) demonstrated that overexpression of an insect antifreeze protein in Arabidopsis enhanced supercooling. Ball et al. (2000), by using IRVT, demonstrated that spatial variation in physical properties of leaves affected the distribution of minimum leaf temperatures and hence the distribution and extent of damage due to freeze-induced dehydration. IRVT has been shown to be a useful tool in exploring how various plant components (biochemical and structural) affect ice nucleation and propagation.
While information on the biochemical and molecular aspects of cold acclimation has greatly increased, knowledge on how these biochemical events affect the freezing process is minimal to nonexistent. In particular the role of proteins and sugars, especially those secreted into the extracellular space, is not understood. Although as Ball et al. (2000) have suggested, they may have a significant effect on the patterns of injury observed. Most studies imply that the freezing of a plant organ is a homogeneous response once ice is initiated, but this may not be the case. Barriers to ice propagation, nonuniform distribution of solutes, and the presence of specific proteins may greatly affect the freezing process.
In this study, IRVT was used to characterize freezing in acclimated and nonacclimated leaves of a winter and a spring canola (Brassica napus) and to determine if cellular constituents play a role in the observed freezing response. Additionally, a novel technique was developed to examine the affect of cellular constituents on the rate of freezing of cell extracts. It is suggested that the use of this technique may lead to a better understanding of the freezing process in the cell walls of plants and how it is affected by the presence of specific metabolites.
RESULTS
Freezing Pattern of Nonacclimated and Acclimated Canola Leaves
IRVT was used to observe the freezing patterns in nonacclimated (LT50 −3°C), partially acclimated (LT50 −7°C), and fully acclimated (LT50 −19°C) leaves of canola cv Express (Fig. 1). The actual temperatures are noted within the figures and reflect the amount of water freezing within the leaf, i.e. large exothermic events reflect large amounts of water freezing while small exothermic events reflect small amounts of water freezing. This is not an absolute since it will partially depend on how fast the heat is dissipated; therefore only qualitative statements can be made. Whether or not the water being frozen had migrated from the symplast to the apoplast in nonacclimated leaves or had frozen intracellularly could not be determined.
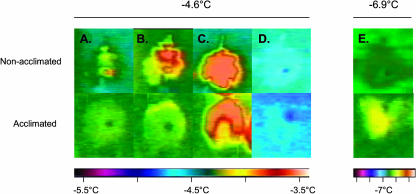
Figure 1.
IRVT visualization of freezing patterns in nonacclimated and acclimated canola (cv Express) leaves. Freezing was induced by INA+ (indicated by the dark spot on the leaf). The chamber was held at −4.6°C, 30 min. After the last exothermic event was observed the temperature was lowered to −6.9°C. −4.6°C: A, In nonacclimated leaves freezing initiated prior to the complete freezing of the INA+ droplet (0.25°C–0.5°C increase in temperature, yellow), while acclimated leaves initiated freezing only after the INA+ droplet completely froze (increase of approximately 0.25°C, green). B, Water rapidly froze throughout the nonacclimated leaves (0.5°C–1°C increase in temperature, yellow to red) compared to only a mild exothermic event observed in acclimated leaves (0.25°C–0.5°C, green to yellow). C, Intense exothermic events were observed in both nonacclimated leaves and acclimated leaves (at least a 1°C increase, red). D, Equilibrium was obtained after approximately 28 min for nonacclimated and after approximately 80 min for acclimated leaves. −6.9°C: E, No exothermic event was detected in nonacclimated leaves; however, in acclimated leaves a mild exothermic event was detected demonstrating the presence of freezable water (increase of 0.25°C–0.5°C, yellow).
At −4.6°C, nonacclimated leaves began to freeze prior to the complete freezing of the ice nucleation active (INA+) bacteria water droplet (Fig. 1A, top). Once freezing was initiated, a rapid significant exothermic event completely spread through the nonacclimated leaves (Fig. 1, B and C, top). In contrast, a slow mild exothermic event was observed in acclimated leaves only after the INA+ droplet had completely frozen (Fig. 1B, bottom). Thereafter, a rapid second exothermic event occurred at the leaf tip producing a horseshoe pattern, after which the entire acclimated leaf froze (Fig. 1C, bottom). There was no apparent impedance for ice growth throughout the entire leaf. Once the leaves reached freezing equilibrium at −4.6°C as represented by a complete dissipation of all exothermic events (Fig. 1D), the temperature was lowered to −6.9°C.
At −6.9°C, no further exothermic events were observed in the nonacclimated leaves; instead they remained isothermal with the chamber temperature indicating the absence of any additional water freezing (Fig. 1E, top). However, a second exothermic event was observed in the acclimated leaves, indicating the freezing of a significant quantity of additional water which was not observed in nonacclimated leaves (Fig. 1E, bottom). The freezing pattern of the partially acclimated leaves was different compared to the nonacclimated leaves, as there was no evidence of localized freezing events as was observed in nonacclimated leaves (data not shown). The time needed to reach freezing equilibrium was much longer for acclimated versus nonacclimated leaves (Table I). Depending on leaf size, acclimated leaves required four times as long to reach freezing equilibrium than nonacclimated leaves while partially acclimated leaves were intermediate.
Table I.
Length of time required to attain equilibrium freezing at −4.6°C for nonacclimated (NAC), partially acclimated (PAC), and acclimated (AC) winter canola (cv Express) leaves is in part a function of tissue water content
| Cultivar | LT50 | Time to Freezing Equilibrium | Average Water Content | Average Dry Weight |
|---|---|---|---|---|
| °C | min | g water g dry wt−1 | g dry wt | |
| Express NAC | −3 | 27.7 ± 2.85 | 5.86 ± 0.66 | 0.0025 ± 0.0003 |
| Express PAC | −7 | 41.8 ± 3.10 | 4.20 ± 0.53 | 0.0059 ± 0.0072 |
| Express AC | −19 | 79.6 ± 6.2 | 2.73 ± 0.43 | 0.0098 ± 0.0002 |
All leaves were of a similar size, and a minimum of six leaves were used for each stage of acclimation. A 10-μL water droplet containing INA+ bacteria was added to each leaf to initiate freezing. The chamber was maintained isothermal until all freezing events attained equilibrium as determined by IRVT. The average water content and dry weight was determined for 1-cm disc sections of NAC, PAC, and AC leaves sampled from seven locations: three were obtained along the midvein from the top to the base of the petiole and four were from the top to the base on either side of the midvein, not including the midvein.
Water content and dry weight was calculated from 1-cm discs sampled from nonacclimated, partially acclimated, and acclimated canola leaves to determine their relationship to the pattern of freezing observed with the IRVT (Table I). In general, average water content decreased and dry weight increased with acclimation, although the distribution was not homogeneous. The water content of nonacclimated leaves varied from 9.85 g water g−1 dry wt for the base of the leaf containing the midvein, to 5.80 g water g−1 dry wt for the tip of the leaf containing the midvein. Areas near the leaf edge not containing the midvein averaged 4.60 g water g−1 dry wt. The dry weight of nonacclimated leaves varied from 0.002 g cm−1 at the tip of leaf to 0.004 g cm−1 at the base of the leaf containing the midvein. The water content of acclimated leaves varied from 2.70 g water g−1 dry wt for the leaf base containing the midvein, to 3.28 g water g−1 dry wt for the tip of the leaf containing the midvein. Leaf areas not including the midvein had an average water content of 2.40 g water g−1 dry wt. The leaf dry weight of acclimated leaves varied from 0.014 g cm−1 at the base containing the midvein, to 0.008 g cm−1 for the leaf tip containing the midvein. Leaf areas not containing the midvein had an average dry wt of 0.009 g cm−1. Partially acclimated leaves had an intermediate water content and dry wt.
Freezing Pattern of Live versus Freeze-Killed Canola Leaves
IRVT was used to observe the freezing patterns of acclimated canola leaves (LT50 −19°C) that were nonfrozen (alive), thawed leaves previously frozen (2°C h−1) to either a nonlethal (−6°C) or an injurious temperature (freeze-killed, −27°C), and leaves killed by placement directly in liquid nitrogen (freeze-killed, N2). The actual temperatures are noted within the figures and reflect the amount of water freezing within the leaf (Fig. 2). When freezing was observed at −3.3°C, a slow mild exothermic event occurred across the entire leaf in both the nonfrozen plants (Fig. 2, A–C, top) and thawed plants previously frozen to −6°C (data not shown). However, in freeze-killed leaves, a large exothermic event immediately occurred as freezing progressed from the point of nucleation across the leaves (Fig. 2, A–C, middle and bottom). After about 90 min at −3.3°C, both the nonfrozen (live) and freeze-killed (−27°C and liquid N2) leaves had reached freezing equilibrium and were isothermal with the chamber (Fig. 2D).
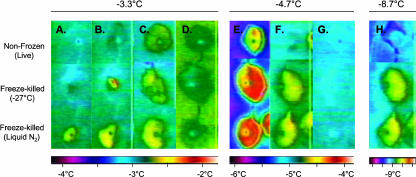
Figure 2.
IRVT visualization of freezing patterns in live and freeze-killed acclimated canola (cv Express) leaves. Freezing was induced by INA+ (indicated by the dark spot on the leaf) to observed freezing in alive nonfrozen leaves (top row) and freeze-killed leaves either previously frozen at 2°C h−1 to −27°C (middle row) or killed by placement directly in liquid N2 (bottom row). The chamber was held at −3.3°C, 30 min after the last exothermic event was observed the temperature was lowered to −4.7°C, then further lowered to −8.7°C. −3.3°C: A–C, After the INA+ droplet completely froze a mild exothermic event occurred throughout the nonfrozen and freeze-killed leaves (0.25°C–0.5°C increase in temperature, green to yellow). D, After approximately 90 min equilibrium was attained for both nonfrozen and freeze-killed leaves. −4.7°C: E, A rapid intense exothermic event occurred in nonfrozen (increase of 0.5°C, yellow) and freeze-killed leaves (at least a 1°C increase, orange to red). F, No further water was observed to be freezing in nonfrozen leaves, while water continued to freeze throughout the freeze-killed leaves (increase of 0.5°C, yellow). G, Equilibrium was obtained after approximately 15 min for the nonfrozen leaves compared to 40 min for the freeze-killed leaves. −8.7°C: H, A very mild exothermic event was observed in nonfrozen leaves (increase of less than 0.25°C, dark blue to green); however, in the freeze-killed leaves a significant exothermic event was detected (0.5°C, yellow).
When the temperature was lowered to −4.7°C, an additional fraction of water froze in leaves of both the nonfrozen plants (Fig. 2E, top) and thawed plants previously frozen to −6°C (data not shown). As well, a large fraction of water froze in the freeze-killed leaves indicated by the rapid significant exothermic event observed via IRVT (Fig. 2E, middle and bottom). Leaves obtained from nonfrozen plants became isothermal after 15 to 20 min, while leaves obtained from freeze-killed plants required 40 min (Fig. 2, F and G). After freezing equilibrium was attained at −4.7°C, the temperature was lowered to −8.7°C.
At −8.7°C, the leaves obtained from nonfrozen plants quickly became either isothermal with the ambient temperature or exhibited a small exothermic event in the middle of the leaf indicating very little additional water was being frozen (Fig. 2H, top). Whereas, leaves obtained from freeze-killed plants again exhibited a significant exothermic event, indicating water was still available for freezing (Fig. 2H, middle and bottom).
Freezing Rate of Cell Sap Extracted from Nonacclimated and Acclimated Canola Leaves
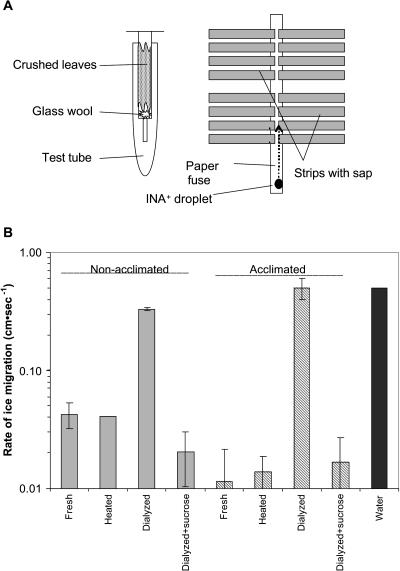
At −3°C cell sap, extracted from nonacclimated and acclimated canola (cv Express) leaves and added to filter paper strips (Fig. 3A), froze at significantly different rates (Fig. 3B). The rate of ice migration was 0.042 cm s−1 for cell sap extracted from nonacclimated leaves compared to 0.011 cm s−1 for cell sap extracted from acclimated leaves (Fig. 3B). Heating the cell sap extract to 90°C for 10 min, followed by centrifugation at 10,000g to remove the coagulated protein, had little or no effect on the freezing rate of cell sap extracted from either acclimated or nonacclimated leaves. However, if the cell sap extracts were dialyzed at 0°C for 24 h (3,500 Mr cutoff) there was a dramatic increase in the rate of freezing. Dialyzed extracts from both nonacclimated and acclimated leaves froze at a similar rate as water (0.50 cm s−1). Interestingly, when 0.5 m of Suc was added to these dialyzed samples, the freezing rates were comparable to that of cell sap extracted from acclimated leaves. Similar results were obtained for nonacclimated and acclimated Puma rye (Secale cereale), Norstar winter wheat (Triticum aestivum), and Elmira winter barley (Hordeum vulgare; data not shown).
Figure 3.
Rate of ice migration (cm s−1) in paper cellulose strips containing extracts from nonacclimated and acclimated canola (cv Express) leaves at −3.5°C. A, To extract the leaf cell sap, tissues were ground with liquid N2, placed in a syringe with glass wool within a Corex tube, and then centrifuged. The extracted cell sap was absorbed to a filter paper strip. One end of this strip was placed perpendicular to a second fuse strip and wetted with double distilled water. An INA+ water droplet was used to initiate freezing. The cell sap strips and fuse strip were placed in a shallow plastic container lined with a thin sheet of plastic, both top and bottom. B, The freezing rate of the cell sap strips was determined at −3.5°C by IRVT. Each cell sap extract was replicated six times. Rate of ice migration (cm s−1) was determined for fresh extracted cell sap; cell sap heated to 90°C for 10 min, then centrifuged to remove coagulated proteins; cell sap dialyzed (3,500 Mr cutoff) against double distilled water at 0°C for 24 h and a dialyzed extract + 0.5 m Suc.
The osmotic potential of the cell sap extracted from nonacclimated and acclimated leaves was 548 and 797 mosmol, respectively. The osmotic potential of the heated cell sap extract was comparable to the nonheated cell sap extract; however, the osmotic potential of the dialyzed samples was only 56 mosmol. The addition of Suc to the dialyzed samples to a final concentration of 0.5 m resulted in an osmotic potential of approximately 550 mosmol.
Sugar analysis revealed the extracts from acclimated leaves of cv Express had a total sugar content of 92.9 mg mL−1, compared to 74.9 mg mL−1 for the cell sap extracted from acclimated cv Quest leaves (Table II). Extracts from nonacclimated Quest leaves had very low levels of sugars, whereas the sugar content was 10 times higher in extracts from nonacclimated Express leaves. This is not too surprising since Quest is a spring type canola that is actively growing at warm temperatures and therefore photosynthates are readily consumed for growth. In contrast, Express is a winter annual that requires a period of vernalization to flower; therefore, growth is slower compared to Quest as Express remains in a vegetative state. Little or no Suc was detected in the nonacclimated leaves of both cultivars. Total sugars of cell extracts from acclimated Quest leaves were 20 times higher and Express leaves were 3 times higher compared to cell sap extracted from nonacclimated leaves. Extracts from acclimated Express had 2-fold higher levels of Suc compared to extracts from acclimated Quest. A similar level of raffinose was detected in extracts from leaves both of acclimated Quest and Express leaves, as well as nonacclimated Express.
Table II.
Sugar analysis of leaf extracts from NAC and AC winter (cv Express) and spring (cv Quest) canola leaves
| Cultivar | Glc | Fru | Suc | Raffinose | Total |
|---|---|---|---|---|---|
| mg mL−1 of extract | |||||
| Quest NAC | 1.2 | 2.1 | 0.0 | 0.0 | 3.1 |
| Quest AC | 24.2 | 28.2 | 12.2 | 10.3 | 74.9 |
| Express NAC | 15.5 | 6.5 | 1.0 | 8.0 | 31.0 |
| Express AC | 28.7 | 22.0 | 31.4 | 10.8 | 92.9 |
Each sample (100 mL) was made to a final volume of 5 mL using double distilled water and passed through a 0.2-μm filter. The sugars were fractionated on a Dionex BioLC 4000 gradient liquid chromatographic system; the detection system was a Dionex pulsed amperometric detector. The carbohydrates were identified and quantitated by comparison with known standards. Statistical analysis of standard curves showed a correlation of 0.994 or better.
Rates of Freezing of Sugar and Protein Solutions
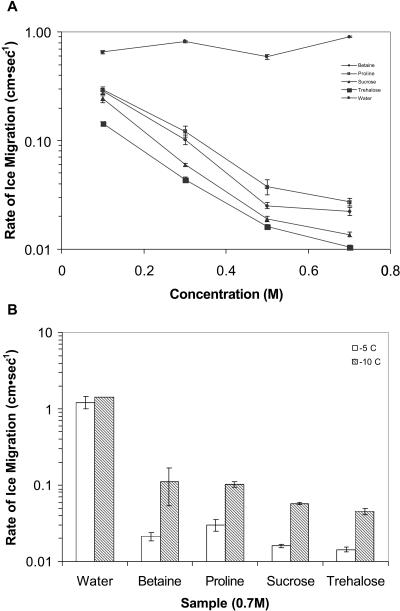
Betaine, Pro, Suc, and trehalose ranging in concentration from 0.0 m to 0.7 m were evaluated for their effect on the rate of ice migration in filter paper strips at −2.5°C (Fig. 4A). With increasing concentration of each compound, the rate of ice migration was dramatically reduced. Trehalose had the greatest effect followed by Suc, betaine, and Pro. At −5°C and −10°C, both 0.7 m trehalose and Suc were equally effective in reducing the rate of ice migration (Fig. 4B). At equimolar concentrations, Pro and betaine were not as effective as Suc and trehalose at reducing the rate of ice migration in comparison to water.
Figure 4.
The effect of betaine, Pro, Suc, and trehalose on the rate of ice migration. A, Concentrations ranging from 0.0 to 0.7 m were evaluated for their effect on the rate of ice migration on filter paper strips at −2.5°C. B, The effect of betaine, Pro, Suc, and trehalose (all at 0.7 m) on the rate of ice migration was evaluated at −5°C and −10°C. The rate of ice migration (cm s−1) on paper cellulose strips was monitored by IRVT and conducted as previously described in Figure 3. Each solution was replicated six times.
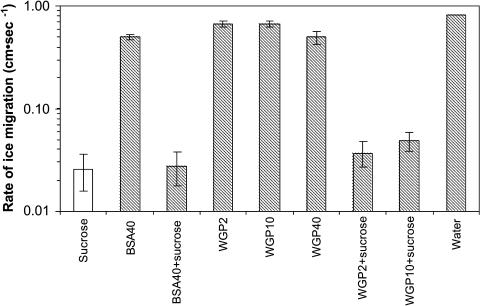
Both bovine serum albumin (BSA), often used as a cryoprotectant, and wheat germ protein (WGP), alone or in combination with Suc, were used to test for the effect of protein on freezing rate. WGP was selected since it is highly hydrophilic, boiling stable, and classified as a late-embryogenesis-abundant protein and therefore is in the same category of a number of the proteins associated with freezing tolerance in plants. Neither BSA nor WGP protein alone had a dramatic effect on the rate of freezing at −3°C (Fig. 5). Solutions of WGP at a concentration of 2 mg mL−1 froze at a rate similar to water. The freezing rate of solutions of WGP at 10 and 40 mg mL−1 was 0.67 and 0.50 cm s−1, respectively. However, when Suc was added to a final concentration of 0.5 m, the freezing rate of the WGP solution was reduced to approximately 0.038 to 0.050 cm s−1 depending on the protein concentration. Solutions of BSA at 40 mg mL−1 froze at a rate comparable to a 40 mg mL−1 solution of WGP. The addition of Suc to a final concentration of 0.5 m reduced the freezing rate of BSA solution to that of 0.5 m Suc alone.
Figure 5.
The effect of BSA and WGP alone and in combination with 0.5 m Suc on the rate of freezing at −3°C. To test the effect of protein on freezing rate, 0.5 m Suc in water and in combination with either BSA or WGP was used. The concentration of both proteins varied from 2 to 40 mg mL−1. The rate of ice migration (cm s−1) on paper cellulose strips was monitored by IRVT and conducted as previously described in Figure 3. Each solution was replicated six times.
The Nucleation Temperature of Leaves and Cell Extracts from Nonacclimated and Acclimated Canola
The temperature at which freezing was initiated in wetted and nonwetted leaves was determined for both Quest and Express canola (Table III). Leaves were obtained from plants that were nonacclimated, partially acclimated under natural conditions, or fully acclimated in a controlled environment chamber. Nonwetted leaves obtained from fully acclimated plants readily supercooled to temperatures as low as −14°C, whereas nonacclimated leaves supercooled only to −9°C or −10°C. Leaves wetted with a fine spray of water supercooled from −6°C to −9°C irrespective if they were acclimated or not. Plants partially acclimated under natural conditions supercooled to similar temperatures as plants acclimated in a controlled environment chamber.
Table III.
The nucleation temperature of leaves and cell extracts from NAC, PAC, and AC winter (cv Express) and spring (cv Quest) canola leaves
| Cultivar | LT50 | Leaves
|
Fresh | Cell Sap Extract
|
Dialyzed + Suc | ||
|---|---|---|---|---|---|---|---|
| Dry | Wet | Heated (90°C) | Dialyzed | ||||
| °C | |||||||
| Express NAC | −3 | −8.6 ± 1.2 | −6.3 ± 0.75 | −10.7 ± 0.5 | −15.8 ± 1.32 | −6.5 ± 0.5 | −12.2 ± 0.5 |
| Express PAC | −7 | −12.5 ± 0.75 | −7.6 ± 0.28 | ||||
| Express AC | −18 | −13.0 ± 0.8 | −8.8 ± 0.85 | −12.2 ± 1.1 | −18.8 ± 0.7 | −10.6 ± 1.0 | −14.2 ± 0.9 |
| Quest NAC | −3 | −10.8 ± 1.5 | −5.5 ± 0.57 | −12.2 ± 1.0 | −18.8 ± 0.6 | −9.5 ± 0.7 | −13.7 ± 0.8 |
| Quest PAC | −6 | −13.1 ± 0.27 | −6.9 ± 0.8 | ||||
| Quest AC | −17 | −14.0 ± 1.2 | −7.2 ± 0.67 | −15.7 ± 1.2 | −18.9 ± 1.7 | −13.3 ± 0.6 | −15.1 ± 1.0 |
The freezing initiation temperature was determined for wetted and nonwetted canola leaves. The ice nucleation temperature was determined for extracted cell sap, heated extract (90°C for 10 min), dialyzed extract, and dialyzed extract + 0.5 m Suc (control −12.6 ± 1.1°C INT).
The ice nucleation temperature of fresh cell sap extracts (control), heated extract samples, and dialyzed extract samples with or without 0.5 m Suc is shown in Table III. Generally cell sap extract from acclimated leaves for both Express and Quest supercooled 2°C more than extracts from nonacclimated leaves. There was close agreement between the nucleation temperature of the leaves and the cell sap extract. For example, nonwetted acclimated leaves of Express supercooled to −13°C compared to −12°C for the cell sap extract. Surprisingly, boiled extract samples tended to supercool more than nonheated extracts. For example, acclimated Express extracts supercooled to −18°C versus only −12°C for the nonheated extract samples. The dialyzed extracts of both cultivars supercooled the least. However, when Suc was added to the dialyzed samples, the nucleation temperature was similar to the cell sap extracted from acclimated leaves. Solutions of 0.5 m Suc alone also supercooled to −12.6°C, which was similar to the dialyzed plus Suc extracts.
Supercooling and Viability
Freezing is generally believed to initiate in the apoplastic spaces of plant tissues. Due to the difference in chemical potential created by a growing ice crystal, water migrates from the symplast to the apoplastic either by diffusion or via aquaporin channels in the plasma membrane (Chaumont et al., 2000). With decreasing temperatures there is an increase in available energy to drive the reaction from liquid water to ice. If a significant degree of supercooling below 0°C occurs, sufficient energy may be available for the growing ice crystal to penetrate the plasma membrane and/or ice formation may occur in the symplast. IRVT provided an excellent means to study the effect of the extent of supercooling prior to ice nucleation on the viability of leaves. As demonstrated earlier, nonwetted, nonacclimated canola leaves (cv Express) can supercool to temperatures as low as −13°C. Nonacclimated canola leaves frozen at and maintained at −3°C for 5 min sustained 6.8% ion leakage, whereas after 10 min leakage increased to 66% (Table IV). Thereafter, ion leakage exceeded 90%, indicating total cell lysis.
Table IV.
Electrolyte leakage following freezing of supercooled (SC) nonacclimated and acclimated canola (cv Express) leaves
| Nonacclimated Canola
|
Acclimated Canola
|
|||||
|---|---|---|---|---|---|---|
| Duration of Freezing
|
Electrolyte Leakage
|
Duration of Freezing
|
Electrolyte Leakage
|
|||
| −3°C | −6°C | −9°C | −12°C | −15°C | ||
| min | % | h | % | |||
| 0 | 6.2 | 0.0 | 7.7 | 5.7 | 5.7 | 6.8 |
| 5 | 6.8 | 0.5 | 8.0 | 4.7 | 8.3 | 41 |
| 10 | 66 | 2.0 | 9.6 | 6.6 | 11 | 46 |
| 20 | 93 | 24 | 8.8 | 16 | 27 | 47 |
| 40 | 92 | |||||
Canola leaves of similar size were excised from both nonacclimated and acclimated plants. In all cases the end of the excised petiole was coated with a silicone grease to prevent water loss and nucleation at the cut surface. Freezing of supercooled nonwetted leaves was initiated with ice crystals and the freezing events were monitored via IRVT. Nonacclimated canola leaves supercooled at −3°C for 5 to 40 min. Fully acclimated leaves were supercooled to −3°C, −6°C, −9°C, −12°C, or −15°C, then held isothermal for 0, 0.5, 2, or 24 h. Afterward, evaluation was conducted by electrolyte leakage.
Fully acclimated nonwetted canola (cv Express) leaves were supercooled to −3°C, −6°C, −9°C, −12°C, or −15°C prior to initiating freezing with the use of ice crystals. The leaves were held isothermal at one of these selected temperatures for 0, 0.5, 2, or 24 h and afterward evaluated for electrolyte leakage. Surprisingly, supercooled, cold acclimated canola leaves when frozen at −9°C sustained little injury as determined by the LT50 results (Table IV). Leaves supercooled to −12°C suffered little injury for up to 2 h, whereas after 24 h ion leakage increased. Freezing of leaves supercooled to −15°C was devastating, resulting in over 40% ion leakage in samples collected 0.5 h after the initiation of freezing.
DISCUSSION
Visualization of Freezing in Nonacclimated and Acclimated Canola Leaves
In this study, nonacclimated canola leaves were killed when frozen at −4.6°C, with freezing observed as a large and rapid exothermic event (Fig. 1). This is due in part to the high water content of nonacclimated leaves (Table I) and the amount of energy available for ice crystal growth (Olien, 1967). In contrast, acclimated leaves that have a lower water content (Table I) initiated freezing much more slowly with a gradual progression to the main midvein (Fig. 1). Acclimated leaves also exhibited a small exothermic and rapid flash freeze that occurred at the leaf edge and progressed in a horseshoe pattern to the base of the leaf. This may be due to the outer edges of the leaf being cooler than the basal section of the leaf. Approximately 80 min was required for freezing of cold acclimated leaves to attain equilibrium at −4.6°C versus only 28 min for nonacclimated leaves. Previous work by Olien (1967) has shown that the time required to freeze the bulk of leaf water can influence the freezing pattern in the whole plant. Water at distal sites readily migrates in the vapor phase to the growing ice crystal. This creates dry sections within the tissue that can impede the rate of ice growth (Single and Marcellos, 1974; Gusta et al., 2000). The time required for freezing to attain equilibrium in leaf tissue is a function of temperature, leaf water content, and permeability of the membrane and cell wall (Reaney, 1989), as well as the osmotic potential of the cell. Other contributing factors may include antifreeze proteins (Antikainen and Griffith, 1997), sugars, and the distance water has to migrate to the ice crystal (Reaney and Gusta, 1999). This study demonstrates how explosive ice growth is in tissue that has high water content. Olien (1967) emphasized the impact water had on the freezing kinetics of tissue water and its impact on the freeze-killing temperature.
Provided that ice is partitioned in the apoplastic spaces, the symplastic water remains in a supercooled state (Pearce and Fuller, 2001). Thus, during freezing the osmotic potential of the supercooled water increases as a function of time and temperature. Water vapor migrates to sites of extracellular ice over time (equilibrium freezing), and thus ions become concentrated and osmotic potential increases. However, our results suggest ice can penetrate the symplast of nonacclimated cells nucleated at −4.6°C, as the whole leaf freezes quickly. When the temperature was lowered to −6.9°C, there was little evidence of additional freezing in the tender cells (nonacclimated); however, a small fraction of water froze in the hardened acclimated cells (Fig. 1). Using NMR spectroscopy, Gusta et al. (1975) demonstrated water in nonacclimated and acclimated herbaceous plants froze similar to a dilute aqueous solution and that over 80% of the freezable water was converted to ice at −8°C. Johansson (1970) suggested cold hardiness is acquired in part by increasing the cell sap concentration and thereby reducing the proportion of water frozen at subfreezing temperatures.
Freezing kinetics of cell sap extracted from leaves suggest dissolved cell solutes with a Mr of less than 3,500 inhibit ice growth. It is well documented that the velocity of the ice crystal growth in dilute solutions and capillaries is determined by simple kinetic and thermodynamic principles (Fletcher, 1970). These same principles have been used to predict freezing in the apoplast and xylem vessels (Reaney, 1989; Reaney and Gusta, 1999). During ice growth, solutes and impurities are concentrated since they are excluded from the growing ice front. During a mild freeze, solutes excluded from ice accumulate in the matrix solution. This matrix structure depresses the freezing point of water and the accumulated solutes depress the freezing point of the matrix further (Fletcher, 1970). The freezing temperature of the cell wall is determined by the solute concentration in the cell wall, the size of the cell wall pores, and the matrix potential of the cell wall (Fletcher, 1970; Reaney and Gusta, 1999). Thus during a mild freeze, the rate of freezing is determined by the rate of water movement through the cell wall as ice does not grow through the cell wall. However, if the temperature is lower than the freezing point depression of the water in the cell wall, then ice rapidly grows through it. In addition, Zimmerman (1982) reported that during freezing, transient pores can form in the plasma membrane allowing for the bulk flow of solutes from the symplast to the apoplast. Olien (1967) as well as Livingston and Henderson (1998) also demonstrated solutes were released from the symplast during a mild freeze and were concentrated in the apoplastic water. Reaney and Gusta (1999) postulated these solutes would reduce the rate of ice growth through the cell wall. These solutes form a fluid zone that surrounds cells as freezing progresses and prevents ice adhesion to the plasma membrane which otherwise can result in lysis (Olien and Smith, 1981). The release of solutes from the symplast may account for the increase in freezing tolerance following a mild nonlethal frost (Trunova, 1965).
Freezing of Live versus Freeze-Killed Canola Leaves
Following a lethal freeze membrane, permeability properties are disrupted or completely lost, resulting in a mixture of water and cell solutes that migrate to the apoplasm (Dexter et al., 1930; Siminovitch and Levitt, 1941; Gusta et al., 1982). The movement of sugars and organic solutes from the symplast to the apoplast would have a direct effect on the freezing rate, as demonstrated by freezing of cell extracts added to filter paper strips (Fig. 3B). Small Mr compounds (Mr cutoff of 3,500) strongly retarded the rate of freezing of cell extracts. This may partially account for why the freeze-killed leaves at −3.5°C froze slowly from the point of nucleation and eventually encompassed the whole leaf. The initial flash freeze at −3.5°C of uninjured leaves (nonfrozen) that was evidenced as a small exothermic event that quickly dissipated (Fig. 2) suggests that this water represents either relatively pure, nonstructured, free liquid water (i.e. water not associated with solutes, cell walls, or other macromolecules) or water vapor, since the event lasts only a matter of seconds. In addition, the amount of water that does freeze is small as the increase in leaf temperature is only minimal. Following the flash freeze −3.5°C, a small mild exothermic event was observed in the nonfrozen leaves, whereas freeze-killed leaves had a significant exothermic event that is indicative of a large quantity of water freezing. Therefore, it appears that in previously uninjured (nonfrozen) leaves water migrates to sites of extracellular ice. A probable cause for the larger quantity of water that freezes at −3.5°C in freeze-killed leaves compared to nonfrozen leaves is due to the loss of water compartmentalization in the leaf. Due to the laceration of cell membranes by ice, water forms one large pool, and therefore ice growth no longer is restricted. These observations are consistent with the hypothesis that the permeability properties of the plasma membrane are altered following a lethal frost. These data also highlight the value of using IRVT to diagnose patterns of injury, compartmentalization of water, and relative amounts of water that are freezing at any particular temperature.
Leaf and Cell Extract Ice Nucleation Activity
Supercooled water in plant tissues is induced to crystallize by either intrinsic or extrinsic heterogeneous ice nucleators (Lindow, 1983). Pseudomonas syringae, an efficient biological ice nucleator, initiates ice formation between −2°C to −5°C. Although intrinsic plant ice nucleators have been suggested, none to date have been isolated and found to be as effective as P. syringae (Chen et al., 1995). Wisniewski et al. (2001), using IRVT, demonstrated that nonwetted herbaceous plants always supercooled to a lower temperature than wetted plants. However, Griffith et al. (1993) demonstrated wetted, nonacclimated rye (Secale cereale) leaves supercooled to −6°C, whereas cold acclimated rye leaves only supercooled to −3°C, which is contrary to the results we obtained for canola and winter cereals in this study. They further reported that populations of ice-nucleating bacteria were below detectable limits using the methods described by Lindow (1983). Our results also demonstrate that nonwetted, cold hardened canola leaves readily supercool to temperatures as low as −13°C to −14°C, while wetted leaves supercool to approximately −7°C (Table III). On average, nonwetted, nonacclimated leaves did not supercool to the same level as the cold acclimated leaves. This may be due in part to the higher water content of nonacclimated leaves (7–8 g water g dry wt−1) versus for the cold hardened leaves (2.5–4 g water g dry wt−1). Our results also suggest small Mr compounds affect the nucleation temperature of cell extracts (Fig. 3). Nondialyzed samples froze on average 2°C to 4°C colder than dialyzed samples. However, the addition of Suc to the dialyzed samples suppressed the nucleation temperature to values comparable to the nondialyzed extracts. Surprisingly, heated extracts supercooled the most. This suggests either certain proteins in their native state may be weak ice nucleators or, if intercellular, heterogeneous nucleators were present at the active site, since ice nucleation was inactivated by heating.
Interestingly, plants grown outside would be expected to have higher populations of ice nucleating bacteria than plants grown in controlled environment chambers since it has been reported that higher populations of INA+ bacteria occur in leaves grown under natural conditions (Lindow, 1983). However, in our study there was no significant difference in the extent of supercooling for these two populations of plants, regardless if they were wetted or nonwetted (Table III). In the case of wetted leaves, the frozen droplets of water containing INA+ bacteria on the leaf surface served as the nucleator and induced freezing in the plant. In the case of the nonwetted leaves, nucleation may have been intrinsic or extrinsic.
Supercooling as Related to Injury
Supercooled tissues escape frost injury as ice does not form in their tissues. However, if ice formation does occur in supercooled tissue, it can be potentially lethal depending on where the ice forms. Injury to the tissue may result from ice formation either within the symplast or via ice growth into the symplast. Since the tissues of herbaceous plants (both tender and hardy) can potentially supercool to temperatures lower than −8°C, nucleation at warmer temperatures must result from the growth of external ice crystals into the internal portions of the plant via stomata or imperfections in the epidermis, inducing the freezing of apoplastic water or water vapor (Wisniewski and Fuller, 1999). Chen (1976) reported wild species of potato tolerated −6 to −8°C if freezing of the leaves was initiated at −2°C but not at −3°C. Wisniewski et al. (2001) placed a single droplet of INA+ bacteria on the surface of tomato leaves and tracked freezing via IRVT. Leaves subjected to 5 min or less of freezing developed necrotic spots or wetting at the site of ice formation in the leaf, suggesting that even though ice had formed within the leaf, small populations of cells must have experienced intracellular ice formation and had been lysed. These results suggest nonacclimated or frost sensitive leaves are injured due to the growth of ice into the symplast rather than due to freeze-induced dehydration effects. In the present study, cells of nonacclimated canola leaves frozen for as little as 10 min suffered complete lysis (40% or greater electrolyte leakage) after a lethal freeze of −3°C (Table IV), suggesting a similar freezing pattern as reported by Wisniewski et al. (2001).
As discussed above, the killing temperature of nonhardy plants such as potatoes is dependent upon the nucleation temperature. Griffith et al. (1993), while studying the frost tolerance of cold acclimated winter rye, demonstrated that rye leaves when spontaneously nucleated at −12°C suffered little or no injury as determined by electrolyte leakage loss. In our study, we observed that cold hardened canola leaves (LT50 −19°C) sustained little injury when nucleated at −9°C (Table IV). At −9°C, ice growth throughout the leaf was extremely rapid in comparison to leaves frozen at −3°C (results not shown). Freezing of leaves supercooled to −12°C resulted in injury only after the leaves were held for 24 h at −12°C but not for leaves frozen for 2 h. Since equilibrium freezing would have occurred within the 2 h interval, it is assumed that leaves frozen for 24 h suffered from freeze-induced desiccation. Gusta et al. (1997) demonstrated cold hardened herbaceous plants (e.g. winter wheat) can tolerate frosts of −24°C when cooled at 2°C h−1; however, a continuous frost of −12°C results in injury after a few days. Plants frozen when supercooled to −15°C were apparently killed via intracellular ice formation resulting from the inability of water to move to the apoplast in a rapid enough manner for equilibrium freezing to occur (Table IV). This would result in rapidly growing ice crystals penetrating the plasma membrane or nucleation occurring directly in the symplast. Results suggest freezing occurred in the apoplast and the movement of water to the apoplast was the critical factor.
CONCLUSIONS
Over the past decade, research on cold hardiness has focused on identifying genes responsible for this trait and how they are regulated (Wisniewski et al., 2003). This effort has resulted in the identification of specific genes or transcription factors that appear to play a significant role; however, global genetic analyses using microarray technologies have also revealed the complexity of the cold acclimation process. The realization that hundreds of genes can be either up- or down-regulated underscores the need to develop a better understanding of how whole plants respond to stress in order to understand the potential impact of any specific gene or sets of genes on the plant's response to freezing stress. Understanding the factors that determine how plants freeze, ice propagates, and plant tissues are injured can provide valuable insight into the protective role of specific proteins and metabolites, as well as the adaptive advantage of the structural composition of plant architecture. In this regard, IRVT has been demonstrated to be a valuable tool in studying the freezing process in whole plants in a nonintrusive manner.
Previously Gusta et al. (1975) demonstrated that the freezing patterns of water were similar in nonacclimated and acclimated winter annuals and that no distinctively different patterns were detected by either thermal analysis or NMR. However in this study, we have established that the freezing process in nonacclimated and cold acclimated plants is distinctly different. We revealed a considerable fraction of unfrozen water at −7°C that was detected only in acclimated leaves. In addition, ice formation in nonacclimated plants was much more rapid compared to acclimated plants where the freezing process occurred in a two-step process. The first step involved a small exothermic event that spread throughout the whole leaf and then quickly dissipated. It is assumed that this was either the freezing of water vapor or the water present on the outside of cell walls. The second step involved the slow migration of intracellular water to extracellular ice and a growing ice front. Additionally, it took acclimated plants significantly longer to reach freezing equilibrium at any specific temperature than nonacclimated plants. The reasons attributable to this differential response appear to be associated generally with water content of the tissue and the amount of solutes present. Nonacclimated plants had a higher water and sugar content (Tables I and II). Sugars and other osmotically active metabolites (Pro and betaine) had the greatest impact on the rate of ice propagation (Figs. 3–5). The novel use of IRVT allowed for the direct visualization and determination of the impact of these components on the freezing process.
Interestingly, it was observed that leaves that were dry had a significant capacity to supercool and that this was reflected in the nucleation temperature of the cell extracts (Table III). These observations further support our previous contention that ice nucleation in herbaceous plants at warm, subzero temperatures is induced by the growth of external ice crystals into the interior of the plant (Wisniewski et al., 2001; Wisniewski and Fuller, 1999). This knowledge represents a novel finding based on the use of IRVT.
Additionally, the use of IRVT allowed us to determine directly the effect of the extent of supercooling prior to freezing on injury and separate out injury that resulted directly from intracellular ice formation versus dehydration (Table IV). Our data indicate that cold acclimated plants can withstand a greater amount of supercooling prior to the initiation of freezing without causing injury than nonacclimated plants. Large exothermic events occur when freezing is initiated in supercooled plants and significant amounts of water must move very rapidly to sites of extracellular ice in order to reach freezing equilibrium. Our data indicate that cold acclimated plants have a greater ability than nonacclimated plants to move this water to sites of extracellular ice without inducing intracellular ice formation. Perhaps specific aquaporins are involved in this ability, although this remains to be determined. In this regard, the overexpression of an aquaporin in baker's yeast (Saccharomyces cerevisae) resulted in greater freeze tolerance (Tanghe et al., 2002).
In summary, this study has revealed distinctly different freezing patterns in acclimated and nonacclimated leaves of canola. Collectively, using IRVT, the data provided in the present report has provided significant new findings on the freezing process in plants and demonstrated a novel method of analyzing the effect of various compounds on the rate of ice propagation. It is suggested that the use of IRVT may be a useful tool to evaluate the effect of genetic manipulations such as the overexpression of specific genes or transcription factors on the freezing process in plants.
MATERIALS AND METHODS
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining permissions will be the responsibility of the requestor.
Cold Acclimation of Plants
Seeds of winter (cv Express) and spring (cv Quest) canola (Brassica napus) were obtained from local research plots. Plants were grown in 15-cm diameter pots in REDI-Earth (W.R. Grace & Co., Ajax, Canada) at 20°C with an 18-h photoperiod (250 μE m−2 s−1 at pot height) until the 3- to 4-leaf stages. The temperature was then reduced to 7°C light/5°C dark for 3 d, 5°C/2°C for 3 d, and finally to 2°C/0°C for 24 d. The photoperiod was changed to 16 h following transfer from 20°C at the same iridescence level. Plants were harvested immediately prior to the reduction of temperature from 20°C (tender, nonacclimated) and after 24 d at 2°C/0°C (acclimated). In addition, plants at the 3- to 4-leaf stages were transferred from 20°C to the natural environment in late April (partially acclimated). Outside day-night temperatures ranged from a high of 12°C to a low of 0°C.
Determination of Freezing Tolerance
The freezing tolerance of canola was determined by the electrolyte leakage test (LT50) as previously described by Sukumaran and Weiser (1972). For all freeze tests, the tissue was briefly misted with fine water droplets, nucleated with ice crystals at −2.5°C, and then left to equilibrate overnight. The following day, the temperature was lowered 2°C h−1 and samples were removed at 2°C intervals. The frozen tissues were allowed to thaw overnight at 4°C and then assayed for loss of electrolytes.
Infrared Video Thermography
Temperature changes and freezing events in both the plant material and cell extracts were monitored with an imaging radiometer (model 760, Inframatrics, North Billerica, MA) with an HgCdTe longwave (8 mm–12 mm) detector, previously described by Wisniewski et al. (1997). Infrared images were recorded on videotape. To achieve the highest resolution of exothermic events, a temperature span of 2°C was selected on the radiometer monitor. The midpoint of this range was lowered manually as the temperature decreased to ensure exothermic events were detected. Color differences are representative of the latent heat of fusion formed when water changes to ice; the greater the amount of freezing water, the greater the degree of exothermic heat loss by the tissue.
Experiments were conducted either in a controlled environment chamber (Conviron P6V-36, Winnipeg, Canada) or within a Tenney Environmental Chamber (Tenney Environmental, Williamsport, PA). The temperature of both chambers can be varied by 0.1°C and held isothermal at a given temperature.
Freezing Pattern of Nonacclimated and Acclimated Canola Leaves
Nonacclimated, partially acclimated, and fully acclimated leaves of winter canola (cv Express) were held at −4.6°C for 30 min in order for the leaves to equilibrate to this temperature. All leaves were of a similar size, and a minimum of 6 leaves were used for each stage of acclimation. A 10-μL water droplet containing ice nucleation active (INA+) bacteria (Cit 7 strain of INA+ Pseudomonas syringae) was added to each leaf to initiate freezing. The chamber was maintained isothermal at this temperature until all freezing events attained equilibrium as determined by IRVT. When the last leaf froze, the temperature of the chamber was maintained at −4.6°C for an additional 30 min and then the temperature was lowered to −6.9°C.
Determination of Water Content and Dry Weight of Express Canola Leaves
Water content was calculated from 1-cm disc sections sampled from nonacclimated, partially acclimated, and fully acclimated canola leaves to determine its influence on temperature nucleation and freezing pattern. The 1-cm discs were obtained with a cork borer from leaves. The leaf discs were weighed immediately, dried at 60°C for 2 d, and then reweighted to obtain the dry weight as described by Gusta et al. (1975). The 1-cm discs were collected from either the base of the leaf containing the midvein, the tip of the leaf containing the midvein, or areas near the leaf edge not containing the midvein.
Freezing of Live versus Freeze-Killed Acclimated Canola Leaves
To determine the freezing pattern of live versus freeze-killed leaves, leaves from cold acclimated canola plants (cv Express; LT50 −19°C) were nonfrozen, previously frozen at 2°C h−1 to either −6 or −27°C, or frozen directly in liquid nitrogen (N2). Frozen leaves were held at 4°C overnight in sealed plastic bags to allow the tissue to thaw and then placed in a chamber maintained at −3.3°C. The freeze-killed plants had the same overall water content as the nonfrozen plants. To each leaf, 10 μL of INA+ bacteria was added to initiate freezing. Freezing events and the time required for leaf tissue to attain temperature equilibrium with ambient temperatures following a freezing event were recorded by IRVT, as described previously by Wisniewski et al. (1997). When a freezing event was detected, the temperature of the chamber was held constant until the leaf tissue reached equilibrium with the chamber temperature. After the freezing event reached equilibrium at −3.3°C, the temperature was lowered to −4.7°C then further lowered to −8.7°C.
Supercooling of Wetted Canola Leaves
Canola (cv Express) leaves of similar size were excised from both nonacclimated and acclimated plants. In all cases the end of the excised petiole was coated with a silicone grease to prevent water loss and nucleation at the cut surface. Leaves were either sprayed on both sides with a fine mist of water containing Tween 20, until the leaves were completely wetted on both sides, or not sprayed. In some studies, a 10-μL droplet of water containing INA+ bacteria was added to the leaf to initiate a point freezing event.
Cell Sap Extraction, Modification, and Freezing
To extract leaf cell sap, plant tissues were ground in a mortar and pestle with liquid N2 and placed in a 5-mL syringe containing a piece of glass wool in the bottom of the tube. The cell sap was extracted by placing the syringe in a 10-mL Corex tube and then centrifuged for 10 min at 10,000g. An aliquot of the cell sap was heated to 90°C for 10 min and then centrifuged at 10,000g for 10 min to remove coagulated proteins (Fig. 3A). In addition, an aliquot of the cell sap was dialyzed (3,500 Mr cutoff) against double distilled water at 0°C for 24 h; to a portion of this aliquot Suc was added to attain a final concentration of 0.5 m. The osmotic potential of the sap was measured using a vapor pressure osmometer (Wescor model 5500; Logan, UT).
The cell sap (2 mL) was absorbed to an 8 cm × 1 cm strip of Whatman (Clifton, NJ) #2 filter paper (Fig. 3A). One end of this strip was placed perpendicular to a second fuse strip and wetted with double distilled water. A 10-μL droplet of water containing INA+ bacteria was placed on the end of the fuse strip to initiate freezing. The cell sap strips and fuse strip were placed in a shallow plastic container lined with a thin sheet of plastic, both top and bottom. The freezing rate of the cell sap strips was determined at −3.5°C. The length of time required to freeze the entire strip was monitored by IRVT. Each cell sap extract was replicated six times.
Sugar Analysis
Cell sap was extracted from nonacclimated and acclimated leaves of Quest and Express as described above. Each sample (100 mL) was made to a final volume of 5 mL using double distilled water and passed through a 0.2-μm filter (Chromatographic Specialties, Brockville, Canada). The sugars were fractionated on a Dionex (Sunnyvale, CA) BioLC 4000 gradient liquid chromatographic system; the detection system was a Dionex pulsed amperometric detector as described previously by Swallow and Low (1994). The carbohydrates were identified and quantified by comparison with known standards. Statistical analysis of standard curves showed a correlation of 0.994 or better.
Ice Nucleation Temperature of Cell Extracts, Sugars, and Proteins
The ice nucleation temperature of cell sap extracts from canola, sugar, and proteins was determined utilizing 10-μL droplets placed on a sheet of thin plastic (Saran Wrap) in a shallow plastic container sealed with thin plastic to reduce evaporation. The chamber temperature was reduced by 0.5°C increments after 20 min at each temperature. The heterogeneous nucleation temperature was determined for either the extracted cell sap, cell sap heated to 90°C for 10 min, dialyzed cell sap, or dialyzed cell sap to which Suc was added to a final concentration of 0.5 m (as described above). The ice nucleation temperature was also determined for 0.5 m Suc in water and in combination with either BSA or WGP. The concentration of both proteins varied from 2 to 40 mg mL−1.
Viability of Supercooled Nonacclimated and Acclimated Canola Leaves
Excised leaves of nonacclimated and acclimated canola leaves (cv Express) were placed within a controlled environment chamber maintained at −3°C, −6°C, −9°C, −12°C, or −15°C. After 30 min the leaves were nucleated at given temperatures to allow equilibration with ice crystals to initiate freezing. Nonacclimated leaves were removed after 0, 5, 10, 20, and 40 min, and cold acclimated leaves were removed after 0, 0.5, 2.0, and 24 h and then held at 4°C for 24 h. To assess injury, electrolyte leakage was determined as described by Sukumaran and Weiser (1972). The leaves were monitored via IRVT to ensure the leaves had supercooled to the selected test temperatures and were unfrozen until nucleated by the ice crystals.
This work was supported by the National Sciences and Engineering Research Council of Canada (NSERC; operating grant to L.V.G.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.028308.
References
- Antikainen M, Griffith M (1997) Antifreeze protein accumulation in freezing-tolerant cereals. Physiol Plant 99: 423–443 [Google Scholar]
- Ashworth EN (1992) Formation and spread of ice in plant tissues. Hortic Rev 13: 215–255 [Google Scholar]
- Ashworth EN, Kieft TL (1995) Ice nucleation activity associated with plants and fungi. In RE Lee, GJ Warren, LV Gusta, eds, Biological Ice Nucleation and Its Applications. APS Press, St. Paul, pp 137–162
- Ball MC, Wolfe J, Canny M, Hofmann M, Nicotra AB, Hughes D (2000) Space and time dependence of temperature and freezing in evergreen leaves. Funct Plant Biol 29: 1259–1272 [DOI] [PubMed] [Google Scholar]
- Carter J, Brennan R, Wisniewski M (2001) Patterns of ice formation and movement in blackcurrant. HortScience 36: 1027–1032 [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize clusters in two sequence subgroups with differential aquaporin activity. Plant Physiol 122: 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P (1976) The study of freezing process, biochemical changes and cold acclimation in Cornus and Solanum species in relation to the frost hardiness and water stress in controlled environments. PhD thesis. University of Minnesota, Saint Paul
- Chen THH, Burke MJ, Gusta LV (1995) Freezing tolerance in plants: an overview. In RE Lee, GJ Warren, LV Gusta, eds, Biological Ice Nucleation and Its Applications. APS Press, St. Paul, pp 115–136
- Dexter ST, Tottingham WE, Garber LF (1930) Preliminary results in measuring the hardiness of plants. Plant Physiol 5: 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher NH (1970) The Chemical Physics of Ice. Cambridge University Press, Cambridge, England
- Griffith M, Ala PI, Yang DSC, Hon W-C, Moffat BA (1992) Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol 10: 593–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M, Marentes E, Ala P, Yang DSC (1993) The role of ice-binding proteins in frost tolerance of winter rye. In PH Li, L Christersson, eds, Advances in Plant Cold Hardiness. CRC Press, Boca Raton, FL, pp 177–186
- Gusta LV, Burke MJ, Kapoor AC (1975) Determination of unfrozen water in winter cereals at subfreezing temperatures. Plant Physiol 56: 707–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusta LV, O'Connor BJ, MacHutcheon MG (1997) The selection of superior winter hardy genotypes using a prolonged freeze test. Can J Plant Sci 77: 97–99 [Google Scholar]
- Gusta LV, Rajashekar C, Chen PM, Burke MJ (1982) Freeze-induced membrane permeability changes in winter rye. Cryo-Letters 3: 27–34 [Google Scholar]
- Gusta LV, Wisniewski M, Nesbitt NT, Gusta ML (2000) Freezing injury in cereals: an overall view and new approaches on increasing the frost tolerance of cereals. In Eighth International Barley Genetics Symposium, Vol 1, October 22–27, 2000, Adelaide University, Department of Plant Science, Adelaide, Australia, pp 260–264
- Huang T, Nicodemus J, Zarka DG, Thomashow MF, Wisniewski M, Duman JG (2002) Expression of an insect (Dendroides Canadensis) antifreeze protein in Arabidopsis thatliana results in a decrease in plant freezing temperature. Plant Mol Biol 50: 333–344 [DOI] [PubMed] [Google Scholar]
- Johansson N-O (1970) Ice formation and frost hardiness in some agriculture plants. Natl Swed Inst Plant Prot 14: 364–382 [Google Scholar]
- Lindow SE (1983) The role of bacterial ice nucleation in frost injury to plants. Annu Rev Phytopathol 21: 363–384 [Google Scholar]
- Livingston III DP, Henderson CA (1998) Apoplastic sugars, fructans, fructan exohydrolase and invertase in winter oat: responses to second-phase cold-hardening. Plant Physiol 116: 403–408 [PMC free article] [Google Scholar]
- Olien CR (1967) Freezing stresses and survival. Annu Rev Plant Physiol 18: 387–408 [Google Scholar]
- Olien CR, Smith MN (1981) Protective systems that have evolved in plants. In CR Olien, MN Smith, eds, Analysis and Improvement of Plant Cold Hardiness. CRC Press, Boca Raton, FL, pp 61–88
- Pearce RS, Fuller MP (2001) Freezing of barley studied by infrared video thermography. Plant Physiol 125: 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaney MJT (1989) The measurement of physiology parameters of cell suspension cultivars relative water content, cell osmotic potential, non-osmotic volume, turner and cell wall modules of elasticity. PhD thesis. University of Saskatchewan, Saskatoon, Saskatchewan, Canada
- Reaney M, Gusta LV (1999) Modeling sequential responses of plant cells to freezing and thawing. In R Margesin, F Schinner, eds, Cold Adapted Organisms: Ecology, Physiology, Enzymology and Molecular Biology. Springer-Verlag, Berlin, pp 119–136
- Siminovitch D, Levitt J (1941) The relation between frost resistance and the physical state of protoplasma. Can J Res Sect C Bot Sci 19: 9–20 [Google Scholar]
- Single WV, Marcellos H (1974) Studies on frost injury to wheat. IV. Freezing of ears after emergence from leaf sheath. Aust J Agric Res 25: 679–686 [Google Scholar]
- Sukumaran NP, Weiser CJ (1972) An excised leaflet test for evaluating potato frost tolerance. HortScience 7: 467–468 [Google Scholar]
- Swallow KW, Low NH (1994) Determination of honey authenticity by anion-exchange liquid chromatography. J AOAC Int 77: 695–702 [PubMed] [Google Scholar]
- Tanghe A, Van Dijck P, Dumortier F, Teunissen A, Hohmann A, Thevelein JM (2002) Aquaporin expression correlates with freeze tolerance in baker's yeast, and overexpression improves freeze tolerance in industrial strains. Appl Environ Microbiol 68: 5981–5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunova TI (1965) Light and temperature systems in the hardening of winter wheat and the significance of oligosaccharides for frost resistance. Sov Plant Physiol 12: 70–77 [Google Scholar]
- Wisniewski M, Bassett C, Gusta LV (2003) An overview of cold hardiness in woody plants: seeing the forest through the trees. HortScience 38: 952–959 [Google Scholar]
- Wisniewski M, Fuller M (1999) Ice nucleation and deep supercooling in plants: new insights using infrared thermography. In R Magnesia, F Schinner, eds, Cold Adapted Organisms: Ecology, Physiology, Enzymology and Molecular Biology. Springer-Verlag, Berlin, pp 105–118
- Wisniewski M, Fuller M, Palta J, Carter J, Gusta L, Griffith M, Duncan J (2001) Factors involved in ice nucleation and propagation in plants: an overview based on new insights gained from the use of infrared thermography. Buvisindi J Agr Sci 14: 41–47 [Google Scholar]
- Wisniewski M, Glenn DM, Fuller MP (2002) Use of a hydrophobic particle film as a barrier to extrinsic ice nucleation in tomato plants. J Amer Soc Hort Sci 127: 358–364 [Google Scholar]
- Wisniewski M, Lindow SE, Ashworth EN (1997) Observations of ice nucleation and propagation in plants using infrared video thermography. Plant Physiol 113: 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workmaster BA, Palta JP, Wisniewski M (1999) Ice nucleation and propagation in cranberry uprights and fruit using infrared thermography. J Amer Soc Hort Sci 124: 619–625 [Google Scholar]
- Zimmerman U (1982) Electric field mediated fusion and electric phenomena. Biochim Biophys Acta 694: 227–277 [DOI] [PubMed] [Google Scholar]