Abstract
Several genes encoding putative glutathione peroxidase have been isolated from a variety of plants, all of which show the highest homology to the phospholipid hydroperoxide isoform. Several observations suggest that the proteins are involved in biotic and abiotic stress responses. Previous studies on the regulation of gpx1, the Citrus sinensis gene encoding phospholipid hydroperoxide isoform, led to the conclusion that salt-induced expression of gpx1 transcript and its encoded protein is mediated by oxidative stress. In this paper, we describe the induction of gpx1 promoter:uidA fusions in stable transformants of tobacco (Nicotiana tabacum) cultured cells and plants. We show that the induction of gpx1 by salt and oxidative stress occurs at the transcriptional level. gpx1 promoter analysis confirmed our previous assumption that the salt signal is transduced via oxidative stress. We used induction of the fusion construct to achieve better insight into, and to monitor salt-induced oxidative stress. The gpx1 promoter responded preferentially to oxidative stress in the form of hydrogen peroxide, rather than to superoxide-generating agents. Antioxidants abolished the salt-induced expression of gpx1 promoter, but were unable to eliminate the induction by H2O2. The commonly employed NADPH-oxidase inhibitor diphenyleneiodonium chloride and catalase inhibited the H2O2-induced expression of gpx1 promoter, but did not affect its induction by salt. Our results led us to conclude that salt induces oxidative stress in the form of H2O2, its production occurs in the intracellular space, and its signal transduction pathway activating the gpx1 promoter is different from the pathway induced by extracellular H2O2.
Gluthatione peroxidases (GPXs) are a family of isozymes that use glutathione to reduce hydrogen peroxide (H2O2) and organic and lipid hydroperoxides, thereby protecting cells against oxidative damage (Flohé and Günzler, 1984). Several forms of these enzymes have been described in great detail in mammalian systems (Ursini et al., 1995). The classical cytosolic GPX is highly active in reducing H2O2 and in its native form is composed of homotetramers. The other isoform, phospholipid hydroperoxide glutathione peroxidase (PHGPX), is active as a monomer and has a higher affinity to lipid hydroperoxides than to H2O2. The mammalian PHGPX isoforms and most of the mammalian GPXs are selenoproteins characterized by the presence of the rare amino acid residue selenoCys in their catalytic site (Brigelius-Flohe, 1999; Arthur, 2000).
In recent years, several genes encoding putative GPXs have been isolated from a variety of plants, including citrus, tobacco, tomato, sunflower, spinach, pea, rice, barley, Chinese cabbage, Avena fatua, and Arabidopsis (Criqui et al., 1992; Holland et al., 1993; Johnson et al., 1995; Eshdat et al., 1997; Sugimoto and Sakamoto, 1997; Sugimoto et al., 1997; Depege et al., 1998; Mullineaux et al., 1998; Roeckel-Drevet et al., 1998; Churin et al., 1999; Li et al., 2000; Jung et al., 2002). All plant cDNAs isolated to date show the highest homology to the PHGPX isoform, and all the encoded proteins have Cys, rather than selenoCys, in their presumed catalytic site. The expected activity of the plant proteins was determined in recombinant proteins tanslated from the cDNAs isolated from citrus, sunflower, tomato, and Chinese cabbage (Beeor-Tzahar et al., 1995; Faltin et al., 1998; Herbette et al., 2002; Jung et al., 2002). Substrate specificity of these enzymes was, as expected, based on gene homology, i.e. the highest activity was demonstrated with lipid hydroperoxides and no activity was obtained with hydrogen peroxide when glutathione served as the reducing agent. In general, the overall activity is rather low compared with those found in the animal kingdom. In recent studies, it has been demonstrated that at least two plant PHGPXs probably represent a novel isoform of thioredoxin peroxidase exhibiting much higher oxidation activity for thioredoxin than for glutathione (Herbette et al., 2002; Jung et al., 2002). It was also demonstrated that H2O2 serves as their preferred substrate.
Several observations suggest that plant PHGPXs are involved in biotic and abiotic stress responses. Increased levels of expression of PHGPX protein and of mRNA were induced by exposure to salt (Avsian-Kretchmer et al., 1999), infection by viral or bacterial pathogens (Levine et al., 1994; Roeckel-Drevet et al., 1998), treatments with heavy metals (Sugimoto and Sakamoto, 1997), oxidative stress (Avsian-Kretchmer et al., 1999; Li et al., 2000), and mechanical stimulation (Depege et al., 1998). It is widely accepted that biotic and abiotic stresses result in the formation of reactive oxygen species (ROS) and lead to an increase in various antioxidant defense mechanisms (Van Breusegem et al., 2001). In the animal kingdom, GPXs serve as key enzymes in reducing H2O2, whereas in plants, different pathways have evolved where the action of superoxide dismutase is followed by APXs as the main scavenging enzymes (Asada, 1994). The role of plant PHGPXs remains to be elucidated. Nevertheless, their induction under a wide range of stresses suggests that they play a specific role in ROS scavenging, such as the removal of lipid hydroperoxides.
In previous studies intended to understand the regulation of gpx1 (formerly named csa), a citrus gene encoding PHGPX, we examined its expression under salt and oxidative stress. We showed that salt treatment increases the amount of PHGPX with a concomitant decrease in overall APX activity (Gueta-Dahan et al., 1997). Moreover, increasing the antioxidant capacity by either preloading cultured citrus cells with external antioxidants or using a cell line with a higher level of constitutive APX activity, eliminated the salt-induced PHGPX expression (Avsian-Kretchmer et al., 1999). Based on these results, we proposed a model in which ROS and peroxides are intermediates in the cascade of events leading from salt exposure to an increase in gpx1 transcript level. The model suggested that when the capacity of the cell to scavenge ROS is high, the induction of gpx1 by salt will be delayed.
The salt-induced increase in steady-state mRNA accumulation may be regulated at the transcriptional and post-transcriptonal levels. In alfalfa, both types of regulation were found in different genes induced by salt (Winicov and Krishnan, 1996). The specific mRNA of tobacco (Nicotiana tabacum) osmotin has been shown to accumulate under both salt stress and abscisic acid (ABA) treatment. This gene was shown to be transcriptionally regulated by salt and post-transcriptionally regulated by ABA and wounding (La Rosa et al., 1992). In our experiments, increased levels of gpx1 mRNA transcript were induced by salt, ABA, and oxidative stress (Avsian-Kretchmer et al., 1999). We have previously suggested that the NaCl-induced increase in gpx1 mRNA is not mediated by ABA and that the ABA-induced increase in the expression of gpx1 is mediated by ROS production (Avsian-Kretchmer et al., 1999).
In this study, we describe the structure of a citrus gene encoding PHGPX and analyze its promoter's pattern of expression. The isolated promoter fused to uidA was inserted into tobacco plants and cultured cells. β-Glucuronidase (GUS) activity in the transformed tissues showed that gpx1 is regulated at the transcriptional level by salt and oxidative stress but not by ABA. Our experiments led to the conclusion that the expression of gpx1 is not tissue specific, and that the oxidative stress induced by salt is essentially in the form of H2O2, which is produced in the intracellular space. NADPH oxidase is involved in the signal transduction activating the gpx1 promoter by H2O2 induction but not in salt-induced activation of this promoter.
RESULTS
gpx1 Genomic Clone and Its Promoter
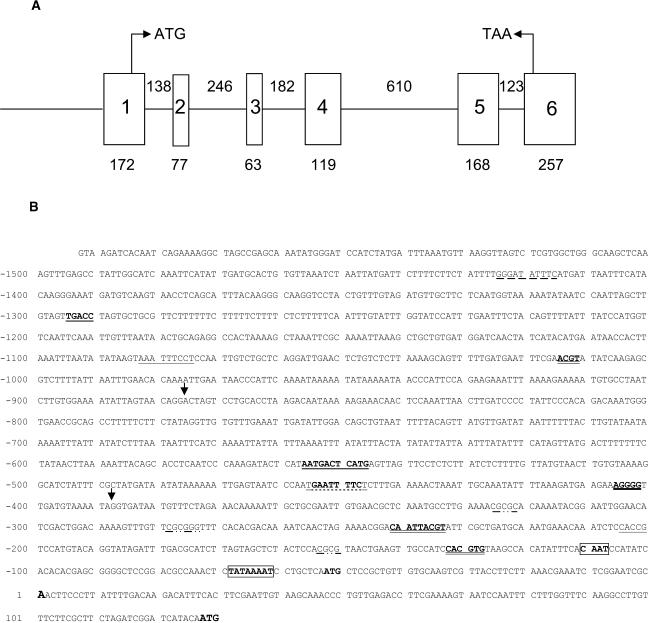
Isolation of a genomic clone corresponding to the cDNA of gpx1 revealed a gene comprised of six exons and five introns (Fig. 1A). Four of the introns were shorter than 200 bp, and the length of intron number 4 was 610 bp. All the introns were spliced following the consensus sequence, having GT and AG at their 5′ and 3′ end, respectively. The putative polyadenylation signal (AATAAA) is located 18 bp upstream of the poly(A) site (Joshi, 1987a). The sequence of the genomic clone revealed an additional ATG (at position −51), which was not found in the longest isolated cDNA, starting at position 1. This ATG codon was in-frame with the one of the cDNA at position 128. A TATA-box and a CAAT-box were identified at positions −162 and −108, respectively. A fragment of 1.6 kb upstream to the 5′ end of the longest cDNA was cloned as the full-length promoter (Fig. 1B). This sequence was analyzed for the presence of known consensus elements described in studies of other genes, as mentioned below, and by the software offered by PlantCare database (Lescot et al., 2002). The putative transcription initiation site (Joshi, 1987b) coincided with the 5′ end of the cDNA (A at position 1) and the putative TATA box was 61 bp upstream, within the range described by Joshi (1987b).
Figure 1.
Structure of the gpx1 gene and its promoter. A, Schematic structure of the gpx1 gene (GenBank accession no. AJ582678). The length of each exon (square) and intron (line) is given. The ATG is located at position 128 bp in exon 1 and the termination TAA at position 33 bp in exon 6. B, Sequence of the gpx1 promoter. The putative transcription start site is at position 1. The various putative cis-acting elements are marked. See text for further explanations. Arrows point to the sites of deletions.
We identified several other consensus elements on the gpx1 promoter. The core sequence ACGT, which is characteristic for ABRE motifs (Uno et al., 2000), exists at positions −129, −234, and −1,011. Only the one at position −129 has the consensus sequence of a G-box (CACGTG; Choi et al., 2000), whereas the two other sequences do not have any of the predicted context. A CE1-like sequence, CACCG, which is suggested to be involved in ABA-induction, is found at position −201 (Niu et al., 2002). A sequence similar to the mammalian binding site for the factor NF-kB (GGGATATTTC), which is related to the oxidative stress response, was identified at position −1,417 with one mismatch (Scandalios et al., 1997). A cis-acting regulatory element involved in methyl jasmonate (MeJ)-responsiveness, CGTCA, which is also part of the as-1 element (Johnson et al., 2001), was found in the minus strand of the DNA at position −176. Several copies of a novel 6-bp CGCG box (Yang and Poovaiah, 2002), which responds to a variety of stresses including salt, MeJ, and H2O2, were located at positions −322, −275, and −150. A heat shock-responsive element consisting of tri-nucleotide repeats arranged in alternating, palindromic orientation separated by 2 bp was identified at position −447 (Guilfoyle, 1997). The WUN-motif aAATTtcct, described as a wound-responsive element in Brassica oleracea, was found at position −1075. A stress-responsive element (STRE) is characterized by the core sequence AGGGG (Ruis and Koller, 1997) and was found at position −402. The consensus sequence RSTGACTMANN, which is present in various ROS-modulated plant gene promoters (Kim et al., 2003), was found at position −547 with one mismatch. Several AT-rich regions exist in the gpx1 promoter, located at −620 to −724 (94% AT), −940 to −964 (96% AT), and −1,078 to −1,118 (88% AT).
Expression of the gpx1 Promoter:uidA Fusion
The expression of GUS by the full-length promoter and two additional deletion constructs was analyzed in tobacco plants to determine tissue specificity and in BY-2 tobacco cells for quantitative measurements of the induction.
Expression of the gpx1 Promoter:uidA Fusion in Tobacco Plants
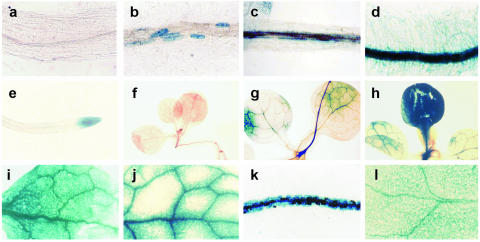
Transgenic tobacco plants containing each one of the three gpx1 promoter:uidA constructs were analyzed for uidA expression of following salt and sorbitol treatments. Data presented in Figure 2 show the patterns of GUS staining in roots and leaves of seedlings transformed with the full-length promoter, which were transferred to a medium containing 0.1 m NaCl or 0.2 m sorbitol for 2 to 4 d. No GUS activity was observed in control roots (Fig. 2A). In roots of plants exposed to NaCl for 48 h, GUS activity was observed in distinct cells but not in the root tips (Fig. 2B). After 72 h, this activity was exhibited all along the roots but expanded to the root hairs only after 96 h (Fig. 2, C and D). In roots treated with sorbitol, only the root-tip was stained after 48 h (Fig. 2E), and roots treated for 96 h were stained all over, similar to those treated with NaCl. In control leaves of seedlings germinating on wet filter paper, no GUS activity was observed (Fig. 2F), but when these seeds were germinated and grown in vitro under sterile conditions on Murashige and Skoog medium without any hormones, a background of blue staining was often observed (not shown).
Figure 2.
Expression of the gpx1 promoter:uidA fusion in tobacco plants. GUS activity was determined as explained in “Materials and Methods.” Root (A) and leaf (F) of control plant; root (B) and leaf (F) of plant exposed for 2 d to 0.1 m NaCl; root of plant exposed for 3 d to 0.1 m NaCl (C); root (D) and leaf (H and I) of plant exposed for 4 d to NaCl; root of plant exposed for 2 d to 0.2 m sorbitol (E); leaf of plant exposed for 4 d to Na2SO4 (J); root (K) and leaf (L) of plant 1 d after wounding (K and L). Length of area photographed in F, G, and H, 7 mm; length in I and J, 2.8 mm; length in C, D, E, K, and L 1.73 mm; length in A and B, 0.7 mm.
In leaves, the overall intensity of GUS staining increased with the duration of the stress and different leaves from the same seedling were stained at different intensities (Fig. 2, G and H). There was no difference in the pattern of staining among NaCl, KCl, and sorbitol treated seedlings (data not shown). The staining of GUS expression in leaves of NaCl-treated seedlings was quite evenly spread over the whole leaf area, whereas in many leaves of Na2SO4-treated seedlings, the pattern of staining showed more pronounced GUS activity around the large veins and less intense activity throughout the leaf area (Fig. 2, I and J). Wounding leaves by squeezing them with forceps resulted in high GUS activity in the wounded leaf, as well as in the nonwounded leaves and roots, which was detected as early as 24 h following the treatment (Fig. 2, K and L).
We analyzed GUS activity in homozygous plants transformed with the two deletion constructs. No activity could be detected in salt-grown plants transformed with the construct containing only the 0.5-kb fragment of the gpx1 promoter. GUS activity could be detected in roots and leaves of salt-grown plants transformed with the 1-kb fragment of the gpx1 promoter, but in most of the plants the intensity of the staining was somewhat lower than that observed with the full-length promoter (data not shown).
Expression of the gpx1 Promoter:uidA Fusion in BY-2 Tobacco Cells
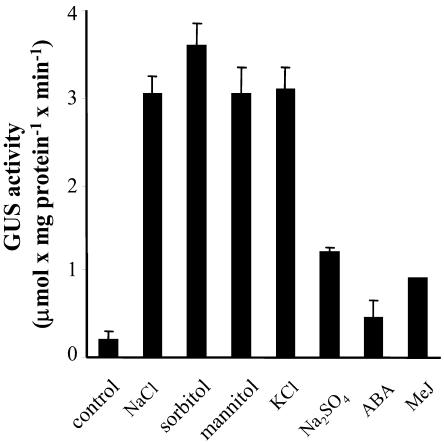
Transformation of BY-2 tobacco cells results in a large number of transformation events, exhibiting a large variation in transformants. The use of cells enables a better quantification of GUS activity and more uniform exposure to stress. The various stresses were usually imposed overnight to 24 h, as this was found the time point to obtain maximal GUS activity. Exposing cells to both NaCl and H2O2 for 4, 7, and 17 h resulted in 0%, 40%, and 90% to 100% of the activity obtained after exposure for 24 h, respectively. GUS activity was determined in control and treated cells by the fluorometric method, as explained in “Materials and Methods.” NaCl, sorbitol, and mannitol induced high levels of GUS activity (Fig. 3), suggesting an osmotic effect. KCl was as effective as NaCl in inducing GUS activity, but Na2SO4 was less so. GUS activity induced by the addition of 100 mm CaCl2 or 200 mm NaNO3 was similar to that induced by Na2SO4, and almost no activity was induced by 200 mm choline chloride (data not shown). It is of interest that ABA and heat treatment (24 h at 37°C), which induce a high level of gpx1 transcript (Avsian-Kretchmer et al., 1999), were ineffective in inducing GUS activity (Fig. 3 and data not shown).
Figure 3.
Effect of salts and osmotic stress on the expression of the gpx1 promoter:uidA fusion in tobacco cells. Tobacco cells toward the end of their fast growth phase were exposed to the various stresses at the indicated concentrations. Analysis of GUS activity was performed as explained in “Materials and Methods.” The concentrations of NaCl, sorbitol, mannitol, KCl, and Na2SO4 were 0.2, 0.4, 0.4, 0.2, and 0.1 m, respectively. The concentration of ABA was 50 μm and that of MeJ, 0.2 mm.
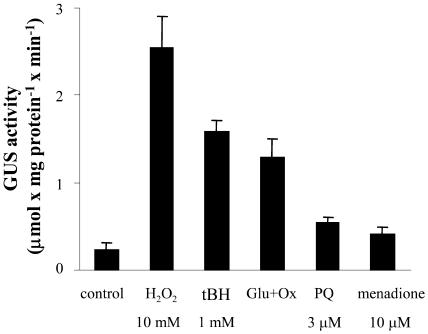
We have previously shown that direct oxidative stress induced by tert-butyl hydroperoxide (tBH), but not by H2O2, increases gpx1-specific mRNA levels to the same extent as NaCl (Avsian-Kretchmer et al., 1999). Results presented in Figure 4 show that H2O2 induced high activity of GUS driven by uidA fused to the gpx1 promoter, even higher than that observed with tBH. It should be noted that cells recovered well from the H2O2 shock, as evidenced by the virtual lack of growth inhibition when they were subcultured for further growth. Lower GUS activity was obtained following treatment with Glc and Glc oxidase. We tested the effects of paraquat and menadione as sources for the generation of superoxide. Paraquat, at a concentration of 3 μm, inhibits the growth of cultured citrus and tobacco cells by about 50% and fails to increase the level of specific gpx1 mRNA or its encoded protein (Gueta-Dahan et al., 1997). At this concentration it induced a very low level of GUS activity (Fig. 4). We treated BY-2 tobacco cells with menadione, at concentrations ranging from 5 to 25 μm and obtained low to total inhibition of growth. At a concentration of 10 μm, which inhibited growth by about 50%, it also induced a very low level of GUS activity, comparable to that obtained with paraquat. Salicylic acid (0.1–1 mm), which has been shown to generate superoxide in tobacco cell suspension culture (Kawano and Muto, 2000), did not induce GUS expression.
Figure 4.
Effect of oxidants on the expression of the gpx1 promoter:uidA fusion in tobacco cells. For details, see Figure 3. The concentration of glucose (Glu) was 10 mm and that of Glc oxidase (Ox), 0.2 units mL−1. PQ, paraquat.
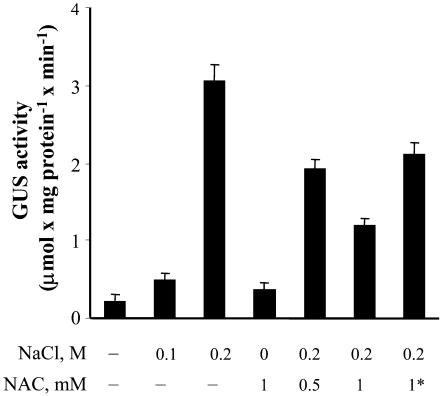
The level of induction of GUS activity reflects the degree of stress imposed on the tissue; 0.1 m NaCl induced a much lower level of GUS activity than 0.2 m NaCl (Fig. 5). Preloading tobacco cells with the antioxidant N-acetyl Cys (NAC) decreased NaCl-induced GUS activity. Loading cells with NAC prior to the addition of NaCl reduced GUS activity more than the simultaneous addition of both, and loading with a higher concentration of NAC resulted in better protection.
Figure 5.
Effect of NAC on the expression of the gpx1 promoter:uidA fusion in tobacco cells. For details, see Figure 3. NAC was added to the cell suspension cultures 1 d prior to the treatment except when indicated by an asterisk, where NAC and NaCl were added simultaneously.
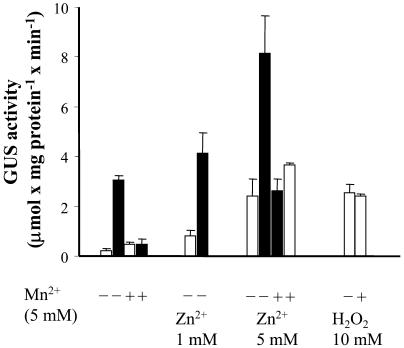
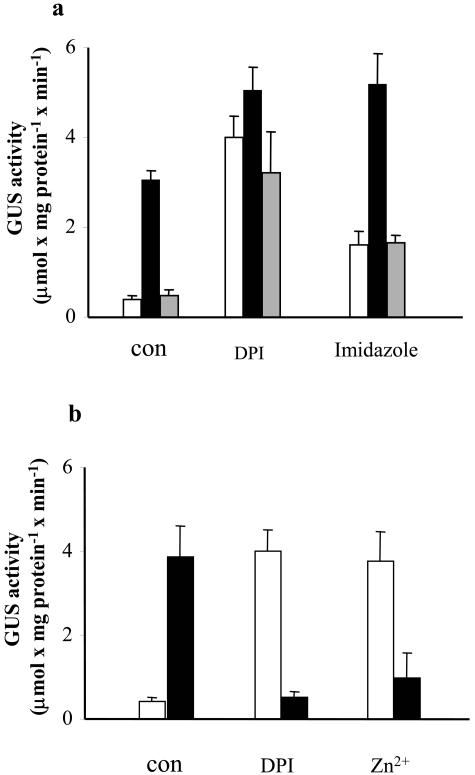
Mn2+ and Zn2+ ions have been shown to be efficient scavengers of ROS in tobacco cell suspension (Kawano et al., 2002). As shown in Figure 6, the addition of 5 mm Mn2+ had no effect on GUS activity, but preloading cells with Mn2+ ions strongly inhibited the NaCl-induced expression of GUS. The action of Mn2+ as an antioxidant in our system was independently proved by applying the fluorometric method and the dye 5-(and-6)-carboxy-2′,7′- dichlorodihydrofluorescein diacetate. We observed a very low fluorescence in control cells which was dramatically increased by salt, whereas in overnight Mn2+-preloaded cells, the addition of salt did not result in an increase of fluorescence, and the level observed was even below that of the control cells (see supplemental data). The addition of Zn2+ alone induced a high expression of GUS, and the addition of Zn2+ and NaCl resulted in a very high level of GUS activity, which was basically the sum of the two stresses. The effects of either Mn2+ or Zn2+ were rather specific, since other bivalent ions, such as Co2+ or Ni2+, did not induce any GUS activity by themselves, and they had a minor effect on the activity induced by NaCl (data not shown). Sorbitol-induced GUS activity was inhibited by NAC and Mn2+ in the same way as NaCl-induced activity (data not shown). It should be noted that Mn2+ failed to inhibit H2O2-induced GUS activity (Fig. 6). It has been suggested that Zn2+, but not Mn2+, acts as an inhibitor of the putative NADPH oxidase in BY-2 tobacco cells (Kawano et al., 2002). To test whether Zn2+-induced GUS activity involves NADPH oxidase, we tested the effect of two known inhibitors of this enzyme, diphenyleneiodonium chloride (DPI) and immidazole. Both inhibitors induced GUS activity when applied alone, although not to he same extent, and as in the case of Zn2+, their induced activity increased upon addition of NaCl (Fig. 7A). In contrast, DPI and Zn2+ dramatically inhibited the activity of GUS induced by H2O2 (Fig. 7B). We analyzed the ability of imidazole, Zn2+ and DPI to produce H2O2 in BY-2 tobacco cells by the fluorometric method using 5-(and-6)-carboxy-2′,7′- dichlorodihydrofluorescein diacetate. None of these agents showed any activity against a positive control obtained by applying NaCl (data not shown). There is no correlation between the extent of induced GUS activity and the degree of growth inhibition obtained by the various chemical agents. Growth of cells was 50% inhibited by 0.2 m NaCl or 10 mm H2O2, and a similar inhibition was obtained by 5 mm Mn2+. The addition of Zn2+ inhibited the growth of cells by about 80%, whereas very few cells survived the treatment with DPI.
Figure 6.
Effect of Mn2+ and Zn2+ ions on the expression of the gpx1a promoter:uidA fusion in tobacco cells. For details, see Figure 3. Mn2+ or Zn2+ ions were added 1 d prior to the treatment. White bars, no NaCl; black bars, 0.2 m NaCl.
Figure 7.
Effect of NADPH oxidase inhibitors on the expression of the gpx1 promoter:uidA fusion in tobacco cells. For details, see Figure 3. A, Effect of inhibitors on NaCl induction. Mn2+ was added 1 d prior to the treatment with NaCl and imidazole or DPI were added 1 h prior to the addition of NaCl. Control, white bars; NaCl, black bars; Mn2+ and NaCl, gray bars. B, Effect of inhibitors on H2O2 induction. DPI or Zn2+ were added 1 h prior to the addition of H2O2. Control, white bars; H2O2, black bars. NaCl, H2O2, Zn2+, Mn2+, imidazole and DPI were added at concentrations of 0.2 m, 10 mm, 5 mm, 5 mm, 10 mm, and 25 μm, respectively.
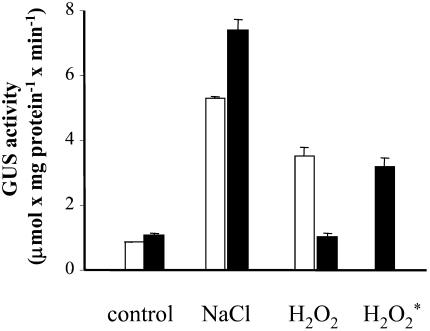
To test whether the inability of DPI to inhibit NaCl-induced expression of gpx1 is due to the intracellular production of H2O2, we added catalase to the cell suspension prior to the addition of either salt or H2O2. The addition of catalase dramatically reduced the H2O2-induced expression of gpx1 but had no effect or even increased the NaCl-induced expression. The addition of catalase 30 min after the addition of H2O2 had only a minor effect on GUS activity (Fig. 8). In parallel, we measured the capacity of the BY-2 cell suspension to remove H2O2 from the medium. Following the addition of 10 to 40 mm H2O2, only about 20% to 25% could be detected in the medium after 2 min, and the level was below 5% after 5 min. Addition of MeJ, which may be involved in signal transduction pathways induced by wounding (Ryan and Moura, 2002), resulted in a moderate level of GUS activity in tobacco cells at a concentration of 200 μm (Fig. 3).
Figure 8.
Effect of catalase on salt- and H2O2-induced expression of the gpx1 promoter:uidA fusion in tobacco cells. For details, see Figure 3. Bovine liver catalase, at a concentration of 130 U mL−1 was added prior to salt or H2O2, except when indicated by an asterisk, where catalase was added 30 min after the addition of H2O2. NaCl and H2O2 were added at 0.2 m and 10 mm, respectively. White bars, no catalase; black bars, with catalase.
We tested the ability of the two deletion constructs to induce GUS activity under a variety of stresses. In tobacco cells transformed with the construct containing the 1-kb fragment of the promoter, GUS activities induced by salt, sorbitol, H2O2, tBH, and Zn2+ were similar to those obtained with the full-length promoter. No GUS activity could be induced in tobacco cells transformed with the 0.5-kb fragment of the promoter.
DISCUSSION
Although genes encoding plant PHGPXs have been found in many species, their function in vivo has not been clearly resolved. Recent reports indicate that the encoded proteins are mainly active as thioredoxin-dependent peroxidases rather than GPXs (Herbette et al., 2002; Jung et al., 2002). The expression of most of the genes has been found to be induced under various stress conditions, where oxidative stress is most probably the common secondary effect. Our intensive studies on the regulation of citrus PHGPX clearly demonstrate that its expression under salt stress is mediated via oxidative stress (Avsian-Kretchmer et al., 1999). To better understand the transduction of salt stress to oxidative stress, we analyzed the regulation of this gene by activating its promoter fused to a reporter gene.
The sequence of the gene gpx1 shows a structure similar to the Arabidopsis gene encoding PHGPX (NM_117229). It consists of six exons and all of the five introns are spliced at conventional sequences. The homologous genes from human (GPX4) and pig heart contain seven exons (Brigelius-Flohe et al., 1994; Kelner and Montoya, 1998) and the nonselenium gene isolated from Chlamydomonas reinhardtii contains five exons (Leisinger et al., 1999). The genomic sequence of the gpx1 gene reveals an ATG 180 bp upstream of the one present in the cDNA (Holland et al., 1993). It is of interest that in rats, humans, pigs, and mice, the genes encoding PHGPX have two in-frame ATGs, both of which can serve as translation start sites to produce a cytosolic and a mitochondrial protein, respectively (Pushpa-Rekha et al., 1995; Nam et al., 1997; Borchert et al., 1999). We previously characterized the citrus PHGPX protein as a cytosolic enzyme based on its detection in the soluble fraction following cell disruption (Ben-Hayyim et al., 1993). In addition, the citrus gpx1 5′ ATG does not conform with the consensus of the plant translation start site (Joshi, 1987b; Joshi et al., 1997) suggesting that the upstream ATG, absent from the cDNA, is not likely to function as a translation initiation site for the protein.
Several putative regulatory elements were identified in the promoter region. The fact that the construct containing the 0.5-kb promoter fragment fused to uidA showed no GUS activity under any of the applied treatments suggests that the cis-acting elements found in the region up to −390 are not sufficient for the regulation of gpx1 expression. These elements include the CGCG box and the core sequences of ABRE motifs. Although osmotic stress induced a high level of GUS activity (Fig. 3), we could not detect drought-responsive-element motif found in drought or the cold-induced RD29A and COR78 genes, which are independent of ABA in Arabidopsis (Stockinger et al., 1997).
Of all the consensus elements identified on the promoter, the STRE at position −402 and the cis-acting element responsive to ROS identified at position −547 may serve as important putative cis-acting elements in regulating gpx1 promoter activity. Both factors exist in the 1- and 1.5-kb promoter fragments, the region which is required for driving GUS activity. The STRE has been characterized in promoters of stress-induced yeast genes. Two copies were located on the catalase T promoter, which was shown to respond to a variety of stresses such as essential nutrient starvation (nitrogen, carbon, sulfur, and phosphorus), external low pH, ethanol, osmotic, and oxidative stress (Ruis and Koller, 1997). This element was also identified in the sequence of the maize cat3 promoter (Scandalios et al., 1997). The other motif responsive to ROS, RSTGACTMANN, has been identified in the promoter of sweet potato stress-inducible peroxidase (Kim et al., 2003), and it shows substantial homology to AP-1, which is present in various ROS-modulated plant and mammalian gene promoters (Kathryn, 1996; Grant et al., 2000). The identification of these elements in the promoter region of gpx1 strongly supports our earlier suggestion that although PHGPX was initially isolated as a salt-induced protein (Ben-Hayyim et al., 1993), it is actually an oxidative stress-induced enzyme (Avsian-Kretchmer et al., 1999).
Similar levels of GUS activity were induced by NaCl, KCl, sorbitol, and mannitol, suggesting that the gpx1 promoter is actually activated by osmotic stress. However, the lower induction of GUS activity obtained in the presence of other salts, such as NaNO3 and Na2SO4, at iso-osmolar concentrations and the lack of specificity for any particular ion indicate that additional factors play a role in this response. We suggest that both osmotic and salt stresses are transduced to an oxidative stress and that the response of the reporter gene is correlated with the degree of oxidative stress imposed. This degree might change with the permeability of the various ions and their distribution within the cells. The fact that salt- and osmotic stress-induced GUS activity were eliminated by preloading the cells with antioxidants, such as NAC and Mn2+ ions (Figs. 5 and 6), provides further support for this notion and strengthens our previous model that salt stress induces gpx1 expression via the production of ROS (Avsian-Kretchmer et al., 1999).
Northern-blot analysis of ABA-treated citrus cells showed a marked increase in the level of the gpx1 transcript, which was interpreted to be mediated by ROS (Avsian-Kretchmer et al., 1999). The most likely explanation for the apparent inconsistency between the high ABA-induced signal of gpx1 transcript, which was detected in the northern-blot analysis and the very low, if any, ABA-induced signal, which was detected here, is the signal observed in the northern-blot analysis represented a compilation of signals derived from several members of a gene family, whereas only one of them was analyzed in this study. Thus, it can be speculated that ABA and salt do not regulate the same promoter. The existence of a gene family for GPX has been shown in most of the plants from which such genes have been isolated (see introduction) and by Southern-blot analysis in citrus (data not shown).
The failure of ABA to activate the gpx1 promoter appears to be in contradiction with recent reports indicating that ABA signaling is mediated by ROS production. It has been shown that ABA treatment increases the production of superoxide in maize leaves (Jiang and Zhang, 2002) and induces stomatal closure and the production of H2O2 in guard cells (Pei et al., 2000). It is possible that ABA is a rather weak inducer of ROS, and/or that the ROS induced by ABA do not activate gpx1, as discussed further on.
The most effective ROS in inducing gpx1 expression seems to be H2O2, which, when added into the medium, needs to be at the rather high concentration of 10 mm (Fig. 4). Lower concentrations of H2O2 (1–5 mm) were less effective (data not shown). The mixture of Glc/Glc oxides, which generate H2O2, induced low GUS activity, most probably due to the low concentration of H2O2 produced under these conditions. It is of interest that the activation induced by paraquat and menadione were rather poor, although both reagents were applied at concentrations which imposed stress, as evidenced by inhibition of cell growth. Paraquat and menadione generate superoxide, which is subsequently dismutated by superoxide dismutase to produce H2O2. In citrus cells, it has been clearly shown that paraquat, but not H2O2, enhances the scavenging capacity of both superoxide dismutase and ascorbate peroxidase (APX; Gueta-Dahan et al., 1997). Thus, the low activation of gpx1 promoter may be explained by the low steady-state level of ROS maintained under these conditions. An alternative explanation could be based on the observation that in yeast, H2O2 and menadione arrest the cell cycle at two different stages, in RAD9-dependent and independent pathways, respectively (Flattery-O'Brien and Dawes, 1998). Thus, although superoxides produced by paraquat or menadione eventually lead to the production of H2O2, they are not perceived in the same manner. Another example of the requirement of a specific form of ROS to induce biological activity was recently reported, where activation of Arabidopsis root-hair plasma-membrane calcium channels required hydroxyl radicals, and H2O2 was ineffective (Foreman et al., 2003). Based on the differential responses of the biological systems to various forms of ROS, it can be suggested that regulation of gpx1 expression responds to H2O2 and that its transduction pathway is not shared with those of paraquat and menadione during induction of oxidative stress. This notion can also explain the inability of salicylic acid to induce the expression of gpx1 promoter fused to uidA (data not shown), although it has been shown that salicylic acid generates superoxide in the same cells (Kawano and Muto, 2000). The high concentration of H2O2 required for the activation of gpx1, similar to the requirement described for initiation of cell death in tobacco (Dat et al., 2003), is most probably due to its very fast elimination by the cells.
In animal systems, Mn2+ ions have been shown to have a protective effect against H2O2-induced cell and tissue injury (Ledig et al., 1991; Varani et al., 1991). The possible protective effect of Zn2+ has been discussed in animal systems as well as in the roots of Zn2+-deficient plants (Bray and Bettger, 1990; Cakmak, 2000). Our results provide unequivocal evidence that Mn2+ ions serve as a very efficient antioxidant. These ions totally eliminated the NaCl-induced GUS activity (Fig. 6) and dramatically reduced the accumulation of H2O2 in the presence of NaCl, even below the level of untreated cells (supplemental material, see www.plantphysiol.org). Thus, we provide further support to a previous study by Kawano et al. (2002), where Mn2+ ions were shown to protect tobacco cells from ROS damage. These authors have also shown the protective effect of Zn2+ ions but suggested that they operated at a different site of action. It was proposed that Mn2+ ions act as a general scavenger, whereas Zn2+ ions inhibit the activity of NADPH oxidase, which is involved in the production of ROS. Our results indicate that Zn2+ ions indeed act on a target which is different from the one suggested for the Mn2+ ions. While Mn2+ ions served as antioxidants and inhibited the NaCl-induced GUS activity, Zn2+ ions alone induced high GUS activity, which was further increased in the presence of salt (Fig. 6). The observation that GUS activity was similarly induced by DPI and to a lesser extent by imidazole, commonly used as specific NADPH oxidase inhibitors (Fig. 7), suggests the role of Zn2+ as an inhibitor of NADPH (Kawano et al. 2002). If DPI, Zn2+, and imidazole indeed act as NADPH oxidase inhibitors, we have to assume that inhibition of this activity imposes a stress which activates gpx1. This stress is different from the one imposed by NaCl, since we could not detect any inhibitor-induced production of H2O2, and the induced GUS activity was not abolished by Mn2+ or NAC (Fig. 7; G. Ben-Hayyim and Y. Gueta-Dahan, unpublished data). The notion of the development of stress conditions due to inhibition of NADPH oxidase is in agreement with recent reports showing that NADPH oxidase activity is required for normal plant cell growth. For example, elongation of root hairs was inhibited in a mutant of Arabidopsis possessing impaired alleles of NADPH oxidase. This mutant accumulated reduced levels of ROS, and the addition of ROS could partly reverse the inhibition of root-hair elongation (Foreman et al., 2003). Maize leaf extension was inhibited by treating the expanding zone with DPI, and the inhibition was reversed by H2O2 (Rodriguez et al., 2002).
The lack of activation of gpx1 promoter by the combination of H2O2 and DPI or Zn2+ versus the high induction by each of these reagents alone is puzzling. If we accept that DPI and Zn2+indeed inhibit NADPH oxidase, we have to assume that: (1) NADPH oxidase takes part in the transduction of H2O2 signal to the activation of gpx1 promoter; and (2) gpx1 promoter also responds to a deprivation of ROS (a stress developed in the presence of DPI, or Zn2+), and that the required level of ROS, which is supplied by NADPH oxidase in the absence of the inhibitors, can be directly derived from H2O2, resulting in a much lower stress (Fig. 7). Alternatively, it is possible that both DPI and Zn2+ induce an unknown NADPH oxidase-independent pathway leading to the induction of gpx1 promoter, which is negatively regulated by H2O2 levels.
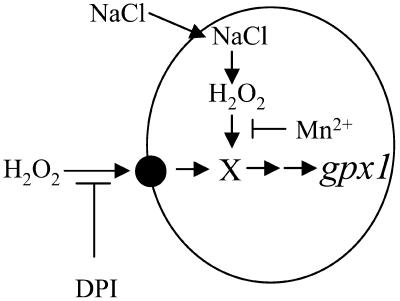
Despite the fact that NaCl stress produces H2O2, there are differences in the responses of H2O2- and NaCl-induced expression of gpx1 promoter to antioxidants, DPI, and catalase (Figs. 7 and 8). The most likely explanation would be that added H2O2 acts as an extracellular signal, which is transduced via a component in the plasma membrane, possibly NADPH oxidase, whereas the H2O2 mediating the salt signal is formed inside the cell. Our suggestions and conclusions from the experiments described here are summarized in a schematic model (Fig. 9). It is proposed that preloaded Mn2+ ions are able to act as antioxidant for the salt and sorbitol signal, when H2O2 is produced inside the cells, and fail to abolish this signal when it is perceived from the extracellular space (Fig. 6). It seems that the intracellular salt-induced production of H2O2 occurs throughout the cytosol and is not restricted to any of the organelles, as indicated by the specific staining with the dye 5-(and-6)-carboxy-2′,7′- dichlorodihydrofluorescein diacetate (supplemental data, see www.plantphysiol.org).
Figure 9.
Scematic diagram for the proposed signaling pathways of NaCl- and H2O2-induced gpx1 expression.
The failure of catalase to reduce H2O2-induced GUS activity when added 30 min after the addition of H2O2 reflect the fact that H2O2 is already absent from the medium and that a short exposure is required for the induction of gpx1 promoter.
Salt activation of gpx1 promoter in leaves and roots of transformed tobacco plants confirms our previous studies showing increased levels of PHGPX protein in leaves and roots of salt-induced citrus plants and cells (Gueta-Dahan et al., 1997). The transformed tobacco plants provide a good system to monitor the kinetics of stress development in the tissues. Exposure of roots to sorbitol or salt for 4 d showed a similar pattern of GUS staining, but a shorter exposure of 2 d showed some interesting differences (Fig. 2, B and E): under sorbitol stress, only the cells at the root tip were stained, whereas under NaCl stress, only certain cells outside the xylem, and none at the root tips, were stained. These results suggest that NaCl is taken up preferentially by certain cells and that in those cells H2O2 is formed and induces the promoter. The high GUS activity observed in sorbitol-treated root tips may reflect the high density of non- or little-vacuolated cells, rather than any particular specificity.
The transformation of plants revealed that wounding is a very strong inducer of gpx1. Studies on the regulation of catalase gene expression in maize have suggested that wound-induced expression may be mediated by MeJ and share a common signal transduction but may also be regulated in a MeJ-independent pathway mediated by ROS (Guan and Scandalios, 2000). H2O2 has been shown to act as a second messenger for the induction of defense genes in tomato plants in response to wounding (Orozco-Cardenas et al., 2001). The strong wound-induced GUS activity driven by the gpx1 promoter (Fig. 2, K and L) and the rather poor activity induced by MeJ (Fig. 3) suggest that ROS is probably responsible for the wound-induced expression of gpx1.
Our analysis of the gpx1 promoter shows that the expression of this gene can monitor the degree of oxidative stress imposed on the cells and gives better insight into the nature of the oxidative stress induced by salt. We have shown that oxidative stress is indeed a mediator in the salt signal and that in the induction of gpx1 it acts at the transcriptional level. It is suggested that salt-induced ROS are predominantly formed as H2O2 and that this process occurs inside the cell. It is likely that NADPH oxidase is involved in the signaling pathway activating the gpx1 promoter by exogenously added H2O2, but not in the signaling pathway activated by NaCl, and that inhibition of its activity imposes stress conditions.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Tobacco (Nicotiana tobacuum) var XHFD8 (Bourgin, 1978) seeds were sterilized and germinated on Murashige and Skoog medium including Nitsch vitamins (M0256; Duchefa Biochemicals, Haarlem, The Netherlands), 3% Suc, 0.08% Caseine Enzymatic Hydrolysate (C7290; Sigma, St. Louis), 2 μg mL−1 kinetin, 0.8 μg mL−1 indole-3-acetic acid, and 0.8% agar (pH 5.8). Tobacco BY-2 cell line (permission to use was received from Dr. T. Nagata, Japan) was grown in 50 mL Murashige and Skoog (M0221; Duchefa Biochemicals) liquid medium containing 0.02% KH2PO4, 3% Suc, 0.01% myo-inositol, 1 μg mL−1 thiamine, and 0.2 μg mL−1 2,4-D (2,4-dichlorophenoxyacetic acid). One milliliter of 7-d-old culture was inoculated to a fresh medium every week.
Isolation of gpx1 Genomic Clone
Valencia (Citrus sinensis) genomic library in Lambda GEM-12 (Promega, Madison, WI) was kindly donated by Dr. A. Koltunow (Commonwealth Scientific and Industrial Research Organization, Australia). The amplified library was screened at high stringency with a 32P-labeled probe derived from the 5′ end of citrus gpx1 cDNA digested with SacI (Sambrook et al., 1989). This clone corresponds to Escherichia coli clone 17, which is 97% identical to the one published (GenBank accession no. X66377) and possess the peptide T-19 rather than T-21 (Holland et al., 1993). Positive single clones were isolated, and relevant genomic fragments were purified and digested with SacI to identify a suitable clone containing the whole gene and its 5′ flanking region. We obtained clone C5 which had a 12-kb insert and a single site of SacI, yielding 5- and 7-kb fragments. Both fragments hybridized to the full-length cDNA probe, whereas only the 7-kb genomic fragment hybridized to the 5′ fragment of the cDNA. Each of the fragments was subcloned into pUC19 at the SacI site resulting in c3-7 and c6-5 plasmids and sequenced. Sequencing of plasmids c3-7 and c6-5 started with pUC-FOR and pUC-REV universal primers, respectively, and primers based on these sequences were used for further sequencing. We sequenced to the 3′ end of the cDNA on the 5-kb fragment and about 1.6 kb upstream of the 5′ end of the cDNA on the 7-kb fragment.
Cloning of the gpx1 Promoter and Deletion Constructs
We isolated a 1.6-kb fragment upstream to the 5′ end of the coding sequence of gpx1 by PCR using the forward primer c3-17 (5′-TTATGTCGACTTAGTCTTGTGGCTGGG-3′) from position −1,526 to −1,510 and the reverse primer c3-16 (5′-TTGGATCCATTGTATGATCCGATCTAG-3′) from position 129 to 111 and to which SalI and BamHI sites were added, respectively. The bold letters represent full homology to the genomic DNA and the italic letters represent the nonhomologous addition used to create a restriction enzyme site (BamHI or SalI underlined) at the ends of the PCR product. The PCR product was obtained by using Pwo DNA polymerase (Boeringer, Mannheim, Germany) and purified. It was digested by BamHI and SalI and ligated to a pUC19 vector digested with the same enzymes to form the plasmid 63-3. The insert was sequenced to verify its identity to the genomic clone.
Two promoter deletion constructs were prepared (see Fig. 1B). The first deletion resulted in a 1,003-bp promoter fragment that was obtained as follows: Plasmid 63-3 was digested with PstI and SpeI to give two products of 600 and 3,700 bp. The bigger product was isolated from a gel and purified, and the cohesive ends were blunted using Klenow (Beoringer). The fragment was then self-ligated using T4 ligase (Beoringer) to form the plasmid 63-2. The second deletion resulted in a 515-bp promoter fragment which was obtained as follows: Plasmid 63-3 was digested with HphI, which produced several fragments of which one fragment of the size of 1.5 kb is composed of a vector fragment downstream to the polylinker cloning sites and a promoter sequence 5′ upstream to the ATG. All other fragments were considerably smaller. DNA fragments were precipitated in ethanol and the cohesive ends were blunted by a fill-in reaction as described above. The 1.5-kb fragment was purified and cut with BamHI at the multiple cloning sites to yield two fragments, a 520-bp fragment of the promoter and about 1,000 bp of the vector. The 520-bp fragment was purified and ligated to pUC19 previously digested with BamHI and HincII to form the plasmid 63-1.
Construction of Promoter:uidA Fusions
Insert of plasmids 63-3, 63-2, and 63-1 were cut out by BamHI and HindIII and further cloned into the Agrobacterium binary vector pBI101-GUSInt previously digested with the same enzymes to form plasmids pBI63-3, pBI63-2, and pBI63-1, respectively. The pBI101-GUSInt vector was designed to exclude the expression of the fused promoter:uidA gene in Agrobacterium and thus eliminate possible background (Gollop et al., 2002). pBI63-3 had a promoter fragment of 1,653 bp upstream of the uidA ATG, which we consider as a full-length promoter, whereas pBI63-2 and pBI63-1 had fragments of 1,003 and 515 bp, respectively. Plasmids pBI63-3, pBI63-2, and pBI63-1 were introduced into Agrobacterium tumefaciens strain EHA105, which is a EHA101 derivate, Agropine-type, Ti-plasmid, pTiBo542 rifr (Li et al., 1992), and single colonies harboring the relevant plasmids were grown and used for transformation.
Agrobacterium-Mediated Transformation
Transformation of Tobacco Plants
Leaves of Nicotiana tobacuum var XHFD8 (Bourgin, 1978) were used for tobacco plant transformation by the leaf disc method (Draper et al., 1988) using A. tumefaciens EHA105 containing the various plasmids. We obtained at least 10 homozygous transgenic lines (R2) for each of the promoter constructs, and those plants were used for determination of GUS activity.
Transformation of BY-2 Tobacco Cell Line
Transformation was performed according to the procedure described by Shaul et al. (1996). Cell cultures were maintained with antibiotics until the supernatant showed no trace of bacteria (3–4 subcultures) and then transferred routinely without antibiotics.
β-Glucoronidase Activity Analysis
Analysis of GUS Activity in Tobacco Seedlings
Sterile 15- to 20-d-old seedlings were used to determine GUS activity under stress conditions. Plants were transferred to media containing 0.1 m NaCl, 0.1 m KCl, 0.05 m Na2SO4, or 0.2 m sorbitol. Plants were wounded by squeezing one leaf with forceps. Qualitative analysis of GUS activity was determined by staining with X-Gluc (X-GlcA-Cyclohexylammonium salt, Duchefa Biochemicals) at the indicated time. Staining solution was prepared by adding 15 μL of a solution of 66.66 mg X-Gluc in 1 mL of N,N-dimethyl-formamide to 1 mL mix containing 0.1 m sodium phosphate buffer pH 7.0, 10 mm EDTA, 50 μm potassium ferricyanide, 50 μm potassium ferrocyanide, and 0.1% Triton X-100. Control and treated seedlings were dipped in staining solution overnight at 25°C in the dark, followed by three washings in 75% ethanol, three washings with 95% ethanol, and three washings with 100% ethanol 1 h each. Plants were left overnight in 100% ethanol and then stored in a solution of Histo-Clear (National Diagnostics, Atlanta) and photographed.
Analysis of GUS Activity in Tobacco Cells
Tobacco cells were exposed to a variety of stresses for 24 h. Cells were routinely subcultured every 7 d, and the treatments were applied at day 6, which is toward the end of their fast growth cycle. Quantitative analysis of GUS activity was performed essentially according to Jefferson et al. (1987) using 4-methyl umbelifferyl-glucoronide (Sigma) as a substrate for the fluorimetric assay. Vacuum-filtered tobacco cells (0.5 g) were ground in 0.5 mL extraction buffer containing 50 mm phosphate, 10 mm EDTA, 0.1% Triton X-100, 0.1% lauryl sarcosine, and 10 mm β-mercaptoethanol pH 7.0. Protein concentration was determined by Bradford (1976), and extracts were kept at −70°C. The reaction was carried out at 37°C in 300 μL extraction buffer containing 150 μg protein and 2 mm 4-methyl umbelifferyl-glucoronide. Fifty-milliliter samples were removed for analysis at 0, 15, 30, 45, and 60 min, and the reaction was terminated by the addition of 950 μL 0.2 m Na2CO3. Fluorescence was measured using Shimadzu (Columbia, MD) spectrofluorimeter RF-540 with excitation at 365 nm and emission at 455 nm. Calibration was done against 4-methyl umbelifferone standards. Mean values of the various time points were calculated, and each treatment was repeated at least three times.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AJ582678 and X66377.
Supplementary Material
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.041921.
References
- Arthur JR (2000) The glutathione peroxidases. Cell Mol Life Sci 57: 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K (1994) Production and action of active oxygen in photosynthetic tissues. In CH Foyer, PM Mullineaux, eds, Causes of Photooxidative, Stress and Amelioration of Defense Systems in Plants. CRC Press, Boca Raton, FL, pp 77–104
- Avsian-Kretchmer O, Eshdat Y, Gueta-Dahan Y, Ben-Hayyim G (1999) Regulation of stress-induced phospholipid hydroperoxide glutathione peroxidase expression in citrus. Planta 209: 469–477 [DOI] [PubMed] [Google Scholar]
- Beeor-Tzahar T, Ben-Hayyim G, Holland D, Faltin Z, Eshdat Y (1995) A stress-associated citrus protein is a distinct plant phospholipid hydroperoxide glutathione peroxidase. FEBS Lett 366: 151–155 [DOI] [PubMed] [Google Scholar]
- Ben-Hayyim G, Faltin Z, Gepstein S, Camoin L, Strosberg AD, Eshdat Y (1993) Isolation and characterization of salt-associated protein in Citrus. Plant Sci 88: 129–140 [DOI] [PubMed] [Google Scholar]
- Borchert A, Schnurr K, Thiele BJ, Kuhn H (1999) Cloning of the mouse phospholipids hydroperoxide glutathione peroxidase gene. FEBS Lett 446: 223–227 [DOI] [PubMed] [Google Scholar]
- Bourgin JP (1978) Valine resistant plants from in vitro selected tobacco cells. Mol Gen Genet 161: 225–230 [Google Scholar]
- Bradford MN (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Bray TM, Bettger WJ (1990) The physiological role of zinc as an antioxidant. Free Radic Biol Med 8: 281–291 [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R (1999) Tissue specific function of individual glutathione peroxidases. Free Radic Biol Med 27: 951–956 [DOI] [PubMed] [Google Scholar]
- Brigelius-Flohe R, Aumann KD, Blocker H, Gross G, Kiess M, Kloppel KD, Maiorino M, Roveri A, Schuckelt R, Usani F (1994) Phospholipid hydroperoxide glutathione peroxidase. Genomic DNA, cDNA, and deduced amino acid sequence. J Biol Chem 269: 7342–7348 [PubMed] [Google Scholar]
- Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146: 185–205 [DOI] [PubMed] [Google Scholar]
- Choi H, Hong J, Ha J, Kang J, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275: 1723–1730 [DOI] [PubMed] [Google Scholar]
- Churin Y, Schilling S, Borner T (1999) A gene family encoding glutathione peroxidase homologues in Hordeum vulgare (barley). FEBS Lett 459: 33–38 [DOI] [PubMed] [Google Scholar]
- Criqui MC, Jamet E, Parmentier Y, Marbach J, Durr A, Fleck J (1992) Isolation and characterization of a plant cDNA showing homology to animal glutathione peroxidases. Plant Mol Biol 18: 623–627 [DOI] [PubMed] [Google Scholar]
- Dat JF, Pellinen R, Beeckman T, Van de Cotte B, Langebartels C, Kangasjarvi J, Inze D, Van Breusegem F (2003) Changes in hydrogen peroxide homoeostasis trigger an active cell death process in tobacco. Plant J 33: 621–632 [DOI] [PubMed] [Google Scholar]
- Depege N, Drevet J, Boyer N (1998) Molecular cloning and characterization of tomato cDNAs encoding glutathione peroxidase-like proteins. Eur J Biochem 253: 445–451 [DOI] [PubMed] [Google Scholar]
- Draper J, Scott R, Armitage P, Walden R (1988) Plant Genetic Transformation and Gene Expression: A Laboratory Manual. Blackwell Scientific Publications, Oxford, pp 97–160
- Eshdat Y, Holland D, Faltin Z, Ben-Hayyim G (1997) Plant glutahtione peroxidases. Physiol Plant 100: 234–240 [Google Scholar]
- Faltin Z, Camoin L, Ben-Hayyim G, Perl A, Beeor-Tzahar T, Strosberg AD, Holland D, Eshdat Y (1998) Cysteine is the presumed catalytic residue of Citrus sinensis phospholipids hydroperoxide glutathione peroxidase over-expressed under salt stress. Physiol Plant 104: 741–746 [Google Scholar]
- Flattery-O'Brien JA, Dawes IW (1998) Hydrogen peroxide causes RAD9-dependent cell cycle arrest in G2 in Saccharomyces cerevisiae whereas menadione causes G1 arrest independent of RAD9 function. J Biol Chem 273: 8564–8571 [DOI] [PubMed] [Google Scholar]
- Flohé L, Günzler WA (1984) Essays of glutathione peroxidase. Methods Enzymol 105: 114–121 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Gollop R, Even S, Colova-Tsolova V, Perl A (2002) Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J Exp Bot 53: 1397–1409 [PubMed] [Google Scholar]
- Grant JJ, Byung-Wook Y, Loake GJ (2000) Oxidative stress and cognate redox signaling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J 24: 569–582 [DOI] [PubMed] [Google Scholar]
- Guan LM, Scandalios JG (2000) Hydrogen peroxide-mediated catalase gene expression in response to wounding. Free Radic Biol Med 28: 1182–1190 [DOI] [PubMed] [Google Scholar]
- Gueta-Dahan Y, Yaniv Z, Zilinskas BA, Ben-Hayyim G (1997) Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in citrus. Planta 203: 460–469 [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ (1997) The structure of plant gene promoters. In JK Setlow, ed, Genetic Engineering, Vol 19. Plenum Press, New York, pp 15–47
- Herbette S, Lenne C, Leblanc N, Julien JL, Drevet JR, Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipids hydroperoxide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem 269: 2414–2420 [DOI] [PubMed] [Google Scholar]
- Holland D, Ben-Hayyim G, Faltin Z, Camoin L, Strosberg AD, Eshdat Y (1993) Molecular characterization of salt-stress-associated protein in citrus: protein and cDNA sequence homology to mammalian glutathione peroxidase. Plant Mol Biol 21: 923–927 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2002) Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215: 1022–1030 [DOI] [PubMed] [Google Scholar]
- Johnson C, Boden E, Desai M, Pascuzzi P, Arias J (2001) In-vivo target promoter-binding activities of a xenobiotic stress-activtaed TGA factor. Plant J 28: 237–243 [DOI] [PubMed] [Google Scholar]
- Johnson RR, Cranston HJ, Chaverra ME, Dyer WE (1995) Characterization of cDNA clones for differentially expressed genes in embryos of dormant and nondormant Avena fatua L. caryopses. Plant Mol Biol 28: 113–122 [DOI] [PubMed] [Google Scholar]
- Joshi CP (1987. a) Putative polyadenylation signals in nuclear genes of higher plants. Nucleic Acids Res 15: 9627–9640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CP (1987. b) An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res 15: 6643–6653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi CP, Zhou H, Huang X, Chiang VL (1997) Context sequences of translation initiation codon in plants. Plant Mol Biol 35: 993–1001 [DOI] [PubMed] [Google Scholar]
- Jung BG, Lee KO, Lee SS, Chi YH, Jang HH, Kang SS, Lee K, Lim D, Yoon SC, Yun D-J, et al (2002) A Chinese cabbage cDNA with high sequence identity to phospholipids hydroperoxide glutathione peroxidase encodes a novel isoform of thioredoxin-dependent peroxidase. J Biol Chem 277: 12572–12578 [DOI] [PubMed] [Google Scholar]
- Kathryn Z (1996) Induction of the mammalian stress response gene GADD153 by oxidative stress: role of AP-1 element. Biochem J 314: 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Kawano N, Muto S, Lapeyrie F (2002) Retardation and inhibition of the cation-induced superoxide generation in BY-2 tobacco cell suspension culture by Zn2+ and Mn2+. Physiol Plant 114: 395–404 [DOI] [PubMed] [Google Scholar]
- Kawano T, Muto S (2000) Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. J Exp Bot 51: 685–693 [PubMed] [Google Scholar]
- Kelner MJ, Montoya MA (1998) Structural organization of the human selenium-dependent phospholipids hydroperoxide glutathione peroxidase gene (GPX4): chromosomal localization to 19p13.3. Biochem Biophys Res Commun 249: 53–55 [DOI] [PubMed] [Google Scholar]
- Kim K-Y, Kwon S-Y, Lee H-S, Hur Y, Bang J-W, Kwak S-S (2003) A novel oxidative stress-inducible peroxidase promoter from sweetpotato: molecular cloning and characterization in transgenic tobacco plants and cultured cells. Plant Mol Biol 51: 831–838 [DOI] [PubMed] [Google Scholar]
- LaRosa PC, Chen Z, Nelson DE, Singh NK, Hasegawa PM, Bressan RA (1992) Osmotin gene expression is posttarnscriptionally regulated. Plant Physiol 100: 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledig M, Tholey G, Megias-Megias L, Kopp P, Wedler F (1991) Combined effects of ethanol and manganese on cultured neurons and glia. Neurochem Res 16: 591–596 [DOI] [PubMed] [Google Scholar]
- Leisinger U, Rufenacht K, Zehnder AJB, Eggen RIL (1999) Structure of glutathione peroxidase homologous gene involved in the oxidative stress response in Chlamydomonas renhardtii. Plant Sci 149: 139–149 [Google Scholar]
- Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S (2002) PlantCare, a database of plant cis-acting regulatory elements and portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30: 325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Li XQ, Liu CN, Ritchie SW, Peng JY, Gelvin SB, Hodges TK (1992) Factors influencing Agrobacterium-mediated transient expression of gusA in rice. Plant Mol Biol 20: 1037–1048 [DOI] [PubMed] [Google Scholar]
- Li W-J, Feng H, Fan J-H, Zhang R-Q, Zhao N-M, Liu J-Y (2000) Molecular cloning and expression of a phospholipid hydroperoxide glutathione peroxidase homolog in Oryza sativa. Biochim Biophys Acta 1943: 225–230 [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Karpinski S, Jimenez A, Cleary SP, Robinson C, Creissen GP (1998) Identification of cDNAS encoding plastid-targeted glutathione peroxidase. Plant J 13: 375–379 [DOI] [PubMed] [Google Scholar]
- Nam S, Nakamuta N, Kurohmaru M, Hayashi Y (1997) Cloning and sequencing of the mouse cDNA encoding a phospholipids hydroperoxide glutathione peroxidase. Gene 198: 245–249 [DOI] [PubMed] [Google Scholar]
- Niu X, Helentjaris T, Bate NJ (2002) Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell 14: 2565–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 446: 731–734 [DOI] [PubMed] [Google Scholar]
- Pushpa-Rekha TR, Burdsall AL, Oleska LM, Chisolm GM, Driscoll DM (1995) Rat phospholipids hydroperoxide glutathione peroxidase. J Biol Chem 270: 26993–26999 [DOI] [PubMed] [Google Scholar]
- Roeckel-Drevet P, Gango G, deLabrouhe T, Dufaure JP, Nicolas P, Drevet JR (1998) Molecular characterization, organ distribution and stress-mediated induction of two glutathione peroxidase-encoding mRNAs in sunflower (Helianthus annuus). Physiol Plant 103: 385–394 [Google Scholar]
- Rodriguez AA, Grunberg KA, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis H, Koller F (1997) Biochemistry, molecular biology, and cell biology of yeast and fungal catalases. In JG Scandalios, ed, Oxidative Stress and Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 309–342
- Ryan CA, Moura DS (2002) Systemic wound signaling in plants: a new perception. Proc Natl Acad Sci USA 99: 6519–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Ma T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Scandalios JG, Guan L, Polidoros AN (1997) Catalases in plants: gene structure, properties, regulation, and expression. In JG Scandalios, ed, Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 343–406
- Shaul O, Mironov V, Bursseen S, van Montagu V, Inze D (1996) Two Arabidopsis cyclin promoters mediate distinctive transcriptional oscillation in synchronized tobacco BY-2 cells. Proc Natl Acad Sci USA 93: 4868–4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Furui S, Suzuki Y (1997) Molecular cloning and characterization of a cDNA encoding putative phospholipids hydroperoxide glutathione peroxidase from spinach. Biosci Biotechnol Biochem 61: 1379–1381 [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Sakamoto W (1997) Putative phospholipids hydroperoxide glutathione peroxidase gene from Arabidopsis thaliana induced by oxidative stress. Genes Genet Syst 72: 311–316 [DOI] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F, Maiorino M, Brigelius-Flohé R, Aumann KD, Roveri A, Schomburg D, Flohé L (1995) Diversity of glutathione peroxidases. Methods Enzymol 252: 38–53 [DOI] [PubMed] [Google Scholar]
- Van Breusegem F, Vranova E, Dat JF, Inze D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161: 405–414 [Google Scholar]
- Varani J, Ginsburg I, Gibbs DF, Mukhopadhyay PS, Sulavik C, Johnson KJ, Weinberg JM, Ryan US, Ward PA (1991) Hydrogen peroxide-induced cell and tissue injury: protective effects of Mn2+. Inflammation 15: 291–301 [DOI] [PubMed] [Google Scholar]
- Winicov I, Krishnan M (1996) Transcriptional and post-transcriptional activation of genes in salt-tolerant alfalfa cells. Planta 200: 397–404 [Google Scholar]
- Yang T, Poovaiah BW (2002) A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem 277: 45049–45058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.