Abstract
We studied the influence of the internal oxygen concentration in seeds of wheat (Triticum aestivum) on storage metabolism and its relation to phloem import of nutrients. Wheat seeds that were developing at ambient oxygen (21%) were found to be hypoxic (2.1%). Altering the oxygen supply by decreasing or increasing the external oxygen concentration induced parallel changes in the internal oxygen tension. However, the decrease in internal concentration was proportionally less than the reduction in external oxygen. This indicates that decreasing the oxygen supply induces short-term adaptive responses to reduce oxygen consumption of the seeds. When external oxygen was decreased to 8%, internal oxygen decreased to approximately 0.5% leading to a decrease in energy production via respiration. Conversely, increasing the external oxygen concentration above ambient levels increased the oxygen content as well as the energy status of the seeds, indicating that under normal conditions the oxygen supply is strongly limiting for energy metabolism in developing wheat seeds. The intermediate metabolites of seed storage metabolism were not substantially affected when oxygen was either increased or decreased. However, at subambient external oxygen concentrations (8%) the metabolic flux of carbon into starch and protein, measured by injecting 14C-Suc into the seeds, was reduced by 17% and 32%, respectively, whereas no significant effect was observed at superambient (40%) oxygen. The observed decrease in biosynthetic fluxes to storage compounds is suggested to be part of an adaptive response to reduce energy consumption preventing excessive oxygen consumption when oxygen supply is limited. Phloem transport toward ears exposed to low (8%) oxygen was significantly reduced within 1 h, whereas exposing ears to elevated oxygen (40%) had no significant effect. This contrasts with the situation where the distribution of assimilates has been modified by removing the lower source leaves from the plant, resulting in less assimilates transported to the ear in favor of transport to the lower parts of the plant. Under these conditions, with two strongly competing sinks, elevated oxygen (40%) did lead to a strong increase in phloem transport to the ear. The results show that sink metabolism is affected by the prevailing low oxygen concentrations in developing wheat seeds, determining the import rate of assimilates via the phloem.
An important aspect of seed development is the accumulation of storage compounds that are to be redistributed during germination. In cereals, the major part of the dry weight of a fully developed seed consists of storage compounds within the endosperm. The main storage component in these seeds is starch (60%–70% of dry weight), but also storage proteins make up a significant proportion (approximately 15%) of seed dry weight.
The synthesis of these storage compounds requires energy. First, uptake of Suc and amino acids into the filial tissue is mediated by H+-symporters (De Jong and Borstlap, 2000; Patrick and Offler, 2001). For rice (Oryza sativa) and wheat (Triticum aestivum), Suc symporters (SUT1) have been described to be specifically active in the aleurone layer (Bagnall et al., 2000; Aoki et al., 2002), whereas transport of amino acids is thought to be mediated by amino acid permeases that have been shown to be present in embryonic tissues of legumes and Arabidopsis (Tegeder et al., 2000; Okumoto et al., 2002). The proton gradient across the plasma membrane needed to energize the symporters is mediated by H+/ATPases that are located in the same cells as where the transporters are found (Bagnall et al., 2000).
After Suc is taken up into the cells of the endosperm, it is metabolized to hexose-phosphates by Suc synthase, hexokinase, and UDP-Glc pyrophosphorylase (James et al., 2003). This sequence of reactions needs PPi, but is otherwise more or less energetically neutral, since UTP needed for hexokinase is produced by UDP-Glc pyrophosphorylase (Geigenberger, 2003a). The first committed step of starch synthesis is the conversion of Glc-1-phosphate and ATP to ADP-Glc and PPi, catalyzed by ADP-Glc pyrophosphorylase (AGPase). In cereal endosperm this reaction is partially extraplastidial, whereas AGPase in all other cereal tissues as well as in noncereal plants is exclusively located in the amyloplast (James et al., 2003). After synthesis in the cytosol, ADP-Glc is imported into the amyloplast, where the Glc-unit is added to the growing glucan chain by starch polymerising reactions. The total energy investment of starch synthesis according to this pathway is therefore 1 ATP (and 1 PPi) per Glc unit. The synthesis of storage proteins (generally prolamins in cereal endosperm) requires more energy. The binding of an amino acid to its tRNA requires one ATP, the coupling of a single amino acid to the peptide chain needs one GTP, and subsequent folding of the polypeptide requires further ATP (Buchanan et al., 2000).
The vast majority of ATP for cellular metabolism is generated via oxidative phosphorylation within the mitochondria. However, since plants lack efficient transport and delivery systems for oxygen, the continuous use of energy, and therefore oxygen, by metabolic processes creates a steep oxygen gradient, particularly throughout dense or bulky tissues. In potato (Solanum tuberosum) tubers, internal oxygen concentrations between 2% and 5% were measured (Geigenberger et al., 2000). In plants, several adaptive responses exist to prevent the tissue from becoming anoxic, which would otherwise lead to a broad set of undesirable effects, such as the induction of glycolysis, a drop in cytosolic pH due to the accumulation of lactate, and fermentative ethanol production (Geigenberger, 2003b). Plant tissue facing a decreasing oxygen tension down-regulates glycolysis and rapidly inhibits respiration in order to reduce oxygen consumption. This is already triggered at oxygen concentrations that are some orders of magnitude higher than the Km of cytochrome oxidase (Km[O2] = 0.14 μm or 0.013% oxygen; Drew, 1997) and does not lead to an increase in cellular redox-state (Geigenberger, 2003b). This indicates that the changes are not a direct effect of the lack of oxygen but are caused by an adaptive response to avoid plant tissues driving itself into anoxia. A second adaptive response to reduced oxygen is the reduction of ATP consumption via the down-regulation of the synthesis of storage compounds (Geigenberger, 2003b) and, where possible, a switch to metabolic pathways that consume less ATP (Bologa et al., 2003).
Not only bulky tissues, like potato tuber, but also seeds were found to be hypoxic. Measurements with very fine oxygen sensors revealed that the oxygen concentration drops well below 1% inside the seeds of pea (Pisum sativum), broad bean (Vicia faba; Rolletschek et al., 2002), and oil-seed rape (Brassica napus; Vigeolas et al., 2003). For several plant species (e.g. soybean [Glycine max] and Arabidopsis) it has been shown that when external oxygen concentrations are reduced below 15%, seed development is impaired. At very low external oxygen concentrations (<5%), seed development becomes even completely inhibited (Kuang et al., 1998; Porterfield et al. 1999). Two explanations for the impairment of seed development at subambient oxygen concentrations were put forward by Geigenberger (2003b): first, the energy state of the seeds becomes too low for nutrient uptake and metabolism of storage products, and second, phloem transport to the seeds suffers from the lack of energy. It has been shown for Ricinus communis that very low oxygen concentrations (<1%) lead to an inhibition of phloem transport because the retrieval of leaked Suc is not adequately energized (van Dongen et al., 2003). Both factors could independently inhibit seed development, but they could also combine to affect each other.
In this study we focus on the short-term effects of low oxygen in seeds of wheat, a monocot species. We first measured the oxygen concentration directly inside seeds, using very small oxygen sensors, and its dependency on changes in external oxygen. Second, we determined the relationship between the oxygen concentration and the energy state of the seeds by measuring nucleotide levels at different ambient oxygen tensions. Third, the changes in metabolic fluxes of carbon into different end products as induced by low and high oxygen were investigated. For this, 14C-labeled Suc was injected into seeds that were exposed to decreased (8%), normal (21%), or increased (40%) oxygen, and the incorporation of label into starch, protein, and cell wall material was determined. Fourth, metabolic intermediates were measured in seeds exposed to changed oxygen concentrations, to reveal possible regulatory sites. Finally, we measured whether the changes in metabolic activity, induced by changing oxygen levels, relate to changes in phloem transport of photoassimilates toward the seeds. For these experiments, 11C-labeling of photoassimilates was used, enabling noninvasive investigation of the effects of changing oxygen in the seeds. These results show that wheat seeds are hypoxic and that the prevailing low oxygen concentration is limiting for energy metabolism in vivo. When oxygen is decreased, storage metabolism decreases, leading to an inhibition of phloem transport toward the seeds without large changes in the levels of metabolites within the seed.
RESULTS
Influence of Changes in External Oxygen on Internal Seed Oxygen Concentration
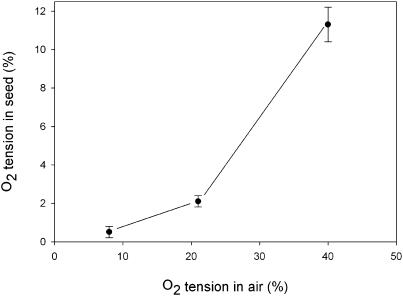
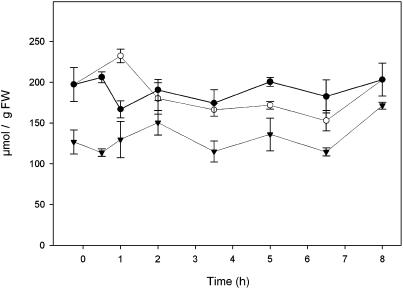
Oxygen concentrations were measured in the endosperm of developing wheat seeds at 20 d after heading (DAH) during the light period. A very small oxygen micro-sensor (tip diameter <30 μm) was inserted into the seeds, while the surrounding oxygen concentration was controlled using premixed air with different oxygen content. At normal external oxygen concentration (21% v/v) seeds were already hypoxic, with the internal oxygen tension gradually decreasing from the outer regions toward the center of the seed (data not shown). The oxygen concentration measured 2 mm below the surface of the seed was about 2.1% (Fig. 1). When the oxygen concentration in the air was decreased to 8% or increased to 40%, the internal oxygen concentration decreased to 0.5% or increased to 11.3%, respectively. These results show that wheat seeds are hypoxic inside and that the relationship between the internal and external oxygen concentration is not linear.
Figure 1.
The oxygen concentration inside wheat seeds that were exposed to subambient (8%), normal (21%), and superambient (40%) oxygen concentrations. Oxygen was measured 2 mm under the seed's surface using a very small oxygen sensor (tip diameter <30 μm). Data represent means ± se of at least five measurements.
Influence of Oxygen on Internal Nucleotide Levels and Energy State of the Seed
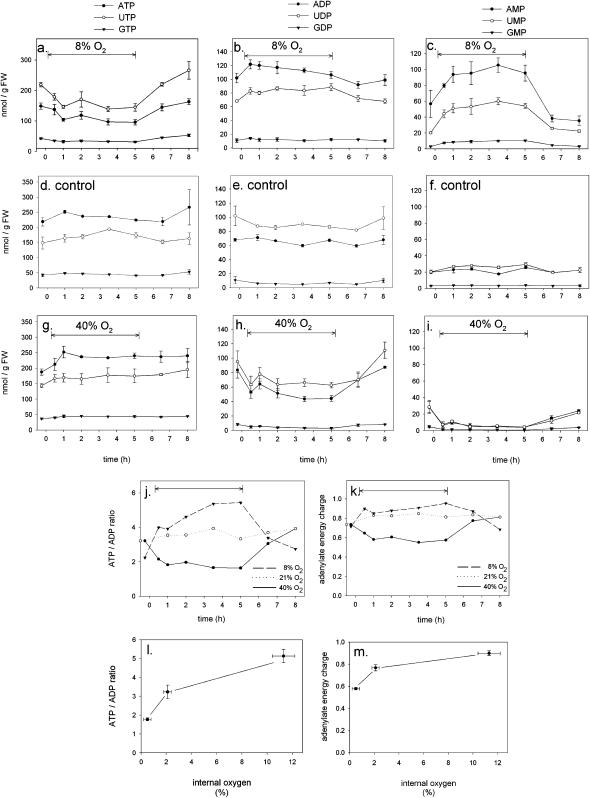
The levels of various nucleotides in the seed were measured during a time-course in which the oxygen around the ear was changed from 21% to 8% or 40%, whereas the rest of the plant was exposed to normal air (21% oxygen). After 5 h, oxygen was returned to ambient. Low oxygen caused ATP, UTP, and GTP levels to decrease, whereas ADP, UDP, and GDP levels increased slightly, and those of AMP, UMP, and GMP increased strongly (Fig. 2, A–C) in comparison to the control experiment (Fig. 2, D–F). The energy status of the wheat seeds, represented by either the ratio of ATP to ADP, or the adenylate energy charge (defined as {[ATP] + 0.5[ADP]}/{[ATP] + [ADP] + [AMP]}; Atkinson, 1977) decreased within 30 min after the oxygen concentration around the seeds was decreased to 8% (Fig. 2, J and K). Similar changes were observed when the ears of rice plants were exposed to 8% oxygen (data not shown).
Figure 2.
Nucleotide levels and energy status of wheat seeds exposed to different oxygen concentrations. The oxygen concentration around the ears was reduced to 8% (A–C), held at ambient concentration (D–F), or increased to 40% (G–I) for a period of 5 h (as indicated by the arrow), whereas the rest of the plant remained at ambient oxygen (21%) during the entire course of the experiment. Data indicate means ± se of measurements on three independent plants. The energy status of the seeds during the course of the experiment was assessed as the ratio of ATP to ADP (J), and as adenylate energy charge (I). Changes induced by 8% oxygen are indicated by • and a solid line, changes induced by 40% oxygen are indicated by ▾ and a dashed line, and the control experiment in which oxygen remained unaltered is indicated with ○ and a dotted line. K and M show the energy status of the seeds against the internal seed concentration as induced by exposure to an external oxygen concentration of 8%, 21%, or 40%, respectively.
Increasing the external oxygen concentration to 40% led to an increase in the energy status of the seeds (Fig. 2, J and K), with nucleoside triphosphate levels being increased, while those of nucleoside mono- and diphosphates dropped dramatically (Fig. 2, G–I). It is noteworthy that the changes of the energy state are not exactly linearly related to the changes in internal oxygen concentration (Fig. 2, L and M). These data show that respiratory energy production is limited by the low internal oxygen concentration inside the seed and that changes induced in the low oxygen concentration range are stronger than at higher oxygen concentrations.
Influence of Oxygen on the Flux of Carbon into the Starch and Protein Storage Pools
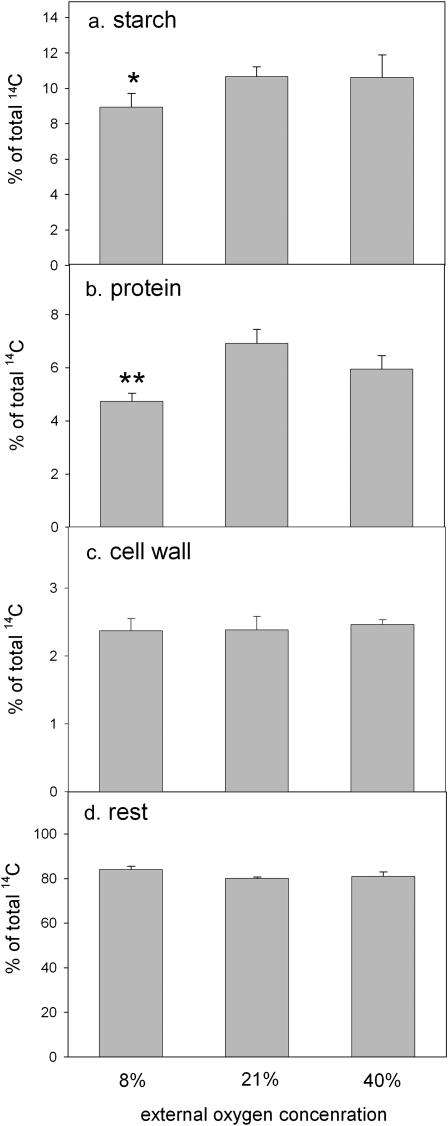
The effect of changing oxygen concentrations on metabolic fluxes of carbon in seeds was investigated by injecting 14C-labeled Suc directly into seeds that were still attached to the plant. During this minimally invasive experiment, the seeds were treated with air containing 8%, 21%, or 40% oxygen. After 3 h, the seeds were harvested and the amount of label incorporated into starch, protein, and cell wall material was determined (Fig. 3). Due to the short incubation time, only a small part of the injected 14C-Suc was metabolized. At normal oxygen, 10.6% of the label was incorporated into starch, and 6.9% of the label was found in the protein fraction. Only a small percentage of label was detected in cell wall material (2.3%). When the oxygen concentration of the air surrounding the seeds was decreased, the flux of carbon into both starch and protein decreased significantly to 8.9% and 4.7%, respectively. No significant change was observed for cell wall synthesis. Increasing the oxygen concentration from 21% to 40% had no significant effect on starch, protein, or cell wall synthesis (Fig. 3). The label detected in various soluble fractions (sugars, hexose phosphates, organic acids) showed no changes in response to oxygen (data not shown).
Figure 3.
The effect of changing external oxygen on the flux of label from 14C-Suc into starch (A), protein (B), cell wall (C), and soluble compounds (D). [14C]Suc was injected directly into the endosperm of seeds, and the percentage of radioactivity incorporated in the different fractions was determined after 3 h of exposure to 8%, 21%, or 40% oxygen. Data represent means ± se of at least six independent determinations. * and ** indicate significantly different values as compared to the 21% O2 treatment at a 90% and 95% confidence interval, respectively, as tested by the Student's t test.
These results implicate oxygen concentration within wheat seeds as an important factor affecting storage metabolism. Both starch and protein synthesis were found to be maximal at normal ambient oxygen concentration.
Influence of Oxygen on Metabolite Levels inside Seeds
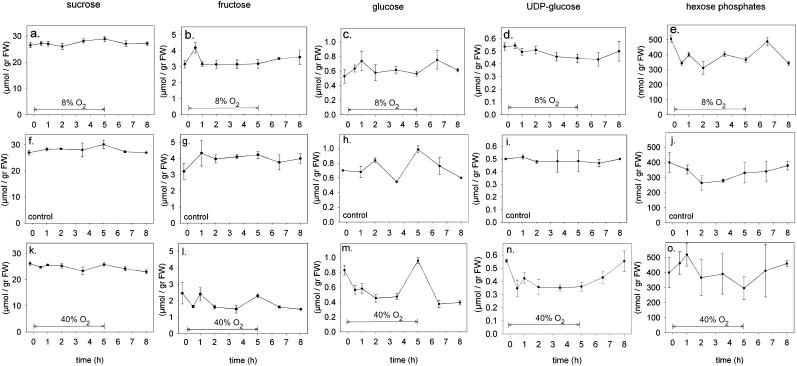
Metabolite levels were measured in a time course after exposing seeds to 8% and 40% external oxygen (Fig. 4). Since these experiments lasted for 8 h and metabolite concentrations might change during the day, a similar time-course was monitored as a control for seeds that remained at ambient (21%) oxygen during the whole experiment (Fig. 4, F–J). Except for some irregular fluctuations of the level of Glc (Fig. 4, C, H, and M) and a slight decrease in the amount of hexose-phosphates during the middle of the experiment (Fig. 4J), the level of metabolites remained constant throughout the entire experiment. Seeds within a time series were selected by similar developmental stage. However, different sets of seeds were slightly different in developmental stage, explaining the small variation between metabolite levels between different time courses (compare Fru in Fig. 4, G and L, or the amino acids in Fig. 5). Decreasing external oxygen to 8% did not lead to significant changes for most of the intermediate metabolites of the Suc to starch synthesis pathway (Fig. 4, A–E). When external oxygen was increased to 40%, there was a clear tendency of sugars and UDP-Glc levels to decrease (Fig. 4, K–O). The decrease was largest for UDP-Glc, and was reverted after oxygen was switched back from 40% to 21%. For rice seeds, similar results were obtained (data not shown). The total amino acids content in the seed did not change with either a decrease or increase in the oxygen concentration surrounding the seeds during the 5-h period (Fig. 5). The relative constancy of metabolite levels in the face of decreased metabolic fluxes at low oxygen suggests that import of assimilates into the seed has been inhibited under this condition.
Figure 4.
Intermediates of the Suc to starch metabolic pathway during the course of an experiment in which oxygen was decreased to 8% (A–E), unchanged (F–J), or increased to 40% (K–O) for the period of 5 h as indicated by the arrow. Suc is shown in A, F, K; Fru in B, G, L; Glc in C, H, M; UDP-Glc in D, I, N; and the hexose-phosphates (Glc-1-phosphate, Glc-6-phosphate, and Fru-6-phosphate) are shown in E, J, O. Data represent the means ± se of at least three measurements for every single point in time.
Figure 5.
Level of total amino acids during the course of an experiment in which ambient oxygen was decreased (8%) ○, increased (40%) •, or unchanged (21%) ▾. Data represent the mean ± se of at least three measurements.
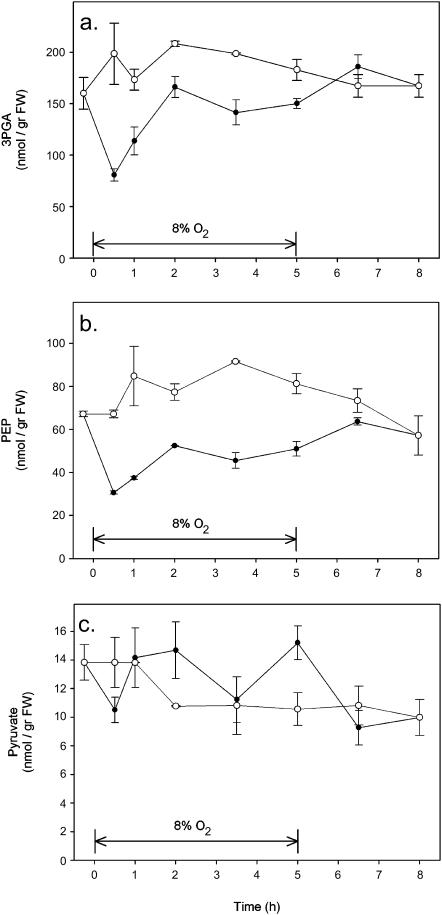
Some key metabolites of the glycolytic pathway were measured to find out whether changes in oxygen concentration affected glycolysis (Fig. 6). Within 30 min after the oxygen concentration was decreased, both 3phosphoglycerate (3PGA) and phosphoenolpyruvate (PEP) levels decreased strongly. Within 2 h, the level of 3PGA recovered completely, whereas PEP recovered only partly (Fig. 6). Changing the oxygen tension from 8% back to 21% had no further effect on the levels of 3PGA and PEP. The concentration of pyruvate did not change on switching to low oxygen. An increase in oxygen concentration to 40% had no effect on the concentration of any of the glycolytic intermediates tested (data not shown). These data show that decreasing the internal oxygen concentration of wheat seeds induced changes in some key metabolites of the glycolytic pathway, which indicates that the glycolytic fluxes changed as an effect of decreasing oxygen.
Figure 6.
Levels of 3PGA (A), PEP (B), and pyruvate (C), three key intermediates of the glycolytic pathway, as measured during an experiment in which ambient oxygen was decreased (8%) •, or remained unchanged 21% ○. Data represent the mean ± se of at least three measurements for every single point in time.
The amount of lactate in the seeds was very low and at the limit of detection in all of the samples (data not shown), indicating that there was no lactic fermentation at the oxygen concentrations that were investigated.
Influence of Oxygen on Phloem Transport toward the Seeds
Phloem transport and assimilate partitioning can be monitored in vivo when photoassimilates are labeled with carbon-11. This radio-isotope is a positron emitter, and the accompanying positron-electron annihilation generates high-energy (0.5 MeV) gamma-radiation easily detected in living tissues. A further advantage of this isotope is that the short half-life (20.3 min.) enables the use of the same plant in a series of experiments.
We monitored transport of 11C-labeled photoassimilates from the flag leaf to the ear and to the plant parts below the node at which the flag leaf was attached. The flag leaf was exposed to air containing 11CO2 with constant specific radioactivity during the entire course of the experiment. About 1 h after the start of the experiment, the first radioactive photoassimilates were detected in the ear (Figs. 7 and 8). With a mean distance of about 60 cm between the flag leaf and the ear, the phloem transport rate of assimilates can be calculated to be about 1.0 cm min−1, which is comparable to previous studies (Wardlaw, 1965). The amount of label detected in the flag leaf reached steady state within 2 h (data not shown), whereas the activity in the ear sometimes oscillated. This variation has been described previously, but no satisfactory explanation has yet been found (Roeb and Britz, 1991).
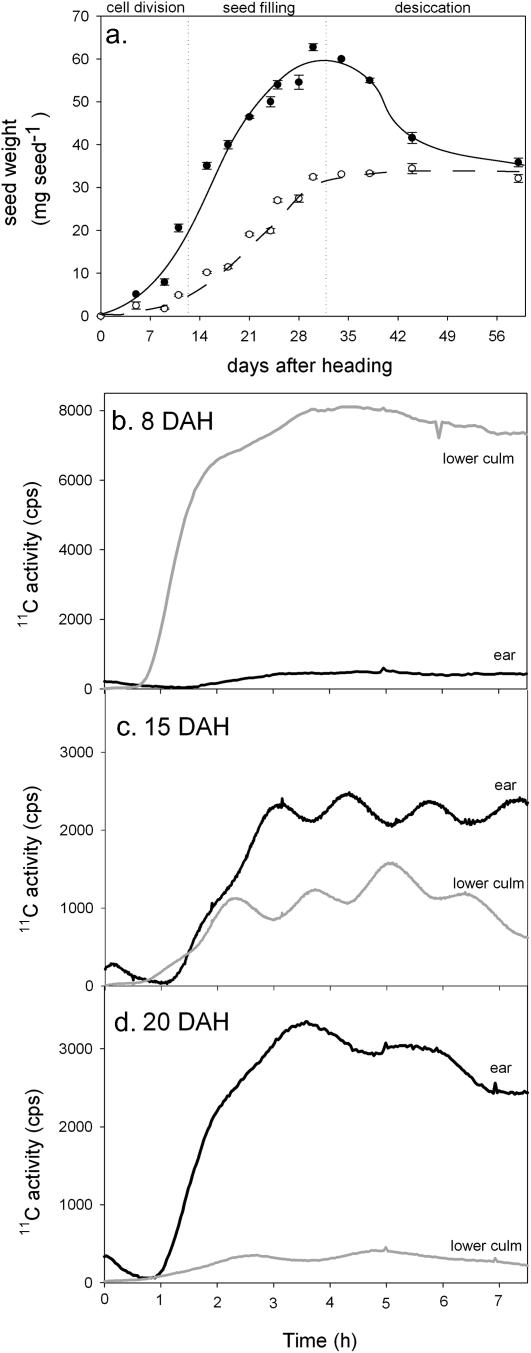
Figure 7.
Seed development in wheat plants and changes in phloem transport. A, Changes in seed fresh weight (•) and dry weight (○) during development. B to D, Phloem transport toward the ear (black line) and a part of the culm below the node at which the flag leaf is attached (gray line). 11C-labeled photoassimilates were measured with radiation detectors placed around the plant. About 2 h of continuous labeling was needed to reach steady state between uptake and release of labeled carbon in the labeling zone. B, Plants 8 DAH, just before seed filling. C, Plants with seeds 15 DAH, early in the seed filling stage. D, Plants with seeds 20 DAH, in the middle of the seed filling stage.
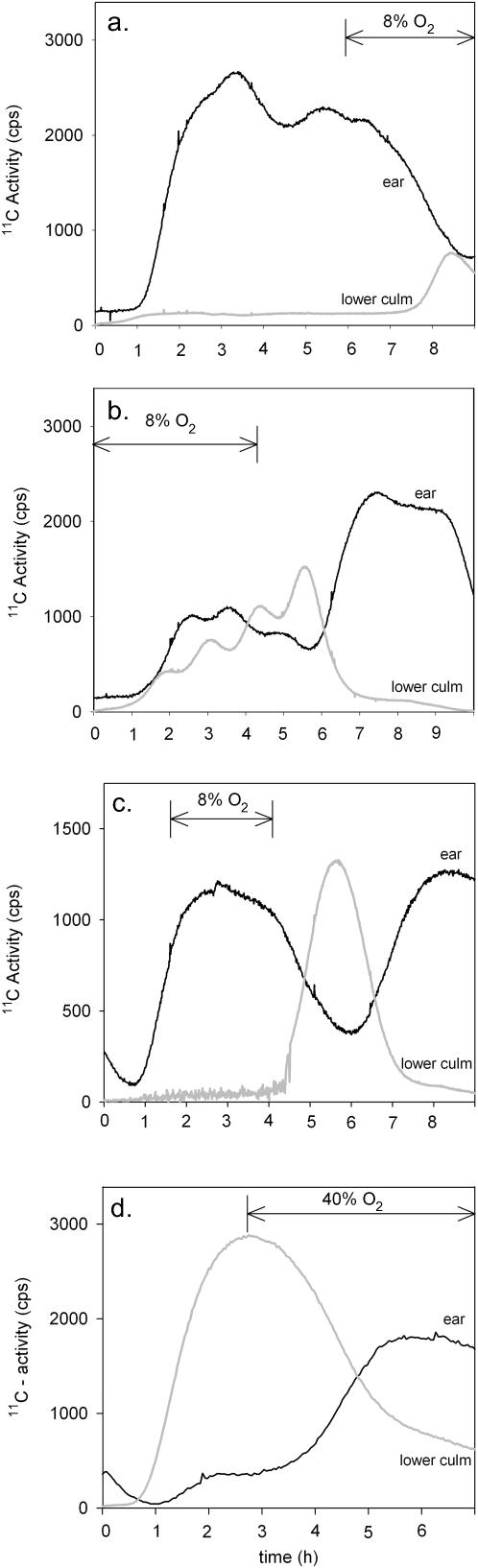
Figure 8.
Effect of seed oxygen supply on the distribution of 11C-labeled assimilates in a wheat plant 20 DAH. A, Transport toward the ear (black line) and lower culm (gray line) was measured when the oxygen concentration of the air around the ear was decreased from 21% to 8% (indicated by the arrow). B, The ear of the same plant as used in A was kept at low oxygen overnight. The next day, a new 11C treatment was started. Oxygen around the ear remained at 8% during the first 5 h of 11C-labeling. Then oxygen was shifted back to 21% (the arrow marks the 8% oxygen treatment). C, The changes in phloem transport induced by a 2-h treatment of low oxygen around the ear. D, Effect of high oxygen around the ear, in a plant trimmed at mid-seed filling (20 DAH) of all leaves except for the flag leaf. Note the reduced transport of label to the ear, and increased transport to the lower stem compared to an untrimmed plant of the same age shown in Figure 7D. Treatment with high (40%) oxygen is indicated by the arrow.
Apart from the variation described above, there is a clear change in the amount of label transported to the different sink regions of the plant during seed development. Shortly after heading, most of the label is transported toward the lower parts of the plant. However, during the seed filling stage (Fig. 7A), the proportion of photoassimilates transported to the seeds increased dramatically (Fig. 7, B–D). Simultaneously, transport toward the lower parts of the plant diminished. These measurements show that the transport profile of labeled assimilates from current photosynthesis in the flag leaf represents total assimilate transport toward the terminal sinks, even though the actual pool of assimilates that reaches the sink will also contain substantial input from turnover of stem carbohydrate reserves. Therefore measurements of labeled assimilates effectively measured transport of both labeled and unlabeled assimilates. The experiments demonstrate that several parts of a plant compete for the same pool of photoassimilates and that the amount of assimilates transported toward the seeds coincides with the demand for nutrients by seed storage metabolism.
A similar relation between sink metabolism and phloem transport toward the seeds was found when storage metabolism was inhibited by a low oxygen treatment of the ear. Within 1 h after reducing oxygen around the ear, phloem transport toward the seeds was reduced (Fig. 8A), whereas transport toward the roots increased. The oxygen concentration around the ear was held at 8% during the night, and the next day oxygen was increased to 21% again. With a similar lag in phloem response, transport rates returned to their original level (Fig. 8B). This experiment has been repeated several times with different plants but with only 2 h of reduced oxygen, showing the same effect on the phloem transport rates (Fig. 8C). In another experiment, a 5-cm stem segment directly below the seeds was treated with 8% oxygen. This had no effect on phloem translocation (data not shown). These experiments show that any effect on phloem import to the seeds when exposed to 8% oxygen is not due to an inhibition of phloem transport function itself.
Increasing the oxygen concentration around the seeds from 21% to 40% had no clear effect on the assimilate partitioning within the plant. Only in some of the experiments, a slight increase in assimilate transport toward the seeds was detected (data not shown). However, when the amount of assimilates transported to the ear was reduced artificially by removing all leaves but the flag leaf (compare Fig. 7D with Fig. 8D), then phloem transport toward the seeds could be stimulated by exposing the ear to 40% oxygen (Fig. 8D). These experiments show that changes in sink metabolism, either developmental or oxygen induced, lead to corresponding changes in assimilate transport toward the seeds by the phloem.
DISCUSSION
Oxygen Falls to Low Levels within Developing Wheat Seeds
The in vivo oxygen concentration within wheat seeds during the middle of the seed filling stage was measured using oxygen sensitive micro-sensors. At this particular developmental stage, seed dry weight was rapidly increasing because of extensive storage metabolism and maximal rates of import of assimilates via the phloem (Fig. 8). The internal oxygen concentration was low (approximately 2%) at ambient (21%) external oxygen and could be changed by changing the concentration of oxygen in the surrounding air (Fig. 1). However, internal oxygen did not decrease proportionally with decreasing oxygen in the surrounding air. This shows that changing the oxygen supply results in a concomitant change of the oxygen consumption by the seeds. Therefore, it can be concluded that the internal oxygen tension at a given external oxygen concentration is determined by the metabolic activity of the seed.
Similar changes in seed oxygen levels have been described for developing seeds of oil-seed rape (Vigeolas et al., 2003), even though they are very different from wheat with regard to the oxygen supply route. Fruit morphology and seed anatomy of rape can be compared to that of pea, a species in which the oxygen gradient through the seed has been described in fine detail (Rolletschek et al., 2002). Seeds of both these plants are embedded in a silique or pod. Although the inner space of the pod is already hypoxic compared to the outside (17% for rape; Vigeolas et al., 2003), it is the seed coat that forms the greatest barrier to oxygen diffusion. The oxygen concentration in the outer region of the cotyledon, just inside the seed coat, is much lower than outside and does not decrease further into the cotyledon (Rolletschek et al., 2002). Furthermore, the green cotyledons of legume seeds were shown to produce oxygen via photosynthesis, which significantly contributed to the internal oxygen tension (Rolletschek et al., 2003). Gifford and Bremner (1981), and more recently Rolletschek et al. (2004) speculated about oxygen delivery to the endosperm via photosynthesis in the maternal pericarp. Nevertheless, both our measurements in wheat seeds, as well as experiments with barley by Rolletschek et al. (2004), show that the endosperm of monocot seeds is hypoxic.
Cereal Seed Energy State Is Limited by the Low Internal Oxygen Concentration
The effect of the internal oxygen concentration on the energy status of wheat and rice seeds was determined by measuring changes in the levels of adenine, uridine, and guanidine nucleotides in experiments where external oxygen was changed from ambient (21%) to 8% or 40%. After 5 h, oxygen was returned to normal levels (Fig. 2). From these data, the adenylate energy charge as well as the ratios of ATP to ADP were calculated to determine the energy available for seed metabolism (Fig. 2, J–M). These parameters, as well as the ratios of UTP to UDP and GTP to GDP, decreased when oxygen was reduced. A similar effect was observed for rice seeds that were exposed to 8% oxygen (data not shown). When oxygen was increased above ambient levels (40%), all parameters for the energy state of the seeds increased in parallel (Fig. 2, J–M), which shows that under normal conditions the oxygen concentration within cereal seeds is limiting for respiration and energy production.
It is striking that the energy state is not linearly related to the internal oxygen concentration. At low internal oxygen concentrations, changes in energy charge were greater than at higher oxygen concentrations (Fig. 2, L and M). Apparently, respiration is strongly down-regulated when the internal oxygen concentration falls below 2%, such that oxygen consumption is reduced. Indeed, the relation between internal and external oxygen is not linear (Fig. 1), with smaller changes at low external concentrations and bigger changes at high oxygen. This adaptive response of respiration to reduced oxygen supply enables the seeds to avoid excessive oxygen consumption at low internal oxygen, which could otherwise ultimately lead to anoxia. A similar response has been described for potato tubers (Geigenberger et al., 2000) as well as for seeds of oil-seed rape (Vigeolas et al., 2003). In all of these cases, the decrease in respiration was clearly separated from the inhibition of cytochrome oxidase and the switch to fermentation, which does not occur until much lower oxygen concentrations.
Starch and Protein Storage Metabolism Are Reduced at Low Internal Oxygen Concentrations within Seeds
For wheat seeds, the main storage compound is starch, which accounts for 60% to 70% of the total dry weight, with storage protein also making up a significant proportion (10%–15%) of total seed dry weight. Experiments from this study show that storage metabolism, but not cell wall synthesis, is inhibited in parallel with the decreased energy state of the seeds during exposure to low (8%) oxygen (Fig. 3). The reduction of protein synthesis is twice that of starch synthesis, which can be explained by the higher energy demand of protein synthesis (see introduction). The high sensitivity of protein synthesis to reduced oxygen also explains the spatial distribution of protein and starch within the endosperm. The main site of storage-protein synthesis in wheat seeds is the subaleurone region, at the outer border of the endosperm where the oxygen concentration is higher than in the center (data not shown). Toward the center of the endosperm, the percentage of starch increases, while the synthesis of storage proteins within the central endosperm cells falls to nearly zero (Pomeranz and Shellenberg, 1961; Gaines et al., 1985; Bechtel, 1989).
Based on experiments with starch-storing potato tubers, it has been suggested that a decrease in internal oxygen is sensed by plants. As an adaptive response, use of oxygen is made more efficient, and metabolic activity is reduced to conserve energy and decrease oxygen consumption (Geigenberger et al., 2000, 2003b; Bologa et al., 2003). Similar adaptations have been described for seeds of oil-seed rape, in which oil is the main storage product (Vigeolas et al., 2003). We have now shown that also in cereal seeds, which are specialized in the storage of starch and protein, respiration as well as storage metabolism are inhibited in response to the low internal oxygen concentrations. Consequently, changes in internal oxygen are disproportionately smaller when seeds were exposed to subambient oxygen, compared to changes induced by superambient oxygen.
Interestingly, changing the oxygen concentration from 21% to 8% did not influence the sugar and amino acid concentration within wheat seeds (Figs. 4 and 5). A lack of changes in metabolite levels was also observed for isolated legume embryos that were exposed to reduced oxygen (Rolletschek et al., 2003) and for seeds of soybean plants of which seed growth rates were increased by changing the light intensity or the external CO2 concentration (Fader and Koller, 1985). Apparently, concomitant with the inhibition of the metabolic flux, the import of assimilates from the mother plant into the seeds is also reduced at low oxygen. In wheat, Suc and amino acids are imported from the seed apoplast into the endosperm via the transfer cells of the aleurone layer (Wang et al., 1994). This transport is mediated by proton symporters (Tegeder et al., 2000; Aoki et al., 2002), which require a proton gradient across the plasmalemma. When the amount of ATP decreases as a result of the low oxygen treatment, less energy becomes available for the H+-ATPases to build the proton motive force that is required for the carriers. Theoretically, this can induce two possible effects. Either the membrane potential depolarizes because the proton pumps run out of energy, or the transporters are directly down-regulated to avoid membrane depolarizations for which the cell cannot further compensate. Either of these explanations would result in a reduced import of Suc and amino acids from the apoplast, which is subsequently expected to influence the rate of phloem transport toward the seeds.
Inhibition of Storage Metabolism by Low Internal Oxygen Inhibits Phloem Allocation to the Seeds
Phloem transport throughout a living plant was monitored in a noninvasive manner after labeling photoassimilates with the radio-isotope carbon-11. Figure 7 shows the changes in phloem transport toward seeds during seed development. Shortly after heading, the amount of photoassimilates transported to the ear was very small (Fig. 7B), but during the seed filling stage (Fig. 7A), the transport of 11C label toward the seeds increased dramatically (Fig. 7, C and D). Conversely, the amount of photoassimilates transported to the roots diminished. This shows that photoassimilates are transported immediately from the flag leaf (source) toward the metabolizing sinks and supports the concept that assimilate partitioning is determined by the competitive interaction of several sinks within a plant (Farrar, 1996; Minchin and Thorpe, 1996).
When seed storage metabolism was reduced by decreasing the oxygen concentration around the ear, the effect on phloem transport from the flag leaf was investigated. For this, ears of wheat plants were kept in a cuvette in which the oxygen concentration was changed, while the rest of the plant remained in normal air (21%). The flag leaf of the plant was placed in a labeling chamber through which 11CO2 was circulated. Within 1 h after reducing oxygen, phloem transport toward the seeds decreased, while transport toward the roots increased in parallel. This effect was reversed when oxygen was returned to 21% (Fig. 8, A–C). As a control, a 5-cm stem segment directly below the seeds was treated with 8% oxygen. This had no effect on phloem translocation (data not shown). Much lower oxygen concentrations (<1%) have been shown to inhibit phloem transport in stems of Ricinus plants by reducing Suc retrieval into the phloem (van Dongen et al., 2003). Apparently, the 8% oxygen concentration as used in the current experiments was not low enough to inhibit transport phloem function. Besides, a reduction of Suc retrieval in the unloading zone is theoretically expected to stimulate nutrient supply to the seeds.
Increasing the oxygen concentration around the ear to superambient levels induced hardly any increase in phloem import. Since almost all of the photoassimilate from the flag leaf is already being delivered to the seeds (Fig. 7D), an increase in phloem import from the flag leaf might not be possible. Interestingly, phloem transport to seeds of plants from which the lower leaves were removed was stimulated when the oxygen concentration around the ear was increased from 21% to 40% (Fig. 8D). Partitioning of assimilates from the flag leaf throughout the rest of the plant changed dramatically when all leaves but the flag leaf were removed, since the ear has to compete with the lower part of the plant for the assimilates produced by the flag leaf. As a result, the supply of photoassimilate to the ear is reduced, and the amount of labeled assimilates transported from the flag leaf to the lower part of the plant increases (compare Fig. 7D with Fig. 8D). Similar, rapid changes in photoassimilate distribution have been reported in a vine when major source leaves or sinks were removed (Pickard et al., 1978). Apparently, increasing of the energy state by superambient oxygen concentrations allows the seeds to compete more strongly for available assimilates in this situation, thus increasing the assimilate flow to the ear.
Implications for the Regulation of Phloem Transport to the Sink
As hypothesized by Münch (1930), phloem transport toward the seeds is driven by a turgor pressure gradient throughout the phloem which drives the bulk flow of assimilates through the phloem. Phloem loading and unloading of assimilates, respectively at the source and sink part of the phloem, causes opposite changes in phloem sap concentration, which is the basis for the turgor pressure gradient along the phloem (for review, see Lalonde et al., 2003; van Bel, 2003).
The experiments described in this paper demonstrate that the metabolic activity, and therefore the consumption of assimilates by the seed, affects the supply of nutrients to the sink. This confirms the supply follows demand model of assimilate unloading proposed by van Dongen et al. (2001). According to this model, which is originally based on experiments with seed coats of legume seeds, the supply of nutrients by the maternal seed coat is determined by the demand of the developing embryo. When nutrients are taken up by the embryo from the apoplast, the concentration of these nutrients in the apoplast decreases, and the concentration gradient across the plasma membrane of parenchyma cells in the seed coat cells increases. The concentration gradient acts as the driving force for unloading of fresh nutrients into the apoplast. For pea, evidence exists that poorly selective pores are present in the plasma membrane of seed coat parenchyma cells to facilitate the unloading of assimilates (de Jong et al., 1996, 1997; van Dongen et al., 2001). Also in wheat, nucellus unloading appears to be a passive process, thus driven by the concentration gradient (Wang and Fisher, 1995). Together with the experiments presented in this study, our study indicates that the metabolic activity of the endosperm acts on the nutrient unloading from the maternal tissue and thereby determines the proportion of assimilates transported by the phloem toward the seeds.
An Overview of the Reasons and Consequences of Hypoxia within Seeds
During the last 2 years, several studies have been published on the oxygen concentration within seeds and the effect oxygen has on seed metabolism and physiology (Table I). Most seeds that were investigated so far had a very similar morphology. Pea and broad bean, both species from the family of the Fabaceae, as well as Arabidopsis and rapeseed (both Brassicaceae) store their seed storage products inside the cotyledons of the embryo, which for itself is capable of photosynthesis and thus produces oxygen. Also the fruit in which the seeds are enclosed has a similar appearance: a pod for Fabaceae species and a silique for Brassicaceae. In contrast, seeds from wheat (this study) and barley (Hordeum vulgare; Rolletschek et al., 2004), which are monocotyledonous species, have a very distinct morphology; nutrients are stored outside the embryo in the endosperm that does not contain chlorophyll, and the fruit wall (pericarp) is only a few cell layers thick and is grown together with the seed coat.
Table I.
Overview of the current knowledge on oxygen availability within seeds and its effect on metabolic fluxes
| Species | Subclass | Main Storage Products | Chlorophyll Present? | Internal [O2] at Ambient Air in the Light | Flux at Reduced Internal O2 | Flux at Increased Internal O2 | Remarks | References |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| Pea | Dicot | Starch, protein | Yes | 10 | N.d. | N.d. | Seed photosynthesis has been shown to contribute significantly to the oxygen supply within the seed. | 1, 2 |
| Broad bean | Dicot | Starch, protein | Yes | 4–10 | Starch ↓ at 0.1% O2 | N.d. | Protein synthesis is mainly located in the outer region, where oxygen, and ATP are relatively high. | 2, 3 |
| Protein = at 0.1% O2 | Starch synthesis dominates in the inner region of the seed, where oxygen and ATP concentrations are lowest. | |||||||
| Arabidopsis | Dicot | Oil, protein | Yes | N.d. | Lipid ↓at reduced external O2 | N.d. | Seed metabolism adapts to reduced oxygen in order to save energy and thereby oxygen. | 4 |
| Protein ↓ at reduced external O2 | ||||||||
| Rapeseed | Dicot | Oil, protein | Yes | 0.8 | Lipid ↓ below 0.8% O2 | Lipid ↑ above 0.8% | The oxygen supply of seeds under normal conditions is strongly limiting for seed metabolism. | 5 |
| Protein ↓ below 0.8% O2 | Protein ↑ above 0.8% | |||||||
| Barley | Monocot | Starch Protein | In the pericarp, but not in the endosperm | 0.2 | N.d. | N.d. | ATP levels and internal seed oxygen correlate with each other, both in a temporal and a spatial manner. | 6 |
| Wheat | Monocot | Starch, protein | In the pericarp, but not in the endosperm | 2.1 | Starch ↓ at 0.5% O2 | Starch = above 2.1% O2 | Using noninvasive 11C-labeleling of assimilates, the internal oxygen concentration within seeds is shown to affect phloem import via a reduction of metabolism. | 7 |
| Protein ↓ at 0.5% O2 | Protein = above 2.1% O2 |
The internal seed oxygen concentrations as given in this table are all measured in the light. The data on the effect of oxygen on metabolic fluxes indicate a decrease (↓), increase (↑), or no change (=) in the incorporation of radioactive label form 14C Suc fed to the tissue while seeds were treated with air containing different oxygen concentrations. Note that the flux experiment on broad bean was performed in darkness in order to reduce internal oxygen. N.d., Not determined. References: (1) Rolletschek et al. (2002); (2) Rolletschek et al. (2003); (3) Borisjuk et al. (2003); (4) Gibon et al. (2002); (5) Vigeolas et al. (2003); (6) Rolletschek et al. (2004); (7) this study.
A comparison of the various studies shows that the internal seed oxygen concentration differs much between the species. However, both high and low oxygen concentrations are measured within species with similar seed and fruit morphology (pea and rapeseed, respectively), indicating that more reasons than morphological characteristics alone must be responsible for the variety in internal seed oxygen. As shown for pea and broad bean, seed photosynthesis contributes significantly to the oxygen supply of the seeds. However, wheat that has no chlorophyll in the endosperm has a higher internal oxygen concentration than seeds of rapeseed that contain chlorophyll in the cotyledons. Therefore, the capability of photosynthetic oxygen production is also not the major determining factor for differences in internal oxygen concentrations. A final explanation is that oxygen consumption is different depending on the predominant metabolic pathways in the seeds. For example, starch synthesis costs much less energy than lipid synthesis does. Therefore, species in which the main storage component is oil will have much higher oxygen consumption than species that store starch. This could explain why oxygen is relatively low in oil-storing rapeseed.
Conclusively, hypoxia is a phenomenon that occurs in seeds of both monocotyledonous and dicotyledonous species. In all species investigated, low internal oxygen reduces seed metabolism in order to prevent the excessive use of oxygen—an adaptation that prevents anoxia. As shown in this study, the consequences of this adaptive response reach beyond the border of the seed and affect assimilate transport throughout the entire plant.
MATERIALS AND METHODS
Plant Material
Wheat plants (Triticum aestivum cv Anza) were grown hydroponically in a phytotron (day/night: 14 h/10 h, 24°C/16°C). The nutrient solution (modified Hoagland, 5 mm MES-KOH, pH 5.4, 1.25 mm Ca[NO3]2, 1.25 mm KNO3, 0.5 mm MgSO4, 0.25 mm KH2PO4, supplemented with micronutrients at a final concentration of 0.1 mm Fe-EDTA, 50 μm H3BO3, 10 μm MnCl2, 1 μm ZnSO4, 1 μm CuSO4, 0.5 μm Na2MoO4) was continuously aerated and changed once every week. If more then one tiller appeared on a plant, the extra tillers were removed so that only one seed-bearing tiller remained. Ears were tagged at the time of heading. Experiments were done in the light. Unless stated otherwise, plants were used 20 DAH, which is in the middle of seed filling when phloem transport of assimilates toward the seeds is at its maximum.
Measurements of Oxygen Tension inside Seeds
The oxygen concentration inside the seeds was measured by inserting a very fine oxygen sensitive optode with a tip diameter less than 30 μm (MicroxTX2, Presens, Regensburg, Germany) into the seeds, as previously described by Rolletschek et al. (2002) and van Dongen et al. (2003). To measure in situ oxygen concentrations in one seed while external oxygen was changed, seeds were detached from the plant and placed in a transparent plastic bag through which air with different oxygen concentrations was circulated. The air supply came from premixed gases (Messer Griesheim GmbH, Magdeburg, Germany) that contained 350 ppm CO2, oxygen concentrations as indicated in the text, with the balance made up by nitrogen.
Flux Analysis
The effect of changing oxygen on the flux of carbon from Suc into starch, protein, and cell wall material within the endosperm was determined as described in detail by Vigeolas et al. (2003). Briefly, 1 μL of a solution containing 115 μm [U-14C]Suc in 20 mm MES, pH 5.7 was injected directly into the endosperm of seeds that were still attached to the plant. To reduce damage to the seeds, a syringe with a very fine needle (470-μm diameter) was used (Hamilton, Neolab Heidelberg). The ear of the plant was covered with a plastic bag through which air with an oxygen concentration of 8%, 21%, or 40% was circulated (as described above). After 3 h, the seeds were frozen in liquid nitrogen and homogenized under liquid nitrogen to a fine powder. The water/ethanol soluble compounds were separated from the insoluble fraction. Starch and protein from the insoluble fraction were enzymatically digested, and label was measured using a liquid scintillation counter. Label in the insoluble fraction that remained after digestion of both starch and protein was classed as cell wall material.
Metabolite Analysis
Metabolites were extracted from the frozen material by trichloroacetic acid extraction as described by Jelitto et al. (1992). Sugars and glycolytic intermediates were measured enzymatically as in Geigenberger et al. (1998). Total amino acids were measured as in Geigenberger et al. (1998). Nucleotides, including UDP-Glc were analyzed by HPLC as described in Geigenberger et al. (1997). Lactate was measured according to Gibon et al. (2002).
Carbon-11 Labeling and Monitoring
11C-labeling was carried out with individual plants within an environmentally controlled cabinet close to the babycyclotron in Jülich, Germany (Japanese Steel BC 1710). Two weeks after heading, plants were transferred to a growth chamber in which the experimental set up was built, and the central 7 cm of the flag leaf enclosed in a cuvette. The ear was enclosed separately in a custom designed cuvette with its own gas circulation system. The leaf and ear were monitored for CO2 uptake and water release for at least 3 d before the first experiment was carried out. Tracer was supplied on 3 consecutive days by feeding 11CO2 to the flag leaf using the continuous labeling mode for about 9 h a day. Radiation detectors were placed around the plant to monitor the 11C activity in individual organs. Data were taken every 40 s and corrected for background radiation and interference from radioactivity in the hot load region. In control experiments, the ear was flushed with air from the growth chamber (about 3 L min−1). Oxygen treatments were performed by mixing the desired amount of CO2 and oxygen to a stream of nitrogen gas. The air stream was humidified by passing through a temperature controlled gas washing bottle.
Acknowledgments
We are grateful to Britta Hausmann and Karin Köhl for excellent plant cultivation in Golm and to John Lunn for critically reading and commenting on the manuscript. Edith Lahon, Sonja Reiland, and Jonas Krebs are acknowledged for their practical assistance in Golm. And we thank Mark Stitt, Uli Schurr, and Sigi Jahnke for stimulating discussions on the results and experiments.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ge 878/1–3).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.040980.
References
- Aoki N, Whitfield P, Hoeren F, Scofield G, Newell K, Patrick J, Offler C, Clarke B, Rahman S, Furbank RT (2002) Three sucrose transporter genes are expressed in the developing grain of hexaploid wheat. Plant Mol Biol 50: 453–462 [DOI] [PubMed] [Google Scholar]
- Atkinson DE (1977) Cellular Energy Metabolism and Its Regulation. Academic Press, NY
- Bagnall N, Wang XD, Scofield GN, Furbank RT, Offler CE, Patrick JW (2000) Sucrose transport-related genes are expressed in both maternal and filial tissues of developing wheat grains. Aust J Plant Physiol 27: 1009–1020 [Google Scholar]
- Bechtel DB (1989) How the structure of the wheat caryopsis could be modified to increase its end-use value. In Y Pomeranz, ed, Wheat is Unique. Structure, Composition, Processing, End-use Properties, and Products. American Association of Cereal Chemists, St. Paul, pp 71–84
- Bologa KL, Fernie AR, Leisse A, Loureiro ME, Geigenberger P (2003) A bypass of sucrose synthase leads to low internal oxygen and impaired metabolic performance in growing potato tubers. Plant Physiol 132: 2058–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisjuk L, Rolletschek H, Walenta S, Panitz R, Wobus U, Weber H (2003) Energy status and its control on embryogenesis of legumes: ATP distribution within Vicia faba embryos is developmentally regulated and correlated with photosynthetic capacity. Plant J 36: 318–329 [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD
- de Jong A, Borstlap AC (2000) Transport of amino acids (L-valine, L-lysine, L-glutamic acid) and sucrose into plasma membrane vesicles isolated from cotyledons of developing pea seeds. J Exp Bot 51: 1663–1670 [DOI] [PubMed] [Google Scholar]
- de Jong A, Koerselman-Kooij JW, Schuurmans JAMJ, Borstlap AC (1996) Characterization of the uptake of sucrose and glucose by isolated seed coat halves of developing pea seeds. Evidence that a sugar facilitator with diffusional kinetics is involved in seed coat unloading. Planta 199: 486–492 [Google Scholar]
- de Jong A, Koerselman-Kooij JW, Schuurmans JAMJ, Borstlap AC (1997) The mechanism of amino acid efflux from seed coats of developing pea seeds as revealed by uptake experiments. Plant Physiol 114: 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC (1997) Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol 48: 223–250 [DOI] [PubMed] [Google Scholar]
- Fader GM, Koller HR (1985) Seed growth rate and carbohydrate pool sizes of the soybean fruit. Plant Physiol 79: 663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JF (1996) Sinks—integral parts of a whole plant. J Exp Bot 47: 1273–1279 [DOI] [PubMed] [Google Scholar]
- Gaines RL, Bechtel DB, Pomeranz Y (1985) Endosperm structural and biochemical differences between a high-protein amphiploid wheat and its progenitors. Cereal Chem 62: 25–31 [Google Scholar]
- Geigenberger P (2003. a) Regulation of sucrose to starch conversion in growing potato tubers. J Exp Bot 54: 457–465 [DOI] [PubMed] [Google Scholar]
- Geigenberger P (2003. b) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6: 247–256 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M (2000) Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem 381: 723–740 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M (1998) Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205: 428–437 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M (1997) Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 201: 502–518 [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, adpglc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221–235 [DOI] [PubMed] [Google Scholar]
- Gifford RM, Bremner PM (1981) Accumulation and conversion of sugars by developing wheat grains. 2. Light requirement for kernels cultured in vitro. Aust J Plant Physiol 8: 631–640 [Google Scholar]
- James MG, Denyer K, Myers AM (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6: 215–222 [DOI] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M (1992) Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing Escherichia coli pyrophosphatase in their cytosol. Planta 188: 238–244 [DOI] [PubMed] [Google Scholar]
- Kuang A, Crispi M, Musgrave ME (1998) Control of seed development in Arabidopsis thaliana by atmospheric oxygen. Plant Cell Environ 21: 71–78 [DOI] [PubMed] [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26: 37–56 [Google Scholar]
- Minchin PEH, Thorpe MR (1996) What determines carbon partitioning between competing sinks? J Exp Bot 47: 1293–1296 [DOI] [PubMed] [Google Scholar]
- Münch E (1930) Die stoffbewegungen in der pflanze. Gustav Fisher, Jena, Germany
- Okumoto S, Schmidt R, Tegeder M, Fischer WN, Rentsch D, Frommer WB, Koch W (2002) High affinity amino acid transporters specifically expressed in xylem parenchyma and developing seeds of Arabidopsis. J Biol Chem 277: 45338–45346 [DOI] [PubMed] [Google Scholar]
- Patrick JW, Offler CE (2001) Compartmentation of transport and transfer events in developing seeds. J Exp Bot 52: 551–564 [PubMed] [Google Scholar]
- Pickard WF, Minchin PEH, Troughton JH (1978) Real-time studies of 11-C translocation in moonflower. 1. Effects of cold blocks. J Exp Bot 29: 993–1001 [Google Scholar]
- Pomeranz Y, Shellenberg JA (1961) Histochemical characterization of wheat and wheat products. II. Mapping of protein distribution in the wheat kernel. Cereal Chem 38: 109–113 [Google Scholar]
- Porterfield DM, Kuang AX, Smith PJS, Crispi ML, Musgrave ME (1999) Oxygen-depleted zones inside reproductive structures of brassicaceae: implications for oxygen control of seed development. Can J Bot 77: 1439–1446 [PubMed] [Google Scholar]
- Roeb G, Britz SJ (1991) Short-term fluctuations in the transport of assimilates to the ear of wheat measured with steady-state C-11 CO2-labeling of the flag leaf. J Exp Bot 42: 469–475 [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53: 1099–1107 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Weber H, Borisjuk L (2003) Energy status and its control on embryogenesis of legumes. Embryo photosynthesis contributes to oxygen supply and is coupled to biosynthetic fluxes. Plant Physiol 132: 1196–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolletschek H, Weschke W, Weber H, Wobus U, Borisjuk L Energy state and ist control on seed development: starch accumulation is associated with high ATP and steep oxygen gradients within barley grains. J Exp Bot (in press) [DOI] [PubMed]
- Tegeder M, Offler CE, Frommer WB, Patrick JW (2000) Amino acid transporters are localized to transfer cells of developing pea seeds. Plant Physiol 122: 319–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel AJE (2003) The phloem, a miracle of ingenuity. Plant Cell Environ 26: 125–149 [Google Scholar]
- van Dongen JT, Laan RGW, Wouterlood M, Borstlap AC (2001) Electrodiffusional uptake of organic cations by pea seed coats. Further evidence for poorly selective pores in the plasma membrane of seed coat parenchyma cells. Plant Physiol 126: 1688–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Schurr U, Pfister M, Geigenberger P (2003) Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol 131: 1529–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigeolas H, van Dongen JT, Waldeck P, Huhn D, Geigenberger P (2003) Lipid storage metabolism is limited by the prevailing low oxygen concentrations within developing seeds of oilseed rape. Plant Physiol 133: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Offler CE, Patrick JW, Ugalde TD (1994) The cellular pathway of photosynthate transfer in the developing wheat-grain. 1. Delineation of a potential transfer pathway using fluorescent dyes. Plant Cell Environ 17: 257–266 [Google Scholar]
- Wang N, Fisher DB (1995) Sucrose release into the endosperm cavity of wheat grains apparently occurs by facilitated diffusion across the nucellar cell-membranes. Plant Physiol 109: 579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw IF (1965) Velocity and pattern of assimilate translocation in wheat plants during grain development. Aust J Biol Sci 18: 269–281 [Google Scholar]