Abstract
We propose the visualization of venous compliance (VC) using a digital red-green-blue (RGB) camera. The new imaging method, which transforms RGB values into VC, combines VC evaluation with blood concentration estimation from the RGB values of each pixel. We evaluate a non-contact plethysmography (NCPG) system for VC based on comparisons with conventional strain gauge plethysmography (SPG). We conduct in vivo measurements using both systems and investigate their differences by evaluating the VC. The results show that the two methods measure different blood vessels and that errors caused by interstitial fluid accumulation are negligible for the NCPG system, whereas SPG is influenced by such errors. Additionally, we investigate the relationship between VC and physical activity using NCPG.
Keywords: venous compliance, non-contact measurement, venous thromboembolism
1. Introduction
The daily monitoring of vital signs, such as blood pressure and heart rate, is important for the maintenance of our health. Recently, some studies have proposed new techniques that use sensors or cameras to measure or quantitatively evaluate vital signs [1,2,3,4,5,6,7] and, thus, facilitate their daily recording. However, the measurement of vascular function, which provides especially important data for disease prevention regarding circulatory conditions, is limited to clinical use because the operation of the associated measurement equipment is difficult for people other than professional medical staff. Therefore, to obtain vascular function data as readily as vital sign data, we require a new measurement system that anyone can easily operate.
Venous compliance (VC) has attracted attention because it provides important vascular function information. VC—i.e., the ability of a blood vessel to stretch—relates to various factors, such as blood vessel disease. Generally, decreased VC is associated with venous thromboembolism and studies show that VC is correlated with physical activity [8,9,10,11]. Moreover, it depends on age [12,13], and gender [14]. For these reasons, VC is an important indicator for health maintenance.
The conventional method for measuring VC utilizes strain gauge plethysmography (SPG) [15,16], in which the volumetric change in a limb or digit, caused by a change in cuff pressure, is measured by a mercury-filled rubber strain gauge attached to the limb or digit. The change in blood volume (CBV) can be measured from this volumetric change. However, SPG suffers from a few crucial problems. First, movement of the limb or digit often causes errors in SPG measurements. Subjects are significantly inconvenienced because their bodies must be kept still for the duration of the measurement. Second, the contact area that can be measured by SPG is limited to parts around which the strain gauge can be wrapped, such as a limb or digit. In addition, the size of the strain gauge must be properly selected to fit the subject’s limb or digit, as inappropriate fitting of the gauge leads to measurement errors. Finally, SPG is affected by the damage of the strain gauge.
To address these problems, we propose a non-contact plethysmography (NCPG) system that uses a red-green-blue (RGB) camera to measure VC [17]. We consider that there are two important differences between NCPG and SPG that may affect the resulting VC measurements. First, the blood vessels measured by the two systems are different. SPG measures all of the vessels within the strain gauge, whereas NCPG measures only the blood vessels located immediately under the skin because visible light cannot travel deeply into the skin tissue. Generally, deep blood vessels also need to be measured for the assessment of the venous system. Therefore, we must investigate whether there is a positive correlation between the VCs evaluated using the two systems. The NCPG method relies on diffuse reflected light, which reflects the absorption of light by the skin chromophore—i.e., the melanin, oxygenated hemoglobin, and deoxygenated hemoglobin. Second, NCPG is not affected by errors due to interstitial fluid accumulation, whereas such errors are possible in SPG measurements. When the upper arm is occluded with a pressure cuff, vein vasodilation is induced by the CBV due to arterial inflow. Even when arterial inflow reaches a plateau, the blood volume of the upper arm will continue to increase owing to the interstitial fluid, which can pass through the small holes of the capillary vessels and accumulate outside the blood vessels. Therefore, SPG measurements reflect not only the vasodilation but also the interstitial fluid accumulation. On the other hand, because hemoglobin does not pass through the small holes of the capillary vessels (see Section 2.5 for further details) NCPG can probe the vasodilation without errors due to interstitial fluid accumulation and, thus, provide a more correct estimation of VC with upper arm occlusion. Therefore, it is important to determine how these differences affect the CBV and VC measurements. In the present study, we compare CBV and VC results measured using the proposed NCPG approach with those obtained with the conventional SPG technique.
Earlier studies [8,9,10,11] have shown a relationship between VC and physical activity. However, these reports were based on data that were measured using SPG. It is not clear whether NCPG would yield a similar result. Therefore, in this study, we categorized subjects into two groups and compared the mean VC of each group using the NCPG method.
Section 2 mainly reviews the core principles involved and the experimental setup of the dual measuring system. Specifically, these principles concern the estimation of blood concentration from the RGB values and the VC imaging method. Moreover, this section details two hypotheses regarding the two systems and the experimental conditions under which the measurements were performed. Section 3 presents and discusses the results. Finally, Section 4 provides brief concluding remarks.
2. Materials and Methods
2.1. NCPG for VC Using an RGB Camera Combined with SPG
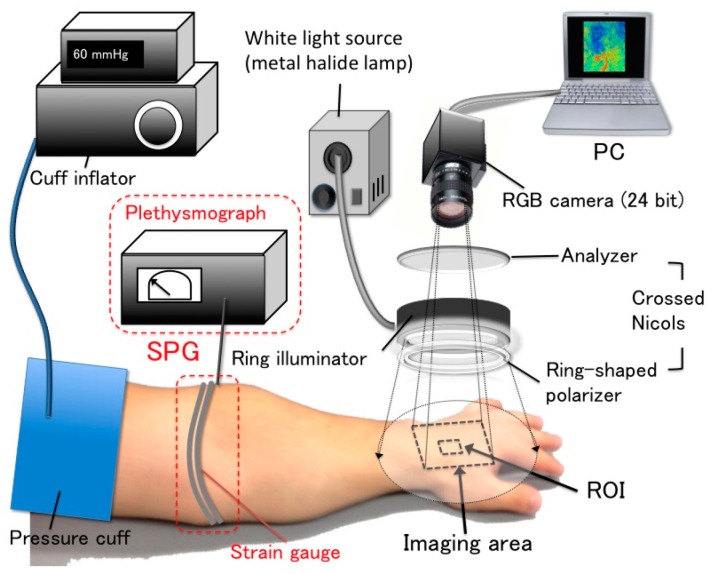
Figure 1 shows the setup of the simultaneous measurement system that combined NCPG and SPG. The NCPG system consisted of a 24-bit RGB charge-coupled device (CCD) camera (DFK23U618, Imaging Source LLC, Charlotte, NC, USA) with a camera lens (Pentax/Cosmica, Japan; f16 mm, 1:1.4), a metal halide lamp (LA-180Me-R4, Hayashi, Tokyo, Japan), a ring illuminator, a set of polarization plates, a computer, a pressure cuff, and a cuff inflator (E-20, D. E. Hokanson Inc., Bellevue, WA, USA). The primary polarization plate (ring-shaped polarizer) and the secondary polarization plate (analyzer) were aligned in the crossed Nicols configuration to reduce specular reflection from the skin surface. The RGB camera was connected to the computer with a USB 3.0 cable. Before using the RGB camera on human skin, the inter-instrument differences in the output of the camera and the spatial non-uniformity of the illumination were corrected for using a standard white diffuser with 99% reflectance (SRS-99-020, Labsphere Inc., North Sutton, NH, USA). The SPG system consisted of a mercury-filled rubber strain gauge and a plethysmograph for data display (EC6, D. E. Hokanson Inc., Bellevue, WA, USA). The strain gauge carried an electric current at the time of measurement. Thus, when the strain gauge (which was wound around the limb) was stretched by the cuff inflation, the change in its electric resistance (which was measured by the plethysmograph) was proportional to the volumetric change in the limb.
Figure 1.
Setup of the simultaneous VC measurement system that consists of an NCPG and an SPG system.
2.2. Estimation Method of Skin Chromophore Concentration from RGB Values
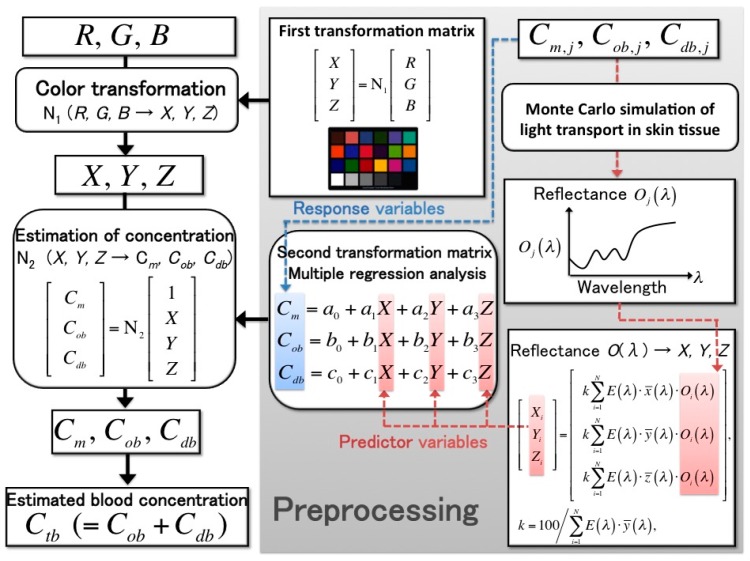
Any CBV in the skin tissue was estimated from the RGB images of human skin acquired using NCPG, whereas the blood volume was estimated from the volumetric change in a limb or digit using SPG. The method for estimating blood concentration from RGB values was proposed by Nishidate et al. [18,19,20]; this process is shown in Figure 2 and can be divided into three steps, as follows:
-
Step 1:We calculated the color transformation matrix N1 that can transform RGB values into Commission Internationale de l’Éclairage XYZ (CIEXYZ) tristimulus values. The matrix N1 was determined in advance using a standard color chart. We measured the RGB values of a standard color chart (ColorChecker®, X-Rite Incorporated, Grand Rapids, MI, USA) consisting of 24 color chips. The diffuse reflectance spectra O(λ) for each chip were supplied with the chart. The CIEXYZ values for the specific illuminant E(λ) were calculated as follows:
where Xi, Yi, and Zi are the CIEXYZ values; , , and are color-matching functions in the CIEXYZ color system; N is the total number of diffuse reflectance spectra; k is a constant whose value results in Y = 100 for a perfect diffuser. In this step, the XYZ value was calculated from each reflectance spectrum for 24 color chips (N = 24). Next, the transformation matrix N1 was determined using multiple regression analysis (MRA), in which the calculated XYZ values were the response variables and the measured RGB values were the predictor variables. The resulting regression coefficients were the elements of N1. If the RGB values of the color chips change by replacing the camera or the light source in the imaging system, the matrix N1 must be recalculated:(1) (2) -
Step 2:The second transformation matrix N2, which was used to transform the XYZ values into blood concentration values, was determined next. The skin chromophore concentrations—i.e., the melanin concentration Cm, the oxygenated blood concentration Cob, and the deoxygenated blood concentration Cdb—represent the light absorption coefficients of the skin tissue. Using various combinations of these three concentrations, we employed a Monte Carlo simulation (MCS) model of the transit of light in human skin to calculate the diffuse reflectance spectra of the skin surface O(λ). The parameters of the MCS used in this study—such as the absorption coefficient, the scattering coefficient, the refractive index, and the layer thickness of skin tissue—were those employed in an earlier study [20]. Moreover, to determine matrix N2, we used the simulation to calculate 300 diffuse reflectance spectra in the wavelength range 400–700 nm at intervals of 10 nm [20]. After the XYZ values were calculated by integrating the diffuse reflectance spectra from Equation (1), which were obtained using MCS, the transformation matrix N2 was determined using MRA:
(3) -
Step 3:
The XYZ values, which were transformed from the measured RGB values using the first matrix, N1, were transformed into the skin chromophore concentrations Cm, Cob, and Cdb using matrix N2. Thus, the total blood concentration was calculated as Ctb = Cob + Cdb. The absorption coefficients of blood for the case of Ctb = 100% were set to those of blood with a 44% hematocrit and 150 g/L of hemoglobin.
Figure 2.
Procedure for estimating the melanin concentration Cm, oxygenated blood concentration Cob, and deoxygenated blood concentration Cdb from RGB values.
2.3. Evaluation of VC
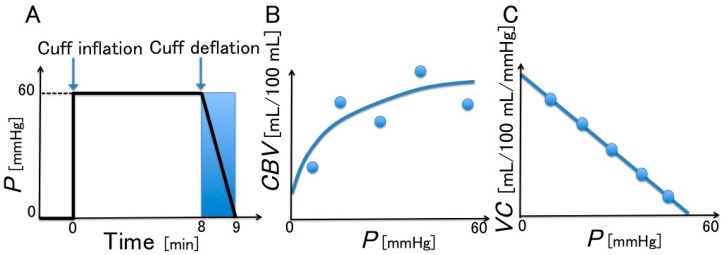
The method proposed by Halliwill et al. [21] for evaluating VC, shown in Figure 3, has been used in some recent studies. The procedure is as follows: as indicated in Figure 3A, after the cuff pressure (which is controlled by a cuff inflator) reaches 60 mmHg, it is maintained at that value for 8 min; it then decreases at a rate of 1 mmHg/s, which approaches the intravenous pressure [15], for 1 min while the volumetric change in the limb (around which the strain gauge is wrapped) is recorded using the plethysmograph. Figure 3B shows the regression equation for the volumetric change in the limb and Figure 3C displays the change in VC obtained by the differentiation of the regression equation.
Figure 3.
Method for the evaluation of VC (Halliwill et al. [21]). (A) Controlled cuff pressure; (B) change in blood volume (CBV) with respect to cuff pressure (P); and (C) venous compliance (VC) with respect to cuff pressure (P).
From the data obtained during the cuff deflation, the relationship between the CBV (mL/100 mL) and the cuff pressure (mmHg), which approximates the intravenous pressure, is obtained using the following quadratic regression equation:
| (4) |
where the CBV obtained using SPG is ΔBV/BVc (ΔBV = BV − BVc) and that using NCPG is ΔCtb/Ctb,c, (ΔCtb = Ctb − Ctb,c); BVc represents the blood volume and Ctb,c the blood concentration at the baseline (time = 0) and BV and Ctb denote these quantities at each measurement time; β0, β1, and β2 are constants; P is the cuff pressure. The VC (mL/100 mL/mmHg) at a particular cuff pressure is obtained from the differential of the regression equation:
| VC = ΔCBV/ΔP = β1 + 2β2P | (5) |
where ΔCBV is the difference in the CBV and ΔP is the difference in the cuff pressure.
2.4. VC Imaging
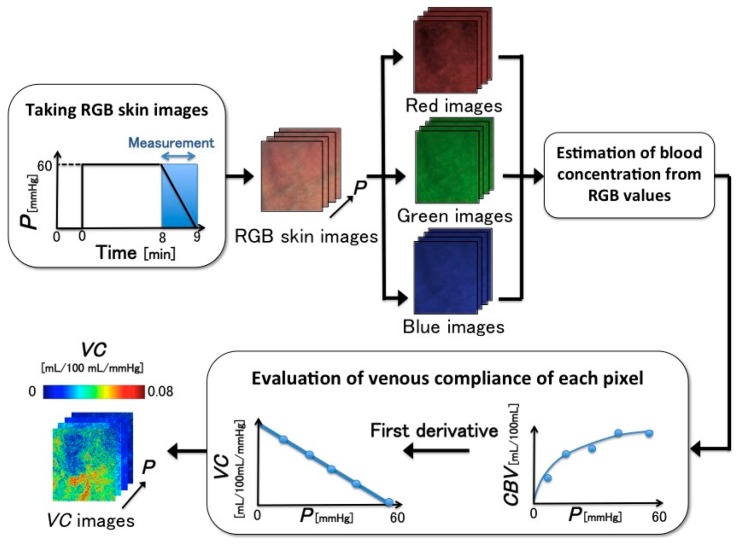
Figure 4 shows the proposed VC imaging process [17]. The spatial distribution of VC could not be observed because no visualization of VC was presented. Thus, we present a new VC imaging method that combines the evaluation of VC with an estimation of the blood concentration from the RGB values of each pixel. The new imaging method can easily obtain VC images from digital RGB images and provide VC measurements using a camera-equipped device, such as a smartphone. These images will help us understand VC intuitively. The new imaging process is as follows. First, during the 1-min period in which the pressure cuff is deflated from 60 mmHg to 0 mmHg, 60 RGB images of the skin are acquired. Secondly, as discussed in Section 2.2, the skin chromophore concentrations—i.e., Cm, Cob, and Cdb—are estimated from the RGB value of each pixel. From this estimation, we can obtain the change of each pixel in terms of blood concentration. Thirdly, the VC of each pixel is determined by the change in the estimated blood concentration according to Equations (4) and (5). The VC images are obtained by calculating the VC of all pixels. This imaging process was programed using MATLAB R2015a (The Mathworks, Inc. Natick, MA, USA).
Figure 4.
Proposed imaging process of VC, which is estimated from RGB images.
2.5. Differences between the Two Measurement Systems
In Section 1, we presented hypotheses concerning important differences that affect the measurement results of the NCPG and SPG methods. First, we considered that the measured blood vessels were different between the two methods and that this difference affects the results. The NCPG measurement is limited to superficial vessels, i.e., vessels near the skin surface. On the other hand, SPG can measure all vessels within the area covered by the strain gauge wound around the limb.
Our second hypothesis is that the NCPG method is not affected by errors in interstitial fluid accumulation, whereas SPG is influenced by such errors. The walls of capillary vessels have small holes (called intercellular clefts) that supply nutrients to the cells. Moreover, fluid components exist inside and outside the blood vessels, namely, the blood plasma and interstitial fluid. Fluids can pass through the holes whereas proteins—such as hemoglobin—cannot. Four pressures, called Starling forces, determine the flow of the fluid [22]. Capillary pressure (Pc) is the pressure in the blood vessel and is responsible for the fluid exiting the blood vessel. The interstitial fluid colloid osmotic pressure (Πi) also enables the flow of the fluid outside the blood vessel. On the other hand, the interstitial pressure (Pi) and plasma colloid osmotic pressure (Πp) enable the flow of the fluid inside the blood vessel. Thus, assuming that inward pressure is positive and outward pressure is negative, the total pressure (Pt) can be expressed as follows:
| (6) |
The direction of the flow is determined mainly by the capillary pressure. If Pc is higher than Pi and Πp, the total pressure is positive (Pt > 0) and the fluid flows outside the blood vessel. On the other hand, if Pc is lower than Pi and Πp, the total pressure is negative (Pt < 0) and the fluid flows inside the blood vessel. The fluid flows inside the venous vessel because Pc is lower and Pt is negative. However, if the vein is occluded from the cuff pressure, the venous pressure becomes higher than usual. Thus, the fluid flows and accumulates outside the blood vessel. Since SPG measures the volumetric change in limbs, SPG measurements are affected by the fluid accumulation in the form of errors that have no relation to vasodilatation. To test these hypotheses, we conducted in vivo experiments using the two systems and compared the results.
2.6. Experimental Conditions
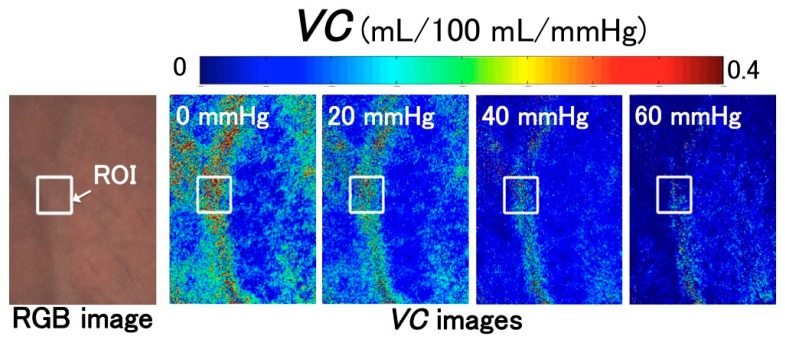
The experimental conditions of the NCPG measurements were as follows: As shown in Figure 1, the imaging area was the back of the subject’s left hand and the image size was 640 × 480 pixels. The lateral resolution of the resulting images was estimated to be 0.56 mm, which is insufficient to visualize the superficial veins. For example, the venula diameter is approximately 100–200 μm. Therefore, the VC images calculated using the method shown in Figure 4 are images in which the VC of superficial veins is spatially averaged. During the experiment, 660 frames of skin images were acquired at one frame per second (fps) by an RGB CCD camera, while 3300 measuring data were measured at five data points per second with the SPG method. Our region of interest (ROI) was 100 × 100 pixels and was placed on an area of the RGB skin image in which the vein could be observed.
The in vivo experiment was conducted on 11 male subjects (Japanese, mean age ± standard deviation: 24 ± 4 years) with no history or evidence of vascular diseases.
In this study, we categorized the subjects into two groups—an active group and a sedentary group—according to their differences in physical activity. For each group, we compared the mean measured VC of the ROI and evaluated it using NCPG. For their categorization, we distributed a questionnaire to all subjects regarding their physical activity. The questionnaire only consisted of two questions (Q1 and Q2) as follows:
Q1 (Past habits): Did you perform any physical activity on a daily basis within the past year?
Q2 (Current habits): Do you perform any physical activity more than two hours per week?
If the subject answered yes to either question, he was categorized into the active group, whereas if he answered no to both questions, he was classified as sedentary. All experimental procedures were approved by Nippon Sport Science University Ethics Committee.
3. Results and Discussion
3.1. Images of Venous Compliances
Figure 5 shows VC images constructed using the method shown in Figure 4. Figure 5 shows the RGB image of the skin and the change in the spatial distribution of VC with respect to the venous pressure, which corresponds to the change in cuff pressure during deflation. These images demonstrate that the area of greatest VC corresponds to the vein shown in the RGB image. Moreover, VC decreases with increasing venous pressure. This can be explained by the fact that the change in vein vasodilation decreases with increasing venous pressure.
Figure 5.
Acquired images showing the change in venous compliance (VC) depending on venous pressure. These images were obtained from the measurement of subject 3. ROI: region of interest. RGB: red-green-blue.
3.2. Difference between the Measurement Results of the Two Systems
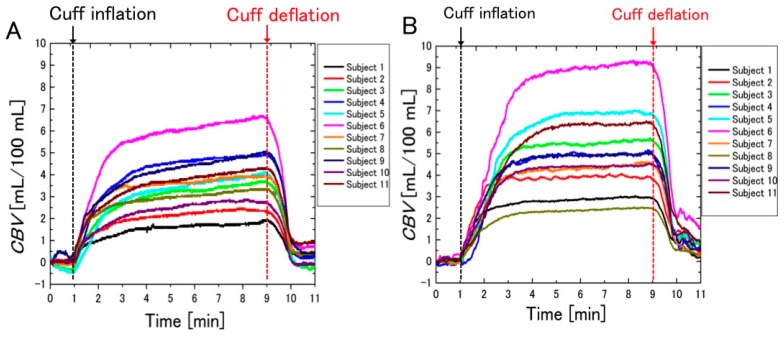
Figure 6 shows the temporal change in blood volume under pressure, measured according to the method of Halliwill et al. using SPG and NCPG. The x-axis denotes time and the y-axis represents the CBV. Figure 6A shows the CBV that was calculated from the volumetric change in the strain gauge using SPG. Figure 6B shows the CBV that was calculated with the NCPG method from the color change of skin images—specifically, from the mean values of the ROI shown in Figure 5.
Figure 6.
Temporal changes in blood volume (CBVs) measured with: (A) strain gauge plethysmography (SPG); and (B) non-contact plethysmography (NCPG) (n = 11).
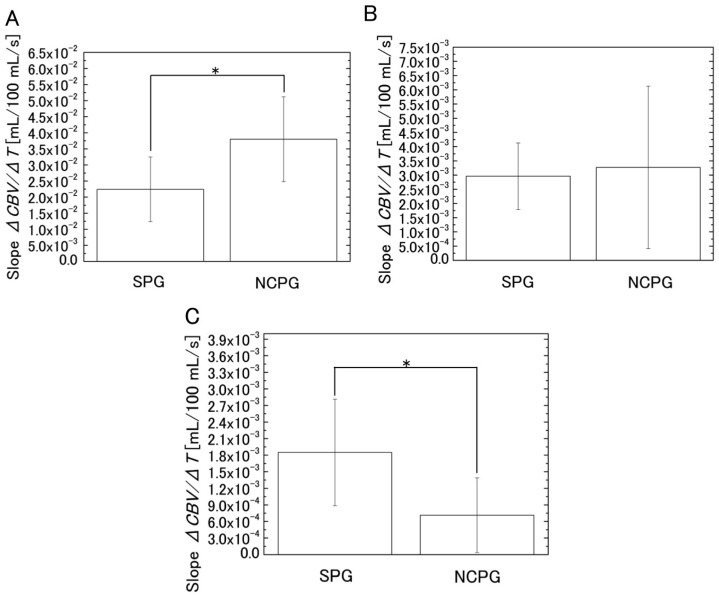
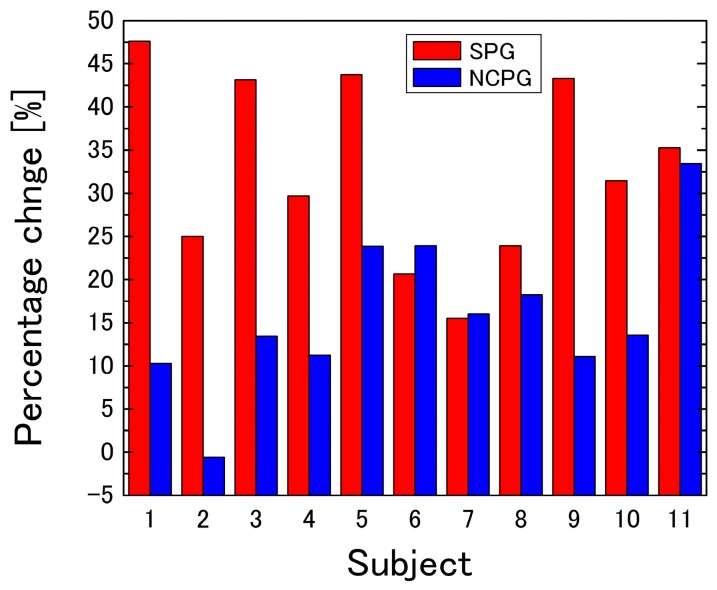
As shown in Figure 6, the CBV increases rapidly after cuff inflation. However, after a 1-min increase, the CBV measured with NCPG shows only a slight increase, whereas that obtained with SPG continues to increase until cuff deflation. To analyze the difference between the two methods, the slopes of the temporal change of the CBV were compared. For this analysis, the measurement time shown in Figure 6 was divided into three segments (segment 1 from 1 to 3 min, segment 2 from 3 to 6 min, and segment 3 from 6 to 9 min). The slopes (ΔCBV/ΔTime) of each segment were calculated from the regression lines of the temporal change of the CBV shown in Figure 6. In addition, after the normality of the mean values for each group was tested according to the Shapiro–Wilk test, the mean values of the slopes of the two systems were compared according to the Student’s t-test and the results are shown in Table 1. Figure 7 shows the estimated slope of the regression line calculated from the CBV measured with SPG and NCPG for each segment. As the results of the Shapiro–Wilk test show, all slopes had normality except for the slopes of NCPG at segment 2, which had no normality. As shown in Table 1, a significant difference was observed between two systems in segments 1 and 3. After the cuff was inflated, the CBVs of both systems increased significantly in segment 1. Then, all of the CBVs of SPG continue to gently increase in segments 2 and 3. On the other hand, as shown in Figure 6B and the standard deviation of NCPG in Figure 7B, some CBVs of NCPG increased significantly until 5 min in segment 2. Then, as shown in Figure 7C, the CBV increase of NCPG was less than that of SPG in segment 3. Moreover, Figure 8 shows the percentage change (%) in CBV between 3 and 9 min (=100 × (CBV9 min − CBV3 min)/CBV3 min) for each subject, and Table 2 shows the results of a t-test comparing the percentage changes obtained with SPG and NCPG. As the table shows, a significant difference of the percentage change was observed between the two systems.
Table 1.
Results of the t-test for the comparison between the slopes obtained with SPG and the NCPG.
| Segment | System | Mean of Slope | SD of Slope | t-Value | p-Value |
|---|---|---|---|---|---|
| Segment 1 | SPG | 2.24 × 10−2 | 1.01 × 10−2 | −3.11 | 5.47 × 10−3 |
| NCPG | 3.80 × 10−2 | 1.32 × 10−2 | |||
| Segment 3 | SPG | 1.85 × 10−3 | 9.63 × 10−4 | 3.21 | 4.38 × 10−3 |
| NCPG | 7.14 × 10−4 | 6.76 × 10−4 |
Figure 7.
Estimated slope (mean ± SD) of the regression line calculated from the change in blood volume (CBV) measured with SPG and NCPG in three segments: (A) segment 1 (from 1 to 3 min); (B) segment 2 (from 3 to 6 min); and (C) segment 3 (from 6 to 9 min). * p < 0.05. SD: Standard deviation (n = 11).
Figure 8.
Percentage change in CBV between 3 and 9 min for each subject.
Table 2.
Results of the t-test for the comparison between the percentage changes obtained with SPG and NCPG.
| System | Mean of Percentage Change | SD of Percentage Change | t-Value | p-Value |
|---|---|---|---|---|
| SPG | 32.7 | 10.8 | 3.98 | 7.38 × 10−4 |
| NCPG | 15.9 | 8.95 |
This discrepancy can be explained in terms of interstitial fluid accumulation. The rapid increase of venous blood pressure that results from cuff inflation leads to the fluid exiting the venous vessel. This flow leads to a volumetric change in the strain gauge that is not associated with vasodilatation, which leads to measurement errors. However, the NCPG method is not affected by such errors because it estimates the concentration of hemoglobin (which cannot exit the venous vessel) from the RGB values of the skin images. Therefore, these results provide strong evidence for the hypothesis presented in Section 1.
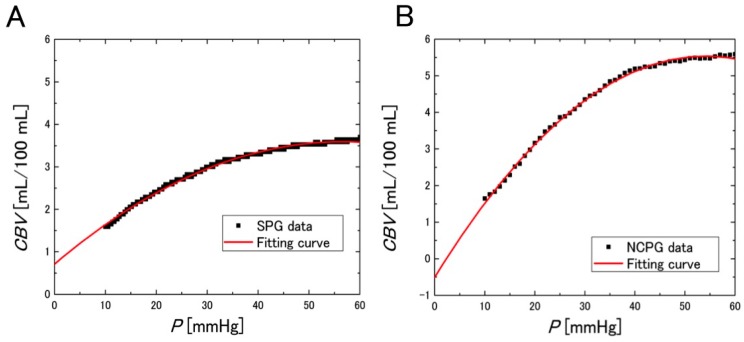
Figure 9 shows the CBV with respect to the cuff pressure during the deflation period and the quadratic regression curve. From the results, highly accurate regression equations were obtained.
Figure 9.
Observed CBV and quadratic regression curve with respect to the cuff pressure P during the deflation period, acquired with: (A) SPG (regression equation: CBV = 0.711 + 0.102·P − 8.99 × 10−4·P2, R2 = 0.996, p < 0.0001); and (B) NCPG (regression equation: CBV = −0.503 + 0.223·P − 2.05 × 10−3·P2, R2 = 0.998, p < 0.0001). The displayed data were obtained from the measurements of subject 3.
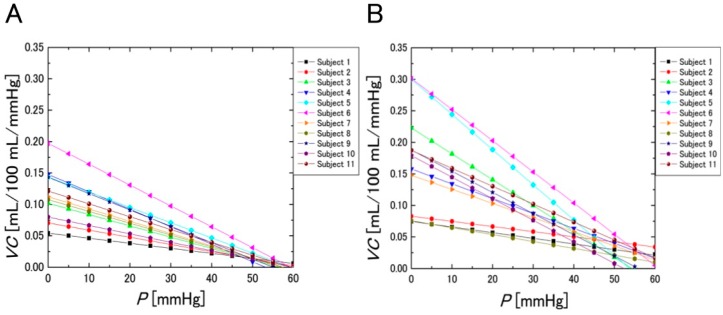
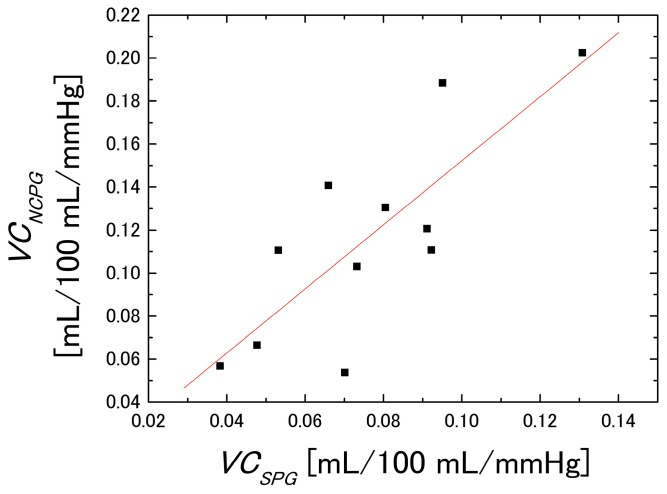
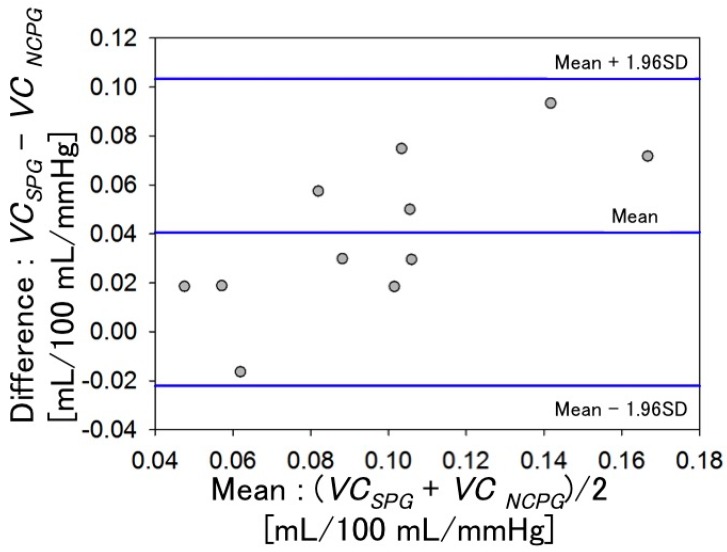
Figure 10 shows the VC evaluated using SPG and the NCPG. These figures were obtained by the primary differentiation of the regression equations with respect to the cuff pressure P. Figure 10A shows the VC obtained with SPG, whereas Figure 10B shows the mean VC evaluated within the ROI using the NCPG. To compare the VC values of the two graphs of Figure 10, we defined the VC value (mL/100 mL/mmHg) at 20 mmHg as the baseline and investigated the correlation between the VC values obtained using the two measurement systems, which is shown in Figure 11. The x-axis denotes the VC values of the subjects measured with SPG (VCPSG) and the y-axis those obtained with NCPG (VCNCPG). Pearson’s product-moment correlation coefficient between the two measurement systems is R = 0.797 (p < 0.005). Although differences exist between the two measurement systems, it can be concluded that there is positive correlation between the VCs evaluated using the two systems. Figure 12 shows the results of the Bland-Altman analysis for two VCs (VCSPG and VCNCPG) measured using the two systems. The x-axis denotes the mean of the two VCs and the y-axis their difference. This result exhibits a difference between the two VCs. As mentioned previously, the difference indicates the difference in the vein types that were measured with the two systems (NCPG: superficial veins vs. (SPG: deep veins + superficial veins + interstitial fluids)). This also suggests a difference between the errors caused by interstitial fluid accumulation in the two systems.
Figure 10.
Change in VC evaluated by: (A) SPG; and (B) NCPG) (n = 11).
Figure 11.
Correlation between VCSPG (VC estimated using SPG) and VCNCPG (VC estimated NCPG (n = 11).
Figure 12.
Bland-Altman comparison of VCSPG and VCNCPG (n = 11).
3.3. Relationship between Physical Activity and VC
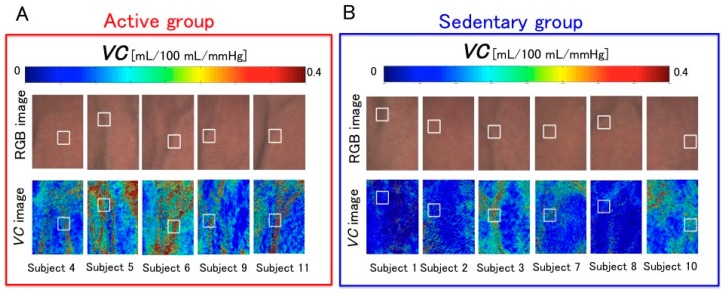
Figure 13 shows the VC images of all subjects at 0 mmHg. In this study, all subjects were categorized into two groups—active and sedentary group—according to their differences in physical activity and the VC of each group was examined. By comparing the images of the two groups, we can observe that the VC values of the active group were relatively higher than those of the sedentary group. Thus, to statistically compare the VC values of the two groups, we defined the ROI and calculated the mean VC value over the ROI. Moreover, after the normality of the mean values for each group was tested according to the Shapiro-Wilk test, they were tested with the Student’s t-test.
Figure 13.
Resulting VC images obtained at 0 mmHg for subjects of: (A) The active group (n = 5); and (B) the sedentary group (n = 6).
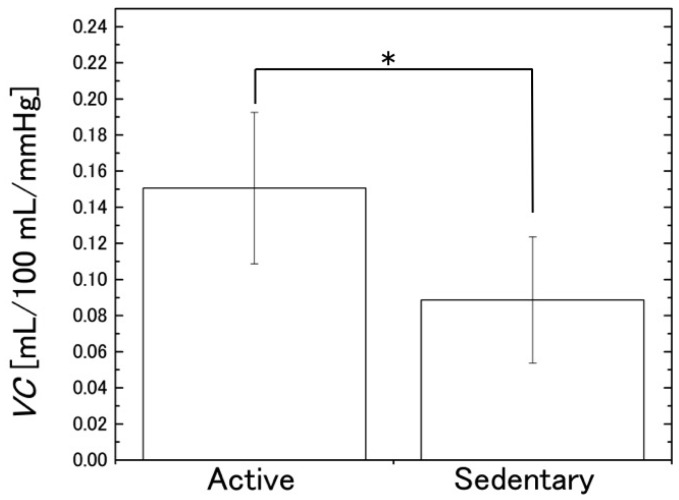
Figure 14 shows the comparison between the mean values of the two groups and Table 3 displays the results of the t-test. These results show that the mean VC in the active group was significantly higher than that in the sedentary group (p = 2.5 × 10−2). Similarly to the findings of earlier studies that used SPG [8,9,10,11], this indicates that VC evaluated with NCPG is associated with physical activity.
Figure 14.
Comparison between the estimated VC values (mean ± SD) of the two subject groups (active and sedentary). * p < 0.05 (n = 11).
Table 3.
Results of t-test for the VC of the active and sedentary groups.
| Group | Number of Subjects | Mean of VC at 20 mmHg | SD of VC at 20 mmHg | t-Value | p-Value |
|---|---|---|---|---|---|
| Active group | 5 | 0.151 | 0.042 | 2.68 | 0.025 |
| Sedentary group | 6 | 0.089 | 0.035 |
4. Conclusions
We achieved non-contact imaging by combining the evaluation of VC with a method that estimates the blood concentration from the RGB values of each pixel. Moreover, we simultaneously measured the VC of subjects using SPG and the proposed NCPG method and investigated the differences between these two systems. From the result shown in Figure 7, we can conclude that measurements performed with the proposed system are not affected by errors caused by interstitial fluid accumulation, whereas measurements with SPG are influenced by such errors. Moreover, we investigated the correlation between the VC values evaluated using the two systems. The results indicated a positive correlation (R = 0.797, p < 0.005) between VCSPG and VCNCPG and, thus, demonstrated that, in addition to SPG (i.e., the conventional VC measurement method), NCPG could be used for the evaluation of VC. Finally, we categorized subjects into two groups according to their differences in physical activity and compared the mean VC of each group using the non-contact system. The results showed that there was a significant difference between the mean VC of the two groups. Therefore, NCPG could investigate the relationship between VC and physical activity.
In future studies, we will investigate the effectiveness of NCPG across a wider range of skin tones. Moreover, we will investigate the relationship between VC and various conditions, such as anemia, septic shock, hypovolemia, hypothermia, and obesity, among others.
Acknowledgments
This work was supported by JSPS KAKENHI, Grant-in-Aid for Young Scientists (B) (grant No. 26820161).
Abbreviations
| VC | Venous compliance |
| SPG | Strain gauge plethysmography |
| NCPG | Non-contact plethysmography |
| RGB | Red-green-blue |
| CBV | Change in blood volume |
| CCD | Charge-coupled device |
| CIEXYZ | Commission Internationale de l’Éclairage XYZ |
| MRA | Multiple regression analysis |
| MCS | Monte Carlo simulation |
| ROI | Region of interest |
Author Contributions
All authors, including Kazuya Nakano, Yuta Aoki, Ryota Satoh, Hiroyuki Suzuki, and Izumi Nishidate, conducted this research. This paper was mainly written by Kazuya Nakano.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wieringa F.P., Mastik F., van der Steen A.F. Contactless multiple wavelength photoplethysmographic imaging: A first step toward “SpO2 camera” technology. Ann. Biomed. Eng. 2005;33:1034–1041. doi: 10.1007/s10439-005-5763-2. [DOI] [PubMed] [Google Scholar]
- 2.McCombie D.B., Shaltis P.A., Reisner A.T., Asada H.H. Adaptive hydrostatic blood pressure calibration: Development of a wearable, autonomous pulse wave velocity blood pressure monitor; Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; Lyon, France. 23–26 August 2007; pp. 370–373. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys K., Ward T., Markham C. Noncontact simultaneous dual wavelength photoplethysmography: A further step toward noncontact pulse oximetry. Rev. Sci. Instrum. 2007;78:044304. doi: 10.1063/1.2724789. [DOI] [PubMed] [Google Scholar]
- 4.Takano C., Ohta Y. Heart rate measurement based on a time-lapse image. Med. Eng. Phys. 2007;29:853–857. doi: 10.1016/j.medengphy.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Verkruysse W., Svaasand L.O., Nelson J.S. Remote plethysmographic imaging using ambient light. Opt. Express. 2008;16:21434–21445. doi: 10.1364/OE.16.021434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez G., Shuzo M., Ushida H., Hidaka K. Continuous blood pressure monitoring in daily life. JAMDSM. 2010;3:179–186. doi: 10.1299/jamdsm.4.179. [DOI] [Google Scholar]
- 7.Poh M.Z., McDuff D.J., Picard R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express. 2010;18:10762–10774. doi: 10.1364/OE.18.010762. [DOI] [PubMed] [Google Scholar]
- 8.Monahan K.D., Dikenno F.A., Seals D.R., Halliwill J.R. Smaller age-associated reductions in leg venous compliance in endurance exercise-trained men. Am. J. Physiol. Heart Circ. Physiol. 2001;281:1267–1273. doi: 10.1152/ajpheart.2001.281.3.H1267. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez J.P., Franke W.D. Age- and fitness-related differences in limbs venous compliance do not affect tolerance to maximal lower body negative pressure in men and women. J. Appl. Physiol. 2004;97:925–929. doi: 10.1152/japplphysiol.01328.2003. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez J.P., Franke W.D. Effect of a 6-mo endurance training program on venous compliance and maximal lower body negative pressure in older men and women. J. Appl. Physiol. 2005;99:1070–1077. doi: 10.1152/japplphysiol.01169.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kawano H., Tanimoto M., Yamamoto K., Gando Y., Sanada K., Tabata I., Higuchi M., Miyachi M. Greater forearm venous compliance in resistance-trained men. Eur. J. Appl. Physiol. 2010;110:769–777. doi: 10.1007/s00421-010-1557-9. [DOI] [PubMed] [Google Scholar]
- 12.Avolio A.P., Chen S.G., Wang R.P., Zhang C.L., Li M.F., O’Rourke M.F. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.CIR.68.1.50. [DOI] [PubMed] [Google Scholar]
- 13.Young C.N., Stillabower M.E., DiSabatino A., Farquhar W.B. Venous smooth muscle tone and responsiveness in older adults. J. Appl. Physiol. 2006;101:1362–1367. doi: 10.1152/japplphysiol.00448.2006. [DOI] [PubMed] [Google Scholar]
- 14.Monahan K.D., Ray C.A. Gender affects calf venous compliance at rest and during baroreceptor unloading in humans. Am. J. Physiol. Heart Circ. Physiol. 2004;286:895–901. doi: 10.1152/ajpheart.00719.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hokanson D.E., Sumner D.S., Strandness D.E. An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans. Biomed. Eng. 1975;22:25–29. doi: 10.1109/TBME.1975.324535. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen T., Hokanson D., Barnes R. Automatic quantitation of venous hemodynamics with an electrically calibrated strain gauge plethysmograph. Med. Instrum. 1977;11:240–243. [PubMed] [Google Scholar]
- 17.Nakano K., Satoh R., Hoshi A., Matsuda R., Suzuki H., Nishidate I. Non-contact imaging of venous compliance in humans using an RGB camera. Opt. Rev. 2015;22:335–341. doi: 10.1007/s10043-015-0041-5. [DOI] [Google Scholar]
- 18.Nishidate I., Sasaoka K., Yuasa T., Niizeki K., Maeda T., Aizu Y. Visualizing of skin chromophore concentrations by use of RGB images. Opt. Lett. 2008;33:2263–2265. doi: 10.1364/OL.33.002263. [DOI] [PubMed] [Google Scholar]
- 19.Nishidate I., Tanaka N., Kawase T., Maeda T., Yuasa T., Aizu Y., Yuasa T., Niizeki K. Noninvasive imaging of human skin hemodynamics using a digital red-green-blue camera. J. Biomed. Opt. 2011;16:086012. doi: 10.1117/1.3613929. [DOI] [PubMed] [Google Scholar]
- 20.Nishidate I., Tanaka N., Kawase T., Maeda T., Yuasa T., Aizu Y., Yuasa T., Niizeki K. Visualization of peripheral vasodilative indices in human skin by use of red, green, blue images. J. Biomed. Opt. 2013;18:061220. doi: 10.1117/1.JBO.18.6.061220. [DOI] [PubMed] [Google Scholar]
- 21.Halliwill J.R., Minton C.T., Joyner M.J. Measurement of limb venous compliance in humans: Technical considerations and physiological findings. J. Appl. Physiol. 1999;87:1555–1563. doi: 10.1152/jappl.1999.87.4.1555. [DOI] [PubMed] [Google Scholar]
- 22.Hall J.E. Guyton and Hall Textbook of Medical Physiology. 11th ed. Elsevier Japan; Tokyo, Japan: 2010. pp. 189–198. [Google Scholar]