Abstract
The biogenesis and function of mature human microRNAs is dependent on the nuclear export of pre-microRNA precursors by Exportin 5 (Exp5). The precursor for the human miR-30 microRNA, which is a 63 nt long RNA hairpin bearing a 2 nt 3′ overhang, forms a specific complex with Exp5 and the Ran-GTP cofactor. Here, we have examined the structural requirements for pre-microRNA binding by Exp5. Our data indicate that pre-miR-30 binding requires an RNA stem of >16 bp and is facilitated by a 3′ overhang. Although a blunt-ended derivative of the pre-miR-30 stem–loop remained capable of binding Exp5, 5′ overhangs were inhibitory. miR-30 variants that had lost the ability to bind Exp5 effectively were not efficiently exported from the nucleus and were also expressed at reduced levels. Furthermore, formation of a pre-microRNA/Exp5/Ran-GTP complex inhibited exonucleolytic digestion of the pre-miRNA in vitro. Together, these data demonstrate that pre-microRNA binding by Exp5 involves recognition of almost all of the RNA hairpin, with the exception of the terminal loop. Moreover, these results argue that Exp5 binding not only mediates pre-microRNA nuclear export but also prevents nuclear pre-microRNA degradation.
INTRODUCTION
MicroRNAs (miRNAs) are a class of ∼21 nt long non-coding RNAs observed in a wide range of metazoan eukaryotes [reviewed by (1)]. Over 200 distinct miRNAs have been reported in human cells. Although the biological role of only a small number of miRNAs has been determined in any species, it is believed that miRNAs play a key role in several biological processes, particularly differentiation and development, by inducing the translational inhibition or degradation of mRNAs bearing partly or fully complementary target sites (1).
While miRNAs are structurally identical to small interfering RNAs (siRNAs), the mediators of RNA interference, they differ in the processing pathway used for their biogenesis (1). miRNAs are encoded in the eukaryotic genome as part of one arm of an ∼80 nt predicted RNA stem–loop structure that in turn forms part of a longer RNA transcript termed a primary or pri-miRNA, that is predominantly single stranded (2). The first step in miRNA processing is the precise excision of the upper part of this RNA stem–loop by a nuclear RNase III enzyme termed Drosha (3). This results in the formation of an ∼65 nt RNA hairpin, bearing a 2 nt 3′ overhang, termed a pre-miRNA (3,4). Pre-miRNAs are specifically bound by the nuclear export factor Exportin 5 (Exp5), acting in concert with the GTP-bound form of the Ran cofactor (5–7). This pre-miRNA/Exp5/Ran-GTP complex then migrates to the cytoplasm where hydrolysis of Ran-GTP to Ran-GDP induces release of the pre-miRNA cargo. After release, the pre-miRNA is further processed by a second RNase III enzyme termed Dicer to release a ∼21 bp RNA duplex intermediate (8–10). One strand of this duplex is then specifically incorporated into the RNA-induced silencing complex (RISC), where it functions to guide RISC to complementary mRNAs.
Exp5, a member of the karyopherin family of nucleocytoplasmic transport factors, has been shown to mediate the nuclear export of several RNA binding proteins and can also act as a minor export factor for transfer RNAs (tRNAs) (11–14). However, the major function of Exp5 seems to be not only the nuclear export of other small structured RNAs such as not only pre-miRNAs (5–7), but also the human Y1 and adenovirus VA1 RNA (15–17). VA1 is a highly structured ∼162 nt long viral non-coding RNA that contains a terminal 18 bp stem, a 3′ overhang of ∼5 nt and a base paired 5′ nucleotide. Using mutated VA1 derivatives as a substrate for nuclear export and in vitro binding assays, it was reported that Exp5 interacted with a cis-acting export element that comprises a double-stranded stem of >14 bp with a base paired 5′ end and a 3–8 nt protruding 3′ end (17).
In this study, we have sought to extend this earlier paper by analyzing the sequence requirements for Exp5-mediated nuclear export and in vitro binding of the 63 nt pre-miRNA for the human miR-30 miRNA. In addition to defining the structural requirements for the binding of a pre-miRNA by Exp5, our data reveal that pre-miR-30 mutants that bind Exp5 poorly in vitro are not only exported inefficiently from the nucleus but also display low levels of steady-state RNA expression, thus suggesting that Exp5 binding may stabilize pre-miRNAs in vivo. Consistent with this hypothesis, we show that formation of a pre-miRNA/Exp5/Ran-GTP complex inhibits the in vitro exonucleolytic cleavage of a pre-miRNA intermediate. These data suggest that in addition to playing a critical role in pre-miRNA nuclear export, Exp5 also serves to stabilize pre-miRNAs in the nucleus and during export.
MATERIALS AND METHODS
Plasmid construction
pSUPER-miR-30 and pCMV-luc-8xmiR-30(P) have been described previously (5,18). Mutants of miR-30 were derived from pSUPER-miR-30 using the QuickChange method (Stratagene), or amplified by PCR, digested with BamHI and HindIII and inserted into pSUPER (19). The plasmid pH1-GFP bears the pGEM-3Zf(+) backbone and the same H1 promoter as pSUPER, and expresses an siRNA targeted to green fluorescent protein (GFP) mRNA. DNA encoding the siRNA was assembled by annealing two oligos: 5′-GATCCCCGAACGGCATCAAGGTGAACTTCAAGAGAGTTCACCTTGATGCCGTTCTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAGAACGGCATCAAGGTGAACTCTCTTGAAGTTCACCTTGATGCCGTTCGGG-3′ and inserting these into pSUPER.
Gel shift analyses
The templates were prepared by PCR with SP6 promoter sequences added as part of the 5′ primer, gel-purified and transcribed by SP6 RNA polymerase (Ambion). After treatment with DNAse, RNAs were purified and quantified by A260nm/A280nm readings, and also run on native and/or denaturing gels for verification of size and purity. A typical RNA binding reaction contained 1× binding buffer (20 mM Tris, pH 7.6, 100 mM KC1, 2 mM MgCl2, 0.1% Tween-20, 40 ng BSA, 30 ng dI–dC, 2 mM DTT, 10% glycerol), 2 U of RNasin (Promega), 100 ng recombinant RanQ69L-GTP and 5–20 ng recombinant Exp5. After incubation with 10 or 40 ng of cold competitor RNA at room temperature for 10 min, ∼10 000 c.p.m. (∼0.2 ng) of a 32P-labeled pre-miR-30 RNA probe was added. The final volume was 10 μl. After a further incubation at room temperature for 30–40 min, 1 μg of heparin and 2 μg of bromophenol blue in 2 μl was added, and the reaction was resolved on a 4–20% TBE gel (Bio-Rad). RanQ69L and Exp5 were gifts of Ian G. Macara. RNA mutants were tested at least three times. Quantification was performed by scanning the gels on a PhosphorImager.
Cell transfection and analysis
293T cells were maintained in DMEM supplemented with glutamine and 10% fetal bovine serum. Transfections of the 293T cells with indicator and effector plasmids were performed as described previously (4) using FuGene 6 (Roche). Dual-luciferase assays were performed according to instructions from Promega.
RNAs were isolated from the nuclear and cytoplasmic fractions of cells with Trizol Reagent (Invitrogen), and northern analyses performed as described in (4,18). To detect miR-30, we used a 5′ 32P-labeled oligo with the sequence 5′-GCAGCTGCAAACATCCGACTGAAAGCCC-3′. To detect the GFP siRNA, we used the oligo 5′-GTACACAAGAACGGCATCAAGG-3′.
Exonuclease T and Dicer cleavage of pre-miR-30
The 53 nt pre-miR-30(19) RNA (Figure 2) was used as a substrate in Exonuclease T (ExoT) cleavage assays. Approximately 20 000 c.p.m. of 32P-labeled RNA was incubated with 2.5 μg Exp5 and 5 μg of RanQ69L-GTP in 15 μl of 1× buffer (NEB buffer 4) at room temperature for 10 min. As a negative control for Exp5/RanQ69L, an equivalent quantity of BSA or RanQ69L-GTP alone was incubated with the RNA. Five units of ExoT (NEB) in 1 μl volume was then added, and incubation continued at room temperature. At 10, 20 or 40 min, 5 μl aliquots were removed and mixed with 100 μl of 20 mM EDTA, 1% SDS, 0.15M NaCl and 50 μg of tRNA, extracted with phenol–chloroform and precipitated with ethanol. Reactions were resolved on a 15% denaturing polyacrylamide gel.
Figure 2.
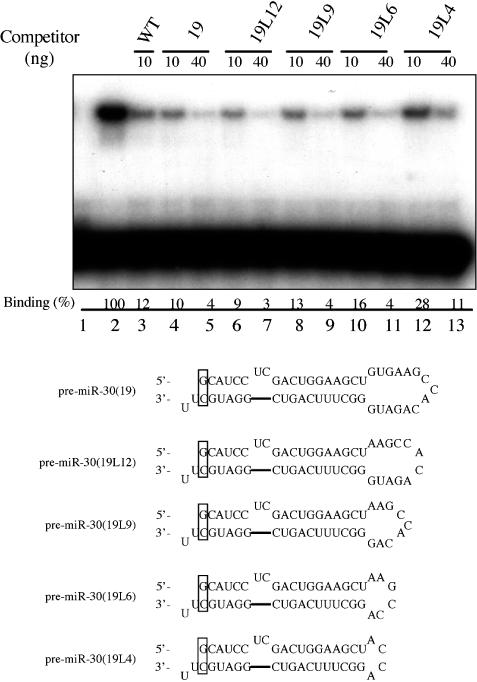
Exp5 binding to pre-miR-30 mutants with different terminal loops. Lane 1, WT pre-miR-30 probe in the absence of Exp5/RanQ69L-GTP; lanes 2–13, with Exp5/RanQ69L-GTP; lanes 3–13, 10 or 40 ng of the indicated unlabeled RNA competitors were added. Binding efficiencies (%) were calculated as the intensities of the shifted bands divided by that of the shifted band in lane 2. Sequences and predicted secondary structures of the loop deletion mutants are shown below the autoradiograph.
The BLOCK-iT Dicer kit (Invitrogen) was used as a source for Dicer and buffer. Each reaction contained ∼5000 c.p.m. of uniformly 32P-labeled pre-miR-30 RNA and 0.1 U of Dicer in 10 μl. For the protection assay, ∼1 μg of Exp5 and ∼2 μg of RanQ69L-GTP were incubated with the labeled RNA at room temperature for 10 min before Dicer was added. Three micrograms of BSA was used as a negative control. After incubation at 37°C for the indicated time, reactions were stopped by the addition of 1 μl of 0.5 M EDTA and 90 μl of 0.3 M sodium acetate containing 50 μg of yeast tRNA. The RNA was then phenol–chloroform extracted, precipitated, resolved on a 15% denaturing TBE gel (Bio-Rad), fixed and finally quantified by PhosphorImager.
RESULTS
Effect of stem length and loop size on pre-miRNA binding by Exp5
The structure of the 63 nt pre-miR-30 miRNA precursor, as produced by nuclear cleavage of the miR-30 pri-miRNA precursor by Drosha, has been reported previously (3). As shown in Figure 1, pre-miR-30 consists of a 22 bp stem, interrupted by a predicted 2 nt bulge in the 5′ arm, linked to a 15 nt terminal loop and a 2 nt 3′ overhang that results from cleavage by the RNase III enzyme Drosha (3). The ‘wild-type’ (WT) pre-miR-30 RNA probe used in in vitro experiments is identical to authentic pre-miR-30 except that the 5′ nucleotide is changed from U to G to accommodate the SP6 RNA polymerase. This mutation necessitates a second change, from A to C, at position 61 to maintain the structural integrity of the pre-miR-30 RNA stem (Figure 1). The 3′ two residue overhang of pre-miR-30 was also changed from 5′-GC-3′ to 5′-UU-3′ to permit identical RNA molecules to be correctly terminated in transfected cells when transcribed under the control of RNA polymerase III (19).
Figure 1.
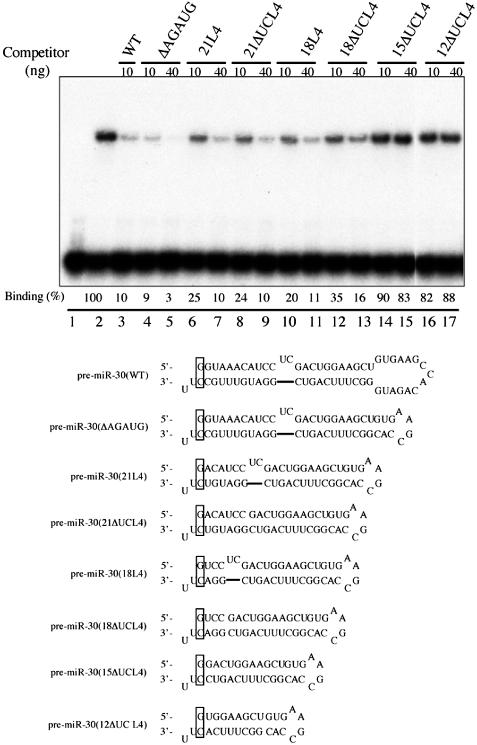
Exp5 binding to pre-miR-30 mutants with different stem lengths. Gel-shift assays were performed as described in Materials and Methods. Lane 1, 32P-labeled WT pre-miR-30 probe in the absence of Exp5/RanQ69L-GTP; lanes 2–17, with Exp5/RanQ69L-GTP; lanes 3–17, 10 or 40 ng of the indicated unlabeled RNAs were used as competitors. Binding efficiencies (%) were calculated as the intensities of the shifted bands, quantified by a PhosphorImager, divided by that of the shifted band in lane 2 (without competitors). The sequences and predicted secondary structures of the WT and mutant pre-miR-30 RNAs are presented below the autoradiograph. Since SP6 RNA polymerase starts transcription with a ‘G’, the corresponding position in pre-miR-30 was changed from ‘U’ to ‘G’, and its base pairing nucleotide was changed from ‘A’ to ‘C’. These changes are indicated by a box. The 2 nt 3′ overhang was also changed to ‘UU’. These changes did not affect Exp5 binding (data not shown).
In initial experiments, a series of stem and/or terminal loop deletion mutants of pre-miR-30 were transcribed in vitro and tested for their ability to compete for binding of the WT pre-miR-30 probe to the Exp5/Ran-GTP heterodimer (subsequently referred to as Exp5) using a previously described gel-shift assay (Figure 1) (5). The pre-miR-30(ΔAGAUG) mutant bears a 5 nt deletion in the terminal loop. This mutation, which is predicted to result in a 3 bp extension of the pre-miR-30 stem and leave a terminal loop of only 4 nt (Figure 1) has been shown previously to cause a severe defect in miR-30 production when introduced into the context of an RNA polymerase-II-driven pri-miR-30 expression plasmid (18). Interestingly, however, this mutation did not inhibit Exp5 binding detectably (Figure 1). Progressive deletion of the RNA stem present in pre-miR-30(ΔAGAUG) from 25 to 21 bp, 18, 15 and finally 12 bp showed that the 21 and 18 bp stems both supported high-affinity binding by Exp5, albeit somewhat less well than pre-miR-30(WT). In the context of the 21 bp stem, the 2 nt ‘UC’ bulge had little or no effect on Exp5 binding, but this bulge did slightly enhance Exp5 binding by the pre-miR-30 derivative with the 18 bp stem (compare lanes 10 and 11 with 12 and 13). The truncation of the pre-miR-30 stem to 15 or 12 bp largely blocked Exp5 binding in this sequence context.
To further analyze the effect of the terminal loop on Exp5 binding by pre-miR-30, we constructed a set of terminal loop deletion mutants in the context of a pre-miR-30 derivative bearing a 19 bp stem (Figure 2). As predicted from the data shown in Figure 1, the 19 bp stem variant bound Exp5 as well as the WT pre-miR-30 stem–loop (Figure 2). Progressive deletion of the terminal loop, from 15, 12, 9, to 6 nt, had no effect on Exp5 binding. However, a final deletion from 6 to 4 nt did result in significant inhibition (compare lanes 10 and 11 with 12 and 13).
Pre-miRNA binding by Exp5 is inhibited by a 5′ overhang
Previous studies analyzing the interaction of Exp5 with the VA1 RNA cargo have revealed that the structure of the terminal stem of VA1 is critical for Exp5 binding and nuclear export and have, in particular, led to the proposal that a base paired 5′ nucleotide and a 3′ overhang of 3–8 nt in length are important for both (17). To test this hypothesis in the context of the smaller pre-miR-30 RNA, we introduced a series of mutations at the base of the pre-miR-30 stem, as described in Table 1. Initially, these were transcribed in vitro and analyzed for binding to Exp5 in the presence of Ran-GTP (Figure 3).
Table 1. Summary of Exp5 binding and in vivo expression of pre-miR-30 mutants.
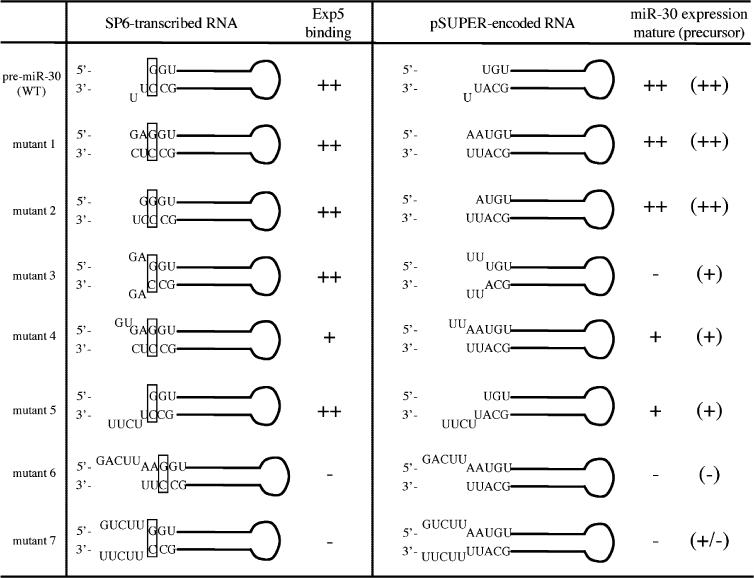
Sequences and predicted secondary structures of ‘WT’ pre-miR-30 and mutant derivatives used in Figures 3 and 4. Only the terminal region is depicted. Column 2 shows RNAs produced by SP6 transcription in vitro; column 3 shows the predicted, corresponding RNAs transcribed from the H1 promoter in vivo. Their relative expression levels in transfected cells were determined by northern blotting (Figure 4A) and reporter assay (Figure 4B). Mathematical signs outside the parentheses indicate the expression levels of mature miRNA, and those within the parentheses indicate that of the pre-miRNA.
Figure 3.
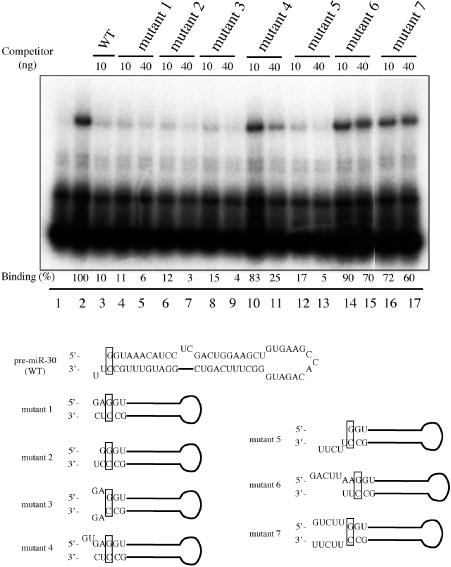
Exp5 binding to pre-miR-30 mutants with different 5′ and 3′ termini. Lane 1, 32P-labeled WT pre-miR-30 probe without Exp5/RanQ69L-GTP; lanes 2–17, with Exp5/RanQ69L-GTP; lanes 3–17: 10 or 40 ng of the indicated unlabeled RNA competitors were added. Binding efficiencies were calculated as in Figures 1 and 2. The terminal sequences and predicted secondary structures of the competitor RNAs used are listed below the autoradiograph as well in columns 1 and 2 of Table 1.
Given the proposal (17) that the presence of a 3′ overhang, and the absence of a 5′ overhang, are important for VA1 binding by Exp5, we were surprised to find that pre-miR-30 variants bearing a blunt end (mutant 1), a 1 nt 3′ overhang (mutant 2) or 2 nt 5′ and 3′ overhangs (mutant 3) all bound Exp5 as readily as the WT pre-miR-30 RNA (Figure 3). However, a pre-miR-30 derivative bearing a 2 nt 5′ overhang and no 3′ overhang (mutant 4) was clearly compromised for Exp5 binding. Two derivatives containing 5 nt 5′ overhangs, one in the absence of a 3′ overhang (mutant 6) and one in the presence of a 5 nt 3′ overhang (mutant 7) appeared to be negative for Exp5 binding (Figure 3). However, a pre-miR-30 derivative bearing a 5 nt 3′ overhang in the absence of any 5′ overhang (mutant 5) bound Exp5 nearly as well as WT. Together, these data validate the previous proposal (17) that Exp5 binding is sensitive to the RNA structure around the base of potential RNA targets but suggest that binding to pre-miR-30 is less easily perturbed than is binding to the VA1 RNA target.
Loss of Exp5 binding results in reduced pre-miRNA expression and function
We then analyzed the pre-miR-30 mutants, described in Table 1, for their ability to be exported from the nucleus and processed appropriately in transfected cells. We have described in (5) a plasmid, pSUPER-miR-30, that is predicted to express a 63 nt RNA that is identical to WT pre-miR-30 except that the 2 nt 3′ overhang is changed to 5′-UU-3′ to accommodate transcription termination by RNA polymerase III. This artificial pre-miR-30 precursor was processed in vivo to give a ∼22 nt RNA that hybridized to a probe for detecting miR-30 (Figure 4A). A series of derivatives of pSUPER-miR-30, designed to give RNA transcripts structurally equivalent to mutants 1 through 7 (Table 1), were also prepared and analyzed in transfected cells. Transfected cells were divided into nuclear and cytoplasmic fractions and pre-miR-30 and mature miR-30 expression analyzed by northern blot. A plasmid expressing an siRNA specific for GFP served as a control for transfection efficiency and RNA recovery (Figure 4A).
Figure 4.
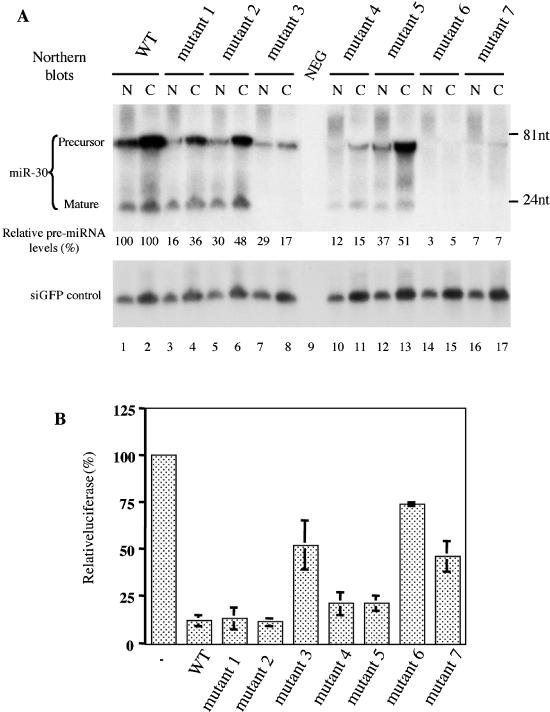
Expression and function of pre-miR-30 mutants in transfected cells. (A) 293T cells were transfected with 1 μg of pH1-GFP and 1 μg of pSUPER-miR-30 (either WT or one of the mutants) per well in 6-well plates. Two days after transfection, RNAs were isolated from nuclear (N) and cytoplasmic (C) fractions and northern analyses performed. Blots were first probed for miR-30, then stripped and probed for the GFP siRNA. Lane 9: total RNA from untransfected 293T cells (NEG). Intensities of the pre-miRNA bands were quantified by a PhosphorImager, with those of the cytoplasmic and nuclear pre-miRNA expressed from pSUPER-miR-30(WT), both set as 100%. The terminal sequences and predicted secondary structures of the pre-miRNA transcripts are depicted in column 3 of Table 1. Positions of DNA markers are shown at the right side of the autoradiograph. (B) 293T cells in 24-well plates were transfected with 5 ng of the pCMV-Luc-8xmiR-30(P) indicator plasmid, 0.5 ng of the Renilla luciferase internal control plasmid pRL-CMV (Promega) and 20 ng pSUPER or pSUPER-miR-30 (WT or mutants 1 to 7). A dual luciferase assay was performed 2 days after transfection. The firefly luciferase activity (normalized against Renilla luciferase activity) observed upon pSUPER co-transfection was set at 100%. Experiments were performed in triplicate with SD indicated.
As shown in Figure 4A, the pre-miR-30(WT) expression plasmid gives rise to readily detectable levels of pre-miR-30 and mature miR-30 RNA in both the nuclear and cytoplasmic fractions derived from transfected 293T cells. The apparent presence of mature miR-30 in the nucleus is unexpected, given that Dicer processing is believed to occur exclusively in the cytoplasm (1). It is possible that the mature miR-30 RNA is re-imported into the nucleus after Dicer cleavage or that the presence of mature miR-30 in the nuclear fraction reflects contamination by cytoplasmic ribosomes attached to the exterior of the nuclear membrane. RISC, together with its miRNA component, is known to be ribosome associated in vivo (1). Mutant 1, bearing a blunt-ended stem, and mutant 2, bearing a 1 nt 3′ overhang, both gave a pattern of pre-miR-30 and miR-30 RNA expression that was comparable to WT. Mutant 3 gave rise to a low but still readily detectable level of both nuclear and cytoplasmic pre-miR-30, thus suggesting that nuclear export was at most modestly impaired, yet yielded very little mature miR-30 (Figure 4A). Mutant 4 also gave rise to a reduced level of both nuclear and cytoplasmic pre-miR-30, but, in contrast to mutant 3, supported a level of mature miR-30 expression that was only slightly less than WT. While mutant 5 essentially gave rise to WT levels of both nuclear and cytoplasmic pre-miR-30, the level of mature miR-30 expression was also lower than that seen with WT (Figure 4A). Finally, mutants 6 and 7 gave rise to only very low levels of either pre-miR-30 or mature miR-30. We note that a very low level of mature miR-30 was detectable with mutants 3, 6 and 7 upon prolonged exposure (data not shown).
We have described in (18) the indicator construct pCMV-Luc-8xmiR-30(P), which contains a firefly luciferase gene linked to eight copies of a target sequence that is perfectly complementary to miR-30. This construct provides a sensitive reagent to detect functional miR-30 expression in co-transfected cells. As shown in Figure 4B, and as reported previously (5), co-transfection of pCMV-Luc-8xmiR-30(P) with the WT pSUPER-miR-30 effector plasmid gave rise to a ∼10 fold inhibition in firefly luciferase expression when compared to the Renilla luciferase internal control. Similarly, mutants 1 and 2, which gave rise to similar levels of mature miR-30 (Figure 4A), also inhibited firefly luciferase expression to an equivalent degree. Mutants 4 and 5, which gave rise to levels of mature miR-30 that were somewhat lower than that detected with WT pSUPER-miR-30 (Figure 4A), gave a readily detectable but slightly attenuated ∼5-fold inhibition of firefly luciferase expression when co-transfected with pCMV-Luc-8xmiR-30(P). Finally, mutants 3, 6 and 7, which gave rise to poor levels of miR-30 production (Figure 4A) inhibited firefly luciferase expression from pCMV-Luc-8xmiR-30(P) by ≤2-fold. Together, these data indicate that the northern analysis, presented in Figure 4A, provides an accurate picture of the level of biologically active miR-30 expressed in transfected 293T cells. The in vitro and in vivo data derived using pre-miR-30 mutants 1 to 7 (Figures 3 and 4) are summarized in Table 1, which also shows the predicted RNA structure adopted by each of these pre-miR-30 derivatives.
Exp5 can protect pre-miRNAs from exonucleolytic digestion
Previously, we have reported that knockdown of Exp5 expression using RNAi prevents the cytoplasmic expression of mature miRNAs, yet does not result in a nuclear accumulation of pre-miRNAs (5). These observations led us to propose that Exp5 might also function to stabilize pre-miRNAs in the nucleus. Analysis of the data summarized in Table 1 shows a good correlation between the ability of pre-miR-30 mutants to bind Exp5 in vitro and the level of expression of these same pre-miR-30 derivatives in transfected cells, as would be predicted if Exp5 binding indeed stabilized pre-miRNA expression.
As a test of the idea that formation of a pre-miRNA/Exp5/Ran-GTP complex protects pre-miRNAs from degradation by cellular exonucleases, we asked if Exp5 binding would be able to prevent the in vitro exonucleolytic digestion of pre-miR-30 by a bacterial exonuclease termed ExoT or RNase T. ExoT is a single-strand-specific 3′ to 5′ exonuclease that normally plays a role in the maturation of bacterial tRNAs, 5S rRNA and 23S rRNA (20). We would, therefore, predict that ExoT would trim the 2 nt 3′ overhang present on pre-miR-30. We used 32P-labeled pre-miR-30(19) (Figure 2) as a substrate here, as preliminary tests found that ExoT readily processed this RNA. As shown in Figure 5A, incubation of the pre-miR-30(19) probe with ExoT resulted in the rapid appearance of an RNA species that migrated slightly more rapidly than the 53 nt long starting material (compare lanes 1 and 5 with lanes 2–4). By comparison to RNA size markers, it was determined that the faster migrating species was 1 nt shorter than the starting substrate (data not shown). In contrast, in the presence of recombinant Exp5 and RanQ69L-GTP, ExoT cleavage of the input pre-miR-30(19) RNA was clearly inhibited, such that even after 40 min of incubation with ExoT, little or no faster migrating RNA was observed (Figure 5A, compare lanes 6–10). Exp5 did not impede ExoT processing of the pre-miR-30(12ΔUCL4) RNA (data not shown), a mutant with a vastly diminished affinity for Exp5 (Figure 1). Therefore, these data demonstrate that Exp5 binding can indeed protect RNA from exonucleolytic digestion.
Figure 5.
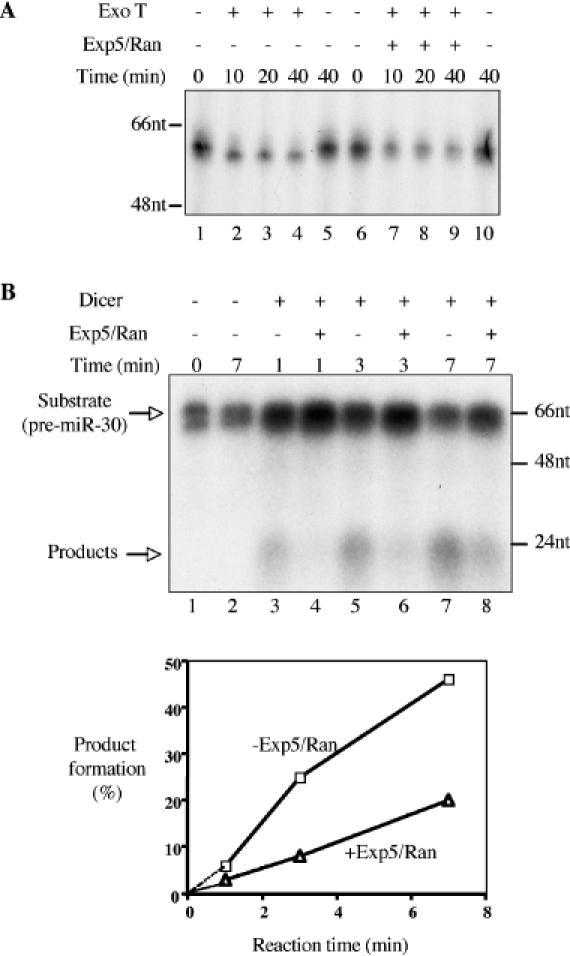
Inhibition of ExoT or Dicer cleavage of pre-miR-30 by Exp5 in vitro. (A) This assay measures the ability of ExoT to remove the 3′ overhang present on the pre-miR-30(19) RNA probe (see Figure 2) in the presence or absence of Exp5 and Ran-GTP. ExoT cleavage assays were performed according to Materials and Methods. For lanes 1, 5, 6 and 10, RNAs were directly mixed with 2× loading buffer (98% formamide, 10 mM EDTA, 0.1% bromophenol blue and 0.1% xylene cyanol) at the indicated time points, without going through extraction or precipitation. Lower RNA levels in lanes 2–4, 7–9 resulted from sample loss during extraction and precipitation. Shown on the left-hand side of the autoradiograph are DNA size markers. (B) Dicer was incubated with radiolabeled pre-miR-30 in the absence (lanes 3, 5 and 7) or presence (lanes 4, 6 and 8) of Exp5/RanQ69L-GTP. At the indicated time points, reactions were stopped and RNA isolated. Lane 1, pre-miR-30 without Dicer, no incubation at 37°C; lane 2, pre-miR-30 without Dicer, after incubation at 37°C. Positions of DNA markers are shown on the right-hand side of the autoradiograph. After quantification, the fraction of product formation was calculated as product intensity/(substrate intensity + product intensity) and is presented below the autoradiograph. Squares represent reactions in the absence of Exp5/Ran-GTP, while triangles represent reactions in the presence of Exp5/Ran-GTP.
The next step in miRNA biogenesis after nuclear export is cytoplasmic processing by Dicer, a member of the RNase III protein family that is believed to bind to the base of pre-miRNAs and then cleave 21–22 nt away on both sides of the pre-miRNA stem, thereby generating an ∼21 bp duplex intermediate bearing two 2 nt 3′ overhangs (3,7,21). If Exp5 binding blocks the ability of ExoT to access the base of the pre-miRNA stem, then one would predict that Exp5 binding might also inhibit Dicer processing of pre-miRNA precursors.
To test this hypothesis, we asked if Exp5 would inhibit processing of the WT pre-miR-30 RNA precursor by recombinant Dicer in vitro. As shown in Figure 5B, the addition of Dicer to pre-miR-30 resulted in the rapid processing of this 63 nt pre-miRNA into products ∼20 nt in size. However, in the presence of recombinant Exp5 and Ran-GTP, Dicer processing was inhibited by ∼70%. Ran-GTP alone had no effect on Dicer processing (data not shown). These data are, therefore, consistent with the hypothesis that Exp5 binding can protect pre-miRNAs from cleavage by cellular RNases. We note that the cytoplasmic hydrolysis of Ran-GTP to Ran-GDP is predicted to induce release of the pre-miRNA cargo from Exp5 in expressing cells, and the data presented in Figure 5B, therefore, argue that this release is, in fact, important for the subsequent processing of pre-miRNAs by cytoplasmic Dicer.
DISCUSSION
In this paper, we have analyzed the interaction between Exp5 and a representative 63 nt pre-miRNA export cargo derived from the human miR-30 gene. The results obtained are generally similar to the results reported previously by the analysis of the interaction of Exp5 with the ∼162 nt VA1 RNA encoded by adenovirus (17). This earlier report concluded that the nuclear export signal in VA1 consisted of a terminal ‘mini-helix’ of >14 bp bearing a bp 5′ nucleotide and a 3′ overhang of 3–8 nt. However, the data presented in this paper demonstrate that Exp5 binding to pre-miR-30 requires a somewhat longer terminal stem than that suggested by mutagenesis of the larger VA1 RNA and also indicate that the binding of pre-miR-30 by Exp5 is less sensitive to minor changes at the base of the RNA hairpin. These differences could result from the different experimental procedures used or could suggest the existence of additional cellular factors that modulate the efficiency of Exp5 binding by the VA1 or pre-miR-30 RNA.
The data presented in Figure 1 argue that pre-miR-30 binding by Exp5 requires a stem of ≥16 bp for detectable binding and ≥18 bp for high-affinity binding by Exp5. Deletion of the naturally occurring 2 nt bulge present in pre-miR-30 did not affect Exp5 binding unless the stem length was suboptimal, in which case deletion of the bulge reduced binding modestly (Figure 1). Modulation of the size of the terminal loop of pre-miR-30 had very little effect on Exp5 binding until reduced from the normal 15 nt size to 4 nt, when a significant effect was noted (Figure 2). This result is interesting in light of our previous observation that reduction in size of the terminal loop to 4 nt strongly inhibits miR-30 production from a longer, pri-miRNA precursor (18). The processing of miR-30 from a pri-miRNA precursor is predicted to require processing by Drosha to give the 63 nt pre-miRNA intermediate (3). The inhibitory effect of terminal loop deletion mutants on miR-30 production from pri-miRNA, but not pre-miRNA precursors, therefore, suggests that the terminal loop may form a part of the processing recognition signal for Drosha. Recent unpublished data from this laboratory are consistent with this hypothesis (data not shown).
Mutants designed to address the importance of the base of the pre-miR-30 stem for Exp5 binding showed that Exp5 could bind pre-miR-30 variants with 5 or 1 nt 3′ overhangs, or with a blunt end, as effectively as WT pre-miR-30 (Figure 3). However, 5 nt 5′ overhangs blocked Exp5 binding while a 2 nt 5′ overhang was also quite inhibitory, especially if no 3′ overhang was present (Figure 3). Therefore, while the 2 nt 3′ overhang naturally generated by Drosha cleavage of pri-miRNAs is clearly optimal for Exp5 binding, minor changes from this ideal are well tolerated, at least in the case of pre-miR-30. Our observation that specific binding of pre-miRNAs by Exp5 requires not only an appropriate structure at the base of the stem but also a stem of ≥18 bp suggests that protein:RNA contacts covering a large proportion of the pre-miRNA surface may be involved in mediating Exp5 recruitment.
We note that our analysis of the in vivo expression and activity of the stem base mutants analyzed for in vitro binding in Figure 3 is subject to the caveat that the pre-miR-30 RNAs analyzed are directly transcribed by RNA polymerase III, rather than processed from a longer RNA polymerase II transcript by Drosha, and therefore have an introduced terminal poly(U) stretch of ∼2 nt. As a result, these artificial RNAs, unlike authentic pre-miR-30, may be subject to binding by the cellular La protein (22). The protein La could, in principle, cause nuclear retention and/or compete with Exp5 for binding (7). In fact, nuclear export of the pre-miR-30 RNA transcribed from pSUPER-miR-30 appears to be somewhat less efficient than export of the WT pre-miR-30 derived by Drosha processing of the pri-miR-30 precursor transcribed from the previously described pCMV-miR-30 plasmid (data not shown). However, all the pre-miR-30 variants are predicted to contain the same 3′ end and, as discussed below, pre-miR-30 expression levels in vivo, in fact, correlate closely with the Exp5 binding affinity in vitro. Therefore, this possible caveat does not appear to be a major concern.
Analysis of the level of in vivo expression of mature miR-30 from the various stem base mutants (Figure 4A) was found to correlate closely with the ability of these mutants to inhibit the expression of an mRNA bearing miR-30 target sites (Figure 4B), thus arguing that the northern analysis presented in Figure 4A accurately measures the level of biologically active miR-30. However, the level of miR-30 expression (Figure 4A) does not fully correlate with the level of Exp5 binding for each pre-miR-30 mutant (Figure 3). In particular, we note that mutant 3, which binds Exp5 efficiently, gave rise to very low levels of mature miR-30, although good levels of the pre-miR-30 were observed (Figure 4A, lanes 7 and 8). Mutant 4 gave rise to clearly reduced levels of mature pre-miR-30 but readily detectable levels of mature miR-30. Finally, mutant 5 gave WT levels of pre-miR-30 but only moderate levels of mature miR-30 (Figure 4A, lanes 10–13). One possible explanation for this discrepancy is that mutant 3, bearing 2 nt 5′ and 3′ overhangs, and mutant 5, bearing a 5 nt 3′ overhang, are exported by Exp5 with good efficiency, but cytoplasmic processing by Dicer, which favors RNA substrates with 2 nt 3′ overhangs (21), is inefficient. Conversely, mutant 4, bearing a 2 nt 5′ overhang, may recruit the Exp5 export factor inefficiently but may then be a good substrate for cytoplasmic Dicer processing. Unfortunately, preliminary in vitro analysis has, so far, not been able to confirm this attractive hypothesis.
A striking observation reported in this paper is that pre-miRNA mutations that reduce affinity for Exp5 also reduce the steady-state level of pre-miR-30 expression in transfected human cells, suggesting that Exp5 may protect pre-miRNAs from degradation. Previous observations have hinted at such a role for Exp5. Thus, the RNAi-mediated knockdown of Exp5 expression inhibited mature miRNA production without causing any nuclear accumulation of the pre-miRNA precursor in human cells (5), and in Xenopus oocyte injection experiments, some pre-miRNAs with lower affinity for Exp5 showed signs of degradation in the nucleus when their export was blocked (7). Using ExoT as a model exonuclease (20), we observed that Exp5, in combination with Ran-GTP, is indeed able to strongly inhibit exonucleolytic digestion of a pre-miR-30 derivative (Figure 5A), presumably by blocking the access of ExoT to the 3′ overhang present on pre-miR-30. Moreover, we were able to demonstrate that Exp5 binding can significantly reduce the efficiency of pre-miRNA cleavage by Dicer (Figure 5B). Furthermore, overexpression of Exp5 enhanced the levels of pre-miR-30 in transfected cells (R. Yi and B. R. Cullen, unpublished data). These data, therefore, directly argue that Exp5 facilitates miRNA biogenesis by not only acting as the nuclear export factor for pre-miRNAs, but also by shielding pre-miRNAs from degradation prior to and possibly during export.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Dr Ian G. Macara (University of Virginia) for reagents used in this research, which was funded by the Howard Hughes Medical Institute and by grant GM071408 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Bartel D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell, 116, 281–297. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y., Jeon,K., Lee,J.-T., Kim,S. and Kim,V.N. (2002) MicroRNA maturation: stepwise processing and subcellular localization. EMBO J., 21, 4663–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y., Ahn,C., Han,J., Choi,H., Kim,J., Yim,J., Lee,J., Provost,P., Rådmark,O., Kim,S. and Kim,V.N. (2003) The nuclear RNase III drosha initiates microRNA processing. Nature, 425, 415–419. [DOI] [PubMed] [Google Scholar]
- 4.Zeng Y., Yi,R. and Cullen,B.R. (2003) MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl Acad. Sci. USA, 100, 9779–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi R., Qin,Y., Macara,I.G. and Cullen,B.R. (2003) Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev., 17, 3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohnsack M.T., Czaplinski,K. and Görlich,D. (2004) Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA, 10, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund E., Güttinger,S., Calado,A., Dahlberg,J.E. and Kutay,U. (2004) Nuclear export of microRNA precursors. Science, 303, 95–98. [DOI] [PubMed] [Google Scholar]
- 8.Grishok A., Pasquinelli,A.E., Conte,D., Li,N., Parrish,S., Ha,I., Baillie,D.L., Fire,A., Ruvkun,G. and Mello,C.C. (2001) Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell, 106, 23–34. [DOI] [PubMed] [Google Scholar]
- 9.Hutvágner G., McLachlan,J., Pasquinelli,A.E., Bálint,É., Tuschl,T. and Zamore,P.D. (2001) A cellular function for the RNA-interference enzyme dicer in the maturation of the let-7 small temporal RNA. Science, 293, 834–838. [DOI] [PubMed] [Google Scholar]
- 10.Ketting R.F., Fischer,S.E., Bernstein,E., Sijen,T., Hannon,G.J. and Plasterk,R.H. (2001) Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev., 15, 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohnsack M.T., Regener,K., Schwappach,B., Saffrich,R., Paraskeva,E., Hartmann,E. and Görlich,D. (2002) Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm. EMBO J., 21, 6205–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brownawell A.M. and Macara,I.G. (2002) Exportin-5, a novel karyopherin, mediates nuclear export of double-stranded RNA binding proteins. J. Cell Biol., 156, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calado A., Treichel,N., Müller,E.-C., Otto,A. and Kutay,U. (2002) Exportin-5-mediated nuclear export of eukaryotic elongation factor 1A and tRNA. EMBO J., 21, 6216–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwizdek C., Ossareh-Nazari,B., Brownawell,A.M., Evers,S., Macara,I.G. and Dargemont,C. (2004) Minihelix-containing RNAs mediate exportin-5-dependent nuclear export of the double-stranded RNA-binding protein ILF3. J. Biol. Chem., 279, 884–891. [DOI] [PubMed] [Google Scholar]
- 15.Gwizdek C., Bertrand,E., Dargemont,C., Lefebvre,J.-C., Blanchard,J.-M., Singer,R.H. and Doglio,A. (2001) Terminal minihelix, a novel RNA motif that directs polymerase III transcripts to the cell cytoplasm. J. Biol. Chem., 276, 25910–25918. [DOI] [PubMed] [Google Scholar]
- 16.Rutjes S.A., Lund,E., van der Heijden,A., Grimm,C., van Venrooij,W.J. and Pruijn,G.J.M. (2001) Identification of a novel cis-acting RNA element involved in nuclear export of hY RNAs. RNA, 7, 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwizdek C., Ossareh-Nazari,B., Brownawell,A.M., Doglio,A., Bertrand,E., Macara,I.G. and Dargemont,C. (2003) Exportin-5 mediates nuclear export of minihelix-containing RNAs. J. Biol. Chem., 278, 5505–5508. [DOI] [PubMed] [Google Scholar]
- 18.Zeng Y. and Cullen,B.R. (2003) Sequence requirements for microRNA processing and function in human cells. RNA, 9, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brummelkamp T.R., Bernards,R. and Agami,R. (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553. [DOI] [PubMed] [Google Scholar]
- 20.Zuo Y. and Deutscher,M.P. (2002) The physiological role of RNase T can be explained by its unusual substrate specificity. J. Biol. Chem., 277, 29654–29661. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H., Kolb,F.A., Jaskiewicz,L., Westhof,E. and Filipowicz,W. (2004) Single processing center models for human Dicer and bacterial RNase III. Cell, 118, 57–68. [DOI] [PubMed] [Google Scholar]
- 22.Wolin S.L. and Cedervall,T. (2002) The La protein. Annu. Rev. Biochem., 71, 375–403. [DOI] [PubMed] [Google Scholar]


