Abstract
In cancer and angiogenesis, coagulation-independent roles of tissue factor (TF) in cell migration are incompletely understood. Immobilized anti-TF extracellular domain antibodies induce cell spreading, but this phenomenon is epitope specific and is not induced by anti-TF 5G9. Spreading on anti-TF is β1 integrin–dependent, indicating functional interactions of the TF extracellular domain 5G9 epitope (a presumed integrin-binding site) and integrins. Recombinant TF extracellular domain supports adhesion of cells expressing αvβ3 or certain β1 integrin heterodimers (α3β1, α4β1, α5β1, α6β1, α9β1) and adhesion is blocked by specific anti-integrin antibodies or mutations in the integrin ligand-binding site. Although several studies have linked TF to cell migration, we here demonstrate that TF specifically regulates α3β1-dependent migration on laminin 5. Expression of TF suppresses α3β1-dependent migration, but only when the TF cytoplasmic domain is not phosphorylated. Suppression of migration can be reversed by 5G9, presumably by disrupting integrin interaction, or by the protease ligand VIIa, known to induce PAR-2–dependent phosphorylation of TF. In both cases, release of α3β1 inhibition is prevented by mutation of critical phosphorylation sites in the TF cytoplasmic domain. Thus, TF influences integrin-mediated migration through cooperative intra- and extracellular interactions and phosphorylation regulates TF's function in cell motility.
INTRODUCTION
Tissue factor (TF) is the cell surface receptor for the serine protease coagulation factor VIIa (VIIa; Ruf and Edgington, 1994). The complex of TF-VIIa activates the coagulation cascade, leading to thrombin generation, fibrin formation, and platelet activation. Local fibrin deposition is frequently observed in malignancy and TF plays an important role in cancer invasion and metastasis (Dvorak et al., 1992; Shoji et al., 1998). TF supports metastatic tumor dissemination (Mueller et al., 1992) by fibrin-dependent pathways (Palumbo et al., 2000), by aiding thrombin-dependent tumor cell survival (Fischer et al., 1995; Ruf and Mueller, 1996; Zain et al., 2000), and through signaling that involves the short TF cytoplasmic domain (Bromberg et al., 1995; Mueller and Ruf, 1998). TF is also found at the leading edge of invasive tumors (Fischer et al., 1999) and in angiogenic endothelial cells (Contrino et al., 1996). In vitro studies documented a close association of TF with cytoskeletal structures (Carson et al., 1994; Ott et al., 1998; Müller et al., 1999) and indicated potential roles in regulating cell motility, such as reverse endothelial cell migration of monocytes (Randolph et al., 1998), enhanced chemotactic fibroblast migration (Siegbahn et al., 2000), and tumor cell adhesion and migration on extracellular ligands for TF (Ott et al., 1998; Fischer et al., 1999). In TF cytoplasmic domain–deleted mice, we have recently provided evidence that the TF cytoplasmic domain can negatively regulate angiogenesis and endothelial sprouting (Belting et al., 2004). However, the molecular interactions by which TF is linked to the migratory machinery of cells remain unclear.
TF participates in multiple cellular effects either indirectly through downstream coagulation activation or directly through TF-associated proteases that may support tumor progression (Hembrough et al., 2003). TF is crucial for the efficiency and specificity of cell signaling by coagulation factors VIIa and Xa that cleave and activate the G-protein–coupled protease-activated receptors (PARs) 1 and 2 (Camerer et al., 2000; Riewald and Ruf, 2001). In part, TF-associated proteases may enhance cell migration by signaling through PARs, which activate small GTPase pathways of relevance for cell migration (Hartwig et al., 1995; DeFea et al., 2000). However, antibodies to TF or other ligands that lack proteolytic activity can support cell spreading, indicating that TF can influence integrin-dependent signaling by protease-independent mechanisms (Ott et al., 1998; Fischer et al., 1999). Whether the TF extracellular domain is important in these effects and whether the TF cytoplasmic domain contributes by signaling is incompletely understood.
A close connection of extracellular proteolysis and cell migration and invasion is well appreciated for cancer invasion, angiogenesis, and vascular remodeling (Mignatti and Rifkin, 1993; Werb, 1997; Carmeliet and Jain, 2000). The fibrinolytic system and matrix-metalloproteinase activation are localized to the leading edge of invasive tumors and orchestrate the complex interplay between matrix remodeling, integrin signaling, and cell motility. Although integrins themselves may directly bind proteases for specific targeting to degrade the extracellular matrix, receptors for proteases are also known to associate with integrins and regulate cell adhesion and migration. For example, the urokinase receptor (uPAR) can interact with a subset of β1 integrin heterodimers, αvβ3 and αMβ2, and supports cell migration by complex mechanisms involving integrin cross-talk and binding of the ligand urokinase to uPAR (Wei et al., 1996; Yebra et al., 1996, 1999; Aguirre Ghiso et al., 1999; Tarui et al., 2001, 2003; Wei et al., 2001). The glycosyl-phosphatidylinositol-anchored uPAR regulates integrin function, at least in part, by altering the association of integrins with caveolin-containing microdomains (Wei et al., 1999). Other examples of integrin-associated proteins are tetraspanin proteins that interact with a subset of integrin heterodimers and regulate their function by intracellular recruitment of signaling cascades (Hemler, 2001). In the present study, we provide novel evidence that TF, a receptor involved in protease binding, regulates α3β1 through cooperative interactions involving the TF intra- and extracellular domains.
MATERIALS AND METHODS
Proteins and Antibodies
Fibronectin, recombinant full-length TF, TF1–218, soluble TF fused with the leucine zipper domain of the transcription factor GCN4 (TFLZ), and isolated leucine zipper domain (LZP) were obtained as described (Ott et al., 1998; Doñate et al., 2000). Monoclonal antibodies (mABs) to TF were previously characterized (Ruf and Edgington, 1991; Ruf et al., 1991a; Huang et al., 1998). IgG and Fab fragments of the humanized version of 5G9 (CNTO859) were kindly provided by Dr. R. Jordan (Centocor, Malvern, PA). Laminin 5 was purified from the rat bladder carcinoma cell line 804G (Hintermann et al., 2001). The following mABs to integrin subunits were used: inhibitory anti-β1 mABs AIIB2 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) and P4C10, noninhibitory anti-β1 mABs P4G11 (Developmental Studies Hybridoma Bank) and TS2/16 (kindly provided by Drs. M. Hemler and T. Springer, Harvard, Cambridge, MA), anti-α1 (FB12, Chemicon, Temecula, CA), anti-α2 (AK7), anti-α3 mABs (anti-49c, PharMingen, San Diego, CA; P1B5; Chemicon; A3X8, kindly provided by Dr. M. Hemler), anti-α4 (P1H4, Chemicon), anti-α5 mABs (anti-49e, PharMingen; P1D6, Chemicon, KH72, kindly provided by Dr. K. Miyake, University of Tokyo), anti-α6 mABs (4F10, Chemicon; GoH3, PharMingen), anti-α9 (Y9A2), anti-αv AV-8 (kindly provided by Dr. B. Felding-Habermann, Scripps Research Institute, La Jolla, CA), anti-αvβ3 LM609 (kindly provided by Dr. D. Cheresh, Scripps), anti-αvβ5 (P1F6, Chemicon).
Cell Lines
HaCaT keratinocytes were a gift from Dr. N. Fusenig (German Cancer Research Center; Boukamp et al., 1988). J82 bladder carcinoma cells and Chinese hamster ovary (CHO) cells were obtained from the American Type Culture Collection (Manassas, VA). CHO cells selected for low levels of α5-expression (B2 variant; Schreiner et al., 1989) was stably transfected to express human α5, site-specific mutants of α5, or other α subunits, as described (Irie et al., 1995; Zhang et al., 1999). A7 melanoma cells (Cunningham et al., 1992; kindly provided by Dr. J. Hartwig, Harvard) were stably transfected with either full-length TF or cytoplasmic domain–deleted TF (truncated after His243). Stable clones were screened for TF expression and clones that expressed levels of the α3 integrin subunit similar to parental A7 cells were selected for further studies.
Adhesion Assays
Cell adhesion assays were performed as previously described (Tarui et al., 2001). Briefly, wells in 96-well Immulon-2 microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated with 100 μl of PBS (10 mM phosphate buffer, 0.15 M NaCl, pH 7.4) containing variable concentrations of proteins for 1 h at 37°C. Remaining protein-binding sites were blocked by incubating with 0.2% bovine serum albumin (Calbiochem, La Jolla, CA) for 1 h at room temperature. Cells (105 cells/well) in 100 μl of HEPES-Tyrode buffer (10 mM HEPES, 137 mM NaCl, 12 mM NaHCO3, 2.5 mM KCl, 0.1% glucose, 0.02% bovine serum albumin) supplemented with 2 mM MgCl2 were added to the wells and incubated at 37°C for 1 h, unless stated otherwise. After nonbound cells were removed by rinsing the wells with the same buffer, bound cells were quantified by measuring endogenous phosphatase activity. Antibodies were used at 250-fold dilution for ascites (as in the case of KH72) and at 10 μg/ml for purified IgG. Data are shown as mean ± SD of three independent experiments.
Staining Procedure for Adherent HaCaT Cells
To visualize the expression of TF relative to integrin heterodimers, HaCaT cells were replated onto laminin 5 or fibronectin coated coverslips for 3 h. During the last 30 min of the adhesion assay, cells were stained with Texas red–conjugated anti-TF 9C3 and—as indicated—with FITC conjugated anti-α2 (clone AK7, Chemicon), anti-α3 (A3X8), or anti-α6 (Clone 4F10, Chemicon). After rapid washes, cell were fixed and mounted for confocal microscopy. Samples were analyzed by sequential scanning, using a Bio-Rad MRC-600 confocal laser scanning microscope (Hercules, CA).
Migration Assays
Cell migration was analyzed using tissue culture–treated 24-well Transwell plates (Costar, Cambridge, MA) with polycarbonate membranes with 8-μm pore size. The lower side of the filter was coated with various concentrations of substrate. Coated filters were placed into a serum-free migration buffer (DMEM supplemented with 10 mM HEPES, 0.5% bovine serum albumin, and 1× penicillin-streptomycin), and cells (100 μl) suspended in the same buffer (8 × 105 J82 cells/ml, 1.2 × 106 HaCaT cells/ml; or 5 × 104 A7 cells/ml) were added to the upper chamber. Cells were incubated at 37°C in 5% CO2 for 20 h in the case of J82 migration on anti-TF antibodies and for 5 and 2 h in the case of HaCaT keratinocytes and A7 cells, respectively. Cells in the upper chamber were removed by wiping, and those that migrated to the lower surface of the filters were fixed and stained with 0.5% crystal violet in 20% ethanol and counted. The result in each well is the mean cell number of 4–8 randomly selected high-magnification microscopic fields from duplicate or triplicate experiments. In some experiments, anti-integrin mABs or anti-TF mABs (10–50 μg/ml) were incubated with cells for 15 min before seeding.
Adenoviral Transduction and Western Blot Analysis
The generation of adenoviruses expressing wild-type, cytoplasmic domain–deleted, and mutated TF has previously been described in detail (Dorfleutner and Ruf, 2003). A7 cells were plated 1 d before transduction. Virus was added to cells in complete medium for 4–6 h, washed twice with phosphate-buffered saline (PBS), and incubated in fresh complete medium for an additional 48 h. Cells were detached with trypsin/EDTA, quenched with serum-containing medium, washed, and resuspended in serum-free medium for adhesion and migration assays as above. An aliquot of cells was set aside at the time of plating to determine TF expression levels and phosphorylation status by Western-blotting, as described (Dorfleutner and Ruf, 2003). In experiments that analyzed the effect of VIIa on cell migration, cells were harvested with EDTA alone, in order to prevent desensitization of the trypsin-cleavable PAR2, the major signaling receptor of VIIa (Camerer et al., 2000; Riewald and Ruf, 2001; Belting et al., 2004). VIIa was added at 50 nM to the cells before plating for the haptotactic migration assay.
RESULTS
TF Antibody–induced Cell Migration and Cell Spreading Is Epitope Dependent
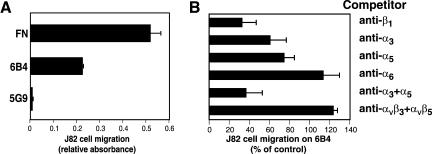
mABs to the TF extracellular domain fall in three majors functional classes (Table 1). 1) mABs (e.g., 6B4, 9C3) that block the VIIa binding site, 2) mABs (5G9) that do not overlap in the epitope with the previous and that do not prevent VIIa binding, but bind to the macromolecular substrate binding exosite in the carboxyl-terminal module of the TF extracellular domain, and 3) nonfunctional blocking mABs, like 10H10, which has a poorly defined epitope that sterically interferes with 5G9 binding (Ruf and Edgington, 1991; Ruf et al., 1991a; Huang et al., 1998). We have previously described that antibodies of the first group support cell migration and cell spreading (see also Figures 1A and 2; Ott et al., 1998). However, the anti-TF antibody 5G9 of the second class failed to support cell migration when immobilized as a haptotactic matrix (Figure 1A) as well as cell spreading (Figure 2). Haptotactic migration of J82 cells on anti-TF mAB 6B4 was inhibited by anti-β1 (Figure 1B), demonstrating integrin dependence. Migration was partially inhibited by anti-α3 or anti-α5 mAB. Anti-α6 and a combination of anti-αvβ3 and αvβ5 mABs did not reduce migration (Figure 1B). The combination of anti-α3 and α5 mABs was similarly effective as the inhibitory anti-β1 mAB (Figure 1B). This indicates that at least two β1 integrin heterodimers interact with TF and contribute to cell migration on immobilized 6B4 (anti-TF) antibody.
Table 1.
Functional characteristics of TF antibodies
| mAB | Epitope | Inhibitory properties |
|---|---|---|
| 6B4 or 9C3 | N-terminal module | Blocks VIIa binding site |
| 5G9 | C-terminal module | Blocks macromolecular substrate binding and integrin cross-talk |
| 10H10 | Steric hindrance/overlap with 5G9 | Noninhibitory |
Figure 1.
Support of TF-dependent cell migration is antibody epitope specific. (A) mAB 5G9 does not support cell migration of J82 cells. Migration was measured in a haptotactic assay overnight (Ott et al., 1998) on FN (10 μg/ml coating concentration), 6B4 or 5G9 (50 μg/ml coating concentration). Similar results were obtained in assays performed for shorter times. (B) Inhibition of J82 cell migration on anti-TF mAB 6B4. Migration was studied in an overnight migration assay with inhibitory mABs added at 50 μg/ml to the upper compartment of the Transwell chamber: mABs to β1 (AIIB2), α3 (P1B5), α5 (P1D6), α6 (GoH3), αvβ3 (LM609), and αvβ5 (P1F6) were added individually or in combination. Results are expressed relative to nonantibody treated cells migrating on 6B4 mAB in the same experiment.
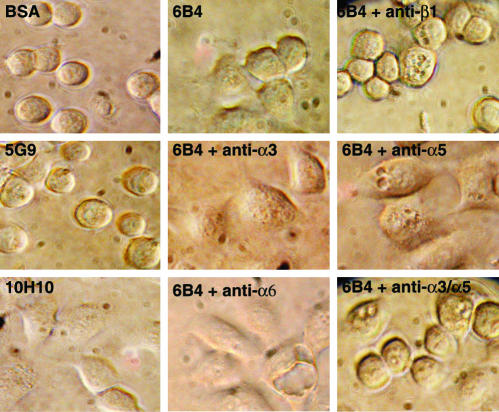
Figure 2.
Cell spreading of HaCaT keratinocytes on anti-TF involves several β1 integrins. Morphology of HaCaT human keratinocytes plated for 3 h on the indicted anti-TF antibodies (50 μg/ml coating concentration) or BSA. Inhibitory mABs to β1 (AIIB2), α3 (P1B5), α5 (P1D6), or α6 (GoH3) were added at 25 μg/ml before plating.
The effect of antibody treatment on cell spreading on anti-TF was further analyzed with HaCaT human keratinocytes, and the morphological changes were followed after plating on different anti-TF antibodies (Figure 2). Although mAB 6B4 and the nonfunction blocking mAB 10H10 induced cell spreading and a flattening of cell morphology, cells firmly attached to mAB 5G9, but retained a round morphology comparable to nonattached cells on plates blocked with BSA. Inhibitory antibodies to the integrin β1 subunit (AIIB2) as well as a combination of anti-α3 and α5 mABs prevented cell spreading on mAB 6B4 for the entire 3-h observation period. Anti-α6, anti-α3, or anti-α5 mAB on their own were not sufficient to inhibit cell spreading on 6B4. These data show that cell spreading is integrin-mediated, but the lack of spreading on anti-TF 5G9 indicates that certain regions of the TF extracellular domain are contributing to functional interactions with integrins.
Interaction of Specific Integrin Heterodimers with Immobilized TF
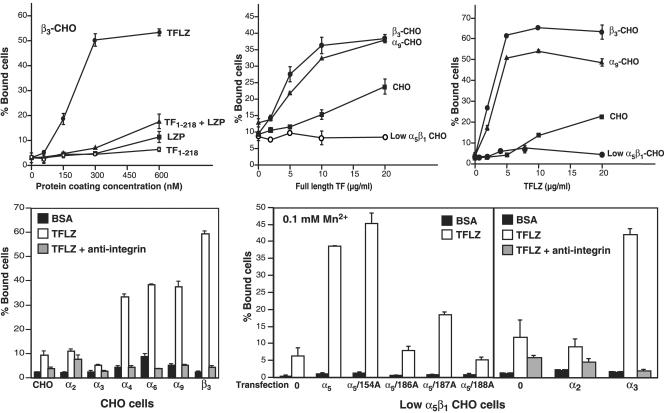
To explore whether the TF extracellular domain may interact with integrins, we used an adhesion assay using CHO K1 cells that are stably transfected with human α subunits to express integrin heterodimers with the hamster β1 subunit or are transfected with human β3 to form a heterodimer with hamster αv (Tarui et al., 2001). We have two forms of soluble TF extracellular domain. TF1–218 supports proteolytic activation of macromolecular substrates by the TF-VIIa complex about fivefold less efficiently than full-length TF solubilized with detergent (Ruf et al., 1991b). Another form of soluble TF (TFLZ) has a carboxyl-terminal leucine zipper homo-dimerization domain fused to the TF extracellular domain (Doñate et al., 2000). TFLZ has activity similar to that of full-length TF. This enhanced activity of TFLZ is independent of dimerization, indicating a slightly different conformation in the carboxyl-terminus that is involved in macromolecular substrate binding. Because mAB 5G9 competes with substrate binding in addition to its integrin-blocking activity (Ruf and Edgington, 1991), we reasoned that integrin interaction may similarly depend on the conformation of the TF extracellular domain.
Figure 3 shows that this was indeed the case. In the presence of 2 mM Mg2+, TFLZ promoted dose-dependent adhesion of CHO cells expressing αvβ3, but cells did not adhere to equimolar concentrations of TF1–218, the isolated LZP alone, or a combination of TF1–218 and LZP (Figure 3A). Thus, the conformation of the TF extracellular domain appears to be of importance for binding of integrin-expressing cells. Coating the plates with full-length TF (solubilized with CHAPS as a detergent) also supported cell adhesion of αvβ3 expressing CHO cells (Figure 3B). In addition, cells expressing specific β1 integrin heterodimers (e.g., α9β1) efficiently adhered to full-length TF. Adhesion of nontransfected CHO cells that express hamster α5β1 integrin was detectable, but a CHO-cell line selected for very low levels of α5β1 expression (Schreiner et al., 1989) did not bind full-length TF. These low level α5β1 cells express hamster αvβ5 and adhere to vitronectin, which indicates that αvβ5 probably does not bind to TF. TFLZ binds integrin similar to immobilized full-length TF (Figure 3C), demonstrating that TFLZ assumes the conformation of full-length TF and therefore is able to appropriately recapitulate the extracellular interactions of full-length TF that lead to integrin-dependent cell adhesion.
Figure 3.
Integrin interaction with immobilized TF. (A) Adhesion of αvβ3-expressing CHO cells to increasing concentrations of immobilized TFLZ or TF1–218. Cell adhesion was studied in the presence of 2 mM MgCl2 with human β3 integrin subunit–transfected CHO cells, as described (Tarui et al., 2001). Adhesion to TFLZ, TF1–218, the leucine zipper domain (LZP) present in TFLZ, or equimolar concentrations of TF1–218 and LZP is shown. (B and C) Adhesion of αvβ3 or α9β1 expressing CHO cells and untransfected CHO cells to full-length TF (B) or TFLZ (C) coated at the indicated concentrations. Adhesion was analyzed in the presence of 2 mM MgCl2. CHO cells express α5β1 and αvβ5. The lack of adhesion of CHO cells selected for very low α5β1 expression (Schreiner et al., 1989) shows that α5β1, but not αvβ5 interacts with TF. (D) Adhesion to TFLZ is blocked to the level of nonspecific binding to BSA by inhibitory antibodies specific for the respective human integrin α-subunits. In the case of untransfected CHO cells, anti-α5 mAB was used as the competitor. Adhesion was analyzed in the presence of 2 mM MgCl2. (E) Interaction of α3β1 and α5β1 with TF. TFLZ binding of low α5β1 CHO cells transfected with human α2, α3, α5, and site-specific mutants thereof was analyzed by adhesion assay in the presence of 0.1 mM MnCl2. The indicated human α-subunits and mutants were introduced into low α5β1 CHO cells by stable transfection. In the right panel specificity is shown by antibody blocking with anti-α5, anti-α2, and anti-α3 mAB, respectively.
Figure 3D shows that in the presence of 2 mM MgCl2, α4β1, α6β1, α9β1, and αvβ3, but not α2β1 or α3β1 interacted with TFLZ. In each case, inhibitory mABs reversed adhesion to background levels observed on BSA–coated plates. Adhesion of untransfected CHO cells was inhibited by anti-α5 antibodies, consistent with a specific interaction of α5β1 and immobilized TF. Adhesion was further studied in the presence of 0.1 mM MnCl2 to activate integrins. Low α5β1 CHO cells (Schreiner et al., 1989) did not appreciably bind to TFLZ in the presence of Mn2+ (Figure 3E). Wild-type human α5 or a nonfunction-blocking mutant (α5/154A) supported binding to TFLZ, whereas function-blocking mutations in the α5 integrin β-propeller structure (α5/186A, 187A, or 188A; Irie et al., 1995) reduced TF binding (Fig. 3E, left panel). These data suggest that, at least in part, the ligand binding pocket of integrin α subunits is involved in TF binding. TFLZ also served as a ligand for Mn2+-activated α3β1, but not for α2β1 (Figure 3E, right panel). These results suggest that TF is similar to uPAR in that both receptors have the potential to interact with a similar group of integrins (α4β1, α3β1, α5β1, α6β1, α9β1, and αvβ3) in dependence of divalent cations Mg2+ or Mn2+ (Tarui et al., 2001).
TF Regulates α3β1-dependent Cell Migration
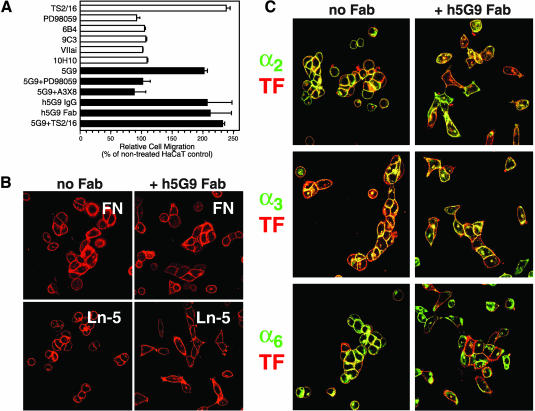
In A7 cells and HaCaT cells, migration on fibronectin is relatively specific for α5β1 and migration on laminin 5 is mediated by α3β1. HaCaT keratinocytes show basal α3β1-dependent migration on laminin 5, but migration can be stimulated by the activating anti-β1 mAB TS2/16 (Figure 4A). TS2/16-stimulated, but not basal, HaCaT migration on laminin 5 is blocked by anti-α3 mAB A3X8 as well as by PD98059, which prevents required ERK1/2 activation (Hintermann et al., 2001), but basal HaCaT migration on laminin 5 is not suppressed by PD98059 (Figure 4A). HaCaT keratinocytes also express relatively high levels of endogenous TF and the role of TF in haptotactic migration of HaCaT cells toward laminin 5 was examined by antibody blocking experiments. Ligation of TF by 6B4 or 9C3, mABs to the VIIa binding site in TF, or the noninhibitory anti-TF 10H10 did not influence migration on laminin 5 (Figure 4A). To test further to what extent the VIIa ligand-binding site on TF is involved in regulating integrin function, we added active site blocked VIIa (VIIai), which is catalytically inactive, but binds with very high affinity to TF (Dickinson and Ruf, 1997). Similar to mABs that block the VIIa binding site, VIIai did not enhance migration of HaCaT cells on laminin 5 (Figure 4A). Contrary to other anti-TF antibodies, 5G9 enhanced haptotactic migration of HaCaT cells toward laminin 5 (Figure 4A).
Figure 4.
Regulation of α3β1-dependent migration by TF. (A) Migration of HaCaT human keratinocytes on laminin 5 is regulated by TF. Migration was analyzed in the presence of the indicated anti-TF mABs 5G9, 6B4, 9C3, or 10H10 (50 μg/ml each), active site-inhibited VIIa (VIIai, 50 nM), migration-blocking mAB to α3β1 (A3X8, 20 μg/ml), IgG and Fab fragments of humanized 5G9 (h5G9; 50 μg/ml), activating anti-β1 mAB TS2/16 (40 μg/ml), or the MEK inhibitor PD98059 (50 μM). Black bars are used to highlight conditions where anti-TF 5G9 was added. (B) Adhesion of HaCaT cells treated for 30 min with or without 50 μg/ml h5G9 Fab fragments before plating on fibronectin or laminin 5 coated coverslips. Cells were stained with Texas red–conjugated anti-TF 9C3 for 30 min before washes and fixation. (C) Localization of TF and integrins in HaCaT keratinocytes plated on laminin 5 in the absence or presence of h5G9 Fab (50 μg/ml) for 3 h. Cells were labeled with FITC-labeled anti-α2 (AK7), anti-α3 (A3X8), or anti-α6 (1F10) together with Texas red–conjugated anti-TF 9C3.
The enhanced migration of HaCaT cells in the presence of mAB 5G9 was α3β1 dependent, because it was blocked by mAB A3X8, which specifically inhibits stimulated α3β1-dependent migration, but not α3β1-dependent adhesion (Hintermann et al., 2001). Blockade of ERK1/2 activation by the MEK inhibitor PD98059 suppressed mAB 5G9-enhanced migration (Figure 4A), consistent with the requirement for ERK activation in TS2/16-stimulated migration on laminin 5 (Hintermann et al., 2001). Interestingly, the activating anti-β1 mAB TS2/16 did not further stimulate 5G9-enhanced α3β1-dependent migration. This may indicate that inefficient migration of HaCaT keratinocytes is caused, in part, by a signaling cross-talk from TF to β1 integrins. To analyze whether bivalent ligation of TF with an IgG is necessary to interfere with the signaling cross-talk, IgG and Fab fragments of humanized mAB 5G9 (CNTO859) were compared. Fab fragments were equally effective in enhancing migration on laminin 5. Thus, occupancy of the 5G9 epitope in the TF extracellular domain, rather than bivalent ligation-dependent signaling, enhanced migration on laminin 5. HaCaT migration on fibronectin was not enhanced by 5G9, indicating that TF specifically suppresses the migratory function of α3β1, but not α5β1.
Treatment with h5G9 Fab did not appreciably influence cell adhesion to laminin 5 or fibronectin, as evidenced by adhesion assays and by similar numbers of cells detected after replating on specific matrix, followed by TF staining (Figure 4B). Figure 4C shows the localization of TF relative to the major α-subunits expressed by HaCaT cells that were plated for 3 h on laminin 5. TF partially colocalized with α2, α3, and α6 integrin (Figure 4C) and staining for α5 demonstrated low expression of this integrin heterodimer, consistent with flow cytometry data of Figure 5A. Although cell adhesion was not reduced in 5G9-treated cells, the cells appeared somewhat more spread out, consistent with a more migratory phenotype. The colocalization of TF with integrins at cell-cell junctions appeared less pronounced in 5G9-treated cells, but this may be indirectly caused by the change in cell morphology and there was no evidence for dominant α-subunit–specific changes in colocalization. Antibody clustering of integrins has been successful to demonstrate a specific colocalization of uPAR with α3 (Wei et al., 2001), but similar experiments did not provide evidence that TF preferentially associates with α2, α3, or α6 upon integrin ligation (unpublished data). The lack of preferential colocalization of TF with a specific β1 integrin heterodimer indicated that the regulation of a3β1-dependent migration was not simply a matter of competitive inhibition of extracellular ligand binding, but rather involved additional dynamically regulated intracellular signaling.
Figure 5.
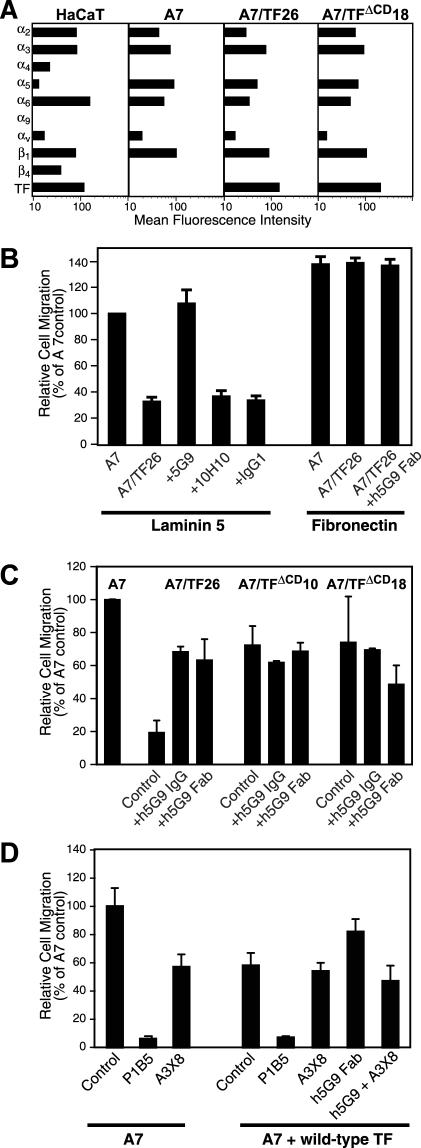
Expression of TF suppresses α3β1-dependent migration. (A) Characterization of integrin repertoire of A7 cells in comparison to HaCaT cells and determination of TF expression levels in HaCaT keratinocytes, A7 cells and A7 clones transfected with full-length TF (A7/TF26) or cytoplasmic domain–deleted TF (TFΔCD 18). Expression was analyzed by flow cytometry using mABs against TF (10H10), α1 (FB12), α2 (AK7), α3 (anti-49c), α4 (P1H4), α5 (anti-49e), α6 (4F10), α9 (Y9A2), and αv (AV-8). (B) Migration of A7 cells or A7 clones transfected with full-length TF (A7/TF26) on laminin 5 or fibronectin in the presence of the indicated mABs added to both compartments of the Transwell chamber at 50 μg/ml. mAb TIB115 was used as a noninteracting, isotype-matched control. A7 cell migration on laminin 5 was set to 100% for each separate experiment. (C) Role of the TF cytoplasmic domain in regulating α3β1-dependent migration. Migration was suppressed in full-length TF-expressing cells, but not in cells transfected with the cytoplasmic domain–deleted TF. Both, IgG and Fab fragments (50 μg/ml) of the humanized version of 5G9 (h5G9) reversed the suppressive effect of full-length TF, but had no effect in cells transfected with cytoplasmic domain–deleted TF. (D) Migration of A7 cell on laminin 5 is α3β1 dependent. Migration of A7 and A7 cells transiently transduced with wild-type TF was analyzed in the absence and presence of 20 μg/ml anti-α3 mAB P1B5 that blocks adhesion and migration to laminin 5 or A3X8 that only blocks stimulated migration.
TF Suppresses α3β1-dependent Migration in a TF Cytoplasmic Domain–dependent Manner
A7 cells which do not express significant TF levels were used to examine the influence of TF expression on α3β1-dependent migration on laminin 5. Figure 5A shows that A7 cells are similar to HaCaT cells in α2, α3, α6, α9, αv, and β1 integrin levels. However, A7 cells do not express α4 and β4 integrin subunits that are present on HaCaT cells. TF reconstituted A7 cells (A7/TF26) and A7 cells that were reconstituted with a TF mutant lacking the cytoplasmic domain (A7/TFΔCD18) did not deviate substantially in their integrin repertoire from A7 cells. TF-transfected A7 cells also expressed levels of TF similar to those found on HaCaT cells. Expression of TF (A7/TF26) suppressed α3β1-dependent migration on laminin 5 compared with TF-negative A7 cells (Figure 5B). Consistent with data in endogenous TF-expressing HaCaT cells, migration of A7/TF26 cells on laminin 5 was enhanced by 5G9, but not by the noninhibitory mAB 10H10 or isotype matched, nonbinding mAB. TF expression did not suppress migration on fibronectin and mAB 5G9 did not enhance fibronectin migration of A7/TF26 cells (Figure 5B). These data exclude the possibility that the 5G9 epitope is involved in interactions that nonselectively suppress cell migration and further emphasize that TF is specifically regulating migration controlled by the α3β1 integrin heterodimer.
The concept that TF is involved in a specific signaling cross-talk with integrin is supported by the finding that suppression of migration on laminin 5 required the TF cytoplasmic domain. The TF cytoplasmic domain is neither required for cell surface expression nor procoagulant function of TF (Mueller and Ruf, 1998). Figure 5C shows experiments with two independent A7 transfectants that express the TF mutant in which the cytoplasmic domain is deleted at expression levels of 25% (clone 10) and 180% (clone 18) relative to A7/TF26. No suppression of migration on laminin 5 was observed and addition of 5G9 IgG or Fab was without effect on cell migration with TF cytoplasmic domain–deleted clones (Figure 5C). Figure 5D documents that A7 cell migration on laminin 5 is α3β1 dependent, because anti-α3 mAB P1B5, which is known to block adhesion and migration on laminin 5 completely (Hintermann et al., 2001), also blocked migration of parental A7 or wild-type TF-expressing A7 cells. As expected, h5G9 Fab stimulated migration of TF-expressing cells was blocked by A3X8 that specifically inhibits stimulated α3β1-dependent migration. Interestingly, A3X8 blocked migration of untransfected A7 cells to levels observed in TF-transfected cells. These data indicate a direct role of TF in negatively regulating migration mediated by stimulated α3β1.
Suppression of α3β1-dependent Migration Is Regulated by TF Cytoplasmic Domain Phosphorylation
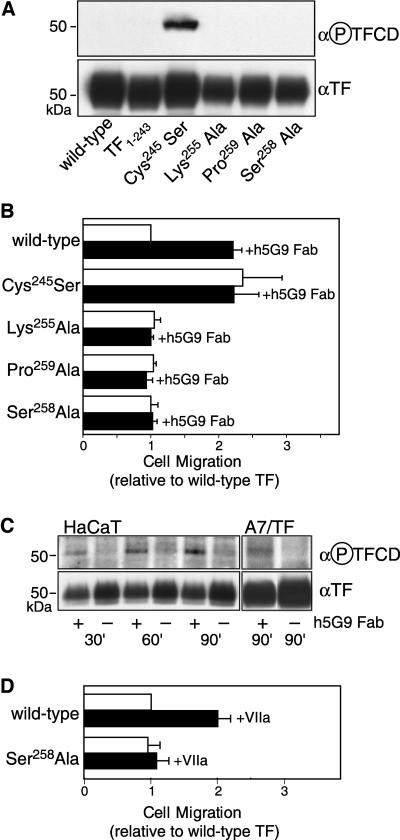
TF has two major cytoplasmic phosphorylation sites, Ser253 and Ser258. Phosphorylation of Ser258 is detectable by a phosphorylation-specific anti-TF antibody (Dorfleutner and Ruf, 2003). Phosphorylation of Ser253 by protein kinase C (PKC) is required for subsequent phosphorylation of Ser258 by an unidentified Pro-directed kinase. In addition to mutations of Ser258, TF phosphorylation is prevented by elimination of the PKC consensus recognition site Lys255 or the recognition site for the Pro-directed kinase at Pro259. TF phosphorylation is negatively regulated by palmitoylation of Cys245, and mutation of this residue results in enhanced phosphorylation relative to wild-type TF (Dorfleutner and Ruf, 2003). In addition, TF-VIIa signaling through PAR2 specifically triggers PKCα-dependent TF cytoplasmic domain phosphorylation (Ahamed and Ruf, 2004). A7 cells were transiently transduced with adenoviral constructs to exclude clonal artifacts of the single wild-type line that we analyzed so far. Adenoviral transduction achieved comparable expression levels of wild-type or cytoplasmic domain–deleted (TF1–243) TF, based on flow cytometry and Western blotting (Figure 6A). Also Cys245 to Ser, Lys255 to Ala, Pro259 to Ala, and Ser258 to Ala mutants showed similar expression levels, but only the Cys245 to Ser mutant was phosphorylated at the beginning of the migration assay as controlled with phospho-specific TF antibody in Western blotting (Figure 6A). TF phosphorylation was presumably induced by cell detachment with trypsin for the migration assay, because trypsin activates PAR2.
Figure 6.
Regulation of α3β1-dependent migration by TF cytoplasmic domain phosphorylation. A7 cells were transduced with adenoviral vectors to express full-length (wild-type), cytoplasmic domain–deleted (TF1–243), or the indicated cytoplasmic domain point mutants of TF. (A) Western blots with anti-TF and phosphorylation-specific TF cytoplasmic domain antibody (α-P-TFCD) of A7 cells transduced with the indicated contructs. Cells were pelleted at the time of plating for the migration assay and analyzed by Western blotting. (B) Migration of A7 cells transduced with the indicated TF constructs in the presence and absence of h5G9 Fab. Migration is expressed relative to wild-type TF-transduced A7 cells in the same experiment. Mean and SD are shown for repeat experiments (n = 3–4). (C) Cytoplasmic domain phosphorylation in HaCaT or TF-transduced A7 cells treated with 5G9 for the indicated times in solution. Immunoprecipitated TF was analyzed by Western blotting with anti-TF and phosphorylation-specific TF cytoplasmic domain antibody (α-P-TFCD). (D) Stimulation of α3β1-dependent migration by VIIa (50 nM) is dependent on phosphorylation of the TF cytoplasmic domain. Note that experiments with VIIa were carried out with EDTA lifted cells to avoid desensitization of VIIa signaling.
In TF-transduced A7 cells cytoplasmic domain–deleted TF did not suppress migration on laminin 5, but full-length TF reduced motility. Cys245 mutated TF did not suppress migration of A7 cells relative to untransfected cells, but cells expressing phosphorylation-deficient mutants (Lys255, Pro259, Ser258 to Ala) showed suppressed migration similar to that of wild-type TF transduced cells (Figure 6B). These data indicate that phosphorylated TF cannot suppress α3β1-dependent cell migration. To address whether 5G9 induces phosphorylation of wild-type TF to release the suppression of migration, we analyzed HaCaT and A7 cells that were treated in suspension with h5G9 Fab for various times. In treated cells, TF cytoplasmic domain phosphorylation was increased relative to controls (Figure 6C). However, phosphorylation was less pronounced in 5G9 mAb-treated cells than the degree of phosphorylation observed in cells expressing Cys245-mutated TF. This indicates that mAB 5G9 treatment transiently induced phosphorylation of TF or that only a subpopulation of TF became phosphorylated. Results with phosphorylation-deficient mutants of TF further demonstrate that phosphorylation of TF is required for mAB 5G9-enhanced migration on laminin 5. Contrary to the results with wild-type TF, mAB 5G9 did not reverse the suppression of laminin 5 migration by any of the phosphorylation deficient mutants (Figure 6B). These data provide evidence that 5G9 releases suppression of migration in dependence of TF cytoplasmic domain phosphorylation. The presented data are consistent with a model in which non-phosphorylated TF cytoplasmic domain suppresses α3β1-dependent cell migration, and that these effects are switched off by transient phosphorylation of Ser258.
TF-VIIa dependent signaling is a physiological agonist pathway by which the TF cytoplasmic domain can become phosphorylated (Ahamed and Ruf, 2004). Based on flow cytometry, A7 cells express PAR2, the relevant G-protein–coupled receptor that triggers TF cytoplasmic domain phosphorylation downstream of TF-VIIa signaling. To avoid desensitization of PAR2 by trypsin detachment, cells were recovered with EDTA to test whether VIIa can reverse the suppressive effect of TF on α3β1-dependent cell migration. Addition of VIIa to A7 cells transduced with wild-type, but not to cells expressing phosphorylation-deficient TF, reversed TF's suppression of A7 migration on laminin 5 (Figure 6D). These data demonstrate a pathway of potential physiological relevance by which TF-mediated suppression of integrin function can be reversed.
DISCUSSION
This study provides new insight into the role of TF in cell migration. Immobilized anti-TF antibodies are known to induce cell spreading and to promote haptotactic cell migration, but these effects are not observed with anti-TF mAB 5G9 to the macromolecular binding exosite in TF. The 5G9 epitope is not cryptic, because cells attach, but do not spread when plated on this antibody. Cell spreading on other anti-TF mABs is further shown to involve β1 integrin heterodimers, suggesting a possible interaction of the TF extracellular domain with integrins. In support of this concept, we find that CHO cells expressing certain β1 integrin heterodimers or αvβ3 interact with purified, immobilized recombinant TF extracellular domain or full-length TF. However, this interaction appears to be sensitive to subtle conformational changes, because one particular form of recombinant TF extracellular domain (TF1–218) inefficiently supported integrin binding. This recombinant protein has also poor macromolecular substrate binding properties, indicating that the macromolecular substrate, but not the VIIa binding site overlap with the region of presumed integrin interaction in the TF extracellular domain. In our previous study, we demonstrated that VIIa can bridge between TF and immobilized extracellular inhibitors to support cell spreading and migration (Fischer et al., 1999). The presented localization of the crucial TF extracellular epitope for integrin cross-talk distant from the VIIa binding sites explains how TF can support cell migration, while simultaneously binding its physiological ligand protease VIIa.
αvβ3-deficient epithelial cells spread on anti-TF mABs dependent on β1 integrins, but blockade of individual α-subunits of relevance for TF interaction failed to prevent cell spreading. However, a combination of anti-α3 and anti-α5 integrin antibodies achieved inhibition of cell spreading. Thus, in somewhat artificial assays of anti-TF induced cytoskeletal changes, multiple integrins appear to functionally interact with TF. It was important to further characterize the relevance of the integrin-TF interaction for cell migration on defined extracellular matrices. Considering the involvement of α3β1 and α5β1, we focused on these two integrin heterodimers. Expression of TF in A7 cells or anti-TF treatment of constitutively TF-expressing HaCaT cells did not influence cell migration on fibronectin. In contrast, expression of full-length TF in A7 cells suppressed α3β1-dependent migration on laminin 5 and anti-TF mAB 5G9 reversed this suppressive effect. In addition, mAB 5G9 enhanced migration of HaCaT cells on laminin 5, which otherwise typically show poor α3β1-dependent migration, unless stimulated with an activating anti-β1 mAB. These data suggest that 5G9 recognizes a TF extracellular domain epitope critically involved in the regulation of α3β1-dependent migration.
Although adhesion of integrin expressing CHO-cells to immobilized TF extracellular domain are consistent with a direct interaction of the TF extracellular domain with integrins, TF does not simply act as a competitive ligand, because inhibition of α3β1-dependent migration was absolutely dependent on the TF cytoplasmic domain. We altered the phosphorylation status of the TF cytoplasmic domain in a set of well characterized mutants (Dorfleutner and Ruf, 2003) and thus provide evidence that the TF cytoplasmic domain when it is not phosphorylated suppresses α3β1 and that TF looses its ability to inhibit α3β1-dependent migration upon phosphorylation. In addition, release of TF's suppression of α3β1-dependent migration by either mAb 5G9 or TF-VIIa signaling was dependent on phosphorylation of the cytoplasmic domain. The cross-talk of TF with α3β1 thus involves both TF's intra- and extracellular domains and is regulated by phosphorylation of the TF cytoplasmic domain.
The urokinase receptor is another protease-binding receptor that interacts with a similar repertoire of integrin subunits as implicated here for binding to TF (Aguirre Ghiso et al., 1999; Tarui et al., 2001; Wei et al., 2001; Tarui et al., 2003). Expression of the glycosyl-phosphatidylinositol–anchored uPAR typically enhances vitronectin adhesion and indirectly promotes cell adhesion through other integrins as well. In addition, coimmunoprecipitation of uPAR with α3β1 indicates that uPAR is in a fairly stable association with this integrin, which is mediated by a highly specific region in the β-propeller structure of the α-chain (Wei et al., 2001; Zhang et al., 2003). We find no evidence that TF alters adhesion or is in a similarly stable association with α3β1. A weaker association of TF with α3β1 may, in part be compensated for by the contribution of the TF cytoplasmic domain that is essential to regulate function of this integrin heterodimer. However, we cannot rule out additional complexity in how TF influences α3β1, because cell migration is regulated through complex networks between different β1 and β3 integrins (Hodivala-Dilke et al., 1998; Hintermann et al., 2001; Schwartz and Ginsberg, 2002).
The concept that the TF cytoplasmic domain acts as a negative regulator of relevant physiological processes has recently received validation by our finding that TF cytoplasmic domain–deleted mice (Melis et al., 2001) exhibit enhanced developmental and tumor angiogenesis (Belting et al., 2004). In addition, phosphorylated TF is specifically associated with neo-angiogenesis in diabetic eye diseases (Belting et al., 2004), providing collateral evidence in vivo that phosphorylation may release suppressive functions of the TF cytoplasmic domain and cause pathology. The present finding that epithelial cell migration is regulated by TF cytoplasmic domain phosphorylation may have implications for angiogenesis, if TF cytoplasmic domain signaling similarly regulates endothelial cell integrin function during sprouting. Integrins are the target for regulatory control in angiogenesis by soluble factors, including semaphorins (Serini et al., 2003) and tissue inhibitors of metalloproteinases (Seo et al., 2003). The presented results for TF add another potential facet to the complex regulation of angiogenesis by demonstrating that a transmembrane protease receptor can regulate integrin function dependent on interactions involving its cytoplasmic and extracellular domains.
The identified cross-talk of TF with integrins is of likely relevance for previous findings suggesting “noncoagulant” roles for TF in tumor cell biology, metastasis, and angiogenesis. Expression of TF in certain tumor cells results in cytoplasmic domain–dependent upregulation of vascular endothelial cell growth factor (VEGF; Zhang et al., 1994; Abe et al., 1999), although this effect appears to be cell type restricted (Bromberg et al., 1999). Loss of αvβ3 leads to upregulation of VEGF and enhanced angiogenesis in vivo (Reynolds et al., 2002). In analogy to the demonstrated signaling cross-talk with α3β1, TF may influence αvβ3 function by altering the ligated state of this integrin and thus VEGF levels in certain tumor cells. TF has also been shown to enhance platelet-derived growth factor (PDGF)-dependent chemotaxis through TF-VIIa mediated signaling (Siegbahn et al., 2000) and TF-VIIa signaling synergizes with PDGF in angiogenesis in TF cytoplasmic domain–deleted mice (Belting et al., 2004). Because integrin and growth factor signaling are connected (Schwartz and Ginsberg, 2002), localizing TF to integrins may help synergize TF-dependent and growth factor signaling pathways. The TF-VIIa complex signals through PAR2 (Camerer et al., 2000; Riewald and Ruf, 2002), a G-protein–coupled receptor suggested to play important roles in cell migration by scaffolding ERK1/2 to the leading edge of cells (DeFea et al., 2000; Ge et al., 2003). TF-VIIa signaling may thus trigger promigratory PAR2 signaling while simultaneously releasing integrin suppression through PAR2-mediated TF phosphorylation. This mechanism may lead to a context-dependent regulation of invasive and metastatic behavior. Transient interaction of integrins with the TF-VIIa complex may further inhibit coagulation by precluding the macromolecular substrate exosite. Thus, this model can explain how TF regulates cell migration in a truly coagulation-independent manner.
Acknowledgments
We thank Jennifer Royce, Cindi Biazak, Pablito Tejada, and Dave Revak for technical assistance. Drs. Hemler, Springer, Cheresh, Miyake, Felding-Habermann, and Jordan kindly provided antibodies, and Drs. Fusenig and Hartwig cell lines. We thank Drs. S. Shattil, Dr. V. Quaranta, and B. Mueller for providing critical comments on the manuscript. This work was supported by National Institutes of Health Grants HL 16411 (W.R.), 60742 (W.R.), and GM 47157 (Y.T.). During these studies, AD was the recipient of a predoctoral fellowship from the Austrian Society of Sciences. The Developmental Studies Hybridoma Bank was the source of several antibodies used in this study.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0640. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–09–0640.
Abbreviations used: mAB, monoclonal antibody; PAR, protease-activated receptor; PKC, protein kinase C; TF, tissue factor; TFLZ, recombinant TF extracellular domain with a carboxyl-terminal leucine zipper domain; TFPI, TF pathway inhibitor; uPAR, urokinase receptor; VIIa, coagulation factor VIIa.
References
- Abe, K. et al. (1999). Regulation of vascular endothelial growth factor production and angiogenesis by the cytoplasmic tail of tissue factor. Proc. Natl. Acad. Sci. USA 96, 8663-8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre Ghiso, J.A., Kovalski, K., and Ossowski, L. (1999). Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 147, 89-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahamed, J., and Ruf, W. (2004). Protease-activated receptor 2-dependent phosphorylation of the tissue factor cytoplasmic domain. J. Biol. Chem. 279, 23038-23044. [DOI] [PubMed] [Google Scholar]
- Belting, M. et al. (2004). Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat. Med. 10, 502-509. [DOI] [PubMed] [Google Scholar]
- Boukamp, P., Petrussevska, R.T., Breitkreutz, D., Hornung, J., Markham, A., and Fusenig, N.E. (1988). Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg, M.E., Konigsberg, W.H., Madison, J.F., Pawashe, A., and Garen, A. (1995). Tissue factor promotes melanoma metastasis by a pathway independent of blood coagulation. Proc. Natl. Acad. Sci. USA 92, 8205-8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg, M.E., Sundaram, R., Homer, R.J., Garen, A., and Konigsberg, W.H. (1999). Role of tissue factor in metastasis: functions of the cytoplasmic and extracellular domains of the molecule. Thromb. Haemost. 82, 88-92. [PubMed] [Google Scholar]
- Camerer, E., Huang, W., and Coughlin, S.R. (2000). Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. USA 97, 5255-5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet, P., and Jain, R.K. (2000). Angiogenesis in cancer and other diseases. Nature 407, 249-257. [DOI] [PubMed] [Google Scholar]
- Carson, S.D., Perry, G.A., and Pirruccello, S.J. (1994). Fibroblast tissue factor: calcium and ionophore induce shape changes, release of membrane vesicles, and redistribution of tissue factor antigen in addition to increased procoagulant activity. Blood 84, 526-534. [PubMed] [Google Scholar]
- Contrino, J., Hair, G., Kreutzer, D.L., and Rickles, F.R. (1996). In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat. Med. 2, 209-215. [DOI] [PubMed] [Google Scholar]
- Cunningham, C.C., Gorlin, J.B., Kwiatkowski, D.J., Hartwig, J.H., Janmey, P.A., Byers, H.R., and Stossel, T.P. (1992). Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255, 325-327. [DOI] [PubMed] [Google Scholar]
- DeFea, K.A., Zalevsky, J., Thoma, M.S., Déry, O., Mullins, R.D., and Bunnett, N. (2000). β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 148, 1267-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, C.D., and Ruf, W. (1997). Active site modification of factor VIIa affects interactions of the protease domain with tissue factor. J. Biol. Chem. 272, 19875-19879. [DOI] [PubMed] [Google Scholar]
- Doñate, F., Kelly, C.R., Ruf, W., and Edgington, T.S. (2000). Dimerization of tissue factor supports solution phase autoactivation of factor VII without influencing proteolytic activation of factor X. Biochemistry 39, 11467-11476. [DOI] [PubMed] [Google Scholar]
- Dorfleutner, A., and Ruf, W. (2003). Regulation of tissue factor cytoplasmic domain phosphorylation by palmitoylation. Blood 102, 3998-4005. [DOI] [PubMed] [Google Scholar]
- Dvorak, H.F. et al. (1992). Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann. NY Acad. Sci. 667, 101-111. [DOI] [PubMed] [Google Scholar]
- Fischer, E.G., Riewald, M., Huang, H.Y., Miyagi, Y., Kubota, Y., Mueller, B.M., and Ruf, W. (1999). Tumor cell adhesion and migration supported by interaction of a receptor-protease complex with its inhibitor. J. Clin. Invest. 104, 1213-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, E.G., Ruf, W., and Mueller, B.M. (1995). Tissue factor-initiated thrombin generation activates the signaling thrombin receptor on malignant melanoma cells. Cancer Res. 55, 1629-1632. [PubMed] [Google Scholar]
- Ge, L., Ly, Y., Hollenberg, M., and DeFea, K. (2003). A β-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2 induced chemotaxis. J. Biol. Chem. 278, 34418-34426. [DOI] [PubMed] [Google Scholar]
- Hartwig, J.H., Bokoch, G.M., Carpenter, C.L., Janmey, P.A., Taylor, L.A., Toker, A., and Stossel, T.P. (1995). Thrombin receptor ligation and activated Rac uncap actin filament barbed end through phosphoinositide synthesis in permeabilized human platelets. Cell 82, 643-653. [DOI] [PubMed] [Google Scholar]
- Hembrough, T.A., Swartz, G.M., Papathanassiu, A., Vlasuk, G.P., Rote, W.E., Green, S.J., and Pribluda, V.S. (2003). Tissue factor/factor VIIa inhibitors block angiogenesis and tumor growth through a nonhemostatic mechanism. Cancer Res. 63, 2997-3000. [PubMed] [Google Scholar]
- Hemler, M.E. (2001). Specific tetraspanin functions. J. Cell Biol. 155, 1103-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann, E., Bilban, M., Sharabi, A., and Quaranta, V. (2001). Inhibitory role of α6β4-associated erbB-2 and phosphoinositide 3-kinase in keratinocyte haptotactic migration dependent on α3β1 integrin. J. Cell Biol. 153, 465-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodivala-Dilke, K.M., DiPersio, C.M., Kreidberg, J.A., and Hynes, R.O. (1998). Novel roles for α3β1 integrin as a regulator of cytoskeletal assembly and as a trans-dominant inhibitor of integrin receptor function in mouse keratinocytes. J. Cell Biol. 142, 1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M., Syed, R., Stura, E.A., Stone, M.J., Stefanko, R.S., Ruf, W., Edgington, T.S., and Wilson, I.A. (1998). The mechanism of an inhibitory antibody on TF-initiated blood coagulation revealed by the crystal structures of human tissue factor, Fab 5G9 and TF-5G9 complex. J. Mol. Biol. 275, 873-894. [DOI] [PubMed] [Google Scholar]
- Irie, A., Kamata, T., Puzon-McLaughlin, W., and Takada, Y. (1995). Critical amino acid residues for ligand binding are clustered in a predicted β-turn of the third N-terminal repeat in the integrin α 4 and α 5 subunits. EMBO J. 14, 5550-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis, E., Moons, L., De Mol, M., Herbert, J.M., Mackman, N., Collen, D., Carmeliet, P., and Dewerchin, M. (2001). Targeted deletion of the cytosolic domain of tissue factor in mice does not affect development. Biochem. Biophys. Res. Commun. 286, 580-586. [DOI] [PubMed] [Google Scholar]
- Mignatti, P., and Rifkin, D.B. (1993). Biology and biochemistry of proteinases in tumor invasion. Physiol. Rev. 73, 161-195. [DOI] [PubMed] [Google Scholar]
- Mueller, B.M., Reisfeld, R.A., Edgington, T.S., and Ruf, W. (1992). Expression of tissue factor by melanoma cells promotes efficient hematogenous metastasis. Proc. Natl. Acad. Sci. USA 89, 11832-11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, B.M., and Ruf, W. (1998). Requirement for binding of catalytically active factor VIIa in tissue factor dependent experimental metastasis. J. Clin. Invest. 101, 1372-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, M., Albrecht, S., Gölfert, F., Hofer, A., Funk, R.H.W., Magdolen, V., Flössel, C., and Luther, T. (1999). Localization of tissue factor in actin-filament-rich membrane areas of epithelial cells. Exp. Cell Res. 248, 136-147. [DOI] [PubMed] [Google Scholar]
- Ott, I., Fischer, E.G., Miyagi, Y., Mueller, B.M., and Ruf, W. (1998). A role for tissue factor in cell adhesion and migration mediated by interaction with actin binding protein 280. J. Cell Biol. 140, 1241-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo, J.S., Kombrinck, K.W., Drew, A.F., Grimes, T.S., Kiser, J.H., Degen, J.L., and Bugge, T.H. (2000). Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 96, 3302-3309. [PubMed] [Google Scholar]
- Randolph, G.J., Luther, T., Albrecht, S., Magdolen, V., and Muller, W.A. (1998). Role of tissue factor in adhesion of mononuclear phagocytes to and trafficking through endothelium in vitro. Blood 92, 4167-4177. [PubMed] [Google Scholar]
- Reynolds, L.E., Wyder, L., Lively, J.C., Taverna, D., Robinson, S.D., Huang, X., Sheppard, D., Hynes, R.O., and Hodivala-Dilke, K.M. (2002). Enhanced pathological angiogenesis in mice lacking β3 and β5 integrins. Nat. Med. 8, 27-34. [DOI] [PubMed] [Google Scholar]
- Riewald, M., and Ruf, W. (2001). Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc. Natl. Acad. Sci. USA 98, 7742-7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewald, M., and Ruf, W. (2002). Orchestration of coagulation protease signaling by tissue factor. Trends Cardiovasc. Med. 12, 149-154. [DOI] [PubMed] [Google Scholar]
- Ruf, W., and Edgington, T.S. (1991). An anti-tissue factor monoclonal antibody which inhibits TF:VIIa complex is a potent anticoagulant in plasma. Thromb. Haemost. 66, 529-533. [PubMed] [Google Scholar]
- Ruf, W., and Edgington, T.S. (1994). Structural biology of tissue factor, the initiator of thrombogenesis in vivo. FASEB J. 8, 385-390. [PubMed] [Google Scholar]
- Ruf, W., and Mueller, B.M. (1996). Tissue factor in cancer angiogenesis and metastasis. Curr. Opin. Hematol. 3, 379-384. [DOI] [PubMed] [Google Scholar]
- Ruf, W., Rehemtulla, A., and Edgington, T.S. (1991a). Antibody mapping of tissue factor implicates two different exon-encoded regions in function. Biochem. J. 278, 729-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf, W., Rehemtulla, A., Morrissey, J.H., and Edgington, T.S. (1991b). Phospholipid independent and dependent interactions required for tissue factor receptor and cofactor function. J. Biol. Chem. 266, 2158-2166. [PubMed] [Google Scholar]
- Schreiner, C.L., Bauer, J.S., Danilov, Y.N., Hussein, S., Sczekan, M.M., and Juliano, R.L. (1989). Isolation and characterization of Chinese hamster ovary cell variants deficient in the expression of fibrinonectin receptor. J. Cell Biol. 109, 3157-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M.A., and Ginsberg, M.H. (2002). Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65-E68. [DOI] [PubMed] [Google Scholar]
- Seo, D.W., Li, H., Guedez, L., Wingfield, P.T., Diaz, T., Salloum, R., Wei, B.Y., and Stetler-Stevenson, W.G. (2003). TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell 114, 171-180. [DOI] [PubMed] [Google Scholar]
- Serini, G. et al. (2003). Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424, 391-397. [DOI] [PubMed] [Google Scholar]
- Shoji, M. et al. (1998). Activation of coagulation and angiogenesis in cancer. Immunohistochemical localization in situ of clotting proteins and vascular endothelial growth factor in human cancer. Am. J. Pathol. 152, 399-411. [PMC free article] [PubMed] [Google Scholar]
- Siegbahn, A., Johnell, M., Rorsman, C., Ezban, M., Heldin, C.-H., and Rönnstrand, L. (2000). Binding of factor VIIa to tissue factor on human fibroblasts leads to activation of phospholipase C and enhanced PDGF-BB-stimulated chemotaxis. Blood 96, 3452-3458. [PubMed] [Google Scholar]
- Tarui, T., Andronicos, N., Czekay, R.P., Mazar, A.P., Bdeir, K., Parry, G.C., Kuo, A., Loskutoff, D.J., Cines, D.B., and Takada, Y. (2003). Critical role of integrin α5β1 in urokinase (uPA)/urokinase receptor (uPAR, CD87) signaling. J. Biol. Chem. 278, 29863-29872. [DOI] [PubMed] [Google Scholar]
- Tarui, T., Mazar, A.P., Cines, D.B., and Takada, Y. (2001). Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J. Biol. Chem. 276, 3983-3990. [DOI] [PubMed] [Google Scholar]
- Wei, Y., Eble, J.A., Wang, Z., Kreidberg, J.A., and Chapman, H.A. (2001). Urokinase receptors promote β1 integrin function through interactions with integrin α3β1. Mol. Biol. Cell 12, 2975-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y., Lukashev, M., Simon, D.I., Bodary, S.C., Rosenberg, S., Doyle, M.V., and Chapman, H.A. (1996). Regulation of integrin function by the urokinase receptor. Science 273, 1551-1555. [DOI] [PubMed] [Google Scholar]
- Wei, Y., Yang, X., Liu, Q., Wilkins, J.A., and Chapman, H.A. (1999). A role for caveolin and the urokinase receptor in integrin-mediated adhesion and signaling. J. Cell Biol. 144, 1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb, Z. (1997). ECM and cell surface proteolysis: regulating cellular ecology. Cell 91, 439-442. [DOI] [PubMed] [Google Scholar]
- Yebra, M., Goretzki, L., Pfeifer, M., and Mueller, B.M. (1999). Urokinase-type plasminogen activator binding to its receptor stimulates tumor cell migration by enhancing integrin-mediated signal transduction. Exp. Cell Res. 250, 231-240. [DOI] [PubMed] [Google Scholar]
- Yebra, M., Parry, G.C.N., Stromblad, S., Mackman, N., Rosenberg, S., Mueller, B.M., and Cheresh, D.A. (1996). Requirement of receptor-bound urokinase-type plasminogen activator for integrin αvβ5-directed cell migration. J. Biol. Chem. 271, 29393-29399. [DOI] [PubMed] [Google Scholar]
- Zain, J., Huang, Y.-Q., Feng, X.-S., Nierodzik, M.L., and Karpatkin, S. (2000). Concentration-dependent dual effect of thrombin on impaired growth/apoptosis or mitogenesis in tumor cells. Blood 95, 3133-3138. [PubMed] [Google Scholar]
- Zhang, F., Tom, C.C., Kugler, M.C., Ching, T.T., Kreidberg, J.A., Wei, Y., and Chapman, H.A. (2003). Distinct ligand binding sites in integrin α3β1 regulate matrix adhesion and cell-cell contact. J. Cell Biol. 163, 177-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X.P., Puzon-McLaughlin, W., Irie, A., Kovach, N., Prokopishyn, N.L., Laferte, S., Takeuchi, K., Tsuji, T., and Takada, Y. (1999). α3β1 adhesion to laminin-5 and invasin: critical and differential role of integrin residues clustered at the boundary between α 3 N-terminal repeats 2 and 3. Biochemistry 38, 14424-14431. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Deng, Y., Luther, T., Müller, M., Ziegler, R., Waldherr, R., Stern, D.M., and Nawroth, P.P. (1994). Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J. Clin. Invest. 94, 1320-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]