Abstract
We used transcription profiling in Candida albicans to investigate cellular regulation involving cAMP. We found that many genes require the adenylyl cyclase Cdc35p for proper expression. These include genes encoding ribosomal subunit proteins and RNA polymerase subunit proteins, suggesting that growth could be controlled in part by cAMP-mediated modulation of gene expression. Other genes influenced by loss of adenylyl cyclase are involved in metabolism, the cell wall, and stress response and include a group of genes of unknown function that are unique to C. albicans. The profiles generated by loss of the adenylyl cyclase regulator Ras1p and a downstream effector Efg1p were also examined. The loss of Ras1p function disturbs the expression of a subset of the genes regulated by adenylyl cyclase, suggesting both that the primary role of Ras1p in transcriptional regulation involves its influence on the function of Cdc35p and that there are Ras1p independent roles for Cdc35p. The transcription factor Efg1p is also needed for the expression of many genes; however, these genes are distinct from those modulated by Cdc35p with the exception of a class of hyphal-specific genes. Therefore transcription profiling establishes that cAMP plays a key role in the overall regulation of gene expression in C. albicans, and enhances our detailed understanding of the circuitry controlling this regulation.
INTRODUCTION
cAMP is an important regulatory molecule in both prokaryotes and eukaryotes. In fungi this molecule has been implicated in a variety of cellular processes. For example, in Magnaporthe grisea cells with mutations in the gene encoding adenylyl cyclase have a reduced vegetative growth rate, are sterile, and are defective in forming appresoria and thus are unable to infect susceptible rice leaves (Choi and Dean, 1997). In Ustilago maydis, mutants of adenylyl cyclase cause constitutively filamentous growth, (Gold et al., 1994), whereas strains with defects in the gene encoding the regulatory subunit of protein kinase A fail to induce tumors in plants (Gold et al., 1997). In Cryptococcus neoformans, the cAMP signaling pathway regulates several important cellular processes including capsule production, melanin formation, mating, and virulence (Alspaugh et al., 1997). In Neurospora crassa, cAMP regulates morphology, conidiation, mating, and stress responses (Lengeler et al., 2000), whereas in Schizosaccharomyces pombe cAMP signaling plays a role in mating, sporulation, gluconeogenesis, and entry into stationary phase (D'Souza and Heitman, 2001).
Perhaps the best studied fungal cAMP signaling network is that of the model eukaryote Saccharomyces cerevisiae (D'Souza and Heitman, 2001), where cAMP is essential for growth and regulates nutrient sensing, stress responses, and pseudohyphal differentiation. Adenylyl cyclase, encoded by the CDC35/CYR1 gene, appears primarily regulated by GTPases. These regulators include a pair of Ras homologues, encoded by RAS1 and RAS2 (Toda et al., 1985), and a Gα subunit encoded by GPA2 (Kubler et al., 1997; Colombo et al., 1998). Loss of adenylyl cyclase activity or loss of Ras function is lethal to the cell, with cells arresting at the G1 phase of the cell cycle in a manner analogous to cells that have been nutrient starved (Toda et al., 1985). A primary target of the yeast adenylyl cyclase is the cAMP-dependent protein kinase (PKA), made up of a regulatory subunit encoded by BCY1 (Matsumoto et al., 1982) and a catalytic subunit encoded by three genes: TPK1, TPK2, and TPK3 (Toda et al., 1987). Downstream of the kinase are a number of transcription regulators. These include the modulators of the stress response pathway Msn2p/Msn4p (Smith et al., 1998) and Sko1p (Pascual-Ahuir et al., 2001) as well as Gis1p involved in postdiauxic shift regulation (Pedruzzi et al., 2000) and Sfl1p (Conlan and Tzamarias, 2001) and Sok2p (Ward et al., 1995) involved in pseudohyphal development.
A regulatory system involving cAMP that appears related to the S. cerevisiae network has been identified in the fungal pathogen Candida albicans. Elements involved in the regulation of C. albicans cellular functions by cAMP include Cdc35p, the adenylyl cyclase that contributes to vegetative growth and is essential for yeast to hypha morphogenesis and virulence of C. albicans (Rocha et al., 2001), and Ras1p, a GTPase required for the formation of hyphae but not pseudohyphae (Feng et al., 1999; Leberer et al., 2001). In addition, there are Tpk1p and Tpk2p, the catalytic subunits of the cAMP-dependent protein kinase, which have redundant functions in growth and stress responses but exhibit functional differences in morphogenesis depending on environmental conditions (Bockmühl et al., 2001; Cloutier et al., 2003). Other components include Pde2p, the high-affinity phosphodiesterase (Bahn et al., 2003), and Efg1p, a transcription factor whose function is apparently controlled, at least in part, by cAMP-dependent protein kinase–directed phosphorylation (Bockmühl and Ernst, 2001).
Although many elements of the cAMP signaling network in C. albicans have been identified and disrupted, the overall structure and regulatory connections of the system are not well understood. Genetic studies are complicated by the diploid nature of the cells, and epistasis experiments involving protein overexpression have led to conflicting observations (Chen et al., 2000; Bockmühl et al., 2001; Leberer et al., 2001). Although Ras1p is implicated in both the MAP kinase and adenylyl cyclase signaling pathways controlling hyphal development (Leberer et al., 2001), loss of Ras function does not have as profound an effect on hyphal formation as does loss of adenylyl cyclase (Feng et al., 1999; Rocha et al., 2001). In S. cerevisiae there are many transcription factors acting downstream of Cdc35p, whereas in C. albicans only Efg1p has so far been convincingly identified as a downstream target of PKA activity (Liu, 2001). The observation that the highly similar adenylyl cyclase proteins of S. cerevisiae and C. albicans provide an essential function in one organism and not in the other suggests that it is the functions of the downstream targets that determine whether cAMP formation is required for viability (Rocha et al., 2001).
To further the understanding of the role of cAMP in C. albicans, we have used transcription profiling to investigate the consequences of the absence of adenylyl cyclase and other key network components. We have studied deletion mutants of CDC35, RAS1, and EFG1 under conditions that direct either yeast or hyphal form growth. This study provides the first comprehensive look at the transcriptional consequences of the deletion of adenylyl cyclase function in a eukaryotic cell and develops the relationship among the components of this regulatory network and its downstream targets.
MATERIALS AND METHODS
Strains and Growth Conditions
The C. albicans strains used in this study were the wild-type clinical isolate SC5314 (Gillum et al., 1984), the Caras1Δ CDH107 (ura3/ura3 caras1::hisG-URA3-hisG/caras1::hisG; Leberer et al., 2001), the Cacdc35Δ CR216 (ura3/ura3 cacdc35::hisG-URA3-hisG/cacdc35::hisG; Rocha et al., 2001), and the efg1Δ HLC52 (ura3/ura3 efg1::hisG-URA3-hisG/efg1::hisG; Lo et al., 1997). Overnight cultures were inoculated from a fresh colony and grown in 1% yeast extract, 2% peptone, 2% dextrose (YPD) medium (pH 6.2–6.5) at 30°C. Overnight cultures were diluted to an OD600 of 0.1 in YPD or YPD + 10% fetal bovine serum (FBS) and grown at 30 or 37°C, respectively, to an OD600 of ∼0.8 (3 generations); this was ∼4–5 h for the wild-type, efg1, and ras1 strains and 8–9 h for the cdc35 strain. Cultures were harvested by centrifugation at 3000 × g for 4 min, and the pellet rapidly frozen in a dry ice/ethanol bath. FBS was from Invitrogen (Carlsbad, CA) and was incubated at 56°C for 30 min before use.
Microarrays: RNA Extraction and Labeling
The microarrays used in this study contained 6002 putative open reading frames based on the preliminary sequence produced by the Stanford Genome Technology Center. These are described in more detail by Nantel et al. (2002) and at http://www.bri.nrc.gc.ca/business/microarraylab/index_e.html. A comprehensive annotation of the C. albicans genome has recently been made available at http://candida.bri.nrc.ca. Isolation of mRNA, labeling, and hybridization of the microarrays were performed as described (Nantel et al., 2002). Transcription profiles for each condition represent the average of at least 4–9 independent hybridizations. These include dye-swap hybridizations (Cy3/Cy5 and Cy5/Cy3) from at least three independently produced RNA preparations.
Data Analysis
Quantitation and normalization of DNA microarrays was performed as described (Enjalbert et al., 2003). To facilitate data interpretation and the number of transcripts that are presented in this article, we used the “one class” algorithm from the Significance Analysis of Microarrays (SAM) package (http://www-stat.stanford.edu/~tibs/SAM/) to isolate genes whose transcripts were significantly modulated by at least 1.4-fold and had a false discovery rate <5%. This dataset was combined with transcription profiles from the yeast-to-hyphae transition induced by serum at 37°C (Nantel et al., 2002; Lee et al., 2004); although we used the average fold variation from both articles, we only used the list of hyphal-modulated genes that were identified in the earlier publication because the results obtained in Lee et al. (2004) were produced on a later version of the Candida array that contained additional genes. Most of the analysis was performed on a collection of 1168 genes whose transcript levels were significantly modulated under at least one of the conditions. Visualization and analysis of the transcript profiles and correlations with signaling pathways in S. cerevisiae was performed in GeneSpring (Silicon Genetics, Redwood City, CA) as described (Enjalbert et al., 2003). Principal Components Analysis was performed in Matlab (The Mathworks, Natick, MA). All of the data can be obtained from http://cbr-rbc.nrc-cnrc.gc.ca/genetics/cAMP/.
Phenotypic Analysis
Strains were tested for Calcofluor White (CFW) (Sigma, Oakville, Ontario, Canada) sensitivity by diluting overnight cultures to an OD600 of 1.0 and plating 3-μl aliquots of 1:10 dilutions on YPD plates containing from 0 to 50 μg/ml CFW. The plates were incubated at 30°C and monitored for 4 d. Sensitivity of the strains to zymolyase was tested by diluting exponentially growing cells to an OD600 of 0.5 in 10 mM Tris-HCl (pH 7.5) containing 0 or 100 μg/ml Zymolyase 100T (ICN, Montreal, Quebec, Canada). The cells were kept at 30°C with shaking and the OD600 was monitored over a period of 2.5 h. For heat shock, early log phase cells grown at 30°C were put in a 50°C waterbath for 0, 10, 20, 30, or 60 min, and 3 μl of a 1:10 dilution of the cells was plated on YPD, grown at 30°C, and monitored for 3 d. For osmotic sensitivity, cells were grown to early log phase and 1:10 dilutions were spotted onto YPD plates containing 1.5 M NaCl. The plates were incubated at 30°C and monitored for 3 d.
RESULTS
In C. albicans, previous studies have shown that loss of components in the cAMP regulatory circuit can have significant effects on growth and morphogenesis (Supplementary Figure 1) as well as on virulence (Whiteway, 2000). When cells that lack CDC35 are grown in conditions generating the yeast form, they tend to aggregate and exhibit a significantly reduced rate of proliferation relative to wild-type cells. In particular, the deletion of adenylyl cyclase blocked the serum induced yeast-to-hyphal transition (Rocha et al., 2001), a process characterized by significant changes in gene expression (Nantel et al., 2002). Therefore we asked both whether cAMP played a role in general control of gene expression, and more specifically in the transcriptional regulation of C. albicans morphological transitions. Deletion mutants of RAS1 and EFG1, two other components of the cAMP regulon, show phenotypes distinct from that of the adenylyl cyclase null mutants. The Ras1 mutant cells show a moderate reduction in the rate of cell proliferation, but do not appear to have any defects in cellular morphology while growing in the yeast form, whereas under hyphal-inducing conditions the cells fail to make true hyphae but can still grow as pseudohyphae (Feng et al., 1999; Leberer et al., 2001). Deletion mutants of EFG1, an effector of the pathway, grow at a normal rate but exhibit somewhat elongated rod-like cells in either yeast or hyphal growth conditions (Lo et al., 1997; Stoldt et al., 1997). Therefore we asked whether these differences were reflected in distinct transcription patterns in these mutant cells.
Transcription Profiling and Cluster Analysis
We initially measured the transcriptional consequences of cells growing with and without adenylyl cyclase. Gene expression profiles of a disruption mutant strain of CDC35 were compared with profiles of wild-type cells during yeast form growth conditions (YPD at 30°C) and also under hyphal inducing conditions (YPD + serum at 37°C). In addition, the transcription profiles of cells undergoing the yeast to hyphal switch were measured by comparing mRNA from the mutant or wild-type cells grown in YPD at 30°C to mRNA from the same cells grown in YPD at 37°C + serum. These profiles were compared with those generated from two other strains containing mutations in genes encoding other components of the cAMP pathway, namely a regulator Ras1p, and an effector Efg1p, under the conditions described above. The complete dataset can be obtained at http://cbr-rbc.nrc-cnrc.gc.ca/genetics/cAMP/.
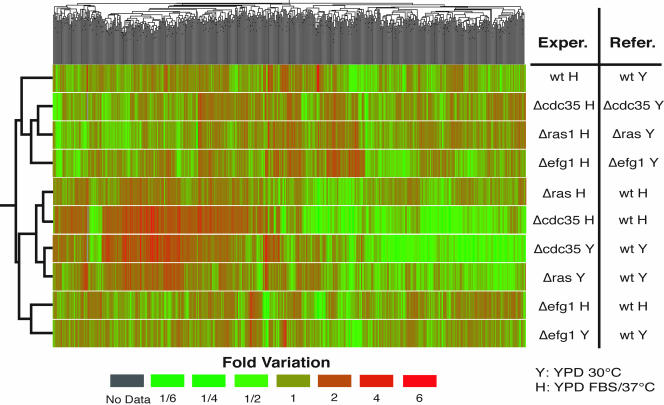
To facilitate presentation of our results, we selected 1168 genes that showed a statistically significant variation in transcript abundance (see Materials and Methods for details) under one of the conditions measured in this study or during the yeast to hyphae transition induced by serum and high temperature in the wild type (Nantel et al., 2002). A hierarchical cluster analysis of the transcript profiles for all experimental conditions in all strains is shown in Figure 1. In such an analysis, genes (X axis) and comparisons (Y axis) that show a similar profile are located closer to each other. Dendrograms serve to represent the extent of profile similarities.
Figure 1.
Cluster analysis of transcription profiles. We selected 1168 transcripts that exhibited a statistically significant variation in abundance of at least 1.4-fold and had a false discovery rate <5% (see Materials and Methods for details). These were separated by two-dimensional hierarchical clustering (Eisen et al., 1998) that groups genes and experiments with similar profiles. Normalized fluorescence ratios between the experimental and reference samples are represented as a green-to-red color scale. Similarity between each gene's transcription profile is represented by the horizontal dendrogram, whereas the vertical dendrogram represents the similarity between experiments.
Among the mutant cells undergoing the yeast to hyphal transition, the cdc35 and ras1 strains clustered closely together and were separate from the profile of the efg1 mutant. In addition, the cdc35 and ras1 profiles were linked together for both yeast and hyphal growth, whereas the efg1 mutant clustered with itself under both growth conditions. Thus under all conditions examined, the ras1 and cyclase profiles are in the same dendrogram subbranch, whereas the profile of the efg1 mutant is distinctly different. Similar results were observed after the separation of each experimental profile with a principal components analysis (PCA), a different classification method that positions each of the comparisons on a three-dimensional graph (Supplementary Figure 2). Morphologically, the three mutants were distinct when growing in YPD at 30°C, and the efg1 and cdc35 mutants both remained nonhyphal when growing at 37°C in the presence of serum. Thus the transcription profiling provides a different picture of the relationships among the elements than did the cellular morphology.
Effects of cAMP on Gene Expression.
When cells of the cdc35 mutant were grown in the yeast form condition and compared with wild-type cells, differences in almost 600 transcripts were revealed. These differences included genes whose mRNA levels were either reduced or elevated in the absence of adenylyl cyclase function (Figure 2A). Among the genes that require CDC35 for their normal transcript levels in yeast cells are those encoding a majority of ribosomal proteins for both the large and small subunits as well as genes for subunits of the RNA polymerase holoenzymes (Supplementary Figure 3A). Another large group of genes whose message abundance is reduced by the loss of CDC35 are associated with metabolic pathways such as the TCA cycle, pyrimidine metabolism, and heme and sterol synthesis (Supplementary Figure 3A). This reduced expression of genes for the transcriptional and translational machinery as well as for central metabolic pathways correlates well with the reduced growth rate exhibited by these cells.
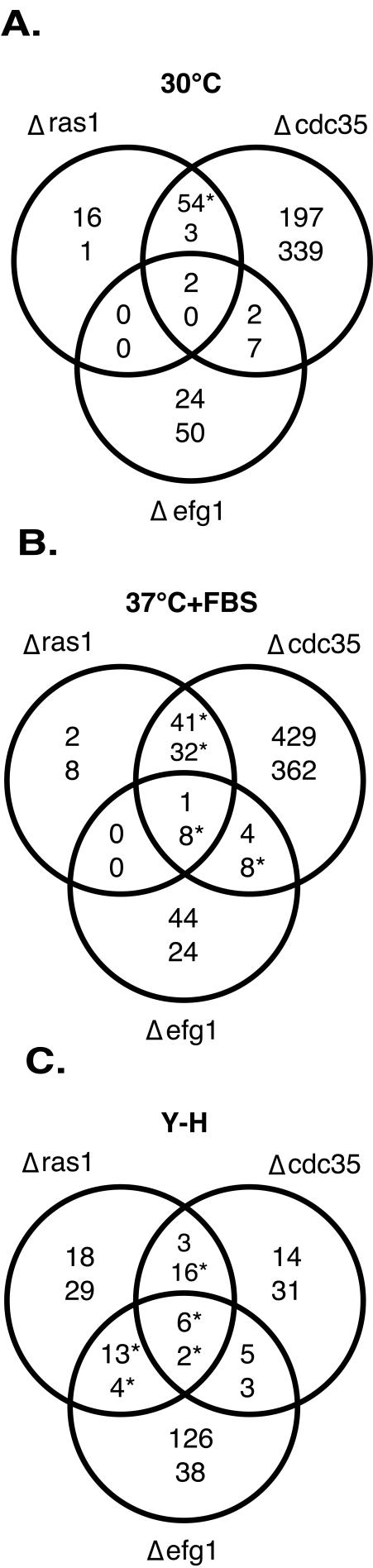
Figure 2.
Comparison of differentially expressed transcripts in the mutants with the wild type. The Venn diagrams show the overlap between groups of genes that show increased (top number) and decreased (bottom number) transcript levels in various experiments. Effects of the ras1, cdc35, and efg1 mutations when compared with the wild type under (A) yeast form conditions, (B) hyphal inducing conditions, or (C) the change in transcript abundance during the yeast-to-hyphal switch in ras1, cdc35, and efg1 mutants (these numbers refer to increases or decreases in the hyphal condition). Statistically significant (p < 10-10) overlaps between gene lists are indicated with an asterisk (*).
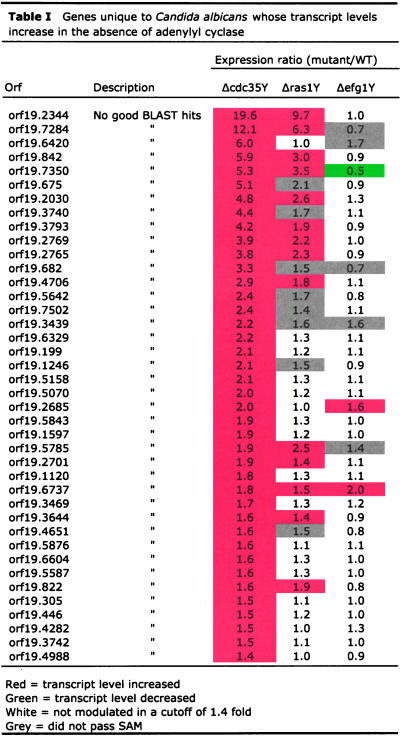
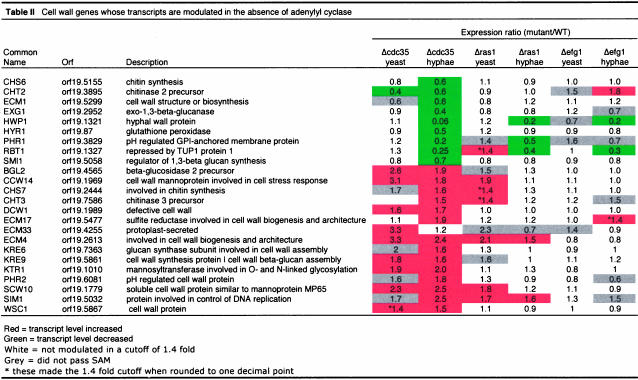
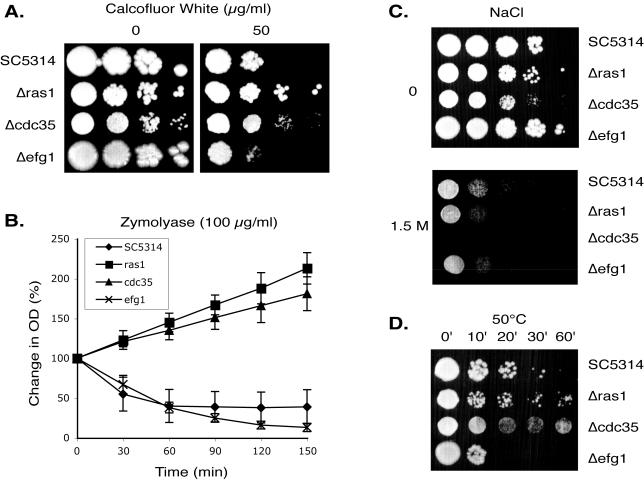
Many transcripts show a higher abundance in the cdc35 mutant. A major group of these genes appear to be both unique to C. albicans and have as yet no known molecular function (Table 1). We have designated three of the most highly influenced genes ASR1, ASR2, and ASR3 for adenylyl cyclase and stress responsive. In addition, a notable group of the transcripts elevated in the absence of cAMP encode proteins involved in the formation and function of the cell wall (Table 2). A variety of phenotypes suggest the wall of the mutant cells is different from that of wild-type cells; the cdc35 mutant tends to aggregate, provides low yields of RNA, and is difficult to transform. As shown in Figure 3, A and B, cdc35 cells are significantly more resistant than wild-type cells to CFW, which binds to chitin, as well as to zymolyase, which is primarily a β1–3 glucanase. Other, smaller groups of genes that are influenced positively and negatively by the loss of cAMP are involved in the cytoskeleton, cell cycle, iron uptake, and multidrug resistance (Supplementary Table 1) and include as well a number of transcription factors (Supplementary Table 2).
Table 1.
Table 2.
Figure 3.
Comparison of sensitivities to CFW, zymolyase, and environmental stress in the wild-type (SC5314) and ras1, cdc35, and efg1 mutants. (A) Comparison of sensitivity to CFW. Strains were spotted on YPD plates containing 0 or 50 μg/ml CFW, and monitored after 3 d of growth. (B) Sensitivity to zymolyase. Strains were incubated in 100 μg/ml zymolase 100T and the OD600nm was monitored for 2.5 h. Data represent mean values ± SD of at least three independent experiments. (C) Sensitivity to osmotic shock. Strains were spotted on YPD plates containing 0 or 1.5 M NaCl. (D) Sensitivity to heat shock. Strains grown at 30°C were immersed in a 50°C waterbath for intervals of 0–60 min and then spotted on a YPD plate and incubated at 30°C. For both stresses, results were monitored after 2 d of growth. Cell concentrations of the cdc35 mutant were adjusted to compensate for different growth rates.
We observed a number of stress response genes influenced by the loss of cAMP. When we compared the list of adenylyl-cyclase–responsive genes during yeast form growth with the stress response data from our group (Enjalbert et al., 2003), we observed a strong correlation between the cdc35 profile and the profile observed in osmotically shocked cells (Supplementary Figure 4A and Supplementary Table 3). In addition, cdc35 cells exhibited an increased sensitivity to osmotic stress (Figure 3C). It should be noted that the treatments used in Enjalbert et al. (2003) were not as stringent as the ones used here because the objective at the time was to produce a mild stress that would not result in excessive cellular mortality. Although we found little correlation between our array data and the stress response data for heat shock, we observed that the cdc35 mutant is slightly more resistant to increased temperature (Figure 3D), perhaps because of changes in the cell wall.
We also compared the cyclase mutant cells to wild-type cells under hyphal growth conditions. As was found in cells growing in the yeast form, the absence of adenylyl cyclase had profound consequences on transcription profiles under these different growth conditions. Over 800 transcripts were affected by the loss of CDC35 when compared with wild type at 37°C + FBS (Figure 2B), and there was a significant correlation between the profiles under both yeast and hyphal conditions in the cdc35 mutant (Supplementary Figure 5A). Among the transcripts modulated in the cdc35 mutant, general metabolic processes remain influenced as they were in the yeast form, although the magnitude of change in transcript levels was often lower in cells growing under hyphal inducing conditions. The genes involved in wall function that were activated in the yeast cells were also up-regulated in hyphal cells. Intriguingly however, there was a group of wall-related genes not identified in yeast growth conditions that were repressed when grown under hyphal conditions (Table 2). Hallmark genes of the cAMP pathway were also modulated in the cdc35 mutant. In the absence of cAMP the transcript level of BCY1 (the negative regulator of PKA) increased as it did in the yeast form, but surprisingly the levels of mRNA for a catalytic subunit TPK2 and the transcription factor EFG1 also increased.
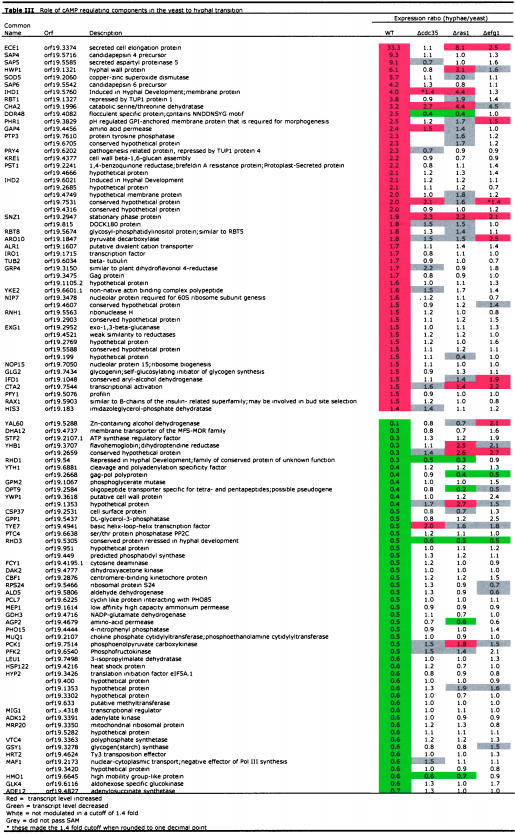
Previous studies have shown that the ability to undergo the yeast-hyphal transition is blocked in cells that lack adenylyl cyclase (Rocha et al., 2001). Our expression profiles show that during the yeast-to-hyphal transition almost all of the genes that were modulated in wild-type cells, including classic hyphal induced genes such as ECE1, HWP1, and SAP4, are no longer responsive to the serum and heat signals in the adenylyl cyclase mutant (Table 3). This suggests that most of the response to a shift from 30 to 37°C + FBS in C. albicans is mediated by the cAMP pathway. However, a few transcripts, including CHA2, GAP4, HMO1, RHD1, RHD3, SNZ1, and orf19.7531, still respond as they did in the wild type (Table 3), suggesting that a cAMP-independent pathway may contribute to morphogenesis under these conditions.
Table 3.
Effects of Other cAMP Network Components on Gene Expression
Ras function has been implicated as a regulator of adenylyl cyclase in fungal systems (Toda et al., 1985), yet the loss of Ras1p function did not have as dramatic an effect on transcription as did loss of adenylyl cyclase. Only 72 transcripts were significantly more abundant in YPD-grown, RAS1-deleted cells than in wild-type, whereas four transcripts were less abundant (Figure 2A). However, the majority of the Ras1p-influenced transcripts are a subset of those that are modulated by cAMP. Many of the transcripts of the Candida-specific genes of unknown function that were more abundant in the absence of CDC35 were also more abundant in the ras1 mutant (Table 1). A number of the cell wall genes whose transcript levels changed in the cdc35 mutant showed the same behavior in the ras1 mutant, and in parallel, ras1 cells were also more resistant than wild-type to treatment with Calcofluor or zymolyase (Figure 3, A and B). As with cdc35 cells, ras1 mutant cells were found to be slightly more resistant to heat shock (Figure 3D); this may also be a feature of changes in the cell wall because there was no strong correlation between data from the ras1 mutant and data from heat shock response. In contrast to cdc35, the ras1 mutant is not sensitive to osmotic stress (Figure 3), and the profiles do not correlate with the osmotic stress response (Supplementary Figure 4B).
Loss of Ras1p function during hyphal inducing conditions modulated the expression of <100 transcripts, most of which were a subset of the transcripts modulated by the loss of CDC35 (Figure 2B). In contrast to the situation during yeast growth conditions, however, numerous transcripts with decreased levels were observed in ras1 cells, and many of these represent hyphal-induced genes.
The ras1 mutant cells showed some differences from the cdc35 mutant cells during analysis of the yeast-to-hyphal switch (Figure 2C). Some of the hyphal-specific genes, such as ECE1, RBT1, and HWP1, whose transcript levels were clearly reduced when ras1 was compared with the wild-type in hyphal conditions, were still partially responsive in ras1 but totally unresponsive in cdc35 mutant cells (Table 3). These genes could have a function in pseudohyphae development, an hypothesis that is supported by the fact that these transcripts were more abundant in the pseudohyphae resulting from the 37°C + FBS treatment of cells lacking the Sit4p phosphatase (Lee et al., 2004).
In the current study we have extended the preliminary analysis (Nantel et al., 2002) of the effects of an EFG1 deletion on transcription profiles. Approximately 85 genes showed significant changes in transcript levels in the efg1 mutant cells compared with wild-type cells growing under yeast growth conditions (Figure 2A). The majority of these Efg1p-modulated transcripts are distinct from those affected by the loss of RAS1 or CDC35. A notable group of decreased transcripts in the efg1 mutant encoded enzymes involved in the glycolysis and gluconeogenesis pathways (Supplementary Figure 3B). As first described (Sohn et al., 2003), the loss of Efg1p was found to modulate transcript levels of genes involved in cell wall maintenance. In our study only a few of the cell wall genes affected by the loss of Cdc35p are also affected by the loss of Efg1p (Table 2). This transcript profile mirrored physiology in that efg1 mutants were moderately sensitive to CFW and zymolyase, rather than resistant as were the ras1 and cdc35 mutants (Figure 3, A and B). We observed a small but significant correlation between the Efg1p-responsive transcripts and genes that were modulated during the white-to-opaque transition (Lan et al., 2002) in C. albicans (Supplementary Figure 5B). This observation can be explained by the fact that levels of Efg1p are greatly reduced in opaque cells (Lan et al., 2002). For the most part, many of the genes whose transcript levels were influenced by Efg1p did not fall into major classes of functionally similar elements, due at least in part to the fact that a large number of the Efg1p-responsive genes have currently unknown functions.
As in the case of cells grown in yeast form conditions, we found that within the group of genes influenced by the loss of EFG1 in hyphal inducing conditions, the majority were distinct from those modulated by either ras1 or cdc35 mutations (Figure 2B). The small number of modulated transcripts that were commonly influenced in the efg1, cdc35, and ras1 mutants identified most of the highly modulated, hyphal-specific genes that were initially used to define this signaling pathway (Table 3).
EFG1 showed its greatest influence when the mutant was undergoing the yeast-to-hyphal switch, where close to 200 genes were affected (Figure 2C). We have previously demonstrated that cells lacking this transcription factor fail to transmit signals that are induced by the presence of serum (Nantel et al., 2002). This may suggest Efg1p modulates a separate pathway that leads to changes in transcript profiles in response to an increase in temperature, and we also observed that the efg1 mutant was sensitive to heat shock (Figure 3D). Approximately 75% of the EFG1-modulated transcript levels increase in abundance compared with wild type (Figure 2C), implying perhaps that Efg1p is primarily a transcriptional repressor during the yeast-to-hyphal transition (Giusani et al., 2002).
DISCUSSION
Both prokaryotic and eukaryotic cells use cAMP as an important regulatory molecule. In prokaryotes the best-studied systems show cAMP functioning as an allosteric regulator of a transcriptional control molecule (Botsford and Harman, 1992), whereas in eukaryotes cAMP provides intracellular regulation through its control of cAMP-dependent protein kinase (Johnson et al., 2001), and it can serve as an extracellular signal in processes such as slime mold chemotaxis (Saran et al., 2002). C. albicans cells become avirulent when they are defective in adenylyl cyclase (Rocha et al., 2001), and so we have used global transcription profiling to examine the intracellular role of cAMP in this important human fungal pathogen.
Our findings establish that modification of cAMP metabolism had dramatic effects on the transcription patterns of C. albicans cells. Changes in the expression patterns of genes involved in transcription, translation, and central metabolic pathways found in cells that lack cAMP can be correlated with modified growth rates. In addition, changes in the expression of genes involved in cell wall metabolism in cyclase-defective cells are reflected by changes in cellular sensitivity to enzymes and compounds that disrupt the wall. As well, the cdc35 mutant cells showed enhanced expression of osmotic stress response genes, though the cells were more sensitive to osmotic shock, perhaps because they could not handle this added challenge in their already activated state. Most dramatically, C. albicans cells that lack adenylyl cyclase function are profoundly defective in the ability to form hyphae, and our study revealed the transcriptional activation of virtually all the genes up-regulated during the yeast-to-hyphal switch is blocked in the cdc35 mutant. Thus the changes in gene expression resulting from the inability to produce cAMP are correlated with significant changes in cellular physiology in this human pathogen. Because cyclase mutants are essentially avirulent in mouse models of systemic infection, it appears that the transcriptional effects resulting from loss of cAMP production impacts significantly on the ability of C. albicans to act as a pathogen.
Major changes in cell behavior due to loss of cAMP regulation are not surprising. Morphogenetic regulation and virulence in C. albicans have also been intimately linked to metabolic control (Tripathi et al., 2002), whereas in S. cerevisiae, cAMP plays a key role in cell cycle regulation (Matsumoto et al., 1982) and loss of adenylyl cyclase activity causes a cell cycle arrest that mimics that of cells responding to nutrient limitation (Kataoka et al., 1985). Cell wall proteins play a key role in C. albicans morphogenesis (Calderone, 2002), and in S. cerevisiae the Ras/cAMP pathway has been shown to be required for maintenance of cell-wall integrity (Tomlin et al., 2000). In addition, osmotolerance is strongly affected by cAMP-dependent protein kinase A in S. cerevisiae (Norbeck and Blomberg, 2000).
Intriguingly, not only known cellular processes appear influenced by cAMP in C. albicans. A large number of genes whose transcript levels are increased in the absence of adenylyl cyclase are of unknown function and so far specific to C. albicans. Further study of this group of unique cAMP-repressed genes may reveal new and specialized functions for adenylyl cyclase in Candida. Several of these cAMP repressed genes also have significantly increased expression levels during osmotic stress; we have chosen three of the most highly influenced genes in this group (orf19.2344, orf19.7284, and orf19.842) and have named them adenylyl cyclase and stress responsive genes, ASR1, ASR2, and ASR3, respectively. Studies are underway to investigate more closely their regulation and cellular roles.
Studies in the yeast S. cerevisiae first suggested that Ras function can act as a regulator of adenylyl cyclase (Toda et al., 1985). Our transcription profiling data in C. albicans support a similar connection between Ras1p and adenylyl cyclase in this somewhat distantly related organism, because the transcriptional consequences of loss of Ras1p and loss of Cdc35p function are similar. However, loss of RAS1 influenced only a subset of the cAMP-responsive genes, and among the genes whose modified regulation was common to both strains, the magnitude of change in expression was typically less for the ras1 mutant. Overall, however, there were few genes whose regulation was uniquely modified in the ras1 mutant, and this result suggests that the primary role of Ras1p in transcription regulation in C. albicans is to function as part of the cAMP regulatory circuit. Because epistatic relationships may change as a function of growth conditions, this observation does not rule out other regulatory patterns under environmental conditions different from those studied here.
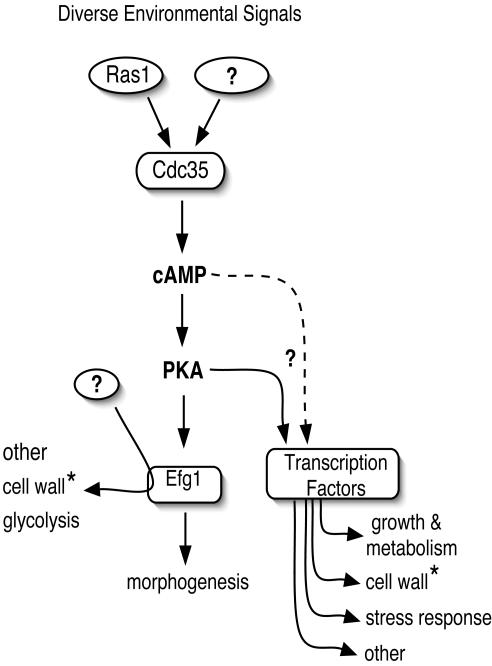
The observation that loss of Ras1p had a less dramatic effect on the transcription profiles than did loss of Cdc35p, both in terms of the number of genes influenced and the magnitude of the induced changes, could be explained if loss of Ras1p only reduced, but did not eliminate, adenylyl cyclase activity. This would be consistent with Cdc35p having other regulators in addition to Ras1p (Figure 4). The C. albicans homolog of Gpa2p, a protein that acts as a regulator of adenylyl cyclase in S. cerevisiae, has been identified, although its role in cAMP signaling in the pathogen appears limited. However, a double mutant of Ras1p and Gpr1p, the C. albicans homolog of the S. cerevisiae glucose receptor that regulates adenylyl cyclase activity (Xue et al., 1998), shows growth characteristics similar to the C. albicans adenylyl cyclase null mutant (our unpublished results).
Figure 4.
Model of the C. albicans cAMP signaling pathway. The schematic representation shows the role of three components of the cAMP pathway: Ras1p, Cdc35p, and Efg1p. The model proposes that Ras1p is a primary regulator of adenylyl cyclase but that there are other regulators of this pathway because the loss of Ras1p modulates only a subset of genes affected by the loss of Cdc35p, and Ras1p defective strains can still produce pseudohyphae. It appears that all three components are involved in the morphogenetic switch, but the cAMP pathway mediates regulation of other cellular processes independent of Efg1p, and these other targets of the cAMP pathway may or may not be regulated through PKA. In addition, we suggest that Efg1p also exerts effects on the transcription of genes outside the cAMP regulatory circuit. The asterisk (*) shows that the cell wall processes affected by cAMP and Efg1 are different.
The transcription factor Efg1p has been identified as a downstream target of the cAMP regulatory circuit based on its role in the process of hyphal development (Bockmühl and Ernst, 2001). However, we found the set of genes whose transcript levels were influenced by the loss of EFG1 to be generally distinct from those influenced by the loss of CDC35 or RAS1. In fact, only the class of genes activated during the yeast-to-hyphal transition responds to the loss of all three elements. Intriguingly, one class of genes transcriptionally affected by loss of the cAMP pathway represents known or putative transcription factors. This fits with the wide impact of the loss of cyclase function on transcription profiles, and the recognition that much of this profile is independent of Efg1p (Figure 4).
Overall, transcription profiling provides a powerful tool to investigate organisms such as C. albicans that are not readily amenable to direct genetic analysis. In this study, we have probed the cAMP regulatory circuit of C. albicans, known to regulate morphogenesis and virulence, and have revealed its function in the regulation of gene expression involved in many cellular processes. This work suggests that there are still regulatory connections that need to be established; in particular the broad transcriptional effects of loss of adenylyl cyclase that are independent of Efg1p suggest that there are further transcriptional regulatory circuits that respond to cAMP.
Supplementary Material
Acknowledgments
We thank H. Lo for generously providing the Efg1 strain, members of the Whiteway lab for helpful discussion, and Ursula Oberholzer and Marc Fortin for critical reading of the manuscript. This project was funded by the Genomics and Health Initiative of the National Research Council of Canada, and Canadian Institutes for Health Research grant MOP-42516. This is National Research Council publication number 46164.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–02–0144. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–02–0144.
The online version of this article contains supplemental material accessible through http://www.molbiolcell.org.
References
- Alspaugh, J.A., Perfect, J.R., and Heitman, J. (1997). Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 11, 3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn, Y.S., Staab, J., and Sundstrom, P. (2003). Increased high-affinity phosphodiesterase PDE2 gene expression in germ tubes counteracts CAP1-dependent synthesis of cyclic AMP, limits hypha production and promotes virulence of Candida albicans. Mol. Microbiol. 50, 391-409. [DOI] [PubMed] [Google Scholar]
- Bockmühl, D.P., and Ernst, J.F. (2001). A potential phosphorylation site for an A-type kinase in the Efg1 regulator protein contributes to hyphal morphogenesis of Candida albicans. Genetics 157, 1523-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmühl, D.P., Krishnamurthy, S., Gerads, M., Sonneborn, A., and Ernst, J.F. (2001). Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol. Microbiol. 42, 1243-1257. [DOI] [PubMed] [Google Scholar]
- Botsford, J.L., and Harman, J.G. (1992). Cyclic AMP in prokaryotes. Microbiol. Rev. 56, 100-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone, R. (2002). Candida and Candidiasis. Washington, DC; ASM Press.
- Chen, J., Zhou, S., Wang, Q., Chen, X., Pan, T., and Liu, H. (2000). Crk1, a novel Cdc2-related protein kinase, is required for hyphal development and virulence in Candida albicans. Mol. Cell. Biol. 20, 8696-8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, W., and Dean, R.A. (1997). The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 9, 1973-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier, M. et al. (2003). The two isoforms of the cAMP-dependent protein kinase catalytic subunit are involved in the control of dimorphism in the human fungal pathogen Candida albicans. Fungal Genet. Biol. 38, 133-141. [DOI] [PubMed] [Google Scholar]
- Colombo, S. et al. (1998). Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 17, 3326-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan, R.S., and Tzamarias, D. (2001). Sfl1 functions via the co-repressor Ssn6-Tup1 and the cAMP-dependent protein kinase Tpk2. J. Mol. Biol. 309, 1007-1015. [DOI] [PubMed] [Google Scholar]
- D'Souza, C.A., and Heitman, J. (2001). Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25, 349-364. [DOI] [PubMed] [Google Scholar]
- Eisen, M.B., Spellman, P.T., Brown, P.O., and Botstein, D. (1998). Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert, B., Nantel, A., and Whiteway, M. (2003). Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol. Biol. Cell 14, 1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Q., Summers, E., Guo, B., and Fink, G. (1999). Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J. Bacteriol. 181, 6339-6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum, A.M., Tsay, E.Y., and Kirsch, D.R. (1984). Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol. Gen. Genet. 198, 179-182. [DOI] [PubMed] [Google Scholar]
- Giusani, A.D., Vinces, M., and Kumamoto, C.A. (2002). Invasive filamentous growth of Candida albicans is promoted by Czf1p-dependent relief of Efg1p-mediated repression. Genetics 160, 1749-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, S., Duncan, G., Barrett, K., and Kronstad, J. (1994). cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8, 2805-2816. [DOI] [PubMed] [Google Scholar]
- Gold, S.E., Brogdon, S.M., Mayorga, M.E., and Kronstad, J.W. (1997). The Ustilago maydis regulatory subunit of a cAMP-dependent protein kinase is required for gall formation in maize. Plant Cell 9, 1585-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, D.A., Akamine, P., Radzio-Andzelm, E., Madhusudan, M., and Taylor, S.S. (2001). Dynamics of cAMP-dependent protein kinase. Chem. Rev. 101, 2243-2270. [DOI] [PubMed] [Google Scholar]
- Kataoka, T., Broek, D., and Wigler, M. (1985). DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell 43, 493-505. [DOI] [PubMed] [Google Scholar]
- Kubler, E., Mosch, H.U., Rupp, S., and Lisanti, M.P. (1997). Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 272, 20321-20323. [DOI] [PubMed] [Google Scholar]
- Lan, C.Y., Newport, G., Murillo, L.A., Jones, T., Scherer, S., Davis, R.W., and Agabian, N. (2002). Metabolic specialization associated with phenotypic switching in Candida albicans. Proc. Natl. Acad. Sci. USA 99, 14907-14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer, E., Harcus, D., Dignard, D., Johnson, L., Ushinsky, S., Thomas, D.Y., and Schroppel, K. (2001). Ras links cellular morphogenesis to virulence by regulation of the MAP kinase and cAMP signalling pathways in the pathogenic fungus Candida albicans. Mol. Microbiol. 42, 673-687. [DOI] [PubMed] [Google Scholar]
- Lee, C.M., Nantel, A., Jiang, L., Whiteway, M., and Shen, S.H. (2004). The serine/threonine protein phosphatase SIT4 modulates yeast-to-hypha morphogenesis and virulence in Candida albicans. Mol. Microbiol. 51, 691-709. [DOI] [PubMed] [Google Scholar]
- Lengeler, K.B., Davidson, R.C., D'Souza, C., Harashima, T., Shen, W.C., Wang, P., Pan, X., Waugh, M., and Heitman, J. (2000). Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64, 746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. (2001). Transcriptional control of dimorphism in Candida albicans. Curr Opin. Microbiol. 4, 728-735. [DOI] [PubMed] [Google Scholar]
- Lo, H.J., Kohler, J.R., DiDomenico, B., Loebenberg, D., Cacciapuoti, A., and Fink, G.R. (1997). Nonfilamentous C. albicans mutants are avirulent. Cell 90, 939-949. [DOI] [PubMed] [Google Scholar]
- Matsumoto, K., Uno, I., Oshima, Y., and Ishikawa, T. (1982). Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 79, 2355-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantel, A. et al. (2002). Transcription profiling of Candida albicans cells undergoing the yeast-to-hyphal transition. Mol. Biol. Cell 13, 3452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbeck, J., and Blomberg, A. (2000). The level of cAMP-dependent protein kinase A activity strongly affects osmotolerance and osmo-instigated gene expression changes in Saccharomyces cerevisiae. Yeast 16, 121-137. [DOI] [PubMed] [Google Scholar]
- Pascual-Ahuir, A., Posas, F., Serrano, R., and Proft, M. (2001). Multiple levels of control regulate the yeast cAMP-response element-binding protein repressor Sko1p in response to stress. J. Biol. Chem. 276, 37373-37378. [DOI] [PubMed] [Google Scholar]
- Pedruzzi, I., Burckert, N., Egger, P., and De Virgilio, C. (2000). Saccharomyces cerevisiae Ras/cAMP pathway controls post-diauxic shift element-dependent transcription through the zinc finger protein Gis1. EMBO J. 19, 2569-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, C.R., Schroppel, K., Harcus, D., Marcil, A., Dignard, D., Taylor, B.N., Thomas, D.Y., Whiteway, M., and Leberer, E. (2001). Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol. Biol. Cell 12, 3631-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran, S., Meima, M.E., Alvarez-Curto, E., Weening, K.E., Rozen, D.E., and Schaap, P. (2002). cAMP signaling in Dictyostelium. Complexity of cAMP synthesis, degradation and detection. J. Muscle Res. Cell Motil. 23, 793-802. [DOI] [PubMed] [Google Scholar]
- Smith, A., Ward, M.P., and Garrett, S. (1998). Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17, 3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, K., Urban, C., Brunner, H., and Rupp, S. (2003). EFG1 is a major regulator of cell wall dynamics in Candida albicans as revealed by DNA microarrays. Mol. Microbiol. 47, 89-102. [DOI] [PubMed] [Google Scholar]
- Stoldt, V.R., Sonneborn, A., Leuker, C.E., and Ernst, J.F. (1997). Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16, 1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda, T., Cameron, S., Sass, P., Zoller, M., and Wigler, M. (1987). Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50, 277-287. [DOI] [PubMed] [Google Scholar]
- Toda, T. et al. (1985). In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40, 27-36. [DOI] [PubMed] [Google Scholar]
- Tomlin, G.C., Hamilton, G.E., Gardner, D.C., Walmsley, R.M., Stateva, L.I., and Oliver, S.G. (2000). Suppression of sorbitol dependence in a strain bearing a mutation in the SRB1/PSA1/VIG9 gene encoding GDP-mannose pyrophosphorylase by PDE2 overexpression suggests a role for the Ras/cAMP signal-transduction pathway in the control of yeast cell-wall biogenesis. Microbiology 146(Pt 9), 2133-2146. [DOI] [PubMed] [Google Scholar]
- Tripathi, G., Wiltshire, C., Macaskill, S., Tournu, H., Budge, S., and Brown, A.J. (2002). Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 21, 5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, M.P., Gimeno, C.J., Fink, G.R., and Garrett, S. (1995). SOK2 may regulate cyclic AMP-dependent protein kinase-stimulated growth and pseudohyphal development by repressing transcription. Mol. Cell. Biol. 15, 6854-6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway, M. (2000). Transcriptional control of cell type and morphogenesis in Candida albicans. Curr. Opin. Microbiol. 3, 582-588. [DOI] [PubMed] [Google Scholar]
- Xue, Y., Batlle, M., and Hirsch, J.P. (1998). GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Galpha subunit and functions in a Ras-independent pathway. EMBO J. 17, 1996-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.