Abstract
In normal human skin fibroblasts (HSFs), fluorescent glycosphingolipid analogues are endocytosed and sorted into two pools, one that is recycled to the plasma membrane and one that is transported to the Golgi complex. Here, we investigated glycosphingolipid recycling in Niemann-Pick type A and C lipid storage disease fibroblasts (NPFs). Cells were incubated with a fluorescent analogue of lactosylceramide (LacCer) at 16°C to label early endosomes (EEs), shifted to 37°C, and lipid recycling was quantified. Using dominant negative rabs, we showed that, in normal HSFs, LacCer recycling was rapid (t1/2 ∼8 min) and mainly rab4-dependent. In NPFs, LacCer recycling was delayed (t1/2 ∼30–40 min), and rab4-dependent recycling was absent, whereas rab11-dependent recycling predominated. Transferrin recycling via the rab4 pathway was similarly perturbed in NPFs. Compared with normal HSFs, EEs in NPFs showed high cholesterol levels and an altered organization of rab4. In vitro extraction of rab4 (but not rab11) with GDP dissociation inhibitor was severely attenuated in NPF endosomal fractions. This impairment was reversed with cholesterol depletion of isolated endosomes or with high-salt treatment of endosomes. These data suggest that abnormal membrane recycling in NPFs results from specific inhibition of rab4 function by excess cholesterol in EEs.
INTRODUCTION
Sphingolipid storage diseases (SLSDs) are a subset of lysosomal storage disorders in which sphingolipids (SLs) accumulate in various tissues in the body, often resulting in neurological (e.g., dementia or developmental delays) and somatic (e.g., hepatomegaly) pathological conditions (for reviews, see Scriver et al., 2001; Jeyakumar et al., 2002). In most of these disorders, the abnormal lipid accumulation is attributable to a defect in a hydrolytic enzyme or activator protein required for the catabolism of a particular lipid. For example, in Niemann-Pick disease, type A (NP-A), there is a deficiency in acid sphingomyelinase, resulting in lysosomal accumulation of sphingomyelin (Schuchman and Desnick, 2001). Cholesterol also often accumulates in these SLSDs (Wood et al., 1985; Puri et al., 1999; Leventhal et al., 2001; Schuchman and Desnick, 2001), presumably because of interactions of stored SLs with cholesterol. In addition, patients with Niemann-Pick disease, type C (NP-C), exhibit intracellular accumulation of cholesterol and SLs, although lysosomal hydrolase activities are normal among these individuals (Patterson et al., 2001). Most patients (>90%) with NP-C disease have defects in NPC1, a late endosomal, transmembrane protein apparently involved in late endocytic trafficking; however, despite extensive studies, the role of NPC1 in cholesterol or SL transport remains controversial (Vanier and Millat, 2003).
Recent studies in our laboratory using a fluorescent analogue {N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)sphingosyl 1-β-D-lactoside; [BODIPY-LacCer)} of lactosylceramide (LacCer), a glycosphingolipid (GSL), in normal human skin fibroblasts (HSFs) and in SLSD fibroblasts have identified GSL trafficking defects in multiple diseases (Chen et al., 1999; Choudhury et al., 2002; Marks and Pagano, 2002). In normal HSFs, the LacCer analogue is endocytosed from the plasma membrane (PM) and transported to the Golgi apparatus through a rab7- and rab9-dependent mechanism, whereas the same lipid accumulates in endosomes and lysosomes in multiple SLSDs. Interestingly, the abnormal transport of LacCer can be corrected with overexpression of wild-type (WT) rab7 or rab9 in several SLSD cell types, and this overexpression dramatically reduces the accumulation of cholesterol in NP-C fibroblasts (Choudhury et al., 2002; Walter et al., 2003).
To better understand these alterations in membrane trafficking, we studied the itinerary of BODIPY-LacCer and showed that the GSL analogue is internalized almost exclusively via caveolae in both normal and SLSD cell types (Puri et al., 2001; Sharma et al., 2003, 2004; Singh et al., 2003). After internalization, the lipid is rapidly transported to EEA1-positive early endosomes (EEs), where it merges with markers for the clathrin pathway (Sharma et al., 2003). There the lipid analogue appears to be fractionated into two pools, one that is transported to the Golgi apparatus (normal HSFs) or late endosomes/lysosomes (SLSD cells), and one that is recycled back to the PM. In the current study, we investigated this recycling pathway in detail. Recycling of fluorescent lipid analogues and lipid probes has been reported, but the rate or extent of recycling varies with the cell type, the method used to measure the signal, and the nature of the lipid probes (Koval and Pagano, 1989; Mayor et al., 1993; Hao and Maxfield, 2000). Studies of protein recycling often use transferrin (Tfn), which exhibits both fast recycling from the EEs to the PM and slower recycling involving transit through the perinuclear recycling endosomes (REs) to the PM (Ullrich et al., 1996; Sheff et al., 1999; Maxfield and McGraw, 2004). At present, there are few quantitative data on the relative fractions of lipids that recycle directly from EEs versus traverse through REs.
Here we examine the recycling process in detail in normal HSFs versus NP-A and NP-C fibroblasts (referred to as Niemann-Pick fibroblasts [NPFs]). We show that the kinetics of recycling are dramatically slowed for both a fluorescent GSL analogue and Tfn in NPFs, relative to normal HSFs. Our results demonstrate that there are selective perturbations in the organization of rab4 on endosomes and in rab4-dependent membrane recycling attributable to the presence of high levels of endosomal cholesterol.
MATERIALS AND METHODS
Cell Culture
Normal HSFs (GM-5659D), NP-C fibroblasts (GM-03123), and NP-A fibroblasts (GM-00112A) were obtained from the Coriell Institute for Medical Research (Camden, NJ). Cells were cultured for ≥72 h in EMEM with 10% fetal calf serum (“standard conditions”) (Martin and Pagano, 1994) or with 5% lipoprotein-deficient serum, to deplete cellular cholesterol (Martin et al., 1993; Puri et al., 1999, 2001).
Lipids and Miscellaneous Reagents
BODIPY-LacCer was synthesized and subsequently complexed with defatted bovine serum albumin (DF-BSA) (Martin and Pagano, 1994) for incubations with cells. Fluorescent Tfn was obtained from Molecular Probes (Eugene, OR). Human Tfn was iodinated and purified as described (Chen et al., 1998). Filipin was obtained from Polysciences (Warrington, PA). mAb against rab4 and EEA1 were obtained from BD Transduction Laboratories (San Diego, CA); polyclonal antisera against rab5 and rab7 (Santa Cruz Biotechnology, Santa Cruz, CA), rab11 (Zymed Laboratories, San Francisco, CA), and EEA1 (Upstate Biotechnology, Lake Placid, NY) were from the indicated vendors. Rab4 polyclonal antiserum for Western blotting was a kind gift from Dr. P. van der Sluijs (Utrecht University School of Medicine, The Netherlands). Secondary antibodies were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). All other reagents were obtained from Sigma (St. Louis, MO).
Constructs and Cotransfection Studies
WT and dominant negative (DN) (S22N) rab4 constructs were a gift from P. van der Sluijs. DN (S25N) rab11 in the pCDNA3.1 vector was generated as described (Choudhury et al., 2002). GFP-WT rab4 (Seachrist et al., 2000) was a gift from H. Radhakrishna (Georgia Institute of Technology, Atlanta, GA). In most of the rab overexpression studies, cells were cotransfected with the rab construct of interest and pDsRed2-Nuc (BD Biosciences Clontech, Palo Alto, CA). The pDsRed2-Nuc construct labeled the nucleus with red fluorescence and served as a reporter for transfected cells. Transfections were carried out using 1 μg of DNA (1:3 DNA mixture of pDsRed2-Nuc plasmid and the rab construct) and 6 μl of Fugene 6 (Roche Applied Science, Indianapolis, IN) for a 35-mm culture dish. Experiments were performed 48 h after transfection.
Fluorescence and Biochemical Assays of LacCer and Tfn Recycling
Normal HSFs or NPFs were incubated with 2.5 μM BODIPY-LacCer or 25 μg/ml Tfn for 1 h at 16°C to selectively label EEs (Sharma et al., 2003). For LacCer, the cells were back-exchanged with 5% DF-BSA to remove any LacCer remaining at the PM and were then incubated (“chased”) for various times at 37°C in HMEM with 5% DF-BSA. This served to remove any lipid that was recycled back to the PM. Similarly, fluorescein isothiocyanate (FITC)-Tfn-loaded cells were acid-stripped (Singh et al., 2003) and then chased in the presence of 10-fold excess unlabeled holo-Tfn. The medium was exchanged in all dishes at each time point, to minimize reinternalization of Tfn transported to the PM. Cells were then observed under the fluorescence microscope, and images were quantified as described below. In one experiment, cells were pretreated with 100 nM wortmannin (WM) for 20 min at 37°C before the incubation with BODIPY-LacCer. All subsequent incubations were also carried out in the presence of WM.
Cells treated with BODIPY-LacCer were dissociated from the culture dish by incubation with trypsin-EDTA for 2–3 min at 37°C, followed by addition of ice-cold buffer and centrifugation. The cell pellets were washed three times with cold phosphate-buffered saline, followed by lipid extraction (Bligh and Dyer, 1959). Fluorescent lipid present in the extracts was quantified with a fluorimeter, and the data were normalized to cell protein. In the case of 125I-Tfn, both the cells and medium were collected (Chen et al., 1998), and the radioactivity in each fraction was measured with a gamma counter.
Immunocytochemical Analysis and Colocalization Studies
Immunocytochemical studies were performed with formaldehyde-fixed cells as described previously (Sharma et al., 2003). For filipin staining, cells were fixed as above and then incubated with 150 μg/ml filipin in phosphate-buffered saline for 30 min at room temperature. Samples were observed by fluorescence microscopy (λex = 360 nm; λem = 460 ± 50 nm). For colocalization of endogenous rab4 with filipin, cells were fixed as above and immunolabeled with rab4 antibodies in blocking buffer containing 150 μg/ml filipin, as described (Neufeld et al., 1999). The organization of endogenous rab4 on EEs was examined by immunolabeling rab4 and EEA1. The organization of endogenous rab11 was determined by immunostaining in HSFs or NPFs after transfection with GFP-rab4 for 8–12 h.
For colocalization of BODIPY-LacCer with EEA1 or endogenous rab11, cells were incubated with 2.5 μM BODIPY-LacCer for 1 h at 16°C, back-exchanged at 10°C with 5% DF-BSA (see above), washed, and warmed for 0, 10, or 40 min at 37°C in the presence of 5% DF-BSA, to remove any fluorescent LacCer recycled back to the PM. Cells were fixed as above, and fluorescence images were acquired. Samples were then immunostained for EEA1 or rab11, and the cells previously photographed for BODIPY-LacCer were relocated and rephotographed for antibody fluorescence. For colocalization with dextran, cells were incubated for 16 h at 37°C with 2 mg/ml cascade blue dextran (Molecular Probes) in complete culture medium, washed, chased for 2 h at 37°C in complete medium, and then pulse-labeled with BODIPY-LacCer as above. Samples were fixed as above, and images at blue and green wavelengths were acquired. Colocalization studies with LysoTracker blue dye (Molecular Probes) were carried out in a manner similar to that for dextran studies, except that 1 μM LysoTracker was incubated with the cells for 40 min at 37°C before pulse-labeling with LacCer.
Isolation of Endosome-enriched Membranes and GDP Dissociation Inhibitor Extraction of Rab Proteins
Subcellular fractionation was performed as described (Lim et al., 2001). Briefly, monolayer cultures of fibroblasts (80–90% confluent) were scraped in homogenization buffer [38 mM potassium aspartate, 38 mM potassium glutamate, 38 mM potassium gluconate, 20 mM 3-(N-morpholino)-propanesulfonic acid, pH 7.2, 5 mM sodium carbonate, 2.5 mM magnesium sulfate] containing protease inhibitors. Cells were homogenized using a ball bearing homogenizer (six passages). The homogenate was centrifuged at 300 × g (5 min) to remove the debris and nuclei, followed by a 500 × g centrifugation (5 min) of the supernatant. A membrane pellet fraction was then generated from the resulting supernatant by centrifugation for 45 min at 27,000 × g. This fraction was enriched five- to sevenfold in rab4, rab5, rab7, and rab11, relative to the homogenate (see supplemental Figure 1, A, B).
GDP dissociation inhibitor (GDI) extraction was performed as described (Cavalli et al., 2001). Endosomal membrane pellets were suspended in EE buffer (30 mM HEPES, 75 mM potassium acetate, 5 mM MgCl2) containing 100 μM ATP, various concentrations of glutathione S-transferase (GST)-GDI, and 500 μM GDP or 500 μM GTPγS, for 20 min at 30°C. Rab bound to GST-GDI was recovered on glutathione-Sepharose 4B beads, and the bound proteins were solubilized with Laemmli sample buffer. Samples were analyzed with SDS-PAGE (15%) and immunoblotted using rab4, rab5, or rab11 polyclonal antiserum. In some experiments, membranes were treated with 5 mM methyl-β-cyclodextrin (mβ-CD), 1 mM mβ-CD/cholesterol (Klein et al., 1995), or 2 M KCl (Faure et al., 1999).
Fluorescence Microscopy
BODIPY-labeled samples were excited at 450–490 nm and viewed at green wavelengths (λem = 520–560 nm). Alexa fluor 488, DsRed, FITC, and Cascade blue fluorescence were viewed at standard excitation and emission wavelengths. Conventional fluorescence microscopy and image processing were carried out with an Olympus IX-70 fluorescence microscope equipped with an Orca 100 CCD camera (Hamamatsu, Bridgewater, NJ) and Metamorph image-processing software (Universal Imaging, Downingtown, PA). Confocal microscopy was performed using a Zeiss 510 microscope with a 100× objective (1.4 NA). In any given experiment, all images were exposed and processed identically for a given fluorophore and were corrected for background fluorescence using unlabeled specimens. For double-label experiments, control samples were labeled identically with the individual fluorophores and exposed identically to the double-labeled samples at each wavelength to verify that there was no crossover at the settings used. Endosome shape factor calculations were performed with a macro written in the Metamorph program.
RESULTS
BODIPY-LacCer Recycling Kinetics in Normal HSFs and NPFs
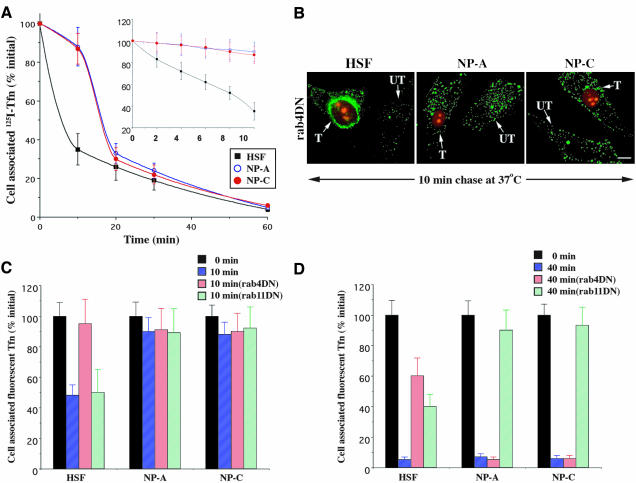
To study lipid recycling, cells are first incubated with BODIPY-LacCer for 1 h at 16°C. This treatment results in endocytosis of the fluorescent lipid from the PM and accumulation in EEs (Sharma et al., 2003). Samples are then chilled to 10°C and incubated with nonfluorescent DF-BSA, to remove any fluorescent lipid that was not internalized. When these cells are subsequently warmed to 37°C, some of the internalized lipid is transported back to the PM because of recycling. This results in the reappearance of fluorescence at the PM, which can be seen directly by fluorescence microscopy (Sharma et al., 2003). The extent of recycling can also be quantified by measuring the loss of cell-associated fluorescence (or the appearance of fluorescence in the bathing medium) when DF-BSA is present during the 37°C incubation.
Figure 1 shows the results of such a recycling experiment with normal HSFs and NPFs. Upon 16°C incubation and back-exchange (“0 min”), numerous fluorescent intracellular vesicles were visible in all three cell types (Figure 1A). In addition, the overall fluorescence intensities of all of the cell types at this time point were approximately the same. When cells were warmed to 37°C for various times, the cell-associated fluorescence was found to decrease more rapidly in control HSFs than in the NPFs. Furthermore, at 40 min, most of the remaining fluorescence in HSFs was at the Golgi apparatus, whereas the lipid in the NPFs was distributed in punctate endosomal structures (Figure 1A). Qualitatively, there was a rapid loss of fluorescence in HSFs during the first 10 min, which was approximately linear (Figure 1B, inset), followed by a slower decrease, whereas only a slow decrease in fluorescence was seen with time at 37°C in NPFs. On the basis of these data, we estimated that the half-time for loss of cell-associated fluorescence due to recycling of BODIPY-LacCer was ∼8 min in HSFs, compared with 30–40 min in NPFs.
Figure 1.
Altered recycling of BODIPY-LacCer in NPFs vs. normal HSFs. (A) Cells were incubated with 2.5 μM BODIPY-LacCer for 1 h at 16°C to selectively label EEs, washed, and back-exchanged with 5% DF-BSA to remove any fluorescent lipid remaining at the PM. The samples were then incubated for 0, 10, or 40 min at 37°C in the presence of 5% DF-BSA to remove any fluorescent LacCer that was recycled back to the PM. G, Golgi apparatus. Bar, 10 μm. (B) Cells were incubated with fluorescent LacCer as in A, and the amount of cell-associated fluorescence was determined by lipid extraction and analysis. Inset, the first 10 min of LacCer recycling was examined in detail. In some experiments (filled symbols), cells were grown in lipoprotein-deficient serum to deplete cholesterol (see Materials and Methods). All values are expressed as percentage of cell-associated fluorescence at 0 min for each cell type and are mean ± SD of three or more independent experiments.
Next, normal HSFs or NPFs were pulse-labeled with the fluorescent lipid as in Figure 1 and the overlap of the cell-associated fluorescent lipid remaining after 0, 10, or 40 min of recycling with markers for EEs (EEA1), REs (rab11), and late endosomes/lysosomes (fluorescent dextran and LysoTracker dye) was quantified (Table 1). Immediately after the 16°C pulse, most of the lipid (87–91%) was in EEA1-positive endosomes, regardless of the cell type. When HSFs were subsequently warmed for 10 min at 37°C, the LacCer signal in EEs decreased to 35%, whereas the overlap with the rab11-positive endosomes increased to 55%, suggesting that part of the lipid pool passed through REs before PM recycling. In contrast, 75–80% of the LacCer in NPFs remained in EEA1-positive structures at 10 min. At 40 min, the percentages of BODIPY-LacCer present in rab11-positive structures (15–25%) were approximately the same for all three cell types. Most of the remaining intracellular fluorescence in normal HSFs at this time point was at the Golgi complex (Figure 1A), whereas 40–45% of the lipid in NPFs was present in punctate structures that were dextran and LysoTracker positive.
Table 1.
Overlap of BODIPY-LacCer with various endosomal markers after internalization from the PM
| Overlap (%)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 min
|
10 min
|
40 min
|
|||||||
| Marker | HSF | NP-C | NP-A | HSF | NP-C | NP-A | HSF | NP-C | NP-A |
| EEA1 | 91 ± 9.1 | 90 ± 11.1 | 87 ± 9.5 | 35 ± 4.0 | 75 ± 8.4 | 79 ± 9.2 | 5 ± 1.5 | 5 ± 1.1 | 5 ± 1.2 |
| Rab11 | 15 ± 2.3 | 12 ± 2.2 | 10 ± 2.2 | 55 ± 7.5 | 18 ± 3.1 | 16 ± 2.5 | 15 ± 2.3 | 20 ± 2.5 | 25 ± 3.0 |
| Dextran | 5 ± 1.0 | 5 ± 2.3 | 5 ± 1.5 | 5 ± 1.5 | 8 ± 2.4 | 8 ± 1.8 | 5 ± 1.2 | 39 ± 2.2 | 45 ± 7.1 |
| LysoTracker | 5 ± 1.2 | 5 ± 1.1 | 5 ± 1.7 | 5 ± 1.4 | 5 ± 1.8 | 6 ± 1.5 | 5 ± 1.3 | 43 ± 2.1 | 42 ± 6.2 |
Normal HSFs, NP-C fibroblasts, or NP-A fibroblasts were pulse-labeled with BODIPY-LacCer as in Figure 1, and the overlap with the indicated endosomal markers was quantified by image analysis. Values are means ± SD (n = 10–20 cells for each condition, in three or more independent experiments).
Rab4-dependent Recycling Is Absent in NP-A and NP-C Fibroblasts
The perturbation in recycling of BODIPY-LacCer in HSFs versus NPFs led us to examine the roles of rab4 and rab11 in the recycling of this lipid, because these rabs play important roles in the recycling of internalized proteins to the PM (van der Sluijs et al., 1992; Daro et al., 1996; Maxfield and McGraw, 2004). The untagged DN constructs of rab4 (S22N, rab4DN) and rab11 (S25N, rab11DN) were cotransfected with a plasmid containing the sequence for DsRed fluorescent protein fused to a nuclear localization signal (DsRed-Nuc). In preliminary studies, we found that >90% of the cells that expressed DsRed-Nuc also overexpressed the rab protein, as identified in immunofluorescence assays with antibodies against rab4 or rab11 (our unpublished results). This strategy allowed us to easily identify transfected cells on the basis of nuclear fluorescence and to quantify the amount of cell-associated BODIPY-LacCer through image analysis without interference from the DsRed signal.
We first examined the effect of DN rab4 or rab11 on the recycling of LacCer at an early time point (10 min) (Figure 2). Cells were cotransfected with the indicated DN constructs and DsRed-Nuc, and 24–48 h later, samples were pulse-labeled with fluorescent LacCer (see Materials and Methods). In normal HSFs, overexpression of DN rab4 caused extensive perinuclear accumulation of the fluorescent LacCer in small punctate structures, whereas relatively low levels of fluorescent lipid were seen in adjacent untransfected cells at this time point. Quantitative analysis indicated almost no loss of cell-associated LacCer fluorescence in the DN rab4-expressing cells (i.e., ∼90% inhibition of recycling), whereas ∼60% of this fluorescence was lost during the first 10 min of chase in untransfected normal cells. In both NP-A and NP-C fibroblasts, there was little loss of LacCer fluorescence between 0 and 10 min of chase, and no obvious effect of DN rab4 overexpression was seen qualitatively or quantitatively (Figure 2A, compare UT vs. T; Figure 2B). When DN rab11 was used, no differences between transfected and untransfected specimens were observed at the 10-min time point in either HSFs or NPFs.
Figure 2.
Effects of DN rab4, DN rab11, and a PI3K inhibitor on LacCer recycling. (A) Normal HSFs or NPFs were cotransfected with the DsRed2-Nuc plasmid and either DN rab4 or DN rab11 constructs. After 48 h, the recycling assay was performed as in Figure 1. Transfected cells were identified on the basis of red fluorescent protein in the nucleus. T, transfected cell; UT, untransfected cell. Bar, 15 μm. (B and C) Fluorescence intensity remaining in the cells after 10 or 40 min of recycling was quantified by image analysis and expressed as a percentage of fluorescence present at 0 min. Values are mean ± SD (n = 60 cells; three independent experiments). The role of PI3Ks on lipid recycling from the EEs was studied using the PI3K inhibitor WM.
After 40 min of chase, most of the intracellular lipid was targeted to the Golgi complex in HSFs, whereas only punctate endosomal staining was observed in NPFs (Figures 1A, 2A, untransfected cells in bottom panels), consistent with our previous studies (Chen et al., 1999; Puri et al., 1999; Choudhury et al., 2002). In normal HSFs overexpressing DN rab4, extensive perinuclear accumulation of BODIPY-LacCer was seen at the 40-min time point (Figure 2A), similar to that seen at 10 min, whereas no obvious effect of DN rab4 was seen in NPFs. In normal cells overexpressing DN rab11, LacCer accumulated in tubular structures distinct from the Golgi apparatus at 40 min, whereas untransfected cells showed Golgi complex staining (Figure 2A). Overexpression of DN rab11 in NPFs induced massive juxtanuclear accumulation of LacCer at 40 min, in contrast to the punctate staining seen in untransfected cells (Figure 2A). Quantitatively, DN rab11 had only a small effect on the loss of LacCer fluorescence from HSFs at the 40-min point (∼85% loss in untransfected cells versus ∼70% loss in transfected cells), whereas the inhibition of recycling was very pronounced in NPFs (∼50% loss in untransfected cells versus ∼5% loss in transfected cells) (Figure 2C).
Taken together, the data in Figure 2 suggest that, after its internalization, BODIPY-LacCer is recycled to the PM in a predominantly rab4-dependent manner in normal HSFs, whereas rab4-mediated recycling is impaired in NPFs. Interestingly, this impaired recycling of LacCer could be restored by overexpression of WT rab4 (supplemental Figure 2), suggesting that the recycling defect was attributable to a deficit of active or properly distributed rab4 on endosomes.
Recycling of Tfn in Normal HSFs and NPFs
We next used labeled Tfn to determine whether protein recycling in NPFs was affected in a manner similar to that seen with the LacCer analogue. Cells were incubated with either 125I- or FITC-labeled Tfn for 30 min at 16°C, as described above. The samples were then washed, acid stripped, and warmed for various times at 37°C, and the amount of cell-associated Tfn was quantified by measurements of radioactivity (Figure 3A) or by quantitative microscopy (Figure 3, C and D). As previously reported for other cell types (Sheff et al., 2002), Tfn recycling in normal HSFs was biphasic, exhibiting both rapid and slow kinetic components (Figure 3A). Interestingly, we found that the rapid component of Tfn recycling was much less pronounced in NPFs, compared with normal HSFs (Figure 3A, inset).
Figure 3.
Recycling of Tfn in normal HSFs versus NPFs. (A) Cells were labeled with 125I-Tfn for 1 h at 16°C and acid-stripped, and the amount of cell-associated Tfn was quantified after various times at 37°C. (B–D) Cells were cotransfected with the DsRed2-Nuc plasmid and either DN rab4 or DN rab11 constructs. After 48 h, the cells were labeled for 1 h at 16°C with Alexa 488-Tfn, washed, acid-stripped, and then incubated for 0, 10, or 40 min at 37°C in the presence of excess unlabeled holo-Tfn (1 mg/ml). The amount of cell-associated Alexa 488-Tfn that remained cell-associated after 10 min (C) and 40 min (D) was quantified by image analysis and expressed as a percentage of the initial fluorescence at 0 min. UT, untransfected; T, transfected. Bar, 20 μm. Values are mean ± SD (n ≥ 60 cells; three independent experiments).
We next examined the effect of DN rab4 or rab11 on Tfn recycling. These experiments were carried out using fluorescent Tfn and image analysis since only a small fraction of the fibroblasts could be transfected, precluding biochemical studies with 125I-Tfn. Cells were pulse-labeled with fluorescent Tfn, and the effect of overexpression of DN rab4 or rab11 was examined. Expression of DN rab4 in normal HSFs resulted in accumulation of the fluorescent Tfn in punctate structures at 10 min, predominantly around the nucleus of the transfected cells, whereas Tfn-positive puncta were widely distributed throughout the cytoplasm in untransfected cells (Figure 3B). In normal HSFs, there was a 50% loss of cell-associated Tfn in the first 10 min and this loss could be blocked by DN rab4 but not DN rab11 (Figure 3C). In contrast, there was little effect of DN rab4 (or DN rab11) in NPFs at this time point (Figure 3, B and C). After 40 min at 37°C, >95% of the initial cell-associated Tfn was lost to the medium due to recycling in all three cell types (Figure 3D). Importantly, this loss was almost completely inhibited in NPFs expressing DN rab11 (but not DN rab4). Together, these results demonstrate that in NPFs (a) rab4-dependent rapid recycling of Tfn is inhibited in a similar manner to that observed using the fluorescent lipid, and (b) most Tfn recycling occurs through the rab11-dependent process in NPFs.
Role of Phosphoinositide 3-Kinases in PM Recycling of LacCer
Phosphoinositide 3-kinases (PI3Ks) are involved in many cellular processes, including the fast recycling pathway from EEs to the PM (Sonnichsen et al., 2000; Hunyady et al., 2002; van Dam et al., 2002). Here we examined the effect of the PI3K inhibitor WM on LacCer recycling in normal HSFs versus NPFs. Cells were pretreated with WM and the recycling experiments were performed as described above. In preliminary studies, we found that WM had no effect on the endocytosis of the LacCer analogue at 16°C in either HSFs or NPFs (see supplemental data, Figure 3). However, WM almost completely blocked the recycling of LacCer at the 10 min time point in normal HSFs (Figure 2B). WM inhibited the recycling of LacCer by ∼65% at 40 min in HSFs, similar to the effect of DN rab4 at this time point. Interestingly, WM treatment had no effect on the extent of LacCer recycling at either time point in NPFs (Figure 2, B and C). These results with WM, along with other data in Figure 2, suggest that PI3K activity is required for the rapid component of LacCer recycling, in agreement with previous studies of protein recycling.
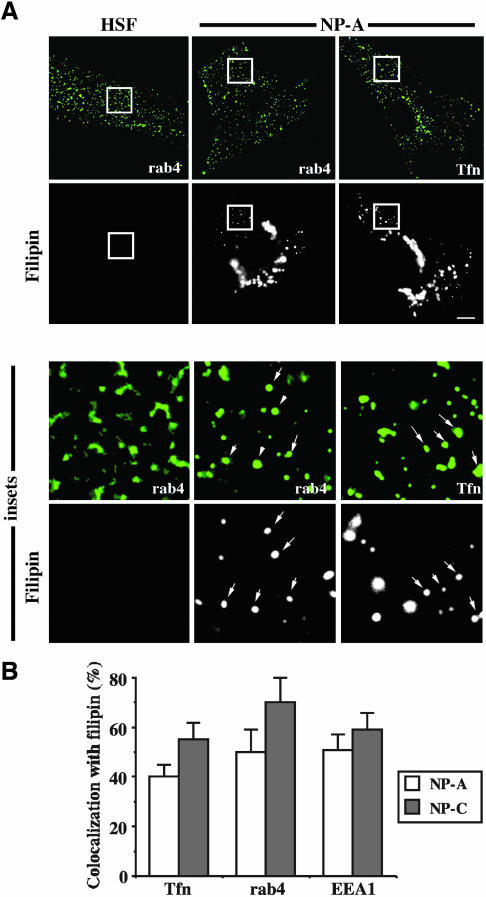
Accumulation of Cholesterol in the Rab4-containing Endosomes of NPFs
It was previously demonstrated that cholesterol accumulates in the late endosomes and lysosomes of NPFs and perturbs GSL transport along the endocytic route (Puri et al., 1999). Since rab4-dependent GSL recycling is impaired in NPFs, we examined cholesterol accumulation in rab4-containing EEs. EEs were labeled with fluorescent Tfn using a 16°C temperature block (Sharma et al., 2003) or by immunolocalization of endogenous rab4 or EEA1, and accumulation of cholesterol was detected with filipin staining (Neufeld et al., 1999). At the exposure settings used, no filipin staining was detected in the EEs of normal HSFs; however, filipin fluorescence was readily detected at the same settings in the rab4-positive endosomes of NP-A cells (Figure 4A) and NP-C cells (our unpublished results). In NPFs, 50–60% of the EEA1-positive structures, 40–55% of the Tfn-positive structures, and 50–70% of the rab4-positive structures were filipin-positive (Figure 4B), suggesting that only a subpopulation of EEs accumulated cholesterol. Using similar experimental conditions, we did not see filipin staining in rab11-positive endosomes (our unpublished results). Interestingly, overexpression of WT rab11 in normal HSFs elevates cholesterol levels in rab11-positive endosomes (Hölttä-Vuori et al., 2002).
Figure 4.
Rab4-containing endosomes in NPFs are enriched in cholesterol, as assessed by filipin staining. Normal HSFs or NPFs either were pulse-labeled with FITC-Tfn for 1 h at 16°C to label the EEs, washed, fixed, and stained with filipin to detect cholesterol, or were fixed and immunolabeled for endogenous rab4 or EEA1, followed by incubation with a secondary antibody conjugated to FITC with filipin present in each step. (A) Fluorescence micrographs showing the distribution of filipin versus rab4 (HSFs) or rab4 and Tfn (NP-A). Note the absence of filipin fluorescence in HSFs, compared with NP-A cells, using the same exposure. Arrows highlight several endosomes that were positive for both filipin and rab4 or FITC-Tfn. Similar results were obtained with NP-C fibroblasts (not shown). Bar, 15 μm. (B) The percentages of Tfn-, rab4-, or EEA1-positive endosomes that were also filipin positive in NPFs were quantified by image analysis. Values are mean ± SD (n ≥ 100 endosomes measured in each of three independent experiments).
Domain Organization of Rab4 Is Perturbed in EEs of NPFs
We next examined the domain organization of endogenous rab4 and rab11 in HSF and NPF endosomes, in a manner analogous to the studies of Sonnichsen et al. (2000). For rab4, cells were co-stained for endogenous rab4 and EEA1 and examined by confocal microscopy (Figure 5). EEA1 was usually present in globular structures and was used to identify EEs. In normal HSFs, EEA1 was often seen to be contiguous with endogenous rab4-positive structures that were tubular in nature; in NPFs, rab4-positive structures were often more globular (Figure 5A). We quantified the changes in rab4-positive endosomes by image analysis, measuring the length of the long and short axes of the rab4-positive structures and computing a “shape factor” that varied from 0 to 1, with the latter representing a perfect sphere. As shown in Figure 5B, rab4 was consistently more globular in NPF versus HSF endosomes. A similar perturbation was seen with GFP-tagged rab4 (supplemental Figure 4). To study rab11 morphology, we used cells that had been transfected with GFP-rab4 for 8–12 h prior to immunostaining endogenous rab11 (supplemental Figure 4). In contrast to rab4, endogenous rab11 on endosomes was predominantly tubular in both HSFs and NPFs (supplemental Figure 4, A and B). These data suggest that the organization of rab4 (but not rab11) was perturbed in the endosomes of NPFs.
Figure 5.
Rab4 organization is perturbed in NPF EEs. Normal HSFs or NPFs were grown under standard conditions (A and B) or in 5% lipoprotein-deficient serum to deplete cellular cholesterol (C and D) (see Materials and Methods). Samples were then fixed and costained with antibodies against rab4 (monoclonal) and EEA1 (polyclonal), and the distribution and shape of endogenous rab4 were examined by immunofluorescence and confocal microscopy. (A and B) In normal HSFs, endogenous rab4 was often found in tubular extensions projecting from EEA1-positive globular structures; in NPFs, rab4 and EEA1 extensively colocalized and were found mainly in globular structures. (C and D) Cholesterol depletion of NPFs resulted in a distribution of rab4 on EEA1-positive endosomes that was nearly identical to that seen in HSFs grown under standard conditions. (B and D) Quantitation of the shape factor (a/b; see text) for endogenous rab4 in HSFs and NPFs. Values are means ± SD from three independent experiments, and at least 100 rab4-positive endosomes were quantified for each data point shown. Bars, 1 μm.
Cholesterol Depletion Restores Normal Lipid Recycling and Rab4 Organization in NPFs
The presence of elevated cholesterol levels in the EEs of NPFs (Figure 4) and the abnormal membrane recycling seen in these cells (Figure 1) led us to examine the possibility that normal recycling might be restored in NPFs by cholesterol depletion. Cells were cultured in 5% lipoprotein-deficient serum for several days (see Materials and Methods), resulting in ∼ 40% reduction of cellular cholesterol (Martin et al., 1993). The recycling of BODIPY-LacCer was then analyzed, as in Figure 1, using cholesterol-depleted cells versus non-depleted control cells. Cholesterol depletion had almost no effect on recycling at 10 min in normal cells, whereas lipid recycling was significantly increased in NPFs, as evidenced by the decrease in cell-associated LacCer (Figure 1B, inset). Furthermore, this enhanced recycling could be blocked by DN rab4 (our unpublished results). At later times (20–40 min), the kinetics and amount of lipid efflux from normal HSFs and NPFs were not affected by cholesterol depletion (our unpublished results). Cholesterol depletion of NPFs also restored the tubular morphological characteristics of rab4 at EEs (Figure 5, C and D), such that they were similar to those seen in HSFs (Figure 5, A and B). Thus, cholesterol depletion partially restored the fast, rab4-dependent recycling in NPFs, as well as the organization of rab4 on EEs. Interestingly, the presence of excess cholesterol in normal HSFs dramatically reduced the fast recycling of LacCer and Tfn at 10 min of chase (supplemental Figure 5), similar to that seen in NPFs (Figure 2).
Inhibition of Rab4 Membrane Retrieval by GDI in NPFs
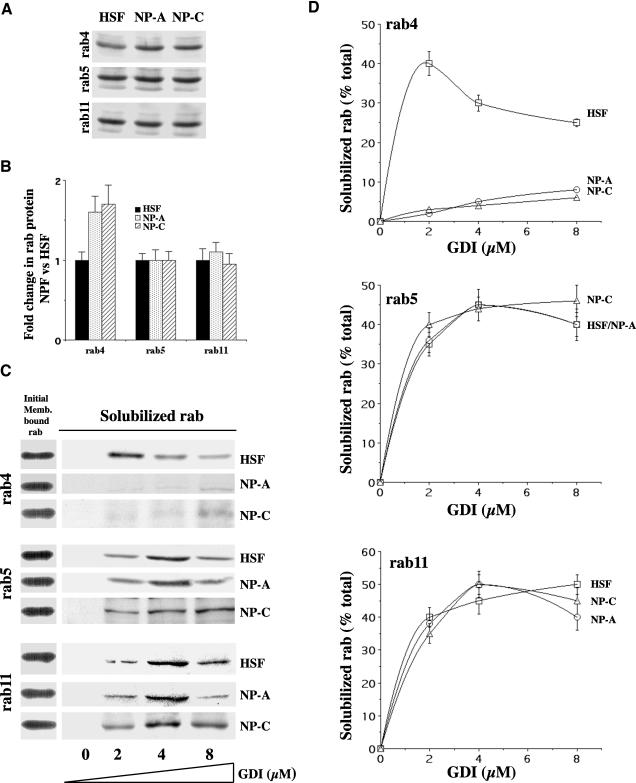
We examined rab4 protein expression in HSFs and NPFs and found that the levels of rab4 were increased 1.5–2-fold in membrane fractions from NPFs, compared with HSFs, although rab5 and rab11 levels were similar among these cell types (Figure 6, A and B). These data indicated that the impairment of rab4 function in NPFs was not a result of reduced rab4 expression. Therefore, we suspected that accumulated cholesterol might affect elements of the machinery involved in regulating rab4 localization and function.
Figure 6.
Rab4, but not rab11, is resistant to extraction from NPF endosomes by GDI. (A) Membrane fractions from HSFs and NPFs were prepared by high-speed centrifugation of postnuclear supernatants. Immunoblots show relative levels of rab proteins after equal membrane protein loading (25 μg/lane). (B) Quantitation of blots (as in A) shows changes in the levels of rab4 protein in NPFs versus HSFs (control). (C) Endosome-enriched membrane pellets were normalized for equal amounts of rab4, rab5, or rab11 and then incubated with 0–8 μM GST-GDI. The GST-GDI–bound rab protein was recovered on glutathione beads and analyzed by immunoblotting with polyclonal antibodies against the indicated rab. Note the resistance of rab4 to extraction by GST-GDI in NPFs versus control HSFs. (D) Quantitation of rab extraction from endosomal membranes by GDI. Blots (as in C) were quantified by densitometry, and results are expressed as a percentage of rab retrieved relative to the total membrane-bound rabs. Results are means ± SD for three experiments.
Normally, after fusion between donor and acceptor compartments, rabs are removed from membranes by rab GDIs and then delivered to donor compartment membranes for reutilization (Pfeffer, 2001; Seabra et al., 2002). We performed in vitro assays to determine whether rab4 retrieval by rab GDI from the endosomal membrane was selectively affected in NPFs. Endosomal membrane fractions were prepared from HSF and NPF homogenates and showed relative enrichment of rab4, rab5, and rab11, compared with other fractions (see supplemental Figure 1, A and B). Using membrane fractions from HSFs and NPFs with normalized levels of each rab, we then measured the ability of GDI to extract each rab in the presence of GDP (500 nM). GDI extraction of rab5 and rab11 from the membranes was similar in HSFs and NPFs; however, rab4 extraction from NPF endosomal membranes was significantly reduced (Figure 6C). Rab4, rab5, and rab11 retrieval by GDI (2–8 μM) ranged from 30 to 50% of each total membrane rab in HSF fractions (Figure 6D). Rab5 and rab11 extraction from NPF membranes was similar to that seen in HSF membranes. However, rab4 GDI extraction from NPF membranes was much lower (0–10% of total rab4) (Figure 6D). For both HSFs and NPFs, rab extraction was almost completely inhibited in the presence of GTPγS (500 nM) (our unpublished results).
Endosomal fractions from NPFs were found to have approximately threefold higher levels of cholesterol, compared with those from HSFs, as shown by TLC analysis of extracted lipids (supplemental Figure 1, C). Therefore, we tested the hypothesis that inhibition of rab4 extraction by GDI is a result of elevated cholesterol levels in endosomal membranes. We treated endosomal fractions from normal HSFs with a cholesterol/mβ-CD complex that increased cholesterol levels in the endosomal membranes by 4.5-fold (supplemental Figure 1, C). These membranes were then washed and GDI extraction of the various rabs from the membrane was assessed as above. Rab4 (but not rab5 or rab11) extraction was almost completely inhibited in normal HSF membranes loaded with excess cholesterol (Figure 7A). Finally, extraction of membrane cholesterol from NP-C endosomal fractions in vitro with mβ-CD reduced the amount of membrane cholesterol by approximately threefold, compared with untreated NPFs (supplemental Figure 1, C). Cholesterol depletion of NP-C (Figure 7B) and NP-A (data not shown) endosomal membranes restored GDI-mediated rab4 extractability to 30–50%, similar to results in normal HSFs (compare rab4 in Figures 7B and 6D). Taken together, the results in Figure 6 and Figure 7, A and B, suggest that, in NPFs, GDI-mediated retrieval of rab4 from endosomes is inhibited by elevated cholesterol levels.
Figure 7.
Effects of cholesterol and high-salt treatments on the GDI-mediated extraction of rab4 from endosomes in vitro. An enriched endosomal membrane fraction was prepared from HSFs or NPFs (see Materials and Methods), and the ability of GDI to extract rab4 from these membranes was quantified as in Figure 6. (A) Normal HSF endosomes were pretreated with 1 mM mβ-CD/cholesterol (increasing cholesterol levels approximately fourfold; see supplemental Figure 1, C) before GDI extraction. Note that GDI extraction of rab4 was selectively impaired and appeared similar to that seen for NPF endosomes (compare rab4 in Figure 6D for NPFs and Figure 7A for HSFs). (B) NP-C endosomal fractions were pretreated with 1 mM mβ-CD (decreasing cholesterol levels ∼2.5–3-fold; see supplemental Figure 1, C) before GDI extraction. Note that normal levels of GDI extraction of rab4 can be observed (compare rab4 in Figure 6D for NPFs and Figure 7B). (C) Endosome-enriched membrane pellets from HSFs and NPFs were briefly treated with 2 M KCl on ice, washed, and normalized for equal amounts of rab4 in treated (+KCl) and untreated (-KCl) membranes. The GDI extraction of rab4 was then quantified as in Figure 6, C and D. Note the extensive extraction of rab4 in KCl-treated NP-C membranes versus untreated membranes. (D) Quantitation of rab4 extraction from the indicated endosomal membrane fraction by GST-GDI was performed as in Figure 6D. Values are means ± SD from three independent experiments each in A, B, and D.
Finally, we tested the possibility that the inhibition of GDI extraction of rab4 in NPF endosomes was a result of altered interactions between rab4 and membrane proteins. To disrupt such interactions, endosomal membranes were pretreated with 2 M KCl for 30–60 s on ice and washed before the GDI extraction experiment. This treatment increased the GDI extraction of rab4 from NPF endosomes by 7–10-fold, whereas little or no effect was seen in HSF endosomes (Figure 7, C and D). No rab4 was solubilized from KCl-treated endosomes in the absence of GDI (Figure 7C). These results suggest that endosomal membrane proteins (e.g., rab effectors) might be involved in modulating the GDI extraction of rab4 from membranes in the presence of high cholesterol.
DISCUSSION
In this study, we examined the recycling of a fluorescent analogue of LacCer in HSFs and NPFs, using a combination of morphological, biochemical, and molecular approaches, and found that recycling was dramatically slowed in NPFs, relative to normal cells. In normal HSFs, LacCer recycling was predominantly a rab4-dependent process, with a half-time for recycling of ∼8 min. In contrast, the half-time for LacCer recycling was much longer (≈ 30–40 min) and rab4-dependent recycling was absent in NPFs. Similar alterations in the recycling kinetics and rab dependence were also observed for Tfn, indicating that the aberration in membrane recycling in NPFs was not limited to the LacCer analogue. We also demonstrated that cholesterol accumulated within rab4-containing EEs in NPFs and that these elevated endosomal cholesterol levels inhibited rab4 extraction by GDI. These results indicate that cholesterol accumulation in NPFs impairs membrane recycling by inhibiting endogenous rab4 retrieval from membranes by GDI.
LacCer Recycling in the Context of Previous Recycling Studies
Previous studies used fluorescent analogues of sphingomyelin (Koval and Pagano, 1989, 1990; Mayor et al., 1993; Hao and Maxfield, 2000), glucosylceramide (Kok et al., 1989), or the amphiphilic FM dyes (Hao and Maxfield, 2000) to examine lipid recycling in several different cell types. In general, those investigations demonstrated that lipid recycling occurs and is a rapid process, and that the itinerary followed by the lipid is very similar or identical to that of Tfn, a well-established marker for protein recycling. In the case of a fluorescent sphingomyelin analog in Chinese hamster ovary cells, the kinetics of lipid and protein recycling are virtually identical (Mayor et al., 1993). The present study differs from the previous ones in several important respects. First, the lipid analogues used previously were thought to enter cells through nonselective internalization or the internalization mechanism was not characterized, whereas the BODIPY-LacCer used in the present study enters cells almost exclusively via caveolar endocytosis (Puri et al., 2001; Sharma et al., 2003, 2004; Singh et al., 2003). Second, previous studies on lipid recycling relied on the kinetics of recycling and colocalization with Tfn to establish the itinerary of the recycling lipids, whereas in the current study we used DN constructs of rab4 and rab11 to inhibit particular steps in the recycling pathway, to allow dissection of the recycling itinerary. We found that the LacCer analogue was recycled predominantly through a rab4-dependent mechanism in normal cells and, as previously shown for Tfn, only the rapid rab4 recycling pathway was sensitive to WM, an inhibitor of PI3K (Sheff et al., 1999; Sonnichsen et al., 2000; Hunyady et al., 2002). The fact that molecules internalized through distinct mechanisms (i.e., caveolae versus coated pits) are recycled almost identically is consistent with our previous work showing that BODIPY-LacCer rapidly merges with markers internalized via clathrin-mediated endocytosis in EEA1-positive EEs of normal HSFs (Sharma et al., 2003).
Selective Perturbation of Rab4 in NPFs by Stored Cholesterol
An unexpected finding in the current study was that the half-times for recycling of both LacCer and Tfn were increased approximately fivefold in NPFs, relative to HSFs (Figures 1 and 3). Similarly, retarded excretion of internalized cholera toxin (initially bound to GM1 ganglioside at the PM) has been demonstrated for NP-C cells (Sugimoto et al., 2001), consistent with a defect in recycling. Here, we showed that impaired recycling resulted from an absence of rab4-dependent membrane recycling from EEs to the PM in NPFs, although rab11-dependent recycling was apparently normal in these cells (Figures 2 and 3). The absence of rab4-dependent recycling was not attributable to a decrease in the amount of rab4, because the levels of rab4 were significantly increased on NPF membranes, compared with HSFs (Figure 6B). Impairment of rab4-dependent recycling is also supported by the fact that LacCer recycling in NPFs was insensitive to WM (Figure 2) and could be restored to normal levels by overexpression of WT rab4 (supplemental Figure 2). Furthermore, the organization of rab4 on EEA1-positive EEs was perturbed in NPFs versus normal HSFs. Namely, the distribution of rab4 on EEs of NPFs frequently had a globular appearance, whereas rab4 was generally present in tubular extensions in normal HSFs (Figure 5, A and B), in agreement with previous studies (Sonnichsen et al., 2000). No perturbation in rab11 distribution was seen in NPFs (supplemental Figure 4).
Finally, a link between stored cholesterol and perturbation of rab4 function and distribution in NPFs was suggested by the following findings: 1) Cholesterol depletion of NPFs enhanced the recycling of LacCer in NPFs (Figure 1B). 2) Excess cholesterol in normal HSFs decreased LacCer recycling (supplemental Figure 5). 3) NPF (but not HSF) EEs exhibited strong filipin staining, indicative of high cholesterol levels (Figure 4). and 4) Cholesterol depletion of NPFs restored the tubular appearance of rab4 on EEA1-positive EEs (Figure 5, C and D).
Perturbation of Rab4 in Vitro by Cholesterol
Interestingly, Lebrand et al. (2002) showed that accumulation of cholesterol in late endosomes induced by treatment with the steroid analogue U18666A increased the membrane association of rab7 (but not rab5) on endosomes and interfered with its extraction by GDI, perhaps as a result of altered membrane fluidity. Here we showed that GDI extraction of rab4 (but not rab5 or rab11) was severely reduced in NPF endosomes (Figure 6, C and D). The inhibition of GDI extraction of rab4 observed in NPF membranes (Figure 6D) could be reversed with reduction of membrane cholesterol levels in vitro (Figure 7B). Conversely, addition of cholesterol to HSF endosomes in vitro selectively inhibited GDI extraction of rab4 (Figure 7A), similar to the impairment of rab4 GDI extraction seen in NPF membranes (Figure 6D). Therefore, our data suggest that the effects of cholesterol on rab4 function and localization observed in NPFs could result from impaired GDI extraction of rab4, which disrupts the normal cycling of this rab protein.
The underlying reason why GDI extraction of rab4 is perturbed by cholesterol is unknown. One possibility is that rab4 is in a GTP-bound state and thus inaccessible to GDI. However, our results do not support this model. First, brief treatment of NPF membranes with high salt (or acute depletion of cholesterol from these membranes) rendered rab4 readily extractable by GDI (Figure 7). Second, elevation of membrane cholesterol levels in normal HSF membranes inhibited the GDI extraction of rab4, mirroring the results in NPF membranes (Figures 6 and 7). Although we cannot exclude the possibility that both of these treatments could induce a rapid change in the GDP/GTP status of rab4, it seems more likely that rab4 is largely bound to GDP on NPF membranes, with extraction by GDI being prevented by other factors. A second possible explanation for the perturbation of rab4 function is that elevated endosomal cholesterol levels cause rab4 to interact more strongly with certain membrane proteins (e.g., its effectors), inhibiting its ability to be released by GDI. Our finding that pretreatment of NPF membranes with high salt greatly enhanced GDI extraction of rab4 supports this hypothesis. Alternatively, elevated cholesterol levels might strengthen a direct interaction between rab4 and endosomal membranes, e.g., through its hypervariable region and/or carboxy-terminal prenyl groups. Pretreatment with KCl could potentially disrupt this interaction and allow greater GDI extraction. It should be noted that isolated endosomes from normal HSFs overloaded with cholesterol in vitro display inhibited GDI extraction of rab4. Therefore, the membrane components responsible for inhibition of GDI solubilization under high-cholesterol conditions appear to be present on membranes from normal cells as well as NPFs. Future studies will be needed to identify the specific membrane constituents responsible for cholesterol-regulated inhibition of GDI extraction of selected rab proteins.
Supplementary Material
Acknowledgments
We thank Dr. P. van der Sluijs for rab4 constructs and the rab4 antibody, Dr. H. Radhakrishna for GFP-WT rab4, and Dr. W. Balch for GST-rab GDI. This work was supported by United States Public Health Service grants GM-60934 and GM-22942 to R.E.P. and by a grant from the Ara Parseghian Medical Research Foundation to R.E.P. and D.L.M.; A.C. was supported by a fellowship from the National Niemann-Pick Disease Foundation and D.K.S. by a Mayo Kendall Fellowship.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–05–0432. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–05–0432.
Abbreviations used: BODIPY-LacCer, N-[5-(5,7-dimethylborondipyrromethenedifluoride)-1-penanoyl]-lactosylsphingosine; DF-BSA, defatted bovine serum albumin; DN, dominant negative; EE, early endosome; FITC, fluorescein isothiocyanate; GDI, GDP dissociation inhibitor; GFP, gene fluorescent protein; GSL, glycosphingolipid; GST, glutathione S-transferase; HSF, human skin fibroblast; LacCer, lactosylceramide; mβ-CD, methyl-Β-cyclodextrin; NP-A, Niemann-Pick disease, type A; NP-C, Niemann-Pick disease, type C; NPF, Niemann-Pick fibroblast; P13K, phosphoinositide 3-kinase; PM, plasma membrane; RE, recycling endosome; SL, sphingolipid; SLSD, sphingolipid storage disease; Tfn, transferrin; WM, wortmannin; WT, wild-type.
The online version of this article contains supplemental material accessible through http://www.molbiolcell.org.
References
- Bligh, E.G., and Dyer, W.J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911-917. [DOI] [PubMed] [Google Scholar]
- Cavalli, V., Vilbois, F., Corti, M., Marcote, M.J., Tamura, K., Karin, M., Arkinstall, S., and Gruenberg, J. (2001). The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol. Cell 7, 421-432. [DOI] [PubMed] [Google Scholar]
- Chen, C.S., Bach, G., and Pagano, R.E. (1998). Abnormal transport along the lysosomal pathway in mucolipidosis, type IV disease. Proc. Natl. Acad. Sci. USA 95, 6373-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.S., Patterson, M.C., Wheatley, C.L., O'Brien, J.F., and Pagano, R.E. (1999). Broad screening test for sphingolipid-storage diseases. Lancet 354, 901-905. [DOI] [PubMed] [Google Scholar]
- Choudhury, A., Dominguez, M., Puri, V., Sharma, D.K., Narita, K., Wheatley, C.W., Marks, D.L., and Pagano, R.E. (2002). Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Invest. 109, 1541-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro, E., van der Sluijs, P., Galli, T., and Mellman, I. (1996). Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc. Natl. Acad. Sci. USA 93, 9559-9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure, J., Vignais, P.V., and Dagher, M.C. (1999). Phosphoinositide-dependent activation of Rho A involves partial opening of the RhoA/Rho-GDI complex. Eur. J. Biochem. 262, 879-889. [DOI] [PubMed] [Google Scholar]
- Hao, M., and Maxfield, F.R. (2000). Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 275, 15279-15286. [DOI] [PubMed] [Google Scholar]
- Hölttä-Vuori, M., Tanhuanpää, K., Möbius, W., Somerharju, P., and Ikonen, E. (2002). Modulation of cellular cholesterol transport and homeostasis by Rab11. Mol. Biol. Cell 13, 3107-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunyady, L., Baukal, A.J., Gaborik, Z., Olivares-Reyes, J.A., Bor, M., Szaszak, M., Lodge, R., Catt, K.J., and Balla, T. (2002). Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J. Cell Biol. 157, 1211-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar, M., Butters, T.D., Dwek, R.A., and Platt, F.M. (2002). Glycosphingolipid lysosomal storage diseases: therapy and pathogenesis. Neuropathol. Appl. Neurobiol. 28, 343-357. [DOI] [PubMed] [Google Scholar]
- Klein, U., Gimpl, G., and Fahrenholz, F. (1995). Alteration of the myometrial plasma membrane cholesterol content with β-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34, 13784-13793. [DOI] [PubMed] [Google Scholar]
- Kok, J.W., Eskelinen, S., Hoekstra, K., and Hoekstra, D. (1989). Salvage of glucosylceramide by recycling after internalization along the pathway of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 86, 9896-9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval, M., and Pagano, R.E. (1989). Lipid recycling between the plasma membrane and intracellular compartments: transport and metabolism of fluorescent sphingomyelin analogs in cultured fibroblasts. J. Cell Biol. 108, 2169-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval, M., and Pagano, R.E. (1990). Sorting of an internalized plasma membrane lipid between recycling and degradative pathways in normal and Niemann-Pick, type A fibroblasts. J. Cell Biol. 111, 429-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrand, C., Corti, M., Goodson, H., Cosson, P., Cavalli, V., Mayran, N., Faure, J., and Gruenberg, J. (2002). Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal, A.R., Chen, W., Tall, A.R., and Tabas, I. (2001). Acid sphingomyelinase-deficient macrophages have defective cholesterol trafficking and efflux. J. Biol. Chem. 276, 44976-44983. [DOI] [PubMed] [Google Scholar]
- Lim, S.N., Bonzelius, F., Low, S.H., Wille, H., Weimbs, T., and Herman, G.A. (2001). Identification of discrete classes of endosome-derived small vesicles as a major cellular pool for recycling membrane proteins. Mol. Biol. Cell 12, 981-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks, D.L., and Pagano, R.E. (2002). Endocytosis and sorting of glycosphingolipids in sphingolipid storage disease. Trends Cell Biol. 12, 605-613. [DOI] [PubMed] [Google Scholar]
- Martin, O.C., Comly, M.E., Blanchette-Mackie, E.J., Pentchev, P.G., and Pagano, R.E. (1993). Cholesterol deprivation affects the fluorescence properties of a ceramide analog at the Golgi apparatus of living cells. Proc. Natl. Acad. Sci. USA 90, 2661-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, O.C., and Pagano, R.E. (1994). Internalization and sorting of a fluorescent analog of glucosylceramide to the Golgi apparatus of human skin fibroblasts: utilization of endocytic and nonendocytic transport mechanisms. J. Cell Biol. 125, 769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield, F.R., and McGraw, T.E. (2004). Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5, 121-132. [DOI] [PubMed] [Google Scholar]
- Mayor, S., Presley, J.F., and Maxfield, F.R. (1993). Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 121, 1257-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld, E.B., et al. (1999). The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 274, 9627-9635. [DOI] [PubMed] [Google Scholar]
- Patterson, M., Vanier, M., Suzuki, K., Morris, J., Carstea, E., Neufeld, E., Blanchette-Machie, E., and Pentchev, P. (2001). Niemann-Pick disease type C: a lipid trafficking disorder. In: The Metabolic and Molecular Bases of Inherited Disease, vol. III, ed. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, New York: McGraw Hill, 3611-3634. [Google Scholar]
- Pfeffer, S.R. (2001). Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11, 487-491. [DOI] [PubMed] [Google Scholar]
- Puri, V., Watanabe, R., Dominguez, M., Sun, X., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (1999). Cholesterol modulates membrane traffic along the endocytic pathway in sphingolipid storage diseases. Nat. Cell Biol. 1, 386-388. [DOI] [PubMed] [Google Scholar]
- Puri, V., Watanabe, R., Singh, R.D., Dominguez, M., Brown, J.C., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2001). Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman, E.H., and Desnick, R.J. (2001). Niemann-Pick disease types A and B: acid sphingomyelinase deficiencies. In: The Metabolic and Molecular Bases of Inherited Disease, vol. III, ed. C.R. Scriver, A.L. Beaudet, W.S. Sly, and D. Valle, New York: McGraw-Hill, 3589-3610. [Google Scholar]
- Scriver, C.R., Beaudet, A.L., Sly, W.S., and Valle, D.D. (eds.) (2001). Lysosomal enzymes. In: The Metabolic and Molecular Bases of Inherited Disease, vol. III, New York: McGraw-Hill, 3371-3894. [Google Scholar]
- Seabra, M.C., Mules, E.H., and Hume, A.N. (2002). Rab GTPases, intracellular traffic and disease. Trends Mol. Med. 8, 23-30. [DOI] [PubMed] [Google Scholar]
- Seachrist, J.L., Anborgh, P.H., and Ferguson, S.S.G. (2000). β2-Adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 275, 27221-27228. [DOI] [PubMed] [Google Scholar]
- Sharma, D.K., Brown, J.C., Choudhury, A., Peterson, T.E., Holicky, E., Marks, D.L., Simari, R., Parton, R.G., and Pagano, R.E. (2004). Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol. Biol. Cell 15, 3114-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, D.K., Choudhury, A., Singh, R.D., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2003). Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564-7572. [DOI] [PubMed] [Google Scholar]
- Sheff, D.R., Daro, E.A., Hull, M., and Mellman, I. (1999). The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff, D.R., Pelletier, L., O'Connell, C.B., Warren, G., and Mellman, I. (2002). Transferrin receptor recycling in the absence of perinuclear recycling endosomes. J. Cell Biol. 156, 797-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R.D., Puri, V., Valiyaveettil, J.T., Marks, D.L., Bittman, R., and Pagano, R.E. (2003). Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 14, 3254-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen, B., De Renzis, S., Nielsen, E., Rietdorf, J., and Zerial, M. (2000). Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto, Y., Ninomiya, H., Ohsaki, Y., Higaki, K., Davies, J.P., Ioannou, Y.A., and Ohno, K. (2001). Accumulation of cholera toxin and GM1 ganglioside in the early endosome of Niemann-Pick C1-deficient cells. Proc. Natl. Acad. Sci. USA 98, 12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, O., Reinsch, S., Urbé, S., Zerial, M., and Parton, R.G. (1996). Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam, E.M., Ten Broeke, T., Jansen, K., Spijkers, P., and Stoorvogel, W. (2002). Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 277, 48876-48883. [DOI] [PubMed] [Google Scholar]
- van der Sluijs, P., Hull, M., Webster, P., Male, P., Goud, B., and Mellman, I. (1992). The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70, 729-740. [DOI] [PubMed] [Google Scholar]
- Vanier, M.T., and Millat, G. (2003). Niemann-Pick disease type C. Clin. Genet. 64, 269-281. [DOI] [PubMed] [Google Scholar]
- Walter, M., Davies, J.P., and Ioannou, Y.A. (2003). Telomerase immortalization upregulates Rab9 expression and restores LDL cholesterol egress from Niemann-Pick C1 late endosomes. J. Lipid Res. 44, 243-253. [DOI] [PubMed] [Google Scholar]
- Wood, P.A., McBride, M.R., Baker, H.J., and Christian, S.T. (1985). Fluorescence polarization analysis, lipid composition, and Na+,K+-ATPase kinetics of synaptosomal membranes in feline GM1 and GM2 gangliosidosis. J. Neurochem. 44, 947-956. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.