Abstract
During ex vivo myoblast differentiation, a pool of quiescent mononucleated myoblasts, reserve cells, arise alongside myotubes. Insulin/insulin-like growth factor (IGF) and PKB/Akt-dependent phosphorylation activates skeletal muscle differentiation and hypertrophy. We have investigated the role of glycogen synthase kinase 3 (GSK-3) inhibition by protein kinase B (PKB)/Akt and Wnt/β-catenin pathways in reserve cell activation during myoblast differentiation and myotube hypertrophy. Inhibition of GSK-3 by LiCl or SB216763, restored insulin-dependent differentiation of C2ind myoblasts in low serum, and cooperated with insulin in serum-free medium to induce MyoD and myogenin expression in C2ind myoblasts, quiescent C2 or primary human reserve cells. We show that LiCl treatment induced nuclear accumulation of β-catenin in C2 myoblasts, thus mimicking activation of canonical Wnt signaling. Similarly to the effect of GSK-3 inhibitors with insulin, coculturing C2 reserve cells with Wnt1-expressing fibroblasts enhanced insulin-stimulated induction of MyoD and myogenin in reserve cells. A similar cooperative effect of LiCl or Wnt1 with insulin was observed during late ex vivo differentiation and promoted increased size and fusion of myotubes. We show that this synergistic effect on myotube hypertrophy involved an increased fusion of reserve cells into preexisting myotubes. These data reveal insulin and Wnt/β-catenin pathways cooperate in muscle cell differentiation through activation and recruitment of satellite cell-like reserve myoblasts.
INTRODUCTION
Satellite cells are skeletal muscle adult stem cells that participate in postnatal muscle growth and regeneration. Although satellite cells are normally quiescent in adult muscle, they are responsible for muscle regeneration after injury and involved in work- or load-induced muscle fiber hypertrophy (Rosenblatt and Parry, 1992; Schultz and McCormick, 1994; De Angelis et al., 1999; Semsarian et al., 1999; Bodine et al., 2001).
Ex vivo models such as myogenic cell lines were isolated from adult mouse muscle and as such are derived from adult satellite cells. At the proliferative stage, myoblasts (activated satellite cells) can be grown extensively in high serum-containing medium and express MyoD, a muscle-specific transcription factor that regulates the differentiation process (Weintraub, 1993). When serum levels are lowered, myoblasts exit the cell cycle and spontaneously differentiate, giving rise to a heterogeneous population of cells. The first and major subpopulation is composed of myotubes, quiescent multinucleated cells expressing muscle-specific structural proteins. The remaining subset is composed of quiescent, mononucleated and undifferentiated cells termed reserve cells. Reserve cells retain the ability to be activated and proliferate after which they can be induced to differentiate, leading again to a new mixed population of myotubes and reserve cells. They also express at least two genes characteristic of skeletal muscle stem cells, namely, myf-5 and cd34 and from these characteristics, reserve cells are similar to satellite stem cells (Kitzmann et al., 1998; Lindon et al., 1998; Yoshida et al., 1998; Beauchamp et al., 2000). Previous studies showed that the effects of insulin and insulin-like growth factor (IGF)-1 and -2 on muscle cells are similar and dual: once activated by insulin or IGFs, the IGF-1 receptor becomes a potent proliferation and differentiation promoting factor. Coolican et al. (1997) showed that mitogenic and myogenic actions of insulin use distinct signaling pathways. The mitogen-activated protein kinase pathway plays a primary role in the mitogenic response and is inhibitory to the myogenic process. In contrast, activation of phosphatidyl inositol 3 kinases (PI3K) and p70S6Kinases is essential for insulin-stimulated differentiation (Coolican et al., 1997). Among downstream targets of PI3K, the serine/threonine protein kinase B/Akt (PKB/Akt), and particularly PKBβ/Akt2 were proposed as essential components for insulin-induced differentiation (Jiang et al., 1999; Vandromme et al., 2001; Sumitani et al., 2002). Once activated by insulin, PKB/Akt specifically phosphorylates various substrates, including Forkhead (FKHD), proapoptotic Bcl-2 family member BAD, and the serine/threonine glycogen synthase kinase 3 (GSK-3) (Cross et al., 1995; for reviews, see Harwood, 2001; Weston and Davis, 2001). Phosphorylation by PKB/Akt inactivates GSK-3 through an N-terminal serine. Generally, phosphorylation of target substrates by GSK-3 has an inhibitory effect (for review, see Doble and Woodgett, 2003). GSK-3 inhibition has an established role in neuronal plasticity and against apoptosis (Grimes and Jope, 2001). In addition, it has been implicated in IGF-induced myotube hypertrophy by regulating the translation factor eukaryotic initiation factor 2B (eIF2B) and by consequence the level of protein synthesis in myotubes (Rommel et al., 2001; Vyas et al., 2002). Further evidence for a role of GSK-3 in muscle differentiation is its implication as a component in Wnt signaling during embryogenesis. Members of the Wnt family bind to different Frizzled (Fz) receptors of the serpentine family and act through distinct pathways (Kawano and Kypta, 2003; Veeman et al., 2003). One group of Wnt signals through the canonical β-catenin/GSK-3 pathway (for review, see Church and Francis-West, 2002). In the absence of Wnt signals, GSK-3 phosphorylates components of the Wnt pathway, targeting β-catenin for ubiquitination and degradation by the proteasome. When the Wnt signaling pathway is activated, GSK-3 no longer phosphorylates β-catenin and the latter accumulates in the cytoplasm and is transduced in the nucleus, where it binds to TCF/Lef-1 transcription factors and induces expression of target genes. For example, Wnt1 and Wnt3, which signal through the canonical Wnt/β-catenin pathway, are secreted by the dorsal neural tube and required for induction of myogenesis during mouse development (Cossu and Borello, 1999). Recently, the implication of Wnt signaling in myogenic determination of adult stem cells has been suggested (Polesskaya et al., 2003). These authors propose a model by which secretion of Wnts that are mostly involved into noncanonical pathways (5a and b and 7a) by activated satellite cells and injured muscle fibers would mobilize resident CD45+ stem cells to become myogenic and thus participate in muscle regeneration. However, no increased expression of these Wnts was observed during muscle regeneration in an extensive study using microarray and quantitative reverse transcription-polymerase chain reaction (Zhao and Hoffman, 2004).
In the present study, we examined the roles of Insulin/IGF-1 and Wnt/β-catenin signaling pathways in the inhibition of GSK-3 during induction of quiescent reserve cell differentiation and in myotube hypertrophy. We show that GSK-3 is poorly phosphorylated in reserve cells and therefore most likely active. Activation of PI3Kinase by insulin or IGF-1 induced the phosphorylation and inactivation of GSK-3 during the induction of myogenesis. We also show that inhibition of GSK-3 by LiCl or SB216763 (SB) mimicked activation of the canonical Wnt pathway known to inhibit GSK-3 and induced nuclear accumulation of β-catenin in C2 myoblasts. GSK-3 inhibition by LiCl or Wnt enhanced the insulin-induced differentiation of reserve cells and increased myotube hypertrophy in both C2 and primary human myoblasts. Finally, we show that when reserve cells are cooperatively activated by insulin and GSK-3 inhibition they are directly recruited into preexisting myotubes.
MATERIALS AND METHODS
Reagents and Cell Culture
DMEM was purchased from Sigma (Saint Quentin Fallavier, France). Fetal calf serum (FCS) was from Eurobio (Les Ulis, France). Lithium chloride (LiCl) anhydrous and cell culture tested insulin were purchased from Sigma. SB216763 was purchased from Calbiochem (Merck Eurolab SA, Fontenay sous Bois, France).
C2.7, a subclone of C2 myoblasts (Yaffe and Saxel, 1977), maintained in a humidified incubator (37°C, 5% CO2), were grown in DMEM supplemented with 2 mM l-glutamine (Invitrogen, Cergy Pontoise, France), 100 units/ml penicillin, and 100 μg/ml streptomycin (Eurobio) and with 15% (vol/vol) FCS:growth medium (GM). For differentiation, confluent cells were refed with DMEM supplemented with 3% (vol/vol) FCS:differentiation medium (DM).
C2inducible cells (C2ind), a subclone of C2 cells dependent on addition of insulin for differentiation (Pinset et al., 1988), were grown in GM containing 10% serum and supplemented with 10-7 M dexamethasone (Sigma). For experiments assaying the conjugated effects of GSK-3 inhibition by LiCl and insulin treatment, cells were incubated in serum-free medium supplemented with dexamethasone.
Primary human myoblasts (PHM) were obtained from needle biopsy sample taken from the paravertebral muscle of a healthy subject, by using a protocol previously described for mouse muscle (Kitzmann et al., 1998). Cells were grown as clonal populations. To obtain homogenous populations of myoblasts, clones were tested individually for differentiation capacity. Positive clones were mixed together to obtain a population termed PHM, which can be grown for up to 10 passages. After transfer into differentiation medium, the majority of cells fuse into multinucleated myotubes after 36–48 h and express muscle-specific markers such as myogenin and troponin T.
Cont-3T3 fibroblast cells were cultured in growth medium composed of DMEM complemented with 10% (vol/vol) newborn calf serum. 3T3-Wnt1 fibroblasts were grown in proliferation medium composed of DMEM complemented with 10% (vol/vol) FCS and supplemented with G418 (Calbiochem) at a final concentration of 200 μg ml-1 (except during experiments).
Isolation of Reserve Cells
C2.7 reserve cells were obtained after differentiation of cultured C2.7 myoblasts. C2.7 cells were cultured for 3 d in GM, before changing into 3% FCS DM. After 4 d in DM, the supernatant of the cultures was diluted twice (vol/vol) with serum-free DMEM and cells were further incubated for 4 d. Finally, after a total of 8 d in differentiation, reserve cells were isolated by short trypsination (0.05% trypsin; 30 s), which specifically removed all myotubes leaving only quiescent undifferentiated reserve cells adherent to the dish (Kitzmann et al., 1998). To study reserve cell activation, cells were isolated and rinsed in serum-free DMEM alone for 4 h to allow respreading on the dish, and then stimulated or not with different molecules (Figure 3) for the indicated times.
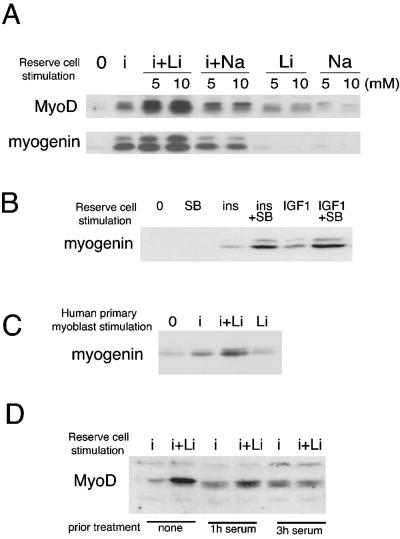
Figure 3.
Insulin and lithium chloride (or SB216763) cooperate to activate and induce the differentiation of quiescent reserve cells. (A) C2.7 reserve cell stimulation: C2.7 myoblasts were cultured in 15% serum proliferation medium for 3 d, and then in 3% serum differentiation medium for 4 d. At that time, the supernatant from these cells was diluted with the same volume of serum-free DMEM and after 2 d, the supernatant was again diluted twice with serum-free DMEM. After a total of 8 d of differentiation, undifferentiated quiescent reserve cells were isolated after removal of the myotubes by limited trypsination. The residual adherent reserve cells were incubated in DMEM for 4 h to allow respreading. Shown are Western blots for MyoD and myogenin after 24-h stimulation of reserve cells by different treatments, as indicated: no treatment (0), or insulin alone at a concentration of 3 μg ml-1 (i), LiCl alone at 5 or 10 mM (Li), or insulin and LiCl 10 mM (i+Li). NaCl (Na or i+Na) was used as a control for LiCl addition. (B) Mouse C2.7 reserve cells were treated as described in A. SB216763 was used instead of LiCl at a concentration of 3 μM (SB). Also shown is the effect of 10 nM IGF-1 instead of insulin. (C) Human primary reserve cell stimulation: human reserve cells purified as described in Materials and Methods were treated with insulin and/or LiCl for 24 h in serum-free DMEM before analysis for myogenin expression. Shown is a representative result repeated in three independent experiments. (D) Mouse C2.7 reserve cells were isolated as for Figure 3A, cultivated in DMEM for 4 h to respread on the dish, and then stimulated with serum, at a final concentration of 15% for the indicated times, to reenter the cell cycle, before 24-h stimulation with insulin alone at 3 μg ml-1 (i) or insulin and LiCl at 10 mM (i+Li). Cells were harvested and analyzed by Western blot for MyoD expression.
Human reserve cells were purified by the following procedure. Primary human myoblasts were grown to confluence in growth medium (DMEM containing 10% FCS and 1% ultroser [Biomedia]) before transfer to differentiation medium (DMEM containing 5% FCS) for 6 d. At that time, myotubes were present together with nonfusing reserve cells. The cultures were trypsinized for 30 s with 0.1% trypsin/0.1 mM EDTA to remove myotubes, leaving only reserve cells attached to the dish. Treatment with insulin and/or LiCl was performed for 24 h in serum-free DMEM.
Wnt-presenting Monolayers
Monolayers expressing Wnt1 were generated after retroviral infection of 3T3J2 fibroblasts (Rheinwald and Green, 1975). Briefly, 20 μg of each plasmid (pMV-7 or pMV-7/Wnt1), were transfected by calcium precipitation technique into GP+E ecotrophic packaging cell line. After 2 wk of selection with G418 at 500 μg/ml, stable transfectants were obtained and the supernatants were collected (Brown and Scott, 1987). Infection of 3T3J2 was performed using the centrifuged supernatant supplemented with 8 μg/ml polybrene for 6 h. Cell lines were then selected as described above, and the polyclonal population was used as Wnt-expressing monolayer. Wnt1 expression was assessed by Western blotting by using the monoclonal antibody anti-Wnt1, clone Mc123 (Euromedex, Mundolshein, France; Brown et al., 1987). Cells were maintained in G418 for selection.
Cocultures
Reserve cells, isolated from myotubes by short trypsination (as described above), were treated with cell dissociation solution nonenzymatic (Sigma) for 5 min before harvesting in serum-free DMEM supplemented with 200 μg/ml bovine serum albumin (BSA) (cell culture tested; Sigma) and counting. Reserve cells were plated on either subconfluent Wnt1-3T3 or Cont-3T3 monolayers at 5 × 104 cells/cm2 in serum-free DMEM. Cells were allowed to attach and spread for 15 h before stimulation with insulin or insulin and LiCl for 24 h and harvesting. To investigate the effects on myotube hypertrophy, C2.7 cells were grown in GM for 3 d. At that time, Cont-3T3 and Wnt1-3T3 were passaged, counted, and 3 × 105 cells were plated in DM (3% FCS) over the confluent C2.7 cells. After 16 h (to allow 3T3 cell respreading), cocultures were stimulated with or without insulin, LiCl, or both. Overlaying Wnt-3T3 cells onto C2.7 cells that had been differentiated for longer than 24 h resulted in variable loss of C2.7 myotube adhesion. For this reason, 3T3-Wnt cells were cocultured onto C2.7 cells only during the first 24 h of differentiation and harvested 24 h later.
Western Blot Analysis
Cells were lysed in 2× Laemmli buffer (100 mM Tris HCl, pH 6.8, 4% SDS, 20% glycerol, 200 mM dithiothreitol, bromphenol blue). Total protein extracts (50 μg; protein concentrations were determined using the Bio-Rad DC kit; Bio-Rad, Hercules, CA) were transferred to nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany) except for the antiphospho-antibodies (Figure 1) where polyvinylidene difluoride membrane (Millipore, Molshein, France) were used. Western blot were performed as described previously (Vandromme et al., 2001). The following primary antibodies were used: polyclonal C20 anti-MyoD (Santa Cruz Biotechnology, Santa Cruz, Germany), 1/400; polyclonal anti-Myf-5 (Santa Cruz Biotechnology), 1/2000; monoclonal anti-myogenin F5D (BD Biosciences, Ozyme, St Quentin-en-Yvelines, France), 1/1000; polyclonal anti-P-473 Akt (Cell Signaling Technology, Ozyme, France), 1/1000; polyclonal anti-P-GSK-3 (Cell Signaling Technology, Beverly, MA), 1/2500; monoclonal anti-pan-GSK-3 (Santa Cruz Biotechnology), 1/1000; polyclonal anti-pan-Akt (Cell Signaling Technology), 1/1000; monoclonal anti β-catenin (BD Biosciences), 1/1000; monoclonal anti-troponin T (Sigma), diluted 1/200; and monoclonal anti-α tubulin (clone DM1A; Blose et al., 1984), 1/10,000.
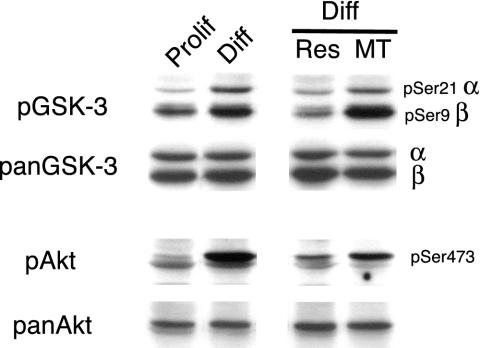
Figure 1.
GSK-3 phosphorylation increases specifically in myotubes during differentiation of C2.7 myoblasts. Proliferative (Prolif) or 3-d differentiated (Diff) C2.7 myoblasts were harvested. The two populations of cells after 3-d differentiation were separated into undifferentiated reserve cells (Res) and myotubes (MT), harvested and analyzed by Western blot for total content of GSK-3α and β (panGSK-3), and for GSK-3 phosphorylation status (pGSK-3). In parallel, extracts were blotted with an antibody antiphospho-Akt directed against serine 473 (pAkt) and for total Akt protein (pan-Akt).
Cell Fractionation
Isolated reserve cells were rinsed twice with cold phosphated-buffered saline (PBS), incubated 10 min, and harvested in hypotonic buffer (10 mM HEPES, pH 7.8, 20 mM NaCl, and protease inhibitors; Sigma), and then centrifuged 15 min at 1000 rpm. The supernatant was centrifuged 15 min at 15,000 × g, this second supernatant (cytoplasmic fraction) was recovered, and protein concentration was determined using the Bio-Rad calibration kit. Proteins were precipitated with trichloroacetic acid and rinsed with diethylether/ethanol [4:1 (vol/vol)]. Proteins (50 μg) were analyzed by Western blot.
The Western blot for β-catenin was quantified using ImageQuant software (Amersham, Biosciences, Orsay, France).
Immunofluorescence and Microinjection
For β-catenin imunofluorescence staining, myoblasts were treated for 3 h with or without insulin and/or 25 mM LiCl before fixation in -20°C methanol for 15 min. β-Catenin distribution was revealed using monoclonal anti β-catenin (BD Biosciences) diluted 1/300 for 1 h at 37°C followed by Alexa 555-conjugated donkey anti-mouse antibody (Molecular Probes, Leiden, The Netherlands). Fluorescence photomicrographs were acquired on a Leica DMR1 fluorescence microscope with 16 and 40× lenses by using a CanonEOS300D digital camera and Adobe PhotoShop 5.0 software.
For microinjection experiments, C2.7 cells or human primary myoblasts were cultured in DM for 2 d to allow differentiation, and isolated myoblasts adjacent to myotubes were microinjected with Texas Red-conjugated Dextran (Molecular Probes), taking care not to microinject any myotubes. Immediately afterward, cells were stimulated with insulin or insulin and LiCl and fixed with formalin 24 h later. Cells were stained for troponin T with monoclonal anti-TropT (1/200; Sigma) and DNA with bisbenzimide 33258 (Hoechst; Sigma) and analyzed by fluorescence microscopy. The percentage of microinjected cells found incorporated in myotubes was calculated and averaged from three different experiments.
Phase Contrast Micrographs and Myotube Hypertrophy Analysis
For morphological analysis of myotubes, cells were fixed with 3.7% formaldehyde (Sigma) in PBS for 7 min and then kept in 0.5% BSA PBS at 4°C. Phase contrast pictures were taken as described previously using Kodak technical pan film (Girard et al., 1991) on a Zeiss Axiovert inverted microscope. For diameter measurements and nuclei quantification, fixed cells were stained with bisbenzimide 33258 (Sigma). To quantify the effect of insulin and LiCl on myotube hypertrophy, we assessed mean diameter of the largest myotubes under control and treated conditions. For each condition, six different 16× microscopic fields were randomly chosen from four independent experiments, and the width of the 10 largest myotubes in each field was measured. Mean values constituted a measure of >200 myotubes for each treatment. Additionally, hypertrophic effects involving fusion were assessed by measuring the mean number of nuclei per myotube for the largest myotubes per field. Again six fields per dish were analyzed for each condition and the 10 largest myotubes in each field counted.
RESULTS
GSK-3 Phosphorylation Increases Specifically in Myotubes during Differentiation
We and others (Jiang et al., 1999; Vandromme et al., 2001; Sumitani et al., 2002) have previously shown the implication of PKB/Akt protein kinases in the process of myogenic differentiation. Because GSK-3 is one of the earliest and best-characterized substrates for PKB/Akt (Cross et al., 1995), we first examined the expression and activity profiles for GSK-3 during myogenic differentiation by using the mouse cell line C2.7 (Yaffe and Saxel, 1977). Protein extracts were prepared from total proliferative myoblasts (Prolif), differentiated cells (Diff), but also, because differentiated cells contain two main subpopulations of cells, from quiescent reserve cells (Res) and differentiated myotubes (MT) (Kitzmann et al., 1998). Total cellular GSK-3α and β content (Figure 1, panGSK-3) and phosphorylation status by using an antiphospho-antibody directed against the N-terminal serine (Figure 1, pSer21 for GSK-3α and pSer9 for GSK-3β) were measured. Phosphorylated GSK-3 corresponds to its inactivated state.
Similar GSK-3 protein levels were observed in both proliferative and differentiated C2.7 stages, as well as in quiescent reserve cells and myotubes (Figure 1, panGSK-3). In contrast, GSK-3 phosphorylation increased during differentiation (Figure 1, compare Prolif and Diff, lane pGSK-3) and was specifically associated with differentiated myotubes and not quiescent undifferentiated reserve cells (Figure 1, compare Res and MT, lane pGSK-3). To correlate this increased GSK-3 phosphorylation with PKB/Akt activity, we also examined the levels and phosphorylation status of PKB/Akt during C2.7 cell differentiation. Whereas PKB/Akt protein levels (measured here as the sum of both Akt1 and Akt2 isoforms) remained unchanged throughout differentiation (Figure 1, compare Prolif, Diff, Res and MT, lane panAkt), PKB/Akt phosphorylation increased during differentiation and in a manner similar to phosphorylated GSK-3, this increase occurred mainly in the subpopulation of differentiated myotubes (Figure 1 compare Prolif, Diff, Res, and MT, lane pAkt).
These results support that GSK-3 is a downstream target of PKB/Akt in the insulin activation pathway leading to skeletal muscle differentiation.
GSK-3 Inhibition Allows Differentiation of the Insulin-dependent C2ind Cell Line
To confirm this conclusion, we exploited the C2ind cell line. This subclone of C2 has lost the capacity to differentiate spontaneously due to an absence of IGF-2 secretion and associated loss of MyoD expression (Pinset et al., 1988; Montarras et al., 1989). Addition of exogenous insulin or IGF to the classical differentiation medium permits C2ind differentiation. C2ind represent a useful means to study the effectors of the insulin signaling pathway, because overexpression of constitutively active downstream components in insulin signaling should mimic the effects of insulin and restore the capacity of the C2ind to differentiate.
As shown in Figure 2, overexpression of a constitutively activated form of hemagglutinin-tagged PKBβ/Akt2 (myristylated membrane-bound PKBβ/Akt2) in C2ind enabled differentiation in a classical 3% serum differentiation medium. After 3 d of differentiation, expression of both myogenin, an early marker of differentiation and troponin T, a late marker of differentiation, were apparent together with myotube formation (Figure 2A, PKBβm). In contrast, wild-type or kinase dead forms of PKBβ/Akt2 did not restore C2ind differentiation (Figure 2A, PKBβwt; unpublished data). This result was consistent with previous studies showing that PKB/Akt is implicated in skeletal muscle differentiation (Jiang et al., 1999; Vandromme et al., 2001; Sumitani et al., 2002).
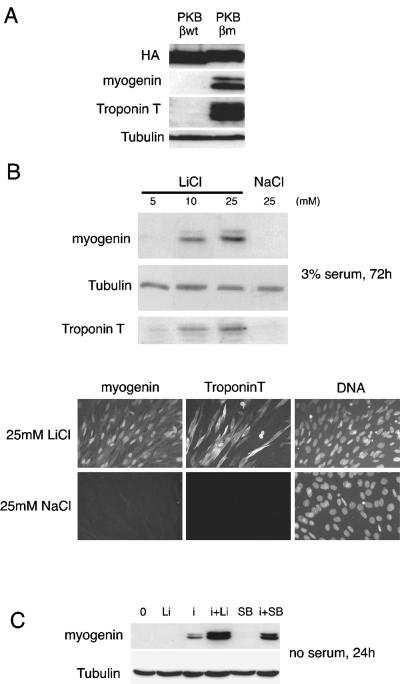
Figure 2.
GSK-3 inhibition restores differentiation of insulin-dependent C2ind in the presence of serum and cooperates with insulin in serum-free conditions. (A) C2ind cells were cultured in a 10% serum growth medium containing 10-7 M dexamethasone and transfected with plasmids coding for different forms of PKBβ/Akt2: wild-type (PKBβWT) and constitutively active (PKBβm) (Vandromme et al., 2001). Then, the medium was changed for a differentiation medium containing 3% serum for 3 d. Cells were harvested and analyzed by Western blot for expression of differentiation markers. (B) C2ind cells were cultured in a 10% serum growth medium containing 10-7 M dexamethasone for 3 d before induction of differentiation in a 3% serum medium for 3 d. Increasing concentrations of LiCl (5, 10, or 25 mM) or NaCl (25 mM) as a control, were added to the differentiation medium and cells subsequently harvested and analyzed by Western blot or fixed for immunofluorescence analysis. In both cases, expression of myogenin was evaluated as markers of differentiation of the C2ind and shown for the Western blot analysis is the expression troponin T a late marker of differentiation. (C) C2ind cells were cultured in a 10% serum growth medium containing 10-7 M dexamethasone for 3 d, and then the medium was changed for serum-free DMEM. C2ind cells were stimulated with LiCl (Li), insulin (i), or a combination of both and in parallel with SB216763 at 3 μM (SB) and a combination of SB216763 and insulin for 24 h before harvesting and Western blot analysis for myogenin expression.
Because PKB/Akt can phosphorylate and inactivate GSK-3, we next investigated whether direct inhibition of GSK-3 in C2ind cells would mimic insulin or IGF stimulation and restored their differentiation. GSK-3 inhibition can be achieved directly, by treatment with LiCl, which is a noncompetitive inhibitor of GSK-3 (Harwood, 2001). As shown in Figure 2B, addition of increasing concentrations of LiCl but not NaCl to the 3% serum DM induced expression of myogenin or troponin-T and enabled differentiation of these cells. The expression of myogenin and troponin T also was clearly induced by LiCl but not NaCl when cells were analyzed by immunofluorescence (Figure 2B, bottom). Thus, GSK-3, in combination with serum factors, participates in the differentiation of C2ind cells presumably by the insulin signaling pathway, because inhibition of GSK-3 by LiCl mimicked stimulation of differentiation by insulin in these cells.
We therefore examined whether GSK-3 inhibition alone via LiCl or SB216763 (SB), a pharmacological inhibitor of GSK-3 (Coghlan et al., 2000; Cross et al., 2001) was sufficient to restore differentiation of C2ind in the absence of serum factors. C2ind cells were grown for 3 d in proliferative medium, and then medium was changed for serum-free DMEM, alone or complemented with insulin, LiCl, or SB for 72 h (Figure 2C, i, or Li or SB). LiCl or SB alone did not induce myogenin expression and thus failed to induce differentiation of the C2ind cells. In contrast and as reported previously (Pinset et al., 1988), insulin alone induced differentiation of C2ind cells. To test whether GSK-3 inhibition may cooperate with insulin to further differentiate C2ind in the absence of serum, confluent C2ind cells were incubated for 24 h in serum-free DMEM complemented with insulin and LiCl or insulin and SB. In the absence of serum, both SB or LiCl with insulin strongly induced myogenin expression compared with insulin alone (Figure 2C, i+Li, i+SB). Therefore, a cooperative effect between insulin and GSK-3 inhibition promotes differentiation of C2ind cells, as assessed by induction of myogenin expression.
GSK-3 Inhibition by Lithium Chloride or SB216763 Cooperate with Insulin in Differentiation of Quiescent Reserve Cells
As shown in Figure 1A, the levels of GSK-3 phosphorylation are very low in reserve C2.7 cells, indicating that GSK-3 is in an active nonphosphorylated state in reserve cells coincident with the low levels of PKB/Akt activity (Figure 1). Such high GSK-3 activity could be linked to the quiescent state and absence of differentiation of C2.7 reserve cells. In addition, C2.7 reserve cells share common characteristics with C2ind cells because they neither express MyoD nor exhibit high levels of myf-5, two myogenic factors that regulate muscle differentiation (Montarras et al., 1989; Kitzmann et al., 1998; Yoshida et al., 1998). Because of these similarities and given the capacity of GSK-3 inhibition to allow C2ind differentiation, we questioned whether inactivation of GSK-3 by LiCl or SB in this population of quiescent undifferentiated cells could lead to their activation and the induction of differentiation.
C2.7 reserve cells were isolated as described in Materials and Methods and stimulated with insulin, LiCl or insulin and LiCl for 24 h (Figure 3A). We then determined the protein levels of two MyoD family genes: MyoD, a marker of reserve cell activation and myogenin, a differentiation marker. Insulin alone induced myogenin expression and to a lesser extent MyoD (Figure 3A, lane i). Lithium chloride alone (Li) at 5 or 10 mM resulted in limited induction of MyoD but little or no myogenin induction even (Figure 3A) when blots were overexposed. However, the combination of insulin and LiCl (i+Li) strongly induced both MyoD and myogenin at both 5 and 10 mM. In contrast, no such effects were observed when sodium chloride (NaCl) was substituted for LiCl, either alone or with insulin (Figure 3A, lanes Na and i+Na), showing that insulin and LiCl cooperate to induce differentiation of C2.7 quiescent reserve cells. A similar induction of myogenin was also seen when GSK-3 was inhibited using the pharmacological inhibitor of GSK-3, SB216763, instead of LiCl together with insulin addition. As shown in Figure 3B, treatment of C2.7 reserve cells with a combination of SB and insulin was highly effective in inducing increased expression of myogenin in comparison with insulin alone. Similar results were observed when insulin was substituted with 10 nM purified IGF-1 (Figure 3B).
The cooperative effect of insulin and LiCl on reserve cell differentiation also was observed using reserve cells isolated from primary cultures of human myoblasts (Figure 3B, compare line i+Li to i or Li alone). Human reserve cells were treated with insulin and/or LiCl for 24 h in serum-free DMEM. It should be noted that human primary reserve cells are too fragile to allow application of the extensive quiescence-inducing protocol used for mouse C2.7 (cf. Materials and Methods). In untreated cells, a low level of myogenin is seen that is similar to that on cells treated with LiCl alone (Figure 3C). However, myogenin levels increased on treatment with insulin, and there was a marked increase over basal levels when cells were treated with both insulin and LiCl. Shown is a representative result repeated in three independent experiments. These results confirm that inhibition of GSK-3 by either LiCl or SB216763 cooperated with the insulin to activate and differentiate reserve cells from mouse or human origin.
Because the response of reserve cells to GSK-3 inhibition is likely to be related to their quiescent G0 state, we examined the consequences of GSK-3 inhibition and insulin stimulation on MyoD expression in C2.7 reserve cells upon entry of these cells into the cell cycle. C2.7 reserve cells were isolated as described above, cultured in serum-free DMEM for 4 h, refed with serum for 1 or 3 h or not, before stimulation with insulin alone (i) or insulin and LiCl (i+Li). Cells were harvested 24 h after and probed for MyoD expression. Whereas a synergistic effect of insulin plus LiCl was observed on the expression of MyoD in cells untreated with serum (Figure 3A), as little as 1 h after reentry into the cell cycle, this cooperation was already less pronounced and is completely lost in cells treated 3 h with serum (Figure 3D). These data show that insulin stimulation and GSK-3 inhibition cooperate in the induction of MyoD only in reserve cells that are synchronized in a quiescent G0 phase of the cell cycle and not in cells that have reentered the cell cycle.
Wnt1 Addition Mimics the Cooperative Effect of Lithium Chloride or SB216763 on Insulin-induced Differentiation of Reserve Cells
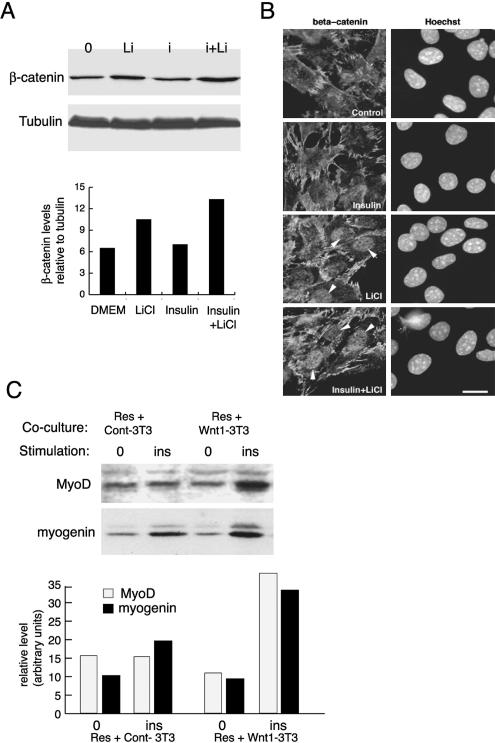
GSK-3 is an essential component of the canonical Wnt signaling pathway: its inhibition upon activation of the Wnt cascade releases its substrate, β-catenin, from a large cytoplasmic complex of proteins, including axin and adenomatous polyposis coli, thereby preventing ubiquitination and degradation of complexed β-catenin by the proteasome (Kühl et al., 2000). To investigate whether LiCl, by inhibiting GSK-3, actually mimics the effects of the Wnt canonical pathway in our model, we tested whether LiCl could lead to the release of soluble β-catenin in the cytoplasm of reserve cells. Treatment of reserve cells with insulin alone did not induce cytoplasmic accumulation of β-catenin in our system. LiCl alone or associated with insulin released a pool of soluble β-catenin from the plasmic membrane to the cytoplasm by 3 h after stimulation (Figure 4A). Densitometric quantification of the bands with respect to tubulin (a marker of soluble cytoplasmic proteins when isolated at 4°C) indicates that LiCl induced a 1.5-fold increase in the quantity of soluble β-catenin and insulin plus LiCl resulted in a 2.0-fold increase showing that these treatments promoted accumulation of soluble β-catenin in the cytosol (Figure 4A). Because the release of β-catenin by activation of the Wnt pathways is known to induce accumulation of β-catenin in the nucleus, where it was shown to be required for Wnt-dependent effects (Cong et al., 2003; for review, see Kawano and Kypta, 2003), we investigated whether treatment with LiCl and insulin was accompanied by nuclear accumulation of β-catenin. For this, C2 myoblasts treated with insulin, LiCl or both were fixed for analysis of β-catenin distribution by immunofluorescence. As shown in Figure 4B, β-catenin is mostly localized to adherent junctions in untreated or insulin-treated cells. In contrast, treatment with LiCl or LiCl plus insulin both induced clear accumulation of β-catenin in the nuclei of treated cells. This effect was semiquantified by counting the percentage of cells showing positive staining for β-catenin in the nucleus, whereas in control or insulin-treated cells, positively stained nuclei were <3%, this value was 46 ± 7% in LiCl-treated myoblasts and 53 ± 9% in LiCl plus insulin-treated cells. These data show that LiCl, like Wnt activation, causes nuclear accumulation of β-catenin.
Figure 4.
Canonical Wnt signaling reproduces the additive effect of lithium chloride on insulin-induced differentiation of reserve cells. (A) Reserve cells prepared as described in Materials and Methods and Figure 3A were incubated for 4 h in DMEM to allow them to spread again on the dish, and then they were stimulated for 3 h with LiCl (Li), insulin (i), or insulin and LiCl together (i+Li). After cell fractionation, soluble cytoplasmic fractions were analyzed by Western blot for levels of β-catenin protein. The same blot was then reprobed for the levels of tubulin. Shown are the blots for soluble β-catenin and tubulin and below a histogram showing the relative levels of β-catenin solubilized with respect to tubulin as determined by densitometric analysis of the blots. (B) Immunofluorescence staining for β-catenin in C2.7 cells treated or not for 3 h with 3 μg ml-1 insulin and/or 25 mM LiCl as indicated. Arrowed are cells showing clear nuclear accumulation of β-catenin. Bar, 10 μM. (C) Reserve cells, prepared as in Figure 3A, were removed from the dish and overlain in DMEM at equal densities on different 3T3 cell monolayers: Wnt1-3T3 cells are 3T3J2 overexpressing Wnt1 and Cont-3T3 are 3T3J2 cells with the empty vector. After spreading, cells were stimulated or not (0) with insulin at 3 μg ml-1 (i) for 24 h. Extracts were analyzed by Western blot for MyoD and myogenin. Quantification of the relative intensities of the bands was done by densitometric scanning of the blot using ImageQuant.
Wnts are secreted glycoproteins that remain trapped in the extracellular matrix and poorly diffuse in the culture medium. Consequently, to test the effect of activation of the Wnt pathway on differentiation of reserve cells, we chose to overlay C2.7 reserve cells (Res) onto 3T3J2 cells overexpressing Wnt1 (Wnt1-3T3), a Wnt protein known to belong to the canonical pathway or, as control 3T3J2 cells transfected with an empty vector (Cont-3T3). Quiescent C2.7 reserve cells (Res) were resuspended in serum-free DMEM before plating onto Control or Wnt1-overexpressing 3T3 cells with or without addition of insulin for 24 h. After harvesting, protein extracts were analyzed by Western blot for MyoD and myogenin (Figure 4C). Coculturing reserve cells with control 3T3 cells or Wnt1-expressing 3T3 cells had no effect on MyoD nor myogenin expression (Figure 4C, Res+Cont-3T3 and Res+Wnt1-3T3, lanes 0). After insulin stimulation, coculturing reserve cells with Cont-3T3 cells showed a slight increase in myogenin expression (Figure 4C, Res+Cont-3T3). In contrast, Wnt1 cooperated with insulin to induce a marked increase in both MyoD and myogenin expression when added to reserve cells cocultured on Wnt1-3T3 (1.7- and 2-fold stimulation of myogenin and MyoD expression, respectively, compared with Cont-3T3+insulin; Figure 4C). These data show that Wnt1-expressing cells, like LiCl treatment, enhanced the differentiating effect of insulin observed on C2.7 reserve cells an effect not seen with 3T3 fibroblasts cells alone.
Inhibition of GSK-3 by Lithium Chloride Cooperates with Insulin to Increase Myotube Size in Differentiation
It has already been shown that GSK-3 plays a role in myotube hypertrophy because inhibition of GSK-3 by the IGF pathway results in derepression of eIF2B and subsequent myotube hypertrophy. Together with an increase in total protein content per myotube, ex vivo muscle hypertrophy also involves an increase in nuclei number per myotube (Rommel et al., 2001), possibly resulting from satellite cell activation and fusion to preexisting myotubes. Because insulin and LiCl-SB/Wnt1 could activate reserve cells and direct them toward the differentiation program (Figures 3 and 4C), we examined whether such treatment could cooperate in the appearance of hypertrophied myotubes.
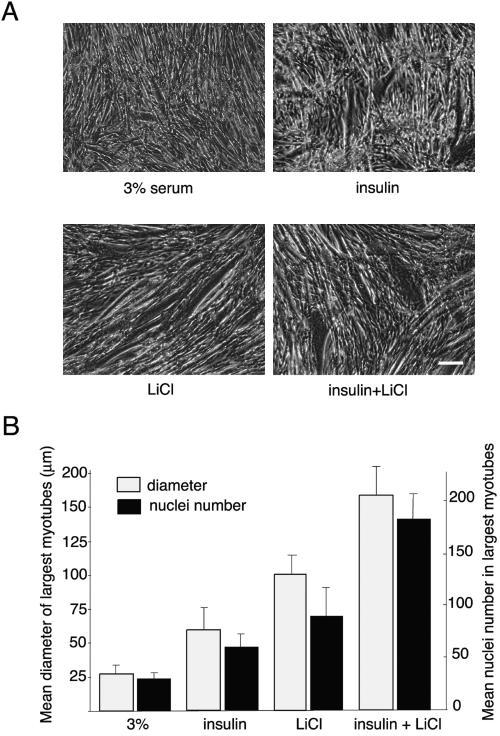
Myoblasts were differentiated into fused myotubes (i.e., for 36–48 h) and then insulin (Figure 5A, insulin), LiCl or both (insulin+LiCl) were added to the differentiation medium for 24 h. The extent of resulting hypertrophy was assessed by measuring the mean diameter of the largest myotubes as detailed in Materials and Methods (Figure 5, A and B). As a control, myotube size was compared with dishes kept in 3% serum for the same period (Figure 5A, 3% serum). Insulin stimulation resulted in an increase in myotube size (Figure 5A, insulin). With LiCl alone, myotubes were also larger than both 3% serum- and insulin-treated cells (Figure 5A, LiCl). These results are consistent with reports showing that insulin alone (Rommel et al., 2001) or LiCl alone (Vyas et al., 2002) can increase myotube size and thus stimulate hypertrophy. However, when insulin and LiCl were used together, the resulting myotubes were considerably larger (∼5-fold greater than 3% serum- and 3-fold than insulin-treated cells) (Figure 5A, insulin+LiCl).
Figure 5.
Insulin and lithium chloride cooperate to promote hypertrophy in differentiating C2.7 myoblasts. (A) C2.7 cells were cultured in a proliferation medium for 3 d and then transferred to differentiation medium for 48 h. At that time, differentiating myoblasts were stimulated or not (control 3%) with insulin at 3 μg ml-1 (insulin), lithium chloride at 10 mM (LiCl), or both of them (insulin+LiCl). Twenty-four hours after stimulation (after a total of 72 h of differentiation), cells were fixed and photographed by phase contrast. Bar, 150 μm. (B) For size measurements and nuclei quantification, cells were fixed with formalin for 7 min and stained with bisbenzimide 33258. The mean diameter of the largest myotubes was evaluated on the basis of the 10 largest myotubes in six different randomly chosen microscopic fields per condition and averaged from four different experiments. To quantify the increase in the fusion process, the cultures also were characterized by counting the maximum number of nuclei present in the 10 largest myotubes as described for the size measurements.
We next investigated whether this increased myotube size resulted from an increase in the fusion process. Differentiated cells treated as described above were analyzed by determining the highest number of nuclei per myotube (Figure 5B). The average number of nuclei in the largest myotubes was 60 ± 15 when increased differentiation was induced by insulin, 95 ± 25 by LiCl (corresponding to a 1.5-fold compared with insulin) and 180 ± 30 when a combination of insulin and LiCl was used (corresponding to a threefold increase over insulin). These results indicate that the increase in myotube size induced by a combination of insulin and lithium chloride is accompanied by an increase in cell fusion. Similar effects on myotube hypertrophy could be obtained when 10 nM purified IGF-1 was substituted for insulin (unpublished data).
Wnt1 Reproduces Lithium Chloride Effects and Cooperates with Insulin to Produce Larger Myotubes
To establish whether a Wnt stimulation would produce similar hypertrophic effects to lithium chloride, and whether cooperation exists between insulin and Wnt pathways, C2.7 cells were cultured in proliferation medium for 3 d. Subsequently, confluent postmitotic C2.7 myoblasts (ready to differentiate) were overlain with Wnt1-expressing or control 3T3 cell lines in 3% serum differentiation medium (Figure 6A, Cont-3T3 and Wnt1-3T3) and after 16 h of recovery, cocultures were stimulated or not with insulin for 24 h (Figure 6A, Cont-3T3+insulin and Wnt1-3T3+insulin) before cell fixation and analysis by phase contrast microscopy.
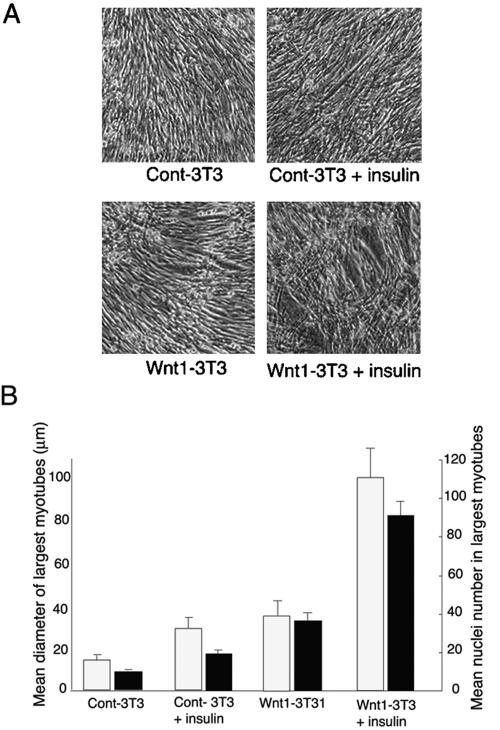
Figure 6.
Wnt1 cooperates with insulin to induce myotube hypertrophy. (A) C2.7 cells were cultured in a proliferation medium for 3 d. At that time Wnt-expressing 3T3J2 cell lines, namely, Cont-3T3 and Wnt1-3T3 were harvested, counted, and 4 × 105 cells plated in 3% differentiation medium on confluent C2.7 cells ready to differentiate. Sixteen hours after plating to allow spreading, cells were stimulated or not with insulin at 3 μg ml-1 (insulin). After 24 h (a total of 40 h of differentiation), cells were fixed and photographed by phase contrast. Bar, 150 μm. (B) For size measurements and nuclei quantification, cells were fixed with formalin for 7 min and stained with bisbenzimide 33258 and assessed as described for Figure 5. The mean diameter of the largest myotubes was evaluated on the basis of the 10 largest myotubes in six different randomly chosen microscopic fields per condition and averaged from four different experiments. To quantify the increase in the fusion process, the cultures also were characterized by counting the maximum number of nuclei present in the 10 largest myotubes as described for the size measurements.
The presence of Wnt1-expressing fibroblasts resulted in differentiation of myoblasts into larger myotubes (Wnt1-3T3) compared with myoblasts cocultured with control 3T3 fibroblast (Cont-3T3). Addition of insulin (Figure 6A, insulin) to the cocultures led to an enhanced hypertrophic response.
These effects also were quantified by measuring the mean increase in myotube diameter and overall number of nuclei per myotube as described for Figure 5B. In the presence of insulin, Wnt1-expressing 3T3 cells more than doubled the mean maximum myotube diameter (Figure 6B), an effect no seen in the absence of insulin. The increase in myotube nuclei in C2.7 myoblasts cultured with Wnt1-3T3 was four-fold more than in cocultures with Cont-3T3cells (Figure 6B). Furthermore, stimulation of the Wnt-1 cocultures with insulin increased the number of nuclei in myotubes to a level fivefold higher than that in control cocultures treated with insulin (Figure 6B, compare Wnt1-3T3+insulin with Cont-3T3+insulin). Differentiating C2.7 in 3% DM in the absence of cocultured 3T3 cells with or without insulin stimulation showed similar differentiation levels (myotube size and nuclei number) to those observed in C2.7 cells cocultured with control 3T3 cells stimulated or not with insulin. Although the increase in myotube diameter and number of nuclei per myotube was lower than for insulin- and LiCl-induced hypertrophy, this is likely to be due to the shorter time these cells spent under differentiation conditions before analysis (cocultures of Wnt-expressing cells were differentiated for 40 h vs. 60–72 h for LiCl treatment). From these data, it seems that the synergy between Wnt1 and insulin mimics the cooperation observed with insulin and LiCl in promoting myotube hypertrophy as defined here as an increase in both myotube size and nuclei per myotube.
Fusion of Reserve Cells to Preexisting Myotubes Participates in Insulin- and Lithium Chloride-induced Hypertrophy of Myotubes
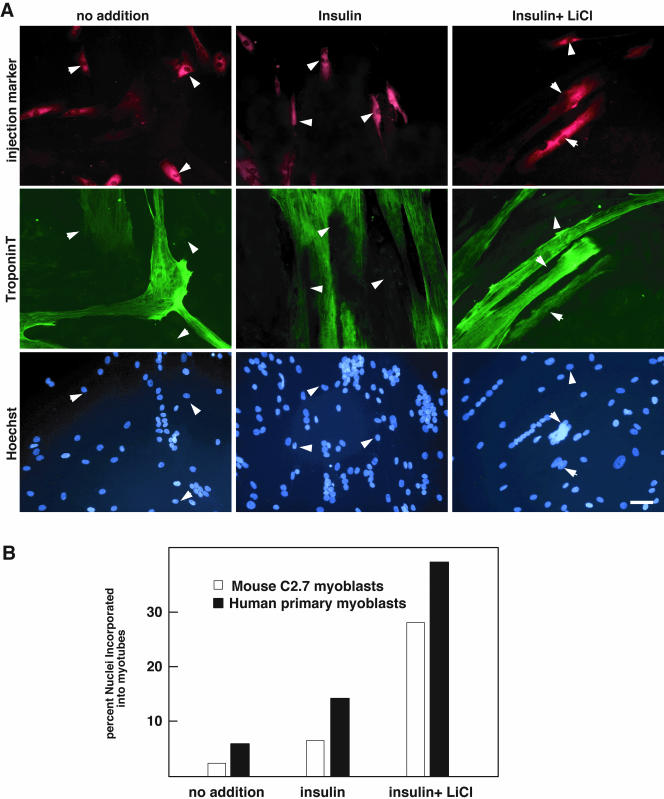
The major increase in myotube size (both in diameter and nuclei number) observed upon treatment of differentiated myoblasts with both insulin and LiCl/Wnt1 could result from an increased fusion between existing myotubes but also frogman activation of reserve cells and fusion to preexisting myotubes during hypertrophy. To examine this, C2.7 cells (Figure 7B) or primary human myoblasts (Figure 7A) were microinjected, after 2 d of differentiation, with inert Texas Red-conjugated dextran, taking care to only microinject mononucleated myoblasts and not myotubes. C2.7 cells or primary human myoblasts were then treated or not with insulin or a combination of insulin and LiCl and fixed after 24 h. Nuclei were labeled with bisbenzimide 33258 and cells were stained for troponin T and analyzed for the resulting distribution of microinjected fluorescent dextran in treated cultures. The results of a typical experiment are shown Figure 7, A and B. They clearly show that in cultures kept in 3% serum or treated with insulin alone, cells injected with fluorescent dextran remained as mononucleated myocytes. Only in the case of treatment with both insulin and LiCl, a significant proportion of injected cells were retrieved in multinucleated myotubes after hypertrophy inducing treatment: in ∼25–35% of injected cells in C2.7 cultures and in primary human myoblasts. These experiments were repeated three times injecting 30–40 mononucleated cells for each treatment, and a summary of the results is shown as percentages in the graph (Figure 7B). These results reveal that the ex vivo hypertrophic response observed when treating differentiated C2.7 cultures or differentiated primary human myoblasts by a combination of insulin and LiCl additionally involves an activation of mononucleated cells and subsequent fusion into myotubes that does not occur with insulin treatment alone. This effect may be related to the differentiating response observed on the population of quiescent reserve cells upon treatment with insulin and LiCl (Figure 3).
Figure 7.
Insulin- and LiCl-induced hypertrophy involves fusion of reserve cells into preexisting myotubes. (A) C2.7 myoblasts (unpublished data) and primary human myoblasts (CHM) were induced to differentiate in low-serum medium for 48 h, a time scale that allows the emergence of both reserve cells and myotubes. Reserve cells only were microinjected with Lucifer yellow to follow their destiny. Cells were stimulated with insulin alone or with a combination of insulin and LiCl for 24 h. After fixation and labeling with bisbenzimide 33258 and troponin T, cells were analyzed by fluorescence microscopy. Percentages of microinjected reserve cells incorporated in myotubes were calculated in each case. (B) Percentages of reserve cells incorporated in myotubes against those that remained mononucleated were calculated for C2.7 (white bars) and PHM (black bars) in each case (no addition, insulin and insulin+LiCl).
DISCUSSION
Different groups have analyzed the role of insulin signaling in differentiation and shown PKB/Akt to play an integral role in myogenesis (Jiang et al., 1999; Vandromme et al., 2001; Sumitani et al., 2002). Here, we focused on the serine/threonine kinase GSK-3, a major substrate phosphorylated and down-regulated by PKB/Akt in a number of cellular systems (Cross et al., 1995). Its inhibition via the PI3K-PKB/Akt signaling cascade has recently been shown to play an important role in IGF-induced myotube hypertrophy (Rommel et al., 2001; Vyas et al., 2002; Pallafacchina et al., 2002) and in overloaded skeletal muscle (Bodine et al., 2001). We show here that GSK-3 activity is down-regulated in differentiating myotubes because the basal phosphorylation of GSK-3 increases between proliferative C2.7 myoblasts and differentiated myotubes but not in reserve cells, further supporting that GSK-3 inhibition plays an integral role in the process of muscle differentiation.
GSK-3 Inhibition Is One Component in the Initiation of Differentiation
The increased phosphorylation of GSK-3 we observed during early myoblast differentiation lead us to question the importance of GSK-3 inhibition to the myogenic program. To examine this, we exploited the C2ind cell system, a subclone of C2 cells dependent on insulin for differentiation (Pinset et al., 1988). Insulin-independent differentiation of C2ind was induced by inhibition of GSK-3 by using either LiCl or SB216763, indicating that GSK-3 inhibition is a downstream effector of IGFR1 in insulin-induced differentiation. However, this effect required basal levels of serum factors present in the differentiation medium and was not observed in serum-free conditions. To determine which serum components complemented GSK-3 inhibition, we screened of a variety of serum compounds, including fibroblast growth factors (FGF1 and FGF6), platelet-derived growth factor, lysophosphatidic acid, and insulin. In quiescent mouse or human reserve cells, only insulin cooperated with GSK-3 inhibition to stimulate differentiation (unpublished observations). These data suggest that inhibition of GSK-3 is necessary but insufficient to induce C2ind differentiation and that the relief observed in the presence of low levels of serum probably reflects the cooperation between GSK-3 inhibition and trace levels of insulin present in the serum (3% in DM).
The cooperation between LiCl and insulin, both inhibitors of GSK-3, is intriguing. A previous report described additive inhibitory effects of LiCl and insulin on GSK-3 activity (Choi and Sung, 2000). Furthermore, this correlated with additive stimulatory effects on both glycogen synthase activity and glycogen synthesis in differentiated L6 myocytes. Here, we have shown the synergistic effects of GSK-3 inhibition with insulin in either activating differentiation of quiescent reserve cells or inducing hypertrophic response and increased fusion in already differentiated C2.7 cells. We also show that in C2ind cells, addition of 10 mM LiCl or SB216763 in DM is sufficient to induce the differentiation response. In contrast, one report described the inhibition of myogenic differentiation in C2 myocytes treated with 30 mM LiCl 24 h after induction in DM (Goichberg et al., 2001). Although the reasons for these differences remain unclear, we have noticed that at high concentrations, LiCl can become increasingly inhibitory to a variety of cellular processes, including cell proliferation and protein synthesis (unpublished observations).
Although the effect of insulin/IGF may involve targets distinct from GSK-3 (Ras/ERK, p38, mTOR, and p70S6K), different signaling cascades converging on the same target may result from the formation of distinct complexes with specific subcellular accessibility and localization. This is the case in classical Wnt signaling where GSK-3 is inhibited by protein sequestration and not phosphorylation. Wnt1, for example, targets and inhibits GSK-3 in a multiprotein complex containing specific GSK-3 substrates (for reviews, see Kawano and Kypta, 2003; Veeman et al., 2003). We have shown here that Wnt1 can mimic to some extent the effects of GSK-3 inhibition by LiCl or SB in synergizing insulin action in induction of reserve cell differentiation and myotube hypertrophy. The effect seems to involve the canonical Wnt signaling family because it was accompanied by nuclear accumulation of β-catenin (Figure 4B). Some cases of cross talk between Wnt and insulin pathways have been reported (Fukumoto et al., 2001; Harwood, 2001), including on β-catenin activity (Papkoff, 1997; Playford et al., 2000; Desbois-Mouthon et al., 2001). The pool of GSK-3 targeted by Wnt is mostly nonphosphorylated by the insulin/PKB pathway (Ding et al., 2000). However, a report by Fukumoto et al., (2001) shows a role of PKB/Akt in the multiprotein complex containing Dvl, Axin, β-catenin, and GSK-3. The authors propose a role of PKB/Akt in the maintenance of the Wnt signal (Fukumoto et al., 2001). One possibility is that inactivation of GSK-3 by phosphorylation through PKB/Akt (insulin pathway), or by other nonphosphorylation events (LiCl/Wnt pathway), targets different subsets of substrates that cooperate to induce reserve cell activation and muscle hypertrophy. We show here that GSK-3 inhibition by LiCl or SB216763, like Wnt1 activation, induces nuclear accumulation of β-catenin. A recent report demonstrated that β-catenin is required as a nuclear coactivator in Wnt signaling (Cong et al., 2003) and β-catenin was shown to be necessary and sufficient to promote myogenesis in carcinomic ES P19 cells (Petropoulos and Skerjanc, 2002). In addition, β-catenin binds to transcriptional coactivators such as CBP/p300. We recently showed that transcriptional control of MyoD induction in activated myoblasts involves the DNA binding of the serum response factor in a multiprotein complex containing CBP and C/EBP (L'honore et al., 2003). It is interesting to speculate that a direct transcriptional activation of MyoD by Wnt may involve β-catenin in such a complex.
Wnt and Insulin Signaling Pathways Have Synergistic Effects to Increase Myotube Differentiation and Hypertrophy
In addition to promoting differentiation of reserve cells, cooperation of GSK-3 inhibition with insulin also induces myotube hypertrophy and cell fusion in both C2.7 cell line and primary human myoblasts. Hypertrophy is defined as an increase in diameter and in total protein content of myotubes. In addition, a number of reports are in favor of a possible implication of satellite cell replication and fusion into existing myotubes during exercise- or load-induced hypertrophy (Coleman et al., 1995; Vyas et al., 2002). Injection of a plasmid encoding IGF-1 into rat latissimus dorsi muscle leads to increased myofiber number with central nuclei 2 months latter, an event that is a hallmark of new muscle formation after satellite cell activation (Semsarian et al., 1999). Moreover, killing satellite cells by irradiation prevents compensatory hypertrophy of rat muscle in vivo (Rosenblatt et al., 1994). In agreement with previous reports suggesting that insulin/IGF-1 promotes proliferation, we observed 5-bromo-2-deoxyuridine incorporation into mononucleated reserve cells upon treatment of differentiated C2 cells with insulin or insulin and LiCl (unpublished observations). In vivo, the hypertrophic effect of IGF-1 alone on skeletal muscles in mice is well documented (Barton-Davis et al., 1998; Musaro et al., 2001). A similar effect was shown on myotube hypertrophy ex vivo (Rommel et al., 2001). Indeed, the authors reported that GSK-3 is phosphorylated and inhibited during ex vivo muscle hypertrophy and that a dominant negative form of GSK-3 induced myotube hypertrophy. These observations clearly implicated GSK-3 as an important modulator in muscle hypertrophy, but did not show whether GSK-3 was a direct target for IGF signaling. Here, we confirmed that GSK-3 is directly implicated in the process of muscle hypertrophy. Our results indicate that GSK-3 could be a potential target of the Wnt signaling pathway independently of, and in addition to, the effect of the IGF/insulin pathway. This would place GSK-3 at a central point in the cooperative cross talk between IGF and Wnt signaling pathways that promote postnatal muscle differentiation and hypertrophy. A synergic interaction of IGF with Shh and basic FGFs has been previously shown to promote somite myogenesis ex vivo (Pirskanen et al., 2000). Others, (Alzghoul et al., 2004), have recently reported that ectopic expression of IGF-1 and Shh in adult mouse muscle induced stronger muscle fiber hypertrophy than either component alone. To our knowledge, no effect of Wnt/b-catenin pathway on muscle hypertrophy has been reported in vivo. A recent report (Polesskaya et al., 2003) described that a subpopulation of hematopoetic-derived CD45+ stem cells from adult skeletal muscle can be mobilized to enter the myogenic program in response to Wnt signaling. Although this finding has interesting implications for use in adult stem cell therapy, it does not demonstrate that such reprogramming plays a significant role in muscle regeneration. Indeed, a recent report by Zammit et al. (2002) showed that activation of myofiber-associated satellite cells is sufficient to fully regenerate skeletal muscle fibers. In this respect, we show here in both established cell lines and primary human myoblasts that the activation of muscle differentiation and hypertrophy involves a cooperative effect of insulin and Wnt-dependent inactivation of GSK-3.
We show here that insulin and Wnt/b-catenin signaling pathways have synergistic effects on myotube hypertrophy and that this process involves the fusion of activated myoblasts into preexisting myotubes. After injection of fluorescent dextran exclusively into mononuclear myocytes present in differentiated cultures, we found incorporation of the dye into multinuclear myotubes when cultures were stimulated with insulin and Wnt. Although further work is required to understand how insulin and Wnt pathways cooperate, our data reveal that these two major signaling pathways can work together to tightly control postnatal myogenesis and myotube size. Due to the complexity and multiplicity of the Wnt signaling pathways, increasing number of receptors and secreted proteins controlling the binding of the Wnt factors to their receptors (sFRP and Dickkopf family of proteins; Kawano and Kypta, 2003; Niehrs, 2004), the positive implication of Wnt in many processes has yet to be confirmed. In the case of myogenesis, even if the roles of Wnts have been studied in detail during development (Borello et al., 1999), it still remains essential to determine which Wnt receptors, among the large family of serpentine Fz receptors, are expressed in reserve cells versus myotubes and in mature muscle. To extend and confirm our conclusions in vivo, it will be interesting to determine whether Wnt/b-catenin signaling, in combination with IGF-1, may also contribute to promote increased muscle hypertrophy in mice.
Acknowledgments
We thank A.M.C. Brown (Rockefeller University, Manhattan, NY) for providing us with the retroviral constructs, Dr. Brian Hemmings (FMI, Basel, Switzerland) for the PKBβ reagents used in this study, and L. Journot (IUGF, Montpellier France) for support to T.B. This work was supported by a grant from European Union Fifth Framework Program (QLK3-CT-2000-01038), and grants from the Association Française contre les Myopathies and Association pour le Recherche sur le Cancer (no. 4459). AR was supported by fellowships from the Ligue Nationale Contre le Cancer and Association pour la Recherche sur le Cancer.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–11–0816. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03–11–0816.
Abbreviations used: C2ind, C2inducible cell line; DM, differentiation medium; GSK-3, glycogen synthase kinase-3; GM. growth medium; IGF, insulin-like growth factor; PI3K, phosphatidyl inositol 3 kinase; PKB/Akt, protein kinase B.
References
- Alzghoul, M.B., Gerrard, D., Watkins, B.A., and Hannon, K. (2004). Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. 18, 221-223. [DOI] [PubMed] [Google Scholar]
- Barton-Davis, E.R., Shoturma, D.I., Musaro, A., Rosenthal, N., and Sweeney, H.L. (1998). Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. USA 95, 15603-15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp, J.R., Heslop, L., Yu, D.S., Tajbakhsh, S., Kelly, R.G., Wernig, A., Buckingham, M.E., Partridge, T.A., and Zammit, P.S. (2000). Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 151, 1221-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine, S.C., et al. (2001). Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell. Biol. 3, 1014-1019. [DOI] [PubMed] [Google Scholar]
- Blose, S.H., Meltzer, D.I., and Feramisco, J.R. (1984). 10-nm filaments are induced to collapse in living cells injected with monoclonal and polyclonal antibodies to tubulin. J. Cell. Biol. 98, 847-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello, U., Buffa, V., Sonnino, C., Melchionna, R., Vivarelli, E., and Cossu, G. (1999). Differential expression of the Wnt putative receptors Frizzled during mouse somitogenesis. Mech. Dev. 89, 173-177. [DOI] [PubMed] [Google Scholar]
- Brown, A.M.C., and Scott, M.R.D. (1987). Retroviral vectors. In: DNA Cloning - A Practical Approach, Vol. III, ed. D.M. Glover, Oxford: IRL Press, 189-212. [Google Scholar]
- Brown, A.M.C., Papkoff, J., Fung, Y.K.T., Shackleforf, G.M., and Varmus, H.E. (1987). Identification of protein products encoded by the proto-oncogene int-1. Mol. Cell. Biol. 7, 3971-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, W.S., and Sung, C.K. (2000). Effects of lithium and insulin on glycogen synthesis in L6 myocytes: additive effects on inactivation of glycogen synthase kinase-3. Biochim. Biophys. Acta 1475, 225-230. [DOI] [PubMed] [Google Scholar]
- Church, V.L., and Francis-West, P. (2002). Wnt signalling during limb development. Int. J. Dev. Biol. 46, 927-936. [PubMed] [Google Scholar]
- Coghlan, M.P., et al. (2000). Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 10, 793-803. [DOI] [PubMed] [Google Scholar]
- Coleman, M.E., DeMayo, F., Yin, K.C., Lee, H.M., Geske, R., Montgomery, C., and Schwartz, R.J. (1995). Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J. Biol. Chem. 270, 12109-12116. [DOI] [PubMed] [Google Scholar]
- Cong, F., Schweizer, L., Chamorro, M., and Varmus, H. (2003). Requirement for a nuclear function of beta-catenin in Wnt signaling. Mol. Cell. Biol. 23, 8462-8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolican, S.A., Samuel, D.S., Ewton, D.Z., McWade, F.J., and Florini, J.R. (1997). The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J. Biol. Chem. 272, 6653-6662. [DOI] [PubMed] [Google Scholar]
- Cossu, G., and Borello, U. (1999). Wnt signaling and the activation of myogenesis in mammals. EMBO J. 18, 6867-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, D.A., Culbert, A.A., Chalmers, K.A., Facci, L., Skaper, S.D., and Reith, A.D. (2001). Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J. Neurochem. 1, 94-102. [DOI] [PubMed] [Google Scholar]
- Cross, D.A., Alessi, D.R., Cohen, P., Andjelkovich, M., and Hemmings, B.A. (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785-789. [DOI] [PubMed] [Google Scholar]
- De Angelis, L., Berghella, L., Coletta, M., Lattanzi, L., Zanchi, M., Cusella-De Angelis, G., Ponzetto, C., and Cossu, G. (1999). Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J. Cell Biol. 147, 869-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois-Mouthon, C., Cadoret, A., Blivet-Van Eggelpoel, M.J., Bertrand, F., Cherqui, G., Perret, C., and Capeau, J. (2001). Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 20, 252-259. [DOI] [PubMed] [Google Scholar]
- Ding, V.W., Chen, R.H., and McCormick, F. (2000). Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J. Biol. Chem. 275, 32475-32481. [DOI] [PubMed] [Google Scholar]
- Doble, B.W., and Woodgett, J.R. (2003). GSK-3, tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto, S., et al. (2001). Akt participation in the Wnt signaling pathway through Dishevelled. J. Biol. Chem. 276, 17479-17483. [DOI] [PubMed] [Google Scholar]
- Girard, F., Strausfeld, U., Fernandez, A., and Lamb, N.J.C. (1991). Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 67, 1169-1179. [DOI] [PubMed] [Google Scholar]
- Goichberg, P., Shtutman, M., Ben-Ze'ev, A., and Geiger, B. (2001). Recruitment of beta-catenin to cadherin-mediated intercellular adhesions is involved in myogenic induction. J. Cell Sci. 114, 1309-1319. [DOI] [PubMed] [Google Scholar]
- Grimes, C.A., and Jope, R.S. (2001). The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 65, 391-426. Review. Erratum in: Prog. Neurobiol. 2001 Dec;65, 497 [DOI] [PubMed] [Google Scholar]
- Harwood, A.J. (2001). Regulation of GSK-3, a cellular multiprocessor. Cell. 105, 821-824. [DOI] [PubMed] [Google Scholar]
- Jiang, B.H., Aoki, M., Zheng, J.Z., Li, J., and Vogt, P.K. (1999). Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA 96, 2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, Y. and Kypta, R. (2003). Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627-2634. [DOI] [PubMed] [Google Scholar]
- Kitzmann, M., Carnac, G., Vandromme, M., Primig, M., Lamb, N.J.C., and Fernandez, A. (1998). The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J. Cell Biol. 142, 1447-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühl, M., Sheldahl, L.C., Park, M., Miller, J.R., and Moon, R.T. (2000). The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 16, 279-283. [DOI] [PubMed] [Google Scholar]
- L'honore, A., Lamb, N.J., Vandromme, M., Turowski, P., Carnac, G., and Fernandez, A. (2003). MyoD distal regulatory region contains an SRF binding CArG element required for MyoD expression in skeletal myoblasts and during muscle regeneration. Mol. Biol. Cell 14, 2151-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindon, C., Montarras, D., and Pinset, C. (1998). Cell cycle-regulated expression of the muscle determination factor Myf5 in proliferating myoblasts. J. Cell Biol. 140, 111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarras, D., Pinset, C., Chelly, J., Kahn, A., and Gros, F. (1989). Expression of MyoD1 coincides with terminal differentiation in determined but inducible muscle cells. EMBO J. 8, 2203-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro, A., McCullagh, K., Paul, A., Houghton, L., Dobrowolny, G., Molinaro, M., Barton, E.R., Sweeney, H.L., and Rosenthal, N. (2001). Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 27, 195-200. [DOI] [PubMed] [Google Scholar]
- Niehrs, C. (2004). Norrin and Frizzled: a new vein for the eye. Dev. Cell 6, 453-454. [DOI] [PubMed] [Google Scholar]
- Pallafacchina, G., Calabria, E., Serrano, A.L., Kalhovde, J.M., and Schiaffino, S. (2002). A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl. Acad. Sci. USA 99, 9213-9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff, J. (1997). Regulation of complexed and free catenin pools by distinct mechanisms. Differential effects of Wnt-1 and v-Src. J. Biol. Chem. 272, 4536-4543. [PubMed] [Google Scholar]
- Petropoulos, H., and Skerjanc, I.S. (2002). Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J. Biol. Chem. 277, 15393-15399. [DOI] [PubMed] [Google Scholar]
- Pinset, C., Montarras, D., Chenevert, J., Minty, A., Barton, P., Laurent, C., and Gros, F. (1988). Control of myogenesis in the mouse myogenic C2 cell line by medium composition and by insulin: characterization of permissive and inducible C2 myoblasts. Differentiation 38, 28-34. [DOI] [PubMed] [Google Scholar]
- Pirskanen, A., Kiefer, J.C., and And Hauschka, S.D. (2000). IGFs, Insulin Shh, bFGF and TGF-b1 interact synergistically to promote somite myogenesis in vitro. Dev. Biol. 224, 189-203. [DOI] [PubMed] [Google Scholar]
- Playford, M.P., Bicknell, D., Bodmer, W.F., and Macaulay, V.M. (2000). Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of beta-catenin. Proc. Natl. Acad. Sci. USA 97, 12103-12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya, A., Seale, P., and Rudnicki, M.A. (2003). Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 113, 841-852. [DOI] [PubMed] [Google Scholar]
- Rheinwald, J.G., and Green, H. (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 6, 331-343. [DOI] [PubMed] [Google Scholar]
- Rommel, C., Bodine, S.C., Clarke, B.A., Rossman, R., Nunez, L., Stitt, T.N., Yancopoulos, G.D., and Glass, D.J. (2001). Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell. Biol. 3, 1009-1013. [DOI] [PubMed] [Google Scholar]
- Rosenblatt, J.D., and Parry, D.J. (1992). Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J. Appl. Physiol. 73, 2538-2543. [DOI] [PubMed] [Google Scholar]
- Rosenblatt, J.D., Yong, D., and Parry, D.J. (1994). Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve 17, 608-613. [DOI] [PubMed] [Google Scholar]
- Schultz, E., and McCormick, K.M. (1994). Skeletal muscle satellite cells. Rev. Physiol. Biochem. Pharmacol. 123, 213-257. [DOI] [PubMed] [Google Scholar]
- Semsarian, C., Wu, M.J., Ju, Y.K., Marciniec, T., Yeoh, T., Allen, D.G., Harvey, R.P., and Graham, R.M. (1999). Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signaling pathway. Nature 400, 576-581. [DOI] [PubMed] [Google Scholar]
- Sumitani, S., Goya, K., Testa, J.R., Kouhara, H., and Kasayama, S. (2002). Akt1 and Akt2 differently regulate muscle creatine kinase and myogenin gene transcription in insulin-induced differentiation of C2C12 myoblasts. Endocrinology 143, 820-828. [DOI] [PubMed] [Google Scholar]
- Vandromme, M., Rochat, A., Meier, R., Carnac, G., Besser, D., Hemmings, B.A., Fernandez, A., and Lamb, N.J.C. (2001). Protein kinase B beta/Akt2 plays a specific role in muscle differentiation. J. Biol. Chem. 276, 8173-8179. [DOI] [PubMed] [Google Scholar]
- Veeman, M.T., Axelrod, J.D., and Moon, R.T. (2003). A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 5, 367-377. [DOI] [PubMed] [Google Scholar]
- Vyas, D.R., Spangenburg, E.E., Abraha, T.W., Childs, T.E., and Booth, F.W. (2002). GSK-3beta negatively regulates skeletal myotube hypertrophy. Am. J. Physiol. 283, C545-C551. [DOI] [PubMed] [Google Scholar]
- Weintraub, H. (1993). The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 75, 1241-1244. [DOI] [PubMed] [Google Scholar]
- Weston, C.R., and Davis, R.J. (2001). Signal transduction: signaling specificity- a complex affair. Science 292, 2439-2440. [DOI] [PubMed] [Google Scholar]
- Yaffe, D., and Saxel, O. (1977). Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 270, 725-727. [DOI] [PubMed] [Google Scholar]
- Yoshida, N., Yoshida, S., Koishi, K., Masuda, K., and Nabeshima, Y. (1998). Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates `reserve cells'. J. Cell Sci. 111, 769-779. [DOI] [PubMed] [Google Scholar]
- Zammit P.S., Heslop, L., Hudon, V., Rosenblatt, J.D., Tajbakhsh, S., Buckingham, M.E., Beauchamp, J.R., and Partridge, T.A. (2002). Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell Res. 281, 39-49. [DOI] [PubMed] [Google Scholar]
- Zhao, P., and Hoffman, E.P. (2004). Embryonic myogenesis pathways in muscle regeneration. Dev. Dyn. 229, 380-392. [DOI] [PubMed] [Google Scholar]