Abstract
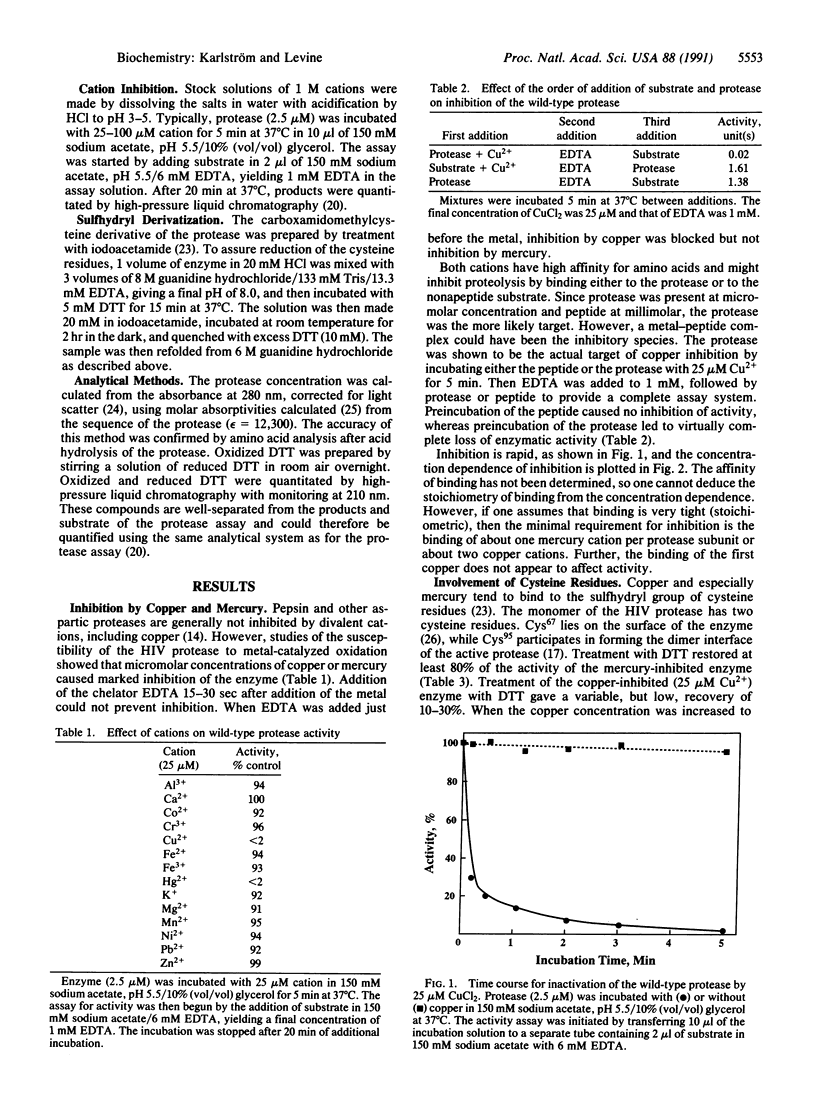
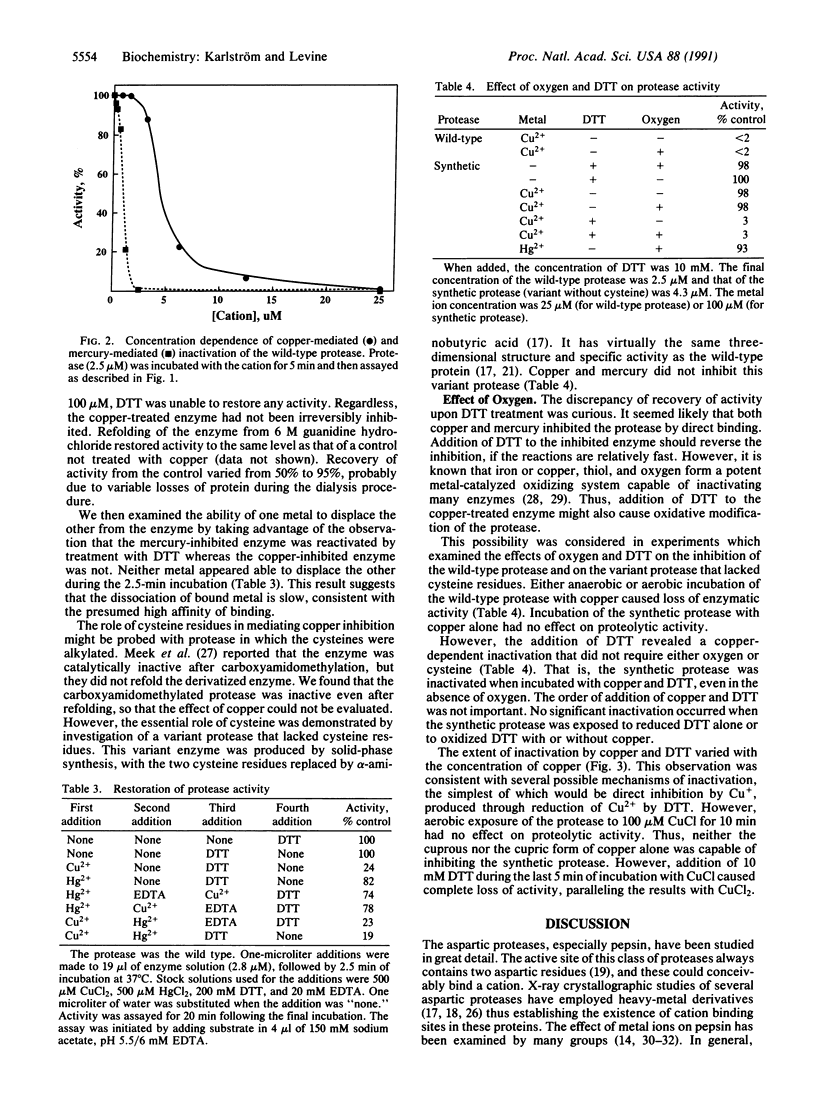
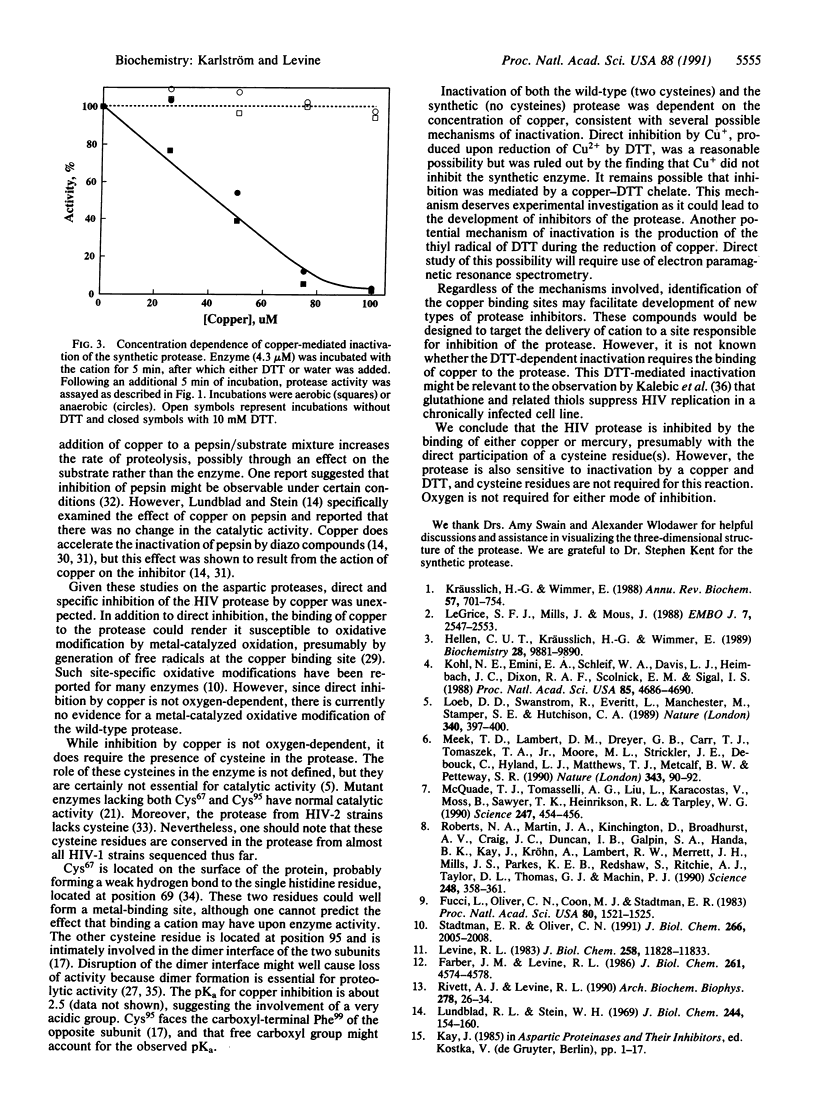
The protease of the human immunodeficiency virus is essential for replication of the virus, and the enzyme is therefore an attractive target for antiviral action. We have found that the viral protease is inhibited by approximately stoichiometric concentrations of copper or mercury ions. Inactivation by Cu2+ was rapid and not reversed by subsequent exposure to EDTA or dithiothreitol. Direct inhibition by Cu2+ required the presence of cysteine residue(s) in the protease. Thus, a synthetic protease lacking cysteine residues was not inhibited by exposure to copper. However, addition of dithiothreitol as an exogenous thiol rendered even the synthetic protease susceptible to inactivation by copper. Oxygen was not required for inactivation of either the wild-type or the synthetic protease. These results provide the basis for the design of novel types of protease inhibitors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreeva N. S., Gustchina A. E., Fedorov A. A., Shutzkever N. E., Volnova T. V. X-ray crystallographic studies of pepsin. Adv Exp Med Biol. 1977;95:23–31. doi: 10.1007/978-1-4757-0719-9_2. [DOI] [PubMed] [Google Scholar]
- Boutelje J., Karlström A. R., Hartmanis M. G., Holmgren E., Sjögren A., Levine R. L. Human immunodeficiency viral protease is catalytically active as a fusion protein: characterization of the fusion and native enzymes produced in Escherichia coli. Arch Biochem Biophys. 1990 Nov 15;283(1):141–149. doi: 10.1016/0003-9861(90)90624-8. [DOI] [PubMed] [Google Scholar]
- Chevion M. A site-specific mechanism for free radical induced biological damage: the essential role of redox-active transition metals. Free Radic Biol Med. 1988;5(1):27–37. doi: 10.1016/0891-5849(88)90059-7. [DOI] [PubMed] [Google Scholar]
- Darke P. L., Leu C. T., Davis L. J., Heimbach J. C., Diehl R. E., Hill W. S., Dixon R. A., Sigal I. S. Human immunodeficiency virus protease. Bacterial expression and characterization of the purified aspartic protease. J Biol Chem. 1989 Feb 5;264(4):2307–2312. [PubMed] [Google Scholar]
- Farber J. M., Levine R. L. Sequence of a peptide susceptible to mixed-function oxidation. Probable cation binding site in glutamine synthetase. J Biol Chem. 1986 Apr 5;261(10):4574–4578. [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte W. E., Jr, Swaminathan S., Mansuri M. M., Martin J. C., Rosenberg I. E., Beveridge D. L. Domain communication in the dynamical structure of human immunodeficiency virus 1 protease. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8864–8868. doi: 10.1073/pnas.87.22.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Kräusslich H. G., Wimmer E. Proteolytic processing of polyproteins in the replication of RNA viruses. Biochemistry. 1989 Dec 26;28(26):9881–9890. doi: 10.1021/bi00452a001. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Ferguson J. B., Fruton J. S. Bifunctional inhibitors of pepsin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2765–2768. doi: 10.1073/pnas.68.11.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalebic T., Kinter A., Poli G., Anderson M. E., Meister A., Fauci A. S. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):986–990. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Kirchgessner M., Beyer M. G., Steinhart H. Activation of pepsin (EC 3.4.4.1) by heavy-metal ions including a contribution to the mode of action of copper sulphate in pig nutrition. Br J Nutr. 1976 Jul;36(1):15–22. doi: 10.1079/bjn19760054. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Mills J., Mous J. Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J. 1988 Aug;7(8):2547–2553. doi: 10.1002/j.1460-2075.1988.tb03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L., Federici M. M. Quantitation of aromatic residues in proteins: model compounds for second-derivative spectroscopy. Biochemistry. 1982 May 25;21(11):2600–2606. doi: 10.1021/bi00540a004. [DOI] [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. II. Characterization of the ascorbate model system. J Biol Chem. 1983 Oct 10;258(19):11828–11833. [PubMed] [Google Scholar]
- Loeb D. D., Swanstrom R., Everitt L., Manchester M., Stamper S. E., Hutchison C. A., 3rd Complete mutagenesis of the HIV-1 protease. Nature. 1989 Aug 3;340(6232):397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Stein W. H. On the reaction of diazoacetyl compounds with pepsin. J Biol Chem. 1969 Jan 10;244(1):154–160. [PubMed] [Google Scholar]
- McQuade T. J., Tomasselli A. G., Liu L., Karacostas V., Moss B., Sawyer T. K., Heinrikson R. L., Tarpley W. G. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990 Jan 26;247(4941):454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- Meek T. D., Dayton B. D., Metcalf B. W., Dreyer G. B., Strickler J. E., Gorniak J. G., Rosenberg M., Moore M. L., Magaard V. W., Debouck C. Human immunodeficiency virus 1 protease expressed in Escherichia coli behaves as a dimeric aspartic protease. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1841–1845. doi: 10.1073/pnas.86.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek T. D., Lambert D. M., Dreyer G. B., Carr T. J., Tomaszek T. A., Jr, Moore M. L., Strickler J. E., Debouck C., Hyland L. J., Matthews T. J. Inhibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature. 1990 Jan 4;343(6253):90–92. doi: 10.1038/343090a0. [DOI] [PubMed] [Google Scholar]
- Miller M., Jaskólski M., Rao J. K., Leis J., Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989 Feb 9;337(6207):576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Rajagopalan T. G., Stein W. H., Moore S. The inactivation of pepsin by diazoacetylnorleucine methyl ester. J Biol Chem. 1966 Sep 25;241(18):4295–4297. [PubMed] [Google Scholar]
- Rivett A. J., Levine R. L. Metal-catalyzed oxidation of Escherichia coli glutamine synthetase: correlation of structural and functional changes. Arch Biochem Biophys. 1990 Apr;278(1):26–34. doi: 10.1016/0003-9861(90)90226-o. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kent S. B. Enzymatic activity of a synthetic 99 residue protein corresponding to the putative HIV-1 protease. Cell. 1988 Jul 29;54(3):363–368. doi: 10.1016/0092-8674(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991 Feb 5;266(4):2005–2008. [PubMed] [Google Scholar]
- Suguna K., Bott R. R., Padlan E. A., Subramanian E., Sheriff S., Cohen G. H., Davies D. R. Structure and refinement at 1.8 A resolution of the aspartic proteinase from Rhizopus chinensis. J Mol Biol. 1987 Aug 20;196(4):877–900. doi: 10.1016/0022-2836(87)90411-6. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]


