Abstract
Fibroblast growth factor (FGF)-1 and -2 have potent biological activities implicated in malignant tumor development. Their autocrine and nonautocrine activity in tumor progression of carcinoma was investigated in the NBT-II cell system. Cells were manipulated to either produce and be autocrine for FGF-1 or -2 or to only produce but not respond to these factors. The autocrine cells are highly invasive and tumorigenic and the determination of specific targets of FGF/fibroblast growth factor receptor (FGFR) signaling was assessed. In vitro studies showed that nonautocrine cells behave like epithelial parental cells, whereas autocrine cells have a mesenchymal phenotype correlated with the overexpression of urokinase plasminogen activator receptor (uPAR), the internalization of E-cadherin, and the redistribution of β-catenin from the cell surface to the cytoplasm and nucleus. uPAR was defined as an early target, whereas E-cadherin and the leukocyte common antigen-related protein-tyrosine phosphatase (LAR-PTP) were later targets of FGF signaling, with FGFR1 activation more efficient than FGFR2 at modulating these targets. Behavior of autocrine cells was consistent with a decrease of tumor-suppressive activities of both E-cadherin and LAR-PTP. These molecular analyses show that the potential of these two growth factors in tumor progression is highly dependent on specific FGFR signaling and highlights its importance as a target for antitumor therapy
INTRODUCTION
Activation of fibroblast growth factor (FGF)/fibroblast growth factor receptor (FGFR) signaling is required to trigger various signal transduction pathways leading to cell proliferation, migration, or survival in a variety of cell types (Powers et al., 2000; Ornitz and Itoh, 2001). The multifunctional growth factors FGF-1 and -2 and their receptors may play a role in autocrine and paracrine growth control of malignant tumors; their overproduction or a constitutive activation of FGF signaling is often associated with cancer (Yoshimura et al., 1998; Chandler et al., 1999; Steele et al., 2001).
Tumor progression is correlated with changes of molecules involved in the adherens junctions between neighboring cells. E-cadherin and β-catenin mediate specific intercellular adhesion and form a complex that maintains epithelial cell polarity and regulates organized epithelial attachment (Nose et al., 1990; McCrea et al., 1991; Steinberg and McNutt, 1999; Gumbiner, 2000). These two molecules control a wide array of cellular behavior and alterations in their expression are correlated with a dedifferentiated and invasive cell phenotype and can promote oncogenicity (Nollet et al., 1999; Goichberg et al., 2001). Specific mutations of β-catenin result in oncogenic transformation (Aoki et al., 2002) and reexpression of E-cadherin in malignant cells has a tumor suppressor effect (Vleminckx et al., 1991). Furthermore, the E-cadherin/β-catenin complex is often linked to growth factor signaling (Hoschuetzky et al., 1994; Gumbiner, 2000).
Activation of receptor tyrosine kinases (RTK), such as FGFR, epidermal growth factor receptor (EGFR), or c-met, induces phosphorylation of tyrosine residues on β-catenin with loss of cadherin-mediated cell adhesion and liberation of β-catenin from the membrane adhesion complex (Hazan and Norton, 1998; Roura et al., 1999). β-catenin is also involved in Wnt signaling that mediates many events in development and may play a role in tumorigenesis (Wodarz and Nusse, 1998; Behrens, 2000). Activation of the Wnt pathway allows the translocation of free β-catenin from the cytoplasm to the nucleus (Holnthoner et al., 2002), where it can interact with members of the Lef/Tcf family of transcription factors leading to the expression of target genes, such as those encoding the urokinase plasminogen activator receptor (uPAR) and c-myc (Hsu et al., 1998). Accumulation of β-catenin in the nucleus may contribute to the development and progression of carcinoma both by dedifferentiation and through proteolytic activity (Mann et al., 1999; Tetsu and McCormick, 1999).
Localized at the plasma membrane, uPAR governs the uPA activation and links its natural inhibitors, plasminogen activator inhibitors (PAI). A balance between these different partners is crucial for the control of uPA activity, which is a determining factor of the invasive process in tumor progression (Dano et al., 1999; Borgfeldt et al., 2001). In addition, uPAR can act as a signaling molecule through interaction with members of the integrin adhesion receptor superfamily (Blasi and Carmeliet, 2002; Rao, 2003).
Tyrosine phosphorylation is one of the critical molecular events regulating physiological and pathological processes. In fact, many oncogenes are either protein tyrosine kinases (PTK), their ligands or their targets (Blume-Jensen and Hunter, 2001; Gisselbrecht, 2003). Tyrosine phosphorylation is also determined by protein tyrosine phosphatases (PTP), which can counterbalance actions of PTKs and act as mediators of cellular adhesion, leading to the hypothesis that some PTP genes might constitute tumor suppressor genes (Hunter, 1989; Beltran and Bixby, 2003). Supporting this hypothesis it has been shown that the expression of PTP in breast tumor cells induced delayed tumor growth and metastasis (Ardini et al., 2000). A member of the transmembrane tyrosine phosphatase family LAR-PTP (leukocyte common antigen-related protein-tyrosine phosphatase) is associated with the E-cadherin/β-catenin complex (Kypta et al., 1996). Current evidence supports a role for LAR-PTP in cadherin complexes where it associates with and dephosphorylates β-catenin, a pathway that may be critical for cadherin complex stability and cell-cell association (Beltran and Bixby, 2003). In NBT-II carcinoma cells, it has been demonstrated that upon EGF-induced cell dissociation, β-catenin is rapidly dephosphorylated by LAR-PTP (Muller et al., 1999). Increased or suppressed LAR-PTP expression is associated with a strong modulation of the tyrosine phosphorylation of tyrosine kinase receptors and of downstream signaling molecules such as fibroblast growth factor receptor substrate 2 (FRS2) and insulin receptor substrate 1 (IRS1), the docking proteins of FGFR and IGFR, respectively (Kulas et al., 1996; Weng et al., 1998; Wang et al., 2000).
In this study, we describe a molecular pattern of genes that are targets of FGF/FGFR signaling involved in the invasive and tumorigenic properties of autocrine NBT-II cells. Exogenously stimulated NBT-II cells with FGF-1 or -2 showed that uPAR is up-regulated as an early event through AP-1 transcriptional control, and E-cadherin and LAR-PTP are down-regulated as later events. The β-catenin, relocalized both in the cytoplasm and in the nucleus after FGF stimulation, whereas its expression level is clearly altered after FGFR1 activation rather than through the FGFR2. All of these results are consistent with cell dissociation and demonstrated that uPAR, the E-cadherin/β-catenin complex and LAR-PTP are targets of FGF signaling involved in the invasive and oncogenic potential of the autocrine NBT-II carcinoma model.
MATERIALS AND METHODS
Reagents
FGF-1 and -2 were gifts from Dr. Tom Maciag (Scarborough, ME) and Dr. Hervé Prats (Toulouse, France), respectively. Heparin was purchased from Choay. Monoclonal anti-E-cadherin, anti-β-catenin, anti-LAR-PTP and polyclonal antiphosphotyrosine antibodies were purchased from Transduction Laboratories (Interchim, France). Monoclonal antibodies against β-actin and protein G-Sepharose were purchased from Sigma (La Verpilliére, France). Protease and phosphatase inhibitor cocktails were purchased from Sigma and Calbiochem (La Jolla, CA), respectively. Polyclonal anti-FRS2 antibodies and protein A-Sepharose beads were purchased from Santa-Cruz Biotechnology (Santa Cruz, CA) and Amersham Biosciences (Orsay, France), respectively. For ECL detection, peroxidase-coupled secondary antibodies were purchased from Amersham. For immunofluorescence staining, Alexas 488– or Alexas 594–conjugated goat anti-mouse IgG, provided by Molecular Probes (Invitrogen, Cergy-Pontoise, France), were used as secondary antibodies.
Cell Lines and Culture
The NBT-II cells were derived from a chemically induced rat bladder carcinoma (Toyoshima et al., 1971). These cells do not produce endogenous FGF-1 or -2, but possess the FGFR-2b high-affinity FGF-1 receptor on their surface; this receptor cannot be activated by FGF2. NBT-II cells engineered to produce FGF-1 are the NSF14 cells (autocrine cells), NBT-II cells engineered to produce FGF-2 are the bGF22 cells (nonautocrine cells), NBT-II-FGFR1 are NBT-II cells that now express the high affinity FGFR1 receptor that can be triggered by exogenous FGF-1 and -2, and NBT-II-FGFR1 cells producing FGF-2 are the cl1 cells (autocrine cells). NBT-II cells with nonfunctional FGFR receptors were generated by stable transfection of NBT-II cells with a dominant-negative form of FGFR1 (ΔN-FGFR1), and these cells producing FGF-1 are cl37 cells (nonautocrine cells). All of these cell lines have been reported previously (Jouanneau et al., 1991; Jouanneau et al., 1997; Billottet et al., 2002). They were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete medium).
For time-course of FGF-stimulation, 1 × 106 cells were seeded in the presence of FGF-1 or -2 (20 ng/ml) together with 50 μg/ml heparin in complete medium.
E-cadherin and β-catenin Indirect Immunofluorescence Staining
Cells, 2 × 104, were seeded on a glass coverslip and grown to subconfluence. Cells were fixed in 4% paraformaldehyde in PBS (phosphate-buffered saline: 136 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4), permeabilized with 0.1% Triton X-100 and blocked in 5% fetal bovine serum. Cells were then incubated with mouse monoclonal anti-E-cadherin antibody (1:25) or anti-β-catenin (1:50) before staining with Alexas 594– or Alexa 488–conjugated goat anti-mouse IgG, respectively. The percentage of cells with nuclear β-catenin was estimated by counting the labeled nuclear β-catenin on two frames of each section (by 2 independent individuals).
Western Blot and Immunoprecipitation Analysis
Subconfluent cells were washed with ice-cold PBS, scraped off into RIPA buffer (1% NP40, 0.5% sodium deoxycholate, and 0.1% SDS, pH 7.4) supplemented with protease and phosphatase inhibitor cocktails. Cell lysates were centrifuged at 10,000 × g for 15 min and the supernatants were collected. Lysate samples containing 100 μg of protein were resuspended in electrophoresis sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.1% bromophenol blue, 1% β-mercaptoethanol), boiled, and finally subjected to electrophoresis in a 10% polyacrylamide gel. Proteins were electroblotted onto a polyvinylidene difluoride membrane (Immobilon, Millipore, Molsheim, France). Membranes were incubated with 5% nonfat milk in Trisbuffered saline supplemented with 0.1% Tween-20 (TBST) and then incubated with the appropriate antibody.
For β-catenin immunoprecipitation, cells were plated at a density of 1 × 106 cells per 75 cm2 the day before. When indicated, cells were pretreated before lysis with sodium orthovanadate (1 mM) for 90 min. Cells were lysed in ice-cold lysis buffer (1% Triton X-100, 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 2.5 mM pyrophosphate, and 1 mM β-glycerophosphate) supplemented with protease and phosphatase inhibitor cocktails. Insoluble material was removed by centrifugation at 12,500 × g for 10 min. One milligram of precleared cell lysate was incubated with 1.25 μg/ml β-catenin antibody and protein G-Sepharose overnight at 4°C. For FRS2 immunoprecipitation, subconfluent cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitor cocktails. Insoluble material was removed by centrifugation at 12,500 × g for 10 min. One milligram of precleared cell lysates was incubated with 1 μg/ml FRS2 antibody and protein A-Sepharose for 2 h at 4°C.
For both immunoprecipitations, the immunocomplexes were then washed three times in the lysis buffer and twice in PBS. The samples were boiled in 2× sample buffer, subjected to electrophoresis and transferred. After blocking, membranes were incubated with the appropriate antibodies in their respective blocking buffer. Before reprobing, membranes were stripped by incubation for 45 min in 62.5 mM Tris-HCl, pH 6.8, 2% SDS and 100 mM β-mercapthoethanol at 65°C. For all blots, proteins were detected through chemiluminescence detection of the peroxydase coupled secondary antibodies with ECL reagent (Amersham).
RT-PCR Amplifications
Total RNA was isolated from cell lines by the RNA PLUS extraction kit (Qbiogene, Illkirch, France). One microgram of total RNA was reverse-transcribed into cDNA using the RNA PCR kit (AMV-RT, Takara Biomedicals, Tokyo, Japan). The resulting cDNAs were amplified using specific primers for the genes of interest (see Table 1) together with primers for the cDNA of the 18S rRNA subunit.
Table 1.
Characteristics of the primers used for RT-PCR and Real-Time PCR amplification
| Tm (°C) | No. of cycles | Product length (bp) | Oligonucleotide primers | ||
|---|---|---|---|---|---|
| ruPA | 55 | 32 | 173 | Sense | 5′GCTATGTGCAAATTGGCCTA3′ |
| Antisense | 5′CTGGTTCTCAACGACAGTGA3′ | ||||
| ruPAR | 65 | 32 | 253 | Sense | 5′GCCCTGGGCCAGGACCTCTG3′ |
| Antisense | 5′GAGAGGTGCAGGATGCACAC3′ | ||||
| rPAI-1 | 55 | 35 | 276 | Sense | 5′CGGCACTGGTAAATCTTTCC3′ |
| Antisense | 5′GGGTCATCCTTCATAGCAAT3′ | ||||
| rc-fos | 62 | 32 | 760 | Sense | 5′TTGATGACTTCTTGTTTCCG3′ |
| Antisense | 5′ACAATCCGAAAAATATCCAG3′ | ||||
| rc-jun | 58 | 35 | 278 | Sense | 5′TTCCATCGCAGCCCCAGCAG3′ |
| Antisense | 5′GGTTTTCACTTTTTCCTCCTA3′ | ||||
| rLAR-PTP | 54 | 35 | 214 | Sense | 5′GCATGTCTACTGGAAGCT3′ |
| 187 | Antisense | 5′AGTAACGGAGTAGGTGGTCT3′ | |||
| r18S | 55 | 18 | 139 | Sense | 5′GGGGAATCAGGGTTCGATT3′ |
| Antisense | 5′GCCTCGAAAGAGTCCTGTA3′ | ||||
| rLAR-PTP (wt) | 58 | 40 | 137 | Sense | 5′CAGAGGAGTCCGAGGACTAT3′ |
| Antisense | 5′TGCCCCTGTGGTAGTAAC3′ | ||||
| rLAR-PTP (exon 13 deleted) | 58 | 40 | 156 | Sense | 5′GCATGTCTACTGGAAGCT3′ |
| Antisense | 5′CTTTGGTGATAGTCGCC3′ | ||||
| HPRT | 58 | 40 | 74 | Sense | 5′GACACTGGAAAACAATGCAG3′ |
| Antisense | 5′GGGTCCTTTTCACCAGCAAG3′ |
r, rat; wt, wild-type.
Real-Time PCR Amplifications
The cDNA were the same as those used for RT-PCR amplifications. We applied the real-time PCR method using the SYBR Green PCR Master Mix as recommanded by the manufacturer (PE Applied Biosystems, Foster City, CA). The relative expression was converted in quantity (pg), and HPRT (hypoxanthine guanine phosphoribosyl transferase) was used as an internal control. Two isoforms of LAR-PTP were expressed in the NBT-II cell system: wild-type LAR-PTP and exon 13–deleted LAR-PTP. The total LAR-PTP quantity was relative to the amount of these two isoforms amplified by two distinct primer sets (see Table 1). The LAR-PTP quantity was estimated by the ratio: the quantity of total LAR-PTP divided by the quantity of HPRT with SE of the mean. The results are representative of three independent experiments.
RESULTS
The E-cadherin/β-catenin Complex Localizes in the Cytoplasm of NBT-II Cells that Are Autocrine for FGF-1 or -2
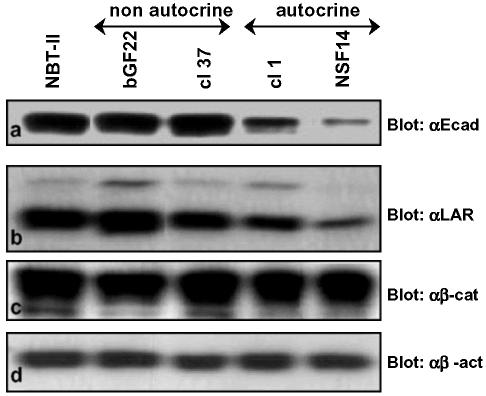
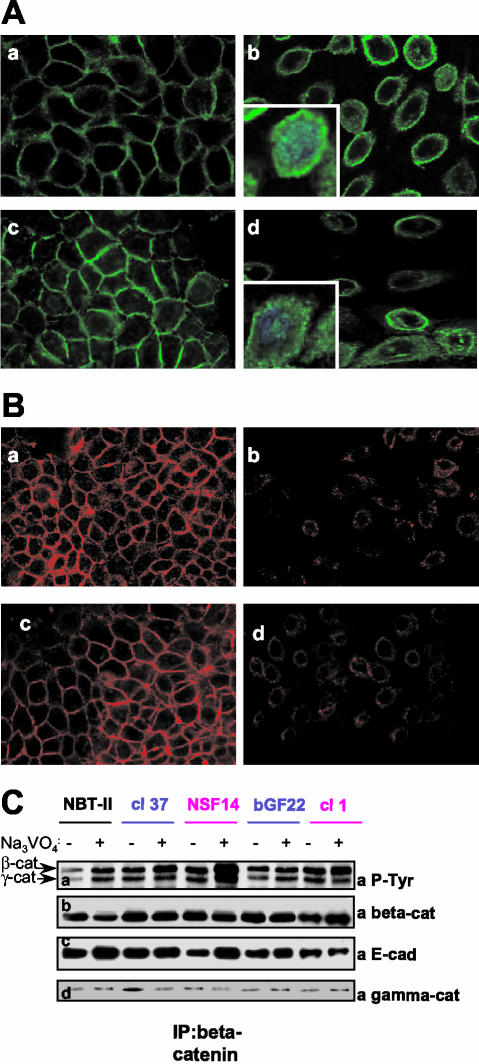
The biological status of the E-cadherin/β-catenin complex was assessed in the NBT-II cell lines producing FGF-1 or -2 in an autocrine or nonautocrine manner. We have previously reported the production and characteristics of these cells (Billottet et al., 2002; see Materials and Methods). NBT-II cells that are autocrine for FGF-1 or -2 possess a transmembrane receptor (FGFR) and produce and secrete the growth factor, whereas the nonautocrine cells produce and secrete the growth factor but have no functional FGFR. The presence of and interaction between E-cadherin and β-catenin were investigated by semiquantitative RT-PCR, Western blotting, immunostaining, and immunoprecipitation. RT-PCR analyses showed that E-cadherin and β-catenin were produced in all cell types, with low levels of E-cadherin expression in cells that are autocrine for FGF-1 or -2 (unpublished data). As evidenced by Western blotting (Figure 1a), NBT-II cells that are nonautocrine for FGF-1 or -2 and parental NBT-II cells expressed similar amounts of E-cadherin, whereas in cells that are autocrine for FGF-1 and -2 the amounts of E-cadherin were lower. Interestingly, the level of β-catenin protein remained the same in all the cells (Figure 1c). Subcellular localization of these molecules was performed using immunofluorescence for E-cadherin and β-catenin. Cells that are nonautocrine for FGF-1 or -2, like parental NBT-II cells, expressed E-cadherin and β-catenin in a uniform pattern along the cell membrane (Figure 2A, a and c, and B, a and c). Conversely, cells that are autocrine for FGF-1 and -2 were dissociated, with predominantly internalized E-cadherin and with β-catenin localized mostly in the cytoplasm around the nucleus and also within the nucleus (Figure 2A, b and d and B, b and d). Immunofluorescence for E-cadherin in autocrine cells was weak and consistent with the Western blots, showing a down-regulation of E-cadherin expression (Figures 1a and 2B, b and d).
Figure 1.
Production of E-cadherin, LAR-PTP, and β-catenin by FGF producing NBT-II cells. Production of E-cadherin (a, αE-cad), LAR-PTP (b, αLAR), β-catenin (c, αβ-cat), and β-actin (d, αβ-act) were detected by Western blotting of total cell extracts. β-actin was used as an internal loading control. For cell nomenclature, see Materials and Methods. Parental cells: NBT-II; FGF-2–producing NBT-II cells: bGF22 (nonautocrine) and cl1 (autocrine); FGF-1–producing NBT-II cells: cl37 (nonautocrine) and NSF14 (autocrine)
Figure 2.
E-cadherin and β-catenin localization and β-catenin immunoprecipitation of FGF-producing NBT-II cells. (A) β-catenin immunostaining (green) was performed on NBT-II (a), NSF14 (b), cl37 (c), and cl1 (d) cells. Nuclei were stained with DAPI (blue) in zoomed (inserted) β-catenin sections. Sections were observed at 630× magnification under a Leica SP2 confocal microscope (RueilMalmaison, France) and acquired with the LSM 5 Image browser software. Z sections were taken in a plane through the nucleus. (B) E-cadherin immunostaining (red) was performed on NBT-II (a), NSF14 (b), cl37 (c), and cl1 (d) cells. Sections were observed at 400× magnification under a Leica SP2 confocal microscope and acquired with the LSM 5 Image browser software. (C) Lysates of control (-) or sodium orthovanadate (Na3VO4)-treated (+) cells, containing an equivalent amount of protein (1 mg), were subjected to immunoprecipitation with a monoclonal anti-β-catenin antibody. Proteins were resolved by SDS-PAGE and probed with the following antibodies: polyclonal antiphosphotyrosine coupled to HRP (a, αP-Tyr), monoclonal anti-β-catenin (b, αβ-cat), monoclonal anti-E-cadherin (c, αE-cad) and monoclonal anti-γ-catenin (d, αγ-cat). For cell nomenclature, see Materials and Methods.
Cell extracts were subjected to immunoprecipitation using β-catenin antibodies. Western blotting of these immunoprecipitates revealed that E-cadherin could also be detected in the β-catenin immunoprecipitates, demonstrating the interaction between E-cadherin and β-catenin, both at the cell membrane and in the cytoplasm (Figure 2C, b and c).
Altogether, these results indicate that parental cells and cells that are nonautocrine for FGF-1 or -2 have a cohesive epithelial phenotype with E-cadherin/β-catenin complexes at the cell membrane. Conversely, cells that are autocrine for FGF-1 or -2, and thereby constitutively activated, have a mesenchymal phenotype associated with a low level of E-cadherin, a pool of E-cadherin/β-catenin complexes within the cytoplasm, and a pool of free β-catenin in the nucleus.
β-catenin and γ-catenin Are Tyrosine Phosphorylated in Autocrine Mesenchymal NBT-II Cells
Although β-catenin is localized both in the cytoplasm and nucleus of NBT-II cells that are autocrine for FGF-1 or -2, its level of expression remains unchanged. Therefore, we determined whether tyrosine phosphorylated β-catenin is present in the mesenchymal and invasive autocrine NBT-II cells. Analyses of the tyrosine phosphorylation of immunoprecipitated β-catenin clearly showed that more β-catenin was phosphorylated in autocrine cells than in parental and nonautocrine cells (Figure 2C, a and b). In all NBT-II–derived cells, the reactivity of β-catenin with the antiphosphotyrosine antibody increased after pretreatment of cells with sodium-orthovanadate, an inhibitor of PTPs. However, immunoprecipitation results obtained with untreated cells show that β-catenin was less tyrosine phosphorylated in parental cell, suggesting that phosphatase activity is greater in these cells. Furthermore, γ-catenin (plakoglobin) that links the tail of E-cadherin to the underlying actin cytoskeleton at cell-cell junctions was phosphorylated in all cells and after the tyrosine phosphorylation of β-catenin (Figure 2C, a and d).
uPAR Is an Early Target Gene of FGF/FGFR Signaling
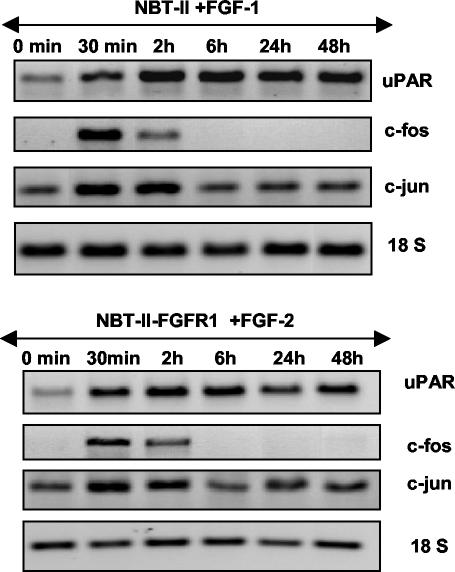
NBT-II carcinoma cells autocrine for FGF-1 or -2 overproduce uPAR. To investigate the impact of FGF-1 and -2 signaling on uPAR gene expression, NBT-II cells and NBT-II-FGFR1 cells were exogenously stimulated by FGF-1 or -2, respectively. In both situations, a time course of stimulation from 0 min to 48 h was performed. The expression of uPAR was assessed by semiquantitative RT-PCR using 18 S RNA as an internal control. From 30 min after stimulation, uPAR expression was increased. This positive modulation is correlated with the increased expression of the transcriptional factors c-fos and c-jun (Figure 3). These results are consistent with the fact that the uPAR promoter possesses AP-1 responsive elements (Dang et al., 1999) and suggests that uPAR is an early target of the FGF-1 and -2 signaling.
Figure 3.
uPAR, c-fos, and c-jun expression upon a time-course of FGF stimulation. RT-PCR for uPAR, c-fos, c-jun, and 18S using RNA of NBT-II and NBT-II-FGFR1 cells, respectively, exogenously stimulated by FGF-1 (top panel) or FGF-2 (bottom panel). RNA 18 S was used as an internal control. For cell nomenclature, see Materials and Methods.
Autocrine Carcinoma Cells Have Enhanced Tumor-associated Properties Correlated with a Decrease of LAR-PTP Tumor-suppressive Activities
We have previously reported that cells that are autocrine for FGF-1 or -2 induced large tumors in nude mice within 2–3 wk, whereas nonautocrine cells induced tumors with a delay similar to those induced by control NBT-II cells (6–7 wk after injection; Billottet et al., 2002). To understand the impact of autocrine FGF/FGFR signaling in this process, we searched for molecular mediators of the tumorigenic behavior.
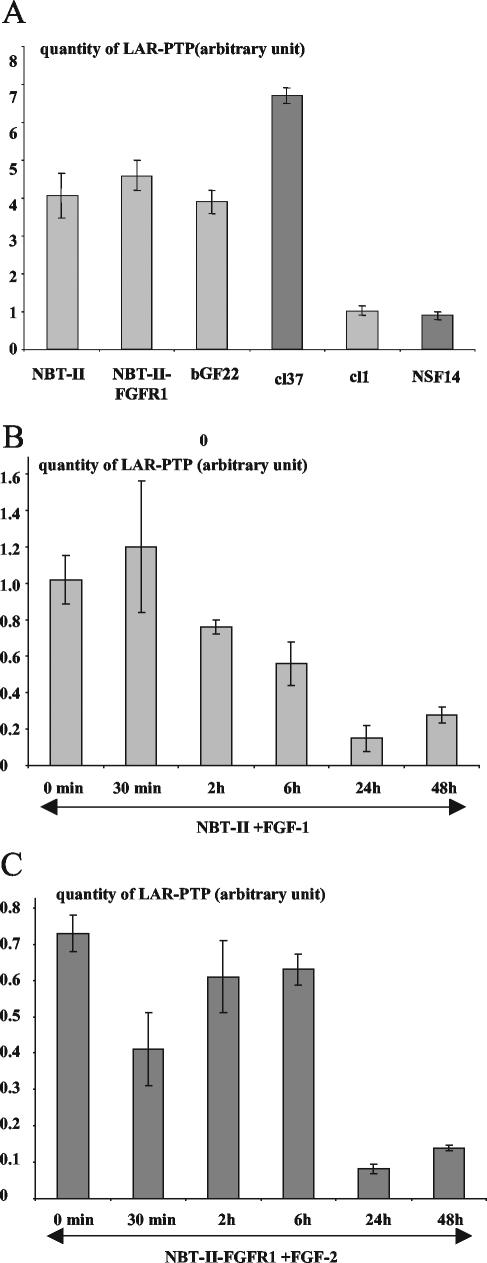
Using the NBT-II cell system, Muller et al. (1999) have reported that the tyrosine phosphatase LAR-PTP can modulate the tyrosine phosphorylation of β-catenin. As reported above we have shown that β-catenin tyrosine phosphorylation depends on FGF signaling; we therefore investigated whether LAR-PTP, like E-cadherin and β-catenin, might be a target of the FGF/FGFR signaling. We first studied the expression and production level of LAR-PTP by real-time RT-PCR (Figure 4A) and by Western blotting (Figure 1b). The results demonstrated that NBT-II parental cells and nonautocrine cells expressed and produced similar amounts of LAR-PTP, whereas this level is greatly reduced in cells that are autocrine for FGF-1 or -2.
Figure 4.
Expression of LAR-PTP by real time RT-PCR. (A) Real time RT-PCR for LAR-PTP using RNA of parental cells (NBT-II), nonautocrine cells (bGF22 and cl37), and autocrine cells (cl1 and NSF14). (B) Time course of real-time RT-PCR for LAR-PTP using RNA of NBT-II cells stimulated by FGF-1. (C) Time course of real-time RT-PCR for LAR-PTP using RNA of NBT-II-FGFR1 cells stimulated by FGF-2. HPRT was used as an internal control and the quantity of LAR-PTP (arbitrary unit) is expressed as a ratio: the quantity of total LAR-PTP divided by the quantity of HPRT, ±SE of the mean. The results were representative of three independent experiments. For cell nomenclature, see Materials and Methods.
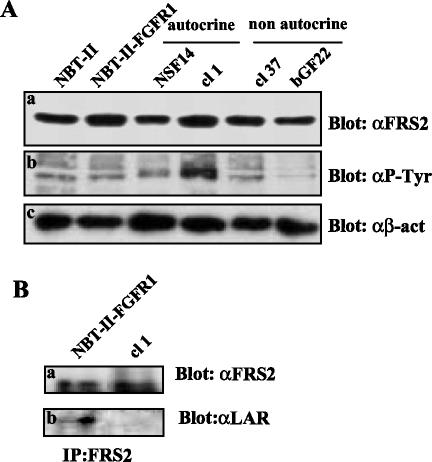
The decrease of E-cadherin, a molecule known as a tumor suppressor, is correlated with an increase in the invasive and tumorigenic potential of autocrine NBT-II cells. In addition, the partial loss of LAR-PTP expression in autocrine NBT-II cells could result in a reduction in the phosphatase activity in these cells. Thus, we hypothesized that in autocrine carcinoma cells, the LAR-PTP protein level and subsequent activity is not sufficient enough to counterbalance the kinase activity resulting from the FGFR signaling. On the basis of this hypothesis, we looked at the status of FRS2, a substrate of LAR-PTP involved in the FGFR signaling (Wang et al., 2000). The level of FRS2 expression by parental cells and FGF producing NBT-II cells was similar (Figure 5Aa), indicating that the FGFR level neither affects nor modulates the FRS2 expression; however, FRS2 was strongly tyrosine phosphorylated in cells that are autocrine for FGF-2 (Figure 5Ab). Interestingly, FRS2 was found to interact with LAR-PTP in parental cells (Figure 5Bb). These results suggest that LAR-PTP is active in parental NBT-II cells. Therefore, in cells that are autocrine for FGF-2, the down-regulation of LAR-PTP could result in an increase of tyrosine phosphorylated FRS2.
Figure 5.
Production of FRS2 in FGF-producing NBT-II cells. (A) Western blotting was performed with total cell extracts of NBT-II, NBT-II-FGFR1, and FGF-producing NBT-II cells with the same amount of protein (100 μg) and the membrane was probed with the following antibodies: polyclonal anti-FRS2 (a, αFRS2), monoclonal antiphosphotyrosine antibody coupled to HRP (b, αP-Tyr), and monoclonal anti-β-actin (c, αβ-act). (B) Lysates of control NBT-II-FGFR1 and NBT-II cells that are autocrine for FGF-2 containing an equivalent amount of protein (500 μg), were subjected to immunoprecipitation with a polyclonal anti-FRS2 antibody. Proteins were resolved by SDS-PAGE and probed with the following antibodies: polyclonal anti-FRS2 (a, α-FRS2) and monoclonal anti-LAR-PTP (b, αLAR). For cell nomenclature, see Materials and Methods.
E-cadherin and LAR-PTP Are Late Targets of FGF/FGFR Signaling
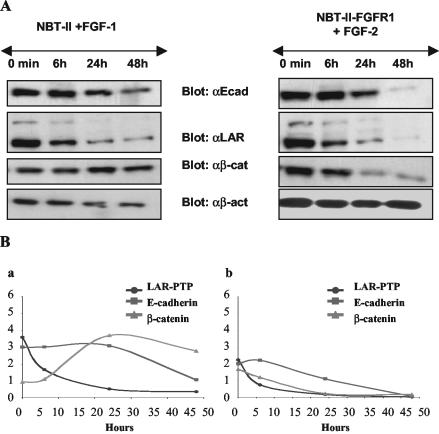
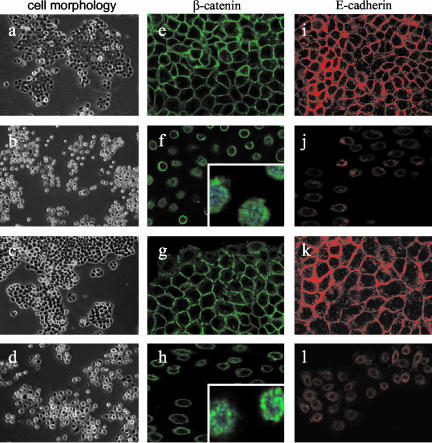
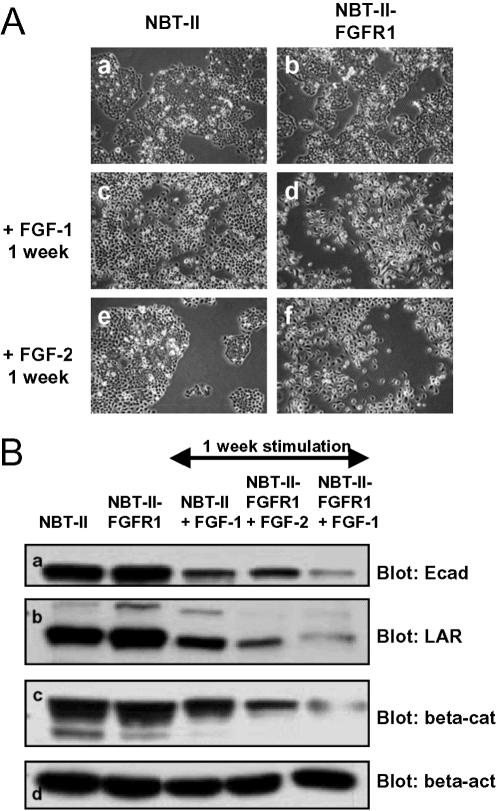
From the data described above, it appears that E-cadherin and LAR-PTP could be either direct or indirect targets of FGF/FGFR signaling. Time lapse videomicroscopy experiments performed >20 h on NBT-II and NBT-II-FGFR1 cells show that from 6 h of FGF treatment, cells loose their epithelial phenotype and undergo an epithelial-mesenchymal transition (EMT; unpublished data). These morphological changes are correlated with a decrease of LAR-PTP after more than 6 h of exogenous stimulation of carcinoma cells by FGF-1 or -2 (Figure 4, B and C). As evidenced from Western blotting of cell protein extracts, the amount of E-cadherin and LAR-PTP protein was inversely correlated with FGF incubation time, between 6 and 24 h of stimulation (Figure 6A). After 48 h of FGF stimulation, cells had a mesenchymal phenotype (Figure 7, b and d), correlated with a relocalization of E-cadherin and β-catenin from the cell membrane to the cytoplasm around the nucleus and the presence of β-catenin in the nucleus (Figure 7, f, h, j, and l). Immunostaining for E-cadherin in cells stimulated for 48 h was weak, consistent with Western blotting and showed a down-regulation of E-cadherin level between 6 and 24 h of stimulation (Figure 6A). Furthermore, the impact of FGF-2 stimulation on the FGFR1 (Figure 6Bb) seemed more potent than that of FGF-1 on FGFR2b (Figure 6Ba). E-cadherin and LAR-PTP had almost disappeared and β-catenin was significantly reduced 48 h after FGF-2 stimulation, whereas the β-catenin level remained high throughout the stimulation period of NBT-II cells with FGF-1. Immunostaining for β-catenin in cells stimulated for 48 h by FGF-1 revealed a high percentage of cells with β-catenin in the nucleus (63 cells with nuclear β-catenin/151 total cells = 42%) compared with cells stimulated for 48 h by FGF-2 (16 cells with nuclear β-catenin/160 total cells = 10%). These results indicate that FGF-1 stimulation through the FGFR2 induces enhanced translocation of β-catenin into the nucleus and preserves its stability, whereas FGF-2 stimulation through the FGFR1 results in the partial loss of β-catenin. To confirm that activation of the FGFR1 is more effective in the down-regulation of the described targets than FGFR2 activation, control NBT-II cells and NBT-II cells that express both the FGFR2b and the FGFR1 receptors, were stimulated with either FGF-1 or -2 for 1 wk. The cells developed a mesenchymal phenotype (Figure 8A) and Western blot analysis on the stimulated cell extracts revealed that FGFR1 activation, through FGF-1 or -2, was more efficient than FGFR2b activation through FGF-1 in reducing E-cadherin, LAR-PTP, and β-catenin levels (Figure 8B).
Figure 6.
E-cadherin, LAR-PTP, and β-catenin production upon a time course of FGF stimulation. (A) E-cadherin (αE-cad), LAR-PTP (αLAR), β-catenin (αβ-cat), and β-actin (αβ-act) were detected by Western blotting of total cell extracts of NBT-II cells exogenously stimulated by FGF-1 (left panel) and total cell extracts of NBT-II-FGFR1 cells exogenously stimulated by FGF-2 (right panel). β-actin was used as an internal loading control. (B) Analyses of Western blots of NBT-II cells stimulated by FGF-1 (a) and NBT-II-FGFR1 cells stimulated by FGF-2 (b) using NIH image 1.63 software. The pixel density of each protein was measured and divided by the pixel density of β-actin for each time point. For cell nomenclature, see Materials and Methods.
Figure 7.
Cell morphology and E-cadherin and β-catenin localization in cells stimulated by FGF for 48 h. Parental NBT-II and NBT-II-FGFR1 cells were cultured for 48 h in complete medium (a and c, respectively) or in complete medium supplemented with FGF-1 for NBT-II cells (b) and FGF-2 for NBT-II-FGFR1 cells (d). Photographs were taken with an inverted microscope at 100× magnification. β-catenin immunostaining (green) and E-cadherin immunostaining (red) were performed on control parental NBT-II cells (e and i) and NBT-II-FGFR1 cells (g and k) in complete medium or stimulated by FGF-1 (f and j) and FGF-2 (h and l), respectively, for 48 h. Nuclei were stained with DAPI (blue) in zoomed (inserted) β-catenin sections. Sections were observed at 400× magnification under a Leica SP2 confocal microscope and acquired with the LSM 5 Image browser software. Z sections were taken in a plane through the nucleus. For cell nomenclature, see Materials and Methods.
Figure 8.
Cell morphology and E-cadherin, LAR-PTP and β-catenin production upon 1 wk of FGF stimulation. (A) NBT-II cells and NBT-II-FGFR1 cells were cultured for 1 wk in complete medium (a and b, respectively), complete medium supplemented with FGF-1 (c and d, respectively), or complete medium supplemented with FGF-2 (e and f, respectively). Photographs were taken with an inverted microscope at 100× magnification. (B) E-cadherin (a, αE-cad), LAR-PTP (b, αLAR), β-catenin (c, αβ-cat), and β-actin (d, αβ-act) were detected by Western blotting of control parental cell extracts and cells stimulated by FGF-1 or -2 for 1 wk. β-actin was used as an internal loading control. For cell nomenclature, see Materials and Methods.
DISCUSSION
Oncogenic transformation may be associated with signaling pathways induced by growth factors and their tyrosine kinase receptors (Blume-Jensen and Hunter, 2001). It has been reported that FGF-1 and -2 are expressed in some human cancers and that autocrine loops for these factors could be linked to aggressive clinical behavior (Yoshimura et al., 1998; Chandler et al., 1999; Powers et al., 2000; Steele et al., 2001). These findings are consistent with constitutive FGF-1 and -2 production directly affecting cell motility, invasiveness, tumorigenicity, and tumor angiogenesis. Furthermore, we suggest that the results obtained do not depend on clonal variation arising after transfection because concordant results were obtained with activated autocrine cells and exogeneously stimulated parental cells.
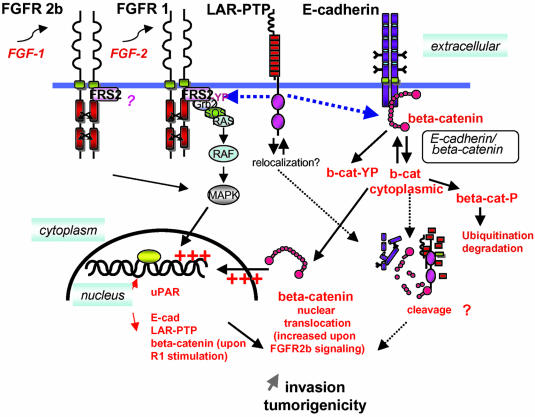
In this study we show that autocrine or paracrine FGF-1 and -2 signaling can influence the invasiveness and tumorigenic potential of carcinoma cells by mechanisms involving more than just changes in cellular adhesion through E-cadherin/β-catenin. A cascade of signaling events also results in the modulation of proteolytic systems and tumor suppressive molecules. (see Figure 9)
Figure 9.
Summary diagram of the signaling events. In the NBTII carcinoma system, activation of either endogenous FGFR2b receptor with FGF-1 or of constitutively expressed FGFR1 by FGF-2, induces the epithelio-mesenchymal transition (EMT) of the cells and thus cell-cell dissociation. The intercell cadherin complexes are disrupted followed by E-cadherin, β-catenin, and free E-cadherin/β-catenin complex internalization. β-catenin is ser/thr phosphorylated after activation and further degraded in the proteasome after ubiquitination. Tyrosinephosphorylated β-catenin can interact with LAR-PTP (Mueller et al., 1999), which in turn could indirectly participate in the β-catenin nuclear signaling after dephosphorylation. LAR-PTP also interacts with activated phosphorylated FRS2. Wang et al. (2000) have demonstrated that such an interaction is transient and may depend on the expression level of the FGFR. In NBT-II cells overexpressing the FGFR1, LAR-PTP is significantly expressed and can be coimmunoprecipitated together with FRS2 by an anti-FRS2 antibody. FRS2 acts as a docking molecular bridge between the FGFR and the downstream signaling protein complexes for activation of the Ras MAP kinase signaling cascade (Hadari et al., 2001). Stimulation through FGFR1 with FGF-1 or -2 ligands is more efficient than FGFR2 signaling in regulating the expression level of E-cadherin, LAR-PTP, and β-catenin (see Figure 8).
uPAR Is an Early Target of FGF/FGFR Signaling
We have previously concluded that NBT-II carcinoma cells that are autocrine for FGF-1 or -2 display similar high invasive behavior and proteolytic activities, including up-regulation in these cells of metalloproteases (MMP-9 and MMP-2), urokinase (uPA) and uPAR, the cell receptor required for activation of the urokinase. (Billottet et al., 2002). Furthermore, in this report, we demonstrate that uPAR is up-regulated upon FGF stimulation of the parental cells and is an early target gene of the FGF/FGFR signaling, most likely through the transcriptional control of c-fos and c-jun, which are concomitantly expressed. This is consistent with the fact that the 5′-flanking region of the uPAR gene promoter contains several putative cis-regulatory elements including two AP-1 responsive elements (Dang et al., 1999).
E-cadherin Is a Late Target of FGF/FGFR Signaling
We have demonstrated that E-cadherin is a late target of the FGF/FGFR signaling. Several years ago, it was shown that reexpression of E-cadherin in malignant cells has a tumor suppressive effect (Vleminckx et al., 1991) and that this tumor suppressor molecule, which is associated with β-catenin can be linked to growth factor signaling such as EGFR signaling (Hoschuetzky et al., 1994). Interaction of E-cadherin with the actin cytoskeleton through α-, β-, and γ-catenins is necessary for the adhesive function of E-cadherin (Gumbiner, 2000) and the E-cadherin/β-catenin complex plays an important role in tumor development (Nollet et al., 1999; Behrens, 2000). We therefore checked the status of E-cadherin and β-catenin in nonactivated cells and both constitutively or exogenously activated cells. Our results demonstrate that autocrine cells have a reduced steady state level of E-cadherin expression and that E-cadherin and β-catenin are found colocalized in the cytoplasm around the nucleus. E-cadherin and β-catenin have almost disappeared from the cell surface of activated cells but can persist at some cell-cell contacts in densely plated cells. The presence of β-catenin in the nucleus of NBT-II cells that are autocrine for FGF-1 or -2 is weak but could be responsible for the activation of target genes such as c-myc, cyclin D1, uPAR, or matrilysin (Hsu et al., 1998). Presence of β-catenin in the nucleus of NBT-II cells that are autocrine for FGF-1 or -2 could result in the nuclear translocation of β-catenin from the tyrosine-phosphorylated β-catenin pool. Unexpectedly, in parental and nonautocrine cells β-catenin is also found to be tyrosine phosphorylated. This could reflect a basal and permanent cytoplasmic pool of phosphorylated β-catenin proteins, not linked to the tail of the transmembrane E-cadherin; although as cells were only cultured for 24 h and did not have a complete cohesive phenotype. In this model, cell density is a strong and important modulator of E-cadherin and β-catenin status. In autocrine cells, high cell density favors partial localization of E-cadherin and β-catenin to the cell membrane, whereas low cell density is associated with a cytoplasmic E-cadherin/β-catenin complex and free β-catenin both in the cytoplasm around the nucleus and in the nucleus (unpublished data).
On the basis of these results, we can conclude that the mesenchymal and invasive phenotype of NBT-II cells that are autocrine for FGF-1 or -2 is correlated with the modulation of the cell adhesion molecules E-cadherin and β-catenin with a loss of adherent E-cadherin.
LAR-PTP Is a Late Target of FGF/FGFR Signaling
The tyrosine phosphorylation status of β-catenin might be a critical molecular event that regulates cellular adhesion of epithelial cells. It has been shown that tyrosine phosphorylation of β-catenin is correlated with a reduction in cellular adhesion and its liberation from the cadherin adhesion complex to the cytoplasm and/or nucleus (Roura et al., 1999). The data presented in this article demonstrates that FGF-1 or -2 treatment reduces cell-cell adhesion and promotes cell scattering in the NBTII carcinoma system associated with tyrosine phosphorylation of several signaling molecules. The presence of tyrosine phosphorylated β-catenin and γ-catenin in autocrine cells could be correlated with the dissociation of the E-cadherin-catenin complexes and thus with their increased invasive and tumorigenic behavior.
LAR-PTP is thought to be a putative tumor suppressor gene that plays an important role in cellular adhesion (Kypta et al., 1996; Beltran and Bixby, 2003). LAR-PTP may dephosphorylate β-catenin, thereby favoring its interaction with E-cadherin and the maintenance of adherens junctions at the plasma membrane (Muller et al., 1999). LAR-PTP is also implicated in the regulation of FGF/FGFR signaling as it interacts with and dephosphorylates FRS2, a FGFR docking protein, resulting in the blockade of downstream signaling via pathways such as the MAPKs and PI3K pathways (Wang et al., 2000).
We have demonstrated that autocrine cells produce a lower level of LAR-PTP protein than parental and nonautocrine cells and that in parental cells LAR-PTP interacts with FRS2. By direct interaction with intracellular signaling molecules such as β-catenin and FRS2, it is possible that LAR-PTP regulates their degree of tyrosine phosphorylation. A low level of LAR-PTP protein could explain the level of tyrosine-phosphorylated β-catenin and FRS2 in autocrine cells. Modulation of LAR-PTP activity in these cells and the possible redistribution of this protein into the cytoplasm may well be a response to FGF/FGFR signaling.
Exogenous FGF stimulation of parental cells results in a time-dependent down-regulation of both LAR-PTP and E-cadherin. Furthermore, immunostaining for E-cadherin and β-catenin revealed that these two proteins are redistributed from the plasma membrane to the cytoplasm where they can still interact (Piedra et al., 2001) and that β-catenin is localized in the nucleus. Altogether, these results show that the E-cadherin/β-catenin complex and LAR-PTP are late targets of FGF/FGFR signaling.
Our results show that at the cellular level there is nothing distinguishing between FGFR2 and FGFR1 activation; however, at the molecular level, FGF signaling through FGFR1 is more efficient at down-regulating E-cadherin, LAR-PTP, and β-catenin. Nevertheless, we cannot exclude the possibility that our results could, at least in part, be dependent on the expression level of the transfected FGFR1 receptor compared with the endogenous FGFR2 density. Endogenous FGFR2 is expressed at 5000 receptors/NBT-II cell (Valles et al., 1990), whereas transfected cells expressed 60,000 FGFR1 receptors/cell (Savagner et al., 1994). The ratio in favor of the FGFR1, which could be activated by both FGF-1 and -2, may favor the FGFR1 signaling cascade; although this seems unlikely, because FGF-1 stimulation through FGFR2b is more efficient than FGFR1 signaling in targeting β-catenin to the nucleus. In BaF3 lymphoid and L6 myoblast cells it has been shown that signaling and biological responses elicited by different FGFRs differ substantially (Wang et al., 1994). Freeman and collaborators have demonstrated that FGFR1 and FGFR2 activation affect the development of prostate cancer differently, showing that equivalent activation of FGFR1 and FGFR2 leads to quantitative differences in activation of downstream targets such as phosphorylation of Erk and expression of tumor-associated osteopontin (Freeman et al., 2003).
In the NBT-II carcinoma model, FGF-1 and -2 signaling, through the FGFR1 and the FGFR2, induce a series of cellular and molecular changes associated with the EMT, which affect the organization of several complexes involving uPAR, E-cadherin, β-catenin, FGFR, and LAR-PTP. These results highlight the importance of FGF/FGFR signaling as a target for antitumor therapy.
Acknowledgments
We thank Dr. T. Maciag and Dr. H. Prats for their FGF-1 and -2 recombinant proteins, respectively, and Dr. R. Mudge and Dr. M. Morgan for useful discussions. This work was supported by the Centre National dela Recherche Scientifique and the Institut Curie, by grants from the Association pour la Recherche sur le Cancer (ARC-9477 and ARC-5902 to J.J.), the Ligue Nationale Française contre le Cancer (National and Paris committees), and the Groupe-ment des Entreprises Françaises contre le Cancer (Gefluc). C.B. is a recipient of ARC fellowship.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–04–0336. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–04–0336.
References
- Aoki, M., Sobek, V., Maslyar, D.J., Hecht, A., and Vogt, P.K. (2002). Oncogenic transformation by beta-catenin: deletion analysis and characterization of selected target genes. Oncogene 21, 6983-6991. [DOI] [PubMed] [Google Scholar]
- Ardini, E., Agresti, R., Tagliabue, E., Greco, M., Aiello, P., Yang, L.T., Menard, S., and Sap, J. (2000). Expression of protein tyrosine phosphatase alpha (RPTPalpha) in human breast cancer correlates with low tumor grade, and inhibits tumor cell growth in vitro and in vivo. Oncogene 19, 4979-4987. [DOI] [PubMed] [Google Scholar]
- Behrens, J. (2000). Control of beta-catenin signaling in tumor development. Ann. NY Acad. Sci. 910, 21-33; discussion 33-35. [DOI] [PubMed] [Google Scholar]
- Beltran, P.J., and Bixby, J.L. (2003). Receptor protein tyrosine phosphatases as mediators of cellular adhesion. Front. Biosci. 8, D87-D99. [DOI] [PubMed] [Google Scholar]
- Billottet, C., Janji, B., Thiery, J.P., and Jouanneau, J. (2002). Rapid tumor development and potent vascularization are independent events in carcinoma producing FGF-1 or FGF-2. Oncogene 21, 8128-8139. [DOI] [PubMed] [Google Scholar]
- Blasi, F., and Carmeliet, P. (2002). uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell. Biol. 3, 932-943. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen, P., and Hunter, T. (2001). Oncogenic kinase signalling. Nature 411, 355-365. [DOI] [PubMed] [Google Scholar]
- Borgfeldt, C., Hansson, S.R., Gustavsson, B., Masback, A., and Casslen, B. (2001). Dedifferentiation of serous ovarian cancer from cystic to solid tumors is associated with increased expression of mRNA for urokinase plasminogen activator (uPA), its receptor (uPAR) and its inhibitor (PAI-1). Int. J. Cancer 92, 497-502. [DOI] [PubMed] [Google Scholar]
- Chandler, L.A., Sosnowski, B.A., Greenlees, L., Aukerman, S.L., Baird, A., and Pierce, G.F. (1999). Prevalent expression of fibroblast growth factor (FGF) receptors and FGF2 in human tumor cell lines. Int. J. Cancer 81, 451-458. [DOI] [PubMed] [Google Scholar]
- Dang, J., Boyd, D., Wang, H., Allgayer, H., Doe, W.F., and Wang, Y. (1999). A region between -141 and -61 bp containing a proximal AP-1 is essential for constitutive expression of urokinase-type plasminogen activator receptor. Eur. J. Biochem. 264, 92-99. [DOI] [PubMed] [Google Scholar]
- Dano, K., Romer, J., Nielsen, B.S., Bjorn, S., Pyke, C., Rygaard, J., and Lund, L.R. (1999). Cancer invasion and tissue remodeling–cooperation of protease systems and cell types. Apmis 107, 120-127. [DOI] [PubMed] [Google Scholar]
- Freeman, K.W., Gangula, R.D., Welm, B.E., Ozen, M., Foster, B.A., Rosen, J.M., Ittmann, M., Greenberg, N.M., and Spencer, D.M. (2003). Conditional activation of fibroblast growth factor receptor (FGFR) 1, but not FGFR2, in prostate cancer cells leads to increased osteopontin induction, extracellular signal-regulated kinase activation, and in vivo proliferation. Cancer Res. 63, 6237-6243. [PubMed] [Google Scholar]
- Gisselbrecht, S. (2003). [Oncogenes and leukemia: history and perspectives]. Med. Sci. (Paris) 19, 201-210. [DOI] [PubMed] [Google Scholar]
- Goichberg, P., Shtutman, M., Ben-Ze'ev, A., and Geiger, B. (2001). Recruitment of beta-catenin to cadherin-mediated intercellular adhesions is involved in myogenic induction. J. Cell Sci. 114, 1309-1319. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. (2000). Regulation of cadherin adhesive activity. J. Cell Biol. 148, 399-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari, Y.R., Gotoh, N., Kouhara, H., Lax, I, and Schlessinger, J. (2001). Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. USA 98, 8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan, R.B., and Norton, L. (1998). The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J. Biol. Chem. 273, 9078-9084. [DOI] [PubMed] [Google Scholar]
- Holnthoner, W., Pillinger, M., Groger, M., Wolff, K., Ashton, A.W., Albanese, C., Neumeister, P., Pestell, R.G., and Petzelbauer, P. (2002). Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J. Biol. Chem. 277, 45847-45853. [DOI] [PubMed] [Google Scholar]
- Hoschuetzky, H., Aberle, H., and Kemler, R. (1994). Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J. Cell Biol. 127, 1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, S.C., Galceran, J., and Grosschedl, R. (1998). Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol. Cell. Biol. 18, 4807-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, T. (1989). Protein-tyrosine phosphatases: the other side of the coin. Cell 58, 1013-1016. [DOI] [PubMed] [Google Scholar]
- Jouanneau, J., Gavrilovic, J., Caruelle, D., Jaye, M., Moens, G., Caruelle, J.P., and Thiery, J.P. (1991). Secreted or nonsecreted forms of acidic fibroblast growth factor produced by transfected epithelial cells influence cell morphology, motility, and invasive potential. Proc. Natl. Acad. Sci. USA 88, 2893-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanneau, J., Plouet, J., Moens, G., and Thiery, J.P. (1997). FGF-2 and FGF-1 expressed in rat bladder carcinoma cells have similar angiogenic potential but different tumorigenic properties in vivo. Oncogene 14, 671-676. [DOI] [PubMed] [Google Scholar]
- Kulas, D.T., Goldstein, B.J., and Mooney, R.A. (1996). The transmembrane protein-tyrosine phosphatase LAR modulates signaling by multiple receptor tyrosine kinases. J. Biol. Chem. 271, 748-754. [DOI] [PubMed] [Google Scholar]
- Kypta, R.M., Su, H., and Reichardt, L.F. (1996). Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J. Cell Biol. 134, 1519-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, B. et al. (1999). Target genes of beta-catenin-T cell-factor/lymphoidenhancer-factor signaling in human colorectal carcinomas. Proc. Natl. Acad. Sci. USA 96, 1603-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea, P.D., Turck, C.W., and Gumbiner, B. (1991). A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254, 1359-1361. [DOI] [PubMed] [Google Scholar]
- Muller, T., Choidas, A., Reichmann, E., and Ullrich, A. (1999). Phosphorylation and free pool of beta-catenin are regulated by tyrosine kinases and tyrosine phosphatases during epithelial cell migration. J. Biol. Chem. 274, 10173-10183. [DOI] [PubMed] [Google Scholar]
- Nollet, F., Berx, G., and van Roy, F. (1999). The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol. Cell. Biol. Res. Commun. 2, 77-85. [DOI] [PubMed] [Google Scholar]
- Nose, A., Tsuji, K., and Takeichi, M. (1990). Localization of specificity determining sites in cadherin cell adhesion molecules. Cell 61, 147-155. [DOI] [PubMed] [Google Scholar]
- Ornitz, D.M., and Itoh, N. (2001). Fibroblast growth factors. Genome Biol. 2, REVIEWS 3005. [DOI] [PMC free article] [PubMed]
- Piedra, J., Martinez, D., Castano, J., Miravet, S., Dunach, M., and de Herreros, A.G. (2001). Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J. Biol. Chem. 276, 20436-20443. [DOI] [PubMed] [Google Scholar]
- Powers, C.J., McLeskey, S.W., and Wellstein, A. (2000). Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 7, 165-197. [DOI] [PubMed] [Google Scholar]
- Rao, J.S. (2003). Molecular mechanisms of glioma invasiveness: the role of proteases. Nat. Rev. Cancer 3, 489-501. [DOI] [PubMed] [Google Scholar]
- Roura, S., Miravet, S., Piedra, J., Garcia de Herreros, A., and Dunach, M. (1999). Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J. Biol. Chem. 274, 36734-36740. [DOI] [PubMed] [Google Scholar]
- Savagner, P., Valles, A.M., Jouanneau, J., Yamada, K.M., and Thiery, J.P. (1994). Alternative splicing in fibroblast growth factor receptor 2 is associated with induced epithelial-mesenchymal transition on rat bladder carcinoma cells. Mol. Biol. Cell 5, 851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, I.A., Edmonson, R.G., Bulmer, J.N., Bolger, B.S., Leung, H.Y., and Davies, B.R. (2001). Induction of FGF receptor 2-IIIb expression and response to its ligands in epithelial ovarian cancer. Oncogene 20, 5878-5887. [DOI] [PubMed] [Google Scholar]
- Steinberg, M.S., and McNutt, P.M. (1999). Cadherins and their connections: adhesion junctions have broader functions. Curr. Opin. Cell Biol. 11, 554-560. [DOI] [PubMed] [Google Scholar]
- Tetsu, O., and McCormick, F. (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422-426. [DOI] [PubMed] [Google Scholar]
- Toyoshima, K., Ito, N., Hiasa, Y., Kamamoto, Y., and Makiura, S. (1971). Tissue culture of urinary bladder tumor induced in a rat by N-butyl-N-(-4-hydroxybutyl)nitrosamine: establishment of cell line, Nara Bladder Tumor II. J. Natl. Cancer Inst. 47, 979-985. [PubMed] [Google Scholar]
- Valles, A.M., Boyer B. Badet, J., Tucker, G.C., Barritault, D., and Thiery, J.P. (1990). Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc. Natl. Acad. Sci. USA 87, 1124-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx, K., Vakaet, L., Jr., Mareel, M., Fiers, W., and van Roy, F. (1991). Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66, 107-119. [DOI] [PubMed] [Google Scholar]
- Wang, J.K., Gao, G., and Goldfarb, M. (1994). Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol. Cell. Biol. 14, 181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Weng, L.P., and Yu, Q. (2000). Specific inhibition of FGF-induced MAPK activation by the receptor-like protein tyrosine phosphatase LAR. Oncogene 19, 2346-2353. [DOI] [PubMed] [Google Scholar]
- Weng, L.P., Yuan, J., and Yu, Q. (1998). Overexpression of the transmembrane tyrosine phosphatase LAR activates the caspase pathway and induces apoptosis. Curr. Biol. 8, 247-256. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., and Nusse, R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59-88. [DOI] [PubMed] [Google Scholar]
- Yoshimura, N., Sano, H., Hashiramoto, A., Yamada, R., Nakajima, H., Kondo, M., and Oka, T. (1998). The expression and localization of fibroblast growth factor-1 (FGF-1) and FGF receptor-1 (FGFR-1) in human breast cancer. Clin. Immunol. Immunopathol. 89, 28-34. [DOI] [PubMed] [Google Scholar]