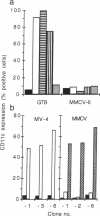
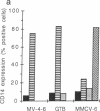
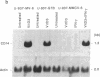
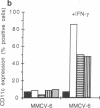
Abstract
Extensive studies suggest a role for the myc protooncogene family in the control of cell proliferation and differentiation in vertebrates. Previously, deregulated expression of exogenous myc genes has been shown to inhibit induced differentiation in Friend erythroleukemia (MEL) cells and in human monoblastic U-937 cells. To examine the nature of the block of phorbol 12-myristate 13-acetate-induced differentiation in v-myc-expressing U-937 cells, we have studied the effect of other inducers utilizing signal pathways distinct from phorbol 12-myristate 13-acetate (i.e., 1 alpha, 25-dihydroxycholecalciferol and retinoic acid). We show that v-myc also inhibits differentiation associated with these inducers. However, the v-myc-associated block of phorbol 12-myristate 13-acetate-, 1 alpha,25-dihydroxycholecalciferol-, and retinoic acid-induced differentiation retinoic acid-induced differentiation can be overcome by adding interferon gamma as a costimulatory factor. Costimulation with interferon gamma restores terminal differentiation, as shown by acquisition of a macrophage phenotype and an irreversible growth arrest in the G0/G1 phase of the cell cycle, but induces only limited differentiation on its own. The differentiation is accomplished without altering the expression or nuclear localization of the v-myc protein. These results argue against the widely held view that down-regulation of myc expression is a general prerequisite for terminal differentiation of hematopoietic cells and suggests that interferon gamma induces a signal(s) that circumvents the v-myc activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Anderson C. L., Guyre P. M., Whitin J. C., Ryan D. H., Looney R. J., Fanger M. W. Monoclonal antibodies to Fc receptors for IgG on human mononuclear phagocytes. Antibody characterization and induction of superoxide production in a monocyte cell line. J Biol Chem. 1986 Sep 25;261(27):12856–12864. [PubMed] [Google Scholar]
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bernards R., Dessain S. K., Weinberg R. A. N-myc amplification causes down-modulation of MHC class I antigen expression in neuroblastoma. Cell. 1986 Dec 5;47(5):667–674. doi: 10.1016/0092-8674(86)90509-x. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Classon M., Henriksson M., Sümegi J., Klein G., Hammarskjöld M. L., Hammaskjöld M. L. Elevated c-myc expression facilitates the replication of SV40 DNA in human lymphoma cells. Nature. 1987 Nov 19;330(6145):272–274. doi: 10.1038/330272a0. [DOI] [PubMed] [Google Scholar]
- Coppola J. A., Cole M. D. Constitutive c-myc oncogene expression blocks mouse erythroleukaemia cell differentiation but not commitment. Nature. 1986 Apr 24;320(6064):760–763. doi: 10.1038/320760a0. [DOI] [PubMed] [Google Scholar]
- Dmitrovsky E., Kuehl W. M., Hollis G. F., Kirsch I. R., Bender T. P., Segal S. Expression of a transfected human c-myc oncogene inhibits differentiation of a mouse erythroleukaemia cell line. Nature. 1986 Aug 21;322(6081):748–750. doi: 10.1038/322748a0. [DOI] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Goyert S. M., Ferrero E., Rettig W. J., Yenamandra A. K., Obata F., Le Beau M. M. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988 Jan 29;239(4839):497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. E., Ralph P., Litcofsky P., Moore M. A. Distinct activities of interferon-gamma, lymphokine and cytokine differentiation-inducing factors acting on the human monoblastic leukemia cell line U937. Cancer Res. 1985 Jan;45(1):9–13. [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Itani T., Kiji Y., Ariga H. Possible function of the c-myc product: promotion of cellular DNA replication. EMBO J. 1987 Aug;6(8):2365–2371. doi: 10.1002/j.1460-2075.1987.tb02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Baldwin A. S., Jr, Sharp P. A. Regulation of heat shock protein 70 gene expression by c-myc. Nature. 1984 Nov 15;312(5991):280–282. doi: 10.1038/312280a0. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Symonds G., Evan G. I., Bishop J. M. Subcellular localization of proteins encoded by oncogenes of avian myeloblastosis virus and avian leukemia virus E26 and by chicken c-myb gene. Cell. 1984 Jun;37(2):537–547. doi: 10.1016/0092-8674(84)90384-2. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. G., Ivhed I., Gidlund M., Pettersson U., Vennström B., Nilsson K. Phorbol ester-induced terminal differentiation is inhibited in human U-937 monoblastic cells expressing a v-myc oncogene. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2638–2642. doi: 10.1073/pnas.85.8.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V., Hogg N., Sim R. B. Ligand binding by the p150,95 antigen of U937 monocytic cells: properties in common with complement receptor type 3 (CR3). Eur J Immunol. 1986 Sep;16(9):1117–1123. doi: 10.1002/eji.1830160915. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Olsson I. L., Breitman T. R. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by retinoic acid and cyclic adenosine 3':5'-monophosphate-inducing agents. Cancer Res. 1982 Oct;42(10):3924–3927. [PubMed] [Google Scholar]
- Olsson I., Gullberg U., Ivhed I., Nilsson K. Induction of differentiation of the human histiocytic lymphoma cell line U-937 by 1 alpha,25-dihydroxycholecalciferol. Cancer Res. 1983 Dec;43(12 Pt 1):5862–5867. [PubMed] [Google Scholar]
- Palmieri S., Kahn P., Graf T. Quail embryo fibroblasts transformed by four v-myc-containing virus isolates show enhanced proliferation but are non tumorigenic. EMBO J. 1983;2(12):2385–2389. doi: 10.1002/j.1460-2075.1983.tb01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J. Deregulated expression of c-myc by murine erythroleukaemia cells prevents differentiation. 1986 Aug 28-Sep 3Nature. 322(6082):848–850. doi: 10.1038/322848a0. [DOI] [PubMed] [Google Scholar]
- Reitsma P. H., Rothberg P. G., Astrin S. M., Trial J., Bar-Shavit Z., Hall A., Teitelbaum S. L., Kahn A. J. Regulation of myc gene expression in HL-60 leukaemia cells by a vitamin D metabolite. Nature. 1983 Dec 1;306(5942):492–494. doi: 10.1038/306492a0. [DOI] [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Griffin J., Kufe D. W. Effects of interferon-gamma on proto-oncogene expression during induction of human monocytic differentiation. J Immunol. 1987 Mar 15;138(6):1954–1958. [PubMed] [Google Scholar]
- Schüle R., Umesono K., Mangelsdorf D. J., Bolado J., Pike J. W., Evans R. M. Jun-Fos and receptors for vitamins A and D recognize a common response element in the human osteocalcin gene. Cell. 1990 May 4;61(3):497–504. doi: 10.1016/0092-8674(90)90531-i. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Versteeg R., Noordermeer I. A., Krüse-Wolters M., Ruiter D. J., Schrier P. I. c-myc down-regulates class I HLA expression in human melanomas. EMBO J. 1988 Apr;7(4):1023–1029. doi: 10.1002/j.1460-2075.1988.tb02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Gallo R. C., Arya S. K., Eva A., Souza L. M., Baluda M. A., Aaronson S. A., Wong-Staal F. Differential expression of the amv gene in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2194–2198. doi: 10.1073/pnas.79.7.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]