Abstract
There are concerns about the meaning of SRH and the factors individuals consider. To illustrate how SRH is contextualized, we examine how the obesity-SRH association varies across age, periods, and cohorts. We decompose SRH into subjective and objective components and used a mechanism-based APC model approach with four decades (1970s-2000s) and five birth cohorts of NHANES data (N=26,184). Obese adults rate their health more negatively than non-obese when using overall SRH with little variation by age, period, or cohort. However, when we decomposed SRH into objective and subjective components, the obesity gap widened with increasing age in objective SRH, but narrowed in subjective SRH. Additionally, the gap narrowed for more recently-born cohorts for objective SRH, but widened for subjective SRH. The results provide indirect evidence that the relationship between obesity and SRH is socially patterned according to exposure to information about obesity and the availability of resources to manage it.
Self-rated health (SRH) is the mostly widely used, validated, single-item indicator of health status across social science research that independently predicts morbidity and mortality (Idler and Angel 1990, Idler and Benyamini 1997). The association between SRH and health has been examined extensively (Ferraro and Yu 1995, Manderbacka, Lundberg and Martikainen 1999, Tessler and Mechanic 1978).
Nonetheless, there are concerns about the meaning of SRH (Au and Johnston 2014). Prior research demonstrates that respondents place themselves into different SRH response categories despite having similar health conditions (Krause and Jay 1994, Prosper, Moczulski and Qureshi 2009). In other words, different groups factor conditions into their SRH assessments differently.
We argue that weight status is a condition that tends to be interpreted differently and may have a variable relationship with SRH. Weight status is important for understanding SRH because of the increasing prevalence of obesity (defined as a body mass index, BMI, at or above 30) in the United States for both genders, all race/ethnic groups, and all levels of socioeconomic status (Flegal, Caroll and Ogden 2010, Zhang and Wang 2004), and because overweight and obese adults report poorer health compared to normal weight adults (Ferraro and Yu 1995, Okosun et al. 2001, Prosper, Moczulski and Qureshi 2009).
Yet it is unclear whether weight status should be treated as a subjective or objective health indicator. Researchers often dichotomize weight status as obese (versus normal or overweight) and interpret it as an objective dimension of health (Singh-Manoux et al. 2006). Other researchers classify it as a lifestyle factor (Prosper, Moczulski and Qureshi 2009). The lack of consensus may be a result of multiple factors. First, obesity and severe obesity (BMI>35) are associated with chronic comorbidities and diseases, but being overweight (i.e., BMI 25 to 29.9) is not consistently associated with health risks (Campos et al. 2006, Masters, Powers and Link 2013). Second, obesity's comorbidities are diffuse and may be related to social stigma, making it difficult to pinpoint excess weight as the source of ill health (Brewis 2014).
Another hint of the variable meanings of SRH and obesity comes from evidence that the obesity-SRH relationship may be changing over time or across birth cohorts. Poor health ratings have not followed lock-step with increasing obesity. Despite the dramatic increase in obesity, individuals’ SRH has improved during the last few decades (Liu and Hummer 2008, Martin et al. 2007), except among young adults (Salomon et al. 2009, Zack et al. 2004). If one interprets SRH as an objective indicator of health, these trends may seem perplexing, and lead to speculations that obesity has become more harmful for young adults’ health than in the past, possibly because of their earlier-age-onset of obesity (Juhaeri et al. 2003, Keyes et al. 2010, Reither, Hauser and Yang 2009). However, an alternative possibility—tested here—is that the way people interpret obesity has changed or varies by age.
We explore the association between obesity and SRH for adults aged 25-64, using multiple waves of the National Health and Nutrition Examination Survey (NHANES). We empirically assess obesity's relationship with both the subjective and objective health components of SRH. Thus, we build on a burgeoning literature that recognizes the multidimensionality of SRH (Ferraro, Farmer and Wybraniec 1997, Hardy, Acciai and Reyes 2014, Kaplan and Baron-Epel 2003). To illustrate this multidimensionality, we examine how the obesity-SRH relationship has changed over time and across age groups. Prior research has examined trends in obesity and SRH across age, cohorts, and periods (e.g., (Martin et al. 2007, Salomon et al. 2009)), but not how the obesity-SRH relationship is modified by age, period and cohort. This paper thus helps clarify the health implications of the obesity epidemic in the United States while conceptually and methodologically contributing to the understanding of SRH.
Background and Theory
Life course theory (Alwin, McCammon and Hofer 2006, Elder 1998) and cumulative disadvantage theory help to frame why the obesity-SRH relationship is likely to vary by age and across birth cohorts. This framework focuses on how early life events and the accumulation of disadvantage over the life course serves to widen health disparities for individuals and groups as they age (DiPrete and Eirich 2006, Ross and Wu 1996). Age, cohort, and period variations in health outcomes (Ferraro and Kelley-Moore 2003, Willson, Shuey and Elder Jr. 2007) are viewed as the result of shifts and variations in early life (dis)advantages and social contexts (Ross and Wu 1996, Zajacova and Burgard 2010).
Past research shows clear associations between weight and poor physical health, including the acquisition of chronic conditions (Bray 2004, Lavie, Milani and Ventura 2009, Must et al. 1999, Stein and Colditz 2004), greater reliance on prescription drugs to manage health conditions (Chang and Lauderdale 2009, Egan, Zhao and Axon 2010), and lower life expectancy (Chang, Pollack and Colditz 2013). However, the linkage between obesity and poor physical health is likely to vary by age, birth cohort, and time period. The health consequences of obesity likely increase with age as the physical wear and tear of obesity accumulates across the life course (Abdullah et al. 2011, Ferraro and Kelley-Moore 2003, Zajacova and Burgard 2010).
For similar reasons, the health consequences of obesity may be particularly detrimental for cohorts born after the rise in childhood obesity. More recent birth cohorts experienced a higher prevalence of obesity in childhood and adolescence (Juhaeri et al. 2003), and obesity in childhood increases the likelihood of adult obesity and its associated health problems (Dietz 1998, Ferraro and Kelley-Moore 2003, Reilly and Kelly 2011). The 1980s birth cohort faces a particularly high risk. They were born during a period of rapid gains in obesity, and their mothers (i.e. women of the late 1950s and 1960s birth cohorts) experienced rapid increases in obesity during their young adulthood exposing them to maternal obesity in utero (Allman-Farinelli et al. 2007, Robinson et al. 2012).
However, it is possible that the obesity-SRH relationship may be weakening over time or across birth cohorts as recent medical advances may have helped to better manage weight-related conditions such as hypertension, diabetes, and high cholesterol and may also reduce the obesity-SRH relationship (Egan, Zhao and Axon 2010). For instance, as of 2010, nearly one-fourth of American adults over the age of 20 were taking statins compared to only 5 percent around 1990 (Kuklina et al. 2013). This increase has contributed to the substantial decline of high cholesterol (Carroll et al. 2005). This may be particularly true for the most-recently born cohorts, since these new treatments were available to them for longer portions of their lives.
Apart from its effects on physical health, obesity may be associated with how people respond to the SRH question. Due to the social stigma associated with obesity (Brewis 2014) and public health or medical messages about the negative health impacts of obesity (Brownell 2005), obese people may rate their health more negatively than a non-obese person with identical health conditions. We refer to this as the “subjective” component to SRH.
The link between obesity and this subjective component of SRH may be particularly strong among young adults. Young and older adults consider different factors when self-assessing their health. Older adults factor functional ability more heavily while younger adults consider lifestyle factors like physical activity, diet, and obesity more heavily (Krause and Jay 1994). One possible explanation is that younger adults have fewer chronic health conditions than older adults, so obese young adults may focus more singularly on their weight when rating their health. Older adults may have several conditions that directly impact their quality of life, so they give their weight comparably less attention.
Additionally, the tendency for obese people to rate their health negatively (even after adjusting for physical health) may have increased over time due to more messages about the negative health consequences of obesity. Mainstream and scientific interest in obesity has grown substantially over the last two decades (Saguy and Almeling 2008).
Messaging and stigma may have had a particularly large impact on recently-born cohorts. Life course theory suggests that adolescent and young adult experiences are particularly impactful (Elder 1998), and it has been argued that today's young adults have had especially negative experiences with obesity. These birth cohorts were ( exposed to messages about the health risks of obesity during particularly impressionable life stages (Reither, Hauser and Yang 2009), and likely to report facing obesity-related discrimination and stigma than older cohorts (Carr and Friedman 2005). As a consequence, they may be particularly likely to incorporate their weight status into their ratings of their own health, over and above expectations based on their physical health conditions. Indeed, recent research on obesity-related cumulative health disadvantages suggests that among adults aged 25 to 39 in the early 1980s, having a high BMI in early adulthood was associated with steep declines in SRH that were not fully explained by baseline health or medical conditions (Zajacova and Burgard 2010).
Research Expectations and Methodological Approach
The research above suggests that the obesity -SRH association depends on whether we are predicting the objective or subjective component of SRH. Obesity is likely to be negatively associated with the objective component of SRH, and this relationship is likely to be stronger for older adults and persons living in earlier time periods. We have less clear expectations about birth cohorts. The association could be stronger among recent birth cohorts due to their earlier onset of obesity; alternatively, it could be weaker due to greater availability of treatments for obesity-related conditions. We expect obesity to be negatively associated with the subjective component of SRH, and we expect this association to be stronger for younger adults and persons living in more recent time periods. Additionally, we expect the association to be stronger for recent birth cohorts due to greater media focus on obesity and earlier onset of obesity.
Assessing these ideas requires measures of both objective and subjective SRH. To accomplish this, we follow the recent work of Hardy and colleagues (2014), who decomposed SRH into two additive parts: (1) an indicator of physical functioning as a function of objective health indicators, and (2) a residual subjective component. We predicted SRH as a function of health conditions, generated predicted values of SRH, which we interpreted as the objective component of SRH. We interpreted the difference between the observed and predicted SRH (i.e., the residual) as the subjective component which indicates the extent that people report better or worse health compared to others with the same constellation of health conditions.
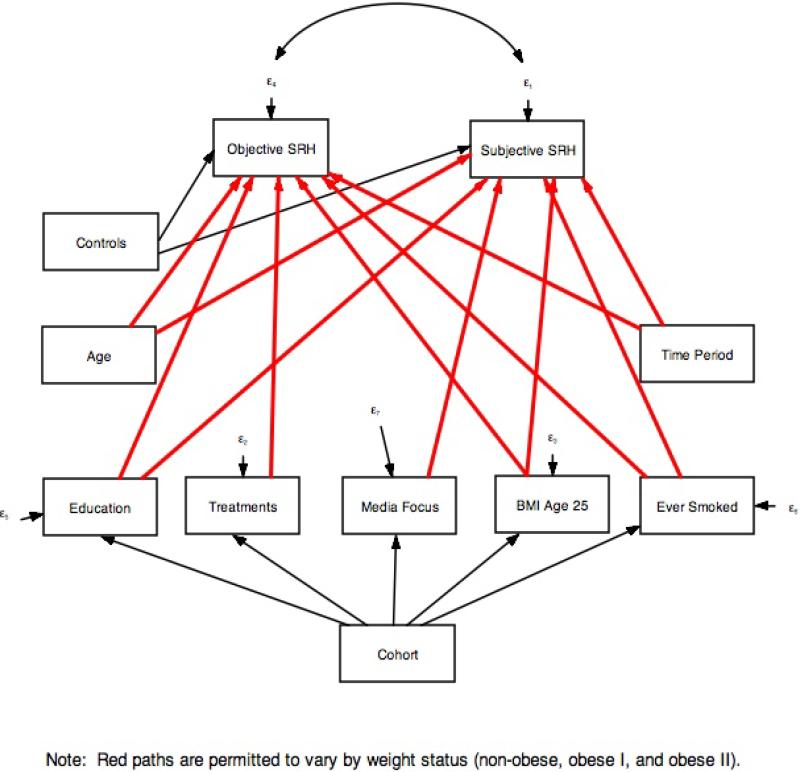
Our research also requires us to disentangle age, period, and cohort effects. While age (A), period (P), and cohort (C) are conceptually distinct concepts (Ryder 1965), including all three in a single model is empirically challenging due to linear dependency (P = C + A). Mechanical approaches for teasing apart APC effects have been developed (Yang and Land 2013), and all—implicitly or explicitly—impose a constraint on at least one of the APC variables to identify the model which influences the results (Bell and Jones 2014, Harding 2009, Pelzer et al. 2015). Rather than employing a mechanical solution, we use Winship and Harding's (2008) mechanism-based approach for identifying APC models. This approach constrains at least one of the APC variables (cohort in our case) as affecting the outcome through a set of theoretically-informed mechanisms. Observed nonlinearities in the relationship between cohort and the cohort mechanisms make it possible to identify the model.
We implemented this approach by estimating the path model shown in Figure 1 using Stata's sem package. Age, time period, and the control variables have direct effects on both components of SRH. Cohort operates indirectly through mechanisms that vary systematically across cohorts and which are likely to influence physical health and/or how people report their health: educational attainment, ever having smoked, BMI at age 25, media focus on obesity at age 25, and the availability of pharmaceuticals for obesity-related conditions at age 25. Paths shown in red vary by weight status, thus allowing the relationship between weight status and SRH to vary by age, period and the cohort mechanisms. The cohort effect is the sum of the paths linking cohort with SRH through each mechanism. .
Figure 1.
Mechanism-based APC Path Model
Data and Measures
We pooled the National Health and Nutrition Examination Survey (NHANES): NHANES II (1976-1980), NHANES III (1990-1994), and the continuous NHANES (1999/2000; 2001/02; 2003/04; 2005/06; 2007/08; 2009/10; and 2011/12). The NHANES is a nationally representative study of health and nutrition of children and adults in the United States conducted by the National Center for Health Statistics (NCHS) since the early 1970s. The NHANES has a complex, multistage probability sample design, and collects demographic, socioeconomic, health and anthropometric data though interviews and examinations. The pooled sample spans thirty-six years and five births cohorts. Although previous National Health Examination Surveys conducted in the 1960s and the NHANES I from the early 1970s were administered, they do not contain full information on SRH or were conducted on specific sub-samples.
The analysis was restricted to non-Hispanic white and non-Hispanic black adults aged 25-64 who completed both the questionnaire and examination portions of the survey, hereafter referred to as whites and blacks. We focused on the working-age population because of concerns regarding changes in health that may induce illness and weight loss in older adults. We excluded adults younger than 25 because we relied on an NHANES question on weight at age 25 (not asked to persons younger than 25). We excluded foreign born adults (n=2,122) to ensure that when we follow birth cohorts over time across cross-sectional surveys, they represent the same (U.S.-born) cohorts, rather than groups whose compositions change due to immigration. Pregnant women were dropped (n=801) due to the confounding between BMI and pregnancy. Underweight adults (BMI<18.5; n=758) and severely obese adults (BMI>50; n=535) were dropped from the sample. The final analytic sample included 26,184 adults after listwise deletion. Sample descriptives are shown in Table 1.
Table 1.
Sample Descriptives
| Mean or Percent | ||
|---|---|---|
| Dependent Variables | ||
| Overall SRH | 3.61 (1.03) | |
| Objective Component of SRH | 3.56 (0.44) | |
| Subjective Component of SRH | .05 (.94) | |
| Weight Category | ||
| Not Obese (BMI<30) | 67.36 | |
| Obese I (BMI 30-34) | 19.04 | |
| Obese II (BMI 35+) | 13.60 | |
| APC Variables | ||
| Age (%) | 25-34 | 24.69 |
| 35-44 | 26.76 | |
| 45-54 | 27.63 | |
| 55-64 | 20.92 | |
| Period (%) | 1976-1977 | 4.47 |
| 1978-1981 | 4.57 | |
| 1990-1992 | 5.37 | |
| 1993-1994 | 5.42 | |
| 1999-2002 | 20.63 | |
| 2003-2006 | 23.50 | |
| 2007-2012 | 36.04 | |
| Cohort (%) | Greatest Generation | 3.05 |
| Silent Generation | 14.32 | |
| Baby Boomers | 51.32 | |
| Generation X | 26.93 | |
| Millennials | 4.38 | |
| Cohort Mechanisms | ||
| BMI at age 25 | 27.44 (4.90) | |
| Ever Smoked (%) | 52.78 | |
| Educational Attainment (years) | 13.39 | |
| Media focus on obesity, age 25 (annual articles in NYTimes) | 9.77 (9.50) | |
| Treatments for obesity-related conditions, age 25 | 21.49 (19.90) | |
| Controls | ||
| Married (%) | 64.23 | |
| Black (%) | 12.95 | |
| Male (%) | 49.60 | |
Data: NHANES II, III, and Continuous
Sample: Non-Hispanic white and black nonpregnant adults age 25-64 (N=26,184) (Standard Deviation)
Self-rated Health
The dependent variable was a response to a single item that asked the respondent to rate their general health (1=Poor, 2=Fair=, 3=Good, 4= Very Good, and 5= Excellent). SHR is decomposed into objective and subjective components. The objective component indicated the respondent's physical health. Using an OLS model, SRH is predicted as a function of the number of respondent-reported health conditions: asthma, anemia, congestive heart failure, heart attack, stroke, bronchitis, emphysema, cancer, hypertension, and diabetes. The Stata “predict” command was used to generate the respondents’ predicted value of SRH. The subjective component of SRH indicates the tendency for respondents to report better or worse health independent of these health conditions. It is the arithmetic difference between the respondent's reported and predicted SRH (i.e. the objective and subjective SRH sum to the reported SRH value).
Weight Status
During the NHANES examination, technicians measured the respondent's height and weight using standardized equipment and procedures. These measures were used to calculate Body Mass Index (BMI= weight in kg/ (height in meters) 2), which we categorized into three groups: non-obese (18.5≤BMI<30.0), obese I (30≤ BMI<35), and obese II (BMI ≥35). We combined obese I and obese II into a single “obese” category (BMI ≥30) for some descriptive analyses.
Age, Period, and Birth Cohort
We treated age, period and cohort as categorical to allow for nonlinear effects. We coded age into ten-year categories (25-34, 35-44, 45-54, 55-64), period into intervals that corresponded with the NHANES survey years (1976-1977, 1978-81,1990-92, 1993-94, 1999-2002, 2003-06, and 2007-12), and five birth cohorts: the Greatest Generation (1907-1927), Silent Generation (1928-1945), Baby Boomers (1946-1964), Generation X (1965-1979), and Millennials (1980 or later) (Taylor et al. 2014). Five-year age and cohort categories produced substantively identical results with less precision due to smaller cell sizes.
Cohort Mechanisms
Cohort mechanisms are the cohort-specific experiences, behaviors, and/or resources that lead to cohort differences in SRH. Cohort effects on the obesity-SRH association likely operate indirectly through these mechanisms. Some cohort mechanisms are likely to influence SRH for all weight categories. Specifically, rising educational levels (Mirowsky and Ross 2008) and declines in smoking (Preston and Wang 2006) are likely to have improved SRH for more recently-born cohorts, but increasing weight in young adulthood is likely to have reduced it. Other cohort mechanisms likely have greater effects on SRH among obese than non-obese people. Media focus on obesity and drug treatments for obesity-related conditions have been increasing which may have influenced how obese people subjectively rate their own health (in the case of media focus on obesity) and the degree to which obesity results in worse physical health (in the case of treatments).
Education in years is approximated from reported categories (less than high school=10, high school graduate=12, some college=14, college graduate=16), and centered at 12 years. A dummy variable for smoking indicated whether the respondent ever smoked (=1). We obtained similar results when we distinguished between current and former smokers. We measured weight in young adulthood with BMI at age 25 (centered at 30), based on self-reported weight at age 25 and measured height at the time of the interview. Before calculating BMI at age 25, we adjusted weight at age 25 for reporting error based on a model that related the respondent's current measured weight to current reported weight and all other analytical variables. We substituted the respondent's reported weight at age 25 into this model (in place of current reported weight) to obtain a predicted value of their measured weight at age 25.
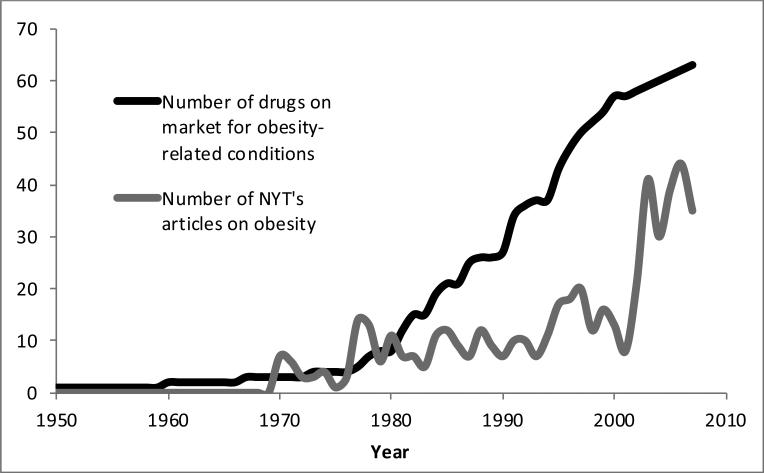
Media focus on obesity was measured as the number of articles published in The New York Times about obesity in the year when the respondent was age 25. We selected The New York Times because it ranks third in circulation among U.S. newspapers with wide readership (Audit Bureau of Circulation 2012), its news service delivers over 200 articles daily to more than two dozen other papers, and its syndicate service provides articles worldwide (The New York Times Syndicate 2012). We systematically reviewed newspaper articles published by The New York Times from 1970 to 2007 using ProQuest fully digitized archives. The search was limited to English language articles excluding advertisements, classified advertisements, stock reports, table of contents, obituaries, marriage announcements, images, credits, reviews, and legal notices using the terms “United States,” “obesity” or “overweight” or “body mass index” or “BMI.” We display the number of articles by year in Figure 2 (grey line), showing that media attention on obesity increased slowly during the late-1980s and 1990s, and then rose in the 2000s.
Figure 2.
Drugs on the U.S. market for obesity-related conditions and annual number of New York Times articles on obesity, by year
Treatment for obesity-related conditions was measured as the number of drugs on the market when the respondent was age 25. We used the mayoclinic.org and medicinenet.com to determine the most common classes of pharmacotherapy approaches to treating type 2 diabetes, high cholesterol, and hypertension, the specific drugs in each class, and the U.S. market-entry date (available from authors). We display the number of these drugs on the market by year in Figure 2 (black line), which shows that these kinds of treatments increased steadily since the late 1980s.
The effects of media focus on obesity and the availability of drugs are likely to be stronger among people with higher levels of education because they more often follow the news and have the resources to take advantage of new treatments (Phelan and Link 2005). To account for this possibility, we included interactions between education and the media and drugs variables.
The cohort mechanisms vary across cohorts but change little by age or period within cohorts. For example, among respondents age 25-34, BMI at age 25, education, exposure to media about obesity and treatments of obesity-related conditions increased rapidly across cohorts, and the percentage that ever smoked declined (Table 2). By contrast, these indicators changed only slightly by age among the Silent Generation, and much less than the observed cohort changes. A small amount of change is to be expected as cohort members die over time, and because cohorts but not individuals are followed across the NHANES data sets. We see similar patterns for the other birth cohorts (not shown).
Table 2.
Cohort Mechanisms (mean or percent) by birth cohort (age 25-34) or age (Silent Generation)
| BMI Age 25 | Percent Ever smoked | Education (years) | Media Focus on Obesity, Age 25 | Treatments for Obesity-related Conditions, Age 25 | |
|---|---|---|---|---|---|
| Cohort, Among those age 25-34 | |||||
| Silent Generation | 26.0 | 63.4 | 12.9 | 2.7 | 3.0 |
| Baby Boomers | 26.4 | 56.8 | 13.0 | 7.3 | 13.3 |
| Generation X | 28.8 | 48.1 | 13.5 | 17.5 | 49.9 |
| Millennials | 30.9 | 48.8 | 13.8 | 39.9 | 62.8 |
| Change (Millennials-Silent) | 4.8 | −14.6 | .9 | 37.2 | 59.8 |
| Age, Among the Silent Generation | |||||
| Age 25-34 | 26.0 | 63.4 | 12.9 | 2.7 | 3.0 |
| Age 35-44 | 25.9 | 66.0 | 12.3 | .0 | 2.0 |
| Age 45-54 | 26.2 | 64.5 | 12.4 | 1.1 | 2.0 |
| Age 55-64 | 26.5 | 62.1 | 13.0 | .8 | 2.3 |
| Difference (55-64 - 25-34) | .5 | −1.2 | .1 | −1.8 | −.7 |
Data and Sample: See Table 1
Controls
We controlled for socio-demographic characteristics, including race/ethnicity (non-Hispanic black=1), gender (male=1), and marital status (married=1). Although our model specified separate intercepts and age, period, and cohort slopes for each of the three weight categories, we controlled for BMI (centered at the mean of each weight category) to account for variations in weight within the three weight categories.
Results
Descriptive Analysis
We first examined obesity prevalence among all age groups, birth cohorts, and time periods available for analysis in our data. As shown in Table 3, all of the generations are observed across multiple years and at varying ages. The Greatest Generation is observed in the earliest years of data when they are in the 45-54 age range and older. We observe the Silent Generation and Baby Boomers in all years and across all age ranges. Generation X respondents are included from 1990 to 2009 from the youngest to the 45-54 age range. Millennials are included in the most recent years in the 25-34 age range.
Table 3.
Age ranges, obesity prevalence, and sample sizes for birth cohorts and age groups
| 25-34 | 35-44 | 45-54 | 55-64 | |
|---|---|---|---|---|
| Year range | ||||
| Greatest Generation | 1976-1979 | 1976-1990 | ||
| Silent Generation | 1976-1979 | 1976-1995 | 1976-1999 | 1990-2009 |
| Baby Boomers | 1976-1993 | 1990-2007 | 1993-2011 | 2001-2011 |
| Generation X | 1990-2011 | 1995-2011 | 2011-2011 | |
| Millennials | 2005-2011 | |||
| Percentage Obese (BM ≥30) | ||||
| Greatest Generation | 16.5 | 19.9 | ||
| Silent Generation | 14.6 | 16.3 | 26.5 | 38.7 |
| Baby Boomers | 15.0 | 31.4 | 37.0 | 40.6 |
| Generation X | 28.8 | 36.5 | 33.4 | |
| Millennials | 30.7 | |||
| Number of Cases | ||||
| Greatest Generation | 569 | 2,426 | ||
| Silent Generation | 370 | 1,368 | 1,792 | 2,679 |
| Baby Boomers | 2,974 | 3,242 | 3,571 | 1,760 |
| Generation X | 2,715 | 1,951 | 92 | |
| Millennials | 728 | |||
Data and Sample: See Table 1
Examining the percentage obese (BMI ≥ 30) confirms previous research (Allman-Farinelli et al. 2007, Keyes et al. 2010, Reither, Hauser and Yang 2009, Robinson et al. 2012). First, within each age group, the percentage obese increased across cohorts with the most recently-born cohorts (i.e. Generation X and Millennials) having the highest prevalence. For example, among respondents aged 45-54, 16.5 percent of the Greatest Generation was obese compared to 26.5 percent of the Silent Generation, 37.0 percent of Baby Boomers, and 33.4 percent of Generation X. Second, within each generation the percentage of members who are obese increased with age. For instance, 15.0 percent of Baby Boomers were obese at age 25-34 compared to 40.6 percent by age 55-64.
We next explored how these trends are related to SRH. Table 4 presents mean SRH by age, period, and birth cohort, and by weight status. Lower mean values indicate worse health. As expected, obese people report worse health than non-obese people, regardless of age, period, or cohort (shown in the first three columns of Table 4, across the rows). Additionally, within weight categories, older people and those born earlier report worse health than younger people and those born more recently.
Table 4.
Mean SRH health by weight category, age, period, and cohort
| SRH (mean) | Gap in SRH (Difference from Non-obese) | ||||
|---|---|---|---|---|---|
| Non-obese | Obese I | Obese II | Obese I | Obese II | |
| Age | |||||
| 25-34 | 3.93 | 3.64 | 3.27 | −.30 | −.66 |
| 35-44 | 3.80 | 3.43 | 3.25 | −.37 | −.55 |
| 45-54 | 3.68 | 3.38 | 3.06 | −.29 | −.62 |
| 55-64 | 3.59 | 3.30 | 2.88 | −.28 | −.71 |
| Period | |||||
| 1976-1977 | 3.74 | 3.29 | 3.03 | −.45 | −.70 |
| 1978-1981 | 3.71 | 3.31 | 3.04 | −.40 | −.67 |
| 1990-1992 | 3.77 | 3.36 | 3.10 | −.41 | −.67 |
| 1993-1994 | 3.78 | 3.52 | 3.22 | −.26 | −.57 |
| 1999-2002 | 3.84 | 3.46 | 3.22 | −.38 | −.63 |
| 2003-2006 | 3.76 | 3.46 | 3.12 | −.29 | −.63 |
| 2007-2012 | 3.73 | 3.40 | 3.03 | −.33 | −.70 |
| Cohort | |||||
| Greatest Generation | 3.29 | 2.90 | 2.55 | −.38 | −.74 |
| Silent Generation | 3.69 | 3.35 | 3.00 | −.34 | −.69 |
| Baby Boomers | 3.76 | 3.40 | 3.08 | −.35 | −.68 |
| Generation X | 3.86 | 3.52 | 3.22 | −.34 | −.64 |
| Millennials | 3.88 | 3.62 | 3.15 | −.26 | −.73 |
Data and Sample: See Table 1
We observe only modest age, period, and cohort patterns in the gap in SRH between non-obese and obese people (shown in the last two columns of Table 4). The SRH gap between the obese (I and II) and non-obese respondents varies little by age, and the SRH gap between non-obese and obese I narrows only slightly over time and across birth cohorts.
However, these weak age, period, and cohort relationships mask offsetting patterns in objective versus subjective SRH. We repeated the descriptive analysis from Table 4, separately for our measures of the subjective and objective components of SRH. For simplicity, we show only the SRH gap between the non-obese and obese II respondents (full results are available upon request). The first column in Table 5 shows the gap in SRH (repeated from the last column of Table 4), and the second and third columns present the gap in the objective and subjective components of SRH, respectively. Note that the gap in SRH is the sum of the gaps in the objective and subjective components.
Table 5.
Gap in SRH (total, objective, and subjective) between obese II (BMI≥35) and non-obese (BMI<30) by age, period, and cohort
| SRH (total) | Objective Component | Subjective Component | |
|---|---|---|---|
| Age | |||
| 25-34 | −.66 | −.13 | −.53 |
| 35-44 | −.55 | −.25 | −.30 |
| 45-54 | −.62 | −.31 | −.31 |
| 55-64 | −.71 | −.41 | −.31 |
| Period | |||
| 1976-1977 | −.70 | −.27 | −.44 |
| 1978-1981 | −.67 | −.25 | −.42 |
| 1990-1992 | −.67 | −.26 | −.41 |
| 1993-1994 | −.57 | −.33 | −.23 |
| 1999-2002 | −.63 | −.27 | −.36 |
| 2003-2006 | −.63 | −.29 | −.34 |
| 2007-2012 | −.70 | −.32 | −.38 |
| Cohort | |||
| Greatest | |||
| Generation | −.74 | −.30 | −.43 |
| Silent Generation | −.69 | −.41 | −.27 |
| Baby Boomers | −.68 | −.35 | −.33 |
| Generation X | −.64 | −.20 | −.44 |
| Millennials | −.73 | −.11 | −.62 |
Data and Sample: See Table 1
The gap in objective SRH between non-obese and obese II respondents steadily widens with age, consistent with the notion that the health impact of obesity worsens with age. Additionally, the gap narrows across birth cohorts, and is close to zero for the Millennial Generation, consistent with the expectation that the effects of obesity on physical health have weakened among more recent cohorts due to greater availability of treatments for obesity-related conditions. Unexpectedly, we observed no consistent period effects in the gap in objective SRH.
We see different patterns in subjective SRH. While the gap widens with age for objective SRH, it narrows for subjective SRH. This suggests that younger adults give greater importance to obesity when rating their own health. Also, whereas the gap narrows across cohorts for objective SRH, it widens for subjective SRH, which is consistent with our expectation that recently-born cohorts would be particularly likely to consider their weight when rating their own health. Finally, the gap in subjective SRH unexpectedly narrows slightly over time and is particularly narrow in the 1990s, which is the time period when negative messages about obesity were starting to penetrate U.S. media and public health discussions (Saguy 2013).
Multivariate Analysis
Age, period, and cohort are highly correlated, so it is possible that the descriptive patterns for the gaps in objective and subjective SRH from Table 5 are spurious or suppressed. To refine our analysis, we estimated the mechanism-based path model shown in Figure 1. The model coefficients are shown in Table 6. The overall model fits well, with a standardize root square residual less than .1 and a coefficient of determination close to 1.0 (Bentler and Raykov 2000). Also, the coefficients for most of the age, period, and cohort mechanism variables differ significantly by weight category, indicated with the † in Table 6. Though one specification is presented, variations of the model produced substantively consistent results. In these sensitivity tests we varied the detail of the age, period, and cohort categories, altered the set of cohort mechanisms (e.g., with only education and BMI at age 25 as mechanisms, without education and education interactions, with marital status as a mechanism, and with greater detail for smoking behaviors), excluded BMI as a control, and allowed all paths to vary by weight category. Additionally, weighted models produced nearly identical results.
Table 6.
Path model of SRH
| Paths to SRH | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Objective Component of SRH | Subjective Component of SRH | |||||||||||||
| Non-Obese | Obese I | Obese II | Non-Obese | Obese I | Obese II | |||||||||
| Age | ||||||||||||||
| 25-34 | .295 | *** | .386 | *** | .351 | *** | † | .187 | *** | .016 | −.038 | † | ||
| 35-44 | .254 | *** | .321 | *** | .255 | *** | † | .040 | −.084 | * | −.063 | † | ||
| 45-54 | .153 | *** | .177 | *** | .153 | *** | −.004 | −.058 | −.047 | |||||
| Period | ||||||||||||||
| 1976-1977 | .109 | *** | .229 | *** | .332 | *** | † | .070 | * | −.086 | −.056 | † | ||
| 1978-1981 | .124 | *** | .222 | *** | .352 | *** | † | .032 | −.093 | −.075 | ||||
| 1990-1992 | .090 | *** | .128 | *** | .254 | *** | † | .038 | .059 | .046 | ||||
| 1993-1994 | .094 | *** | .176 | *** | .246 | *** | † | .004 | .015 | .066 | ||||
| 1999-2002 | .062 | *** | .079 | *** | .173 | *** | † | .102 | *** | .037 | .091 | |||
| 2003-2006 | .013 | .078 | *** | .080 | *** | † | .054 | * | .013 | .074 | ||||
| Cohort Mechanisms | ||||||||||||||
| BMI age 25 (centered) | −.005 | *** | −.005 | ** | −.006 | *** | .001 | .009 | * | .008 | * | |||
| Ever smoked | −.056 | −.075 | −.111 | † | −.152 | *** | −.079 | ** | −.079 | * | † | |||
| Education (centered) | .022 | *** | .031 | *** | .034 | *** | † | .132 | *** | .116 | *** | .084 | *** | † |
| Treatments for obesity-related conditions | .002 | *** | .004 | *** | .007 | *** | † | |||||||
| × education | −.0005 | *** | −.0004 | ** | −.0005 | ** | ||||||||
| Media focus on obesity, age 25 | −.002 | .000 | −.006 | * | ||||||||||
| × education | −.0016 | *** | −.0034 | *** | −.0012 | † | ||||||||
| Controls | ||||||||||||||
| BMI (centered) | −.007 | *** | −.018 | *** | −.011 | *** | † | −.004 | −.024 | * | −.021 | *** | † | |
| Married | .024 | *** | .024 | *** | .024 | *** | .127 | *** | .168 | *** | .064 | † | ||
| Black | −.012 | −.012 | −.012 | −.171 | −.171 | −.171 | ||||||||
| Male | .069 | *** | .069 | *** | .069 | *** | −.010 | −.010 | −.010 | |||||
| Intercept | 3.276 | *** | 3.068 | *** | 2.903 | *** | −.105 | ** | −.175 | *** | −.227 | *** | ||
| R-sq | .109 | .203 | .266 | .125 | .116 | .077 | ||||||||
| Paths to Cohort Mechanisms | BMI age 25 | Ever Smoked | Education | Media Focus on Obesity, Age 25 | Treatments for obesity-related conditions | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Birth Cohort | ||||||||||
| Greatest Generation | −5.50 | *** | .13 | *** | −2.32 | *** | −39.94 | *** | −61.81 | *** |
| Silent Generation | −5.05 | *** | .14 | *** | −1.27 | *** | −39.35 | *** | −60.75 | *** |
| Baby Boomers | −4.46 | *** | .06 | ** | −.40 | *** | −32.64 | *** | −51.42 | *** |
| Generation X | −2.61 | *** | −.03 | −.14 | −25.38 | *** | −18.07 | *** | ||
| Intercept | 1.45 | *** | .50 | *** | 1.44 | *** | 39.94 | *** | 62.79 | *** |
| R-sq | .05 | .02 | .08 | .73 | .84 | |||||
Standardized root mean squared residual=.097
Coefficient of determination=.936
p<.001
p<.01
p<.05
Coefficients significantly differ by weight category p<.05
Data and Sample: See Table 1
Because the intercepts and coefficients for age, period, and the cohort mechanisms vary by weight category, it is difficult to interpret the results directly. Therefore, we use predicted values generated from the model by substituting mean values for all controls and the other APC variables into the full SEM model while varying age, period, or cohort, and weight category.
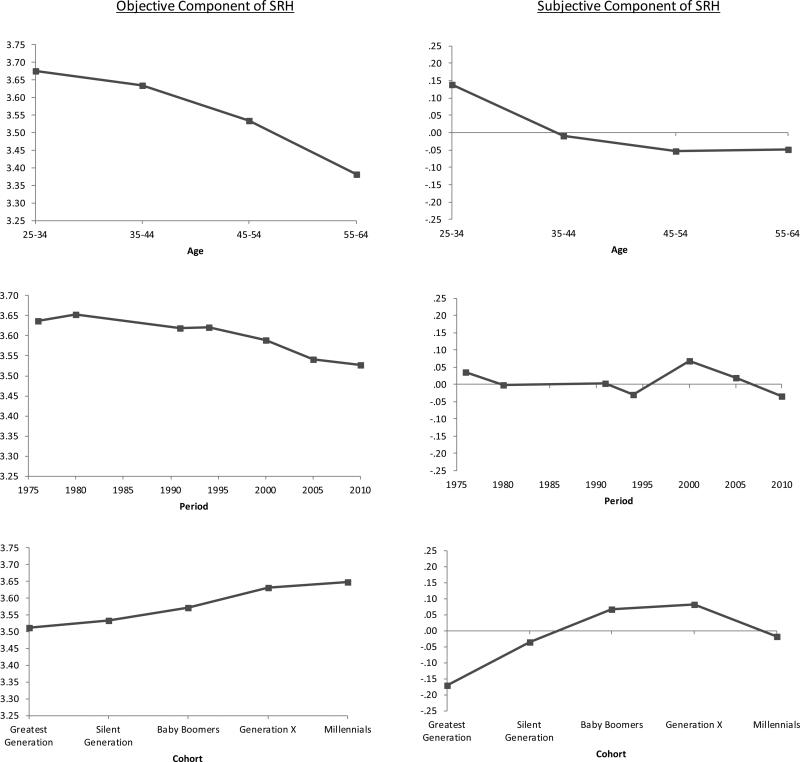
The predicted relationships for non-obese persons provide a baseline for comparison with obese persons in Figure 3. The first column of Table 7 also displays the age, period and cohort effects along with their significance level (i.e., difference between the first and last age, period and cohort categories) for non-obese persons. For example, the age effect on objective SRH for the non-obese is −.295 (p<.001), meaning that objective SRH is nearly one-third of a point lower for 55-64 year-olds compared to those in the 25-34 age group. Both subjective and objective SRH declined significantly with age, but the association was stronger for objective SRH (see top panel of Figure 3). Over time, objective and subjective SRH declined significantly, although the change was small in magnitude compared to age differences. Finally, both objective and subjective SRH increased significantly across cohorts (lower panel of Figure 3).
Figure 3.
Predicted Self-rated Health Among non-obese Persons (BMI<30); based on models shown in Table 6; higher values indicate better health
Table 7.
Predicted Values for Age, Period, and Cohort Effects Derived from Path Model in Table 6
| Non-Obese (BMI<30) | Obese I (BMI 30-34) | Obese II (BMI 35+) | Gap from non-obese | All Groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obese I | Obese II | |||||||||||
| Objective Component of SRH | ||||||||||||
| Age Effects (55-64 minus 25-34) | −.295 | *** | −.386 | *** | −.351 | *** | −.091 | ** | −.056 | −.263 | *** | |
| Period Effects (2010 minus 1976) | −.109 | *** | −.229 | *** | −.332 | *** | −.119 | ** | −.223 | *** | −.125 | *** |
| Cohort Effects (Millennials minus Greatest) | .136 | *** | .248 | *** | .449 | *** | .112 | * | .313 | *** | .193 | *** |
| Cohort pathways | ||||||||||||
| BMI age 25 | −.027 | *** | −.028 | ** | −.035 | *** | −.001 | −.008 | −.007 | |||
| Ever smoked | .007 | *** | .010 | *** | .015 | *** | .002 | .007 | ** | .008 | *** | |
| Education | .051 | *** | .072 | *** | .079 | *** | .020 | * | .028 | * | .055 | *** |
| Treatments for obesity-related conditions, Age 25 | .152 | *** | .234 | *** | .435 | *** | .082 | .282 | *** | .190 | *** | |
| × education | −.048 | *** | −.040 | ** | −.044 | ** | .008 | .004 | −.053 | *** | ||
| Subjective Component of SRH | ||||||||||||
| Age Effects (55-64 minus 25-34) | −.187 | *** | −.016 | .038 | .171 | ** | .224 | ** | −.086 | ** | ||
| Period Effects (2010 minus 1976) | −.070 | * | .086 | .056 | .157 | * | .126 | −.057 | + | |||
| Cohort Effects (Millennials minus Greatest) | .154 | ** | .141 | −.057 | −.012 | −.211 | + | .107 | * | |||
| Cohort pathways | ||||||||||||
| BMI age 25 | .007 | .047 | * | .044 | * | .040 | .037 | .022 | + | |||
| Ever smoked | .020 | *** | .010 | * | .010 | * | −.010 | * | −.009 | + | .020 | *** |
| Education | .306 | *** | .270 | *** | .195 | *** | −.036 | + | −.111 | *** | .296 | *** |
| Media focus on obesity, age 25 | −.089 | .008 | −.240 | * | .097 | −.150 | −.090 | |||||
| × education | −.090 | *** | −.194 | *** | −.066 | −.104 | * | .023 | −.141 | *** | ||
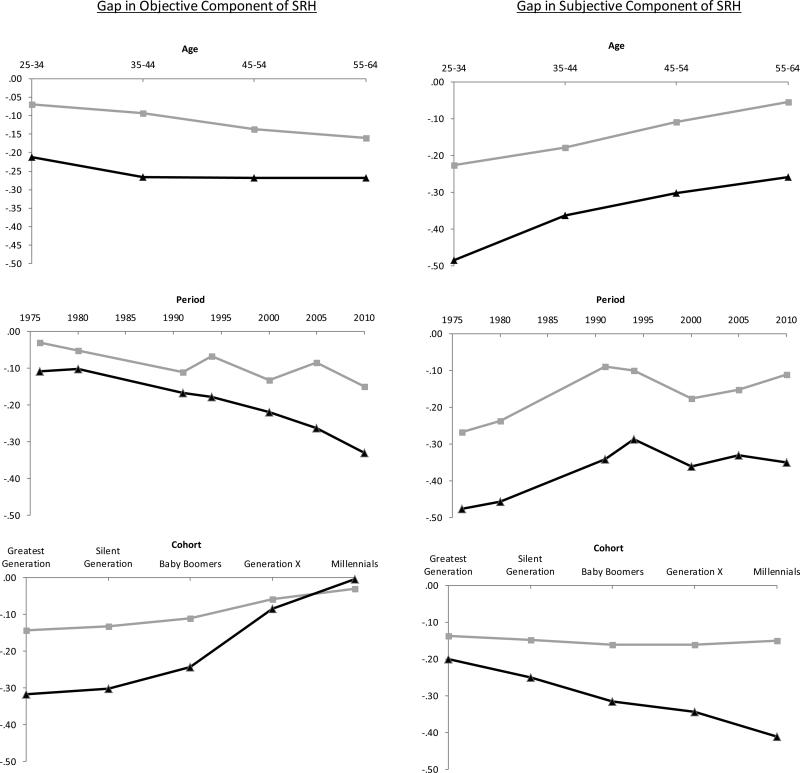
The predicted values in Figure 4 depict the predicted gap in SRH between Obese I and Obese II and non-obese persons by age, period and cohort. It is important to understand that negative values in Figure 4 indicate worse health for the obese compared with the non-obese. The x-axis intersects at zero at the top of the charts, so health gaps appear as values dipping below the axis. Additionally, the second and third columns of Table 7 display the age, period, and cohort effects on SRH for those in the obese I and obese II weight categories, and the fourth and fifth columns display the gap from non-obese persons. In general, negative gaps indicate divergence from the non-obese, and positive gaps indicate convergence. For example, the period effect on objective SRH for those in the obese II group is −.332, meaning that SRH declined by about a third of a point from 1976 to 2010. The gap from non-obese (last column) is −.223, meaning that it declined more (by .223 points) for obese II than non-obese respondents.
Figure 4.
Predicted Gap in Self-rated Health from non-obese Persons (BMI<30); grey line = obese I (BMI 30-34); black line = obese II (BMI=35+); higher values indicate better health; based on models shown in Table 6
The age patterns were consistent with our expectations (top panel of Figure 4). Independent of changes over time or in the cohort mechanisms, the gap in objective SRH widened steadily from the youngest to the oldest age group, but was significant only for those in the obese I category and was modest in size, only .091 points. In other words, physical health worsened with age for all three weight groups, but slightly more rapidly among those in the heavier weight categories. In contrast, the gap in subjective SRH between non-obese respondents and those in both of the obese weight categories significantly narrowed with age. This is consistent with the notion that young adults, compared with older adults, are more likely to consider obesity when rating their own health net of their actual physical health conditions.
The patterns by time period were inconsistent with our expectations (middle panel of Figure 4) that the gap would narrow for objective SRH and widen for subjective SRH. Instead, the gap in objective SRH widened significantly. Physical health declined faster over time among obese than non-obese persons. Additionally, the gap in subjective SRH narrowed slightly, but only significantly for the obese I group.
Independent of these age and period effects, the results (based on the relationship of the cohort mechanisms with the outcomes) suggested that objective SRH converged across cohorts (lower panel of Figure 4). As shown in Table 7, this convergence is due to the fact that, although objective SRH increased across birth cohorts for all weight categories, it increased more among the obese such that they reached parity with the non-obese among the most recently-born cohorts. In contrast, subjective SRH worsened more among the obese II category relative to the non-obese (difference is marginally significant; p = .08). This is consistent with the idea that recently-born cohorts are more likely to consider their weight when rating their own health compared with earlier cohorts.
To help interpret the cohort effects, we estimated all indirect paths connecting cohort to SRH through each of the cohort mechanisms (shown in Table 7). The increasing availability of treatments for obesity-related conditions accounts for most of the convergence in objective SRH between Obese II and non-obese respondents. This factor was associated with better objective SRH for all weight categories, and the effect was much greater for heavier people, as would be expected. It increased SRH by .435 points among those in the obese II group compared to .152 among the non-obese.
Surprisingly, BMI at age 25 did not play a role in explaining cohort differences in objective SRH. BMI at age 25 increased across birth cohorts, and it was related to worse objective SRH, but the effects were no worse for respondents who were currently obese than for non-obese respondents. Moreover, its negative effects were overshadowed by the positive effects of rising education and the availability of treatments for obesity-related conditions. Estimating the model separately by weight status or dummy coding BMI to reflect obesity did not change the results.
In contrast to the objective component of SRH, the gap in subjective SRH widened across cohorts due to educational attainment. Education increased across cohorts, and was associated with better health ratings for all weight groups. However, the association was stronger for the leaner weight groups than those in the heaviest weight category. Media focus on obesity was also associated with worse subjective SRH among obese II respondents, but did not contribute significantly to the growing gap in subjective SRH.
Discussion
Despite the strengths of SRH as a measure of health, there are concerns about the factors individuals consider when rating their health. In this study we examine how the obesity-SRH association varies across age groups, periods, and cohorts while using a mechanism-based approach to estimate APC models.
Our results highlighted the multidimensionality of SRH. By decomposing SRH into objective (physical health) and subjective (evaluative) components (Hardy, Acciai and Reyes 2014), patterns emerged that were obscured by the overall SRH measure. Specifically, when assessing the difference in overall SRH between non-obese and obese adults, we found that obese adults rate their health more negatively than non-obese and little variation in this result by age, period, or cohort. But when we examined the SRH gap using the objective and subjective dimensions of SRH, the gap widened with increasing age for objective SRH, but narrowed in the case of subjective SRH. Additionally, the gap narrowed for more recently-born cohorts in the case of objective SRH, but widened in the case of subjective SRH. Overall, , the results suggest that the link between obesity and objective SRH is stronger among older adults and those born in earlier time periods and the link between obesity and subjective SRH is stronger for younger adults and more recently-born cohorts.
These results bolster research supporting the multidimensionality of SRH (Jylha 2009, Layes, Asada and Kephart 2012) as “subjective and evaluative” (Kaplan and Baron-Epel 2003, Shields and Shooshtari 2001, Singh-Manoux et al. 2006). In our sample, different cohorts and people at various ages emphasized obesity differently.
The results also show that the relationship between obesity and objective and subjective SRH is socially patterned. With the exception of our expectations about time period, the results confirmed many of our expectations. In the case of objective health, obesity was a more important predictor for older than younger adults (who have accumulated health problems over years of exposure to obesity), and for earlier-born cohorts than younger cohorts (more adept at managing obesity-related health conditions). Interestingly, recently-born cohorts do not suffer the greatest health risks of obesity due to greater prevalence of childhood and early adult obesity: BMI at age 25 did not significantly mediate cohort effects on objective SRH. Instead, widespread availability of pharmaceutical treatment options for obesity-related conditions accounted for much of the convergence in objective SRH across birth cohorts, although we caution that unmeasured factors associated with the introduction of new drugs may have also contributed to the convergence. In the case of subjective health, obesity was more important for younger than older adults (who may focus more singularly on this condition) and for recent than earlier cohorts. This cohort effect operated through rising levels of education.
The fundamental causes of disease (FC) theory provides a framework for understanding the emergence of a health risk like obesity. FC suggests that health gradients emerge as knowledge about health risks and treatment increases, but flatten when treatment options saturate the population (Link et al. 2008, Omran 1971, Phelan et al. 2004). Arguably, the accessibility of treatment options for obesity-related diseases has reached a threshold minimizing the impact of obesity on objective SRH across cohorts. With more pharmaceutical treatment options, recently-born cohorts are able to manage the comorbidities associated with obesity that impact their objective SRH.
FC also suggests that those with the most resources (i.e. education) or social status will be the most likely to adopt new health-related ideas (Link 2008). In other words, the definition of a condition as a health risk is likely to instigate differential responses, with the highest status being early adopters of new ideas. Consistent with this idea, our results show that more recently-born, highly-educated cohorts are more likely to emphasize the role of obesity on their subjective SRH than earlier cohorts.
The contribution of this study to the literature on SRH and obesity should be considered within its limitations. First, we singularly measure SRH based on one set of conditions. Nevertheless, objective SRH includes the range of chronic health conditions consistently available across the data. Sensitivity tests including additional predictors of SRH (smoking behaviors and medications) obtained nearly identical results, but do not preclude the importance of potentially omitted conditions such as mental health. Though objective health indicators are often the strongest predictors, SRH captures dimensions of a respondent's mental well-being which may be objective (clinically-diagnosed) or subjective (Shields and Shooshtari 2001, Singh-Manoux et al. 2006). Because mental illness is not included in the prediction of SRH, it is considered a subjective component. Future decompositions of SRH may consider mental health as a third component. Similarly, because different groups base their SRH on different criteria and the meaning attributed to conditions varies (Hardy, Acciai and Reyes 2014, Krause and Jay 1994, Lindeboom and Van Doorslaer 2004, Shmueli 2003) decomposing SRH on the same conditions across age, period, or cohorts raises concerns of reporting heterogeneity (Dowd and Zajacova 2010). The reporting heterogeneity may then be reflected as measurement error in subjective SRH. To alleviate these concerns, we included respondent death within five years of the NHANES survey in the SRH prediction model as mortality arguably has the same meaning across groups. The substantive findings remain unchanged.
Additionally, despite thirty-six years of cross-sectional data, ideally we would follow adults longitudinally to assess how SRH assessments change over time and across cohorts as obesity increases by directly asking if their weight status affects their health. BMI, a reliable body fatness tool (CDC 2011), may be less precise than measures strongly associated with objective health such as waist-to-hip ratios , but respondents generally know their body weight, so BMI category may be more readily factored into SRH evaluations.
Finally, population heterogeneity adds complexity to the obesity-SRH association. The prevalence of obesity and SRH vary by subpopulations; therefore, the obesity-SRH association likely varies. Blacks have higher BMIs (Ogden et al. 2006) and report poorer health than whites (Cagney, Browning and Wen 2005, Farmer and Ferraro 2005); and women typically assess their health status more negatively than men and obesity has more negative implications for women's SRH than men's (Katz, McHorney and Atkinson 2000, Okosun et al. 2001). Moreover, whites have more favorable health in young adulthood (including lower BMIs) and experience a slower SRH decline than blacks (Zajacova and Burgard 2010). However, we are unable to test the role of population heterogeneity on our findings due to limited sample sizes when the data is divided by cohort, BMI, and gender or race.
Our findings on the obesity-SRH association remain noteworthy. Prior SRH research highlighted the need “to both be clear about what kind of health we are talking about, and be ready for the possibility that different types of health behave in very different ways” ((Apouey and Clark 2014), p. 22). By separating subjective from objective SRH and considering age, period, and cohort effects, we aimed to address these concerns. Indeed, the association between obesity and SRH's subjective and objective components are distinct and operate differently by age and cohorts suggesting important shifts in U.S. adults’ conceptualizations of health over the past four decades. Future research on the obesity-SRH association must account for the differential role of treatments and educational attainment for various age groups and birth cohorts.
Biography
Claire E. Altman is an assistant professor in Health Sciences and the Truman School of Public Affairs at the University of Missouri. Her research broadly focuses on population health and immigrant well-being.
Jennifer Van Hook is Professor of Sociology and Demography at the Pennsylvania State University. Her research explores the social, cultural, and economic incorporation of immigrants and their children. Her current work focuses on how school, family, and community contexts influence food choices, nutrition, weight, and weight-related health outcomes of Mexican immigrants and their children.
Marianne Hillemeier is Professor of Health Policy and Administration and Demography at the Pennsylvania State University, and she is Head of the Department of Health Policy and Administration. Her research interests focus on disparities in health by socioeconomic status, race/ethnicity, and geographic location.
Contributor Information
Claire E. Altman, University of Missouri.
Jennifer Van Hook, The Pennsylvania State University.
Marianne Hillemeier, The Pennsylvania State University.
References
- Abdullah Asnawi, Wolfe Rory, Stoelwinder Johannes U., De Courten Maximilian, Stevenson Christopher, Walls Helen L, Peeters Anna. The Number of Years Lived with Obesity and the Risk of All-Cause and Cause-Specific Mortality. International Journal of Epidemiology. 2011;40(4):985–96. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- Allman-Farinelli MA, Chey T, Bauman AE, Gill T, James WPT. Age, Period and Birth Cohort Effects on Prevalence of Overweight and Obesity in Australian Adults from 1990 to 2000. European journal of clinical nutrition. 2007;62(7):898–907. doi: 10.1038/sj.ejcn.1602769. [DOI] [PubMed] [Google Scholar]
- Alwin Duane F., McCammon Ryan J., Hofer Scott M. Studying Baby Boom Cohorts within a Demographic and Developmental Context: Conceptual and Methodological Issues. In: Whitbourne SKE, Willis SL, editors. The Baby Boomers Grow Up: Contemporary Perspectives on Midlife. Lawrence Erlbaum Associates Publishers; Mahwah, NJ: 2006. [Google Scholar]
- Apouey Bénédicte, Clark Andrew E. Winning Big but Feeling No Better? The Effect of Lottery Prizes on Physical and Mental Health. Health Economics. 2014 doi: 10.1002/hec.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au Nicole, Johnston David W. Self-Assessed Health: What Does It Mean and What Does It Hide? Social Science & Medicine. 2014;121:21–28. doi: 10.1016/j.socscimed.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Audit Bureau of Circulation Circulation Averages for the Six Months Ended: 9/30/2012. 2012 http://abcas3.accessabc.com/ecirc/newssearchus.asp.
- Bell Andrew, Jones Kelvyn. Don't Birth Cohorts Matter? A Commentary and Simulation Exercise on Reither, Hauser, and Yang's (2009) Age–Period–Cohort Study of Obesity. Social Science & Medicine. 2014;101:176–80. doi: 10.1016/j.socscimed.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Bentler Peter M., Raykov Tenko. On Measures of Explained Variance in Nonrecursive Structural Equation Models. Journal of Applied Psychology. 2000;85(1):125. doi: 10.1037/0021-9010.85.1.125. [DOI] [PubMed] [Google Scholar]
- Bray George A. Medical Consequences of Obesity. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2583–89. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- Brewis Alexandra A. Stigma and the Perpetuation of Obesity. Social Science & Medicine. 2014;118:152–58. doi: 10.1016/j.socscimed.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Brownell Kelly D. The Chronicling of Obesity: Growing Awareness of Its Social, Economic, and Political Contexts. Journal of Health Politics, Policy and Law. 2005;30(5):955–64. doi: 10.1215/03616878-30-5-955. [DOI] [PubMed] [Google Scholar]
- Cagney Kathleen A., Browning Christopher R., Wen Ming. Racial Disparities in Self-Rated Health at Older Ages: What Difference Does the Neighborhood Make? The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(4):S181–S90. doi: 10.1093/geronb/60.4.s181. [DOI] [PubMed] [Google Scholar]
- Campos Paul, Saguy Abigail, Ernsberger Paul, Oliver Eric, Gaesser Glenn. The Epidemiology of Overweight and Obesity: Public Health Crisis or Moral Panic? International Journal of Epidemiology. 2006;35(1):55–60. doi: 10.1093/ije/dyi254. [DOI] [PubMed] [Google Scholar]
- Carr Deborah, Friedman Michael A. Is Obesity Stigmatizing? Body Weight, Perceived Discrimination, and Psychological Well-Being in the United States. Journal of Health and Social Behavior. 2005;46(3):244–59. doi: 10.1177/002214650504600303. [DOI] [PubMed] [Google Scholar]
- Carroll MD, Lacher DA, Sorlie PD, et al. Trends in Serum Lipids and Lipoproteins of Adults, 1960-2002. JAMA. 2005;294(14):1773–81. doi: 10.1001/jama.294.14.1773. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- CDC. About Bmi for Adults. Vol. 2011. National Centers for Health Statistics; Atlanta: 2011. [Google Scholar]
- Chang Su-Hsin, Pollack Lisa M., Colditz Graham A. Life Years Lost Associated with Obesity-Related Diseases for Us Non-Smoking Adults. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Virginia W, Lauderdale Diane S. Fundamental Cause Theory, Technological Innovation, and Health Disparities: The Case of Cholesterol in the Era of Statins. Journal of Health and Social Behavior. 2009;50(3):245–60. doi: 10.1177/002214650905000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz W. Health Consequences of Obesity in Youth: Childhood Predictors of Adult Disease. Pediatrics. 1998;101(3):518–25. [PubMed] [Google Scholar]
- DiPrete Thomas A., Eirich Gregory M. Cumulative Advantage as a Mechanism for Inequality: A Review of Theoretical and Empirical Developments. Annual Review of Sociology. 2006:271–97. [Google Scholar]
- Dowd Jennifer Beam, Zajacova Anna. Does Self-Rated Health Mean the Same Thing across Socioeconomic Groups? Evidence from Biomarker Data. Annals of epidemiology. 2010;20(10):743–49. doi: 10.1016/j.annepidem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan Brent M., Zhao Yumin, Neal Axon R. Us Trends in Prevalence, Awareness, Treatment, and Control of Hypertension, 1988-2008. Journal of the American Medical Association. 2010;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- Elder Glen H. The Life Course as Developmental Theory. Child development. 1998;69(1):1–12. [PubMed] [Google Scholar]
- Farmer Melissa M., Ferraro Kenneth F. Are Racial Disparities in Health Conditional on Socioeconomic Status? Social Science & Medicine. 2005;60(1):191–204. doi: 10.1016/j.socscimed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Ferraro Kenneth F., Yu Yan. Body Weight and Self-Ratings of Health. Journal of Health and Social Behavior. 1995 [PubMed] [Google Scholar]
- Ferraro Kenneth F., Farmer Melissa M., Wybraniec John A. Health Trajectories: Long-Term Dynamics among Black and White Adults. Journal of Health and Social Behavior. 1997;38(1):38–54. [PubMed] [Google Scholar]
- Ferraro Kenneth F., Kelley-Moore Jessica A. Cumulative Disadvantage and Health: Long-Term Consequences of Obesity? American Sociological Review. 2003;68(5):707. [PMC free article] [PubMed] [Google Scholar]
- Flegal Katherine, Caroll Margaret, Ogden Cynthia. Prevalence and Trends in Obesity among Us Adults, 1999-2008. Journal of the American Medical Association. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Harding David J. Recent Advances in Age-Period-Cohort Analysis. A Commentary on Dregan and Armstrong, and on Reither, Hauser and Yang. Social Science & Medicine. 2009;69(10):1449–51. doi: 10.1016/j.socscimed.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Hardy Melissa A., Acciai Francesco, Reyes Adriana M. How Health Conditions Translate into Self-Ratings a Comparative Study of Older Adults across Europe. Journal of Health and Social Behavior. 2014;55(3):320–41. doi: 10.1177/0022146514541446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler Ellen L, Angel Ronald J. Self-Rated Health and Mortality in the Nhanes-I Epidemiologic Follow-up Study. American Journal of Public Health. 1990;80(4):446–52. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler Ellen L., Benyamini Yael. Self-Rated Health and Mortality: A Review of Twenty-Seven Community Studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Juhaeri June Stevens, Jones Daniel W., Arnett Donna. Associations of Aging and Birth Cohort with Body Mass Index in a Biethnic Cohort. Obesity Research. 2003;11(3):426–33. doi: 10.1038/oby.2003.58. doi: 10.1038/oby.2003.58. [DOI] [PubMed] [Google Scholar]
- Jylha Marja. What Is Self-Rated Health and Why Does It Predict Mortality? Towards a Unified Conceptual Model. Social Science & Medicine. 2009;69(3):307–16. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Kaplan Giora, Baron-Epel Orna. What Lies Behind the Subjective Evaluation of Health Status? Social Science & Medicine. 2003;56(8):1669–76. doi: 10.1016/s0277-9536(02)00179-x. [DOI] [PubMed] [Google Scholar]
- Katz David A., McHorney Colleen A., Atkinson Richard L. Impact of Obesity on Health-Related Quality of Life in Patients with Chronic Illness. Journal of general internal medicine. 2000;15(11):789–96. doi: 10.1046/j.1525-1497.2000.90906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes Katherine M., Utz Rebecca L., Robinson Whitney, Li Guohua. What Is a Cohort Effect? Comparison of Three Statistical Methods for Modeling Cohort Effects in Obesity Prevalence in the United States, 1971-2006. Social Science & Medicine. 2010;70(7):1100–08. doi: 10.1016/j.socscimed.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause Neal M., Jay Gina M. What Do Global Self-Rated Health Items Measure? Medical care. 1994:930–42. doi: 10.1097/00005650-199409000-00004. [DOI] [PubMed] [Google Scholar]
- Kuklina Elena V., Carroll Margaret D., Shaw Kate M., Hirsch Rosemarie. Trends in High Ldl Cholesterol, Cholesterol-Lowering Medication Use, and Dietary Saturated-Fat Intake: United States, 1976-2010. Vol. 117. National Center for Health Statistics; Hyattsville, MD: 2013. [PMC free article] [PubMed] [Google Scholar]
- Lavie Carl J, Milani Richard V, Ventura Hector O. Obesity and Cardiovascular Diseaserisk Factor, Paradox, and Impact of Weight Loss. Journal of the American College of Cardiology. 2009;53(21):1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Layes Audrey, Asada Yukiko, Kephart George. Whiners and Deniers–What Does Self-Rated Health Measure? Social Science & Medicine. 2012;75(1):1–9. doi: 10.1016/j.socscimed.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Lindeboom Maarten, Van Doorslaer Eddy. Cut-Point Shift and Index Shift in Self- Reported Health. Journal of health economics. 2004;23(6):1083–99. doi: 10.1016/j.jhealeco.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Link Bruce G. Epidemiological Sociology and the Social Shaping of Population Health. Journal of Health and Social Behavior. 2008;49(4):367–84. doi: 10.1177/002214650804900401. [DOI] [PubMed] [Google Scholar]
- Link Bruce G., Phelan Jo C., Miech Richard, Leckman Westin Emily. The Resources That Matter: Fundamental Social Causes of Health Disparities and the Challenge of Intelligence. Journal of Health and Social Behavior. 2008;49(1):72–91. doi: 10.1177/002214650804900106. [DOI] [PubMed] [Google Scholar]
- Liu Hui, Hummer Robert A. Are Educational Differences in Us Self-Rated Health Increasing?: An Examination by Gender and Race. Social Science & Medicine. 2008;67(11):1898–906. doi: 10.1016/j.socscimed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderbacka Kristiina, Lundberg Olle, Martikainen Pekka. Do Risk Factors and Health Behaviours Contribute to Self-Ratings of Health? Social Science & Medicine. 1999;48(12):1713–20. doi: 10.1016/s0277-9536(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Martin Linda G., Schoeni Robert F., Freedman Vicki A., Andreski Patricia. Feeling Better? Trends in General Health Status. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62(1):S11–S21. doi: 10.1093/geronb/62.1.s11. [DOI] [PubMed] [Google Scholar]
- Masters Ryan K., Powers Daniel A., Link Bruce G. Obesity and Us Mortality Risk over the Adult Life Course. American Journal of Epidemiology. 2013;177(5):431–42. doi: 10.1093/aje/kws325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky John, Ross Catherine E. Education and Self-Rated Health Cumulative Advantage and Its Rising Importance. Research on Aging. 2008;30(1):93–122. [Google Scholar]
- Must A, Spandan EH, Coakley AE, Colditz Field, G., Dietz WH. The Disease Burden Associated with Overweight and Obesity. Journal of the American Medical Association. 1999;282:1523–529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of Overweight and Obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Okosun Ike S., Choi Simon, Matamoros Tara, Dever GE. Obesity Is Associated with Reduced Self-Rated General Health Status: Evidence from a Representative Sample of White, Black, and Hispanic Americans. PREVENTIVE MEDICINE. 2001;32(5):429–36. doi: 10.1006/pmed.2001.0840. [DOI] [PubMed] [Google Scholar]
- Omran Abdel R. The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. The Milbank Memorial Fund Quarterly. 1971;49(4):509–38. [PubMed] [Google Scholar]
- Pelzer Ben, te Grotenhuis Manfred, Eisinga Rob, Schmidt-Catran Alexander W. The Non-Uniqueness Property of the Intrinsic Estimator in Apc Models. Demography. 2015;52(1):315–27. doi: 10.1007/s13524-014-0360-3. [DOI] [PubMed] [Google Scholar]
- Phelan Jo C, Link Bruce G, Diez-Roux Ana, Kawachi Ichiro, Levin Bruce. Fundamental Causes” of Social Inequalities in Mortality: A Test of the Theory. Journal of Health and Social Behavior. 2004;45(3):265–85. doi: 10.1177/002214650404500303. [DOI] [PubMed] [Google Scholar]
- Phelan Jo C., Link Bruce G. Controlling Disease and Creating Disparities: A Fundamental Cause Perspective. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(Special Issue 2):S27–S33. doi: 10.1093/geronb/60.special_issue_2.s27. [DOI] [PubMed] [Google Scholar]
- Preston Samuel H., Wang Haidong. Sex Mortality Differences in the United States: The Role of Cohort Smoking Patterns. Demography. 2006;43(4):631–46. doi: 10.1353/dem.2006.0037. [DOI] [PubMed] [Google Scholar]
- Prosper Marie-Hortence, Moczulski Vanessa L., Qureshi Azhar. Obesity as a Predictor of Self-Rated Health. American Journal of Health Behavior. 2009;33(3):319–29. doi: 10.5993/ajhb.33.3.10. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Kelly J. Long-Term Impact of Overweight and Obesity in Childhood and Adolescence on Morbidity and Premature Mortality in Adulthood: Systematic Review. International Journal of Obesity. 2011;35(7):891–98. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- Reither Eric N., Hauser Robert M., Yang Yang. Do Birth Cohorts Matter? Age-Period-Cohort Analyses of the Obesity Epidemic in the United States. Social Science & Medicine. 2009;69(10):1439–48. doi: 10.1016/j.socscimed.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson WR, Keyes KM, Utz RL, Martin CL, Yang Y. Birth Cohort Effects among Us-Born Adults Born in the 1980s: Foreshadowing Future Trends in Us Obesity Prevalence. International Journal of Obesity. 2012;37(3):448–54. doi: 10.1038/ijo.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross Catherine E., Wu Chia-Ling. Education, Age, and the Cumulative Advantage in Health. Journal of Health and Social Behavior. 1996:104–20. [PubMed] [Google Scholar]
- Ryder Norman B. The Cohort as a Concept in the Study of Social Change. American Sociological Review. 1965:843–61. [PubMed] [Google Scholar]
- Saguy Abagail C. What's Wrong with Fat? Oxford University Press; New York: 2013. [Google Scholar]
- Saguy Abigail C., Almeling Rene. Fat in the Fire? Science, the News Media, and the “Obesity Epidemic. Sociological Forum. 2008;23(1):53–83. [Google Scholar]
- Salomon Joshua A., Nordhagen Stella, Oza Shefali, Murray Christopher J. L. Are Americans Feeling Less Healthy? The Puzzle of Trends in Self-Rated Health. American Journal of Epidemiology. 2009;170(3):343–51. doi: 10.1093/aje/kwp144. doi: 10.1093/aje/kwp144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields Margot, Shooshtari Shahin. Determinants of Self-Perceived Health. Health Reports. 2001;13(1):35–52. [PubMed] [Google Scholar]
- Shmueli Amir. Socio-Economic and Demographic Variation in Health and in Its Measures: The Issue of Reporting Heterogeneity. Social Science & Medicine. 2003;57(1):125–34. doi: 10.1016/s0277-9536(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux Archana, Martikainen Pekka, Ferrie Jane, Zins Marie, Marmot Michael, Goldberg Marcel. What Does Self Rated Health Measure? Results from the British Whitehall Ii and French Gazel Cohort Studies. Journal of epidemiology and community health. 2006;60(4):364–72. doi: 10.1136/jech.2005.039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Cynthia J, Colditz Graham A. The Epidemic of Obesity. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2522–25. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- Taylor Paul, Doherty Carroll, Parker Kim, Krishnamurthy Vidya. Millennials in Adulthood: Detached from Instutions, Networked with Friends. Pew Research Center; Washington, DC: 2014. [Google Scholar]
- Tessler Richard, Mechanic David. Psychological Distress and Perceived Health Status. Journal of Health and Social Behavior. 1978;19(3):254–62. [PubMed] [Google Scholar]
- The New York Times Syndicate . The New York Times News Service. New York: 2012. ( https://www.nytsyn.com/about/news-service.) [Google Scholar]
- Willson Andrea E., Shuey Kim M., Elder Glen H., Jr. Cumulative Advantage Processes as Mechanisms of Inequality in Life Course Health. American Journal of Sociology. 2007;112(6):1886–924. [Google Scholar]
- Winship Christopher, Harding David J. A Mechanism-Based Approach to the Identification of Age-Period-Cohort Models. Sociological methods & research. 2008;36(3):362–401. [Google Scholar]
- Yang Yang, Land Kenneth C. Age-Period-Cohort Analysis: New Models, Methods, and Empirical Applications. Chapman & Hall/CRC Press; New York: 2013. [Google Scholar]
- Zack Matthew M, Moriarty David G, Stroup Donna F, Ford Earl S, Mokdad Ali H. Worsening Trends in Adult Health-Related Quality of Life and Self-Rated Health-United States, 1993-2001. Public Health Reports. 2004;119(5):493. doi: 10.1016/j.phr.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova Anna, Burgard Sarah A. Body Weight and Health from Early to Mid-Adulthood a Longitudinal Analysis. Journal of Health and Social Behavior. 2010;51(1):92–107. doi: 10.1177/0022146509361183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Qi, Wang Youfa. Trends in the Association between Obesity and Socioeconomic Status in U.S. Adults: 1971 to 2000. Obesity Research. 2004;12(10):1622–32. doi: 10.1038/oby.2004.202. [DOI] [PubMed] [Google Scholar]