Abstract
Introduction
Natriuretic peptides have a well-recognized role in cardiovascular homeostasis. Recently, higher levels of B-type natriuretic peptide (BNP) have also been associated with decreased risk of diabetes in middle-aged adults. Whether this association persists into older age, where the pathophysiology of diabetes changes, has not been established, nor have its intermediate pathways.
Methods
We investigated the relationship between N-terminal (NT)-proBNP and incident diabetes in 2359 older adults free of cardiovascular disease or chronic kidney disease in the Cardiovascular Health Study.
Results
We documented 348 incident cases of diabetes over 12.6 years of median follow-up. After adjusting for age, sex, race, body mass index, systolic blood pressure, anti-hypertensive treatment, smoking, alcohol use, and LDL, each doubling of NT-proBNP was associated with a 9% lower risk of incident diabetes (HR=0.91 [95% CI: 0.84–0.99]). Additional adjustment for waist circumference, physical activity, estimated glomerular filtration rate or C-reactive protein did not influence the association. Among putative mediators, HDL and triglycerides, adiponectin, and especially homeostasis model assessment of insulin resistance, all appeared to account for a portion of the lower risk associated with NT-proBNP.
Conclusion
In older adults without prevalent cardiovascular or kidney disease, higher NT-proBNP is associated with decreased risk of incident diabetes even after adjustment for traditional risk factors. These findings suggest that the metabolic effects of natriuretic peptides persist late in life and offer a potential therapeutic target for prevention of diabetes in older people.
1.0 Introduction
Brain (B-type) natriuretic peptide (BNP) is one of the three characterized human natriuretic peptides.1, 2 It is produced primarily by cardiomyocytes and has well-known effects on heart and vascular function. Released in response to ventricular stretch, BNP promotes vasodilation and natriuresis.2 In the clinical setting, BNP is used to assess the presence and severity of heart failure, in which it is pathologically elevated.2
In addition to its effects on cardiovascular homeostasis, accumulating data show that natriuretic peptides (NPs) have metabolic effects. NP receptors are expressed in adipose tissue and skeletal muscle, and NPs have been shown to promote adipocyte browning, foster adipokine secretion and increase oxidative capacity in muscle.3–8 In clinical studies, higher levels of N-terminal proBNP (NT-proBNP), a cleavage product of proBNP, are associated with less obesity and metabolic syndrome,9–12 and a decreased risk of incident type 2 diabetes.13–16 Moreover, studies of genetic variants in the natriuretic peptide precursor A (NPPA) and B (NPPB) loci suggest that mild, lifelong elevations in natriuretic peptides may protect against type 2 diabetes.15, 17
Such epidemiological evidence linking lower natriuretic peptide levels to increased risk of incident diabetes comes primarily from middle-aged cohorts. As compared with middle-aged individuals, however, the pathophysiology of diabetes in older adults has an important component of aging-related decline in skeletal muscle mass and quality, which results in peripheral insulin resistance.18, 19 With aging there is also central fat redistribution, accompanied by a decrease in brown fat in favor of expansion of white fat,20, 21 findings similarly associated with diabetes.22 Because natriuretic peptides have been shown in experimental models to promote fatty acid oxidation and mitochondrial biogenesis in skeletal muscle3 and to foster adipocyte browning,7, 8 their favorable metabolic properties could be especially relevant in older age. Prior work from our group has indeed documented a positive association of circulating NT-proBNP with various measures of insulin sensitivity, although a relationship for genetically determined levels could not be demonstrated.23 As diabetes is especially common in adults ≥65, with roughly one third in the U.S. affected,24 assessing whether the previously reported association applies to older people is of particular importance. In this study, we investigate whether lower NT-proBNP is associated with increased risk of incident diabetes in a prospective study of community-dwelling older adults.
2.0 Methods
2.1 Study population
The Cardiovascular Health Study (CHS) is a longitudinal population-based cohort study investigating risk factors for cardiovascular disease (CVD) in older adults.25 Participants age 65 and older residing in the community were identified using Medicare eligibility lists and recruited from 4 field centers in the United States (Washington County, MD; Allegheny County, PA; Forsyth County, NC; Sacramento County, California). An initial cohort of 5,201 predominantly white individuals was recruited in 1989–90 (original cohort), and an additional cohort of 687 mostly African-American individuals was recruited in 1992–93 (supplemental cohort). Participants received standardized health evaluations at site clinics as previously described.25, 26 All centers obtained institutional review board approval for the study and participants gave informed consent.
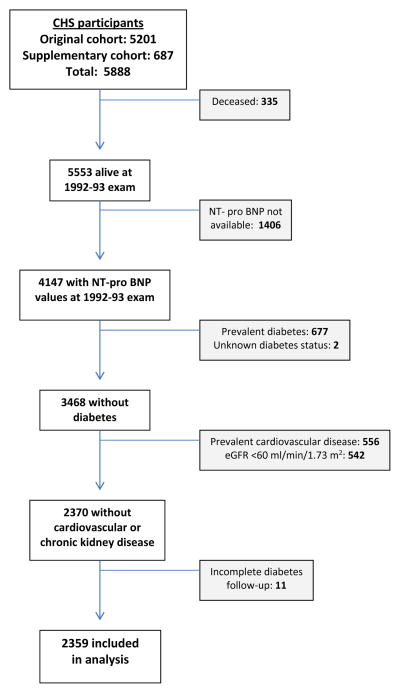
For the present analysis, we used the 1992–93 visit as our baseline exam to allow incorporation of Centers for Medicare and Medicaid claims data for ascertainment of diabetes, information on which was available starting in 1991. As detailed in Figure 1, of the 5,533 CHS participants alive at the 1992–93 visit, 4,147 had NT-proBNP measurements available. Participants with available NT-proBNP measurements were younger and more commonly female, had less hypertension, diabetes, and self-reported fair/poor health status, and exhibited lower prevalence of cardiovascular disease, in comparison to those without available NT-proBNP measurements. Of participants with available NT-proBNP values, we excluded individuals who had prevalent diabetes mellitus (DM) or unknown DM status and those without diabetes follow-up after 1992–93. Since cardiovascular disease and chronic kidney disease can result in pathologic elevations in NT-proBNP levels,2 those with prevalent atherosclerotic cardiovascular disease (CVD) (myocardial infarction and stroke), heart failure (HF), atrial fibrillation and estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 were also excluded. Therefore, a total of 2,359 individuals were included in the analysis (2,059 original cohort, 300 supplemental cohort)(Figure 1).
Figure 1.
Number of participants after exclusion by each criteria.
2.2 Measurement of NT-proBNP
NT-proBNP was measured in serum specimens collected from both the original and supplemental cohort in 1992–93, which were stored at −70°C to −80°C and thawed before testing. NT-proBNP measurements were made on the Elecsys 2010 system (Roche Diagnostics, Indianapolis, Indiana). The analytical measurement range was 5 to 35,000 pg/ml, and the coefficient of variation was 2–5%.27
2.3 Measurement of covariates
Demographic, lifestyle and clinical characteristics were recorded during field center visits using standardized questionnaires along with blood pressure and anthropometric assessments, as previously described.25 Laboratory specimen collection and biochemical testing procedures were performed as reported elsewhere.28 Estimated glomerular filtration rate (eGFR) was calculated using cystatin C.29 Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as fasting glucose(mmol/l)×fasting insulin(mU/l)/22.5.30 Total adiponectin was measured in serum as previously described.31 All laboratory measurements were taken from the 1992–1993 visit.
2.4 Ascertainment of diabetes
Glucose was measured on blood specimens collected during annual clinic visits in 1992–93, 1994–95, 1996–97, 1998–99, and 2005–06. Information on prescription medications taken in the previous 2 weeks was collected annually using standardized methods at clinic visits for the first 10 years, and by telephone contact thereafter. Medicare claims data for inpatient (hospital, skilled nursing facility, or home health) and outpatient (outpatient or carrier) health care services were available beginning in 1991. Hypertension was defined by systolic blood pressure ≥140 and/or diastolic blood pressure ≥90 mm Hg or by self-report and use of antihypertensive medication. Diabetes was defined as single measure of fasting glucose ≥126 mg/dL (7.0 mmol/L), nonfasting glucose ≥200 mg/dL (11.1 mmol/L), or use of insulin or oral hypoglycemic medication, or the existence of ≥ 2 inpatient or ≥ 3 inpatient or (≥ 1 inpatient and ≥ 1 outpatient) or Medicare claims during a 2-year time period which included a diagnostic International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 250.xx.
2.5 Statistical analysis
Baseline covariates were examined by quartiles of NT-proBNP. Cox models were used to calculate adjusted relative risks for incident diabetes by quartile of NT-proBNP and by NT-proBNP doubling. The functional form of the association between NT-proBNP and new-onset diabetes was evaluated with generalized additive models, and non-linearity assessed with the gain statistic. The proportional hazards assumption was tested using Schoenfeld’s goodness-of-fit; this revealed no material violations.
To adjust for confounding, models were constructed that included relevant covariates based on previously demonstrated relations with outcomes in CHS or other cohorts. The first model included age, sex and race. This was followed by addition of clinical and laboratory variables, including body mass index (BMI), systolic blood pressure, use of anti-hypertensive medication, smoking, alcohol consumption, and LDL cholesterol. Additional adjustment for waist circumference, leisure-time physical activity, cystatin-based eGFR or C-reactive protein was then undertaken. Next, we explored the impact of factors documented or presumed to be influenced by BNP that could therefore, at least in part, act as potential causal intermediates in the relationship of BNP with diabetes. Three separate models therefore added HDL cholesterol and triglycerides, adiponectin, or HOMA-IR to assess their effect on the association of interest.
First-order interactions of NT-proBNP with age, sex, race, BMI, physical activity level, and hypertension status were examined. In addition, sensitivity analysis excluded participants with NT-proBNP ≥190 pg/ml, levels at which an elevated risk of heart failure has been previously observed in the CHS cohort.27 Analyses were conducted with Stata version 13.0 (Statacorp, College Station, TX). A two-sided P value <0.05 was considered statistically significant.
3.0 Results
The median age of the study sample was 74 (range, 64 to 103) years, with men constituting 33.8% and African Americans 15.6%. The distribution of baseline characteristics by quartiles of NT-proBNP is presented in Table 1. There was an increase in age, hypertension, fair or poor self-reported health, HDL cholesterol, C-reactive protein and total adiponectin across higher quartiles of NT-proBNP. By contrast, there were smaller proportions of men and African Americans with higher quartiles of NT-proBNP, as well as lower levels of adiposity, LDL cholesterol, triglycerides, eGFR, and HOMA-IR.
Table 1.
Baseline characteristics by quartile of NT-proBNP
| NT-proBNP | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value |
|---|---|---|---|---|---|
| Range, pg/ml | ≤60.6 | >60.6–111.5 | >111.5–203.8 | >203.8 | |
| Age, years | 72 ± 3 | 73 ± 4 | 74 ± 5 | 76 ± 5 | <0.001 |
| Male sex, % | 45.3 | 34.6 | 27.1 | 28.4 | <0.001 |
| Black race, % | 24.7 | 16.3 | 12.4 | 8.8 | <0.001 |
| BMI, kg/m2 | 27.1 ± 4.2 | 26.7 ± 4.5 | 25.8 ± 4.4 | 25.5 ± 4.6 | <0.001 |
| Waist circumference, cm | 97.8 ± 11.5 | 95.9 ± 12.7 | 93.4 ± 12.8 | 93.2 ±13.7 | <0.001 |
| Physical activity, kcal | 1601 ± 1644 | 1574 ± 1861 | 1672 ± 1963 | 1447 ± 1639 | 0.27 |
| Systolic blood pressure, mmHg | 129 ± 18 | 134 ± 19 | 136 ± 20 | 142 ± 22 | <0.001 |
| Anti-hypertensive medication, % | 34.1 | 34.2 | 38.8 | 47.9 | <0.001 |
| Hypertension status, % | 41.9 | 48.4 | 53.1 | 62.7 | <0.001 |
| Alcohol >7 drink/week, % | 13.1 | 14.1 | 16.6 | 13.2 | 0.63 |
| Current smoker % | 9.8 | 9.5 | 10.3 | 10.5 | 0.60 |
| Self-reported health fair/poor, % | 12.3 | 12.5 | 13.9 | 17.5 | 0.009 |
| LDL cholesterol, mg/dl | 133 ± 33 | 129 ± 31 | 127 ± 31 | 124 ± 35 | <0.001 |
| HDL cholesterol, mg/dl | 54 ± 14 | 56 ± 14 | 58 ± 15 | 58 ± 15 | <0.001 |
| Triglycerides, mg/dl | 142 ± 75 | 131 ± 64 | 128 ± 68 | 126 ± 62 | <0.001 |
| eGFR, ml/min/1.73 m2 | 84 ± 15 | 81 ± 14 | 80 ± 13 | 78 ± 14 | <0.001 |
| CRP, mg/l | 3.9 ± 5.3 | 4.4 ± 8.9 | 4.4 ± 7.3 | 4.7 ± 8.8 | 0.10 |
| Total adiponectin, mg/l | 11.3 ± 5.8 | 14.0 ±.6.7 | 15.7 ± 7.6 | 17.7 ± 9.1 | <0.001 |
| HOMA-IR | 3.1 ± 1.8 | 2.5 ± 1.4 | 2.4 ± 1.5 | 2.2 ± 1.3 | <0.001 |
Continuous variables presented as mean ± standard deviation.
BMI= Body mass index. LDL= Low density lipoprotein.
CRP= C-reactive protein.
eGFR= Estimated glomerular filtration rate.
HOMA-IR= Homeostatic model assessment of insulin resistance
HDL= High density lipoprotein.
NT-ProBNP= N-terminal Pro B-type Natriuretic Peptide.
During 12.6 median years of follow-up, 348 new cases of diabetes occurred. As shown in Table 2, the incidence of diabetes was progressively lower across increasing quartiles of NT-proBNP. The relative risk estimates for incident diabetes by quartiles and by continuous levels of NT-proBNP are also presented in Table 3. The incidence of diabetes was inversely associated with BNP. Generalized additive models did not reveal significant departure from a linear relationship (p=0.45). In the model adjusting for age, sex, race, systolic blood pressure, hypertension medication, smoking status, alcohol consumption, and LDL, every doubling of NT-proBNP concentration was associated with a 9% lower risk of new-onset diabetes. Additional adjustment for waist circumference, physical activity, eGFR or C-reactive protein did not meaningfully affect the risk estimate. There was no statistically significant difference between quartiles of NT-proBNP in risk of diabetes despite the consistent trend towards lower relative risk of diabetes with increasing quartiles in all models.
Table 2.
Association of NT-proBNP with incident diabetes
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Overall | |
|---|---|---|---|---|---|
| Events/Individuals at risk (n) | 105/590 | 95/590 | 85/590 | 63/589 | 348/2359 |
| Incidence (per 1000 py)* | 14.0 | 13.3 | 12.2 | 10.5 | 12.6 |
| HR vs. referent (95% CI), p Value | HR per doubling (95% CI), p Value | ||||
| Model 1† | 1.00 (Ref.) | 0.99 (0.75 – 1.31) p=0.93 | 0.92 (0.68 – 1.24) p=0.59 | 0.82 (0.59 – 1.13) p=0.24 | 0.93 (0.85 – 1.01) p=0.10 |
| Model 2‡ | 1.00 (Ref.) | 0.95 (0.71 – 1.26) p=0.71 | 0.93 (0.69 – 1.25) p=0.62 | 0.76 (0.54 – 1.07) p=0.11 | 0.91 (0.84 – 0.99) p=0.039 |
p=0.060 for trend across quartiles.
Adjusted by age, sex, race.
Adjusted by age, sex, race, body mass index, systolic blood pressure, antihypertensive medication, smoking status, alcohol consumption, and low density lipoprotein cholesterol.
CI=Confidence interval. HR=Hazard ratio. PY= Person-years.
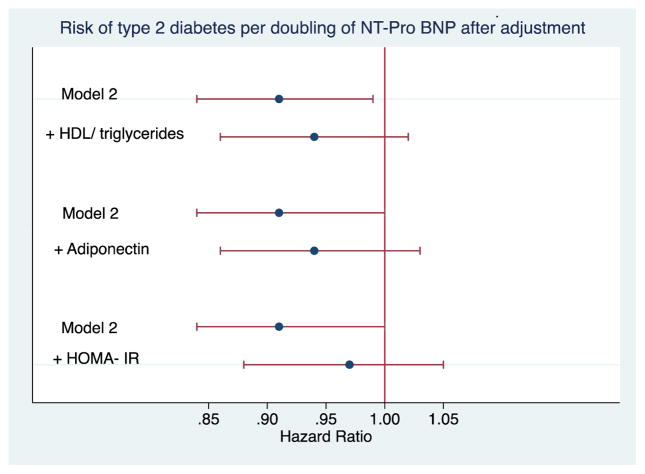
Figure 2 displays the results of additional adjustment for potential causal intermediates of the relationship between NT-proBNP and incident diabetes. Addition of HDL cholesterol and triglycerides or adiponectin to the confounder-adjusted model modestly attenuated the association, and statistical significance was lost. Adjustment for HOMA-IR in addition to potential confounders more substantially attenuated the association, moving it closer to null.
Figure 2.
Impact of additional adjustment for putative mediators of the relationship between NT-proBNP and diabetes. Model 2 is the same as in Table 3, and includes adjustment for age, sex, race, body mass index, systolic blood pressure, smoking, alcohol, and LDL cholesterol. Data available in n=2,359 participants for HDL cholesterol and triglycerides; n=2,343 participants for adiponectin; and n=2,347 participants for HOMA-IR. Horizontal bars represent 95% confidence intervals.
Assessment for effect-modification did not reveal an interaction between NT-proBNP and age, sex, race, BMI, physical activity or hypertension status. Exclusion of participants with NT-proBNP ≥190 pg/ml did not materially alter the risk estimate for continuous NT-proBNP level (per doubling) in relation to incident diabetes.
4.0 Discussion
4.1 Principal Findings
The present report shows that in a cohort of community-dwelling older adults without prevalent cardiovascular disease or chronic kidney disease, higher levels of NT-proBNP were associated with a lower risk of incident diabetes. Our study demonstrates that this relationship was independent of various potential confounders and consistent across a variety of subgroups.
Although analysis by quartiles of NT-proBNP suggested an inverse association with incident diabetes, a significant relationship was only observed when NT-proBNP levels were modeled continuously, likely due to the less robust power associated with categorization. Furthermore, the association, which was marginally non-significant after adjustment for demographic covariates, became statistically significant only after additional adjustment for potential clinical and laboratory confounders. This finding reflects the positive association of NT-proBNP with hypertension, which acted as a negative confounder of NT-proBNP’s inverse relationship with diabetes.
As hypothesized, the association was weakened after adjustment for several putative mediators. This included HDL cholesterol and triglycerides, lipid subfractions that are closely linked to insulin resistance,32 as well as adiponectin and, particularly, HOMA-IR itself.
4.2 Previous Studies
Previous prospective studies have examined the association between natriuretic peptides and incident diabetes in predominantly middle-aged populations.13–17 Among Finnish participants in the FINRISK97 and HEALTH 2000 longitudinal studies, with mean (geometric) ages of ~46 and ~53 years, respectively, every SD increment in NT-proBNP was associated with an 18% lower risk of incident diabetes.14 This inverse relationship was replicated among participants in the EPIC-Norfolk study, ages 39 to 79 years, which documented a 21% lower adjusted risk of diabetes per SD increment in log-transformed NT-proBNP.17 The Malmo Diet and Cancer study also reported an inverse association between NT-proBNP and development of diabetes among participants with a mean age of ~57 years, but this fell short of statistical significance (adjusted odds ratio [OR] per SD increment, 0.92 [95% CI, 0.80–1.06]).13 There was, however, a significant association between mid-regional atrial natriuretic peptide and new-onset diabetes (adjusted OR per SD increment, 0.85 [95% CI 0.73–0.99]).13
Recent reports from U.S. cohorts have also provided support for an inverse association. The Women’s Health Study documented a significant relationship among middle-aged women, with a 22% lower adjusted risk of diabetes per SD increment in logarithm-transformed NT-proBNP.15 Likewise, the Atherosclerosis Risk in Communities (ARIC) study reported a significant 35% decrease in adjusted risk of incident diabetes in comparison of extreme quartiles among men and women with a mean age of ~62 years, although there was flattening of the association starting at levels within the third quartile.16 Such findings linking natriuretic peptides with onset of diabetes have been buttressed by analyses of genetic variants in natriuretic peptide precursor (NPP) genes, NPPA and NPPB, associated with higher levels of NT-proBNP. Two separate studies have reported such variants to be associated with reduced risk of diabetes,15, 17 providing population-level evidence for the potentially causal influence of relative natriuretic peptide deficiency on the development of diabetes.
Lately, the Multi-Ethnic Study of Atherosclerosis also found an inverse association between NT-proBNP and incident diabetes among individuals ages 45–84.33 Comparison of the upper versus the lowest three sextiles of baseline NT-proBNP was associated with a 32% lower adjusted risk of this outcome, but attenuation of the association occurred at levels toward the upper limit of the fifth sextile (>150 pg/ml),33 consistent with similar flattening of NT-proBNP’s associations with metabolic risk factors as concentrations reached a higher range.11, 12 The same study33 reported a U-shaped association for subsequent 3-year interval change in NT-proBNP and diabetes. Taken together with the ARIC results, these findings are consistent with the adverse metabolic impact of pathologic elevations of NT-proBNP into the higher range, as compared with physiologic elevations.
The present results extend these previous findings to an older population with a mean age of 74 years, showing that after exclusion of cardiovascular and kidney disorders apt to confound any inverse association of physiologic natriuretic peptide levels with diabetes,34, 35 such protective association holds for individuals late in life. Unlike previous reports, there was no evidence of attenuation of the association at the upper range of the NT-proBNP distribution, but the smaller number of incident diabetes cases may have limited the current study’s ability to detect such an effect. Regardless, the current findings building on our separate report23 of inverse associations between NT-proBNP and various measures of insulin resistance are important. Not only is diabetes more prevalent in older adults than in any other age group, 24 but it is also characterized by distinct pathogenetic features, including aging-related decline in beta-cell function and loss of skeletal muscle mass with attendant skeletal muscle insulin resistance.18 Moreover, abnormal glucose regulation is well documented to heighten risk of cardiovascular disease and mortality in elders, much as it does in middle-aged adults.36, 37 Hence, our findings have implications for harnessing natriuretic peptides and related pathways as potential therapeutic targets in the segment of the population with the greatest burden of disorders of glucose regulation.
4.3 Potential Mechanisms
The mechanisms behind the association between NT-proBNP and decreased risk for diabetes are not well understood. Recently discovered effects of NPs on metabolism and energy expenditure raise the intriguing possibility that NPs play a causal role in protecting against obesity and dysmetabolism, and may affect pathways that are involved in age-related insulin resistance and dysglycemia.6, 38 It was discovered over a decade ago that adipocytes express natriuretic peptide receptor A (NPR-A) and that BNP induces lipolysis in cultured adipocytes.5 Subsequent experiments demonstrated that transgenic mice overexpressing BNP are protected from obesity and insulin resistance, and have less ectopic and visceral fat accumulation when fed a high-fat diet.3 More recent work has documented that activation of NPR-A on adipocytes by BNP also induces transcription of genes, including PGC1-α and UCP1, that enhance energy expenditure and adipocyte browning.7 With aging, there are changes in adipose tissue distribution associated with an adverse metabolic profile, including an increase in visceral fat and loss of brown adipose tissue.21, 22, 39 Thus, it is possible that BNP may help to protect against such changes in humans. Although we adjusted for waist circumference, which had no meaningful impact on the observed association, we lacked reliable measures of adipocyte browning to support or refute this specific hypothesis.
Another mechanism by which BNP might decrease the risk of diabetes is through effects on skeletal muscle. ANP and BNP are released from the heart during exercise40, 41 and in response to exercise training, obese individuals up-regulate NPR-A, PGC1-α and genes involved in oxidative phosphorylation.42 Studies in rodents show that long-term exposure to increased BNP increases oxidative capacity in skeletal muscle and protects against skeletal muscle atrophy.3, 8 Such skeletal muscle effects may be especially important in older adults, who experience decline in skeletal muscle mass and quality,19 and are therefore particularly susceptible to skeletal muscle insulin resistance.18 We examined levels of self-reported physical activity in our analyses as a surrogate for skeletal muscle health, as well as a stimulus for natriuretic peptide secretion, but did not find these to influence our findings. We lacked direct measures of skeletal muscle mass or quality, however, and therefore had limited ability to evaluate the interrelationship between natriuretic peptides, skeletal muscle, and metabolic risk in our study.
Finally, BNP may exert some of its metabolic effects is through release of adipokines. BNP is positively correlated with adiponectin,43–45 and in vitro studies of cultured human adipocytes show that ANP and BNP enhance adiponectin secretion via NPR-A. ANP infusion also raises adiponectin plasma levels in healthy adults and those with heart failure.4, 46 These findings have led to speculation that the metabolic effects of BNP may be partially mediated by adiponectin. Indeed, our data show a positive association between NT-proBNP and suggest some, but not complete, attenuation of the association between BNP and incident diabetes by adiponectin in older adults. A recent cross-sectional study showed that the association between higher BNP and a favorable fat distribution profile persisted even after adjustment for adipokine levels.43
4.4 Strengths and Weaknesses
Our study strengths include its prospective, population-based design, its long-term follow-up, and its focus on adults late in life. The present investigation is also subject to several limitations. NT-proBNP was not available for all participants at the 1992–93 visit. Participants with missing NT-proBNP measurements tended to be less healthy than those with available values. This is, however, precisely the population we sought to exclude to avoid confounding by pathologic elevations of NT-proBNP. The outcome measure was based on a combination of glucose measurements, medication use, and CMS diagnostic codes. Although this affords good accuracy for diabetes diagnosis, lack of additional measures at follow-up, such as glycated hemoglobin, may have decreased sensitivity. The resulting misclassification, however, would generally tend to bias the association of interest toward the null. Finally, the results were based on a single measure of NT-proBNP, which has been shown to have some degree of intraindividual variability.47 While use of genetic variants influencing plasma NT-proBNP can provide a better measure of long-term levels, we lacked sufficient power in the present analysis to undertake this type of evaluation, which will require meta-analytic approaches for meaningful investigation specifically in elders.
5.0 Conclusions
In conclusion, our findings show that higher circulating NT-proBNP levels are associated with lower risk of incident diabetes in older adults. These results add to the growing evidence that the heart acts, through release of natriuretic peptides, as an important endocrine organ in metabolic homeostasis, even in the setting of advanced age. Further studies are necessary to assess whether direct pharmacologic administration of natriuretic peptides, or interventions to raise their levels, represent effective new strategies for the prevention or treatment of diabetes.
Acknowledgments
Sources of Funding
This work was supported by R01 HL-094555, as well as by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Footnotes
Jorge Kizer is the guarantor of this manuscript and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript
Financial Disclosures
None
Author Contributions: EFB and JRK designed the study and wrote the manuscript. MLB contributed to study design, performed all data analyses and reviewed/edited manuscript.
LD, JHI, FK, KJM, DSS, IHD and CRD contributed to study concept and discussion and reviewed/edited manuscript. JSG, JAD, RPT helped with study concept.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Nishikimi T, Kuwahara K, Nakao K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. Journal of cardiology. 2011;57:131–140. doi: 10.1016/j.jjcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Daniels LB, Maisel AS. Natriuretic peptides. Journal of the American College of Cardiology. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cgmp/cgmp-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, Okazaki H, Asai M, Nagamachi Y, Maeda N, Shintani Y, Minamino T, Asakura M, Kishimoto I, Funahashi T, Tomoike H, Kitakaze M. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. Journal of the American College of Cardiology. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: A new lipolytic pathway in human adipocytes. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 6.Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nature reviews Endocrinology. 2014;10:157–163. doi: 10.1038/nrendo.2013.234. [DOI] [PubMed] [Google Scholar]
- 7.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 mapk to induce the brown fat thermogenic program in mouse and human adipocytes. The Journal of clinical investigation. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plante E, Menaouar A, Danalache BA, Broderick TL, Jankowski M, Gutkowska J. Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia. 2014;57:1257–1267. doi: 10.1007/s00125-014-3201-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 10.Musani SK, Vasan RS, Bidulescu A, Liu J, Xanthakis V, Sims M, Gawalapu RK, Samdarshi TE, Steffes M, Taylor HA, Fox ER. Aldosterone, c-reactive protein, and plasma b-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: Findings from the jackson heart study. Diabetes care. 2013;36:3084–3092. doi: 10.2337/dc12-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, Maisel A, Peralta CA, Jacobs DR., Jr The associations between metabolic variables and nt-probnp are blunted at pathological ranges: The multi-ethnic study of atherosclerosis. Metabolism: clinical and experimental. 2014;63:475–483. doi: 10.1016/j.metabol.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez OA, Duprez DA, Daniels LB, Maisel AS, Otvos JD, Peralta CA, Lima JA, Bahrami H, Jacobs DR., Jr The association between n-terminal pro b-type natriuretic peptide and lipoprotein particle concentration plateaus at higher n-terminal pro b-type natriuretic peptide values: Multi-ethnic study on atherosclerosis. Metabolism: clinical and experimental. 2015;64:857–861. doi: 10.1016/j.metabol.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, Morgenthaler NG, Nilsson P, Newton-Cheh C, Wang TJ, Melander O. Low plasma level of atrial natriuretic peptide predicts development of diabetes: The prospective malmo diet and cancer study. The Journal of clinical endocrinology and metabolism. 2012;97:638–645. doi: 10.1210/jc.2011-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salomaa V, Havulinna A, Saarela O, Zeller T, Jousilahti P, Jula A, Muenzel T, Aromaa A, Evans A, Kuulasmaa K, Blankenberg S. Thirty-one novel biomarkers as predictors for clinically incident diabetes. PloS one. 2010;5:e10100. doi: 10.1371/journal.pone.0010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett BM, Cook NR, Chasman DI, Magnone MC, Bobadilla M, Rifai N, Ridker PM, Pradhan AD. Prospective evaluation of b-type natriuretic peptide concentrations and the risk of type 2 diabetes in women. Clinical chemistry. 2013;59:557–565. doi: 10.1373/clinchem.2012.194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazo M, Young JH, Brancati FL, Coresh J, Whelton S, Ndumele CE, Hoogeveen R, Ballantyne CM, Selvin E. Nh2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes. 2013;62:3189–3193. doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfister R, Sharp S, Luben R, Welsh P, Barroso I, Salomaa V, Meirhaeghe A, Khaw KT, Sattar N, Langenberg C, Wareham NJ. Mendelian randomization study of b-type natriuretic peptide and type 2 diabetes: Evidence of causal association from population studies. PLoS medicine. 2011;8:e1001112. doi: 10.1371/journal.pmed.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS. Diabetes in older adults. Diabetes care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 20.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 22.Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC, Richard D. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18f-fdg-detected bat in humans. The Journal of clinical endocrinology and metabolism. 2011;96:192–199. doi: 10.1210/jc.2010-0989. [DOI] [PubMed] [Google Scholar]
- 23.Kim F, Biggs ML, Kizer JR, Brutsaert EF, de Fillipi C, Newman AB, Kronmal RA, Tracy RP, Gottdiener JS, Djousse L, de Boer IH, Psaty BM, Siscovick DS, Mukamal KJ. Brain natriuretic peptide and insulin resistance in older adults. Diabetic medicine: a journal of the British Diabetic Association. 2016 doi: 10.1111/dme.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the united states, 1988–2012. JAMA: the journal of the American Medical Association. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 25.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: Design and rationale. Annals of epidemiology. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 26.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the cardiovascular health study. Annals of epidemiology. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 27.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated n-terminal pro-b-type natriuretic peptide testing. Journal of the American College of Cardiology. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the cardiovascular health study. Clinical chemistry. 1995;41:264–270. [PubMed] [Google Scholar]
- 29.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin c measured by automated immunoassay: A more sensitive marker of changes in gfr than serum creatinine. Kidney international. 1995;47:312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Kizer JR, Arnold AM, Benkeser D, Ix JH, Djousse L, Zieman SJ, Barzilay JI, Tracy RP, Mantzoros CS, Siscovick DS, Mukamal KJ. Total and high-molecular-weight adiponectin and risk of incident diabetes in older people. Diabetes care. 2012;35:415–423. doi: 10.2337/dc11-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparks JD, Sparks CE, Adeli K. Selective hepatic insulin resistance, vldl overproduction, and hypertriglyceridemia. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez OA, Duprez DA, Bahrami H, Peralta CA, Daniels LB, Lima JA, Maisel A, Folsom AR, Jacobs DR. Changes in n-terminal pro-b-type natriuretic peptide and incidence of diabetes: The multi-ethnic study of atherosclerosis (mesa) Diabetes & metabolism. 2015;41:378–386. doi: 10.1016/j.diabet.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarnow L, Gall MA, Hansen BV, Hovind P, Parving HH. Plasma n-terminal pro-b-type natriuretic peptide and mortality in type 2 diabetes. Diabetologia. 2006;49:2256–2262. doi: 10.1007/s00125-006-0359-4. [DOI] [PubMed] [Google Scholar]
- 35.Bruno G, Barutta F, Landi A, Cavallo Perin P, Gruden G. Nt-probnp linking low-moderately impaired renal function and cardiovascular mortality in diabetic patients: The population-based casale monferrato study. PloS one. 2014;9:e114855. doi: 10.1371/journal.pone.0114855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith NL, Barzilay JI, Kronmal R, Lumley T, Enquobahrie D, Psaty BM. New-onset diabetes and risk of all-cause and cardiovascular mortality: The cardiovascular health study. Diabetes care. 2006;29:2012–2017. doi: 10.2337/dc06-0574. [DOI] [PubMed] [Google Scholar]
- 37.Brutsaert EF, Shitole S, Biggs ML, Mukamal KJ, deBoer IH, Thacker EL, Barzilay JI, Djousse L, Ix JH, Smith NL, Kaplan RC, Siscovick DS, Psaty BM, Kizer JR. Relations of postload and fasting glucose with incident cardiovascular disease and mortality late in life: The cardiovascular health study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2016;71:370–377. doi: 10.1093/gerona/glv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: New findings and future developments for diabetes research. Diabetes care. 2014;37:2899–2908. doi: 10.2337/dc14-0669. [DOI] [PubMed] [Google Scholar]
- 39.Graja A, Schulz TJ. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontology. 2015;61:211–217. doi: 10.1159/000366557. [DOI] [PubMed] [Google Scholar]
- 40.Konig D, Schumacher YO, Heinrich L, Schmid A, Berg A, Dickhuth HH. Myocardial stress after competitive exercise in professional road cyclists. Medicine and science in sports and exercise. 2003;35:1679–1683. doi: 10.1249/01.MSS.0000089248.37173.E7. [DOI] [PubMed] [Google Scholar]
- 41.Niessner A, Ziegler S, Slany J, Billensteiner E, Woloszczuk W, Geyer G. Increases in plasma levels of atrial and brain natriuretic peptides after running a marathon: Are their effects partly counterbalanced by adrenocortical steroids? European journal of endocrinology/European Federation of Endocrine Societies. 2003;149:555–559. doi: 10.1530/eje.0.1490555. [DOI] [PubMed] [Google Scholar]
- 42.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. The Journal of clinical investigation. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neeland IJ, Winders BR, Ayers CR, Das SR, Chang AY, Berry JD, Khera A, McGuire DK, Vega GL, de Lemos JA, Turer AT. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. Journal of the American College of Cardiology. 2013;62:752–760. doi: 10.1016/j.jacc.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wannamethee SG, Welsh P, Whincup PH, Sawar N, Thomas MC, Gudnarsson V, Sattar N. High adiponectin and increased risk of cardiovascular disease and mortality in asymptomatic older men: Does nt-probnp help to explain this association? European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2011;18:65–71. doi: 10.1097/HJR.0b013e32833b09d9. [DOI] [PubMed] [Google Scholar]
- 45.Vila G, Grimm G, Resl M, Heinisch B, Einwallner E, Esterbauer H, Dieplinger B, Mueller T, Luger A, Clodi M. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes. 2012;61:2592–2596. doi: 10.2337/db11-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birkenfeld AL, Budziarek P, Boschmann M, Moro C, Adams F, Franke G, Berlan M, Marques MA, Sweep FC, Luft FC, Lafontan M, Jordan J. Atrial natriuretic peptide induces postprandial lipid oxidation in humans. Diabetes. 2008;57:3199–3204. doi: 10.2337/db08-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fradley MG, Larson MG, Cheng S, McCabe E, Coglianese E, Shah RV, Levy D, Vasan RS, Wang TJ. Reference limits for n-terminal-pro-b-type natriuretic peptide in healthy individuals (from the framingham heart study) The American journal of cardiology. 2011;108:1341–1345. doi: 10.1016/j.amjcard.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]