Abstract
Objective
To examine the hypothesized mediating role of cognitive processing speed (CPS) in the relationship between cardiovascular disease (CVD) and executive functioning (EF). This study investigates whether the processing speed hypothesis in aging also explains the unique contribution that CPS may have to EF deficits in CVD patients.
Method
21 older adults with a history of CVD and 73 older adults with no history of CVD completed a neuropsychological assessment, including multiple measures of CPS and EF. Structural equation models examined the indirect associations between CVD and six EF task outcomes via a CPS factor. Competing indirect links were assessed using the product-of-coefficients (α*β) approach with bias-corrected bootstrap confidence intervals.
Results
CVD was significantly, negatively related to CPS (β = -.239, 95% CI [-.457, -.021]). CPS was significantly, positively related to an EF composite score (β = .566, 95% CI [.368, .688]). CVD was significantly, negatively related to the EF composite score (β = -.137, 95% CI [-.084, -.211]). The indirect links from CVD to the individual measures of the EF composite score via CPS were all significant. Tasks of cognitive flexibility and inhibition were most adversely affected by CVD via CPS.
Conclusions
The present study demonstrates that the processing speed hypothesis in aging extends to older adult patients with CVD. Reduced CPS significantly underlies the link between CVD status and poorer EF. Individuals with CVD demonstrated poorer CPS and EF than those without CVD, and CPS was specifically implicated as a CVD-related mechanism leading to worse EF.
Keywords: processing speed, cardiovascular disease, structural equation modeling, executive functioning, neuropsychological assessment
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the United States, accounting for 610,000 deaths each year (Centers for Disease Control, 2013). Its prevalence dramatically increases as people reach advanced age, such that CVD is present in over 70% of individulas 75 years or older. This is particularly alarming given that the proportion of older adults in today’s societies is rising substantially due to falling birth rates, societal development, and increased longevity (Bloom, 2011; Lutz, Sanderson, & Scherbov, 2008). Globally, it is estimated that the number of individuals aged 60 and over will reach two billion in 2050 (United Nations, 2013). In the United States, it is estimated that there will be nearly twice as many older adults over 65 than children under 15 (Centers for Disease Control, 2013; Cohen, 2003). In addition to its effects on physical well-being, CVD causes significant psychological, social, and economic hardship (Haan et al., 1997; Mangano, 1995; Miller & Missov, 2001; Smith & Mensah, 2003; Rich, 1997). It is estimated that the annual direct cost of CVD in the United States exceeds $300 billion (Mozaffarian et al., 2015).
Many CVD patients develop cognitive problems, including consistent observations of slowed cognitive processing speed (CPS) and impaired executive functioning (EF; Cohen et al., 1999; DeRight, Jorgensen, & Cabral, 2015; Gunstad et al., 2005; Jefferson, Poppas, Paul, & Cohen, 2009; Norlund et al., 2007; Paul et al., 2005; van Buchem et al., 2014). For example, Jefferson and colleagues (2009) found that CVD patients with low cardiac output (< 4.0 L/min) demonstrated significantly worse EF compared to CVD patients with normal cardiac output (≥4.0 L/min). These authors hypothesized that subcortical areas critical to EF (e.g., frontal areas) were negatively affected by the reduced blood flow of the CVD patients with low cardiac output. Because the overall domain of CPS appears to be represented by a system of integrated brain networks, disruption of coordination between these networks (i.e., due to hypoperfusion and white matter damage) likely results in slowed CPS. In fact, research by Turken et al. (2008) found that CPS is closely associated with the structural integrity of major white matter tracts that run along the anterior-posterior axis of the brain, allowing fronto-posterior network interactions. Thus, in addition to the well-known declines in cognition due to healthy aging, the presence of CVD alone may lead to cognitive decrements due to its effects on major white matter tracts.
While the cognitive sequelae have historically received less attention than the emotional and psychosocial effects of CVD, there is compelling evidence that cognitive functioning is an important determinant of health status, quality of life, and functional ability (Baltes, Wahl, & Schmid-Furstoss, 1990; Bastone & Kerns, 1995; Depp & Jeste, 2006; Reichstadt, Depp, Palinkas, Folsom, & Jeste, 2007; Rowe & Kahn, 1997; Royall, Chiodo, & Polk, 2000). In fact, even subtle cognitive problems among CVD patients affect their ability to benefit from treatment (e.g., cardiac rehabilitation) and may portend more serious problems like vascular dementia (Cohen et al., 1999; Cohen et al., 2002).
CPS is of particular interest to research on normal age-related cognitive decline because it is a fundamental component of many of the brain’s other cognitive functions (Rypma & Prabhakaran, 2009; Salthouse, 1996). In fact, slowed CPS significantly contributes to declines in performance in other cognitive domains, including mutiple components of EF (Finkel, Reynolds, McArdle, & Pedersen, 2007; Gold, Powell, Xuan, Jicha, & Smith, 2010; Kennedy & Raz, 2009; Salthouse & Coon, 1993; Whiting & Smith, 1997). In healthy adults, for example, CPS ability declines by more than 50% between the ages of 25 and 65 on the Symbol Search subtest of the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 2008). Therefore, the accurate assessment of CPS ability may serve as a sensitive predictor of changes in higher-order cognitive abilities, and an early marker of brain dysfunction (Duering et al., 2014; Eckert, 2011; Salthouse & Ferrer-Caja, 2003; Tam, Lam, Huang, Wang, & Lee, 2015). Indeed, measures of CPS are frequently employed to detect cognitive changes associated with both normal age-related and pathological changes in brain integrity (Lezak, Howieson, Bigler, & Tranel, 2012).
CPS has long been considered to have a pervasive role in cognitive aging (Birren & Fisher, 1995; Salthouse, 1996; Tucker-Drob, Johnson, & Jones, 2009), and these effects are likely exacerbated by CVD. The relationship between age-related cognitive decline and CPS is often referred to as the processing speed hypothesis and implies a mediational indirect effect of age on cognitive functioning through CPS. The cognitive decrements due to CVD are highly correlated with aging, leading previous research to conceptualize CVD as an aging accelerator (Lakatta & Levy, 2003a; Lakatta & Levy, 2003b; O’Rourke & Hashimoto, 2007). Yet, to our knowledge, no prior research has investigated the processing speed hypothesis in CVD patients. Thus, despite the pivotal role EF and CPS occupy in the functional abilities of older adults with CVD, it is unknown to what degree CPS mediates the relationship between CVD and EF.
Given how fundamental CPS is to performance in higher-order cognitive domains like EF, and, consistent with the processing speed hypothesis, it follows that CVD may incur risk for EF deficits via CPS. Because CVD affects CPS and white matter connections associated with CPS, it is important that we investigate the degree to which CVD influences EF through CPS. In the present study, we examined linkages between CVD, CPS, and six components of EF in a cross-sectional sample of older adults. Specifically, our first aim was to test the associations between CVD status, CPS, and EF. Second, we tested a mechanistic model to confirm the direct links between CVD, and EF via CPS in this sample. We hypothesize that CVD status and EF will be indirectly associated via the CPS mediator.
Methods
Participants
Individuals with CVD (n = 21) were patients recruited from outpatient cardiology clinics in the Providence, Rhode Island area as part of a National Institutes of Health-funded study on the effects of CVD on brain functioning. Ages ranged from 59 to 86 years (M age = 72.14; SD = 8.87). Mean level of education was 15.71 years (SD = 2.61). In addition to receiving direct care from a cardiologist, CVD patients must have had a history of cardiac disease that affected the structural integrity or other functioning of the heart. They underwent a cardiac exam to confirm the diagnosis of CVD. Other inclusion criteria were that participants be right-handed English speakers over 50 years of age, with normal or corrected vision and hearing at the time of testing. Patients with CVD were excluded if they had history of comorbid neurological disease (e.g., Alzheimer’s Disease, stroke, multiple sclerosis), traumatic brain injury (with loss of consciousness), substance abuse that resulted in hospitalization, diagnosis of current psychiatric illness, or any magnetic resonance imaging (MRI) contraindications (e.g., contraindicated metal implants).
A community sample of 73 healthy older adults was recruited via the same cardiac clinics (e.g., spouses), newspaper ads, and flyers. Ages ranged from 50 to 85 years (M age = 63.11; SD = 8.10). Mean level of education was 15.81 years (SD = 1.95). Inclusion and exclusion criteria were the same as patients with CVD, except the confirmed absence of CVD was required (confirmed by cardiac exam).
All participants provided written informed consent prior to study participation and were monetarily compensated. The present study was approved by Institutional Review Boards of Butler Hospital and Brown University (Providence, Rhode Island).
Procedures
All participants completed a neuropsychological assessment that was supervised by a licensed clinical neuropsychologist. The assessment took place in a quiet and private room. Responses to all neuropsychological assessment measures were collected via paper and pencil as per standardized procedures delineated in their respective manuals. All participants underwent a cardiac exam, which included an electrocardiogram test, an interview with a cardiologist, and a FMRI assessment subsequently.
Measures
For all measures, raw scores were converted to age-corrected z-scores using the prescribed procedures and normative samples of the measures from which the subtests were obtained.
CPS tasks
The Symbol Search subtest of the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, 1997) and the Coding subtest of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, Tierney, Mohr, & Chase, 1998) were used to measure CPS. Symbol Search is often included in clinical neuropsychological assessments to measure CPS. This is a self-paced task during which examinees are allotted two minutes to complete as many items as possible. The task requires rapid comparisons to determine if a set of five target geometric designs include one of two exemplars, which are positioned just to the left of the targets. If one of the exemplars is present in the set of target designs, participants draw a line through a “YES” box; if neither of the two exemplars is found in the group of target designs, a line is drawn through a “NO” box. Participants were instructed to work as quickly and accurately as possible. The Coding subtest of the RBANS is similar to Symbol Search in that it is frequently used to measure CPS in clinical neuropsychological assessments. In this task, participants were instructed to fill in missing digits corresponding to shapes in a key located at the top of a page as quickly as possible for 90 seconds. Confirmatory factor analyses (CFA) were conducted to derive a latent factor of CPS based on age-adjusted z-scores of the WAIS-III Symbol Search and RBANS Coding.
EF tasks
The current study administered selected subtests of the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001) to measure six components of EF. Each of the following subtests and trials were administered and scored according to established procedures.
The Number-Letter Switching trial of the D-KEFS Trail Making Test was administered to measure cognitive flexibility. This trial requires examinees to switch back and forth between connecting numbers and letters (i.e., 1, A, 2, B, etc., to 16, P) as quickly as possible until finished or 240 seconds have passed. The time, in seconds, required to finish the trial constitutes the participants score.
The Letter Fluency and Category Fluency trials, respectively, of the D-KEFS Verbal Fluency Test were also administered. On Letter Fluency, the participant is provided a phonemic cue (e.g., words beginning with a certain letter) and the participant has 60 seconds to produce as many words beginning with that letter with some exceptions (e.g., no names, proper nouns, or numbers). On Category Fluency, the participant is provided a semantic cue (e.g., animals) and given 60 seconds to produce as many words from that category as possible. Participants also completed the Category Switching trial of this subtest. Category Switching asks participants to switch back and forth between naming two separate semantic categories; participant scores were based on the number of correct items generated.
The Inhibition and Inhibition/Switching trials of the Color-Word Interference Test measured inhibition. On the Inhibition trial, the participant is presented with a page containing the words of various colors that are printed incongruently in a different color of ink. The participant is asked to say the color of the ink in which each word is printed as quickly as possible without making mistakes. The Inhibition/Switching trial is very similar to the Inhibition trial but half of the words are enclosed within a box. Participants are once again told to name the color of ink a word is printed in, except if it is printed in a box. If the word is printed in the box, the participants are asked to read the word (and not name the ink color). Performance is measured by completion time.
Demographics
CVD was coded as 0 = no CVD and 1 = CVD. Sex was used as a covariate in the model and coded as 0 = males and 1 = females.
Statistical Analyses
Data were analyzed using Mplus Version 7.31 (Muthén & Muthén, 2012). Missing data were tested and determined to be missing at random (MAR; Little & Rubin, 2002). Estimation of missing data were conducted using full information maximum likelihood (FIML). Structural equation modeling (SEM) was used to examine the indirect associations between CVD and six EF task outcomes via a CPS factor. Statistical fit criteria suggested by Hu and Bentler (1999) assessed model fit. Competing indirect effects were assessed with the product-of-coefficients (α*β) approach (Fritz & MacKinnon, 2007) using the bias-corrected bootstrap (10,000 replications) confidence intervals (Preacher & Hayes, 2008).
Results
Descriptive Analyses
Table 1 contains demographic and descriptive data. Table 2 summarizes the bivariate zero-order correlations among the modeled variables.
Table 1.
Demographic and Descriptive Information of Study Participants
| CVD Patients (n = 21) | Healthy Controls (n = 73) | ||
|---|---|---|---|
|
|
|||
| t | |||
| Age | 72.14(8.87) | 63.11(8.10) | 4.41*** |
| Years of Education | 15.71(2.61) | 15.81(1.95) | -0.18 |
| WTAR | 108.62(9.65) | 109.61(8.48) | -0.46 |
| χ2 | |||
| Ethnicity | 85% Caucasian | 98% Caucasian | 11.00* |
| Sex | 14 males | 23 males | 8.45** |
Note. Age-corrected standard scores used in analyses of the cognitive measures. WTAR = Wechsler Test of Adult Reading.
p < .05;
p < .01;
p < .001.
Table 2.
Bivariate Zero-Order Correlations of Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Coding | -- | |||||||||
| 2. Symbol Search | .59** | -- | ||||||||
| 3. Number-Letter Switching | .61** | .46** | -- | |||||||
| 4. Letter Fluency | .27** | .16 | .41** | -- | ||||||
| 5. Category Fluency | .41** | .20 | .47** | .64** | -- | |||||
| 6. Category Switching | .38** | .26* | .30** | .37** | .52** | -- | ||||
| 7. Inhibition | .49** | .46** | .49** | .43** | .41** | .42** | -- | |||
| 8. Inhibition/Switching | .44** | .32** | .41** | .39** | .41** | .39** | .70** | -- | ||
| 9. Age | -.24* | -.13 | -.17 | .03 | -.21 | -.15 | -.19 | -.26* | -- | |
| 10. Sex | .25* | .13 | .18 | .28** | .22* | .37** | .32** | .18 | -.09 | -- |
Note.
p < .05;
p < .01
Measurement Model
The latent factor of CPS consisted of two age-corrected CPS measures: 1) WAIS-III Symbol Search and 2) RBANS Coding. Loading factors were significant at the level p < .01, two tailed, and larger than .4. Within the overall structural model, fit was excellent: χ2(8) = 11.150, p = .193; CFI = 0.990; RMSEA = 0.065; SRMR = 0.041 (Hu & Bentler, 1999).
Cardiovascular Disease, Cognitive Processing Speed, and Executive Functioning
Figure 1 presents an SEM evaluating the direct associations between CVD, CPS, and the EF composite score. Sex was included as a covariate to control its variance. CVD was significantly, negatively related to CPS (β = -.239, 95% CI [-.457, -.021]). CPS was significantly, positively related to the EF composite score (β = .566, 95% CI [.368, .688]). CVD was significantly, negatively related to the EF composite score (β = -.137, 95% CI [-.084, -.211]). Next, we tested and found that the direct effect from CPS to the individual measures of the EF composite score were all significant (Table 3; Figure 2). The direct links from CVD to the individual measures of the EF composite were all nonsignificant (Table 3). The indirect links from CVD to the individual measures of the EF composite score were all statistically significant (Table 4).
Figure 1.
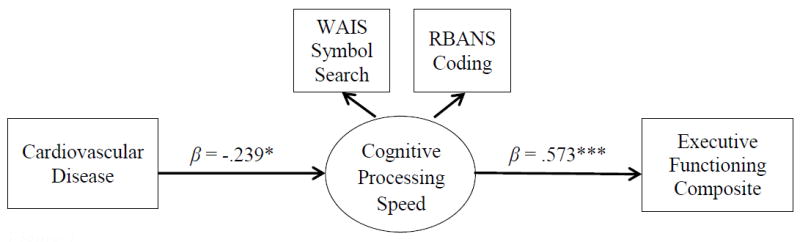
Direct paths between CVD, CPS, and the EF composite score. Sex entered as a covariate but removed for clarity. Standardized betas are presented. *p < .05; **p < .01; ***p < .001.
Table 3.
Parameter Estimates of Direct Effects
| Direct Associations | B (SE) | β | 95% CI | R2 |
|---|---|---|---|---|
| CVD → CPS | -.297 (.150) | -.239 | [-.457, -.021]* | .057 |
| CVD → EF composite | -.246 (.118) | -.137* | [-.084, -.211]* | |
| CVD → Number-Letter Switching | .096 (.221) | .044 | [-.142, .230] | |
| CVD → Letter Fluency | .263 (.312) | .092 | [-.116, .299] | |
| CVD → Category Fluency | -.048 (.257) | -.017 | [-.186, .152] | |
| CVD → Category Switching | -.193 (.245) | -.072 | [-.244, .101] | |
| CVD → Inhibition | .225 (.249) | .102 | [-.110, .313] | |
| CVD → Inhibition/Switching | .075 (.192) | .039 | [-.149, .227] | |
| CPS → EF composite | .867 (.183) | .566 | [.368, .688]*** | |
| CPS → Number-Letter Switching | 1.214 (.263) | .695 | [.519, .871]*** | .473 |
| CPS → Letter Fluency | .586 (.317) | .254 | [.031, .477]* | .119 |
| CPS → Category Fluency | .919 (.346) | .399 | [.189, .609]*** | .186 |
| CPS → Category Switching | .722 (.278) | .334 | [.124, .544]** | .231 |
| CPS → Inhibition | .980 (.239) | .549 | [.373, .725]*** | .341 |
| CPS → Inhibition/Switching | .744 (.206) | .481 | [.329, .632]*** | .235 |
Note. Model fit: χ2 (8) = 11.150, p = .193; CFI = 0.990; RMSEA = 0.065; SRMR = 0.041.
p <.05;
p < .01;
p < .001.
Figure 2.
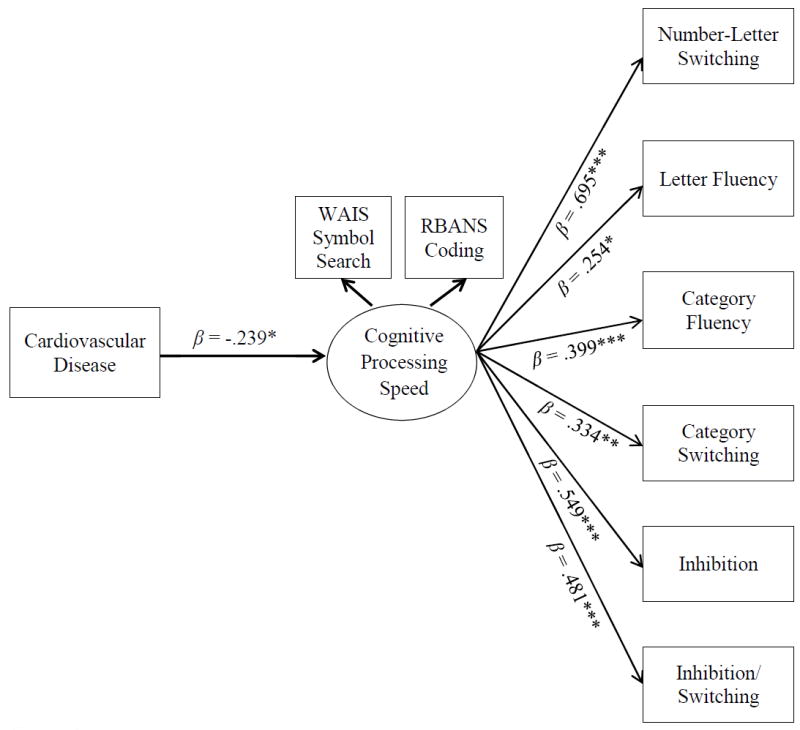
Direct paths between CVD, CPS, and constituent D-KEFS subtests of the EF composite score. Sex was entered as a covariate but removed from the figure for clarity. Indirect pathways are not shown for clarity. Standardized betas are presented. *p < .05; **p < .01; ***p < .001.
Table 4.
Parameter Estimates of Indirect Effects
| Indirect Associations | A*B (SE) | α*β | 95% CI |
|---|---|---|---|
| CVD → CPS → Number-Letter Switching | -.360 (.194) | -.166 | [-.825, -.029]* |
| CVD → CPS → Letter Fluency | -.174 (.120) | -.061 | [-.549, -.008]* |
| CVD → CPS → Category Fluency | -.273 (.150) | -.095 | [-.677, -.036]* |
| CVD → CPS → Category Switching | -.214 (.120) | -.080 | [-.558, -.031]* |
| CVD → CPS → Inhibition | -.291 (.156) | -.131 | [-.692, -.036]* |
| CVD → CPS → Inhibition/Switching | -.221 (.118) | -.115 | [-.533, -.026]* |
Note. Model fit: χ2 (8) = 11.548, p = .173; CFI = 0.989; RMSEA = 0.069; SRMR = 0.041
p < .05.
The indirect effects of CVD on each measure of EF via CPS were contrasted against one another to determine if significant differences existed. Table 5 contains the results of these analyses. Number-Letter Switching was more adversely affected by CVD via CPS than was Category Fluency and Category Switching. Letter Fluency was more adversely affected by CVD via CPS than was Inhibition and Inhibition/Switching. No other contrasts of differences in indirect effects were statistically significant.
Table 5.
Contrasts of Indirect Effects
| Contrast | β | 95% CI |
|---|---|---|
| 1 vs. 2 | -.069 | [-.288, .034] |
| 1 vs. 3 | -.140 | [-.417, -.007]* |
| 1 vs. 4 | -.186 | [-.585, -.005]* |
| 1 vs. 5 | -.087 | [-.441, .049] |
| 1 vs. 6 | -.146 | [-.517, .002] |
| 2 vs. 3 | -.070 | [-.266, .005] |
| 2 vs. 4 | -.117 | [-.451, .025] |
| 2 vs. 5 | -.913 | [-1.783, -.281]* |
| 2 vs. 6 | -.716 | [-1.434, -.159]* |
| 3 vs. 4 | -.047 | [-.304, .087] |
| 3 vs. 5 | .052 | [-.076, .307] |
| 3 vs. 6 | -.006 | [-.206, .120] |
| 4 vs. 5 | .099 | [-.005, .342] |
| 4 vs. 6 | .040 | [-.121, .304] |
| 5 vs. 6 | -.059 | [-.343, .066] |
Note. 1 = Number-Letter Switching; 2 = Letter Fluency; 3 = Category Fluency; 4 = Category Switching; 5 = Inhibition; 6 = Inhibition/Switching.
p < .05.
Discussion
The current study’s primary aim was to examine the role of CVD in a sample of older adult patients as it related to EF and the potential mediating effect of CPS. Specifically, in line with our hypotheses, we found that CVD patients underperformed on CPS and EF measures to a greater degree than did a demographically comparable sample of older adults without the CVD condition. Further, we found that reduced CPS was negatively linked to EF.
The current study contributes to the extant literature by extending the processing speed hypothesis in aging to encompass older adults with CVD, such that CPS deficits specific to this population are significantly associated with poorer EF. Consistent with our expectations and prior literature, the current results also show that CVD and EF are mediated by CPS. This finding held for a composite measure of EF as well as the individual EF measures from which it was comprised.
These findings replicate prior research suggesting that CVD is associated with worse EF outcomes (e.g., Moulaert, Verbunt, van Heugten, & Wade, 2009), potentially implicating cardiovascular health as a unique contributor to the aging-EF relationship. Similarly, present findings show that CVD is inversely related to CPS, such that individuals with CVD demonstrated poorer CPS than those without CVD. This is consistent with prior literature that shows that CVD is associated with declines in CPS (Cohen et al., 1999). Finally, reduced CPS significantly underlied the link between CVD status and poorer EF. These findings are consistent with prior research on non-patient samples that has shown age-related declines in CPS are associated with EF declines (Salthouse, 1996) and suggest that CVD significantly influences age-related declines in EF above and beyond normal aging without the presence of CVD.
The second aim of the current study was to test if CVD was indirectly associated with individual components of EF through the CPS mediator. This was done to determine whether or not the expected association between CVD with the EF composite score was driven by a single EF component. It was hypothesized that CVD would be inversely associated with each component of EF. As the EF composite consisted of six measures, we tested the indirect links from CVD to each of the EF sub-domains and all were significant. These results demonstrate that CVD has a negative influence on multiple components of EF (i.e., cognitive flexibility, inhibition, and phonemic and semantic fluency) and that CPS mediated each. Comparisons of the indirect effects of CVD on particular components of EF via CPS with one another showed that D-KEFS Number-Letter Switching and Letter Fluency were most adversely affected.
The contribution of this work to extant research is two-fold. First, it provides support for the processing speed hypothesis in an older adult population with CVD. Results implicate CVD as a potential mechanism of observed declines in CPS (Salthouse, 1996). Although previous studies of the effects of age on cognition have primarily focused on healthy aging, this study provides further support for the importance of the role of CPS in EF of older adults with CVD. Extending the processing speed hypothesis to CVD patients is particularly important given the large proportion of older adults with CVD and because this population is expected to grow in coming years.
Second, findings suggest that individuals with CVD would benefit from neuropsychological assessments that include CPS as a potential marker of accelerated cognitive decline in other domains. CPS assessments are brief and may efficiently and effectively indicate risk in CVD prior to the administration of a larger battery that includes EF measures. The evidence provided here suggests that CVD may be a marker for increased risk of declines in CPS, and subsequently, EF above and beyond typical aging. CVD may accelerate cognitive aging and may place individuals with a history of CVD at a higher risk of suffering from the resulting health and life disparities associated with declines in CPS and EF.
The specific neurobiological mechanisms of these effects need further study. Autoregulatory mechanisms are thought to protect the brain from systemic vascular dysfunction. Yet, there is increasing evidence that chronic CVD causes cerebrovascular dysfunction in the elderly, which ultimately leads to brain abnormalities (e.g., white matter hyperintensities) and cognitive impairment as observed on neuropsychological tests and magnetic resonance imaging (Aberg, 1974; Barclay, Weiss, Mattis, Bond, & Blass, 1988; Cohen & Gunstad, 2010; Cohen et al., 1999; Gunstad et al., 2005; Moser et al., 1999; Paul et al., 2005; Vogels et al., 2007). Cardio and cerebrovascular mechanisms of cognitive decline are likely exacerbated by CVD and lead to slowed CPS and subsequent EF declines. It is thought that reduced cardiac output and other systemic perfusion abnormalities contribute to these negative cognitive effects, but the process by which this occurs is not well understood (De Reuck, 1996; Lindsay, Herbert, & Rockwood, 1997; Petty, Parker, & Parker, 1992; Tatemichi et al., 1994). Findings from the current study show CVD affects cognitive functioning via CPS. It has been well-documented that white matter hyperintensities have strong effects on CPS and in CVD populations, and future research that investigates the role of brain abnormalities on the cognitive mechanisms observed in this study is warranted.
Acknowledgments
This work was supported by a grant from the National Institutes of Health Heart, Lung, and Blood Institute (L.H.S., R01 HL084178).
Footnotes
The authors do not report any conflicts of interest.
References
- Aberg T. Effect of open-heart surgery on intellectual function. Scandinavian Journal of Thoracic and Cardiovascular Surgery. 1974;15:1–63. [PubMed] [Google Scholar]
- Baltes MM, Wahl HW, Schmid-Furstoss The daily life of elderly Germans: Acitivity patterns, personal control, and functional health. Journal of Gerontology. 1990;45(4):173–179. doi: 10.1093/geronj/45.4.P173. [DOI] [PubMed] [Google Scholar]
- Barclay LL, Weiss EM, Mattis S, Bond O, Blass JP. Unrecognized cognitive impairment in cardiac rehabilitation patients. Journal of the American Geriatrics Society. 1988;36(1):22–28. doi: 10.1111/j.1532-5415.1988.tb03429.x. [DOI] [PubMed] [Google Scholar]
- Bastone EC, Kerns RD. Effects of self-efficacy and perceived social support on recovery-related behaviors after coronary artery bypass graft surgery. Annals of Behavioral Medicine. 1995;17(4):324–330. doi: 10.1007/BF02888597. [DOI] [PubMed] [Google Scholar]
- Birren JE, Fisher LM. Aging and speed of behavior: Possible consequences for psychological functioning. Annual Review of Psychology. 1995;46:329–353. doi: 10.1146/annurev.ps.46.020195.001553. [DOI] [PubMed] [Google Scholar]
- Bloom DE. 7 billion and counting. Science. 2011;333(6042):562–569. doi: 10.1126/science.1209290. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. The state of aging and health in America. Atlanta, GA: Centers for Disease Control and Prevention, US Dept. of Health and Human Services; 2013. [Google Scholar]
- Cohen RA, Gunstad J. Neuropsychology and cardiovascular disease. Oxford: Oxford University Press; 2010. [Google Scholar]
- Cohen RA, Moser DJ, Clark MM, Aloia MS, Cargill BR, Stefanik S, Forman DE, et al. Neurocognitive functioning and improvement in quality of life following participation in cardiac rehabilitation. American Journal of Cardiology. 1999;83(9):1374–1378. doi: 10.1016/S0002-9149(99)001034. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Paul RH, Ott BR, Moser DJ, Zawacki TM, Stone W, Gordon N. The relationship of subcortical MRI hyperintensities and brain volume to cognitive function in vascular dementia. Journal of the International Neuropsychological Society. 2002;8:743–752. doi: 10.1017/S1355617702860027. [DOI] [PubMed] [Google Scholar]
- Cohen JE. Human population: The next half century. Science. 2003;302(5648):1172–1175. doi: 10.1123/science.1088665. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Depp CA, Jeste DV. Definitions and predictors of successful aging: A comprehensive review of larger quantitative studies. American Journal of Geriatric Psychiatry. 2006;14(1):6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- De Reuck JL. Evidence for chronic ischaemia in the pathogenesis of vascular dementia: From neuroPATH to neuroPET. Acta Neurologica Belgica. 1996;96(3):228–231. [PubMed] [Google Scholar]
- DeRight J, Jorgensen RS, Cabral MJ. Composite cardiovascular risk scores and neuropsychological functioning: A meta-analytic review. Annals of Behavioral Medicine. 2015;49(3):344–357. doi: 10.1007/s12160-014-9681. [DOI] [PubMed] [Google Scholar]
- Duering M, Gesierich B, Seiler S, Pirpamer L, Gonik M, Hofer E, Dichgans M, et al. Strategic white matter tracts for processing speed deficits in age-related small vessel disease. Neurology. 2014;82(22):1946–1950. doi: 10.1212/WNL.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA. Slowing down: Age-related neurobiological predictors of processing speed. Frontiers in Neuroscience. 2011;5(25) doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22(3):558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiology of Aging. 2010;31(3):512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, MacGregor KL, Paul RH, Poppas A, Jefferson AL, Todaro JF, Cohen RA. Cardiac rehabilitation improves cognitive performance in older adults with cardiovascular disease. Journal of Cardiopulmonary Rehabilitation. 2005;25(3):173–176. doi: 10.1097/00008483-200505000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Selby JV, Quesenberry CP, Jr, Schmittdiel JA, Fireman BH, Rice DP. The impact of aging and chronic disease on use of hospital and outpatient services in large HMO 1971-1991. Journal of the American Geriatrics Society. 1997;45(6):667–674. doi: 10.1111/j.1532-5415.1997.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of Aging. 2007;28(3):477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation. 2003a;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation. 2003b;107:346–354. doi: 10.1161/01.CIR.0000048893.62841.F7. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 5. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Lindsay J, Hérbert R, Rockwood K. The Canadian Study of Health and Aging: Risk factors for vascular dementia. Stroke. 1997;28(3):526–530. doi: 10.1161/01.STR.28.3.526. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. 2. Hoboken, NJ: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- Lutz W, Sanderson W, Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451(7179):716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- Mangano DT. Cardiovascular morbidity and CABG surgery—A perspective: Epidemiology, costs, and potential therapeutic solutions. Journal of Cardiac Surgery. 1995;10(S4):366–368. doi: 10.1111/j.1540-8191.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Miller LW, Missov ED. Epidemiology of heart failure. Cardiology Clinics. 2001;19(4):547–555. doi: 10.1016/S0733-8651(05)702423. [DOI] [PubMed] [Google Scholar]
- Moser DJ, Cohen RA, Clark MM, Aloia MS, Tate B, Stefanik S, Forman DE, Tilkemeier PL. Neuropsychological functioning among cardiac rehabilitation patients. Journal of Cardiopulmonary Rehabilitation. 1999;19(2):91–97. doi: 10.1097/00008483-199903000-00002. [DOI] [PubMed] [Google Scholar]
- Moulaert VRMP, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: A systematic review. Resuscitation. 2009;80(3):297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Turner MB, et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus 7.1 (Computer Program) Version 7.1. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Nordlund A, Rolstad S, Klang O, Lind K, Hansen S, Wallin A. Cognitive profiles of mild cognitive impairment with and without vascular disease. Neuropsychology. 2007;21(6):706–712. doi: 10.1037/0894-4105.21.6.706. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: A clinical perspective. Journal of the American College of Cardiology. 2007;50(1):1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- Paul RH, Gunstad J, Poppas A, Tate DF, Foreman D, Brickman AM, Cohen RA, et al. Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovascular Disease. 2005;20(2):129–133. doi: 10.1159/000086803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty LA, Parker JR, Parker JC., Jr Hypertension and vascular dementia. Annals of Clinical & Laboratory Science. 1992;22(1):34–39. [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Reichstadt J, Depp CA, Palinkas LA, Folsom DP, Jeste DV. Building blocks of successful aging: A focus group study of older adults’ perceived contributors to successful aging. American Journal of Geriatric Psychiatry. 2007;15(3):194–201. doi: 10.1097/JGP.0b013e318030255f. [DOI] [PubMed] [Google Scholar]
- Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. Journal of the American Geriatrics Society. 1997;45(8):968–974. doi: 10.1111/j.1532-5415.1997.tb02968.x. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. The Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Royall DR, Chiodo LK, Polk MJ. Correlates of disability among elderly retirees with “subclinical” cognitive impairment. Journal of Gerontology: MEDICAL SCIENCES. 2000;55A(9):M541–M546. doi: 10.1093/gerona/55.9.M541. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37(2):207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Coon VE. Influence of task-specific processing speed on age differences in memory. Journal of Gerontology. 1993;48(5):245–255. doi: 10.1093/geronj/48.5.p245. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Pyschology and Aging. 2003;18(1):91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Smith SM, Mensah GA. Population aging and implication for epidemic cardiovascular disease in Sub-Saharan Africa. Ethnicity & Disease. 2003;13(S2):S77–80. [PubMed] [Google Scholar]
- Tam HMK, Lam CLM, Huang H, Wang B, Lee TMC. Age-related difference in relationships between cognitive processing speed and general cognitive status. Applied Neuropsychology: Adult. 2015;22(2):94–99. doi: 10.1080/23279095.2013.860602. [DOI] [PubMed] [Google Scholar]
- Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: Frequency, patterns, and relationship to functional abilities. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57(2):202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: A longitudinal examination of age-associated declines in reasoning and processing speed. Developmental Psychology. 2009;45(2):431–446. doi: 10.1037/a0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo J, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2012 Revision, Highlights and Advance Tables (Working Paper No ESA/P/WP.228) New York, NY: 2013. [Google Scholar]
- van Buchem M, Biessels G, Brunner la Rocca H, De Craen A, Van der Flier W, Ikram MA, Daemen MJ, et al. The heart-brain connection: A multidisciplinary approach targeting a missing link in the pathophysiology of vascular cognitive impairment. Journal of Alzheimer’s Disease. 2014;42(S4):443–451. doi: 10.3233/JAD-141542. [DOI] [PubMed] [Google Scholar]
- Vogels RLC, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. European Journal of Heart Failure. 2007;9(10):1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition. New York, NY: Pearson; 1997. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. New York, NY: Pearson; 2001. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Fourth Edition. New York, NY: Pearson; 2008. [Google Scholar]
- Whiting WL, IV, Smith AD. Differential age-related processing limitation in recall and recognition tasks. Psychology and Aging. 1997;12(2):216–224. doi: 10.1037/0882-7974.12.2.216. [DOI] [PubMed] [Google Scholar]


