Abstract
Introduction
The physiologic vitamin D (VD), 1α,25(OH)2 D3 (1,25D) is a local paracrine/autocrine effecter of fetal lung maturation. By stimulating alveolar type II cell and lipofibroblast proliferation and differentiation, parenterally administered 1,25D has been shown to enhance neonatal lung maturation; but due to the potential systemic side effects of the parenteral route, the translational value of these findings might be limited. To minimize the possibility of systemic toxicity, we examined the effects of VD on neonatal lung maturation, when delivered directly to lungs via nebulization.
Methods
One-day-old rat pups were administered three different doses of 1,25D and its physiologic precursor 25(OH)D (25D), or the diluent, via nebulization daily for 14 days. Pups were sacrificed for lung, kidneys, and blood collection to determine markers of lung maturation, and serum 25D and calcium levels.
Results
Compared to controls, nebulized 25D and 1,25D enhanced lung maturation as evidenced by the increased expression of markers of alveolar epithelial (SP-B, leptin receptor), mesenchymal (PPARγ, C/EBPα), and endothelial (VEGF, FLK-1) differentiation, surfactant phospholipid synthesis, and lung morphology without any significant increases in serum 25D and calcium levels.
Conclusions
Inhaled VD is a potentially safe and effective novel strategy to enhance neonatal lung maturation.
Keywords: Prematurity, Childhood asthma, Lung development, Bronchopulmonary dysplasia, Active vitamin D
Introduction
Despite all advances, chronic lung disease of prematurity also known as bronchopulmonary dysplasia (BPD), characterized by the disruption in normal lung development, remains common [1]. In the last 2 decades, with the improved survival of extremely premature infants, BPD prevalence has actually increased ranging between 26 and 30 % [2]. Although multiple risk factors such as inflammation, exposure to hyperoxia, and volutrauma have been implicated in pathogenesis, immature lung due to prematurity provides the platform for the development of BPD. Although antenatal steroids have been used for decades to enhance fetal lung maturation, they do not prevent BPD; overall, there is no clinically effective safe postnatal intervention that enhances lung maturation.
1α,25(OH)2D3 (1,25D), the physiologically active form of vitamin D (VD), has been shown to be a critically important local paracrine/autocrine factor that affects lung maturation by stimulating surfactant protein and phospholipid synthesis and by modulating alveolar type II (ATII) cell and lipofibroblast (LIF) proliferation and apoptosis [3–7]. Fetal rat lung fibroblasts produce 1,25D, with the production rate peaking at 20–21 days of rat gestation [6]. Vitamin D receptor (VDR) is expressed on ATII cells at a time corresponding to the start of ATII cell differentiation and surfactant secretion, and its expression increases with maturation [4]. 1,25D and its metabolites, in particular, 3-epi-1,25D, have been shown to stimulate ATII cell and LIF proliferation and differentiation, possibly, by enhancing alveolar epithelial–mesenchymal interactions [3].
There is high prevalence of VD deficiency among women of child-bearing age and during pregnancy [8]. Although the evidence remains somewhat controversial, based on several epidemiological studies, it is likely that VD deficiency increases the risk of childhood asthma and wheezing [9, 10], which are also potential long-term respiratory morbidities among BPD survivors even into their adulthood [11]. Moreover, a higher maternal VD intake during pregnancy or in early life has been shown to correlate inversely with childhood asthma [12]. Importantly, using a rodent model, we have recently mechanistically linked perinatal VD deficiency to increased airway hyper-responsiveness, and we showed that optimal VD supplementation prevented VD deficiency-associated childhood asthma phenotype [13].
Although parenterally administered physiologic VD steroid hormone 1,25D accelerates lung maturation, due to the potential systemic side effects such as hypercalcemia as seen with both parenteral and oral forms [14], and the invasive approach, i.e., intraperitoneal approach, used in previous studies, the translational value of these findings remains somewhat limited. To circumvent the possibility of systemic side effects, in this study, using a neonatal rat model, we examined if inhaled VD could effectively and safely enhance lung maturation.
We hypothesized that VD delivered directly to lungs via nebulization enhances neonatal lung maturation and avoids potential systemic side effects. Specifically, we determined if nebulized VD, both in its active (1,25D) and circulating inactive-25(OH) D (25D) forms, enhances neonatal lung maturation without increasing serum VD (25D) and calcium levels.
Materials and Methods
All animal studies were performed in accordance with the National Institutes of Health guidelines and approved by the Los Angeles Biomedical Research Institute Animal Care and Use Committee. 25D (Catalog # 17938) and 1,25D (Catalog # 1530) were purchased from Sigma-Aldrich (St. Louis, MO).
Animal Protocol and Study Design
Pregnant embryonic day (E) 19 Sprague–Dawley rat dams were obtained from Charles River Laboratories (Hollister, CA) and were allowed to deliver spontaneously at term; pups breast feed ad libitum. On postnatal day 1, pups were divided into 3 main groups: (1) Control, administered diluent in 1 ml saline via nebulization over 30 min; (2) 25D, further subdivided into 100, 500, or 1000 ng/kg groups; and (3) 1,25D, subdivided into 1, 10, or 50 ng/kg groups. 25D and 1,25D were administered in 1 ml saline via nebulization over 30 min. Starting on postnatal day 1, pups were exposed to the assigned treatments once a day for 14 days, and nebulization was performed in an enclosed plastic container (10.5 × 6.5 × 4.5 inches) using Lincare nebulizer (Pari Respiratory Equipment, Inc., Midlothian, VA). Pups were killed on postnatal day 14. Upon death, lungs, kidneys, and blood were collected for subsequent analysis.
Serum 25D and Calcium Levels
Serum samples were kept frozen at −70 °C until analysis, when samples were initially moved to −25 °C for 6 h and then thawed at 4 °C until complete dissolution was achieved. Serum calcium levels were measured by a colorimetric assay (BioVision, K380-250; sensitivity 0.4–100 ng/ml) using manufacturer’s instructions. Serum 25D levels were measured by ELISA (EagleBioscience, VID31-K01, sensitivity 1.6 ng/ml) using manufacturer’s protocol.
Lung Morphology
Hematoxylin and eosin-stained lung sections were examined by a blinded investigator to determine radial alveolar counts and mean liner intercept using the previously described methods [13, 15]. Briefly, randomly selected nonoverlapping fields from sections obtained from similar regions of each lung for each treatment group were examined. Each field was viewed at 200-fold magnification, scanned with a digital camera, and projected on a video monitor. For each field, the number of alveoli was counted visually and expressed as per square millimeter [16].
Choline Incorporation
Choline incorporation in saturated phosphatidylcholine assay. [3H]choline (NEN Dupont, Boston, MA) incorporation into saturated phosphatidylcholine, which is the major surface-active lipid substrate of surfactant, responsible for maintaining the stability of the alveoli, was determined in cultured lung explants by following the previously described protocol [16].
Triglyceride Uptake Assay
Triolein uptake, a key marker of alveolar LIF function, was used to quantitate triglyceride uptake by fetal rat lung explants using the previously described protocol [16]. Briefly, lung explants were cultured in Waymouth’s medium, which at the beginning of the triglyceride uptake assay was replaced with DMEM containing 20 % adult rat serum mixed with [3H] triolein (5 μCi/ml). The explants were incubated in 5 % CO2–air balance for 4 h at 37 °C. At the termination of the incubation, the medium was decanted, the explants were rinsed two times with 1 ml of ice-cold PBS, and the tissue was thoroughly homogenized. An aliquot of the tissue homogenate was taken for protein assay, and the remaining tissue homogenate was extracted to determine its neutral lipid content.
Western Analysis
The isolated lungs were flash-frozen in liquid nitrogen and then homogenized and sonicated in four volumes of ice-cold lysis buffer containing 50 mM β-glycerophosphate (pH 7.4), 150 mM NaCl, 1.5 mM EGTA, 1 mM EDTA, 1 % Triton X-100, 100 mM NaF, 2 mM Na3VO4, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 2 μg/ml pepstatin A. After centrifugation at 13,200 g for 15 min at 4 °C, the supernatant was used for Western blot analysis to determine protein levels of VDR; epithelial differentiation markers (surfactant protein) SP-B and leptin receptor; mesenchymal differentiation markers peroxisome proliferator-activated receptor (PPAR)-γ, CCAAT/enhancer-binding protein (C/EBP) α; and endothelial differentiation markers VEGF and its receptor FLK-1. The total protein concentration of the supernatant was measured by the Bradford method, using bovine serum albumin as the protein standard. Aliquots of the supernatant, each containing 30 μg of protein, were separated by SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding sites were blocked with Tris-buffered saline (TBS) containing 5 % nonfat dry powdered milk (wt/vol) for 1 h at room temperature. After a brief rinse with TBS containing 0.1 % Tween 20 (TBST), the protein blots were incubated in 1:250 diluted anti-VDR antibody (sc-1008), 1:500 diluted anti-PPARγ antibody (sc-7196), 1:200 diluted anti-SP-B antibody (sc-13978), 1:200 diluted anti-C/EBPα antibody (sc-61), 1:200 diluted anti-leptin receptor antibody (sc-8325), 1:250 diluted anti-VEGF antibody (sc-507), 1:200 diluted anti-FLK-1 antibody (sc-6251; all of them from Santa Cruz), and 1:10,000 diluted anti-GAPDH monoclonal antibody (MAB-374; Millipore) overnight at 4 °C. After three washes in TBST, the protein blots were exposed to appropriate secondary antibody for 1 h at room temperature. And then following three further washes in TBST, the blots were exposed to X-ray film using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL) and developed. The relative densities of the protein bands were determined with UNSCAN-IT software (Silk Scientific, Orem, Utah) and normalized to that of GAPDH. VDR and PPARγ protein levels were also measured in kidney, a major extrapulmonary VDR-and PPARγ-expressing organ for possible systemic effects [17].
Immunohistochemistry
For tissue immunofluorescence staining for the relevant proteins, rat lungs were inflated in situ with 4 % paraformaldehyde in phosphate buffer at a standard inflation pressure of 20 cm H2O for 4 h at 4 °C. The lungs were subsequently transferred to PBS containing 30 % sucrose (wt/vol) until equilibrated in the cold (4 °C). After fixation, 5-μm paraffin sections were treated three times with Histo-Clear (National Diagnostics, Atlanta, GA) for 5 min and then rehydrated by a sequential ethanol wash. Sections were then washed two times for 10 min with PBS and blocked for 1 h in PBS–5 % normal goat serum-0.2 % Triton X-100. Sections were incubated with primary antibodies for 1 h at room temperature and then with the appropriate secondary antibody for 1 h, also at room temperature. Antibodies included (for all antibodies, source was the same as for Western blot analysis except for p180, which was from MMS-645R, Covance) VDR (primary antibody 1:100; secondary antibody 1:100), PPARγ (primary antibody 1:100; secondary antibody 1:150), SP-B (primary antibody 1:100; secondary antibody 1:100), p180 (primary antibody 1:350; secondary antibody 1:200), VEGF (primary antibody 1:100; secondary antibody 1:100), and FLK-1 (primary antibody 1:100; secondary antibody 1:100). Sections were washed with PBS and then mounted on glass slides with ProLong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA) for visualization under a fluorescence microscope.
Statistical Analysis
All data values were expressed as mean ± SE, and a p < 0.05 was considered to represent statistically significant difference between the control and experimental groups.
Results
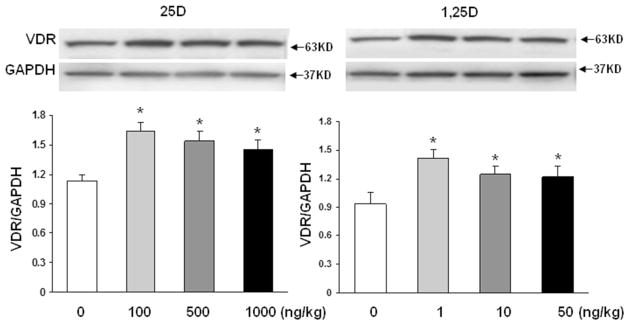
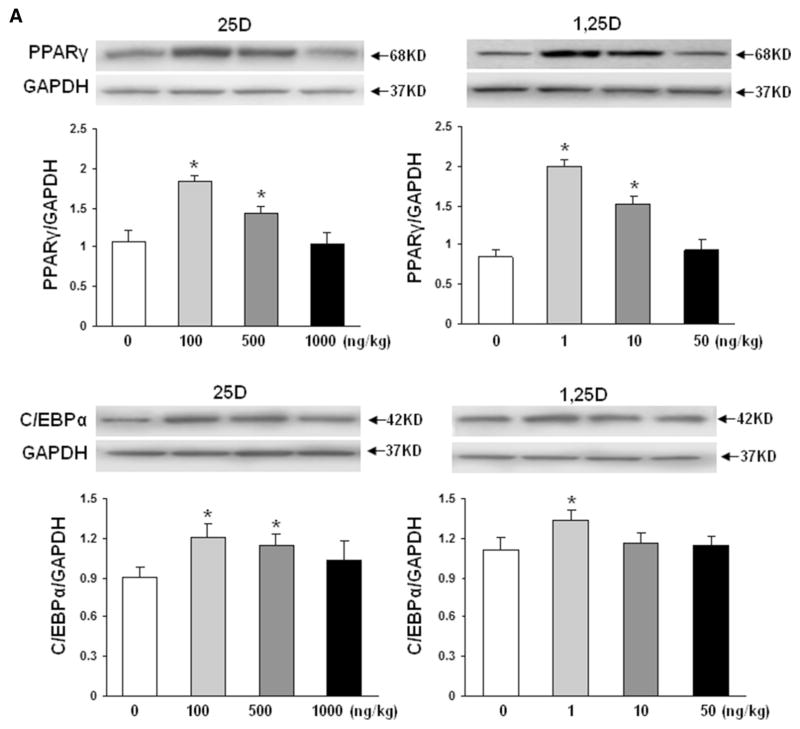
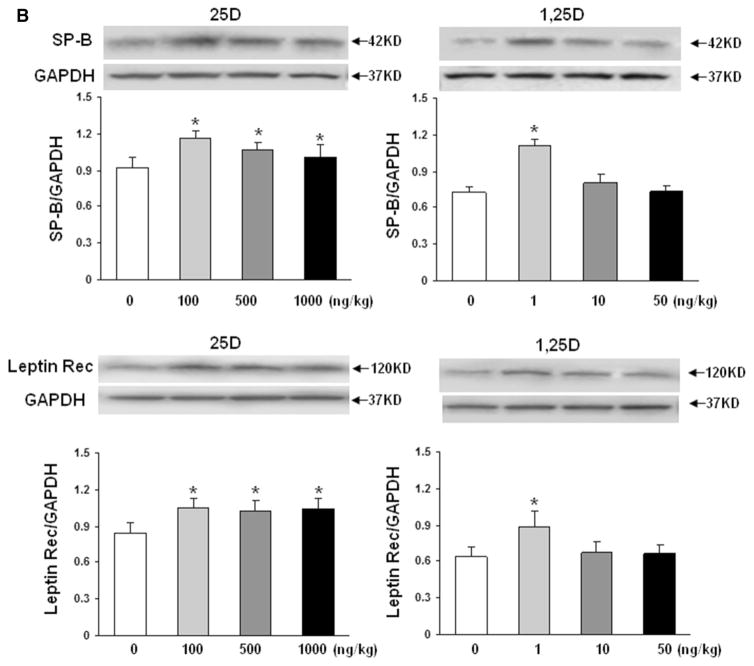
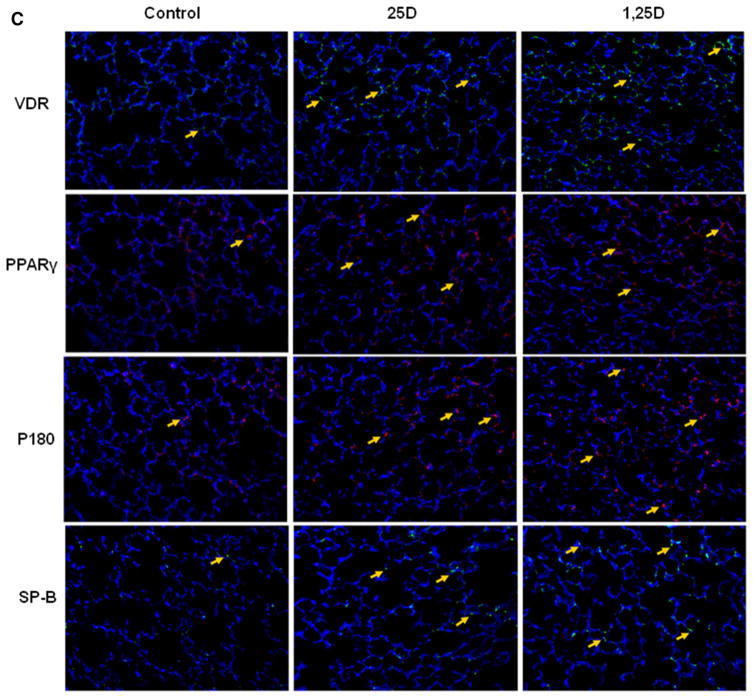
To confirm the validity of the proposed paradigm, we initially examined the effect of nebulized 25D and 1,25D on lung VDR protein levels (Fig. 1). Compared to controls, 2 weeks of once daily nebulized 25D (100, 500 and 1000 ng/kg) and 1,25D (1, 10, and 50 ng/kg) administrations, from postnatal day 1 to 14, resulted in significantly increased VDR protein levels (n = 4). Interestingly, the magnitude of this effect was similar with 25D and 1,25D, and all doses examined. Determination of key mesenchymal markers (Fig. 2a) of alveolar differentiation indicated that after 2 weeks of daily administration of various doses of 25D and 1,25D resulted in significantly increased protein levels of PPARγ and C/EBPα, with maximal increases seen with 100 ng/kg of 25D and 1 ng/kg of 1,25D for both markers. Similarly, compared to controls, nebulized 25D and 1,25D resulted in increased ATII cell differentiation markers (Fig. 2b), surfactant protein B (SP-B), and leptin receptor, again, with maximal increases seen with 100 ng/kg of 25D and 1 ng/kg of 1,25D. Surfactant protein C was studied, but no difference was seen between the control-and VD-treated groups (data not shown). The effect of VD on VDR and mesenchymal and epithelial differentiation markers (Fig. 2c) was corroborated by immunofluorescence staining for VDR, and the relevant mesenchymal (PPARγ) and epithelial (p180 and SP-B) differentiation markers, which showed increased expression in both 25D and 1,25D groups compared to the control group.
Fig. 1.
Effect of nebulized 25D and 1,25D on whole-lung VDR level. 2 weeks of daily administration of different doses of 25D and 1,25D from postnatal day 0 to 14 resulted in significantly increased expression of VDR dose-dependently; *p < 0.05 versus control; n = 4
Fig. 2.
Effect of nebulized 25D and 1,25D on lipofibroblast (a) and alveolar epithelial type II cell (b) differentiation markers. 2 weeks of daily administration of different doses of 25D and 1,25D from postnatal day 0 to 14 resulted in significantly increased protein levels of PPARγ and C/EBPα (a), with maximal increases seen with 100 ng/kg of 25D and 1 ng/kg of 1,25D. *p < 0.05 versus control. n = 4. Similarly, SP-B and leptin receptor (b), two key ATII cell differentiation markers, protein levels increased with 100 ng/kg of 25D and 1 ng/kg of 1,25D. *p < 0.05 versus control. n = 4. Effect of nebulized 25D and 1,25D on key alveolar differentiation markers was also confirmed by immunofluorescence staining of the 25D- and 1,25D-exposed lung sections (c). Compared to the controls, significantly increased expression of VDR, lung mesenchymal differentiation marker PPARγ, and lung epithelial differentiation markers p180 and SP-B were seen with both 25D and 1,25D. n = 4
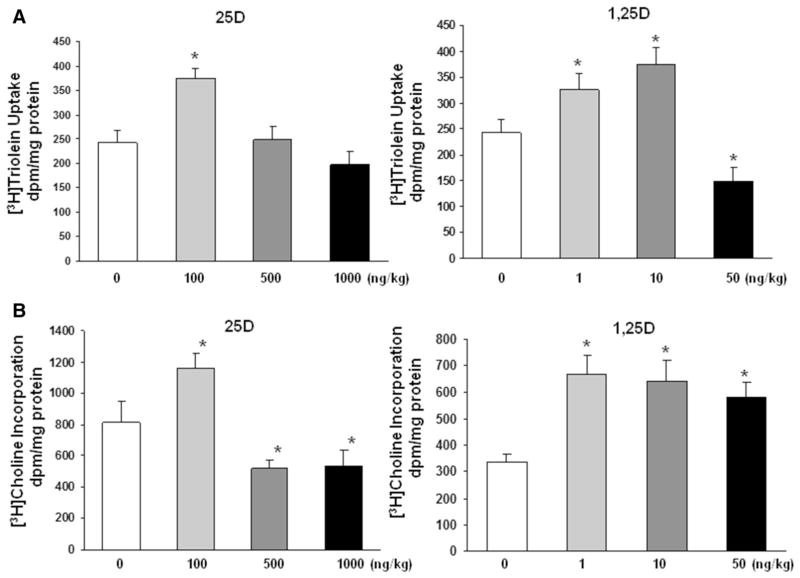
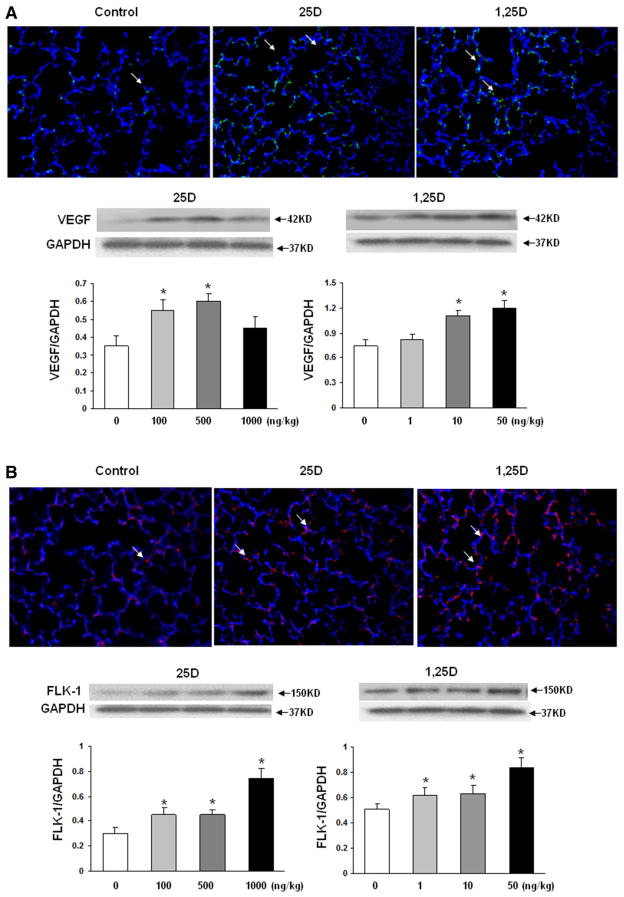
To further confirm the effect of nebulized 25D and 1,25D on lung maturation, surfactant phospholipid synthesis was assessed by determining triglyceride uptake and de novo surfactant phospholipid synthesis by lung explants, based on triolein uptake (Fig. 3a) and choline incorporation (Fig. 3b) into saturated phosphatidylcholine, respectively. Triolein uptake increased significantly with 100 ng/kg of 25D and, 1 and 10 ng/kg of 1,25D, whereas choline incorporation increased significantly only with 100 ng/kg of 25D, while higher doses, i.e., 500 and 1000 ng/kg of 25D, decreased it significantly. In contrast, all 3 doses of 1,25D increased choline incorporation into saturated phosphatidylcholine. Since vascular maturation is a critical component of alveolar differentiation, next, the effect of nebulized VD on endothelial differentiation marker VEGF and its kinase insert domain receptor (FLK-1) was determined. Nebulized 25D and 1,25D significantly increased VEGF (Fig. 4a) and FLK 1 (Fig. 4b) protein levels. In general, all doses of 25D and 1,25D studied increased VEGF and FLK-1 protein levels.
Fig. 3.
Effect of nebulized 25D and 1,25D on surfactant phospholipid synthesis. Triglyceride uptake and de novo surfactant phospholipid synthesis by cultured lung explants, based on triolein uptake (a) and choline incorporation (b) into saturated phosphatidylcholine, respectively, are shown. Triolein uptake showed significant increase with 100 ng/kg of 25D and 1 and 10 ng/kg of 1,25D, and a significant decrease with 50 ng/kg of 1,25D. Choline incorporation increased significantly with 100 ng/kg of 25D and decreased significantly with 500 and 1000 ng/kg of 25D; in contrast, it increased significantly with all doses of 1,25D studied. *p < 0.05 versus control for both. n = 6
Fig. 4.
Effect of nebulized 25D and 1,25D on endothelial cell differentiation markers. 2 weeks of daily administration of different doses of 25D and 1,25D from postnatal day 0 to 14 resulted in significantly increased expression of VEGF (a) and FLK 1 (b) with all doses of 25D and 1,25D examined, except with 1000 ng/kg of 25D and 1 ng/kg of 1,25D. *p < 0.05 versus control (lower panels). Compared to the controls, increased expression of VEGF and FLK-1, with both 25D and 1,25D, was confirmed with immunofluorescence (representative images are shown in the top panels); n = 4
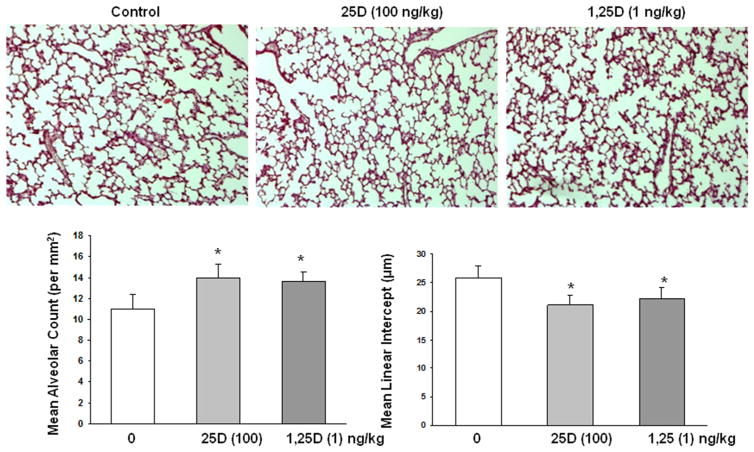
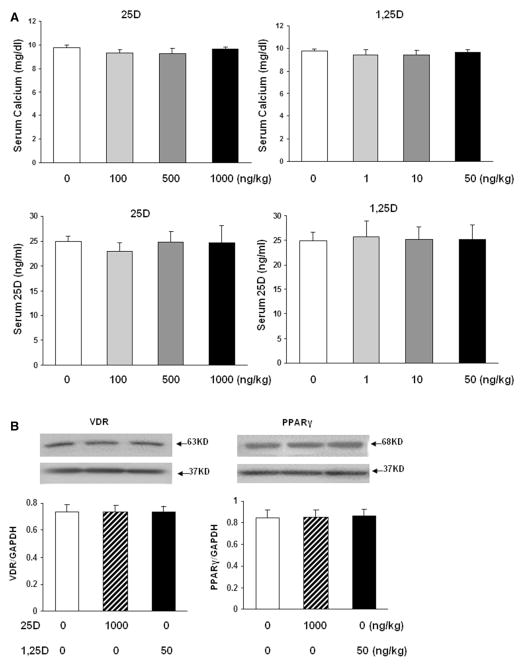
Furthermore, determination of the effects of nebulized 25D and 1,25D on lung morphometry showed increased alveolarization, as indicated by increased alveolar counts and corresponding decrease in mean linear intercepts (Fig. 5). To assess the possibility of potential systemic side effects of VD inhalation, we determined serum calcium and 25D levels (Fig. 6). Overall, there was no effect of 25D or 1,25D inhalation on either serum calcium or 25D (Fig. 6a) levels. Lastly, as a proof of principle, to further demonstrate the absence of any other systemic side effects of inhaled VD, VDR and PPARγ expressions in kidney, which is a major extrapulmonary VDR- and PPARγ-expressing organ, was determined. Compared to the controls, there was no effect on protein levels of either VDR or PPARγ, even with the highest nebulized doses of 25D (1000 ng/kg) and 1,25D (50 ng/kg) used in these studies (Fig. 6b).
Fig. 5.
Effect of nebulized 25D and 1,25D on lung morphometry. 2 weeks of daily administration of 25D (100 ng/kg) and 1,25D (1 ng/kg) from postnatal day 0 to 14 resulted in increased alveolarization, as determined by the alveolar count and mean linear intercept; mean alveolar count increased significantly (11.2 ± 1.1, 14.1 ± 1.3, 13.7 ± 0.8; control, 25D, and 1,25D groups, respectively under ×10 magnification: *p < 0.05 vs. control for both 25D and 1,25D groups), while mean linear intercept decreased significantly (25.9 ± 2, 21 ± 1.8, 22 ± 1.9 μm; control, 25D, and 1,25D groups, respectively under ×10 magnification: *p < 0.05 vs. control for both 25D and 1,25D groups). n = 6
Fig. 6.
Assessment of potential systemic side effects of vitamin D inhalation. Effect of nebulized 25D and 1,25D on serum calcium and 25D levels (a) and on VDR and PPARγ protein levels in kidney (b). 2 weeks of daily administration of 25D or 1,25D from postnatal day 0 to 14 had no effect on both serum calcium and 25D (a) levels. n = 4. Similarly, 2 weeks of daily administration of nebulized 25D (1000 ng/kg) or 1,25D (50 ng/kg) from postnatal day 0 to 14 had no effect on whole-kidney VDR or PPARγ protein levels. n = 3
Discussion
Vitamin D has been convincingly shown to modulate rodent lung development [3–7]. We previously showed that by effecting LIF and ATII cell differentiation and proliferation/apoptosis, intraperitoneal administration of VD stimulates perinatal lung maturation in rat. To further explore the translational opportunities of this finding, in the present study, using a neonatal rat model, we determined whether VD, delivered via nebulization, in either its active hormonal (1,25D) or inactive precursor (25D) form, enhances lung maturation without increasing potential systemic side effects, e.g., hypercalcemia with its associated potential cardiac (arrhythmias), renal (nephrocalcinosis), metabolic (hypophosphatemia, hypomagnesemia), gastrointestinal (decreased gut motility), and central nervous system complications, seen with both parenteral and oral forms [14]. We found that both 25D and 1,25D, when administered via inhalation, once daily, from postnatal day 1 to 14, enhanced rat lung maturation, as determined by increased protein levels of alveolar epithelial and mesenchymal (including endothelial) differentiation markers. This was accompanied by increased surfactant phospholipid production, as well as enhanced alveologenesis. Neither nebulized 25D nor 1,25D affected serum calcium or 25D levels, or expression of target genes in an extrapulmonary VDR- and PPARγ-expressing organ such as the kidney [17]. In general, among the various doses and preparations examined, nebulized 100 ng/kg of 25D and 1 ng/kg of 1,25D were most effective in stimulating lung maturation. These data for the first time show enhanced postnatal lung maturation using inhaled VD.
Extending the previous findings by our group of enhanced postnatal lung maturation with intraperitoneally administered VD [3], this study specifically demonstrates the lung maturational effect of nebulized VD; enhanced LIF differentiation, indicated by increased protein levels of PPARγ and C/EBPα, and increased triglyceride uptake; enhanced ATII cell differentiation, indicated by increased SP-B, leptin receptor, and p180 levels; and enhanced endothelial differentiation, indicated by increased VEGF and FLK-1 levels. Increased surfactant production is indicated by increased surfactant phospholipid synthesis and triglyceride uptake; however, these effects were dose-dependent; a significant increase in triglyceride uptake was seen with 100 ng/kg of 25D, and 1 and 10 ng/kg of 1,25D. Choline incorporation into saturated phosphatidylcholine, a marker of surfactant phospholipid synthesis, showed significant increase with all doses of 1,25D examined, but only with 100 ng/kg of 25D, with a significant decrease seen with 500 and 1000 ng/kg of 25D, similar to what we have previously reported in an in vivo model of perinatal VD deficiency-associated asthma [13]. Of note, although VD’s effects on alveolar epithelial cells and fibroblasts in the developing lung are relatively well reported, there is limited information on its effects on the pulmonary endothelial cells. However, in line with our finding of increased lung VEGF and FLK-1 protein levels with nebulized VD, 1,25D has been previously shown to up-regulate VEGF expression in cultured human umbilical cord vein endothelial cells [18], increase vascular smooth muscle proliferation via VEGF-mediated pathway [19], decrease the risk of preeclampsia in pregnancy by up-regulating VEGF [20], and increase survival, oxygenation, and alveolarization in a neonatal LPS-induced lung injury model, again via VEGF-mediated pathway [21]. In general, comparable stimulation of lung maturation with relatively lower doses of 1,25D versus 25D is not unexpected since it is the active hormonal form of VD, whereas 25D needs to be metabolized (likely locally) to active 1,25D form before it can stimulate lung maturation by targeting VD-responsive genes.
Recent observational studies have shown a high prevalence of maternal VD deficiency and its positive correlation with infant’s VD levels [22–24]. Although data are controversial, experimental animal and epidemiological human studies suggest an association between maternal VD deficiency and the development of childhood asthma and other common respiratory morbidities, e.g., respiratory infections [25–27]. This is not unexpected since many animal studies suggest a role of VD in cellular growth, proliferation, and differentiation, including it effects during various stages of rodent lung development [3, 6, 28, 29]; consequently, VD deficiency has been suggested to predispose to neonatal lung morbidity [29]. Since prior work using systemic VD administration during the antenatal, perinatal, and postnatal periods showed improved lung maturation, structure, and function [3, 4, 6, 21, 30–32], it is not surprising that nebulized VD also showed similar effects.
The absence of any effect of inhaled VD on serum calcium and 25D levels as well as on the expression of target genes in extrapulmonary VD-expressing organ—kidney—reassuringly indicates that therapeutically effective doses of VD can be delivered by inhalation without any likely systemic side effects. This is particularly relevant since there is accumulating recent evidence that systemic VD levels and their effects following orally or parenterally administered VD are highly genotype-dependent, i.e., regulated by variations in the expressions of multiple VD pathway-related genes, in particular, those affecting its absorption and systemic circulation in free versus bound state [33–35]; local delivery of VD is likely to circumvent many of these confounding variables.
Pulmonary immaturity is the main contributor to preterm infant mortality and short- and long-term pulmonary morbidities [1, 2], justifying search for a safe and effective lung-maturing agent. However, despite intense research over the last several decades, no such agent has been discovered [36]. Although VD’s role in lung maturation has been known for over 2 decades, it’s only recently that there has been an increased awareness of the critical significance of VD’s numerous nonskeletal effects including its role in embryogenesis in general and organ-specific development such as that of the brain and especially lung [6, 29, 30, 37, 38]. Furthermore, low VD levels during pregnancy are associated with reduced placental development that likely predisposes to preterm birth [39], which per se leads to premature lungs and the associated consequences especially BPD. Other than preventing prematurity, administration of antenatal steroids is the only effective intervention to promote fetal lung maturation; however, it has not been shown to affect BPD; moreover, there is no clinically effective intervention to promote lung maturation postnatally. In fact, when administered postnatally, steroids are associated with significant side effects, necessitating continued search for an effective and safe agent that enhances lung maturation postnatally [40].
Given the extensive evidence for VD’s biological role in fetoneonatal lung maturation, complemented by the data included in this study, we propose that inhaled VD is a promising therapeutic modality to enhance lung maturation postnatally. Although the lung injury prevention efficacy of this approach is yet to be determined and the long-term impact of stimulating lung maturation in the neonatal period remains to be tested, if proven to be effective and safe, this strategy would be extremely cheap and likely devoid of side effects known to be associated with systemic VD administration. Clearly, human studies to determine the safe and effective dose of natural VD or its synthetic analogs are needed to translate this promising therapeutic modality from bench to bedside. The known differences in bioactivities of 25D and 1,25D and the fact that comparable pulmonary maturational effects were seen with a much lower (1/100) dose of 1,25D versus 25D, our study provides some guidance on therapeutic dosing of VD in future translational studies. It is important to point out that given a number of potential benefits associated with aerosolized drug delivery, a search for an optimal delivery system for the spontaneously breathing, as well as for the ventilated neonates, continues [41–44]. Nevertheless, as a proof of principle, employing the nebulization system used in this study, improvement in respiratory outcomes has been demonstrated in both clinical and experimental settings, indicating an effective pulmonary delivery of the nebulized medications with the approach utilized in this study [17, 45]. In conclusion, we propose that nebulized VD offers a novel and possibly a safer postnatal lung maturation and potentially injury protection strategy.
Acknowledgments
Grant Support NIH (HD51857, HD058948, HL107118, HD071731; HL127237); TRDRP (17RT-0170, and 23RT-0018).
Footnotes
Compliance with Ethical Standards
Conflicts of Interest The authors declare that they have nothing to declare.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100(3):145–157. doi: 10.1002/bdra.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai R, Shin E, Fonseca S, et al. 1alpha,25(OH)2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L496–L505. doi: 10.1152/ajplung.90539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin L, Dufour ME, Nguyen TM, Garabedian M. Maturational changes induced by 1 alpha,25-dihydroxyvitamin D3 in type II cells from fetal rat lung explants. Am J Physiol. 1993;265:L45–L52. doi: 10.1152/ajplung.1993.265.1.L45. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen M, Trubert CL, Rizk-Rabin M, et al. 1,25-Dihydroxyvitamin D3 and fetal lung maturation: immunogold detection of VDR expression in pneumocytes type II cells and effect on fructose 1,6 bisphosphatase. J Steroid Biochem Mol Biol. 2004;89–90(1–5):93–97. doi: 10.1016/j.jsbmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TM, Guillozo H, Marin L, et al. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol. 1996;271(3):L392–L399. doi: 10.1152/ajplung.1996.271.3.L392. [DOI] [PubMed] [Google Scholar]
- 7.Rehan VK, Torday JS, Peleg S, et al. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxy vitamin D3: production and biological activity studies in pulmonary alveolar type II cells. Mol Genet Metab. 2002;76(1):46–56. doi: 10.1016/s1096-7192(02)00022-7. [DOI] [PubMed] [Google Scholar]
- 8.Hollis BW, Wagner CL. Vitamin D deficiency during pregnancy: an ongoing epidemic. Am J Clin Nutr. 2006;84(2):273. doi: 10.1093/ajcn/84.1.273. [DOI] [PubMed] [Google Scholar]
- 9.Zosky GR, Hart PH, Whitehouse AJ, et al. Vitamin D deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Ann Am Thorac Soc. 2014;11(4):571–577. doi: 10.1513/AnnalsATS.201312-423OC. [DOI] [PubMed] [Google Scholar]
- 10.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 11.Islam JY, Keller RL, Aschner JL, et al. Understanding the Short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192(2):134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yurt M, Liu J, Sakurai R, et al. Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. Am J Physiol Lung Cell Mol Physiol. 2014;307(11):L859–L867. doi: 10.1152/ajplung.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogiatzi MG, Jacobson-Dickman E, DeBoer MD. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99(4):1132–1141. doi: 10.1210/jc.2013-3655. [DOI] [PubMed] [Google Scholar]
- 15.Knudsen L, Weibel ER, Gundersen HJ, et al. Assessment of air space size characteristics by intercept (chord) measurement: an accurate and efficient stereological approach. J Appl Physiol. 2010;108(2):412–421. doi: 10.1152/japplphysiol.01100.2009. [DOI] [PubMed] [Google Scholar]
- 16.Rehan VK, Wang Y, Sugano S, et al. In utero nicotine exposure alters fetal rat lung alveolar type II cell proliferation, differentiation, and metabolism. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L323–L333. doi: 10.1152/ajplung.00071.2006. [DOI] [PubMed] [Google Scholar]
- 17.Morales E, Sakurai R, Husain S, et al. Nebulized PPAR-gamma agonists: a novel approach to augment neonatal lung maturation and injury repair in rats. Pediatr Res. 2014;75(5):631–640. doi: 10.1038/pr.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong W, Gu B, Gu Y, et al. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J Steroid Biochem Mol Biol. 2014;140:56–62. doi: 10.1016/j.jsbmb.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardus A, Parisi E, Gallego C, et al. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int. 2006;69(8):1377–1384. doi: 10.1038/sj.ki.5000304. [DOI] [PubMed] [Google Scholar]
- 20.Grant WB. Role of vitamin D in up-regulating VEGF and reducing the risk of pre-eclampsia. Clin Sci. 2009;116(12):871. doi: 10.1042/CS20080562. [DOI] [PubMed] [Google Scholar]
- 21.Mandell E, Seedorf G, Gien J, et al. Vitamin D treatment improves survival and infant lung structure after intra-amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2014;306(5):L420–L428. doi: 10.1152/ajplung.00344.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gur G, Abaci A, Koksoy AY, et al. Incidence of maternal vitamin D deficiency in a region of Ankara, Turkey: a preliminary study. Turk J Med Sci. 2014;44(4):616–623. doi: 10.3906/sag-1304-107. [DOI] [PubMed] [Google Scholar]
- 23.Marwaha RK, Tandon N, Chopra S, et al. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011;106(9):1383–1389. doi: 10.1017/S000711451100170X. [DOI] [PubMed] [Google Scholar]
- 24.Xiao JP, Zang J, Pei JJ, et al. Low maternal vitamin D status during the second trimester of pregnancy: a cross-sectional study in Wuxi, China. PLoS One. 2015;10(2):e0117748. doi: 10.1371/journal.pone.0117748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camargo CA, Jr, Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 26.Hart PH, Lucas RM, Walsh JP, et al. Vitamin D in fetal development: findings from a birth cohort study. Pediatrics. 2015;135(1):e167–e173. doi: 10.1542/peds.2014-1860. [DOI] [PubMed] [Google Scholar]
- 27.Foong RE, Bosco A, Jones AC, et al. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am J Respir Cell Mol Biol. 2015;53(5):664–675. doi: 10.1165/rcmb.2014-0356OC. [DOI] [PubMed] [Google Scholar]
- 28.Zosky GR, Berry LJ, Elliot JG, et al. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183(10):1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 29.Lykkedegn S, Sorensen GL, Beck-Nielsen SS, et al. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am J Physiol Lung Cell Mol Physiol. 2015;308(7):L587–L602. doi: 10.1152/ajplung.00117.2014. [DOI] [PubMed] [Google Scholar]
- 30.Edelson JD, Chan S, Jassal D, et al. Vitamin D stimulates DNA synthesis in alveolar type-II cells. Biochim Biophys Acta. 1994;1221(2):159–166. doi: 10.1016/0167-4889(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 31.Marin L, Dufour ME, Tordet C, et al. 1,25(OH)2D3 stimulates phospholipid biosynthesis and surfactant release in fetal rat lung explants. Biol Neonatol. 1990;57(3–4):257–260. doi: 10.1159/000243200. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TM, Guillozo H, Marin L, et al. 1,25-dihydroxyvitamin D3 receptors in rat lung during the perinatal period: regulation and immunohistochemical localization. Endocrinology. 1990;127(4):1755–1762. doi: 10.1210/endo-127-4-1755. [DOI] [PubMed] [Google Scholar]
- 33.Chishimba L, Thickett DR, Stockley RA, et al. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65(5):456–462. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 34.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beam KS, Aliaga S, Ahlfeld SK, et al. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J Perinatol. 2014;34(9):705–710. doi: 10.1038/jp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eyles D, Burne T, McGrath J. Vitamin D in fetal brain development. Semin Cell Dev Biol. 2011;22(6):629–636. doi: 10.1016/j.semcdb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Christakos S, DeLuca HF. Minireview: vitamin D: is there a role in extraskeletal health? Endocrinology. 2011;152(8):2930–2936. doi: 10.1210/en.2011-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans KN, Nguyen L, Chan J, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75(6):816–822. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 40.Zia MT, Vinukonda G, Vose LR, et al. Postnatal glucocorticoid-induced hypomyelination, gliosis, and neurologic deficits are dose-dependent, preparation-specific, and reversible. Exp Neurol. 2015;263:200–213. doi: 10.1016/j.expneurol.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazela J, Polin RA. Aerosol delivery to ventilated newborn infants: historical challenges and new directions. Eur J Pediatr. 2011;170(4):433–444. doi: 10.1007/s00431-010-1292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Syedain ZH, Naqwi AA, Dolovich M, Somani A. In vitro evaluation of a device for intra-pulmonary aerosol generation and delivery. Aerosol Sci Technol. 2015;49(9):747–752. doi: 10.1080/02786826.2015.1067670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiemersma S, Minocchieri S, van Lingen RA, Nelle M, Devadason SG. Vibrating membrane devices deliver aerosols more efficient than standard devices: a study in a neonatal upper airway model. J Aerosol Med Pulm Drug Deliv. 2013;26(5):280–286. doi: 10.1089/jamp.2012.0993. [DOI] [PubMed] [Google Scholar]
- 44.Das GK, Anderson DS, Wallis CD, Carratt SA, Kennedy IM, Van Winkle LS. Novel multi-functional europium-doped gadolinium oxide nanoparticle aerosols facilitate the study of deposition in the developing rat lung. Nanoscale. 2016;8(22):11518–11530. doi: 10.1039/c6nr00897f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gappa M, Gärtner M, Poets CF, von der Hardt H. Effects of salbutamol delivery from a metered dose inhaler versus jet nebulizer on dynamic lung mechanics in very preterm infants with chronic lung disease. Pediatr Pulmonol. 1997;23(6):442–448. doi: 10.1002/(sici)1099-0496(199706)23:6<442::aid-ppul8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]