Abstract
Cognitive performance is characterized by at least two distinct life course trajectories. Many cognitive abilities (e.g. “effortful processing” abilities including fluid reasoning, and processing speed) improve throughout early adolescence and start declining in early adulthood, while other abilities (e.g. “crystallized” abilities like vocabulary breadth) improve throughout adult life, remaining robust even at late ages. Although schooling may impact performance and cognitive “reserve”, it has been argued that these age patterns of cognitive performance are human universals. Here we examine age patterns of cognitive performance among Tsimane forager-horticulturalists of Bolivia, and test whether schooling is related to differences in cognitive performance over the life course to assess models of active vs. passive cognitive reserve. We used a battery of eight tasks to assess a range of latent cognitive traits reflecting attention, processing speed, verbal declarative memory and semantic fluency (n=919 individuals, 49.9% female). Tsimane cognitive abilities show similar age-related differences as observed in industrialized populations: higher throughout adolescence and only slightly lower in later adulthood for semantic fluency, but substantially lower performance beginning in early adulthood for all other abilities. Schooling is associated with greater cognitive abilities at all ages controlling for sex, but has no attenuating effect on cognitive performance in late adulthood, consistent with models of passive cognitive reserve. We interpret the minimal attenuation of semantic fluency late in life in light of evolutionary theories of post-reproductive lifespan, which emphasize indirect fitness contributions of older adults through the transfer of information, labor and food to descendant kin.
Keywords: cognitive performance, fluid vs. crystallized intelligence, cognitive reserve, aging, education, Tsimane
A growing consensus among psychologists suggests that human cognitive performance is not a generalized ability, but is instead composed of multiple inter-related abilities marked by distinct life course trajectories (Salthouse 2010; Salthouse 2004; Craik & Bialystok 2006). “Fluid” intelligence represents the ability to problem-solve and reason in novel situations, “crystallized” intelligence represents the ability to effectively apply acquired knowledge and learned skills (Carroll 1993; Cattell 1971), while some related abilities such as processing speed and working memory arguably may not fit the classical “fluid” vs. “crystallized” distinction (Schaie 1989; Zimprich & Mascherek 2010). Fluid and other associated cognitive abilities (hereafter referred to as “effortful processing” abilities sensu Tucker-Drob & Salthouse 2011) often decline from the mid-twenties or thirties onward, while crystallized abilities often improve up through the sixth decade of life (Bugg et al. 2006; Horn & Cattell 1967). Performance on effortful processing tasks not only declines earlier in life but also more rapidly than performance on tasks drawing more from crystallized abilities (Harada et al. 2013). These distinct age patterns have been demonstrated for both sexes in large-scale studies (Schaie 2005; Tucker-Drob 2011; McArdle et al. 2002; Singh-Manoux & Kivimaki 2012; Bugg et al. 2006; Wang & Kaufman 1993), including recent Weschler Adult Intelligence Scale IV and Weschler Memory Scale IV normative samples (Salthouse 2010). Despite more nuanced taxonomies for classifying cognitive abilities and subtle differences in rates of change, these broad age patterns generally appear to be consistent across and within populations. Given their consistent replication these age patterns have been identified as universals of human cognitive aging (Salthouse 2009; Park et al. 2002; Hedden et al. 2002; Park & Gutchess 2006).
Despite these claims of universality, the extent to which these patterns exist among pre-industrialized populations with limited exposure to formal schooling remains understudied. An adequate attempt to describe and explain “universal” age patterns of human cognitive performance should include studies of pre-industrialized populations, and should highlight the costs and benefits of different cognitive abilities with age under socio-ecological conditions more representative of human evolutionary history (e.g. energy-limited, subsistence production, higher fertility, pathogen exposure). Humans lived as hunter-gatherers for the vast majority of their evolutionary history, and thus natural selection on cognitive aging and development, and its associated neural substrates, occurred in the context of a foraging lifestyle. Yet virtually nothing is known about how cognition develops and declines among traditional subsistence-level populations with limited schooling. For example, a pattern where all cognitive abilities senesce concurrently with physical declines would contradict existing evolutionary theories of aging that emphasize that post-reproductive lifespan evolved to transfer difficult-to-acquire resources and information to descendant kin (Kaplan & Robson 2002; Lee 2003; Gurven 2012); based on this logic, one should instead expect some cognitive abilities (especially crystallized) to persist into late adulthood.
While literacy and schooling are nearly universal in industrialized populations, this was not the case throughout the vast majority of human history. Most studies find strong impacts of higher education on cognitive performance (Alley et al. 2007), though such studies generally only examine differences between secondary and post-secondary education, as primary schooling is compulsory in most industrialized populations. Current consensus of the effects of schooling on cognitive performance is based on a narrow range of populations where compulsory schooling is commonplace in a competitive skills-based wage economy (Ardila et al. 2000; Baker et al. 2015; Ardila & Rosselli 2007), and where certain socio-ecological factors (e.g. reduced nutrient availability, high pathogen burden, high fertility and demand for childcare) pose fewer constraints to accruing human capital in controlled settings. Some researchers have argued that observed age patterns of cognitive performance may be specific to the Western, educated, industrialized, rich and democratic (i.e. WEIRD societies, Henrich et al. 2010) and may not generalize to a broader range of populations. If alternative socio-ecologies favor different kinds of skills at different ages, then age profiles of cognitive performance might vary from those documented in WEIRD societies. Furthermore, the extent to which cognitive performance is affected by differential exposure to schooling at the lower tail of the schooling distribution is unclear. Identifying human-typical patterns of cognitive development and decline in the absence of schooling, as was typical for most of human history, is therefore difficult given the complicated and potentially reinforcing effects of modern environments.
Cognitive Performance and Schooling
While pathways by which schooling affects cognitive task performance are still not clearly defined, it is clear that literacy, numeracy and abstract logic are often necessary for occupational successin industrialized populations, unlike in traditional subsistence societies (Furnham & Cheng 2013). Schooling plays a critical role in directly cultivating many of the cognitive skills that underpin performance on intelligence tests and eventual economic success in adulthood (Ceci, 1991). Across all ages, those with higher educational attainment develop various cognitive skills more rapidly than those with minimal schooling (Alley et al. 2007). Positive associations between schooling and cognitive performance (Lam et al. 2013; Alley et al. 2007; Deary et al. 2007; Schoon 2010), and between both and adult income and job performance are well established (Feinstein & Bynner 2004; Schmidt & Hunter 2004; Cheng & Furnham 2012). Cohort studies tracking school attendance and reverse causality due to dropout have also consistently reported strong positive associations between schooling and multiple domains of cognitive performance (Brinch & Galloway 2012; Carlsson et al. 2015).
In addition to direct effects of schooling on cognitive outcomes, participation in the structured, routinized, controlled learning environment of formalized education also fosters “non-cognitive” traits associated with concentration, improved attention span, and self-regulation that predict success in both academic, professional and social environments (Blair & Raver 2014; Komarraju et al. 2013). Formalized schooling places demands on executive function – the ability to avoid distractions, focus attention, hold relevant information in working memory, and to regulate impulsive behaviors. A well-developed working memory and the ability to control attention enables individuals to focus and process information more efficiently, which further supports the retention of knowledge (Ruchkin et al. 2003; Bull & Scerif 2001). The ability to avoid distractions and stay on-task for extended periods of time (i.e. “grit”; Duckworth et al. 2007) affects a range of cognitive, scholastic, occupational and social outcomes. Schooling also rewards high performance on exams, thereby instilling test-taking motivations, including effort and persistence--traits that may not exist for individuals with minimal or no exposure to timed testing (Eklöf 2010).
Cognitive Decline: Active vs. Passive Reserve
Many cognitive abilities decline with age, although such declines are usually less steep for more educated individuals (Alley et al. 2007). This consistent attenuation in age-related decline has raised new questions regarding how differential schooling earlier in life might affect later-life cognition. “Cognitive reserve” reflects the ability for individuals to be buffered from some aspects of cognitive aging (Le Carret et al. 2003; Barulli & Stern 2013; May 2011). The extent to which schooling plays a role in mitigating age-related cognitive decline has been vigorously debated (Leibovici et al. 1996; Anstey & Christensen 2000; Van Dijk et al. 2008; Zahodne et al. 2011; Tucker-Drob et al. 2009; Nyberg et al. 2012), and the mechanisms remain poorly understood.
The “active reserve” model focuses on brain plasticity for recruiting alternative cognitive capacities and reorganizing cognitive networks to counter age-dependent cognitive degradation. Proponents argue that schooling encourages more efficient cognitive processing and neural networks, which facilitates cognitive reorganization and buffers against effects of normal cognitive aging (Stern 2002). This model suggests that more educated individuals will (a) outperform their less educated counterparts throughout adult life, and (b) show a slowing of the typical age-related patterns of cognitive decline. In support of this model, greater schooling has been associated with lower rates of cognitive decline in healthy adults (e.g. Lyketsos et al. 1999; Butler et al. 1996; Farmer et al. 1995) and with slower transition from mild cognitive impairment to dementia (Poletti et al. 2011).
The “passive reserve” model focuses on building physical reserve capacity (e.g. neuronal network density, synapse count) and clinical impairment beyond a threshold level of brain pathology (Satz 1993). This model also predicts that greater schooling leads to higher cognitive performance, but unlike the active reserve model, predicts no difference in rate of cognitive decline as a function of schooling (Stern 2002). Instead, greater schooling should be associated with enhanced retention of physical cognitive reserves, delayed attainment of brain pathology, and delayed onset of cognitive decline. However, longitudinal studies consistently show no significant attenuating effect of schooling on rate of cognitive decline at older ages (Tucker-Drob et al. 2009; Van Dijk et al. 2008; Karlamangla et al. 2009; Alley et al. 2007; Zahodne et al. 2011).
Most studies of schooling’s effects on the age pattern of cognitive performance compare adults with relatively high educational attainment or children varying in secondary schooling but who have all received compulsory primary schooling (reviewed in Ceci 1991). Variability in cognitive performance throughout adulthood at low levels of schooling may be instructive for helping to distinguish the relative impacts of cumulative exposures that incrementally improve cognitive performance over time, versus attitudes, expectation formation and other schooling-related factors whose impacts on cognitive performance may be learned with even low levels of schooling.
The Present Study
To shed light on cognitive performance and “reserve” in a unique non-WEIRD socio-ecology characterized by low levels of schooling, we report age patterns of cognitive performance among Tsimane forager-horticulturalists of Bolivia. Despite the low overall level of schooling, we test whether variation in schooling is associated with a) cognitive performance and b) age-related differences in cognitive performance using a cross-sectional study design. Both active and passive reserve models predict that more educated individuals will outperform their less educated counterparts at all ages, but only the active reserve model predicts that greater levels of schooling are associated with a shallower rate of cognitive decline; the passive reserve model predicts no relationship between schooling and rates of decline.
To our knowledge, this is the largest study (n=919 individuals, 49.9% female) of cognitive performance in a traditional small-scale population. Not all Tsimane villages have had access to schools, and those that do vary in the duration of their establishment and efficacy of the teachers. Thus, schooling is not heavily confounded with age cohort in the current sample, as might be expected if schools were uniformly established across the Tsimane territory at the same time. We are therefore able to leverage regional variation to disentangle availability and quality of schooling and age in our analytic approach despite the cross-sectional design, which makes the Tsimane an ideal population in which to study the effects of schooling on cognitive development and aging.
Method
Study Population
The Tsimane are a semi-sedentary population of ~15,000 forager-horticulturalists living in the Bolivian Amazon. They inhabit 90+ villages ranging in size from 50–500 individuals. They cultivate plantains, rice, corn, and sweet manioc in small swiddens, and regularly fish and hunt. Traditional foods comprise >90% of the calories in the diet, with the remainder purchased from market stores, or obtained from trade with merchants (Martin et al. 2012). Tsimane live in extended family clusters, where the majority of food and labor sharing occurs. Mortality has been traditionally high, with approximately 20% of children dying before age 5 (Gurven et al. 2007). The Tsimane rarely use modern contraceptives and total fertility rate is high (9.1 births per woman; McAllister et al. 2012). The population growth rate is also high at 3.6% per year. Mean (SD) age of first birth for men and women is 22.8 (4.2), and 18.6 (2.9) years, respectively (ibid).
Many villages now have elementary schools (up to 5th grade) taught by bilingual (Spanish-Tsimane) teachers. For over three decades, bilingual Tsimane teachers (all young men) were trained by Protestant missionaries, who produced all instructional materials (Godoy et al. 2007). Up until 2000, no village had a middle or high school, although several large villages near town had educational programs one week per month where teenagers and adults who completed elementary school could obtain up to a high school diploma. Unlike in most of Bolivia where indigenous children were taught in Spanish, Tsimane children were taught in their own language, and curricula prioritized Tsimane literacy. However, since the early 2000’s, more teachers are Bolivian nationals, and curricula have shifted to emphasize more Spanish fluency and to conform more with standards set by the Bolivian Ministry of Education. Secondary schools now exist in several larger villages near the closest market town of San Borja, and young adults are starting to become high school graduates (5% of adults aged 18–25). However, in general school attendance rates are either low or inconsistent for many students and overall adult literacy rate is low (18%). We suspect that much variability in educational attainment, especially up to the fifth grade level, is based on the number and types of schools in a participant’s natal village, and labor demands in the family that might otherwise affect attendance (Bock 2002a). Fluency in the Tsimane language is universal as Tsimane remains the native language (unrelated to Spanish); roughly 65% of adults in our sample are at least moderately fluent in Spanish. While Spanish is taught in schools, Tsimane also gain Spanish fluency from greater interaction with Bolivian nationals.
Cognitive Battery and Recruitment
A sample of 919 Tsimane (49.9% female) aged 8–88 (mean±SD = 33.85 y±18.58) completed a battery of eight tasks (described in greater detail below) to assess a wide range of latent cognitive traits, based in part on those used by the Mexican Health and Aging Study (Mejía-Arango et al. 2015) and the Cross-Cultural Cognitive Examination dementia screening (Glosser et al. 1993). These tasks largely assess verbal declarative memory (short-term learning and recall), attention (visual scan, digit forward), psychomotor speed (visual scan), and semantic fluency (category fluency). Subjects were participants of annual biomedical surveillance by the Tsimane Health and Life History Project (THLHP) between January 2008 to July 2009 (see Trumble et al. 2015). Given the THLHP’s focus on aging, all village members age 40+ are eligible for complete biomedical surveillance including behavioral and cognitive assessment, whereas younger individuals receive medical attention but further data collection exists only on a sub-sample (~30%). From a THLHP census population of 2,768 individuals age 8+ across the 34 villages sampled in this study, 2,003 (72%) were seen by the THLHP; excluded are those who (in descending order of relative frequency) were: visiting other villages, engaged in multi-day foraging excursions or other subsistence work (e.g. hunting) entailing travel away from the village, and those who did not wish to participate. Of the 439 adults age 40+ who received medical exams by THLHP physicians, 345 (79%) participated in the cognition study. Of the 1,020 Tsimane age 8–24 yrs across the 34 villages, 338 (33%) were sampled to in the cognition study. The main reason for excluding this age category from the cognition study was logistical: the bilingual Tsimane researchers conducting the cognition study were also conducting other interviews and their time was thus limited. Over a third of participants (31.0% males, 38.4% females)) had no schooling (Figure S1). While years of schooling ranged from 0–12 (mean±SD= 2.38±2.80; median=2), nearly 60% of the sample had ≤ 2 years (Figure S2). It is worth noting, however, that a year of schooling among Tsimane may contain significant gaps in attendance and is unlikely to be equivalent to a year of schooling in industrialized societies.
Cognitive tests were conducted in the Tsimane language by a trained Tsimane research assistant with multiple years of experience conducting interviews as part of the THLHP. Tests were conducted at participants’ homes or elsewhere in the village in a private location. Interviews were conducted entirely in the Tsimane language, with the exception of the Spanish numbers in the digit forward test.
Consent was provided at three levels: Tsimane government (Gran Consejo Tsimane), village leadership, and study participants. All procedures were approved by the IRB at the XXXXX(Deleted to insure anonymity).
Immediate and Delayed Recall
Verbal memory was assessed by immediate and delayed recall tasks (Unsworth et al. 2009). Participants were read a list of eight Tsimane nouns three times and then asked each time to repeat the list immediately in any order. After 10 minutes participants were also asked to recall in any order as many of the eight words from the original list as possible.
Digit Forward
Attentional capacity was assessed by digit span tasks. Digit forward tasks require participants to store and sequentially update a list of single-digit numbers and then retrieve the list from working memory at the end of a trial (Maylor et al., 1999). Participants repeat a series of digits increasing in length until failure on two consecutive trials. This test was first administered in the Tsimane language. Some Tsimane numbers have multiple syllables (e.g. yavatidye’ (seven), arajtac (nine)), and so a second version using Spanish numbers was conducted. Even though Spanish fluency varied in the sample, Spanish numbers up to 10 are understood by all Tsimane. In a third spatial span task, the interviewer touched a pre-determined sequence of numbered boxes on a poster, which participants were asked to replicate. This task draws upon spatial processing and mental mapping in addition to working memory.
Visual Scan
A visual scan task required participants to locate in two minutes as many instances as possible of a target symbol in an array of jumbled symbols. Visual scanning requires discrimination between target stimuli and distractors, and executive processing skills such as psychomotor speed, sustained attention and interference avoidance (Glosser et al. 1993).
Semantic Fluency
Semantic fluency tasks assess semantic memory in two locally salient domains: animals and fish (Ardila et al. 2006). Participants had two minutes to generate as long a list of items in each category as possible. Performance on semantic fluency tasks is considered a good measure of crystallized intelligence (Carroll 1993; Ekstrom et al. 1976) based on the ability to retrieve information from long term memory, and to filter distractions and limit responses to relevant objects.
Data Analysis
Data analysis was conducted in R version 3.1.2 and SAS 9.2. Individual performance in each cognitive test was first converted to Z-scores, allowing direct comparisons across tests with different scoring protocols. Z-scores for separate tasks were also averaged to create composite measures for “effortful processing” ability (immediate and delayed recall, digit forward, and visual scan) and semantic fluency (animals and fish).
Multivariate linear regression models were used to assess relationships between cognitive performance, age and years of schooling controlling for sex and Spanish fluency. Interaction terms between age and schooling were added to estimate the effect of schooling on age-related decline in cognitive performance. Because of possible non-linear age patterns of cognitive performance, age2 terms are included in all models. Generalized additive models (GAMs; R package mgcv) were used to examine age trends in composite effortful processing and semantic fluency domains, while controlling for sex, Spanish fluency, and schooling, i.e. Y|X1,X2,X3 = B0+S1(X1)+X2+X3, where S(X1) is a nonparametric smooth function (spline) for age, and X2 and X3 are sex and schooling, respectively.
To better isolate age patterns of cognitive performance at early and later life stages, we used segmented linear regression (R package segmented) to estimate (a) age of peak performance, (b) the slope of age-related differences (i.e.”decline”) for those older than this peak, and (c) the age-related slope of “increase” before this peak. Segmented linear models permit identification of transition points that may otherwise be obscured when using quadratic functions or non-parametric estimates from GAMs (Fortenbaugh et al. 2015). Additional regressions of pre-adult and early adult cognitive performance are restricted to ages <25 yrs, while those focused on middle-age and older adult cognitive performance are limited to ages 40+.
Inter- and Intra-Village Variation
Because villages varied in access to and quality of teachers and schools, each of the 34 communities in this study was treated as a random effect in a mixed effects model. We also make adjustments due to non-independence from biological kinship amongst individuals in our sample, using detailed genealogy and census records on all 919 participants (Gurven et al. 2007). Amongst 919*918/2=421,821 possible unique dyads, 677 are parent-offspring dyads, and 1,510 are sibling dyads; 97.4% reflect non-kin relationships (Wright’s coefficient of relatedness r=0), 1.6% reflect 0<r≤0.125, 0.6% reflect 0.125<r≤0.25, and 0.4% reflect r≥0.25. To adjust for shared kinship we add two random intercept terms for members of the same 139 matrilines and 143 patrilines in all mixed effects regression models.
Results
Descriptive Statistics
Summary statistics on cognitive performance and covariates by age and sex are shown in Table 1. On average, males are more educated than females (Mann-Whitney U, z= 2.28, p = 0.023; Figure S1) and speak Spanish more fluently, and so sex and Spanish fluency are included as controls in all models. However, highest grade achieved and Spanish fluency were similar between young males and females <25 yrs (grade: 3.1 yrs; Spanish competence: 2.3 (out of 4), Table 1; Figures S2, S3); adult men had slightly higher educational attainment and Spanish fluency than women (schooling: 3.1 vs. 2.1 ages 25–39, 1.9 vs. 1.2 ages 40+; Spanish: 2.7 vs. 2.0 ages 25–39, 2.5 vs. 2.1 ages 40+; Table 1). For both sexes, highest educational attainment is lower with age (Table 1; Figure S2), but Spanish fluency varies minimally with age (Table 1; Figure S3). Even controlling for schooling and Spanish fluency, males outperform females in all tasks. There was no significant age difference between sexes (t(914.21) = −0.52, p = 0.60) (Table 1).
Table 1.
Descriptive sample statistics by age and sex
| Variable | AGE < 25 | AGE 25–39 | AGE 40+ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MALE | FEMALE | MALE | FEMALE | MALE | FEMALE | |||||||
|
| ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (yrs) | 14.1 | 4.3 | 14.9 | 4.6 | 33.3 | 4.0 | 31.8 | 4.3 | 54.1 | 11.2 | 53.1 | 11.3 |
| Highest Grade (0–12) | 3.1 | 2.5 | 3.1 | 2.4 | 3.1 | 3.6 | 2.1 | 2.8 | 1.9 | 3.0 | 1.2 | 2.1 |
| Spanish fluency (0–4) | 2.3 | 1.2 | 2.3 | 1.0 | 2.7 | 1.0 | 2.0 | 1.2 | 2.5 | 1.2 | 2.1 | 1.2 |
| Average Short-Term Learning (0–8) | 5.1 | 1.2 | 4.9 | 1.2 | 5.4 | 1.2 | 5.1 | 1.2 | 4.7 | 1.1 | 4.5 | 0.8 |
| Long-Term Recall (0–8) | 6.2 | 1.2 | 6.4 | 1.3 | 6.5 | 1.1 | 6.1 | 1.2 | 5.6 | 1.3 | 5.5 | 1.4 |
| Tsimane Digit Forward (0–18) | 10.4 | 1.9 | 9.9 | 1.8 | 11.1 | 2.0 | 9.9 | 1.9 | 10.2 | 2.1 | 9.3 | 1.8 |
| Spanish Digit Forward (0–18) | 10.7 | 2.1 | 10.5 | 2.3 | 11.6 | 2.0 | 10.1 | 2.1 | 10.8 | 1.9 | 9.6 | 2.0 |
| Spatial Forward (0–7) | 4.2 | 1.1 | 4.1 | 1.0 | 4.4 | 1.1 | 4.0 | 1.2 | 4.0 | 0.9 | 3.5 | 0.9 |
| Visual Scan (#Correct-#Wrong) | 34.4 | 14.7 | 33.6 | 14.9 | 39.3 | 14.5 | 28.8 | 15.2 | 20.6 | 16.7 | 14.3 | 14.0 |
| Total # Animals in 2 mins | 22.2 | 6.0 | 19.9 | 5.6 | 27.1 | 5.9 | 22.7 | 5.3 | 25.9 | 6.2 | 23.5 | 5.2 |
| Total # Fish in 2 mins | 19.1 | 5.3 | 17.0 | 5.1 | 24.2 | 5.0 | 20.4 | 4.9 | 23.7 | 5.3 | 21.5 | 5.1 |
Table 2 shows the sex-specific Pearson correlations in performance among all cognitive tasks, and two composites: “semantic fluency” and “effortful processing” for all other domains. Most correlations between “effortful processing” hover between 0.2 and 0.5, and 0.6 among the two semantic fluency tasks. Long term recall, spatial forward and visual scan are most strongly correlated with the “effortful processing” composite. In the complete sample, “effortful processing” and “semantic fluency” composites are uncorrelated with each other; correlations among “effortful processing” and “semantic fluency” composites are significant only for those <20 yrs (r=0.16, p=0.007, controlling for sex), and for schooled adults (1–2 yrs: r=0.35,; 3+ yrs: r=0.21, p’s<0.0001, controlling for age and sex). The average correlation among all cognitive tasks vary little by age group: (<20 yrs: r=0.226 (n=279); 20–39 yrs: r=0.210 (n=291); 40–59 yrs: r=0.175 (n=252); 60+ yrs: r=0.191 (n=77), controlling for sex) or by level of schooling: (0 yrs: r=0.191 (n=306); 1–2 yrs: r=0.245 (n=227); 3+ yrs: r=0.235 (n=364). Thus, we find little evidence that the cognitive abilities assessed in this study change consistently in their degree of independence from zero to low levels of schooling, or across the lifespan (see Tucker-Drob 2009 and citations therein).
Table 2.
Pearson correlations between cognitive tasks, all ages combined. Correlations above the diagonal reflect females (n=461). Correlations below the diagonal reflect males (n=458).
| Short Term Recall | Long Term Recall | Tsim Digit Forward | Span Digit Forward | Spatial Forward | Visual Scan | Animal fluency | Fish fluency | “Effortful Processing” | “Semantic fluency” | |
|---|---|---|---|---|---|---|---|---|---|---|
| Short Term Recall | - | 0.425*** | 0.160** | 0.201*** | 0.213*** | 0.192*** | 0.079^ | 0.064 | 0.387*** | 0.054 |
| Long Term Recall | 0.429*** | - | 0.232*** | 0.240*** | 0.244*** | 0.387*** | 0.141* | 0.131* | 0.572*** | 0.157** |
| Tsim Digit Forward | 0.206*** | 0.213*** | - | 0.570*** | 0.387*** | 0.301*** | 0.080^ | 0.042 | 0.601*** | 0.075^ |
| Span Digit Forward | 0.257*** | 0.248*** | 0.505*** | - | 0.383*** | 0.364*** | 0.079^ | 0.032 | 0.639*** | 0.058 |
| Spatial Forward | 0.199*** | 0.299*** | 0.192*** | 0.274*** | - | 0.510*** | −0.016 | −0.058 | 0.698*** | −0.046 |
| Visual Scan | 0.252*** | 0.438*** | 0.233*** | 0.229*** | 0.354*** | - | −0.098+ | −0.151** | 0.728*** | −0.144* |
| Animal fluency | 0.141* | 0.092+ | 0.173** | 0.183*** | 0.01 | 0.026 | - | 0.612*** | 0.017 | 0.859*** |
| Fish fluency | 0.036 | 0.007 | 0.113+ | 0.120** | −0.005 | −0.059 | 0.636*** | - | −0.043 | 0.865*** |
| “Fluid” | 0.495*** | 0.655*** | 0.544*** | 0.576*** | 0.611*** | 0.693*** | 0.121* | 0.017 | - | −0.03 |
| “Semantic fluency” | 0.100+ | 0.074 | 0.158** | 0.165** | 0.005 | −0.002 | 0.876*** | 0.871*** | 0.074 | - |
Note:
p<0.0001,
p<0.001,
p<0.01,
p<0.05,
p<0.10
Age Patterns of Cognitive Performance
Tables 3 and 4 test the relationship between age, schooling, and Spanish fluency on cognitive performance for the full sample, younger individuals (<25 years old), and middle-aged and older adults (aged 40+). In the full sample, age is significantly positively associated with performance on all cognitive measures, while the age2 term is significantly negatively associated with cognitive performance, demonstrating lower age-related performance at older ages in all abilities (all p’s < 0.001).
Table 3.
Multivariate regressions predicting performance on each fluid subtask for all individuals. Community, matriline and patriline are random effects in the model. Parameter estimates are regression coefficients in raw units of the predictor variables. Cognitive task performance is in z-score units.
| Short Term Recall | Long Term Recall | Tsimane Digit Forward | Spanish Digit Forward | Spatial Digit Forward | Visual Scan | “Effortful Processing” | Animals | Fish | Categorical Fluency | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.022*** | 0.023*** | 0.023*** | 0.019** | 0.012* | 0.016** | 0.018*** | 0.070*** | 0.068*** | 0.072*** |
| Age2 | −0.0004*** | −0.0004*** | −0.0003*** | −0.0003*** | −0.0002** | −0.0004*** | −0.0003*** | −0.0007*** | −0.0007*** | −0.0007*** |
| Sex | 0.195*** | 0.055 | 0.350*** | 0.379*** | 0.321*** | 0.305*** | 0.292*** | 0.457*** | 0.441*** | 0.453*** |
| Years of Schooling | 0.031** | 0.038** | 0.079*** | 0.066*** | 0.102*** | 0.086*** | 0.060*** | 0.029* | 0.022† | 0.030* |
| Spanish Fluency | −0.427*** | 0.213*** | −0.004 | 0.027 | 0.042 | 0.073† | 0.072** | 0.050 | 0.054 | 0.084† |
|
| ||||||||||
| Variance Between Communities | 0.419 | 0.176 | 0.106 | 0.187 | 0.277 | 0.403 | 0.239 | 0.290 | 0.272 | 0.289 |
| Variance Within Communities | 0.807 | 0.912 | 0.935 | 0.914 | 0.848 | 0.773 | 0.448 | 0.855 | 0.846 | 0.833 |
p ≤ .1,
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001.
Table 4.
Multivariate regressions predicting cognitive performance for (a) individuals aged <25 (n=351), and (b) aged 40+ (n = 376). Community residence, matriline and patriline are random effects.
| AGE < 25 | Short Term Recall | Long Term Recall | Tsimane Digit Forward | Spanish Digit Forward | Spatial Digit Forward | Visual Search | “Effortful Processing” | Animals | Fish | Category Fluency |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.028** | 0.015 | 0.022* | 0.005 | 0.017† | 0.027*** | 0.013** | 0.068*** | 0.066*** | 0.065*** |
| Male | 0.149 | −0.053 | 0.267** | 0.113 | 0.163† | 0.168* | 0.170*** | 0.446*** | 0.435*** | 0.453*** |
| Highest Grade | 0.035 | 0.050* | 0.096*** | 0.128*** | 0.134*** | 0.065*** | 0.084*** | 0.030 | −0.008 | 0.019 |
| Spanish Fluency | −0.470 | 0.279*** | 0.137* | 0.077 | 0.022 | 0.091† | 0.126*** | 0.086 | 0.148* | 0.179** |
|
| ||||||||||
| Variance Between Communities | 0.431 | 3.44e-12 | 1.07e-08 | 0.182 | 0.257 | 0.408 | 0.178 | 0.434 | 0.321 | 0.436 |
| Variance Within Communities | 0.840 | 0.910 | 0.888 | 0.934 | 0.848 | 0.679 | 0.446 | 0.775 | 0.783 | 0.753 |
| AGE 40+ | Short Term Recall | Long Term Recall | Tsimane Digit Forward | Spanish Digit Forward | Spatial Digit Forward | Visual Search | “Effortful Processing” | Animals | Fish | Category |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | −0.022*** | −0.032*** | −0.013** | −0.015** | −0.010* | −0.033*** | −0.021*** | −0.013** | −0.005 | −0.009* |
| Male | 0.194* | 0.093 | 0.396*** | 0.551*** | 0.454*** | 0.349*** | 0.377*** | 0.379*** | 0.382*** | 0.354*** |
| Years of Schooling | 0.003 | 0.0001 | 0.040† | 0.010 | 0.045* | 0.052** | 0.026** | 0.015 | 0.021 | 0.026 |
| Spanish Fluency | −0.255*** | 0.203** | −0.104 | −0.019 | 0.107 | 0.073 | 0.083* | 0.051 | 0.005 | 0.053 |
|
| ||||||||||
| Variance Between Communities | 0.354 | 0.1627 | 0.210 | 0.290 | 0.292 | 0.366 | 0.286 | 0.288 | 0.246 | 0.258 |
| Variance Within Communities | 0.7138 | 0.9375 | 0.938 | 0.843 | 0.784 | 0.732 | 0.426 | 0.864 | 0.870 | 0.841 |
p ≤ .1,
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001.
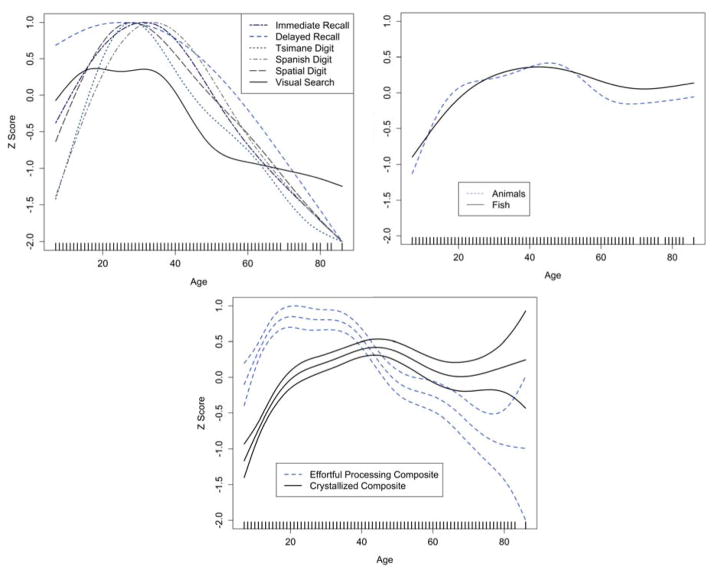
Because of these non-linear effects we use GAMs to provide greater resolution on age patterns of cognitive performance. Controlling for sex, schooling and Spanish fluency, many abilities show peaks during early adulthood (20’s and 30’s), followed by a considerable plateau throughout mid-adulthood before witnessing declines throughout later life (Figures 1). While the age profiles of many tasks are similar, delayed recall and visual scan tasks show slightly earlier peaks than other abilities, and visual scan shows fewer age-related differences at later ages (Figure 1). On the other hand, semantic fluency performance improves consistently throughout early life and mid adulthood before showing slightly lower performance at later ages (Figure 1).
Figure 1.
Smoothed curve of the effect of age on cognitive performance, controlling for sex, education and Spanish fluency, based on GAM (see text). Upper and lower lines of each curve represent 95% confidence intervals. Rug marks on the X-axis indicate the range of unique ages in the sample (range: 8–88 yrs). In all models, age represented a significant smoothing term (p’s < 0.001). Sex, education, and Spanish fluency added as controls to the models.
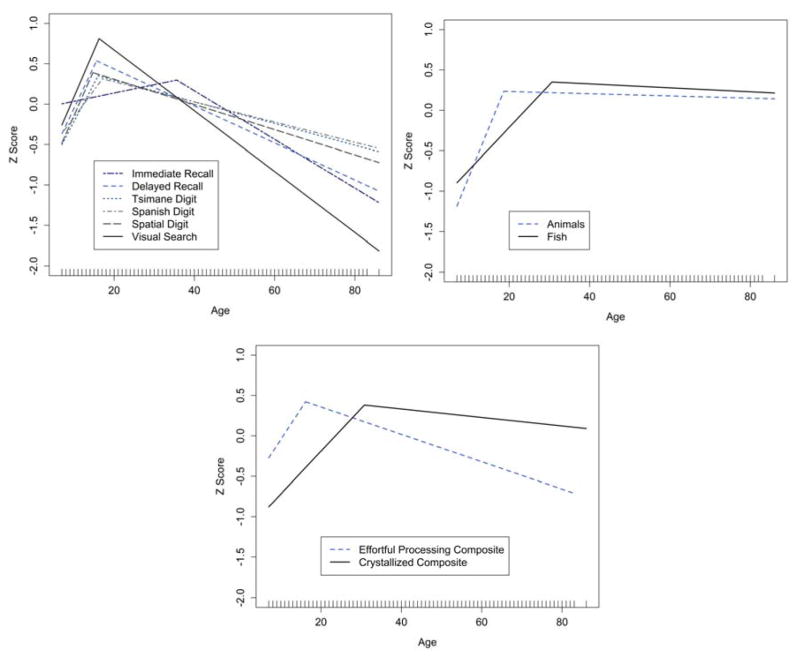
We employ segmented multivariate linear regressions to estimate the ages of peak performance, and differences in performance at ages earlier and later than the peak (Table 5). Most “effortful processing” tasks peak earlier (average: 16.2 years) than semantic fluency tasks (average: 30.6 years, p < 0.001) (Figure 2, Table 5). These peak ages are somewhat earlier than those displayed in GAMs (Figure 1). “Effortful processing” abilities improve significantly from early age until mid-adolescence (β = 1.409, p < 0.001) and then show lower performance thereafter (β = −0.314, p < 0.001). Semantic fluency abilities improve significantly from early age until mid-adulthood (β = 1.042, p < 0.001) and are only slightly lower among older adults (β = −0.081, p < 0.001).
Table 5.
Segmented linear regression predicting peak age of cognitive performance across subtasks (n=919 individuals)
| Cognitive Task | Age of peak (yrs) | Slope of increase to peak | Slope of decline from peak | Decline per decade |
|---|---|---|---|---|
| Immediate Word Recall | 34.6 | 0.237*** | −0.558*** | −0.300 |
| Delayed Word Recall | 15.6 | 1.961*** | −0.425*** | −0.229 |
| Tsimane Digit Forward | 16.1 | 1.731** | −0.250** | −0.135 |
| Spanish Digit Forward | 17.0 | 1.272* | −0.231* | −0.124 |
| Spatial Forward | 14.7 | 2.107** | −0.289** | −0.155 |
| Visual Scan | 16.3 | 2.149*** | −0.700*** | −0.377 |
| Composite “Effortful Processing” | 16.2 | 1.409*** | −0.314*** | −0.169 |
|
| ||||
| Category Fluency | ||||
| Semantic Fluency (Animals) | 30.8 | 2.404*** | −0.016*** | −0.009 |
| Semantic Fluency (Fish) | 30.7 | 0.974*** | −0.046*** | −0.025 |
| Composite | 30.6 | 1.042*** | −0.081*** | −0.044 |
= p < 0.05,
= p < 0.01,
= p <0.001
Note: Slopes are reported as standardized betas. Expected decline in performance is in standard deviation units per decade for each subtask and composite measure.
Figure 2.
Estimated age of peak cognitive performance for all tasks. (a) composite “effortful processing” components, (b) category fluency subtasks, and (c) composite scores. Rug marks on the X-axis represent the range of unique ages in the sample.
Effects of schooling on acquisition and age of peak performance
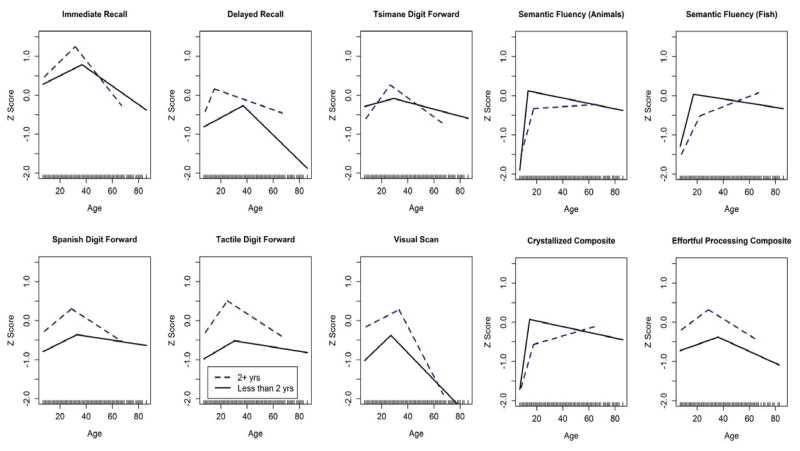
We use segmented linear regressions to consider whether schooling plays a significant role in the cognitive performance of adolescents and thereby on the peak age of cognitive performance. Performance in “effortful processing” abilities peaks 10.6 years earlier and 1.21 SD higher for individuals with 2+ vs. <2 years of schooling (Figure 3). Across most ages, those with low or no schooling perform significantly below the population average. Performance on semantic fluency tasks exhibits a different pattern than the other tasks: those with 2+ years of schooling display rapid improvement throughout adolescence followed by minimal change or even slight increase with age (Figure 3). Those with <2 years of schooling exhibit a similar pattern at early ages, but then show slightly lower performance from age 20 onward. Comparing Tsimane with no schooling to those with some (1–2 years) or more (3+ years) shows similar dose-response patterns across different cognitive tasks (Figures S4).
Figure 3.
Segmented linear regression predicting cognitive performance across two levels of educational attainment (<2 years vs. 2+ years).
Years of schooling and Spanish fluency are consistently positively associated with cognitive performance in “effortful processing” tasks, but only Spanish fluency is associated with performance in semantic fluency tasks (Table 4). For individuals <25 years old, there is a significant negative interaction between age and schooling on “effortful processing” composite performance and numerous sub-tasks (Table 6); educated individuals perform higher than uneducated individuals at earliest ages, but then their performance proceeds at a slightly slower rate towards a higher peak than observed in less educated age-matched peers. There is no significant age*schooling interaction on semantic fluency performance (Table S6a).
Table 6.
Schooling, and rates of cognitive performance: acquisition (<25 yrs) and decline (>40 yrs)
| AGE <25 | Short Term Recall | Long Term Recall | Tsimane Digit Forward | Spanish Digit Forward | Spatial Digit Forward | Visual Search | “Effortful Processing” | Animals | Fish | Category Fluency |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.046*** | 0.036* | 0.022 | 0.015 | 0.047*** | 0.055*** | 0.034*** | 0.074*** | 0.054** | 0.062*** |
| Male | 0.144 | −0.053 | 0.267** | 0.112 | 0.158† | 0.158* | 0.165*** | 0.445*** | 0.437*** | 0.454*** |
| Years of Schooling | 0.165* | 0.197** | 0.095 | 0.200* | 0.348*** | 0.275*** | 0.232*** | 0.071 | −0.088 | 0.004 |
| Spanish Fluency | −0.465*** | 0.279*** | 0.137* | 0.079 | 0.027 | 0.103† | 0.129*** | 0.088 | 0.146* | 0.178** |
| Age* Grade | ||||||||||
| Level Interaction | −0.007† | −0.008* | 0.0001 | −0.004 | −0.011** | −0.011*** | −0.008*** | −0.002 | 0.004 | 0.001 |
|
| ||||||||||
| Variance Between Communities | 0.434 | 0.004 | 2.87e-13 | 0.184 | 0.243 | 0.371 | 0.165 | 0.440 | 0.314 | 0.434 |
| Variance Within Communities | 0.835 | 0.904 | 0.888 | 0.933 | 0.838 | 0.671 | 0.438 | 0.774 | 0.782 | 0.753 |
| AGE 40+ | Short Term Recall | Long Term Recall | Tsimane Digit Forward | Spanish Digit Forward | Spatial Digit Forward | Visual Search | “Effortful Processing” | Animals | Fish | Category Fluency |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | −0.019** | −0.032*** | −0.011* | −0.012* | −0.011* | −0.030*** | −0.019*** | −0.009† | −0.006 | −0.008† |
| Male | 0.184* | 0.093 | 0.390*** | 0.541*** | 0.457*** | 0.339*** | 0.373*** | 0.368*** | 0.385*** | 0.350*** |
| Years of Schooling | 0.144† | −0.004 | 0.116 | 0.149 | 0.005 | 0.173† | 0.088 | 0.168 | −0.025 | 0.077 |
| Spanish Fluency | −0.246*** | 0.203** | −0.099 | −0.011 | 0.104 | 0.081 | 0.087* | 0.060 | 0.002 | 0.056 |
| Age* Grade | ||||||||||
| Level Interaction | −0.003† | 0.0001 | −0.002 | −0.003 | 0.001 | −0.002 | −0.001 | −0.003 | 0.001 | −0.001 |
|
| ||||||||||
| Variance Between Communities | 0.348 | 0.162 | 0.204 | 0.288 | 0.293 | 0.368 | 0.285 | 0.290 | 0.243 | |
| Variance Within Communities | 0.712 | 0.938 | 0.938 | 0.841 | 0.784 | 0.730 | 0.425 | 0.861 | 0.870 | |
p ≤ .1,
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001.
Effect of schooling on rate of cognitive decline
To test predictions of the passive and active models of cognitive reserve, we include an age*schooling term in the sub-sample of adults aged 40+ (n = 376) (Tables 4, 5b, 6b). A significant main effect of schooling on performance but no significant age*schooling term would be consistent with no buffering effect of schooling on cognitive decline, as predicted by the passive reserve model. A positive and significant age*schooling term would instead support the active reserve model, suggesting that there is an attenuating effect of schooling on cognitive decline at later ages. A third possibility, where a positive main effect of schooling and a negative age*schooling term, would instead suggest that the protective effects of schooling are smaller at late ages.
We find a positive effect of schooling on several tasks, including short term recall and visual scan, no effect on other tasks or on the composite measures, and no significant age*schooling term for any measure (Table 6), consistent with the passive reserve model. To explore non-linear effects of schooling on rates of “effortful processing” cognitive decline, we divide the sample into low (<2 years) and moderate to high (2+ years) schooling, and similarly performed multivariate linear regression (Table 7). In order to determine whether differences in schooling produce distinct patterns of decline, we conducted a t-test for significant differences in slope with age. Results show that the slopes with age are similar across both groups (t(342) = 1.093, p = 0.275), confirming that there is no significant difference in rates of decline for those with minimal or moderate schooling. A positive effect of Spanish fluency on “effortful processing” composite performance is only observed among individuals with less than two years of schooling (Table 7).
Table 7.
Mixed effects regressions predicting performance among adults age 40+ on z-scores of fluid composite by education level with community residence, matriline and patriline as random effects.
| Education | ||||
|---|---|---|---|---|
| < 2 yrs (n = 229) | 2+ years (n = 117) | |||
|
| ||||
| B | SE | B | SE | |
| Intercept | 0.222 | 0.190 | 0.786** | 0.280 |
| Age | −0.020*** | 0.003 | −0.018*** | 0.005 |
| Male | 0.299*** | 0.065 | 0.340*** | 0.080 |
| Spanish Fluency | 0.140** | 0.030 | −0.029 | 0.042 |
|
| ||||
| Variance Between Communities | 0.233 | 0.054 | 0.348 | 0.075 |
| Variance Within Communities | 0.424 | 0.025 | 0.384 | 0.029 |
Note. Sample comprised of all individuals aged 40+, broken into two levels of educational attainment.
= p < 0.05,
= p < 0.01,
= p <0.001
Community differences, schooling and cognitive performance
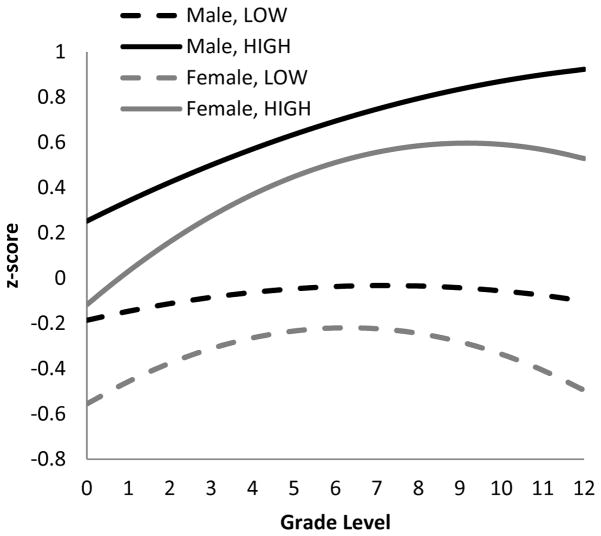
In all models, substantial variance in performance was captured by the random effect for community ID. Communities vary in the length of time schools have existed, in the quality of schools (e.g. building construction, classroom materials), and in the quality of teachers. To explore variable relationships between educational attainment in different villages, we grouped village schooling level into three categories: low (with no or relatively recent schools appearing in the past decade), moderate (schools have existed for about two decades) and high (schools have existed for longer than two decades), and interacted this variable with education. We also consider quadratic schooling terms to explore potential non-linear relationships between schooling and cognitive performance. Controlling for age, age2 and Spanish fluency, we find that the effects of grade on “effortful processing” composite performance are greatest among those with lower levels of schooling, and diminish among those at higher educational levels (significant negative quadratic term), especially among women (Table 8, Models 2 & 3; Figure 4). The effects of schooling, however, vary considerably depending on village residence. Coming from a village with low school quality is associated with 0.4–0.5 lower z-scores in performance, on average, and the increase in performance with grade level is smaller in these villages (significant interaction terms between grade and village school level). Similar effects are found when examining each cognitive task separately, but interactions of village schooling history and grade is significant only for visual scan and verbal recalls (Table S1). Schooling does not bears any non-linear relationships with semantic fluency (Table 8, S1). Yet more remote villages with the least history of schooling show roughly 0.4 z-score units greater semantic fluency.
Table 8.
Schooling effects as a function of village history and quality of schooling (captured by the variable “Village School level”). (n=844). Models include random effect terms for community, matriline and patriline.
| “Effortful Processing” Tasks Composite | Category Fluency Composite | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Predictor | est. | est. | est. | est. | est. | est. |
| Intercept | −0.372*** | −0.563*** | −0.599*** | −1.839*** | −1.830*** | −1.843*** |
| Age | 0.017*** | 0.020*** | 0.020*** | 0.071*** | 0.071*** | 0.071*** |
| Age2 | −0.000*** | −0.000*** | −0.000*** | −0.001*** | −0.001*** | −0.001*** |
| Sex | 0.269*** | 0.271*** | 0.355*** | 0.444*** | 0.444*** | 0.476*** |
| Grade | 0.060*** | 0.125*** | 0.156*** | 0.025† | 0.011 | 0.026 |
| Grade2 | −0.006*** | −0.008*** | 0.001 | −0.000 | ||
| Spanish | 0.084*** | 0.154*** | 0.151*** | 0.034 | 0.058 | 0.058 |
| Spanish2 | −0.020 | −0.019† | −0.005 | −0.005 | ||
| Sex*Grade | −0.064* | −0.031 | ||||
| Sex*Grade2 | 0.006* | 0.003 | ||||
| Village Education Level | ||||||
| (low vs. high) | −0.495*** | −0.432*** | −0.438*** | 0.416** | 0.407** | 0.404** |
| (moderate vs. high) | −0.072 | −0.043 | −0.052 | 0.195 | 0.191 | 0.187 |
| Grade*Village School Level | ||||||
| (low vs. high) | −0.035 | −0.049* | −0.049* | −0.025 | −0.022 | −0.022 |
| (moderate vs. high) | −0.050** | −0.052** | −0.049** | 0.052† | 0.053† | 0.055† |
Figure 4.
“Effortful processing” cognitive performance, schooling and village history of schooling (only “low”, “high” shown here). Based on Model 3 in Table 8.
Discussion
Consistent with many studies documenting distinct age patterns of cognitive abilities (Craik & Bialystok 2006; Schaie 2005; Hedden & Gabrieli 2004; McArdle et al. 2002; Wilson et al. 1999), we find that effortful cognitive performance spanning working memory, attention and psychomotor speed domains peaked in early adulthood and thereafter performance was lower than in tasks oriented around semantic fluency (Figures 1, 2). The present study is the first to replicate this general cognitive aging profile in a traditional subsistence population with low levels of schooling. We find ages of peak performance in early-mid adulthood, and greater age-related differences in performance on effortful processing tasks tapping working memory, short- and long-term verbal declarative memory, visual search and spatial memory (Table 5). For short- and long-term verbal memory, declines manifest at an average recall of 4.1% and 3.7% fewer words per decade after age of peak performance, respectively. These age-related differences, while modest, are consistent with other studies in industrialized populations indicating earlier onset of decline in abilities related to cognitive-processing and memory in healthy adult samples (Baltes & Lindenberger 1997; Germine et al. 2011; Halberda et al. 2012; Hartshorne & Germine 2015; Murre et al. 2013). For example, a recent study of cognitive performance in the UK showed 10-yr cross-sectional declines at ages 45–49 ranging from −2.9% to −3.6% in men, and −6.5% to −11.4% in women (Singh-Manoux et al. 2012: Table 2); cross-sectional rates of decline among age-matched Tsimane in analogous tasks are −4.6% in men (ranging from −0.1% to −9.1%) and −4.5% in women (ranging from −1.9% to −8.8%).
Overall we find no significant attenuating effect of relatively low levels of schooling on age-related differences in cognitive performance in later adulthood, consistent with the passive reserve model. In our sub-sample of adults aged 40+, those with greater schooling exhibited similar age-related differences in performance as their less-schooled counterparts. This finding supports recent longitudinal studies that failed to document the presence of active reserve among Western adults with higher levels of education than observed here (Tucker-Drob et al. 2009; Van Dijk et al. 2008; Karlamangla et al. 2009; Alley et al. 2007; Zahodne et al. 2011) and is also consistent with studies suggesting that, at least for some cognitive abilities, lower performance at late age may represent reductions in an individual’s ability to sustain investment in metabolically expensive faculties in the face of persistent challenges (Trollor et al. 2005; Minoshima et al. 1997). Instead we find significant positive main effects of schooling on cognitive performance over much of the life course, consistent with passive models positing improved cerebral and neural reserves among those with greater schooling (Stern 2002). Further, these effects are greater in villages with a longer history of schooling, where teachers are more experienced and schools are generally of higher quality. For semantic fluency, schooling and Spanish fluency were weakly associated with greater performance. However, only for semantic fluency was performance higher in remote villages that only recently obtained schools. We suspect this is because there is more active hunting and fishing in these more remote villages (Gurven et al. 2006), and so greater first-hand and second-hand experience with a larger diversity of animals and fish.
We also show that schooling is associated with greater performance at earliest ages in cognitive skills such as working memory, spatial memory, and short- and long-term verbal recall, particularly throughout adolescence and early adulthood (Figures 3, S4). Because these cognitive skills are not directly taught in school, these results may be due, in part, to the reinforcing effects of non-cognitive skills that are associated with increased exposure to schooling, such as improved attentional control, increases in self-regulatory abilities, and benefits to executive functioning (Blair & Raver 2014; Komarraju et al. 2013). In particular, literate individuals performed significantly better on the visual scan task as they tended to track the page from left to right in a systematic pattern, while non-literate participants did not follow any organized routine. It is possible that Tsimane adolescents and young adults who have accumulated more experience in the classroom may be better equipped to succeed on tests of cognitive ability in part because of greater motivation to perform, sustained attention to focus on each cognitive task in the face of distractions, and expectations generated from formal teacher-pupil interactions (Eklöf 2010). Yet despite the limited range of schooling among Tsimane, we see dose-dependent improvements in performance across levels of schooling (Figures 3, 4, S4), suggesting that there are additional positive effects of schooling on “effortful processing” performance beyond the cultivation of non-cognitive supporting skills. These effects were independent of age, Spanish fluency and village history of schooling.
Why do age profiles of cognitive performance vary by domain?
Evolutionary Perspective on Cognitive Aging
The genetic architecture underlying cognitive performance has been shaped by a number of evolutionary forces, where roughly 28–51% of the variance in intelligence is due to the cumulative effect of thousands of minor-acting common genetic variants (Arslan & Penke 2015). Gene by environment dynamics have likely played an important role in shaping the expression of cognitive traits (Penke & Jokela 2016), and its developmental trajectory (Briley & Tucker-Drob 2015). While the evolutionary and behavioral genetics literature has helped address questions about inter-individual variability, heritability, stability and development, little attention has been placed on the functional logic of distinct age trajectories of cognitive traits.
If aging were a general process, somatic and cognitive function should decline at similar rates, in sync with declines in “reproductive value”, as suggested by Hamilton (1966). Common-cause models of aging often highlight the association between declines in cognitive and physical abilities (Clouston et al. 2013; Christensen et al. 2001; Krall et al. 2014). For instance, Rosano et al. (2005) report a robust association between declines in executive control and declines in physical functioning in samples of healthy aging adults. Other work supporting common-cause models focusing on general reasoning and memory tasks indicates that declines in physical abilities such as gait speed and strength are often good predictors of cognitive deficiencies in elderly adults (Atkinson et al. 2007). Our findings are instead consistent with recent work that disaggregates performance on cognitive tasks (e.g. Hartshorne & Germine 2015). They support the idea that there is no single age at which individuals perform at peak levels on all, or even most, cognitive tasks. Additionally, for many domains of cognitive performance, the functioning of older adults remains particularly strong throughout later life (Hartshorne & Germine 2015; Harada et al. 2013; Cavanaugh & Blanchard-Fields 2006), despite general declines in physical capabilities such as strength, muscle mass, and gait speed (Auyeung et al. 2014; Cooper et al. 2011), and despite increased risk of frailty-induced depression (Stieglitz et al. 2014). The delayed senescence of some cognitive domains relative to others, and the distinction between declines in physical and cognitive performance in later adult life requires explanation. Though speculative, we offer one general account that might help provide an ultimate-level explanation for differences in age-related decline among cognitive domains.
The evolved human life history is characterized by large, encephalized brains, extended life span, downward intergenerational resource transfers and mid-life surplus production (Kaplan et al. 2000). It has been proposed that these features were co-evolved responses to a feeding niche consisting of nutrient-dense resources such as hunted game, extracted roots and other resources that are difficult to acquire. Peak efficiency in food production among hunter-gatherers and forager-horticulturalists occurs in the fourth and fifth decades of life, well after peak physical capabilities are attained (Walker & Hill 2003; Gurven & Kaplan 2006; Gurven et al. 2006). The relatively slow acquisition of foraging and other skills is linked to the development of cognitive abilities, experience and on-the-job training (Schniter et al. 2015). Acquiring and storing extensive complex ecological knowledge (i.e. crystallized intelligence, Bock 2002b; Reyes-García et al. 2009) is a prerequisite for successful food production. Similarly, substantial subsistence endeavors like hunting and stalking prey, careful planning of planting, harvesting and burning schedules, all require developed executive function and other fluid abilities, and the integration of knowledge from past experience. The accumulation and maintenance of a wide range of social relationships (i.e. social capital) also requires diverse skills that together impact another domain of delayed production affecting ego and descendent fitness (Whiten & Byrne 1997; Dunbar & Shultz 2007).
Understanding the origins of particular age profiles of cognitive development and senescence requires attention to the skills and fitness-related consequences thereof in the kinds of environments more similar to those of our ancestors. That many cognitive age profiles among Tsimane are similar to those in industrialized populations despite their remoteness and very distinct socio-ecology suggests that changes in the cognitive landscape throughout life relate in important ways to age-related changes in resource production. In all human populations, older, post-reproductive individuals maintain roles as providers and investors in the embodied capital of their descendent kin. This often involves direct or indirect instruction, advising, conflict mediation, storytelling and information sharing, especially when their own resource productivity is increasingly diminished (Kline 2014; Gurven et al. 2012; Schniter et al. 2015; Stieglitz et al. 2013).
If natural selection shaped cognition to promote the transition from knowledge acquisition (i.e., investment in wide range of fluid and crystallized capabilities) in early life, to production and investment in descendant kin (i.e., crystallized capabilities) throughout adulthood, then some effortful cognitive domains might be subject to degradation at a more rapid rate, while others preserved well into late age, depending on their relative contributions to fitness. If informational transfer provided dependable and measurable benefits to descendent kin above and beyond the costs associated with decreased productivity, selection should have retained those cognitive skills that are implicated directly in knowledge retention, memory retrieval, and information transmission, despite declining physical abilities and more fluid cognitive abilities. Those retained would include category fluency and other domains of crystallized abilities. Recent genetic evidence seems to support evolutionary scenarios where older adults are equipped to impact descendant kin. Schwarz et al. (2016) provide evidence of the relatively rapid proliferation of protective alleles unique to the human lineage that help maintain cognitive function at late ages, such as the immunoregulatory receptor CD33, ApoE and PON1.
Several studies are consistent with our “trade-off” perspective to understanding cognitive aging. Reser (2009) reports that metabolic activity in the hippocampus and neocortex becomes increasingly more costly with age. Normal aging has been consistently associated with atrophy in general cerebral capacity, driven largely by reductions in both gray and white matter (Pakkenberg et al. 2003; Lemaitre et al. 2012). These effects are largely present in the prefrontal regions (Tisserand & Jolles 2003; Allen et al. 2005), which are implicated heavily in speed of mental processing, working memory, and overall executive control. The large metabolic cost of simultaneously preserving multiple fluid domains likely places a significant constraint on metabolic investment in older adults. As productivity declines in middle adulthood, it may be less beneficial to sustain high investment across all brain regions; young adults may be more inclined to pay such high cost activation of expensive brain networks because they stand to benefit greatly over a long time period from the learning that accompanies it. Upon reaching adulthood, humans living in environments characteristic of ancestral populations would have already sampled diverse aspects of the local ecology, and internalized much of the social and ecological information that they need to become efficient producers and social contributors. Once an individual reaches mid- to late-adulthood, the maintenance costs associated with many effortful processing capabilities may surpass their expected marginal returns to increased productivity and/or fitness. The consistent tradeoff between investment in more fluid abilities during early adulthood and the reliance on crystallized information storage in later life may represent a functionally efficient compromise. The resulting age trajectories of cognitive skills affect production in predictable ways. For example, in modern industrialized societies, peak ages in productivity for jobs that reward experience, managerial ability and other crystallized skills are relatively late in adulthood, despite declines in fluid abilities (Skirbekk 2008, 2004).
Limitations and Future Directions
The cross-sectional design prevents assessment of individual life course trajectories. Cross-sectional data can also be problematic when there are cohort effects, although longitudinal data often suffers from bias due to retest practice effects artificially inflating cognitive change over time (Rabbitt et al. 2001). Recent evaluations confirm that cross-sectional studies with minimal cohort differences may offer more valid inferences about age-related changes than longitudinal studies biased by retest effects (Salthouse 2009). Indeed, retesting among Tsimane has been shown to lead to improvement in at least one cognitive task (Ravens colored progressive matrices), especially among more educated individuals (Davis 2014). Thus, our results concerning differential rates of cognitive decline from our one-shot cross-sectional design are at least consistent with those in cross-sectional studies in other human populations, and in non-human populations where cohort differences are non-existent (Herndon et al. 1997; Salthouse 2009). Here we argue that schooling plays an important role in the acquisition of cognitive skill at young ages; if older adults are less educated than their younger counterparts due to more limited access to educational opportunities, then naturally this raises concerns about the availability of formal schooling across communities and across ages. In the current sample, however, there is considerable community-level variability in access to schools and the duration of their establishment, and so schooling is not heavily confounded with age cohort. Nevertheless, future research focused on documenting age-related differences in cognition in similar populations should take into consideration the potential reinforcing effects of education on performance, particularly among adolescents and young adults. It is also possible that cognitive buffering might require more schooling than the low levels observed in the Tsimane population.
Despite these limitations, the current results provide important information that will help improve our understanding of normative changes in cognition across the life course. Our results support existing patterns of cognitive aging across the life cycle in samples of healthy individuals and extend existing research by documenting the effects of educational attainment on both the acquisition of cognitive skills and the rate of cognitive declines with age. To our knowledge, the current study is among the first to replicate age-related patterns of cognitive performance within a small-scale human society characterized by limited exposure to formalized schooling, and is one of a few attempts to provide an evolutionary framework for the distinct age profiles of different cognitive abilities.
Supplementary Material
Acknowledgments
We thank the Tsimane for participating, and THLHP personnel for collecting and coding data. Research was supported by grants to MG and HK from the NIH/NIA (R01AG024119, R56AG024119) and the NSF (BCS-0422690).
Footnotes
Author contributions: MG, HK designed research; MG, HK performed research; MG, EF, BT analyzed data; BB organized datasets; MG, EF wrote the paper, with assistance from BT, JS, HD.
References
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Alley D, Suthers K, Crimmins EM. Education and cognitive decline in older Americans: Results from the AHEAD sample. Research on Aging. 2007;29:73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Ardila a, Ostrosky-Solis F, Rosselli M, Gómez C. Age-related cognitive decline during normal aging: the complex effect of education. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2000;15:495–513. [PubMed] [Google Scholar]
- Ardila A, Ostrosky-Solís F, Bernal B. Cognitive testing toward the future: The example of Semantic Verbal Fluency (ANIMALS) International Journal of Psychology. 2006;41:324–332. doi: 10.1080/00207590500345542. [DOI] [Google Scholar]
- Ardila A, Rosselli M. Illiterates and Cognition: The Impact of Education. International Handbook of Cross-Cultural Neuropsychology. 2007:181–198. [Google Scholar]
- Arslan RC, Penke L. Zeroing in on the Genetics of Intelligence. Journal of Intelligence. 2015;3(2):41–45. [Google Scholar]
- Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, … Kritchevsky SB. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- Auyeung TW, Lee SWJ, Leung J, Kwok T, Woo J. Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatrics & gerontology international. 2014;14(Suppl 1):76–84. doi: 10.1111/ggi.12213. [DOI] [PubMed] [Google Scholar]
- Baker DP, Eslinger PJ, Benavides M, Peters E, Dieckmann NF, Leon J. The cognitive impact of the education revolution : A possible cause of the Flynn Effect on population IQ. Intelligence. 2015;49:144–158. doi: 10.1016/j.intell.2015.01.003. [DOI] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Closing the achievement gap through modification of neurocognitive and neuroendocrine function: results from a cluster randomized controlled trial of an innovative approach to the education of children in kindergarten. PloS one. 2014;9:e112393. doi: 10.1371/journal.pone.0112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J. Evolutionary demography and intrahousehold time allocation: School attendance and child labor among the Okavango Delta Peoples of Botswana. American Journal of Human Biology. 2002a;14(2):206–221. doi: 10.1002/ajhb.10040. [DOI] [PubMed] [Google Scholar]
- Bock J. Learning, Life History, and Productivity: Children’s Lives in the Okavango Delta, Botswana. Human Nature. 2002b;13(2):161–198. doi: 10.1007/s12110-002-1007-4. [DOI] [PubMed] [Google Scholar]
- Briley DA, Tucker-Drob EM. Comparing the Developmental Genetics of Cognition and Personality over the Life Span. Journal of Personality. 2015 doi: 10.1111/jopy.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinch CN, Galloway TA. Schooling in adolescence raises IQ scores. Proceedings of the National Academy of Sciences. 2012;109:425–430. doi: 10.1073/pnas.1106077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Zook Na, DeLosh EL, Davalos DB, Davis HP. Age differences in fluid intelligence: contributions of general slowing and frontal decline. Brain and cognition. 2006;62:9–16. doi: 10.1016/j.bandc.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bull R, Scerif G. Executive functioning as a predictor of children’s mathematics ability: inhibition, switching, and working memory. Developmental neuropsychology. 2001;19:273–293. doi: 10.1207/S15326942DN1903_3. [DOI] [PubMed] [Google Scholar]
- Butler SM, Ashford JW, Snowdon DA. Age, education, and changes in the Mini-Mental State Exam scores of older women: Findings from the Nun Study. Journal of the American Geriatrics Society. 1996;44(6):675–681. doi: 10.1111/j.1532-5415.1996.tb01831.x. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Dahl GB, Öckert B, Rooth DO. The effect of schooling on cognitive skills. The Review of Economics and Statistics. 2015;97:533–547. doi: 10.1162/REST. [DOI] [Google Scholar]
- Carroll JB. Human Cognitive Abilities. 1993 doi: 10.1017/CBO9780511571312. [DOI] [Google Scholar]
- Cattell RB. Abilities: Their Structure, Growth and Action. Oxford, England: Houghton Mifflin; 1971. [Google Scholar]
- Cavanaugh JC, Blanchard-Fields F. Adult Development and Aging. Belmont, CA: Thomson Learning; 2006. [Google Scholar]
- Ceci SJ. How much does schooling influence general intelligence and its cognitive components? A reassessment of the evidence. Developmental Psychology. 1991;27:703–722. doi: 10.1037//0012-1649.27.5.703. [DOI] [Google Scholar]
- Cheng H, Furnham A. Childhood cognitive ability, education, and personality traits predict attainment in adult occupational prestige over 17years. Journal of Vocational Behavior. 2012;81(2):218–226. [Google Scholar]
- Christensen H, Mackinnon AJ, Korten A, Jorm AF. The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychology and Aging. 2001;16:588–599. doi: 10.1037//0882-7974.16A588. [DOI] [PubMed] [Google Scholar]
- Clouston SAP, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, … Hofer SM. The Dynamic Relationship Between Physical Function and Cognition in Longitudinal Aging Cohorts. Epidemiologic Reviews. 2013;35:33–50. doi: 10.1093/epirev/mxs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Hardy R, Aihie Sayer A, Ben-Shlomo Y, Birnie K, Cooper C, … Kuh D. Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PloS one. 2011;6:e27899. doi: 10.1371/journal.pone.0027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends in Cognitive Sciences. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Davis HE. PhD. University of New Mexico; Albuquerque, NM: 2014. Variable Education Exposure and Cognitive Task Performance. [Google Scholar]
- Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13–21. doi: 10.1016/j.intell.2006.02.001. [DOI] [Google Scholar]
- Duckworth AL, Peterson C, Matthews MD, Kelly DR. Grit: perseverance and passion for long-term goals. Journal of Personality and Social Psychology. 2007;92(6):1087. doi: 10.1037/0022-3514.92.6.1087. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Eklöf H. Skill and will: test-taking motivation and assessment quality. Assessment in Education: Principles, Policy & Practice. 2010;17(4):345–356. [Google Scholar]
- Ekstrom RB, French JW, Harman HH. Manual for Kit of Factor-Referenced Cognitive Tests 1976 [Google Scholar]
- Farmer ME, Kittner SJ, Rae DS, Bartko JJ, Regier DA. Education and change in cognitive function: The epidemiologic catchment area study. Annals of epidemiology. 1995;5(1):1–7. doi: 10.1016/1047-2797(94)00047-w. [DOI] [PubMed] [Google Scholar]
- Feinstein L, Bynner J. The importance of cognitive development in middle childhood for adulthood socioeconomic status, mental health, and problem behavior. Child development. 2004;75:1329–1339. doi: 10.1111/j.1467-8624.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- Fortenbaugh FC, DeGutis J, Germine L, Wilmer JB, Grosso M, Russo K, Esterman M. Sustained Attention Across the Life Span in a Sample of 10, 000: Dissociating Ability and Strategy. Psychological Science. 2015 doi: 10.1177/0956797615594896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnham A, Cheng H. Factors influencing adult earnings: Findings from a nationally representative sample. Journal of Socio-Economics. 2013;44:120–125. doi: 10.1016/j.socec.2013.02.008. [DOI] [Google Scholar]
- Germine LT, Duchaine B, Nakayama K. Where cognitive development and aging meet: face learning ability peaks after age 30. Cognition. 2011;118:201–210. doi: 10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Glosser G, Wolfe N, Albert ML, Lavine L, Steele JC, Calne DB, Schoenberg BS. Cross-Cultural Cognitive Examination: Validation of a Dementia Screening Instrument for Neuroepidemiological Research. Journal of the American Geriatrics Society. 1993;41(9):931–939. doi: 10.1111/j.1532-5415.1993.tb06758.x. [DOI] [PubMed] [Google Scholar]
- Godoy R, Seyfried C, Reyes-García V, Huanca T, Leonard WR, McDade T, … Vadez V. Schooling’s contribution to social capital: Study from a native Amazonian society in Bolivia. Comparative Education. 2007;43(1):137–163. [Google Scholar]
- Gurven M. Human survival and life history in evolutionary perspective. In: Mitani J, Call J, Kappeler P, Palombit R, Silk JB, editors. The Evolution of Primate Societies. Chicago: University of Chicago Press; 2012. pp. 293–314. [Google Scholar]
- Gurven M, Kaplan H, Gutierrez M. How long does it take to become a proficient hunter? Implications for the evolution of delayed growth. Journal of Human Evolution. 2006;51:454–470. doi: 10.1016/j.jhevol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Zelada Supa A. Mortality experience of Tsimane Amerindians: regional variation and temporal trends. American Journal of Human Biology. 2007;19:376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Hooper PL, Gomes C, Kaplan H. From the womb to the tomb: The role of transfers in shaping the evolved human life history. Experimental Gerontology. 2012;47:807–813. doi: 10.1016/j.exger.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven MD, Kaplan HS. Determinants of time allocation to production across the lifespan among the Machiguenga and Piro Indians of Peru. Human Nature. 2006;17(1):1–49. doi: 10.1007/s12110-006-1019-6. [DOI] [PubMed] [Google Scholar]
- Halberda J, Ly R, Wilmer JB, Naiman DQ, Germine L. Number sense across the lifespan as revealed by a massive Internet-based sample. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11116–11120. doi: 10.1073/pnas.1200196109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WD. The molding of senescence by natural selection. Journal of Theoretical Biology. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Harada CN, Love MCN, Triebel KL. Normal cognitive aging. Clinics in geriatric medicine. 2013;29(4):737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne JK, Germine LT. When Does Cognitive Functioning Peak? The Asynchronous Rise and Fall of Different Cognitive Abilities Across the Life Span. Psychological Science. 2015:1–11. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hedden T, Park DC, Nisbett R, Ji LJ, Jing Q, Jiao S. Cultural variation in verbal versus spatial neuropsychological function across the life span. Neuropsychology. 2002;16:65–73. doi: 10.1037/0894-4105.16.1.65. [DOI] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A. The weirdest people in the world? Behavioral and Brain Sciences. 2010;33:61–83. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behavioural brain research. 1997;87(1):25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta psychologica. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster JB, Hurtado AM. A theory of human life history evolution: Diet, intelligence, and longevity. Evolutionary Anthropology. 2000;9(4):156–185. [Google Scholar]
- Kaplan HS, Robson AJ. The emergence of humans: the coevolution of intelligence and longevity with intergenerational transfers. Proceedings of the National Academy of Sciences. 2002;99:10221–10226. doi: 10.1073/pnas.152502899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. American journal of epidemiology. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline MA. How to learn about teaching: An evolutionary framework for the study of teaching behavior in humans and other animals. Behavioral and Brain Sciences. 2014:1–70. doi: 10.1017/S0140525X14000090. [DOI] [PubMed] [Google Scholar]
- Komarraju M, Ramsey A, Rinella V. Cognitive and non-cognitive predictors of college readiness and performance: Role of academic discipline. Learning and Individual Differences. 2013;24:103–109. doi: 10.1016/j.lindif.2012.12.007. [DOI] [Google Scholar]
- Krall JR, Carlson MC, Fried LP, Xue QL. Examining the Dynamic, Bidirectional Associations Between Cognitive and Physical Functioning in Older Adults. American journal of epidemiology. 2014;180:838–846. doi: 10.1093/aje/kwu198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Eng GK, Rapisarda A, Subramaniam M, Kraus M, Keefe RSE, Collinson SL. Formulation of the age-education index: measuring age and education effects in neuropsychological performance. Psychological assessment. 2013;25:61–70. doi: 10.1037/a0030548. [DOI] [PubMed] [Google Scholar]
- Le Carret N, Lafont S, Mayo W, Fabrigoule C. The effect of education on cognitive performances and its implication for the constitution of the cognitive reserve. Developmental neuropsychology. 2003;23:317–337. doi: 10.1207/S15326942DN2303_1. [DOI] [PubMed] [Google Scholar]
- Lee RD. Rethinking the evolutionary theory of aging: Transfers, not births, shape senescence in social species. PNAS. 2003;100(16):9637–9642. doi: 10.1073/pnas.1530303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovici D, Ritchie K, Ledesert B, Touchon J. Does Education Level Determine the Course of Cognitive Decline? Age Ageing. 1996;25:392–397. doi: 10.1093/ageing/25.5.392. [DOI] [PubMed] [Google Scholar]
- Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Normal age-related brain morphometric changes: Nonuniformity across cortical thickness, surface area and grey matter volume? Pubmed. 2012;33:1–15. doi: 10.1016/j.neurobiolaging.2010.07.013.Normal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: an 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area study. American Journal of Psychiatry. 1999;156(1):58–65. doi: 10.1176/ajp.156.1.58. [DOI] [PubMed] [Google Scholar]
- Martin MA, Lassek WD, Gaulin SJ, Evans RW, Woo JG, Geraghty SR, … Gurven MD. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Maternal & child nutrition. 2012;8(3):404–418. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Experience-dependent structural plasticity in the adult human brain. Trends in Cognitive Sciences. 2011;15:475–482. doi: 10.1016/j.tics.2011.08.002. [DOI] [PubMed] [Google Scholar]
- McAllister L, Gurven M, Kaplan H, Stieglitz J. Why do women have more children than they want? Understanding differences in women’s ideal and actual family size in a natural fertility population. American Journal of Human Biology. 2012;24(6):786–799. doi: 10.1002/ajhb.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology. 2002;38:115–142. doi: 10.1037//0012-1649.38.1.115. [DOI] [PubMed] [Google Scholar]
- Mejía-Arango S, Wong R, Michaels-Obregón A. Normative and standardized data for cognitive measures in the Mexican Health and Aging Study. Salud publica de Mexico. 2015;57:s90–s96. doi: 10.21149/spm.v57s1.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Annals of neurology. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Murre JMJ, Janssen SMJ, Rouw R, Meeter M. The rise and fall of immediate and delayed memory for verbal and visuospatial information from late childhood to late adulthood. Acta Psychologica. 2013;142:96–107. doi: 10.1016/j.actpsy.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Lövdén M, Riklund K, Lindenberger U, Bäckman L. Memory aging and brain maintenance. Trends in Cognitive Sciences. 2012;16(5):292–305. doi: 10.1016/j.tics.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJG, Nyengaard JR, Regeur L. Aging and the human neocortex. Experimental gerontology. 2003;38:95–99. doi: 10.1016/S0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Park D, Gutchess A. The cognitive neuroscience of aging and culture. Current Directions in Psychological Science. 2006;15:105–108. doi: 10.1111/j.0963-7214.2006.00416.x. [DOI] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and aging. 2002;17:299–320. doi: 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Penke L, Jokela M. The evolutionary genetics of personality revisited. Current Opinion in Psychology. 2016;7:104–109. http://dx.doi.org/10.1016/j.copsyc.2015.08.021. [Google Scholar]
- Poletti M, Emre M, Bonuccelli U. Mild cognitive impairment and cognitive reserve in Parkinson’s disease. Parkinsonism & related disorders. 2011;17:579–586. doi: 10.1016/j.parkreldis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Diggle P, Smith D, Holland F, Mc Innes L. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia. 2001;39(5):532–543. doi: 10.1016/s0028-3932(00)00099-3. [DOI] [PubMed] [Google Scholar]
- Reser JE. Alzheimer’s disease and natural cognitive aging may represent adaptive metabolism reduction programs. Behavioral and brain functions : BBF. 2009;5:13. doi: 10.1186/1744-9081-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-García V, Broesch J, Calvet-Mir L, Fuentes-Peláez N, McDade TW, Parsa S, … Martínez-Rodríguez MR. Cultural transmission of ethnobotanical knowledge and skills: an empirical analysis from an Amerindian society. Evolution and Human Behavior. 2009;30(4):274–285. [Google Scholar]
- Rosano C, Simonsick EM, Harris TB, Kritchevsky SB, Brach J, Visser M, … Newman AB. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005;24(1–2):8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Grafman J, Cameron K, Berndt RS. Working memory retention systems: A state of activated long-term memory. Behavioral and Brain Sciences. 2003;26(06):709–728. doi: 10.1017/s0140525x03000165. [DOI] [PubMed] [Google Scholar]
- Salthouse Ta. What and When of Cognitive Aging. Current Directions in Psychological Science. 2004;13:140–144. doi: 10.1111/j.0963-7214.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. When does age-related cognitive decline begin? Neurobiology of aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse Ta. Selective review of cognitive aging. Journal of the International Neuropsychological Society : JINS. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273–295. doi: 10.1037/0894-4105.7.3.273. [DOI] [Google Scholar]
- Schaie KW. Perceptual speed in adulthood: cross-sectional and longitudinal studies. Psychology and Aging. 1989;4(4):443. doi: 10.1037//0882-7974.4.4.443. [DOI] [PubMed] [Google Scholar]
- Schaie KW. What Can We Learn From Longitudinal Studies of Adult Development? 2005;2 doi: 10.1207/s15427617rhd0203_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FL, Hunter J. General mental ability in the world of work: occupational attainment and job performance. Journal of personality and social psychology. 2004;86:162–173. doi: 10.1037/0022-3514.86.1.162. [DOI] [PubMed] [Google Scholar]
- Schniter E, Gurven M, Kaplan HS, Wilcox NT, Hooper PL. Skill ontogeny among Tsimane forager-horticulturalists. American Journal of Physical Anthropology. 2015 doi: 10.1002/ajpa.22757. [DOI] [PubMed] [Google Scholar]
- Schoon I. Childhood cognitive ability and adult academic attainment: evidence from three British cohort studies. Longitudinal and Life Course Studies. 2010;1:241–258. [Google Scholar]
- Schwarz F, Springer SA, Altheide TK, Varki NM, Gagneux P, Varki A. Human-specific derived alleles of CD33 and other genes protect against postreproductive cognitive decline. Proceedings of the National Academy of Sciences. 2016;113(1):74–79. doi: 10.1073/pnas.1517951112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ: British Medical …. 2012;7622:1–8. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirbekk V. Age and individual productivity: A literature survey. Vienna yearbook of population research. 2004:133–153. [Google Scholar]
- Skirbekk V. Age and productivity potential: A new approach based on ability levels and industry-wide task demand. Population and Development Review. 2008;34:191–207. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society : JINS. 2002;8:448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- Stieglitz J, Gurven M, Kaplan H, Hooper PL. Household Task Delegation among High-Fertility Forager-Horticulturalists of Lowland Bolivia. Current Anthropology. 2013;54(2):232. doi: 10.1086/669708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz J, Schniter E, Von Rueden C, Kaplan H, Gurven M. Functional disability and social conflict increase risk of depression in older adulthood among Bolivian forager-farmers. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2014:gbu080. doi: 10.1093/geronb/gbu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex; a journal devoted to the study of the nervous system and behavior. 2003;39:1107–1128. doi: 10.1016/S0010-9452(08)70880-3. [DOI] [PubMed] [Google Scholar]
- Trollor N, Sachdev S, Haindl W, Brodaty H, Wen W, Walker M. Regional cerebral blood flow deficits in mild Alzheimer’s disease using high resolution single photon emission computerized tomography. Psychiatry and Clinical Neurosciences. 2005;59:280. doi: 10.1111/j.1440-1819.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Trumble BC, Stieglitz J, Thompson ME, Fuerstenberg E, Kaplan H, Gurven M. Testosterone and male cognitive performance in Tsimane forager-horticulturalists. American Journal of Human Biology. 2015;27(4):582–586. doi: 10.1002/ajhb.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM. Differentiation of cognitive abilities across the life span. Developmental Psychology. 2009;45(4):1097. doi: 10.1037/a0015864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM. Global and domain-specific changes in cognition throughout adulthood. Developmental psychology. 2011;47:331–343. doi: 10.1037/a0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: a longitudinal examination of age-associated declines in reasoning and processing speed. Developmental psychology. 2009;45:431–446. doi: 10.1037/a0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Salthouse TA. Individual differences in cognitive aging. In: Chamorro-Premuzic T, von Stumm S, Furnham A, editors. The Wiley-Blackwell Handbook of Individual Differences. Vol. 2. Hoboken, NJ: Wiley-Blackwell; 2011. p. 242. [Google Scholar]
- Unsworth N, Redick TS, Heitz RP, Broadway JM, Engle RW. Complex working memory span tasks and higher-order cognition: A latent-variable analysis of the relationship between processing and storage. Memory. 2009;17:635–654. doi: 10.1080/09658210902998047. [DOI] [PubMed] [Google Scholar]
- Van Dijk KRa, Van Gerven PWM, Van Boxtel MPJ, Van der Elst W, Jolles J. No protective effects of education during normal cognitive aging: results from the 6-year follow-up of the Maastricht Aging Study. Psychology and aging. 2008;23:119–130. doi: 10.1037/0882-7974.23.1.119. [DOI] [PubMed] [Google Scholar]
- Walker R, Hill K. Modeling growth and senescence in physical performance among the Ache of eastern Paraguay. Ameican Journal of Human Biology. 2003;15:196–208. doi: 10.1002/ajhb.10135. [DOI] [PubMed] [Google Scholar]
- Wang J, Kaufman AS. Changes in Fluid and Crystallized Intelligence Across the 20- to 90-Year Age Range on the K-Bit. Journal of Psychoeducational Assessment. 1993;11:29–37. [Google Scholar]
- Whiten A, Byrne RW. Machiavellian Intelligence II: Extensions and Evaluations. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in Cognitive Function in Older Persons From a Community Population. Archives of neurology. 1999;56:1274–1279. doi: 10.1001/archneur.56.10.1274. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SW, Manly JJ. Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. Journal of the International Neuropsychological Society. 2011;17(06):1039–1046. doi: 10.1017/S1355617711001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich D, Mascherek A. Five views of a secret: does cognition change during middle adulthood? European Journal of Ageing. 2010;7(3):135–146. doi: 10.1007/s10433-010-0161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.