Abstract
Arcuate nucleus (ARC) neurons sense the fed/fasted state and regulate hunger. Agouti-related protein (ARCAgRP) neurons are stimulated by fasting, and once activated, they rapidly (within minutes) drive hunger. Pro-opiomelanocortin (ARCPOMC) neurons are viewed as the counterpoint to ARCAgRP neurons. They are regulated in an opposite fashion and decrease hunger. However, unlike ARCAgRP neurons, ARCPOMC neurons are extremely slow in affecting hunger (many hours). Thus, a temporally analogous, rapid ARC satiety pathway does not exist or is presently unidentified. Here, we show that glutamate-releasing ARC neurons expressing oxytocin receptor, unlike ARCPOMC neurons, rapidly cause satiety when chemo- or optogenetically manipulated. These glutamatergic ARC projections synaptically converge with GABAergic ARCAgRP projections on melanocortin-4 receptor (MC4R)-expressing satiety neurons in the paraventricular hypothalamus (PVHMC4R neurons). Importantly, transmission across the ARCGlutamatergic→PVHMC4R synapse is potentiated by the ARCPOMC neuron-derived MC4R agonist, α-MSH. This excitatory ARC→PVH satiety circuit, and its modulation by α-MSH, provides new insight into regulation of hunger/satiety.
Introduction
The ARC is located adjacent to the median eminence, which lacks a blood brain barrier. Unique properties that likely involve tanycytes allow circulating factors controlling energy balance such as leptin, insulin and various gut-secreted hormones to access ARC neurons1, 2. Importantly, the ARC contains two key opposing regulators of energy balance: ARCPOMC and ARCAgRP neurons. ARCPOMC neurons release the MC4R agonist α-MSH and promote negative energy balance, whereas ARCAgRP neurons release the MC4R antagonist AgRP, as well as the inhibitory transmitters GABA and NPY, to do the opposite. Evidence supporting this model is extremely robust. Ablation of ARCPOMC neurons3, 4, or genetic deficiency of POMC5, 6 or MC4R7–10 results in hyperphagia and massive obesity. Ablation of ARCAgRP neurons, on the other hand, causes starvation11, while opto- or chemogenetic stimulation of ARCAgRP neurons drives intense feeding12, 13. Indeed, the antagonistic “yin-yang” function of these two neurons is a constant feature of essentially all proposed models of homeostatic hunger/satiety regulation14–16. At odds with this widely held view, however, is the finding that opto- and chemo-genetic activation of ARCPOMC neurons fails to decrease feeding over a period of less than 8–12 hours of stimulation4, 12, 17. This is in striking contrast to the potent effect on hunger observed just minutes following ARCAgRP neuron stimulation12, 13, 18. This lack of rapid effect strongly suggests that ARCPOMC neurons, by themselves, are not the full counterpoint to ARCAgRP neurons. Based on this, we hypothesized that a functionally important, presently unknown neural component of the ARC-based homeostatic satiety system is missing from current models.
The paraventricular nucleus of the hypothalamus (PVH) is an important effector site for hunger/satiety regulation by ARCPOMC and ARCAgRP neurons. ARCPOMC and ARCAgRP neurons send dense projections to the PVH19, 20, injection of MC4R ligands and NPY into the PVH potently affect feeding20, 21, and, as determined via Cre/lox gene knockout studies, the hyperphagia-preventing actions of MC4Rs are mediated specifically by MC4Rs on PVH neurons8, 22. Importantly, ARCAgRP neurons selectively, monosynaptically engage and consequently inhibit PVHMC4R neurons23 and optogenetic stimulation of ARCAgRP terminals in the PVH stimulates intense feeding within minutes23, 24. Of note, the ability of ARCAgRP terminal stimulation in the PVH to increase feeding is markedly blunted by concurrent light/ChR2 stimulation of the downstream PVHMC4R neurons23. Thus, PVHMC4R satiety neurons are important effectors of the ARC-based melanocortin system and as such exert marked control over hunger/satiety.
The ability of ARCAgRP neurons to rapidly affect feeding relates to their release of the fast-acting inhibitory transmitters GABA and NPY18, 24. Blockade of either NPY1 receptors or GABAA receptors markedly attenuates the rapid feeding response to optogenetic ARCAgRP terminal stimulation in the PVH24. Inhibition of melanocortin receptors via Ay-mediated ectopic expression of agouti, on the other hand, is without effect12. Similarly, in mice unable to release NPY or GABA from ARCAgRP neurons, chemogenetic stimulation of ARCAgRP neurons no longer affects feeding over a 2-hour period18. However, in these same mice, AgRP, working through MC4Rs, induces feeding but only after 4 hours of stimulation18. Thus, the ability of ARCAgRP neurons to rapidly affect feeding depends upon release of the fasting-acting transmitters, GABA and NPY. AgRP (and subsequent MC4R inhibition) effects feeding but only after a protracted period. Of interest, the reduced feeding seen following prolonged stimulation of ARCPOMC neurons is absent in Ay mice12, suggesting that it is mediated by αMSH (and not GABA or glutamate). Consistent with the role of GABA in mediating rapid effects of AgRP neurons, light/ChR2-evoked release of transmitter from ARCAgRP→PVH projections generates robust GABAergic IPSCs in PVH neurons23–25. In striking contrast, similar light/ChR2-evoked release studies in ARCPOMC→PVH projections are largely ineffective in generating either IPSCs or EPSCs in PVH neurons26. In total, the above findings are consistent with αMSH, as opposed to GABA and glutamate, being the relevant effector released by ARCPOMC neurons.
Given that rapid effects of ARCAgRP neurons are mediated by release of fast-acting transmitters and that AgRP (MC4R antagonist) mediates only slow effects, and that PVH-projecting ARCPOMC neurons appear to release αMSH (MC4R agonist) but not fast-acting transmitters, we hypothesized that this transmitter profile of ARCPOMC neurons accounts for their slow and not fast suppression of feeding. Related to this, given that rapid effects of ARCAgRP neurons are mediated by inhibitory transmitters, we hypothesized that a rapidly acting ARC satiety neuron, if it existed, would be excitatory and release the fast-acting transmitter, glutamate, onto PVH satiety neurons. Of interest, it was recently discovered that, by virtue of their expression of Nav1.7 (Scn9a), PVH neurons summate excitatory inputs over greatly extended timescales, allowing near-perfect integration of inputs magnifying their effects on neuronal firing27. Remarkably, genetic deletion of Nav1.7 in PVH neurons decreased their firing due to diminished summation of excitatory inputs and this resulted in massive obesity27. Thus, glutamatergic input from some presently unknown source plays a key role in regulating the activity of PVH satiety neurons, and subsequently hunger/satiety.
The majority of neurons in the ARC are GABAergic; far fewer are glutamatergic28. This raises the possibility that vesicular glutamate transporter 2 (ARCVglut2) neurons, or one of the few subsets of ARCVglut2 neurons, has a specific function – for example, satiety. In the present study, we pursue the above-mentioned hypotheses and investigate the role of ARCVglut2 neurons in regulating hunger/satiety.
Results
ARCVglut2 neurons, unlike ARCPOMC neurons, rapidly promote satiety
To compare and contrast the temporal dynamics by which ARCPOMC neurons increase satiety, as well as another process they control - interscapular brown adipose tissue (iBAT) thermogenesis16, Cre-dependent AAV-DIO-hM3Dq13 was injected into the ARC of Pomc-Cre BAC transgenic mice29 (Fig. 1a). Temperature transponders were implanted subcutaneously above iBAT to assess thermogenesis. Clozapine-N-oxide (CNO) effectively activated ARCPOMC neurons as evidenced by increased firing and induction of Fos (Supplementary Fig. 1a–d). As previously observed4, 12, ARCPOMC neuron stimulation failed to rapidly decrease dark-cycle feeding (i.e., within 4 hours; Fig. 1b), but did decrease feeding after 24 and 48 hours (Supplementary Fig. 1e). In striking contrast, ARCPOMC neurons rapidly induced iBAT thermogenesis (Fig. 1c). This result demonstrates that ARCPOMC neurons, as a group, are capable of generating rapid responses. To ensure that failure to rapidly regulate satiety was not due to inadequate Cre expression by Pomc-Cre BAC transgenic mice (e.g., in a specific subset of POMC neurons), we generated Pomc-IRES-Cre knockin mice (Supplementary Fig. 1f). Consistent with Cre being driven by the endogenous Pomc allele, Cre activity was restricted to the ARC, and within the ARC, to neurons immunopositive for POMC (Supplementary Fig. 1f–h). We next bilaterally transduced ARCPOMC neurons in Pomc-IRES-Cre mice with AAV-DIO-hM3Dq (Supplementary Fig. 1i). CNO administration effectively activated hM3Dq-expressing ARCPOMC neurons as assessed by induction of Fos (Supplementary Fig. 1j). Stimulation of ARCPOMC neurons in Pomc-IRES-Cre mice, however, again failed to rapidly suppress dark-cycle feeding (Fig. 1d). Thus, ARCPOMC neurons do not rapidly suppress feeding.
Figure 1. Regulation of feeding by ARCPOMC versus ARCVglut2 neurons.
a, Experimental schematic. b, d, e, Effect of CNO/hM3Dq stimulation of ARCPOMC neurons (b, d) or ARCVglut2 neurons (e) on dark-cycle feeding. c, Effect of CNO/hM3Dq stimulation of ARCPOMC neurons on iBAT temperature. f, Effect of CNO/hM4Di inhibition of ARCVglut2 neurons on light-cycle feeding. b–f, Data are presented as mean ± SEM. Mice from multiple litters. Repeated measures two-way ANOVA followed by Sidak’s multiple comparisons test (b, n = 5 animals; Treatment F(1,4) = 0.3265, P = 0.5983; time F(4,16) = 89.82, P < 0.0001; interaction F(4,16) = 3.037, P = 0.0485. 1h, P = 0.4831; 2h, P = 0.9980; 3h, P = 0.4831; 4h, P = 0.0610; c, n = 4 animals. Treatment F(1,3) = 8.931, P = 0.0582; time F(5,15) = 8.008, P = 0.0008; interaction F(5, 15) = 6.494, P = 0.0021. 30 min, ****P < 0.0001; 1h, ****P < 0.0001; 2h, **P = 0.0011; 3h, *P = 0.0102; 4h, ***P = 0.0008; d, n = 4 animals; Treatment F(1,3) = 0.7301, P = 0.4557; time F(4,12) = 75.69, P < 0.0001; interaction F(4, 12) = 1.475, P = 0.2703; e, n = 4 animals. Treatment F(1,3) = 63.28, P = 0.0041; time F(4,12) = 40.61, P < 0.0001; interaction F(4, 12) = 10.40, P = 0.0007. 1h, **P = 0.0040; 2h, ****P < 0.0001; 3h, ****P < 0.0001; 4h, ****P < 0.0001; f, n = 6 animals. Treatment F(1,5) = 16.55, P = 0.0096; time F(4,20) = 24.66, P < 0.0001; interaction F(4, 20) = 7.808, P = 0.0006. 1h, ***P = 0.0002; 2h, **P = 0.0016; 3h, ****P < 0.0001; 4h, ****P < 0.0001).
To determine if any ARC glutamatergic neurons rapidly induce satiety, we injected AAV-DIO-hM3Dq into the ARC of [Vglut2 (Slc17a6)-IRES-Cre] mice28. Vglut2 is the major glutamate synaptic vesicle transporter in the hypothalamus, hence Vglut2-IRES-Cre mice target relevant glutamatergic ARC neurons28. Small volumes (2–5 nl) were injected to avoid expression in neighboring, non-ARC areas (Supplementary Fig. 2a, b). hM3Dq/CNO stimulation of ARCVglut2 neurons, in contrast to ARCPOMC neurons, rapidly and markedly suppressed feeding (Fig. 1e). To determine if ARCVglut2 neurons are necessary, as well as sufficient for satiety, we transduced ARCVglut2 neurons with AAV-DIO-hM4Di13 (Supplementary Fig. 2a, c). CNO markedly inhibited hM4Di-expressing ARCVglut2 neuron firing as evidenced by decreased firing (Supplementary Fig. 2d). Importantly, hM4Di/CNO-mediated inhibition of ARCVglut2 neurons rapidly increased light-cycle feeding (Fig. 1f). Thus, ARCVglut2 neurons rapidly and bidirectionally regulate satiety.
Fasting decreases ARCVglut2 neuron activity
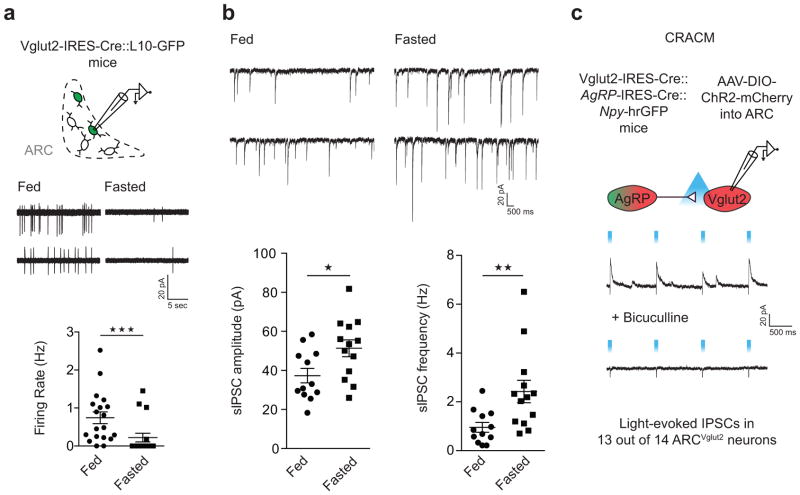
Given their role in promoting satiety, ARCVglut2 neurons should be activated by feeding and inhibited by fasting. In agreement with this, we found that the firing rates of ARCVglut2 neurons in brain slices from fasted mice, compared with fed mice, were considerably lower – indeed 13 of 17 neurons were silent (Fig. 2a). Consistent with reduced ARCVglut2 neuron activity, fasting significantly increased both frequency and amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs; Fig. 2b). The fasting-induced increase in sIPSCs raises the possibility that ARCVglut2 neurons, like ARCPOMC neurons24, are negatively regulated by GABAergic ARCAgRP neurons. To probe connectivity between ARCAgRP and ARCVglut2 neurons, we performed ChR2-assisted circuit mapping (CRACM)30, 31. To this end, we stereotaxically injected AAV-DIO-ChR2-mCherry into the ARC of Agrp-IRES-Cre::Vglut2-IRES-Cre::Npy-hrGFP mice and recorded light-evoked inhibitory poststynaptic currents (IPSCs) in ARCVglut2 neurons (mCherry-positive, GFP-negative; Fig. 2c). Light-evoked IPSCs, which were completely blocked by the GABAAR antagonist bicuculline, were detected in 13 of 14 ARCAgRP→ARCVglut2 neurons (Fig. 2c). Thus, ARCVglut2 neurons, which are inhibited by fasting (Fig. 2a), receive strong GABAergic inhibitory input from hunger-inducing ARCAgRP neurons, which are themselves activated by fasting32.
Figure 2. Effects of fasting on ARCVglut2 neurons and their inhibition by ARCAgRP neuron afferents.
a, Top, schematic shows experimental approach used in (a) and (b). a, Representative traces (middle) and summary (bottom) of fasting effects on the firing rate of ARCVglut2 neurons as assessed using cell-attached recordings. Number of animals: Fed/fasted, N = 3/3. Sample size (cells): Fed/fasted, n = 19/17; Fed (M = 0.7437, s.d. = 0.6771) versus fasted (M = 0.2208, s.d. = 0.4725): Two-tailed Mann-Whitney test: U = 61, ***P = 0.0007. b, Representative traces (top) and summary (bottom) of fasting effects on sIPSCs in ARCVglut2 neurons. Number of animals, fed/fasted, N = 3/3; sample size (cells), fed/fasted, n = 12/13. Unpaired two-tailed t-test: sIPSC amplitude: Fed (M = 37.33, s.d. = 12.95) versus fasted (M = 51.34, s.d. = 15.5): t(23) = 2.442, *P = 0.0227; sIPSC frequency: Fed (M = 0.9563, s.d. = 0.6858) versus fasted (M = 2.423, s.d. = 1.667): t(23) = 2.830, **P = 0.0095. Data are presented as mean ± SEM. c, ARCAgRP→ARCVglut2 CRACM. Top, schematic of the connection tested. ARCAgRP and ARCVglut2 neurons were transduced with a Cre-dependent ChR2 tagged with mCherry (AAV-DIO-ChR2-mCherry). ChR2-expressing ARCAgRP neurons were identified by co-expression of mCherry and hrGFP (note that NPY expression in the ARC marks all AgRP neurons47). ARCVglut2 neurons were identified by expression of mCherry and absence of hrGFP. Bottom, representative traces of light-evoked IPSCs before and after bath application of bicuculline (GABAA receptor antagonist). Vh = 0 mV was used to prevent movement of Na+ and Ca2+ through ChR2 which was expressed in the patched ARCVglut2 neurons, in addition to the afferent ARCAgRP neurons.
ARCVglut2→PVH projections, unlike ARCPOMC→PVH projections, are effective in glutamatergic transmission
To determine if ARCVglut2 neurons project to sites where ARCAgRP and ARCPOMC neurons project and regulate hunger, we injected the anterograde tracer, AAV-DIO-Synaptophysin-mCherry, into the ARC of Vglut2-IRES-Cre mice and performed immunohistochemistry for AgRP and POMC (Supplementary Fig. 3a–f). ARCVglut2, ARCAgRP and ARCPOMC neurons projected to many of the same sites, including the PVH. Some ARCVglut2 terminals in the PVH contained POMC but many did not (Supplementary Fig. 3d). Thus, ARCVglut2 projections converge with hunger-regulating ARCAgRP and ARCPOMC projections on downstream targets.
To compare ARCPOMC→PVH and ARCVglut2→PVH synaptic function, we performed CRACM on unidentified PVH (uPVH) neurons. We stereotaxically injected AAV-DIO-ChR2 into the ARC and recorded from unidentified PVH (uPVH) neurons in brain slices containing ChR2-expressing ARC terminals. When Pomc-Cre or Pomc-IRES-Cre mice were used, we failed to detect light-evoked excitatory postsynaptic currents (EPSCs) in any of 71 ARCPOMC→uPVH neurons tested (Fig. 3a, b). Given that ARCPOMC neurons express GABAergic markers (GAD isoforms)33, 34, we also assessed if any PVH-projecting ARCPOMC neurons synaptically release GABA. Remarkably, we failed to detect light-evoked IPSCs in any of the 71 ARCPOMC→uPVH neurons tested (data not shown). In contrast, when Vglut2-IRES-Cre mice were used, we readily detected robust light-evoked EPSCs in many (12/30) ARCVglut2→uPVH neurons (Fig. 3c). The light-evoked EPSCs were mediated by glutamate as they were blocked by the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) antagonist CNQX (Fig. 3c). Thus, ARCVglut2 neurons, unlike ARCPOMC neurons, synaptically release glutamate onto PVH neurons. This raises the important possibility that the PVH is an important effector site of rapidly-acting ARCVglut2 satiety neurons.
Figure 3. ARCVglut2→PVH projections, unlike ARCPOMC→PVH projections, are effective in glutamatergic transmission.
a–c, ARC→PVH CRACM. Top, schematics showing connection being tested. ARCPOMC or ARCVglut2 neurons were transduced with Cre-dependent ChR2. Below, representative traces showing assessment of light-evoked EPSCs. c, Bottom, representative traces of light-evoked EPSCs before and after bath application of CNQX (AMPAR antagonist)
RNA-seq assessment of ARCVglut2 satiety neurons
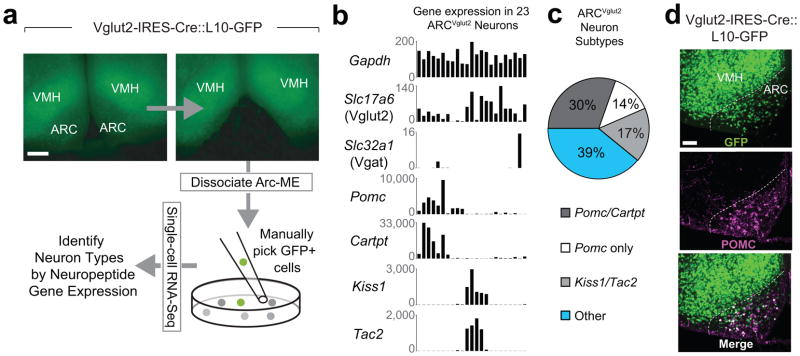
ARCVglut2 neurons are a heterogeneous population that includes ARCPOMC neurons33, 34 and fertility-regulating ARCKiss1 neurons35 though other subtypes may exist as well. To identify subtypes of ARCVglut2 neurons, we profiled their gene expression using single-cell RNA-seq. Specifically, we isolated GFP-labeled neurons from the ARC of Vglut2-IRES-Cre::L10-GFP (loxSTOPlox-L10-GFP) mice (Fig. 4a) and sequenced their mRNA using the Smart-Seq2 RNA-seq method36. Since ARC neuron types are generally known for the neuropeptides they express, our first analysis focused on neuropeptide genes. A clustering analysis of 23 ARCVglut2 neurons based on their expression of 90 neuropeptide genes showed three subpopulations (Supplemental Fig. 4a–c and Supplemental table 1). Specifically, we found that 44% were POMC neurons (the majority of which co-expressed cocaine- and amphetamine-regulated transcript peptide; Cartpt), 17% were Kiss1/Tac2 neurons and 39% lacked a strong marker and so are annotated as “Other” neurons (Fig. 4b, c; Supplementary Fig. 4b). Consistent with the RNA-seq data, approximately 38% of ARCVglut2 neurons are POMC neurons as assessed by immunohistochemistry (Fig. 4d; 105.4 ± 9.3 of 274.1 ± 10.3 GFP-positive cells were POMC-positive; mean ± SEM; counts from a total of 9 section, 3 sections from each of n = 3 mice; mice from multiple litters).
Figure 4. RNA-seq analysis of ARCVglut2 neurons.
a, Schematic and representative ARC dissection images for single-cell RNA-seq of GFP-positive ARCVglut2 neurons. Scale bar represents 200 μm and applies to all images. b, Detection of housekeeping (Gapdh), glutamatergic (Slc17a6; Vglut2), GABAergic (Slc32a1; Vgat), and selected neuropeptide (Pomc, Cartpt, Kiss1, and Tac2) transcripts in ARCVglut2 neurons. Expression values are in units of RPKM (reads per kilobase gene model per million mapped reads). c, Percentages of ARCVglut2 neurons that are Pomc/Cartpt, Pomc only, Kiss1/Tac2 or “Other” neurons. d, Co-localization of POMC and GFP immunoreactivity in the ARC from a Vglut2-IRES-Cre::L10-GFP mouse. Scale bar represents 100 μm and applies to all images.
Given the absence of glutamatergic transmission from ARCPOMC→PVH neurons (Fig. 3a, b), we wondered if the Pomc-positive and Pomc-negative subtypes of ARCVglut2 neurons differ in their expression of genes involved in synaptic transmission. We assigned ARCVglut2 neurons to Pomc-positive and Pomc-negative subsets based on their expression of Pomc, and then ranked genes according to the percentage of each ARCVglut2 subset expressing the gene above a calculated expression threshold37. The analysis revealed that two genes encoding presynaptic molecules needed for fast, Ca2+-triggered neurotransmitter release, Cplx1 (complexin 1) and Syt1 (synaptotagmin 1)38, 39, were the 12th and 15th, respectively, most enriched genes in Pomc-negative ARCVglut2 neurons relative to Pomc-positive ARCVglut2 neurons. We then compared expression levels of these two genes by the Pomc-positive and Pomc-negative subsets of ARCVglut2 neurons, as well as the expression levels of Vglut2 (Slc17a6), which confers capacity to synaptically release glutamate. Of interest, Cplx1, Syt1 and Vglut2 gene expression were reduced in Pomc-positive versus Pomc-negative ARCVglut2 neurons by 80%, 78% and 71%, respectively (Supplementary Fig. 4d). The much lower expression of Cplx1, Syt1 and Vglut2, by Pomc-positive ARCVglut2 neurons, compared with Pomc-negative ARCVglut2 neurons, may explain their ineffective glutamatergic transmission onto PVH neurons. However, our results do not exclude the possibility that higher translational activity or greater protein stability could compensate for the lower transcript levels in Pomc-positive ARCVglut2 neurons relative to Pomc-negative ARCVglut2 neurons.
Glutamatergic ARC satiety neurons are marked by expression of the oxytocin receptor
Since a subset of ARCVglut2 neurons is Kiss1-positive, we addressed whether ARCKiss1 neurons regulate satiety. Kiss1-Cre mice35 were used for this purpose. First, we injected AAV-DIO-ChR2 into the ARC and performed CRACM. Of note, we detected light-evoked EPSCs in a small subset (2/14) of ARCKiss1→uPVH neurons (Supplementary Fig. 5a), indicating that glutamatergic ARCKiss1 afferents are a minority of the ARCVglut2 neuron afferents to PVH. Next, we bilaterally transduced ARCKiss1 neurons with AAV-DIO-hM3Dq (Supplementary Fig. 5b). CNO administration effectively activated hM3Dq-expressing ARCKiss1 neurons as assessed by Fos induction (Supplementary Fig. 5c). Stimulation of ARCKiss1 neurons, however, failed to rapidly suppress dark-cycle feeding (Supplementary Fig. 5d). Thus, ARCKiss1 (as well as the ARCPOMC) neurons are not the subset that rapidly induces satiety.
Of interest, our RNA-seq analysis revealed that some ARCVglut2 neurons express the gene encoding the oxytocin receptor (Oxtr; Fig. 5a). Notably, we failed to detect Oxtr expression in Kiss1-positive ARCVglut2 neurons (Fig. 5a) or Vglut2-negative ARC neurons we profiled using single-cell RNA-Seq (unpublished data). Given these findings and a previous study demonstrating that intra-ARC injections of oxytocin rapidly suppresses feeding40, we wondered whether ARCOxtr marks the subset of ARCVglut2 neurons that rapidly induce satiety when stimulated. To further investigate ARCOxtr neurons, we obtained Oxtr-Cre mice41. Consistent with GENSAT’s expression atlas, Cre activity was restricted to a small number of ARC neurons (Fig. 5b). Also, confirming our RNA-seq analysis, approximately 50% of ARCOxtr neurons expressed POMC as assessed by immunohistochemistry (Fig. 5b). To characterize the effect of oxytocin on ARCOxtr neurons, we recorded from fluorescently labeled cells in brain slices from Oxtr-Cre::tdTomato mice and found that bath-applied oxytocin markedly increased the firing rate of synaptically isolated ARCOxtr neurons (i.e., in presence of CNQX, D-AP5 and bicuculline; Fig. 5c). Thus, Oxtr-Cre targets a small population of ARC neurons that are directly activated by oxytocin. Some of these are a subset of ARCPOMC neurons while others are not.
Figure 5. Oxtr expression marks ARCGlutamatergic neurons that rapidly promote satiety.
a, Oxtr expression in ARCVglut2 neurons as determined by RNA-seq. b, Co-localization of POMC protein in tdTomato-positive ARCOxtr neurons. Approximately 50% of ARCOxtr neurons expressed POMC. c, Representative trace (top) and summary (bottom) of oxytocin’s effect on ARCOxtr neuron firing rate. Scale bars, 50 pA, 30 sec. 6 slices from 3 mice; mice from multiple litters; cells, n = 6. Baseline (M = 1.261, s.d. = 0.8192) versus Oxytocin (M = 2.991, s.d. = 1.495): Paired two-tailed t-test, t(5) = 4.253, **P = 0.0081. d, CRACM of ARCOxtr→PVH connectivity. Below, representative traces showing light-evoked EPSCs. e, Effect of CNO/hM3Dq stimulation of ARCOxtr neurons on dark-cycle feeding (n = 6 animals). f, Left, experimental schematic. Right, effect of ARCOxtr terminal photostimulation in the PVH on dark-cycle feeding (n = 5 animals). e, f, Data are presented as mean ± SEM. Repeated measures two-way ANOVA followed by Sidak’s multiple comparisons test. e, Mice from multiple litters, n = 6 animals. Treatment F(1,5) = 26.25, P = 0.0037; time F(4,20) = 63.88, P < 0.0001; interaction F(4, 20) = 15.31, P < 0.0001.1h, P = 0.1760; 2h, **P = 0.0025; 3h, ****P < 0.0001; 4h, ****P < 0.0001. f, Mice from multiple litters, n = 4 animals. Repeated measures two-way ANOVA: Treatment F(1,3) = 36.87, P = 0.0090; time F(4,12) = 24.90, P < 0.0001; interaction F(4, 12) = 14.04, P = 0.0002. Sidaks multiple comparisons test: 1h, P = 0.1276; 2h, *P = 0.0195; 3h, ***P = 0.0001; 4h, ****P < 0.0001.
Next, we probed connectivity of ARCOxtr neurons to unidentified PVH (uPVH) neurons. Following AAV-DIO-ChR2 delivery into the ARC of Oxtr-Cre mice, we detected light-evoked glutamatergic EPSCs in a subset (7/21) of ARCOxtr→uPVH neurons (Fig. 5d). Given that the majority of ARC neurons are GABAergic28, we also assessed if any PVH-projecting ARCOxtr neurons synaptically release GABA. Remarkably, we failed to detect light-evoked IPSCs in any of the ARCOxtr→uPVH neurons tested (Supplementary Fig. 6a). Thus, no PVH-projecting ARCOxtr neurons are GABAergic. Given that ARCPOMC neurons are incapable of light-evoked synaptic release of glutamate in the PVH (Fig. 3a, b), these excitatory ARCOxtR afferents must come from ARCOxtr neurons that lack POMC.
To assess the effect of ARCOxtr stimulation on dark-cycle feeding, we bilaterally transduced ARCOxtR neurons with AAV-DIO-hM3Dq (Supplementary Fig. 6b). CNO administration effectively activated ARCOxtr neurons as assessed by Fos induction (Supplementary Fig. 6c). Of note, stimulation of ARCOxtr neurons rapidly suppressed dark-cycle feeding (Fig. 5e). In addition, stimulation of ARCOxtr neurons decreased refeeding in mice that were fasted for 24 hours (Supplementary Fig. 6d). To determine if the PVH is a downstream effector site for ARCOxtR neurons, we optogenetically activated ChR2-expressing ARCOxtr terminals in the PVH (Fig. 5f). This terminal stimulation rapidly suppressed dark-cycle feeding (Fig. 5f and Supplementary Fig. 6e). Thus, as with hunger-promoting inhibitory ARCAgRP neurons, the PVH is an important effector site for satiety-promoting glutamatergic ARCOxtr neurons.
Excitatory ARCVglut2 and inhibitory ARCAgRP projections synaptically converge on PVHMC4R neurons
To see if glutamatergic ARC neurons, like ARCAgRP neurons23, preferentially engage PVHMC4R neurons, we performed CRACM experiments using Mc4r-t2a-Cre mice23. In these studies, AAV-DIO-GFP was injected into the PVH to visualize PVHMC4R neurons and Cre-independent AAV-Chr2 was injected into the ARC (see methods). Light evoked, AMPAR-mediated EPSCs could be detected in the majority (14/21) of ARCGlutamatergic→PVHMC4R neurons (Fig. 6a, d and Supplementary Fig. 7a). In contrast, only a small subset (1/7) of ARCGlutamatergic→PVHMC4R-negative neurons received light-evoked EPSCs (Fig. 6d and Supplementary Fig. 7b). Thus, ARCGlutamatergic neurons selectively engage PVHMC4R neurons.
Figure 6. ARCGlutamatergic and ARCOxtr neurons preferentially engage PVHMC4R neurons.
a–c, CRACM of ARC→PVH connectivity. Top, schematics show connections being tested. Below, representative traces showing assessment of light-evoked EPSCs. d, Percentage of PVHMC4R+ (MC4R-positive) or PVHMC4R− (MC4R-negative) neurons with light-evoked EPSCs. e, Convergent CRACM of ARCAgRP and ARCOxtr afferents onto PVH neurons. Schematic shown on the top right. Left, representative traces of light-evoked ARCAgRP-mediated IPSCs (top)/ARCOxtr-mediated EPSCs (bottom) recorded from the same uPVH neuron. Right, Venn diagram summary for all 10 neurons receiving input.
To test if ARCOxtR neurons also preferentially engage PVHMC4R neurons, we generated Oxtr-Cre::Mc4r-t2a-Cre mice. AAV-DIO-GFP was injected into the PVH to visualize PVHMC4R neurons and AAV-DIO-ChR2-mCherry was injected into the ARC. We detected light-evoked EPSCs in the vast majority (17/20) of ARCOxtr→PVHMC4R neurons (Fig. 6b, d) and, again only in a small subset (3/10) of PVHMC4R-negative neurons (Fig. 6d and Supplementary Fig. 7c). We also tested ARCPOMC→PVHMC4R connectivity in Pomc-Cre::Mc4r-t2a-Cre mice. AAV-DIO-GFP was injected into the PVH to visualize PVHMC4R and AAV-DIO-ChR2-mCherry was injected into the ARC. In agreement with our earlier results, we failed to detect light-evoked EPSCs in all but one PVHMC4R neuron (Fig. 6c, d). These CRACM studies demonstrate that, like ARCAgRP neurons23, excitatory ARCOxtr neurons, preferentially engage PVHMC4R neurons.
Due to the high connectivity of both ARCAgRP→PVHMC4R (ref.23) and ARCOxtr→PVHMC4R neurons (Fig. 6b, d), we reasoned that convergence of ARCAgRP input and ARCOxtr input onto the same downstream PVH neurons was likely. To assess this possibility, we transduced both ARCAgRP and ARCOxtr neurons in Agrp-IRES-Cre::Oxtr-Cre mice with AAV-DIO-ChR2 and recorded light-evoked IPSCs (ARCAgRP input) and light-evoked EPSCs (ARCOxtr input) from the same downstream uPVH neurons. This was accomplished by alternating between the holding potential of 0 mV (ARCAgRP input) and −70 mV (ARCOxtr input), respectively (Fig. 6e). Of 16 uPVH neurons tested, 2 received only ARCAgRP input, 2 received only ARCOxtr input, and 6 received dual input from both (Fig. 6e). Thus, ARCAgRP and ARCOxtr afferents often converge on single PVH neurons.
α-MSH post-synaptically increases glutamatergic transmission onto PVHMC4R neurons
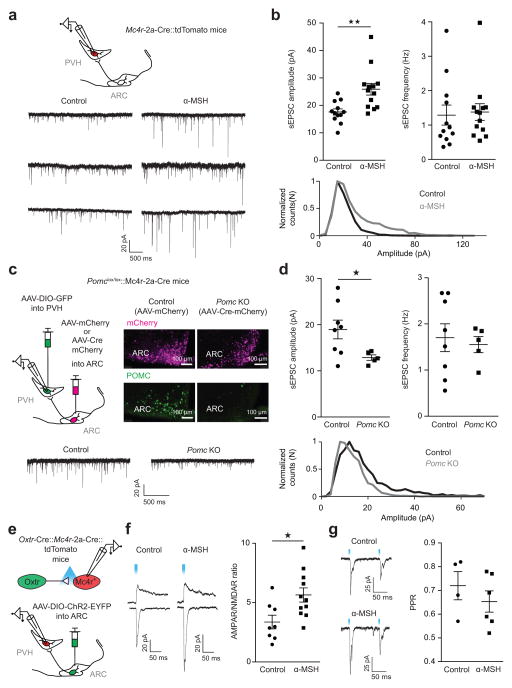
Given that PVHMC4R neurons are a key downstream site for ARCVglut2 neurons, we wondered if the fast acting glutamatergic and slow acting α-MSH satiety pathways interact via α-MSH-mediated effects on plasticity of excitatory inputs onto PVHMC4R neurons. This possibility is of interest because i) ARCPOMC neurons heavily innervate the PVH where α-MSH promotes satiety15, 16, and ii) α-MSH/MC4R action in other contexts can affect synaptic plasticity42, 43. To address this possibility, we recorded spontaneous excitatory postsynaptic currents (sEPSC) from PVHMC4R neurons identified in Mc4r-t2a-Cre::tdTomato [Ai9 (Rosa26-CAG-loxSTOPlox-tdTomato)] mice and tested the affect of the POMC-derived MC4R agonist α-MSH on sEPSCs in PVHMC4R neurons. We found that ex vivo treatment with α-MSH (250 nM)42 markedly increased the number of large-amplitude sEPSCs and the mean amplitude of sEPSCs (Fig. 7a, b). In contrast, the frequency of sEPCSs in PVHMC4R neurons was not affected by α-MSH (Fig. 7a, b). Given that the postsynaptic neurons express the MC4R, a receptor for α-MSH, this raises the important possibility that α-MSH potentiates glutamatergic transmission via a postsynaptic mechanism. Consistent with this, we found that α-MSH increased by 2-fold the ratio of the amplitude of electrically-evoked AMPAR- to N-methyl-D-aspartate receptor (NMDAR)-EPSCs (AMPAR/NMDAR ratio) – a measure of postsynaptic plasticity (Supplementary Fig. 8a). In contrast, we failed to detect changes in glutamate release probability by α-MSH treatment, as assessed by paired-pulse ratios (PPRs) of electrically-evoked EPSCs – further arguing against a presynaptic mechanism (Supplementary Fig. 8b).
Figure 7. α-MSH post-synaptically increases excitatory input onto PVHMC4R neurons.
a, Top, experimental schematic. a, b, Representative traces (a, bottom) and summary (b) of α-MSH effects on sEPSCs. 8 slices from 4 mice; mice from multiple litters; sample size (cells): control/α-MSH, n = 12/13. sEPSC amplitude: Control (M = 17.6, s.d. = 3.923) versus α-MSH (M = 25.88, s.d. = 7.668): Unpaired two-tailed t-test, t(23) = 3.353, **P = 0.0028. sEPSC frequency: Control (M = 1.29, s.d. = 1.011) versus α-MSH (M = 1.379, s.d. = 0.8736): Two-tailed Mann-Whitney test: U = 65, P = 0.4951 b, Bottom, sEPSC amplitude distribution showing that α-MSH significantly increased the frequency of large-amplitude sEPSCs (Kolmogorov-Smirnov test, P < 0.0001). c, Left, experimental schematic. Right, POMC and mCherry immunolabeling in control and Pomc knockout (Pomc KO) mice. c, d, Representative traces (c, Bottom) and summary (d) of Pomc KO effects on sEPSCs. Number of slices: Control/Pomc KO, N = 4/4. Number of animals: Control/Pomc KO, N = 2/2. Sample size (cells): Control/Pomc KO, n = 8/5; sEPSC amplitude: Control (M = 18.96, s.d. = 5.721) versus Pomc KO (M = 12.88, s.d. = 1.293): Unpaired two-tailed t-test, t(11)= 2.304, *P = 0.0417. sEPSC frequency: Control (M = 1.703, s.d. = 0.853) versus Pomc KO (M = 1.553, s.d. 0.3897): Unpaired two-tailed t-test: t(11) = 0.3653, P = 0.7218. Bottom, sEPSC distribution showing decreased frequency of large-amplitude sEPSCs in Pomc KO mice (Kolmogorov-Smirnov test, P < 0.0001). e, Experimental schematic. f, g, Representative traces (left) and summary (right) of α-MSH effects on light-evoked AMPAR/NMDAR ratios (f, slices from 3 mice; mice from multiple litters. Sample size (cells): Control/α-MSH, n = 8/11; Control (M = 3.365, s.d. = 1.622) versus α-MSH (M = 5.642, s.d. = 1.980): Unpaired two-tailed t-test: t(17) = 2.661, *P = 0.0165) and on light-evoked Paired-Pulse ratios (PPRs) (g, Slices from 3 mice; mice from multiple litters. Sample size (cells): control/α-MSH, n = 4/6. Control (M = 0.72, s.d. = 0.1186) versus α-MSH (M = 0.6533, s.d. = 0.1093): Unpaired two-tailed t-test: t(8) = 0.9150, P = 0.387).
We next determined if absence of endogenous α-MSH, in vivo, affects glutamatergic synaptic activity onto PVHMC4R neurons. Towards these ends, we generated Pomclox/lox mice (Supplementary Fig. 8c) and crossed them to Mc4r-t2a-Cre mice to generate Pomclox/lox::Mc4r-t2a-Cre mice. In these mice, Pomc deletion (POMC knock out; POMC KO) was achieved by delivery of AAV-Cre-mCherry into the ARC (Fig. 7c). Visualization of PVHMC4R neurons was achieved by delivery of AAV-DIO-GFP into the PVH (Fig. 7c). AAV-Cre-mCherry effectively deleted POMC expression as revealed by POMC immunohistochemistry (Fig. 7c). We found that the number of large-amplitude sEPSCs and, consistent with this, the mean amplitude of sEPSCs in PVHMC4R neurons in POMC KO mice was markedly reduced (Fig. 7c, d). In contrast, we failed to detect changes in the frequency of sEPSCs in PVHMC4R neurons in POMC KO mice (Fig. 7c, d). Together, these results demonstrate that α-MSH released by ARCPOMC neuron terminals postsynaptically augments glutamatergic synaptic activity in PVHMC4R neurons.
To determine if glutamateric transmission across the ARCOxtr→PVHMC4R synapse is specifically increased by α-MSH, we delivered AAV-DIO-ChR2-EYFP into the ARC of Oxtr-Cre::Mc4r-t2a-Cre::tdTomato mice and recorded light-evoked EPSCs from PVHMC4R neurons (tdTomato-positive; Fig. 7e). We found that α-MSH significantly increased the AMPAR/NMDAR ratios of light-evoked EPSCs (Fig. 7f). In contrast, the PPRs of light-evoked EPSCs in ARCOxtr→PVHMC4R neurons were not affected by α-MSH (Fig. 7g). These findings demonstrate that excitatory ARCOxtr→PVHMC4R inputs are postsynaptically potentiated by α-MSH.
Discussion
Current models for homeostatic control of feeding lack a rapidly-acting ARC-based satiety mechanism that is temporally analogous to that provided by rapidly-acting, hunger-promoting ARCAgRP neurons. In the present study we report the discovery of this missing component, namely glutamate-releasing neurons in the ARC that express the oxytocin receptor. We demonstrate that chemogenetic activation/inhibition of ARCVglut2/Oxtr neurons, in contrast to ARCPOMC neurons, rapidly and robustly decreases/increases hunger, respectively. Furthermore, we show that the PVH is an important effector site for ARCVglut2/Oxtr neurons. They project to and specifically induce EPSCs in PVHMC4R neurons, and optogenetic stimulation of their terminals in the PVH rapidly decreases feeding. Using a “convergent CRACM” approach to simultaneously test for dual input, we show that ARCVglut2/Oxtr satiety neurons and inhibitory ARCAgRP hunger neurons converge onto the same downstream PVHMC4R neurons (Supplemental Fig. 9). In contrast to the hunger-promoting system where slow- (AgRP) and rapid-acting signals (GABA and NPY) are conveyed by one group of neurons (ARCAgRP neurons)18, 24, the counteracting satiety-promoting system is split into two parallel-projecting neurons, the previously known ARCPOMC neurons which deliver slow-acting α-MSH, and the previously unknown ARCVglut2/Oxtr neurons which release the rapid-acting transmitter, glutamate (Supplemental Fig. 9). Importantly, these two parallel-projecting satiety neurons interact as α-MSH released by ARCPOMC neurons postsynaptically increases glutamatergic transmission across the ARCVglut2/Oxtr→PVHMC4R synapse (Supplemental Fig. 9). This missing rapid-acting component, working in concert with ARCPOMC neurons, provides the full “yang” to the ARCAgRP neuron’s “yin”.
Many ARCPOMC neurons express glutamatergic (Vglut2) or GABAergic (GAD isoforms) markers33, 34 suggesting that they are capable of synaptically releasing glutamate and/or GABA. Despite this, a recent study26 established that ARCPOMC→PVH projections are largely ineffective in releasing glutamate or GABA in response to light/ChR2-evoked depolarization (light-evoked currents could be detected in only 3/66 of PVH neurons). In the present study, we confirm the ineffectiveness of synaptic transmission by ARCPOMC→PVH projections. Indeed, we observed no light/ChR2-evoked glutamatergic currents and no light/ChR2-evoked GABAergic currents in all 71 PVH neurons sampled. In striking contrast, using Vglut2-IRES-Cre mice to express ChR2 in all ARCVglut2 neurons, we observed light/ChR2-evoked glutamatergic currents in a substantial subset (40%) of PVH neurons. Thus, ARCVglut2→PVH projections, unlike ARCPOMC→PVH projections, are effective in rapid glutamatergic synaptic transmission. Since the PVH contains neurons that promote satiety8, 19–24 and since PVH neurons efficiently integrate and summate excitatory synaptic inputs to control neuron excitability27, we believe our findings to be significant, and suggest an important, unique role for glutamatergic transmission from POMC-negative ARCVglut2 neurons. The reason why POMC-positive ARCVglut2 PVH projections are ineffective in synaptic transmission is presently unknown, but could be related to their much lower expression of genes involved in fast, Ca2+-triggered glutamate release38, 39, Vglut2, Syt1 and Cplx1 (Supplementary Fig. 4).
It is noteworthy that ARCVglut2 neurons express the oxytocin receptor and that Oxtr-Cre mice target glutamatergic ARC neurons that exert strong, rapid control over satiety. It has previously been established that exogenous administration of oxytocin acutely decreases food intake in mice40, monkeys44 and humans45. Given that i) acute suppression of feeding occurs with intra-ARC injection of oxytocin40, ii) oxytocin exerts a direct stimulatory effect on ARCOxtr neurons and iii) ARC neurons are highly accessible to circulating factors, our finding provide a potential basis for the satiety-inducing effect of exogenously administered oxytocin. This possibility is of interest as oxytocin is being investigated as an anti-obesity drug46. Given their accessibility to circulating factors and that ARCVglut2/Oxtr neurons exert hitherto unknown strong control over satiety, they may also provide neural mechanisms for other previously observed effects for which the basis is either unknown or incompletely understood – examples include but are not limited to regulation of satiety/hunger by cannabinoids or orally administered GLP-1R agonists. Further studies will be needed to address these possibilities.
Given that both ARCVglut2 and ARCPOMC neurons project to the PVH, the site where hunger-suppressing MC4Rs reside8, 22, 23, we examined how these two “parallel” satiety-promoting projections interact. While MC4R agonists are capable of directly increasing membrane potential and firing of PVHMC4R neurons in ex vivo brain slices, the magnitude of these increases in PVHMC4R neurons that project to the central lateral parabrachial nucleus (cLPBN), the downstream site through which PVHMC4R neurons mediate satiety, is generally small23. This raises the possibility that the direct effect of αMSH/MC4Rs on neuron excitability plays a smaller role in regulating cLPBN-projecting PVHMC4R neuron activity. Based on this, and importantly on the fact that opto- or chemogenetic stimulation of ARCPOMC neurons is unable to rapidly reduce feeding (Fig. 1 and refs. 4, 12, 17), we suggest that ARCPOMC neurons do not work in isolation but instead decrease hunger by increasing the strength of excitatory synaptic transmission onto PVHMC4R satiety neurons (Supplementary Fig. 9). Using an ex vivo CRACM approach, to selectively monitor the strength of ARCVglut2/Oxtr→PVHMC4R synapses, we show that α-MSH postsynaptically upregulates glutamatergic transmission across this projection. This is consistent with two prior studies demonstrating that MC4Rs affect excitatory synaptic transmission via postsynaptic mechanisms – albeit in opposite directions depending upon cellular context42, 43. Our observation suggests that αMSH released by ARCPOMC neurons sets the gain on ARCVglut2 excitatory input to downstream PVHMC4R satiety neurons – and hence satiety. This plasticity is likely important given that the vast majority of PVHMC4R neurons receive functionally consequential monosynaptic excitatory input from ARCVglut2 neurons. Thus, relevant “substrate” exists for αMSH/MC4R-mediated plasticity. Overall, our work provides a novel mechanism for α-MSH/MC4R regulation of satiety, namely plasticity of excitatory input onto PVHMC4R neurons.
Online methods
Animals
All animal care and experimental procedures were approved by the National Institute of Health and Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Mice were housed at 22–24 °C with a 12 h light (06:00)/dark (18:00) cycle with standard mouse chow (Teklad F6 Rodent Diet 8664) and water provided ad libitum. Pomc-Cre29, Vglut2-IRES-Cre28, Npy-hrGFP47, L10-GFP25, Agrp-IRES-Cre48, Kiss1-Cre35, Oxtr-Cre41, tdTomato49, Mc4r-t2a-Cre23 mice were maintained on a mixed background and have been described previously. All Cre driver and Cre-reporter mice were used in the heterozygous state. For all behavioral studies male mice between 8–12 weeks were used. For electrophysiological studies male and female mice between 5–12 weeks were used.
Generation of mice
Pomc-IRES-Cre mice
Mice were generated using recombineering techniques as previously described28. Briefly, a selection cassette containing an internal ribosomal entry sequence (IRES) linked to Cre-recombinase and an Frt-flanked kanamycin resistance gene was targeted just downstream of the stop codon of the Pomc gene, so that Cre-recombinase expression was driven by the endogenous gene. A targeting plasmid containing the Cre-containing selection cassette and 4 kb genomic sequence upstream and downstream of the Pomc stop codon was isolated and used for embryonic stem cell targeting. Correctly targeted clones were identified by long range PCR and injected into blastocysts. Chimeric animals generated from blastocyst implantation were then bred for germline transmission of the altered Pomc-allele. Germline-expressing Flpe recombinase transgene mice50 were then used to remove the neomycin selection cassette.
Pomclox mice
A BAC clone containing the mouse Pomc genomic sequence was used to generate Pomclox mice using recombineering. To insert the first loxP sequence in intronic region between exon 2 and 3 located at ~922 bases downstream of the start codon, a PCR amplicon containing a loxP-kanamycin/neomycin-loxP cassette from the pSV-Cre plasmid was amplified with a forward primer containing 71 bp of Pomc homologous sequence upstream of the XhoI site and 19 bp of lox-kanamycin/neomycin-loxP and a reverse primer containing 71 bp of Pomc homologous sequence downstream of the XhoI site and 21 bp corresponding to the 3′ end of the lox-kanamycin/neomycin-loxP cassette. The PCR amplicon was transformed into electrocompetent EL350 bacteria that had been previously transfected with the Pomc BAC clone. Homologous recombination in the EL350 cells was induced as described previously33 to insert the lox-kanamycin/neomycin-loxP cassette into the Pomc BAC at the XhoI site. The kanamycin/neomycin cassette was removed, leaving behind one loxP sequence. The modified BAC was then transformed into EL250 bacteria, and the second loxP sequence was inserted at ~640 bases downstream of the stop codon by generating a second PCR amplicon containing a loxP-frt-kanamycin-frt cassette from the pL451 plasmid using a forward primer containing 70 bp of Pomc homologous sequence upstream of the SalI site and 19 bp of loxP-frtkanamycin-frt and a reverse primer containing 69 bp of Pomc homologous sequence downstream of the SalI site and 19 bp corresponding to the 3′ end of the loxP-frt-kanamycin-frt cassette. Homologous recombination was induced in the EL250 cells containing the modified Pomc BAC to insert the loxP-frtkanamycin-frt cassette into the SalI site and concomitant deletion of the restriction site. The targeting construct spanning ~4 kb upstream of the first loxP sequence and 4 kb downstream of the second loxP sequence was lifted out of the modified BAC and electroporated into W4 ES cells. Positively identified clones were injected into blastocysts, resulting in a number of 100% chimeric founders. The founders were bred to female mice bearing a germline-expressing Flpe recombinase transgene50 to obtain germ-line transmission of the Pomclox allele and to remove the frt-kanamycin-frt cassette.
Validation of Pomc-IRES-Cre mice
Pomc-IRES-Cre mice were crossed with tdTomato reporter mice. Cre activity in Pomc-IRES-Cre::tdTomato mice was highly restricted to the ARC, and was only seen in ARCPOMC neurons (Supplementary Fig. 1g, h). Thus, fidelity of Cre expression in Pomc-IRES-Cre mice is superior to Pomc-Cre BAC transgenic mice51.
Brain tissue preparation
Animals were terminally anesthetized with 7% chloral hydrate diluted in saline (350 mg/kg) and transcardially perfused with phosphate-buffered saline (PBS) followed by 10% neutral buffered formalin (PFA). Brains were removed, stored in the same fixative for 2 hours, transferred into 20% sucrose at 4°C overnight, and cut into 30 μm sections on a freezing microtome (Leica) coronally into four equal series. A single series of sections per animal was used in the histological studies.
To determine the number of POMC-expressing ARCVglut2 neurons, three sections, which covered the ARC, from each animal were used. POMC immunoreactive ARCVglut2 cells from one hemisphere were manually counted. As previously reported, the expression level of Slc17a6 (Vglut2) across the ARC52 and in ARCPOMC neurons in particular53 is not affected by fasting. These findings suggest that the number of POMC-expressing ARCVglut2 neurons as reported here (Fig. 4b–d) is not affected by the fed/fasted state.
Immunohistochemistry
Brain sections were washed in PBS with Tween-20, pH 7.4 (PBST) and blocked in 3% normal donkey serum/PBST for 1 h at room temperature. Then, brain sections were incubated overnight at room temperature in blocking solution containing primary antiserum (rat anti-mCherry, Life Technologies M11217, 1:1,000; rabbit anti-POMC precursor, Phoenix Pharmaceuticals H-029-30, 1:3,000, goat anti-AgRP, Neuromics GT15023, 1:3,000, chicken anti-GFP, Life Technologies A10262, 1:1,000, goat anti-Fos, Calbiochem PC38, 1:1,000). The next morning sections were extensively washed in PBS and then incubated in Alexa-fluorophore secondary antibody (Molecular Probes, 1:1,000) for 1 h at room temperature. After several washes in PBS, sections were mounted on gelatin-coated slides and fluorescence images were captured with Olympus VS120 slide scanner microscope.
Electrophysiology
Animals were deeply anesthetized, decapitated and brains were quickly removed into ice-cold cutting solution consisting of (in mM): 72 sucrose, 83 NaCl, 2.5 KCl, 1 NaH2PO4, 26 NaHCO3, 22 glucose, 5 MgCl2, 1 CaCl2, oxygenated with 95% O2/5% CO2, measured osmolarity 310 – 320 mOsm/l. 300-μm-thick coronal sections were cut with a Leica VT1000S vibratome and incubated in oxygenated cutting solution at 34 °C for 45 min. Slices were transferred to oxygenated aCSF (126 mM NaCl, 21.4 mM NaHCO3, 2.5 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.4 mM CaCl2, 10 mM glucose) and stored in the same solution at room temperature (20–24 °C) for at least 60 min prior to recording. A single slice was placed in the recording chamber where it was continuously superfused at a rate of 3–4 ml per min with oxygenated aCSF. Neurons were visualized with an upright microscope (SliceScope, Scientifica) equipped with infrared-differential interference contrast and fluorescence optics. Borosilicate glass microelectrodes (5–7 MΩ) were filled with internal solution.
Loose-seal, cell-attached recordings (seal resistance, 20–50 MΩ) were made in voltage clamp mode with aCSF as internal solution and holding current maintained at Vh = 0 mV.
To assess the effect of CNO on hM3Dq-expressing ARCPOMC neurons, 5- to 7-week-old Pomc-Cre mice were injected with AAV8-DIO-hM3Dq-mCherry into the ARC 2–3 weeks before recording. To assess the effect of CNO on hM4Di-expressing ARCVglut2 neurons, 5- to 7-week-old Vglut2-IRES-Cre mice were injected with AAV8-DIO-hM4Di-mCherry into the ARC 2–3 weeks before recording. After acquisition of stable loose-seal cell-attached recordings for 3–5 min, aCSF solution containing CNO (10 μM) was perfused into the brain slice preparation.
To assess the effect of oxytocin on ARCOxtr neurons, loose-seal cell-attached recordings were performed on fluorescently labeled ARC cells in Oxtr-Cre::tdTomato mice. CNQX (10 μM), D-AP5 (50 μM) and bicuculline (10 μM) were added to the aCSF to synaptically isolate ARCOxtr neurons. After acquisition of stable recordings for 3–5 min, aCSF solution containing oxytocin (200 nM) was perfused into the brain slice preparation.
To assess effects of fasting on ARCVglut2 neurons, recordings were performed on fluorescently labeled ARC cells in Vglut2-IRES-Cre::L10-GFP mice. Two groups of animals were provided with fresh cages 24 hours prior to euthanasia. The fed group had ad libitum access to food, while no food was provided to the fasted group.
sIPSCs in ARCVglut2 neurons were recorded in whole-cell voltage-clamp mode, with membrane potential clamped at Vh = −70 mV using a CsCl-based internal solution consisting of (in mM): 140 CsCl, 1 BAPTA, 10 HEPES, 5 MgCl2, 2 Mg-ATP, and 0.3 Na2-GTP (pH 7.35 adjusted with NaOH; 295 mOsm · kg−1) in presence of D-AP5 and CNQX.
For CRACM experiments, recordings were obtained using a Cs+-based low Cl− internal solution consisting of (in mM): 135 CsMeSO3, 10 HEPES, 1 EGTA, 4 MgCl2, 4 Na2-ATP, 0.4 Na2-GTP, 10 Na2-phosphocreatine (pH 7.3 adjusted with CsOH; 295 mOsm · kg−1; ECl = −70 mV). Light-evoked EPSCs and IPSCs were recorded in whole-cell voltage-clamp mode, with membrane potential clamped at Vh = −70 mV and 0 mV, respectively. CNQX and D-AP5 or bicuculline were included to isolate glutamatergic/GABAergic currents. The light-evoked EPSC/IPSC detection protocol consisted of four blue light pulses (473 nm wavelength, 5 msec) administered 1 s apart during the first 4 s of an 8-s sweep, repeated for a total of 30 sweeps. Evoked EPSCs/IPSCs with short latency (≤6 ms) upon light stimulation were considered as light-driven. As discussed by others, such currents are most likely monosynaptic31. Number of animals used for CRACM experiments: ARCAgRP→ARCVglut2, n = 3; ARCPOMC (Pomc-Cre)→uPVH, n = 2; ARCPOMC (Pomc-IRES-Cre)→uPVH, n = 5; ARCVglut2→uPVH, n = 2; ARCKiss1→uPVH, n = 2; ARCOxtr→uPVH, n = 2; ARCGlutamatergic→PVHMC4R or PVHMC4R-negative, n = 2; ARCPOMC→PVHMC4R, n = 2; ARCOxtr→PVHMC4R or PVHMC4R-negative, n = 2.
For CRACM experiments in which recordings were made from PVHMC4R neurons, the PVHMC4R neurons were labeled with AAV8-DIO-GFP injected into the PVH of Mc4r-t2a-Cre mice (Fig. 6a, Supplementary Fig. 7a, b), Oxtr-Cre::Mc4r-t2a-Cre (Fig. 6b, Supplementary Fig. 8c) or Pomc-Cre::Mc4r-t2a-Cre (Fig. 6c). For these experiments mCherry-tagged ChR2 was used. The Vglut2-IRES-Cre allele could not be used to selectively express ChR2 in ARCVglut2 neurons (Fig. 6a and Supplementary Fig. 7a, b) since most neurons in the PVH express Vglut2 and Cre activity from the Mc4r-t2a-Cre allele is necessary to selectively visualize PVHMC4R neurons. Instead, ARC neurons were transduced with Cre-independent AAV-ChR2-mCherry and synaptic blockers were used to isolate glutamatergic EPSCs. Note that no Cre activity could be detected in the PVH of Oxtr-Cre mice. Thus, Cre activity from the Mc4r-t2a-Cre allele visualized PVHMC4R neurons in Oxtr-Cre::Mc4r-t2a-Cre mice transduced with AAV-DIO-GFP (Fig. 6b and Supplementary Fig. 7c) or in Oxtr-Cre::Mc4r-t2a-Cre::tdTomato (Fig. 7e–g) mice. As previously reported23, no Cre activity could be detected in the ARC of Mc4r-t2a-Cre::tdTomato mice.
For monitoring α-MSH-induced synaptic changes, recordings were performed on fluorescently labeled PVH cells in Mc4r-t2a-Cre::tdTomato mice. Brain slices were incubated in aCSF containing α-MSH (250 nM) for at least 2–3 h at room temperature (20–24°C) before recordings were made. Acute application of α-MSH during recordings failed, on average, to affect sEPSC amplitude or frequency during 12 min of application. For all experiments examining the effects of α-MSH incubation, recordings from control cells kept in aCSF were interleaved with recordings from cells undergoing α-MSH treatment.
Spontaneous EPSCs (sEPSCs) and electrically-evoked EPSCs were recorded at Vh = −70 mV using a Cs+-based internal solution (see above) in presence of bicuculline. A patch pipette filled with aCSF was placed within 20–40 μm from the cell body of the patched neuron, and EPSCs were evoked electrically by single monopolar square pulse stimulation. Test pulses of 0.1 ms duration (150 – 400 μA) were given at intervals of 15 s. Paired-pulse ratios (PPRs) of electrically-evoked EPSCs were obtained by applying a pair of synaptic stimuli 50 ms apart. PPRs of light-evoked EPSCs were recorded at Vh = −70 mV and obtained by applying a pair of light stimuli 100 ms apart. PPRs were determined by dividing the amplitude of the second EPSC by the amplitude of the first EPSC averaged over 5 min epochs of 20 EPSCs.
AMPAR/NMDAR ratios were recorded using an internal solution consisting of (in mM): 135 CsMeSO3, 10 HEPES, 0.2 EGTA, 4 MgCl2, 4 Na2-ATP, 0.4 Na2-GTP, 10 TEA, 0.1 spermine (pH 7.3 adjusted with CsOH; 295 mOsm · kg−1) in presence of bicuculline. Electrically-evoked AMPAR/NMDAR ratios were recorded at Vh = +40 mV and AMPAR-mediated EPSCs were isolated by adding D-AP5 to block NMDAR–mediated synaptic currents. The NMDAR-mediated EPSCs were calculated by subtracting the AMPAR-mediated EPSCs from the EPSCs measured in the absence of D-AP5. The electrically-evoked AMPAR/NMDAR ratios were calculated by dividing the peak amplitudes. Light-evoked AMPAR/NMDAR ratios were recorded in presence of bicuculline and were calculated as the ratio of the peak amplitude of the light-evoked EPSC at Vh = −70 mV (AMPAR-mediated EPSCs) to the magnitude of the light-evoked EPSC recorded at Vh = +40 mV at 25–50 ms after light stimulation (NMDAR-mediated EPSCs). Access resistance (<30 MΩ) was continuously monitored by a voltage step and recordings were accepted for analysis if changes were <15%.
All recordings were made using a Multiclamp 700B amplifier, and data were filtered at 2 kHz and digitized at 10 kHz. To photostimulate channelrhodopsin2-positive fibers, a laser or LED light source (473 nm) was used. The blue light was focused onto the back aperture of the microscope objective, producing wide-field exposure around the recorded cell of 10–15 mW per mm2. The light power at the specimen was measured using an optical power meter PM100D (Thorlabs). The light output was controlled by a programmable pulse stimulator, Master-8 (A.M.P.I.) and pClamp 10.2 software (Axon Instruments). All recordings were analyzed offline using Clampfit 10.
Single-cell RNA-seq
Single-cell RNA sequencing was performed as previously described23. Briefly, ARCVglut2 neurons were identified based on their fluorescence (GFP-positive in Vglut2-IRES-Cre::L10-GFP mice). Brain slices were sectioned as for electrophysiological experiments (see above) and the ARC was micro-dissected, and dissociated with papain. Individual GFP-positive ARC cells were isolated from suspension by micropipette, then washed, frozen at −80 °C, and lysed for cDNA synthesis and PCR amplification. To control for mRNA contamination during cell picking, an equivalent volume of cell-picking buffer was sampled and processed with each batch of single-cell samples. Amplified cDNA was analyzed by quantitative PCR (qPCR) for expression of the housekeeping gene expression, Actb. Single-cell samples showing low or undetectable levels of Actb expression were excluded from further use, as were batches of samples in which the cell-picking buffer showed evidence of mRNA contamination (i.e., Actb-positive). Barcoded RNA-seq libraries were constructed with a commercially available kit (Ovation Rapid Library Kit NuGen, California, USA) and sequenced in multiplex by Illumina HiSeq (50-base single-end reads). Pass-filter reads were aligned to the mouse genome (mm10) and quantified by TopHat/Bowtie2/Cufflinks software in units of RPKM (reads per kilobase exon per million fragments mapped).
Cluster analysis
Each of the 23 ARCVglut2 neurons was represented by the expression of 90 neuropeptide genes (Supplemental table 1). Of the 92 neuropeptide genes provided (gene list from www.neuropeptides.nl54), we excluded Oxt and Avp from our analysis due to cross-contamination. To cluster the neurons to groups we first reduced the representation of each neuron to 3 dimensions. Dimensionality reduction was performed with the tSNE algorithm, using free and open source software written in Matlab (distributed under the FreeBSD License. https://lvdmaaten.github.io/tsne/). The software first performs a dimensionality reduction using standard Matlab principal component analysis. The first 10 principal components for each neuron were then fed into the tSNE procedure (with perplexity parameter set to 2). Following this procedure each neuron was represented by 3 tSNE dimensions. Next, we performed clustering using ‘kmeans’ procedure to find the four major groups. All analysis was done in Matlab.
Viral injections
Stereotaxic injections were performed using previously described procedures23. Mice were anesthetized with xylazine (5 mg per kg) and ketamine (75 mg per kg) diluted in saline (0.9%) and placed into a stereotaxic apparatus (KOPF). For postoperative care, mice were injected intraperitoneally with meloxicam (0.5 mg per kg). A micromanipulator (Grass Technologies, model S48 stimulator) was used to deliver the viruses at 2–5 nl per min and the pipette was withdrawn 5 min after injection. AAV1-FLEX-ChR2(H134R)-mCherry, AAV9-FLEX-ChR2(H134R)-EYFP, AAV9-CAG-ChR2(H134R)-mCherry (University of Pennsylvania School of Medicine), AAV8-DIO-hM3Dq-mCherry, AAV8-DIO-hM4Di-mCherry, AAV8-hSyn-mCherry-Cre, AAV5-hSyn-mCherry (University of North Carolina Vector Core), AAV8-DIO-synaptophysin-mCherry (Virovek, Inc) were injected into the ARC (coordinates, bregma: AP: −1.35 mm, DV: −6.00 mm, ML: ±0.2 mm). Mice with ‘missed’ injections, incomplete ‘hits’ or expression outside the ARC were excluded from analysis after post hoc examination of mCherry/GFP expression. AAV8-DIO-GFP (University of North Carolina Vector Core) was injected into the PVH (coordinates, bregma: AP: −0.75 mm, DV: −4.85 mm, ML: −0.20 mm).
Optic fiber implantation
Fiber implantation was performed using previously described procedures23. Optical fibers (200 μm diameter core; Thor Labs) were implanted unilaterally over the PVH (coordinates, bregma: AP: −0.70 mm, DV: −4.35 mm, ML: 0 mm). Fibers were fixed to the skull using dental acrylic and mice were allowed 2 weeks for recovery before acclimatization to the recording chambers for 1 week.
Food intake studies
All animals were singly housed for at least 2.5 weeks following surgery and handled for 10 consecutive days before the assay to acclimate mice to the experimental procedure. Feeding studies were performed in home cages with ad libitum food access to chow. The day before the experiment, mice were provided with fresh cages to avoid leftover of food spilling in the bedding. CNO was administered at 1 mg per kg of body weight. Saline was delivered at the same volume to maintain consistency in the studies. Mice with ‘missed’ injections, incomplete ‘hits’ or expression outside the ARC were excluded from analysis after post hoc examination of mCherry expression.
For light-cycle measurements, animals were injected with either saline or CNO at 9:00 AM and food intake was monitored 1, 2, 3 and 4 h after i.p. injection from 9:00 AM to 1:00 PM. For refeeding experiments, mice were provided with fresh cages at 9:00 AM the day prior the experiment and no food was provided. Following 24 hour fasting, mice were injected with either saline or CNO at 8:45 the following day and food intake was monitored 1, 2, 3 and 4 h after i.p. injection from 9:00 AM to 1:00 PM. For dark-cycle measurements, animals were injected with either saline or CNO at 6:00 PM and food intake was monitored 1, 2, 3 and 4 h after i.p. injection from 6:00 PM to 10:00 PM. For 24 and 48 hours food intake measurements, animals were injected with either saline or CNO three times per day.
For in vivo optogenetic studies, fiber optic cables were firmly attached to the implanted fiber optic cannulae with zirconia sleeves. Light pulse trains (5 ms pulses of 20 Hz; 3 s on, 1 s off) were programmed using a waveform generator that provided input to a blue light LED (473 nm). The light power exiting the fiber optic cable was adjusted so that it had 5–8 mW measured using an optical power meter. After completion of photostimulation experiments, mice were perfused and the approximate locations of fiber tips were identified.
iBAT temperature
iBAT temperature was measured using a previously described method55. Temperature transponders (IPTT-300, Bio Medic Data Systems) were implanted subcutaneously above the iBAT pad between the scapula under anesthesia. Animals were allowed 1 week recovery and acclimation was performed with saline injection for 5 consecutive days. Data were collected using a handheld reader system (DAS-7006/7r; Bio Medic Data System) at time points indicated.
Statistical analysis
Statistical analyses were performed using Prism 6.0 (GraphPad) software. No statistical method was used to predetermine sample size. Sample sizes were chosen similar to those reported in previous publications13, 23, 25, 28. Randomization and blinding methods were not used. All data presented met the assumptions of the statistical test employed. Experimental animals were excluded if histological validation of the injection site demonstrated absence of reporter expression. This was established prior to data collection. N values represent final number of validated animals. For examining differences in the distribution of sEPSC amplitudes, we used MATLAB (Mathworks) to generate normalized frequency distributions and compared them using a Kolmogorov-Smirnov test.
Supplementary Material
Acknowledgments
This research was funded by the following NIH grants to B.B.L.: R01 DK075632, R01 DK096010, R01 DK089044, P30 DK046200, P30 DK057521; to J.M.R.: F32 DK103387; to J.N.C., AHA Postdoctoral Fellowship 14POST20100011. Y.M. was supported by a Charles A. King postdoctoral fellowship. Y.L. was supported by an EMBO postdoctoral fellowship. We thank members of B.B.L. lab for helpful discussions.
Footnotes
Author Contributions
H.F. and B.B.L. designed the experiments and analyzed data. J.N.C. performed the single cell RNA-seq experiments with help from Y.M.. H.F. and A.M.V. conducted the behavioral and histological studies with help from J.M.R. and Z.Y.. H.F. performed the electrophysiological experiments with help from J.C.M. and Y.L.. J.Y. generated Pomc-IRES-Cre mice. B.P.S. generated Pomclox mice. H.F. and B.B.L. wrote the manuscript with comments from all of the authors.
The authors declare no competing financial interests.
Data availability
RNA-Seq data are available at Gene Expression Omnibus (GEO), accession number GSE81789. The authors declare that all other relevant data supporting the findings of this study are available on request.
References
- 1.Langlet F. Tanycytes: a gateway to the metabolic hypothalamus. J Neuroendocrinol. 2014;26:753–760. doi: 10.1111/jne.12191. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Xu AW, et al. Effects of hypothalamic neurodegeneration on energy balance. PLoS Biol. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan C, et al. Acute and Long-Term Suppression of Feeding Behavior by POMC Neurons in the Brainstem and Hypothalamus, Respectively. J Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krude H, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 6.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 7.Huszar D, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 8.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 10.Yeo GS, et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 11.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 12.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krashes MJ, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers MG, Jr, Olson DP. Central nervous system control of metabolism. Nature. 2012;491:357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- 15.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–378. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161:133–145. doi: 10.1016/j.cell.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch M, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagnol D, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowley MA, et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 21.Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- 22.Shah BP, et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A. 2014;111:13193–13198. doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfield AS, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18:863–871. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krashes MJ, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atasoy D, et al. A genetically specified connectomics approach applied to long-range feeding regulatory circuits. Nat Neurosci. 2014;17:1830–1839. doi: 10.1038/nn.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branco T, et al. Near-Perfect Synaptic Integration by Nav1.7 in Hypothalamic Neurons Regulates Body Weight. Cell. 2016;165:1749–1761. doi: 10.1016/j.cell.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 32.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- 33.Jarvie BC, Hentges ST. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J Comp Neurol. 2012;520:3863–3876. doi: 10.1002/cne.23127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521:3287–3302. doi: 10.1002/cne.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cravo RM, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picelli S, et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- 37.Usoskin D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 38.Sudhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blakely RD, Edwards RH. Vesicular and plasma membrane transporters for neurotransmitters. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maejima Y, et al. Oxytocinergic circuit from paraventricular and supraoptic nuclei to arcuate POMC neurons in hypothalamus. FEBS Lett. 2014;588:4404–4412. doi: 10.1016/j.febslet.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Nakajima M, Gorlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159:295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen Y, Fu WY, Cheng EY, Fu AK, Ip NY. Melanocortin-4 receptor regulates hippocampal synaptic plasticity through a protein kinase A-dependent mechanism. J Neurosci. 2013;33:464–472. doi: 10.1523/JNEUROSCI.3282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blevins JE, et al. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2015;308:R431–438. doi: 10.1152/ajpregu.00441.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawson EA, et al. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 2015;23:950–956. doi: 10.1002/oby.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blevins JE, Baskin DG. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: Insights from rodents, nonhuman primates and humans. Physiol Behav. 2015;152:438–449. doi: 10.1016/j.physbeh.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Pol AN, et al. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- 51.Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153:1219–1231. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jovanovic Z, Tung YC, Lam BY, O’Rahilly S, Yeo GS. Identification of the global transcriptomic response of the hypothalamic arcuate nucleus to fasting and leptin. J Neuroendocrinol. 2010;22:915–925. doi: 10.1111/j.1365-2826.2010.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henry FE, Sugino K, Tozer A, Branco T, Sternson SM. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. Elife. 2015;4 doi: 10.7554/eLife.09800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burbach JP. Neuropeptides from concept to online database http://www.neuropeptides.nl. Eur J Pharmacol. 2010;626:27–48. doi: 10.1016/j.ejphar.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 55.Kong D, et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.