Abstract
Objective
The main aim of this study was to use familial risk analysis to examine the association between attention deficit hyperactivity disorder (ADHD) and substance use disorders (SUDs) attending to sex effects and the specificity of alcohol and drug use disorder risks.
Methods
Subjects were derived from two longitudinal case-control family studies of probands aged 6 to 17 years with and without DSM-III-R ADHD of both sexes and their first degree relatives followed from childhood onto young adult years. Cox proportional hazard models were used to estimate rates of ADHD and SUDs (any SUD, alcohol dependence, and drug dependence). Logistic regression was used to test both co-segregation and assortative mating.
Results
Our sample included 404 probands (ADHD: 112 boys and 96 girls; Control: 105 boys and 91 girls) and their 1,336 relatives. SUDs in probands increased the risk for SUDs in relatives irrespective of ADHD status. The risk for dependence to drug or alcohol in relatives was non-specific. There was evidence that even in the absence of a SUD in the proband, ADHD by itself increased the risk of SUDs in relatives. Proband sex did not moderate the familial relationship between ADHD and SUDs. There was evidence of co-segregation between ADHD and SUD.
Conclusions
Findings indicate that various independent pathways are involved in the transmission of SUD in ADHD and that these risks were not moderated by proband sex. ADHD children and siblings should benefit from preventive and early intervention strategies to decrease their elevated risk for developing a SUD.
Keywords: familial risk, substance use disorder, attention deficit hyperactivity disorder, co-segregation
INTRODUCTION
A significant and bidirectional association between attention deficit hyperactivity disorder (ADHD) and substance use disorders (SUDs) has been well documented in clinical and epidemiological studies (Charach et al., 2011; Lee et al., 2011; van de Glind et al., 2014; van Emmerik-van Oortmerssen et al., 2012; Wilens et al., 2011). While these findings suggest that the two disorders are linked, the nature of the association remains unclear. Since both ADHD and SUDs are known to be familial disorders (Faraone and Biederman, 1994; Merikangas et al., 1998), family studies can elucidate the nature of this association. To date there have been a limited number of family studies investigating the relationship between ADHD and SUD (Cantwell, 1972; Groenman et al., 2013; Morrison and Stewart, 1971).
We previously reported on the familial risk associated with ADHD and SUDs in boys with and without ADHD followed into young adulthood (Biederman et al., 2008). In that study, ADHD and alcohol dependence were found to be independently transmitted whereas the relationship between ADHD and drug dependence was most consistent with variable expressivity of a common risk between the disorders. However, since the sample only included boys uncertainties remain as to whether these findings extend to girls with ADHD.
Consistent with the above findings are the results from two large cohort samples from the longitudinal Swedish national registry. Both studies found first degree relatives of ADHD probands to have an elevated risk for SUDs when compared to relatives of controls (Skoglund et al., 2015; Sundquist et al., 2015). However, the evaluation of the nature of the risk for SUDs was limited by the data available in the registries since SUD was only detected in family members if they presented for treatment for their SUD (Skoglund et al., 2015; Sundquist et al., 2015) or had legal consequences associated with their drug use disorder (Sundquist et al., 2015). Since the majority of people with SUD never present for treatment, the familial risk for SUDs associated with ADHD may have be underestimated using a sample derived from registries (Ali et al., 2015). Furthermore, this study did not examine the specificity of the association between ADHD and SUDs.
In contrast, a recent study by Groenman et al (Groenman et al., 2013) found no association between ADHD and SUDs in a familial risk analysis examining the risk for SUDs in ADHD siblings. However, this study was limited by the relatively young age of the probands and siblings (mean: 17 years) and the absence of inclusion of parents. Thus, more research is needed to help further clarify the nature of the association between ADHD and SUDs attending to the limitations of the extant literature. Such insights could inform the development of early intervention strategies aimed at mitigating the risk for developing a SUD in children with ADHD.
The main aim of this study was to re-examine the association between ADHD and SUDs attending to proband sex, addressing the specificity of the association, and evaluating the risk in all first-degree biological relatives. To this end we conducted a familial risk analysis of two well characterized longitudinal samples of boys and girls with and without ADHD ascertained from pediatric and psychiatric sources followed up into young adulthood using operational definitions of substance abuse and dependence. We examined the following research questions: 1) whether the risk for SUD in probands breeds true in relatives; 2) whether these risks are specific to alcohol or drugs; 3) whether these risks are independent of ADHD; and 4) whether these risks are moderated by proband sex. To the best of our knowledge, this represents the largest and most comprehensive familial risk analysis examining the association between ADHD and SUDs and the only one that has examined proband sex effects and specificity of the risk in moderating this association.
METHODS
Detailed study methodology has been previously described. (Biederman et al., 2006; Biederman et al., 2010b) (Busch et al., 2002) Subjects were derived from two identically designed longitudinal case-control family studies of ADHD. These studies recruited male and female probands aged 6 to 17 years with DSM-III-R ADHD (N=140 boys, N=140 girls) and without ADHD (i.e. Controls, N=120 boys, N=122 girls) from pediatric and psychiatric clinics and their first degree relatives (parents and siblings).
Potential subjects were excluded if they had been adopted, their nuclear family was not available for study, if they had major sensorimotor handicaps, psychosis, autism, inadequate command of the English language, or a Full Scale IQ less than 80. ADHD subjects met full DSM-III-R diagnostic criteria for ADHD at the time of referral and all had active symptoms of the disorder at recruitment. Parents and adult offspring provided written informed consent to participate, and parents provided consent for offspring under the age of 18. Children and adolescents provided written assent to participate. The human research committee at Massachusetts General Hospital approved this study.
ASSESSMENT PROCEDURES
Psychiatric assessments in probands, siblings, and parents >18 years were completed using the Structured Clinical Interview for the DSM-IV (SCID) (First et al., 1997; Spitzer et al., 1990) supplemented with modules from the Schedule for Affective Disorder and Schizophrenia for Children (K-SADS-E), (Orvaschel, 1994) to assess childhood diagnoses. Parents were only assessed at baseline. Youth <18 years of age were assessed at baseline and at follow up with the K-SADS-E. All diagnostic assessments were conducted by highly trained and closely supervised raters. Raters were blind to the ascertainment of the families. Direct interviews were conducted with subjects >12 years of age and indirect interviews were conducted with their mothers. We combined data from direct and indirect interviews by considering a diagnostic criterion positive if it was endorsed in either interview.
Board-certified child and adult psychiatrists who were blind to the subject’s ADHD status, referral source, and all other data resolved diagnostic uncertainties. All potential diagnoses of SUD were reviewed. To assess the reliability of our overall diagnostic procedures, we computed kappa coefficients of agreement by having blinded board-certified child and adult psychiatrists diagnose subjects from audiotaped interviews made by the assessment staff. Based on 500 assessments from interviews of children and adults, the median kappa coefficient was 0.98. The kappa coefficient for ADHD was 0.88 and for SUD was 1.0. Socioeconomic status (SES) was measured using the 5-point Hollingshead scale (Hollingshead, 1975).
STATISTICAL ANALYSIS
For this analysis we used data on probands and siblings at the 10 year follow up (parents were only assessed at baseline). Comparisons were made between probands with and without ADHD with and without any SUD (any alcohol or drug abuse or dependence), alcohol dependence or drug dependence. We first compared differences between ADHD and control probands using the Wilcoxon Rank Sum test, Student’s T test, and Chi Squared tests.
In the first set of analyses we separated the sample into four groups of first degree relatives defined by the probands’ ADHD and SUD status (i.e. neither, ADHD alone, SUD alone, both ADHD and SUD). Using Cox proportional hazard models, we compared the relatives of the four proband groups on estimated percentages of ADHD and SUDs. We used Cox models to adjust for the varying age at onset of SUDs. In these models, survival time is age at onset for those with a disorder and age at interview for those without the disorder (i.e., the censored individuals). Using logistic regression, co-segregation was established if the presence of ADHD in the relative was significantly associated with SUD in the same relative within the subset of families having a proband with both ADHD and SUD. Logistic regression was also used to test assortative mating by using all relatives of ADHD probands and examining whether ADHD in one parent is associated with SUD in the other parent. In the second set of analyses, we repeated the analytical approach described above except that we used alcohol dependence to define the proband group and as an outcome in the relatives. In the third set of analyses, we used drug dependence to define the proband groups and as an outcome in the relatives. To account for the nonindependence of family members, we used the Huber (Huber, 1967) correction to produce robust variances for all statistical tests using family members. All tests were two-tailed with alpha set at 0.05. We calculated all statistics using STATA, version 13.0. Data are expressed as mean ± standard deviation (SD) unless otherwise specified.
RESULTS
As previously described (Biederman et al., 2010a; Biederman et al., 2012), there were no significant differences between participants who were lost-to-follow-up and those who remained in the study on baseline characteristics of age, race, GAF score, intactness of the family, or psychiatric outcomes (all p values > 0.05). For the follow-up study of boys, both ADHD and control probands who were lost-to-follow-up had a lower mean SES than those successfully reassessed (Biederman et al., 2010a). Our final sample included 404 probands (ADHD: 112 boys and 96 girls; Control: 105 boys and 91 girls) and their 1,336 first degree relatives (ADHD: 208 mothers, 206 fathers, 282 siblings; Control: 196 mothers, 190 fathers, 254 siblings).
We found small but significant differences in the age of probands and the age of mothers of probands with and without ADHD (both p values < 0.05) (Table 1). We also found ADHD families had a significantly lower SES compared to comparison families (p=0.001). Thus, all analyses corrected for proband age and SES. Analyses first examined whether proband sex had any interaction effect on the relationship between proband ADHD and SUD status and risk of ADHD and SUD in relatives. Since we found no significant interaction effects (all p values > 0.05), all subsequent analyses were assessed across a collapsed sex group.
Table 1.
Demographic Characteristics of Attention Deficit Hyperactivity Disorder (ADHD) and Comparison Probands and Their First-Degree Relatives
| ADHD Probands |
Comparison Probands |
Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Age (years) | ||||||||
| N | Mean | SD | N | Mean | SD | Test | Df | p | |
| Age at last assessment (years) | |||||||||
| Probands | 208 | 21.6 | 3.5 | 196 | 22.7 | 3.5 | t=3.1 | 402 | 0.002 |
| Mothers | 208 | 40.0 | 5.3 | 196 | 41.9 | 5.3 | t=3.6 | 402 | <0.001 |
| Fathers | 206 | 42.8 | 6.2 | 190 | 44.0 | 5.9 | t=1.9 | 394 | 0.05 |
| Siblings | 282 | 21.8 | 6.4 | 254 | 22.0 | 6.2 | t=0.3 | 534 | 0.8 |
| Family Socioeconomic Status | 208 | 1.8 | 0.9 | 196 | 1.5 | 0.8 | z=−3.2 | 1 | 0.001 |
| N | % | N | % | ||||||
| Proband sex (male) | 208 | 112 | 54 | 196 | 105 | 54 | χ2=0.003 | 1 | 1.0 |
FAMILIAL RISK ANALYSIS OF ANY SUD
Four groups of first-degree relatives were used to examine the familial risk between any SUD (any alcohol or drug abuse or dependence) and ADHD: 1) relatives of 101 comparison probands without any SUD (Comparison, N=322); 2) Relatives of 95 comparison probands with any SUD (Comparison+Any SUD, N=318); 3) Relatives of 91 ADHD probands without any SUD (ADHD, N=307), and 4) relatives of 117 probands with both any SUD and ADHD (ADHD + Any SUD, N=389).
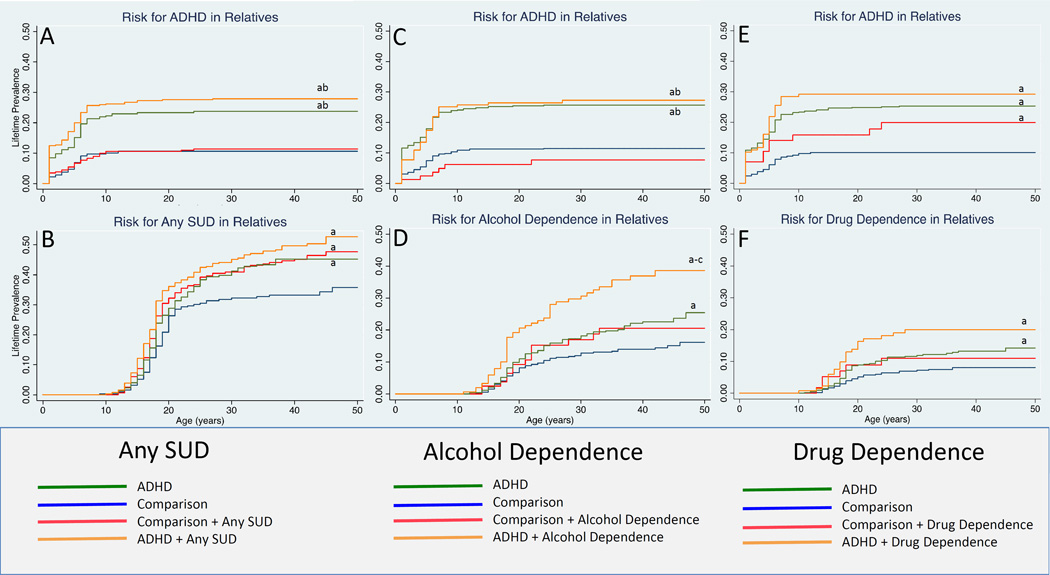
Figure 1A shows that the age-adjusted rates of ADHD in the ADHD+Any SUD and ADHD groups of relatives were significantly higher in relation to the Comparison group (28% and 24% vs. 11%; hazard ratio (HR): 3.1; 95% Confidence Interval (CI): 2.1, 4.6; p<0.001; HR: 2.3; 95% CI: 1.5, 3.5; p<0.001, respectively). We also found that both the ADHD+Any SUD and ADHD groups had higher age-adjusted rates of ADHD compared to the Comparison+Any SUD group (28% and 24% vs. 11%; HR: 2.6; 95% CI: 1.7, 4.0; p<0.001; HR: 1.9; 95% CI: 1.2, 3.0; p=0.004, respectively). There was no significant difference in the rates of ADHD between the ADHD+Any SUD group and the ADHD group (28% vs. 24%; HR: 1.4; 95% CI: 0.99, 1.9; p=0.06).
Figure 1.
Risk for substance use and attention deficit hyperactivity disorders in relatives by proband diagnosis.
a p<0.05 vs. comparison subjects
b p<0.05 vs. comparison subjects plus substance use disorder subjects
c p<0.05 vs. subjects with ADHD
The ADHD+Any SUD, ADHD, and Comparison+Any SUD groups all had higher age-adjusted rates of any SUD in relation to the Comparison group (Figure 1B; 45%, 37%, 41% vs. 30%; HR: 1.7; 95% CI: 1.3, 2.3; p<0.001; HR: 1.4, 95% CI: 1.0, 1.8; p=0.039; HR: 1.5; 95% CI: 1.2, 2.0; p=0.002). There were no other significant findings (all p values >0.05).
There was evidence of cosegregation in the ADHD+Any SUD group (58% SUD in subjects with ADHD vs. 40% SUD in subjects without ADHD; p=0.003). There was no evidence of assortative mating in the ADHD group: comparing ADHD in mothers vs. SUD in fathers: p=0.78; comparing ADHD in fathers vs. SUD in mothers: p=0.78).
FAMILIAL RISK FOR ALCOHOL DEPENDENCE
The familial risk analysis of alcohol dependence and ADHD used four groups: 1) Relatives of 172 comparison probands without alcohol dependence (Comparison, N=556); 2) Relatives of 24 comparison probands with alcohol dependence (Comparison+Alcohol Dependence, N=84); 3) Relatives of 162 probands with ADHD without any alcohol dependence (ADHD, N=541); 4) Relatives of 46 probands with ADHD and alcohol dependence (ADHD+Alcohol Dependence, N=117).
Both the ADHD+Alcohol Dependence and ADHD groups had higher age-adjusted rates of ADHD in relation to the Comparison group (Figure 1C, 27%, 26% vs. 11%; HR: 2.7; 95% CI: 1.8, 4.2; p<0.001; HR: 2.4; 95% CI: 1.7, 3.2; p<0.001, respectively) and in relation to the Comparison+Alcohol Dependence group (27%, 26% vs. 7%; HR: 3.8; 95% CI: 1.6, 9.4; p=0.003; HR: 3.3; 95% CI: 1.4, 7.8; p=0.006, respectively). There was no significant difference in rates of ADHD between the Comparison+Alcohol Dependence group and the Comparison group (7% vs. 11%; HR: 0.7; 95% CI: 0.3, 1.7; p=0.45).
Both the ADHD+Alcohol Dependence and ADHD groups had higher age-adjusted rates of alcohol dependence in relation to the Comparison group (Figure 1D, 32%, 18% vs. 12%; HR: 2.9; 95% CI: 1.9, 4.4; p<0.001; HR: 1.6; 95% CI: 1.1, 2.2; p=0.01, respectively). The ADHD+Alcohol Dependence group also had higher age-adjusted rates of alcohol dependence in relation to the Comparison+Alcohol Dependence group (32% vs. 17%; HR: 2.0; 95% CI: 1.1, 3.7; p=0.02) as well as in relation to the ADHD group (32% vs. 18%; HR: 1.9; 95% CI: 1.3, 2.7; p=0.002).
FAMILIAL RISK FOR DRUG DEPENDENCE
The familial risk analysis of drug dependence and ADHD used four groups: 1) Relatives of 178 comparison probands without any drug dependence (Comparison, N=580); 2) Relatives of 18 comparison probands with drug dependence (Comparison+Drug Dependence, N=60); 3) Relatives of 165 ADHD probands without any drug dependence (ADHD, N=559); 4) Relatives of 43 ADHD probands with drug dependence (ADHD+Drug Dependence, N=137).
The ADHD+Drug Dependence group, the ADHD group, and the Comparison+Drug Dependence group all had higher age-adjusted rates of ADHD in relation to the Comparison group (Figure 1E, 29%, 25%, 19% vs. 10%; HR:3.2; 95% CI: 2.1, 5.0; p<0.001; HR: 2.6; 95% CI: 1.9, 3.6; p<0.001; HR: 2.1; 95% CI: 1.004; 4.3; p=0.049, respectively). There were no other significant associations (all p values >0.05).
The ADHD+Drug Dependence group and the ADHD group had higher age-adjusted rates of Drug Dependence in relation to the Comparison group (Figure 1F, 18%, 11% vs. 7%; HR: 2.9; 95% CI: 1.6, 5.3; p=0.001; HR: 1.7; 95% CI: 1.04; 2.65; p=0.034, respectively). No other associations were significant (all p values >0.05).
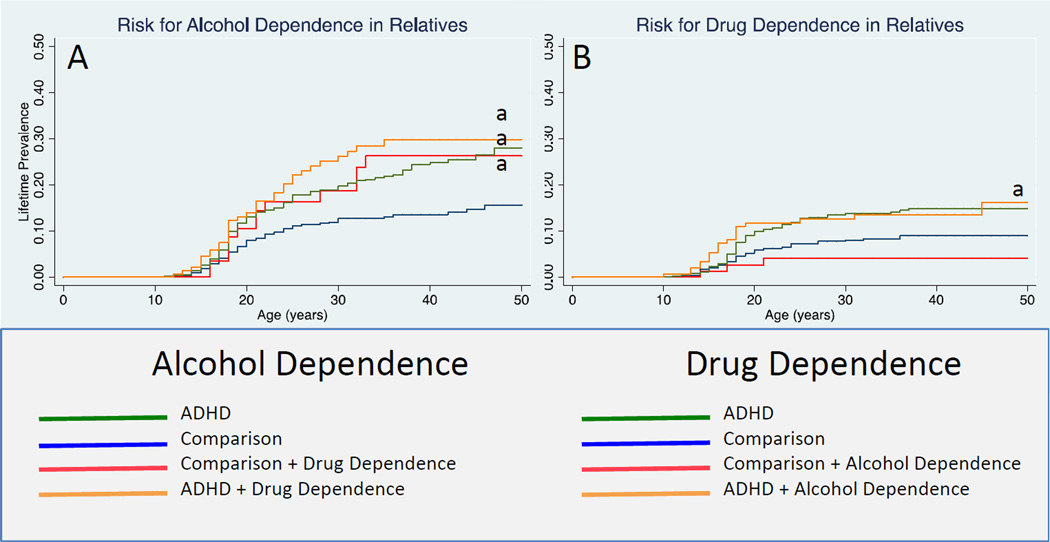
SPECIFICITY OF RISK FOR ALCOHOL AND DRUG DEPENDENCE
For alcohol dependence, we found that relatives of probands with ADHD +Drug dependence, ADHD, and Comparisons+Drug Dependence all had higher age-adjusted rates of alcohol dependence in relation to the Comparison group (Figure 2G; 25%, 20%, 22% vs. 12%; HR: 2.3; 95% CI: 1.4, 3.7; p=0.001; HR: 1.8; 95% CI: 1.3, 2.6; p<0.001; HR: 1.8; 95% CI: 1.1, 3.0; p=0.03, respectively). We found similar results for drug dependence (Figure 2H). Relatives of probands with ADHD had higher age-adjusted rates of drug dependence in relation to the Comparison group (12% vs. 8%; HR: 1.7; 95% CI: 1.0, 2.7; p=0.03). All other results were not significant (all p values >0.05) although we found a trend to significance between the ADHD+Alcohol Dependence group and the Comparison group (13% vs. 8%; HR: 1.9; 95% CI: 0.97, 3.8; p=0.06).
Figure 2.
Risk for alcohol dependence and drug dependence in relatives by proband diagnosis
a p<0.05 vs. comparison subjects
DISCUSSION
Results from this familial risk analysis found that: 1) SUDs in probands increased the risk for SUDs in relatives irrespective of ADHD status; 2) the risk for dependence to drug or alcohol in relatives was non-specific; 3) ADHD in probands predicted SUD in relatives even in the absence of SUD in the proband; 4) There was evidence for co-segregation between ADHD and SUDs; and 5) Sex did not moderate the familial relationship between ADHD and SUDs.
The finding that SUDs are transmitted in families of probands with and without ADHD is consistent with a large literature documenting an increased risk for SUDs in families of subjects with SUDs (Merikangas et al., 1998). The findings showing that proband’s alcohol or drug dependence predicted both types of SUD in relatives are consistent with the hypothesis that what is inherited is the vulnerability to develop a SUD and not a specific type of SUD. This is both consistent (Nurnberger et al., 2004) and inconsistent (Bierut et al., 1998; Merikangas et al., 1998) with the extant literature and more work is needed to further elucidate whether there is specificity in the transmission of SUD and the reasons for the direction the expression of the vulnerability takes in some people.
The finding that ADHD in the proband predicted risk for SUDs in relatives independently of proband SUD status is consistent with findings were reported by Skoglund and colleagues’ using data from a large Scandinavian cohort registry study (Skoglund et al., 2015). These investigators also found that first degree relatives of probands with ADHD were at increased risk for SUD (OR 2.2 and 1.8) independently of SUD in the proband. These findings support the hypothesis that etiological risk factors associated with ADHD itself confer an increased risk for SUD.
Our findings that ADHD in the proband predicted risk for SUDs in relatives independently of proband SUD status are inconsistent with those of Groenman et al, (Groenman et al., 2013) in an adolescent sample and with our previous work reporting findings from the same sample as that used in the current analysis in mid adolescent years (Biederman et al., 2009; Milberger et al., 1998). These discrepant results are likely related to the young age of participants in both studies in that they were not through the age of risk for developing a SUD. (Biederman et al., 2009; Groenman et al., 2013; Milberger et al., 1998). Although our previous findings in young adult male ADHD probands (Biederman et al., 2008) were consistent with those of the current analysis, significance could not be established in all analyses, most likely due to limited statistical power. More work is needed to confirm the finding that ADHD in a proband is a risk for SUD in relatives independently of SUD status in the proband.
Co-segregation, which reflects the tendency for closely linked genes and genetic markers to be inherited together, was present between ADHD and SUDs in the absence of assortative mating. This finding is consistent with the hypothesis that some forms of ADHD and SUD may represent a distinct genetic subtype of either ADHD or SUD although our analysis did not differentiate between genetic and environmental familial influences. Notably, our data do not suggest that ADHD+SUD is genetically more severe than ADHD alone because the two groups did not differ in their risks for either ADHD or SUD. This calls for studies of ADHD and SUD samples using genetic risk score methodology to confirm this finding.
The finding that proband sex did not moderate the familial relationship between ADHD and SUD is consistent with our previous work with this same sample and the work of Ottosen and colleagues that both demonstrated no sex differences in the risk for SUD associated with ADHD. (Wilens et al., 2011) (Ottosen et al., 2016) To our knowledge this is the first familial risk analysis examining the association between ADHD and SUD that documents absence of sex effects indicating that the associations between ADHD and SUDs are not moderated by or accounted by sex of the proband and are due to ADHD itself.
Our finding showing that various pathways are involved in the transmission of SUD in ADHD families is novel. These include the risk associated with SUD itself, the risk conferred by ADHD, and the risk conferred by the co-segregation of ADHD and SUD. Although the way in which these different risk factors interact with each other and with environmental factors remain to be explored, our findings do suggest that genes responsible for the risk for SUDs overlap with those responsible for the risk for ADHD. It is likely that such genes contribute to the well documented comorbidity between SUDs and ADHD. (van de Glind et al., 2014)
Further understanding as to the nature of the association between ADHD and SUD has important clinical implications. The presence of multiple etiological pathways emphasizes the complexity of the risk for SUD in individuals with ADHD and their family members, and begins to explain the elevated risk of SUDs in youth with ADHD. (Charach et al., 2011; Lee et al., 2011) Clinicians should be encouraged to screen for ADHD and SUD in siblings and parents when assessing a child with ADHD. Furthermore, since ADHD typically onsets at least a decade before the onset of SUD, ADHD children and their siblings would benefit from preventive and early intervention strategies aimed at mitigating this risk.
Strengths of this study include the large sample size including equal numbers of probands of both sexes, the inclusion of all first degree relatives, the long length of follow up such that probands and siblings were evaluated for SUDs after they transitioned through the peak age of risk for a SUD, and the reliance on operationalized definitions of ADHD and SUDs through structured interviews.
Despite these strengths our study needs to be viewed in light of some methodologic limitations. Parents were only assessed at baseline and it is possible that additional cases of SUDs emerged in the parents during the follow-up period. This concern is somewhat mitigated by the fact that parents had passed the peak age of risk for the development of a SUD (Falk et al., 2008) and by the use of Cox models that calculated age-adjusted rates of SUD. The SUD prevalence may have also been underestimated in probands and relatives since subjective rather than objective measures were used to assess for substance use disorders. We have previously demonstrated however that clinical data is very sensitive to SUD even in the absence of urine toxicology testing (Gignac et al., 2005). Additionally, co-segregation and assortative mating could not be evaluated in analysis of the alcohol and drug dependence groups due to the smaller sample size of these groups. Our familial analysis examining the relationship between ADHD and SUD did not control for other known risk factors for the development of SUD that commonly co-occur with ADHD. Future studies could benefit from a more comprehensive examination of all risk factors for SUD in ADHD. Since the sample was referred and mostly Caucasian, our findings may not generalize to community samples and other ethnic groups.
Despite these considerations, results from this large familial risk analysis show that multiple etiological pathways are involved in the manifestation of SUDs in subjects with ADHD, the risk for SUD is not specific to a particular type of substance of abuse, and the risk is not moderated by sex of the proband. Taken together, these finding provide novel insights as to the nature and complexity of the association between ADHD and SUDs.
Highlights for Submission.
Multiple pathways are involved in the manifestation of SUD in subjects with ADHD
ADHD in probands predicted SUD in relatives even in the absence of SUD in proband
There is evidence of co-segregation between ADHD and SUD
The risk for dependence to drug or alcohol in relatives was non-specific
Sex did not moderate the familial relationship between ADHD and SUD
Acknowledgments
Dr. Faraone is supported by the K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, University of Bergen, Bergen, Norway, the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no 602805, the European Union’s Horizon 2020 research and innovation program under grant agreement No 667302 and NIMH grant 5R01MH101519. The data used in the current familial risk analysis were collected with support from the NIH awards R01 HD036317 and R01 MH050657 to Dr. Biederman.
Financial Disclosures
Amy Yule, MD: Dr. Amy Yule received grant support from the American Academy of Child and Adolescent Psychiatry Pilot Research Award for Junior Faculty supported by Lilly USA, LLC in 2012. She received grant support from the Massachusetts General Hospital Louis V. Gerstner III Research Scholar Award from 2014 to 2016. Dr. Yule is currently receiving funding through the American Academy of Child and Adolescent Psychiatry Physician Scientist Program in Substance Abuse K12DA000357-17.
Stephen Faraone, PhD: In the past year, Dr. Faraone received income, potential income, travel expenses and/or research support from Rhodes, Arbor, Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, NeuroLifeSciences and NACE. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received income or research support from: Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health, Oxford University Press: Schizophrenia: The Facts and Elsevier: ADHD: Non-Pharmacologic Interventions. He is principal investigator of www.adhdinadults.com.
Timothy Wilens, MD: Dr. Timothy Wilens receives or has received grant support from the following sources: NIH(NIDA).Dr. Timothy Wilens is or has been a consultant for: Euthymics/Neurovance, NIH(NIDA), Ironshore, Sunovion, TRIS; US National Football League (ERM Associates), U.S. Minor/Major League Baseball; Bay Cove Human Services and Phoenix House (Clinical Services). Dr. Timothy Wilens has a published book: Straight Talk About Psychiatric Medications for Kids (Guilford Press); and co/edited books ADHD in Children and Adults (Cambridge Press), and Massachusetts General Hospital Comprehensive Clinical Psychiatry (Elsevier). Dr. Wilens is co/owner of a copyrighted diagnostic questionnaire (Before School Functioning Questionnaire). Dr. Wilens has a licensing agreement with Ironshore (BSFQ Questionnaire). Dr. Wilens is Chief, Division of Child and Adolescent Psychiatry and (Co) Director of the Center for Addiction Medicine at Massachusetts General Hospital.
Joseph Biederman, MD: Dr. Joseph Biederman is currently receiving research support from the following sources: The Department of Defense, Food & Drug Administration, Lundbeck, Merck, Neurocentria Inc., PamLab, Pfizer, Shire Pharmaceuticals Inc., SPRITES, Sunovion,, and NIH. Dr. Biederman’s program has received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH.
In 2016, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses, and from Avekshan, Alcobra and AACAP. He has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. In 2015, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses, and from Avekshan. He received research support from Ironshore, Magceutics Inc., and Vaya Pharma/Enzymotec. In 2014, Dr. Joseph Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals Inc. In 2013, Dr. Joseph Biederman received an honorarium from the MGH Psychiatry Academy for a tuition-funded CME course. He received research support from APSARD, ElMindA, McNeil, and Shire. In previous years, Dr. Joseph Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol Myers Squibb, Cambridge University Press, Celltech, Cephalon, The Children’s Hospital of Southwest Florida/Lee Memorial Health System, Cipher Pharmaceuticals Inc., Eli Lilly and Co., Esai, Fundacion Areces (Spain), Forest, Fundación Dr.Manuel Camelo A.C., Glaxo, Gliatech, Hastings Center, Janssen, Juste Pharmaceutical Spain, McNeil, Medice Pharmaceuticals (Germany), Merck, MGH Psychiatry Academy, MMC Pediatric, NARSAD, NIDA, New River, NICHD, NIMH, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shionogi Pharma Inc, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc., Veritas, and Wyeth.
Role of funding source: The funding sources had no role in the study design, had no role in the collection, analysis, or interpretation of data, had no role in the writing of the report, and had no role in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Amy Yule was the primary author of the manuscript, disseminated the major findings and coordinated among co-authors. MaryKate Martelon provided statistical support, and analyzed the data from the data set. Stephen Faraone provided statistical consultation, as well as critical review of the final manuscript. Nicholas Carrellas completed the literature searches and assisted in the drafting and revisions of the text, tables and figures. Timothy Wilens provided logistical and financial support, as well as administrative oversight. He played a key role in the interpretation of findings and drafting the manuscript. Joseph Biederman was the PI of the study which originated the data. He provided general oversight of the study, training of the interviewers, quality control, and financial support, in addition to interpretation of the findings and drafting of the manuscript.
MaryKate Martelon and Nicholas Carrellas: The authors have no conflict of interest relevant to this article to disclose.
REFERENCES
- Ali MM, Teich JL, Mutter R. The role of perceived need and health insurance in substance use treatment: implications for the Affordable Care Act. J Subst Abuse Treat. 2015;54:14–20. doi: 10.1016/j.jsat.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux M, Mick E, Spencer T, Wilens T, Silva J, Snyder L, Faraone SV. Young Adult Outcome of Attention Deficit Hyperactivity Disorder: A Controlled 10 year Follow-Up Study. Psychological Medicine. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010a;177(3):299–304. doi: 10.1016/j.psychres.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Monuteaux MC, Fried R, Byrne D, Mirto T, Spencer T, Wilens TE, Faraone SV. Adult psychiatric outcomes of girls with attention deficit hyperactivity disorder: 11-year follow-up in a longitudinal case-control study. Am J Psychiatry. 2010b;167(4):409–417. doi: 10.1176/appi.ajp.2009.09050736. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Monuteaux MC, Mick E, Clarke A, Ten Haagen K, Faraone SV. Familial risk analysis of the association between attention-deficit/hyperactivity disorder and psychoactive substance use disorder in female adolescents: a controlled study. J Child Psychol Psychiatry. 2009;50(3):352–358. doi: 10.1111/j.1469-7610.2008.02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, O'Connor KB, Hyder LL, Faraone SV. Predictors of persistence in girls with attention deficit hyperactivity disorder: results from an 11-year controlled follow-up study. Acta Psychiatr Scand. 2012;125(2):147–156. doi: 10.1111/j.1600-0447.2011.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Wilens TE, Fraire MG, Purcell CA, Mick E, Monuteaux MC, Faraone SV. Familial risk analyses of attention deficit hyperactivity disorder and substance use disorders. Am J Psychiatry. 2008;165(1):107–115. doi: 10.1176/appi.ajp.2007.07030419. [DOI] [PubMed] [Google Scholar]
- Bierut L, Dinwiddie S, Begleiter H, Crowe R, Hesselbrock V, Numberger J, Porjdd Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking. Archives of General Psychiatry. 1998;55(11):982–988. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- Busch B, Biederman J, Cohen LG, Sayer JM, Monuteaux MC, Mick E, Faraone SV. Correlates of ADHD among children in pediatric and psychiatric clinics. Psychiatric Services. 2002;53(9):1103–1111. doi: 10.1176/appi.ps.53.9.1103. [DOI] [PubMed] [Google Scholar]
- Cantwell D. Psychiatric illness in the families of hyperactive children. Archives of General Psychiatry. 1972;27:414–417. doi: 10.1001/archpsyc.1972.01750270114018. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry. 2011;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Falk D, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and drug use and disorders: findings from the National Epidemiologic Survey of Alcohol and Related Conditions (NESARC) Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2008;31(2):100–110. [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J. Is attention deficit hyperactivity disorder familial? Harv Rev Psychiatry. 1994;1(5):271–287. doi: 10.3109/10673229409017090. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]
- Gignac M, Wilens TE, Biederman J, Kwon A, Mick E, Swezey A. Assessing cannabis use in adolescents and young adults: what do urine screen and parental report tell you? J Child Adolesc Psychopharmacol. 2005;15(5):742–750. doi: 10.1089/cap.2005.15.742. [DOI] [PubMed] [Google Scholar]
- Groenman AP, Oosterlaan J, Rommelse N, Franke B, Roeyers H, Oades RD, Sergeant JA, Buitelaar JK, Faraone SV. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108(8):1503–1511. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- Huber PJ. The Behavior of Maximum Likelihood Estimates Under Nonstandard Conditions Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability; 1967. pp. 221–233. [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin Psychol Rev. 2011;31(3):328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K, Stolar M, Stevens D, Goulet J, Preisig M, Fenton B, Zhang H, O'Malley S, Rounsaville B. Familial transmission of substance use disorders. Archives of General Psychiatry. 1998;55(11):973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Milberger S, Faraone S, Biederman J, Chu M, Wilens T. Familial risk analysis of the association between attention-deficit/hyperactivity disorder and psychoactive substance use disorders. Arch Pediatr Adolesc Med. 1998;152:945–951. doi: 10.1001/archpedi.152.10.945. [DOI] [PubMed] [Google Scholar]
- Morrison JR, Stewart MA. A family study of the hyperactive child syndrome. Biological Psychiatry. 1971;3:189–195. [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O'Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61(12):1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. Schedule for Affective Disorder and Schizophrenia for School-Age Children Epidemiologic Version. Ft. Lauderdale: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- Ottosen C, Petersen L, Larsen JT, Dalsgaard S. Gender Differences in Associations Between Attention-Deficit/Hyperactivity Disorder and Substance Use Disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(3):227–234. e224. doi: 10.1016/j.jaac.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Skoglund C, Chen Q, Franck J, Lichtenstein P, Larsson H. Attention-deficit/hyperactivity disorder and risk for substance use disorders in relatives. Biol Psychiatry. 2015;77(10):880–886. doi: 10.1016/j.biopsych.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R-Non-Patient Edition (SCID-NP, Version 1.0) Washington, D.C.: American Psychiatric Press; 1990. [Google Scholar]
- Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Attention-deficit/hyperactivity disorder and risk for drug use disorder: a population-based follow-up and co-relative study. Psychol Med. 2015;45(5):977–983. doi: 10.1017/S0033291714001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Glind G, Konstenius M, Koeter MW, Oortmerssen KvE-v, Carpentier P-J, Kaye S, Degenhardt L, Skutle A, Franck J, Bu E-T. Variability in the prevalence of adult ADHD in treatment seeking substance use disorder patients: Results from an international multi-center study exploring DSM-IV and DSM-5 criteria. Drug and alcohol dependence. 2014 doi: 10.1016/j.drugalcdep.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Emmerik-van Oortmerssen K, van de Glind G, van den Brink W, Smit F, Crunelle CL, Swets M, Schoevers RA. Prevalence of attention-deficit hyperactivity disorder in substance use disorder patients: A meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012;122(1–2):11–19. doi: 10.1016/j.drugalcdep.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, Biederman J. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry. 2011;50(6):543–553. doi: 10.1016/j.jaac.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]