Abstract
Phosphocholine (PCho) is an essential metabolite for plant development because it is the precursor for the biosynthesis of phosphatidylcholine, which is the major lipid component in plant cell membranes. The main step in PCho biosynthesis in Arabidopsis thaliana is the triple, sequential N-methylation of phosphoethanolamine, catalyzed by S-adenosyl-l-methionine:phosphoethanolamine N-methyltransferase (PEAMT). In screenings performed to isolate Arabidopsis mutants with altered root system architecture, a T-DNA mutagenized line showing remarkable alterations in root development was isolated. At the seedling stage, the mutant phenotype is characterized by a short primary root, a high number of lateral roots, and short epidermal cells with aberrant morphology. Genetic and biochemical characterization of this mutant showed that the T-DNA was inserted at the At3g18000 locus (XIPOTL1), which encodes PEAMT (XIPOTL1). Further analyses revealed that inhibition of PCho biosynthesis in xpl1 mutants not only alters several root developmental traits but also induces cell death in root epidermal cells. Epidermal cell death could be reversed by phosphatidic acid treatment. Taken together, our results suggest that molecules produced downstream of the PCho biosynthesis pathway play key roles in root development and act as signals for cell integrity.
INTRODUCTION
Phosphatidylcholine (PtdCho) is a major phospholipid present in mammalian, plant, yeast, and some prokaryote cell membranes. PtdCho has been demonstrated to play critical roles both as a structural component in cell membranes and in cellular signaling. In mammalian cells, the perturbation of PtdCho homeostasis caused by mutations, inhibitors, or nutrient deficiency retards cell growth and leads to cell death (Cui and Vance, 1996; Cui et al., 1996; Yen et al., 1999; Ramos et al., 2000). In plants, PtdCho is the most abundant phospholipid in cell membranes, accounting for up to 60% of total membrane lipids (Datko and Mudd, 1988a; Prud'homme and Moore, 1992; Moreau et al., 1998). It has been shown that the main biosynthetic pathway for PtdCho production in plants is the CDP-choline pathway, which is also found in yeast and other eukaryotes (Datko and Mudd, 1988b; Bolognese and McGraw, 2000). This pathway depends on phosphocholine (PCho) acting as a precursor in the synthesis of PtdCho. Plants produce PCho via a triple, sequential N-methylation of phosphoethanolamine (PEA), reactions performed by a single enzyme, S-adenosyl-l-methionine:phosphoethanolamine N-methyltransferase (PEAMT) (Datko and Mudd, 1988b; Munnik et al., 1998; Bolognese and McGraw, 2000; McNeil et al., 2000; Nuccio et al., 2000; Smith et al., 2000). Three loci encoding putative PEAMTs (At1g48600, At1g73600, and At3g18000) have been identified in the Arabidopsis thaliana genome database. Complementation studies of the Saccharomyces cerevisiae opi3 mutant affected in PtdCho synthesis demonstrated that at least one of these loci, At3g18000 (XIPOTL1), indeed encodes an enzyme with PEAMT activity (McGraw and Henry, 1989; Bolognese and McGraw, 2000). Some authors suggest that PCho could be synthesized via an untested metabolic step in Arabidopsis: the conversion of choline (Cho) to PCho, catalyzed by a Cho kinase (Kinney and Moore, 1987; Bolognese and McGraw, 2000). Silencing of the PEAMT gene family in transgenic Arabidopsis lines was reported to cause temperature-sensitive male sterility, decreased Cho production, and hypersensitivity to salt stress (Mou et al., 2002).
PtdCho is not only a structural component of plant membranes, it can also be converted into phosphatidic acid (PA) and free Cho by phospholipase D (PLD). This metabolic step could become increasingly important considering that recent studies using plant cell cultures suggest that PA is a new signal molecule acting as a secondary messenger, which plays diverse roles in plant development (Munnik et al., 1998; Katagiri et al., 2001; Munnik, 2001; Potocky et al., 2003). Recent reports point to the novel PLDζ1 as a sustrate-specific enzyme responsible for the breakdown of PtdCho into PA and Cho (Qin and Wang, 2002). It has been demonstrated that inhibition, overexpression, or silencing of this enzyme alters Arabidopsis root hair development (Ohashi et al., 2003). Therefore, PEAMT is not only the key enzyme in PtdCho biosynthesis but also could be important for the production of Cho and PA in plants. In spite of the relevance of PEAMT, there are no descriptions of knockout mutants in any of the loci that encode for a PEAMT and the potential effects that single gene defects could cause in Arabidopsis. This study describes the isolation of the insertional mutant xipotl and its allele from the Salk collection, S_036291, both of them affected in XPL1. Mutant plants showed drastic alterations in root architecture components, including root elongation and lateral root and root hair formation and development. We also provide evidence that cell death in some epidermal cells observed in xipotl is caused by deficiency in PA production.
RESULTS
Isolation of an Arabidopsis Mutant Affected in Root Development
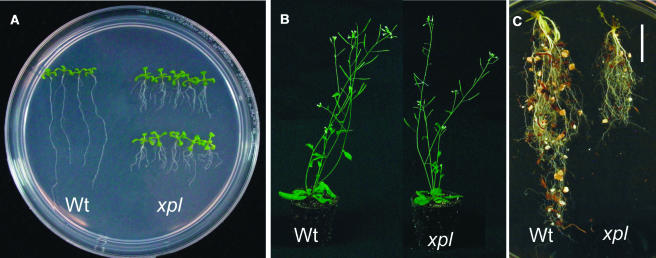
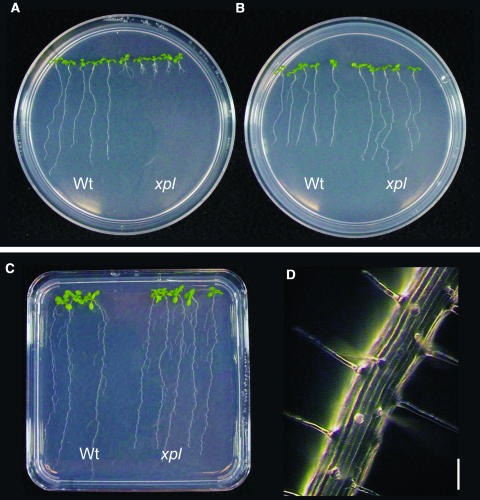
Screening of Arabidopsis plants from a T-DNA–mutagenized collection resulted in the isolation of several mutant seedlings with altered root architecture. Among them, line 287 showed a remarkable phenotype in the root system (Figure 1). This line showed evident differences in root system architecture at both early (8 d after germination [dag]) and late (40 dag) developmental stages (Figure 1). At early stages of development, the primary root of line 287 was approximately four times shorter than in wild-type plants and had longer lateral roots than the wild type (Figure 1A). Line 287 is fully fertile and at a late developmental stage, no major developmental changes in the aerial part of the plant were observed, except for a slight delay in the growth of some floral stems (Figure 1B). However, the root system of adult plants of line 287 was found to be significantly shorter (3 cm in length) than in wild-type plants (7 cm in length) (Figure 1C). Crosses with wild-type plants showed that the mutation affecting line 287 was recessive, with a segregation pattern corresponding to a single affected gene (data not shown). As we will describe below, line 287 was named xipotl, a Nahuatl term that means swelling or tumefaction, because of the swelling observed in epidermal cells of this mutant.
Figure 1.
Effect of XPL1 Disruption on Arabidopsis Morphology.
(A) Root architecture differences between 8-dag xipotl (xpl) and wild-type plants.
(B) and (C) Comparison of aerial phenotype (B) and mature roots (C) between 40-dag mutant and wild-type plants. Bar in (C) = 1 cm.
xipotl Is an Insertional Mutant in the At3g18000 Locus
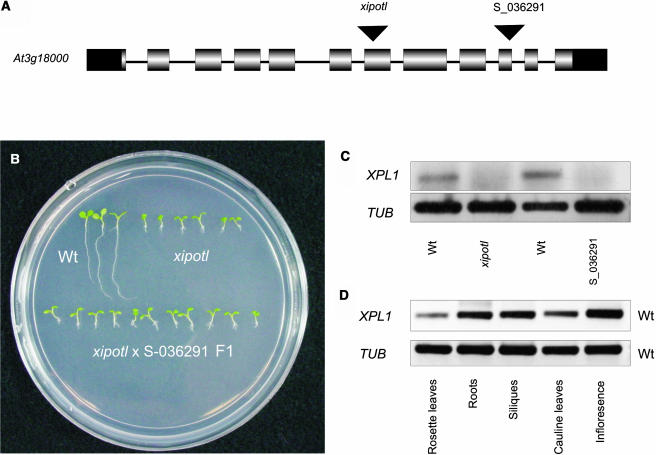
To determine whether the T-DNA insertion cosegregated with the observed xipotl phenotype, 100 mutant seedlings from the F2 progeny of a cross between xipotl and the wild type were tested for the presence of the T-DNA encoded kanamycin resistance gene. Growth in kanamycin-containing media and PCR analysis showed that in all tested mutant seedlings the resistance gene was present, indicating that the T-DNA cosegregated with the mutant phenotype (data not shown). Using the thermal asymmetric interlaced-PCR technique, we determined that the T-DNA present in the xipotl mutant was inserted in exon 7 of the At3g18000 locus that encodes for XIPOTL1.
A search in the Salk T-DNA insertion collection identified a line (mutant S_036291) with an insertion in exon 10 of XPL1 (Figure 2A). Phenotypic analysis showed that line S-036291 presents a similar phenotype to that observed for xipotl in terms of reduced primary root length, increased lateral root formation, and reduced number of root hairs. To determine whether the T-DNA insertion in XPL1 in xipotl and line S_036291 was responsible for the observed mutant phenotype, we performed reciprocal crosses between the two mutants. Analysis of more than 30 seedlings from each of the crosses demonstrated that all F1 plants showed the xipotl phenotype, confirming that the gene affected in both mutants is the same (Figure 2B). PCR-based genotyping of the F2 progeny of a cross between xipotl and the wild type using oligonucleotides specific for XPL1 and a primer specific for the right or left border of the T-DNA showed that all mutant seedlings were homozygous for the T-DNA insertion in XPL1, whereas wild-type seedlings were either heterozygous for the T-DNA insertion or lacked the T-DNA (data not shown).
Figure 2.
Genetic and Molecular Analysis of xipotl and S_036291 Seedlings and XPL1 Expression.
(A) Schematic representation of XPL1 genomic organization, indicating the insertion at exon 7 in xipotl and at exon 10 in S_036291.
(B) Plants (8 dag) of the F1 population from the cross between homozygous xipotl and homozygous S_036291 mutant plants.
(C) Detection of XPL1 transcripts by RT-PCR in wild-type Columbia 0 (Col 0), xipotl, and S_036291 plants (lane 1) and the tubulin expression control (lane 2).
(D) Tissue-specific RT-PCR analysis of XPL1 expression in wild-type Col 0 plants (lane 1) and the tubulin expression control (lane 2).
To determine if XPL1 expression was affected in xpl1 and S_036291 mutants, total RNA from 10-d-old mutant and wild-type plants was extracted, and gene-specific primers were designed for transcript detection by RT-PCR. Transcripts of XPL1 were not detected in xipotl and S_036291 plants, whereas XPL1 transcription was detected in wild-type plants (Figure 2C).
To establish whether XPL1 is expressed in different plant tissues or only in roots, gene-specific primers were designed, total RNA was extracted from rosette leaves, roots, siliques, cauline leaves, and infloresences of wild-type plants, and transcript detection was performed by RT-PCR. Transcripts of XPL1 were detected in all of these tissues (Figure 2D), being apparently less abundant in leaves than in roots, infloresences, and silique tissues.
xipotl Is Affected in Primary Root Elongation and Root Epidermal Cell Development
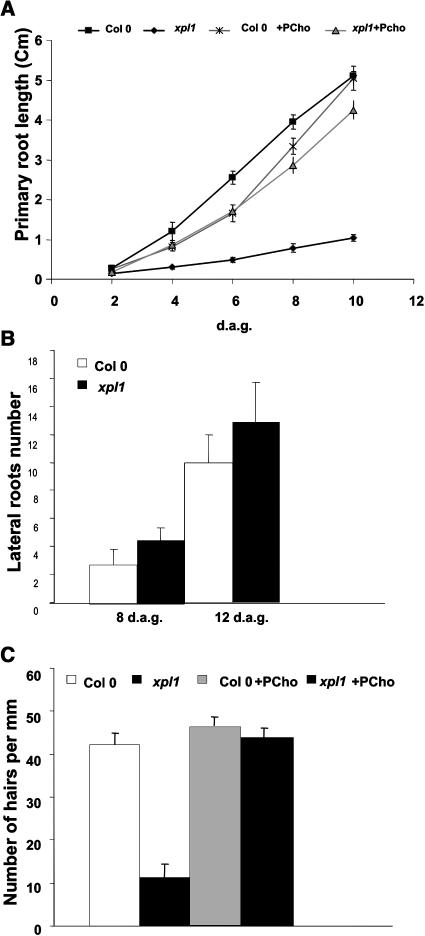
To determine whether xipotl has the same primary root elongation rate at early stages of development as the wild type, the kinetics of primary root growth were analyzed. It was observed that as early as 4 dag, the growth rate of the primary root of xipotl is significantly reduced, although it keeps elongating, albeit at a slower rate, than the wild type (Figure 3A). It was also observed that at 8 dag, xipotl had a slightly greater number of lateral roots than the wild type (Figure 3B). However, considering the length of the primary root, the mutant at this stage had a much greater lateral root (LR) density (5.6 LR/cm) than the wild type (0.68 LR/cm).
Figure 3.
The Effect of XPL1 Disruption on Arabidopsis Root Architecture.
Comparison of root architecture traits between wild-type and mutant plants: primary root length kinetics (A), lateral root number (B), and root hair number (C). Alterations in roots are restored by exogenous PCho ([A] to [C]).
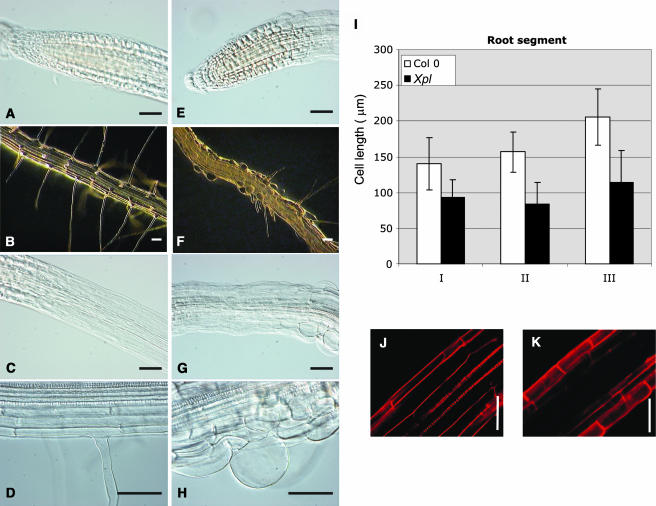
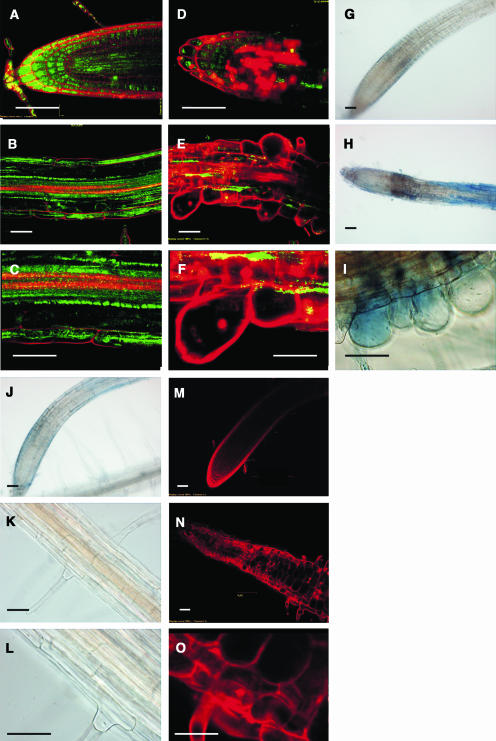
Microscopic analysis showed that the roots of xipotl had few root hairs and evident abnormalities in epidermal cells. Root hair number in mutant plants was determined to be four times lower in comparison to the wild type (Figure 3C). After a more detailed analysis of xipotl roots, it was observed that even though the root meristematic region appeared relatively normal, it was shorter than in the wild type (Figures 4A and 4E). Moreover, in some regions of the elongation and differentiation zones, the epidermal cells, and probably also cortical and endodermal cells had abnormal morphologies (Figures 4F to 4H), in comparison to those of the control (Figures 4B to 4D). Extreme alterations in root hair formation and development were observed in the differentiation zone, where not only zones lacking root hairs or having short root hairs were found but also globular shaped and collapsed cells were observed (Figure 4H).
Figure 4.
Comparison of Cellular Anomalies between Wild-Type and Mutant Plants.
Microscopic analysis of wild-type ([A] to [D]) and mutant plants ([E] to [H]). Nomarski optics images of the meristem ([A] and [E]), elongation zone ([C] and [G]), and root hair cells ([D] and [H]). Cell length quantification of three different root sections (I): apical elongation zone (I), middle zone (II), and basal zone (III) of wild-type and mutant primary roots. Stereoscopic images of 8-dag plants of a root section at the differentiation zone ([B] and [F]). Confocal images of PI-stained root sections showing cell length differences between wild-type (J) and mutant (K) plants. Bars in (A) to (H), (J), and (K) = 50 μm.
The short primary root phenotype observed in xipotl could be a result of reduced cell division or a reduction in cell size. Because the primary root meristem appears normal, we analyzed whether cell elongation was affected in xipotl by measuring the epidermal cell size in three root regions: the elongation zone and the proximal and distal regions of the differentiation zone of the primary root in wild-type and mutant plants (Figure 4I). These measurements show that, in the three root regions, the cells of the xipotl mutant are on average 50% shorter than those of the wild type (Figures 4J and 4K).
Rescue of the Mutant Phenotype by PCho and Cho
PEAMT is the enzyme responsible for the synthesis of PCho. Therefore, to confirm that the phenotype of xipotl was caused by the lack of PCho or one of the metabolites derived from this compound, we germinated xipotl seeds in normal MS media and MS media supplemented with 100 μM PCho and analyzed their root architecture traits. It was found that primary root growth is restored by addition of PCho to the media (Figure 3A). In contrast with the difference in the root system architecture observed between the wild type and xipotl in MS media alone (Figure 5A), no significant differences were observed in MS media supplemented with PCho (Figure 5B). Furthermore, a closer examination revealed that all cellular alterations, including root hair formation (Figure 3C) and abnormal morphology of epidermal cells (Figure 5D), were corrected by supplying exogenous PCho to xipotl. Besides a slight reduction in primary root growth (Figure 3A), no significant changes were observed for wild-type seedlings grown in PCho-containing media compared with controls grown in unsupplemented media.
Figure 5.
xipotl Phenotype Rescue by Exogenous PCho and Cho.
Morphology of 10-dag wild-type and mutant plants (xpl) grown on solid MS media (A), MS media supplemented with PCho (B), and MS media supplemented with Cho (C). Stereoscopic image (D) of a root section from a PCho-rescued mutant plant. Bar in (D) = 50 μm.
It has been reported that the salt hypersensitivity of PEAMT silenced lines is reversed by the addition of Cho (Mou et al., 2002). To test whether Cho can also suppress the xipotl phenotype, we sowed mutant and wild-type seeds on complete solid MS media supplemented with Cho. It was found that the mutant phenotype was also completely restored by adding Cho to the media (Figure 5C; data not shown).
Taken together, these results show that a reduction in the synthesis of PCho and/or other compounds derived from it, such as PtdCho, impairs normal root development. The finding that Cho also restores normal root development in the xipotl1 mutant suggests that Cho can be converted into PCho in planta via a Cho phosphorylation reaction.
Inhibition of PCho Biosynthesis Induces Death in Epidermal and Root Hair Cells
It has been well documented for mammalian cells that perturbation of PtdCho homeostasis is related to cell death (Cui and Vance, 1996; Cui et al., 1996; Yen et al., 1999; Ramos et al., 2000). Therefore, it is possible that the aberrant morphology of root epidermis and root hair cells in xipotl seedlings could, at least partially, be the result of cell death. To address this question, we analyzed 8-d-old mutant and wild-type plants for cell death by double fluorescent staining with propidium iodide (PI) and fluorescein diacetate (FDA). PI is a DNA binding dye used to confirm loss of cell membrane integrity and cell viability because it can only penetrate membrane-damaged cells and fluoresces red in the nuclei of dead cells, and PI also stains cell walls independently of cell viability (Kirik et al., 2001; Chaves et al., 2002). FDA is a nonfluorescent molecule that passes through the cell membrane and once inside is cleaved at the diacetate group by esterases of viable cells producing fluorescein, a green fluorescent molecule. Overlap of both stains appears yellow (Celenza et al., 1995).
Microscopic analysis revealed that in the roots of 8-d-old wild-type seedlings, all cells at the meristematic, elongation, and distinct differentiation zones, with the exception of the xylem, were alive because FDA was actively converted into fluorescein producing a green color, whereas PI stained only the cell walls, and no staining was observed inside the cells (Figures 6A to 6C). By contrast, numerous epidermal and cortical dead cells, as evidenced by PI staining inside the cell and lacking green fluorescence, were observed in the meristem and elongation zones of xipotl roots (Figures 6D and 6E). Major damage was observed in certain segments along the differentiation zone, where the nuclei of most of the globular-shaped cells were stained red, and no FDA staining was observed (Figure 6F).
Figure 6.
Cell Death on Root Cells Caused by XPL1 Disruption and by Treatments with 1-Butanol.
Confocal images of wild-type ([A] to [C]) and mutant ([D] to [F]) root sections stained with PI and FDA. Nomarski optics images of Evans blue–stained root sections of wild-type (G) and mutant ([H] and [I]) plants. Nomarski optics images of Evans blue–stained root sections of mutant plants grown on PCho-supplemented MS medium ([J] to [L]). Confocal images that show meristem organization and cell integrity of wild-type plants 3 d after being transferred to 2-butanol (M) and 1-butanol ([N] and [O]). Bars in all panels = 50 μm.
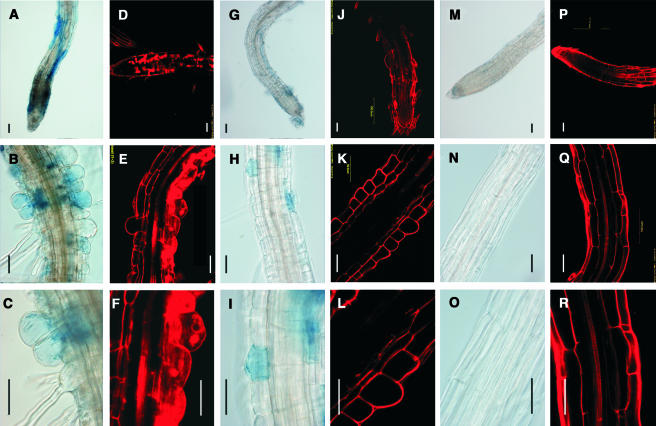
To confirm these results, we stained wild-type and mutant seedlings with Evans blue. This dye reveals cell death because of its ability to enter only those cells with a completely permeable plasma membrane (Mergemann and Sauter, 2000). This analysis revealed the absence of stained cells in the wild-type roots (Figure 6G). Conversely, we found large patches of stained cells in the elongation zone of the mutant plant roots (Figure 6H), and along the differentiation zone we observed that most of the globular-shaped cells were dead (Figure 6I). Importantly, the cell death patterns revealed by Evans blue and the double fluorescent staining were in close agreement.
Because addition of PCho restores normal root growth in xipotl, we tested whether this treatment also eliminates the abnormal cell death observed in this mutant. Staining with Evans blue showed that addition of exogenous PCho completely eliminates cell death concomitant with the restoration of normal morphology of all cell types in the xipotl root (Figures 6J to 6L). These results confirm that a reduction in the synthesis of PCho, or a compound derived from its metabolism, triggers cell death in some root cell layers.
PA Reverts Cell Death in the Mutant Phenotype
PLD converts structural phospholipids, such as PtdCho, phosphatidylethanolamine (PtdEA), and phosphatidylglycerol, into PA and a free head group, Cho, ethanolamine, or glycerol, respectively. Because PCho is an intermediary of a pathway that leads to the synthesis of PtdCho and also of PA as a result of the action of PLD on PtdCho (Munnik et al., 1998; Munnik, 2001; Meijer and Munnik, 2003), it is possible that a reduction in the level of some of these final products may be responsible for the cell death observed in the roots of xipotl. Of particular interest is PA, which has recently been shown to be involved in pollen tube elongation and root hair development (Ohashi et al., 2003; Potocky et al., 2003).
Previous studies established that PLD has a unique ability to transfer the phosphatidyl group of phospholipids to a primary alcohol (1-butanol) rather than water, producing phosphatidyl alcohol and inhibiting the production of PA (Potocky et al., 2003). As an initial approach to test whether PA could be involved in the cell death observed in xipotl, we transferred 5-d-old wild-type seedlings, germinated in solid MS media, to plates of solid MS media supplemented with 0.5% of 1-butanol or 2-butanol, the latter a secondary alcohol that cannot be used by PLD as a substrate. Five days after transfer, plants were stained with PI and analyzed by Nomarski or confocal microscopy. Whereas plants transferred to 2-butanol did not stop growing and showed no evident alterations in root morphology or cell death (Figure 6M), the roots of those transferred to 1-butanol stopped growing after 3 d of treatment, and their root morphology was altered (Figures 6N and 6O). In 1-butanol–treated seedlings, primary root meristem organization was lost, cell length was shortened and morphology became abnormal, lateral root and root hair formation were inhibited, and cell death was induced (Figures 6N and 6O; data not shown). Because some of the effects of treatment with the PA biosynthesis inhibitor, 1-butanol, are reminiscent of the xipotl phenotype, these results suggest that some of the root defects observed in this mutant could be because of a reduction in the production of PA.
To further explore the possibility that mutations in XPL1 could reduce PA production and that this reduction might be responsible for some of the phenotypic alterations in xipotl, we tested the effect of different concentrations of exogenous PA on the growth, morphology, and cell death of xipotl seedlings. With this aim in mind, control and mutant seeds were germinated and grown in solid media in the presence of PA and stained with PI or Evans blue. In contrast with what was observed for mutant plants grown in solid media lacking PA, which showed the cell death pattern described before (Figures 7A to 7F), those grown in media with 28 μM PA showed a decrease in cell death and morphological cell abnormalities (Figures 7G to 7L). Treatments with 280 μM PA led to a total recovery of the cell death phenotype and a complete disappearance of globular-shaped epidermal cells (Figures 7M to 7R). However, PA treatment did not restore normal primary root growth or root hair formation. In wild-type plants grown on 28 μM PA, the root phenotype was normal except that root hairs were shorter than those of untreated plants; treatment with 280 μM PA further inhibited root hair and primary root elongation compared with that of the untreated control (data not shown). These results show that treatment with PA can alleviate cell death and some morphological abnormalities observed in the epidermal cells of xipotl and that high concentrations of PA can inhibit root growth and epidermal cell differentiation in wild-type Arabidopsis plants.
Figure 7.
PA Rescues the Cell Death Phenotype of xipotl Roots.
Nomarski optics ([A] to [C]) and confocal ([D] to [F]) images showing the cell death pattern of mutant plants stained with Evans blue and PI, respectively. Confocal and Nomarski optics images that show partial and complete reversion of the cell death phenotype in mutant plants grown on MS medium supplemented with 28 μM PA ([G] to [L]) and 280 μM PA ([M] to [R]). Bars in all panels = 50 μm.
xipotl Plants Have a Significant Decrease in PtdCho Content
To determine whether mutations in XPL1 cause a reduction in the biosynthesis of PCho, PtdCho, and Cho, we measured the total content of these molecules in the root and leaves of xipotl seedlings. When PCho and Cho were quantified, we found a consistent, albeit statistically nonsignificant, decrease of between 5 and 7% in the content of these two compounds in leaves and roots of xipotl compared with that present in wild-type plants (Table 1). Moreover, when the content of PtdCho in roots and leaves of mutant and wild-type plants was determined, significantly lower levels of PtdCho were detected in xipotl tissues (Table 1). Roots of xpl1 plants showed an ∼23% decrease in PtdCho content in comparison with those of wild-type plants, whereas the difference in leaves was 18%. Although the contribution of XPL1 for the biosynthesis of PtdCho is significant in root and aerial tissues, the reduction in the synthesis of PtdCho in xpl1 has an evident effect on root but not aerial tissue development. This suggests that the other members of the PEAMT gene family play a more important role in the synthesis of PCho and PtdCho in aerial tissues than XPL1. This is consistent with the differential expression shown by the three PEAMT genes revealed by microarray experiments (L. Nussaume, L. Herrera-Estrella, and K.G. Raghothama, unpublished data). However, to demonstrate this statement, detailed studies of the expression of each of the members of the PEAMT gene family are needed.
Table 1.
PCho, Cho, and PtdCho Determination in xpl1 and Wild-Type Plants (nmol g−1 Fresh Weight)
| Molecule | Col 0 Roots | xpl1 Roots | Col 0 Leaves | xpl1 Leaves |
|---|---|---|---|---|
| PCho | 14.6 ± 1.0a | 13.6 ± 0.7a | 54.0 ± 2.0b | 50.1 ± 1.5b |
| Cho | 41.7 ± 2.0a | 35.6 ± 2.5a | 63.5 ± 2.5b | 59.8 ± 3.0b |
| PtdCho | 114.9 ± 3.9a | 89.5 ± 3.5b | 115.3 ± 7.8a | 94.7 ± 5.2b |
Data are means and se (±) for three independent assays and were statistically analyzed in the SPSS 10 program (SPSS, Chicago, IL). Duncan's method was used to determine whether the differences between mutant and wild-type plants were statistically significant with a 95% confidence coefficient. The univariate analysis showed significant differences in the content of PtdCho between Col 0 and xpl1 tissues by grouping values in distinct subsets (a or b).
XPL1-Specific Expression Revealed by the Whole Mount in Situ Hybridization Technique
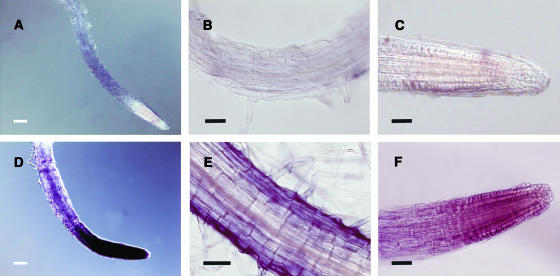
Because the defects observed in xipotl mainly affect the elongation and differentiation zones of the root, it was important to determine whether XPL1 transcription was restricted to a specific region or cell layer in Arabidopsis roots. Therefore, we analyzed the XPL1 tissue-specific expression pattern by the whole mount RNA in situ hybridization technique in 7-d-old wild-type seedlings. When seedlings hybridized with the sense probe were incubated overnight in the presence of the phosphatase substrate, no hybridization signal was observed (Figures 8A to 8C), whereas in the seedlings hybridized for the same time with the antisense probe, a high level of XPL1 transcripts was detected in the meristem and elongation zones of the root (Figure 8D), and a less intense signal was detected in differentiated epidermal cells (Figure 8E). To observe XPL1 expression in specific cell layers in the root meristem, the time of incubation was reduced to 30 min. We observed that XPL1 transcripts accumulate in the epidermal, cortical, and endodermal cells of the meristematic region and to a lower extent in the elongation zone and columella cells (Figure 8F). No expression was detected in the same cell layers in sense-hybridized seedlings (Figure 8C). Aerial parts of the seedling were devoid of signal except for a weak signal observed at the shoot apical meristem and the hydothodes (data not shown). These results show that XPL1 transcripts are present in high levels in the root regions where cell death and abnormalities are observed.
Figure 8.
XPL1 Expression Pattern Revealed by Whole Mount RNA in Situ Hybridization.
The presence of XPL1 transcripts is visible as a purple precipitate. Nomarski optics images of different regions of 7-d-old Col 0 seedlings hybridized with the sense ([A] to [C]) or antisense ([D] to [F]) probes for XPL1. The expression pattern observed in the root meristem and elongation zone (D) and differentiated epidermal cells of seedlings incubated over night with the phosphatase substrates (E) compared with their respective controls ([A] and [B]). The expression pattern of XPL1 observed in specific cell layers of the elongation zone and the root meristem (F), and the respective control (C), in seedlings incubated for 1 h with the phosphatase substrates.
DISCUSSION
Important advances have been achieved in the identification of genes involved in the development of the Arabidopsis root system. Genes required for root hair development, auxin signaling, and transcription factors involved in root cell differentiation and maintenance have been isolated (Reire et al., 1994; Min Lee and Schiefelbein, 1999; Schiefelbein, 2000; reviewed in López-Bucio et al., 2003). However, many of the signaling pathways regulating root development remain to be identified.
Here, we describe the isolation of xipotl, an Arabidopsis mutant affected in root development. xipotl is characterized by a reduced primary root growth and root hair formation and alteration in the morphology of root epidermal cells. The gene responsible for this phenotypic alteration was identified as XPL1 by (1) isolating the border sequences of the T-DNA inserted in the original line in which the xipotl phenotype was identified, (2) the lack of complementation with an independent line that harbors an insertion in XPL1, and (3) the restoration of normal root growth and development by an exogenous supply of PCho, the product of the reaction catalyzed by the enzyme encoded by XPL1.
In the Arabidopsis genome database, three loci encoding putative PEAMTs are reported: At1g48600, At1g73600, and At3g18000. If these three loci indeed encode active PEAMTs, the finding that mutations in XPL1 result in a drastic alteration in the development and growth of the root system suggests that the function of the members of this gene family is not completely redundant. RT-PCR analysis showed that XPL1 is expressed in roots, leaves, siliques, and flowers, suggesting that the corresponding gene participates in the synthesis of PtdCho in different organs. However, the finding that mutations in XPL1 only produce a visible phenotype in roots suggests that XPL1 is limiting in providing PCho and/or PCho derived products during root development. Moreover, in situ hybridization experiments showed that in Arabidopsis seedlings XPL1 is preferentially expressed in the meristematic zone and epidermal cells of the Arabidopsis roots, indicating that PtdCho synthesis in these tissues is more severely affected in xpl1. Therefore, we propose that the lack of functional redundancy among the members of the PEAMT gene family is probably because of differential expression of the three PEAMT genes. In agreement with this, microarray experiments showed that XPL1 expression in roots is 3.65 times and 12.84 times higher than in leaves of 5- and 14-d-old plants, respectively, and that the expression of At1g48600 and At1g73600 is higher (10.88 and 6.08 times, respectively) in leaves than that of XPL1 (L. Nussaume, L. Herrera-Estrella, and K.G. Raghothama, unpublished data).
Silencing of the PEAMT gene family in Arabidopsis by sense and antisense expression of PEAMT using the 35S promoter has been reported to result in a phenotype characterized by pale green rosette leaves in juvenile stages, early senescence in the late reproductive stages, short siliques, reduced male fertility, and salt hypersensitivity (Mou et al., 2002). Two important differences were noted between these phenotypes of PEAMT silenced lines and xipotl: (1) we did not find major alterations in the aerial part of the plant and although we did not quantify pollen or seed production, no obvious decrease in fertility was detected in the two lines we characterized with T-DNA insertions in XPL1, and (2) in contrast with PEAMT silenced lines that have a 60% reduction in PCho content in leaves, we only observe a 7% decrease in total PCho content in xipotl leaves. These differences could be explained by the finding that XPL1 is mainly expressed in the root meristem and root epidermal cells.
Mou et al. (2002) did not report alterations in root development in the PEAMT silenced lines. This difference could be attributable to a lack of a detailed analysis of the root system by these authors or to the fact that in these silenced lines PEAMT transcripts were still detected by RT-PCR. It is quite possible that complete silencing of the PEAMT gene family would be lethal for the plant given that PtdCho is a major component of the plasma membrane. Nevertheless, the finding that partial silencing of the entire family of genes encoding for PEAMT results in alterations in leaf and reproductive organs suggests that PEAMT genes other than XPL1 are responsible for the major synthesis of PCho in these parts of the Arabidopsis plant. These observations support the notion that PEAMT genes are not functionally redundant and that XPL1 plays a major role in root growth and development.
Metabolic Implications of xipotl
In contrast with other eukaryotes, whereas the main route for the synthesis of PtdCho is the stepwise methylation of PtdEA, evidence in different species shows that PtdCho can be synthesized in plants via two parallel pathways involving sequential methylations of PEA (P-base) or Ptd-base. In tobacco (Nicotiana tabacum) leaves, the first methylation occurs exclusively at the P-base level, and 83 to 92% of the second and 65 to 85% of the third methylations are also performed at the P-base, with the remainder occurring at the Ptd-base. The three methylations at the P-base level are catalyzed by PEAMT. Because PEAMT is feedback inhibited by PtdCho and Cho and induced in response to salt stress, it has been proposed that PEAMT is the key regulatory enzyme in the PtdCho biosynthetic pathway, in which the first methylation step is the major control point in the entire pathway (McNeil et al., 2000, 2001). Mutations in XPL1 lead to a root phenotype that can be reverted by addition of PCho, showing that the P-base pathway of synthesis of PtdCho plays a fundamental role in roots and that XPL1 is needed to produce sufficient PtdCho to maintain normal root growth and epidermal cell integrity.
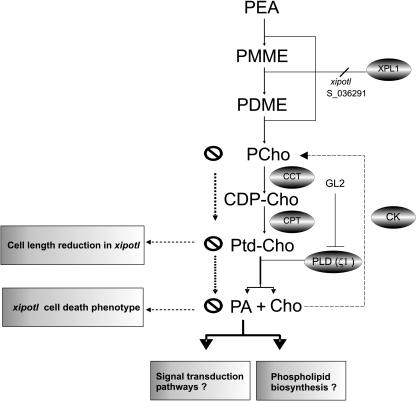
The finding that not only PCho but also Cho can restore normal root growth and cell morphology in the xipotl mutant coupled with the fact that there are no reported pathways to produce PtdCho directly from Cho suggest that PCho can be produced from Cho by a phosphorylation reaction performed, most probably, by a choline kinase (CK). The presence of genes encoding CKs in plants has been demonstrated by heterologous expression of soybean (Glycine max) CK cDNAs in yeast and Escherichia coli (Monks et al., 1996). Our results support the existence in Arabidopsis, at least in the root system, of a pathway in which PEAMT plays a critical role in the synthesis of PCho, PtdCho, and Cho and the alternative conversion of Cho into PCho (Figure 9).
Figure 9.
Model Proposed for PCho and PtdCho Biosynthesis in Arabidopsis.
The main PCho biosynthesis pathway involves a triple sequential methylation performed by XPL1. PCho is the essential precursor for PtdCho production via the action of PCho cytidylyltransferase (CCT) and choline phosphotransferase (CPT). PtdCho is converted to PA and Cho by the action of PLD. PLDζ1 is a PtdCho substrate-specific enzyme that is negatively regulated by GLABRA2 (GL2), inhibiting PA biosynthesis via PtdCho as the precursor. Cho is converted to PCho by an alternate biochemical step (dashed arrow) performed by a proposed CK. PMME, phospho-monomethylethanolamine; PDME, phospho-dimethylethanolamine; CDP-Cho, cytidylylphosphocholine.
Implications of PEAMT in Cell Growth and Cell Death
It has been described that in mammalian cells, growth and integrity are directly influenced by changes in cell membrane composition occurring when PtdCho biosynthesis has been perturbed by mutations, inhibition, or nutrient deficiency (Cui et al., 1996). We also found that the reduced primary root growth in XPL1 Arabidopsis mutants is related to a 50% reduction in cell elongation. Because PEAMT plays a pivotal role in the PtdCho biosynthetic pathway in plants, reduced root growth and epidermal cell death in xipotl could also be because of perturbation in the synthesis of PtdCho in the root meristem. However, the observation that PA can alleviate cell death suggests that some of cellular phenotypes observed in xipotl are not because of a direct effect in the reduction of PtdCho levels (Figure 9).
Cell death has been classified into physiological cell death and nonphysiological cell death (Vaux and Korsmeyer, 1999). Physiological cell death refers to the process of programmed cell death, in which the organism kills its own cells as part of its developmental programs, whereas nonphysiological cell death includes cell death caused by external events, such as lack of nutrients, toxic susbtances, mutations in essential enzymes, or altered expression of a gene product that might be toxic to cells. Although little is known about the signal pathways involved in triggering cell death, it has been shown that programmed cell death can be induced by alterations in phospholipid, sphingolipid, and fatty acid biosynthesis (Obeid et al., 1993; Allan, 2000; Mou et al., 2000).
Studies focused on phospholipid signaling show that PtdCho not only plays an important role as an essential component in cell membranes but also as a PLD substrate for the biosynthesis of PA (Exton, 1994; Wang, 1999, 2001, 2002; Wang and Wang, 2001). Several reports show that PA plays a role in plant defense (Lee et al.,1997; Laxalt and Munnik, 2002) and stress responses (Frank et al., 2000; Katagiri et al., 2001; Munnik, 2001; Arisz et al., 2003). More recently, it was reported that PA also plays an important role in signaling pathways related to cell growth and integrity, such as pollen tube growth (Potocky et al., 2003) and root hair formation and maintenance (Den Hartog et al., 2001; Ohashi et al., 2003).
Two pathways have been proposed for the biosynthesis of PA in plants: the PLD pathway and a phospholipase C pathway. The latter involves the conversion of phosphatidylinositolbisphosphate to diacylglycerol by phospholipase C and the conversion of diacylglycerol to PA by diacylglycerol kinase (Munnik, 2001).
Our results show that an upstream inhibition of the PtdCho pathway leads to cell death in the Arabidopsis root epidermis (Figure 9). However, to test whether the lack of PtdCho per se was responsible for the observed cell death in xipotl or if this was related to the role of PtdCho as the substrate for PA production, we performed experiments in which PA but not Cho production by PLD was blocked by treatment with 1-butanol. These experiments showed that the inhibition of PA production by PLDs resulted in an arrest of root growth and epidermal cell death, suggesting that the reduction in PA production could be involved in the observed cell death in xipolt. However, we observed that the effects of 1-butanol are more drastic than those observed in the xipotl phenotype.
This difference could be the result of inhibiting PA formation by all PLDs by 1-butanol, and not only that specific for PtdCho or to the effects of 1-butanol on the activation of PLDs that could be involved in microtubule reorganization as recently proposed by Dhonukshe et al. (2003). Nevertheless, the finding that PA can revert the cell death phenotype and some morphological anomalies in the epidermal cells of xipotl confirms that PA is required for cell integrity, at least in this cell type. Moreover, an important finding by Zhang et al. (2003) shows that induced cell death, by exogenous H2O2 on Arabidopsis wild-type and PLDδ-null protoplasts, is partially inhibited by exogenous PA, supporting the notion that the inhibition of PA biosynthesis in specific cell layers is directly involved in the xipotl cell death phenotype.
GLABRA2 (GL2) is a homeobox transcription factor expressed predominantly in hairless epidermal cell layers and thought to negatively regulate root hair development (Reire et al., 1994). Recently it has been reported that GL2 directly represses the expression of the Arabidopsis gene encoding PLDζ1 and that this PLD is required for normal root hair development (Ohashi et al., 2003). Because PLDζ1 is a PtdCho substrate-specific PLD (Qin and Wang, 2002), it is possible that GL2 regulates trichoblast cell integrity and root hair development by modulating PA production (Figure 9). The finding that a mutant affected in PtdCho biosynthesis is affected in root hair development and epidermal cell integrity confirms that root hair formation involves the modulation of phospholipid signaling. In addition, the finding that PA reverts the cell death phenotype in the xipotl mutant provides direct evidence that this phospholipid is required for epidermal cell integrity, which could be preferentially produced by PLDζ1 in epidermal cells. However, the finding that treatment with PA does not revert the lack of root hair formation in xipotl suggests that not only PA but also PtdCho as a critical component of the lipid bilayer is required for root hair development. Because xipotl is affected in a key enzyme in the synthesis of PtdCho, these results also suggest that the PtdCho-specific PLDζ1 pathway of PA synthesis is active in roots and plays an important role in root cell development (Figure 9).
We may conclude that PA is a molecule involved in signal transduction pathways related to cell death and, in accordance with the results of Ohashi et al. (2003), support its pivotal role in root hair formation and development. However, because of the complexity of the molecule and the low permeability of PA to different root cell layers, we cannot exclude the possibility that PA is involved in other components of the xipotl phenotype. Nevertheless, further studies are needed to unravel in detail the precise function of each phospholipid in the Arabidopsis membrane composition and process-specific signal transduction pathways.
METHODS
Plant Growth Conditions
Wassilewskija (Ws) and Col 0 are the Arabidopsis thaliana ecotypes of xipotl and S_036291 seedlings, respectively. Mutant and wild-type seeds were surface sterilized with 95% (v/v) ethanol for 5 min and 20% (v/v) bleach for 7 min. After five washes in distilled water, seeds were germinated and grown on agar plates containing complete Murashige and Skoog (solid MS) medium, which depending on the type of experiments, was supplemented with different chemicals. Plates were placed vertically at an angle of 65° to allow root growth along the agar surface and to allow unimpeded growth of the hypocotyl into the air. For plant growth, we used a plant growth cabinet (Percival Scientific, Perry, IA), with a photoperiod of 16 h light/8 h darkness, under a light intensity of 300 μmol m−2 s−1 and temperature of 22 to 24°C.
Mutant Isolation and Genetic Analysis
Screening of 38,000 seedlings of the Sussman and Amassino T-DNA mutant collection was performed on solid MS medium. A selected homozygous xipotl mutant plant was backcrossed four times with the wild-type Ws ecotype to produce a pure mutant line. To determine the segregation pattern of the xipotl phenotype, the F2 population derived from the cross between xipotl and Ws was analyzed. A typical 3:1 recessive segregation was observed. To relate the mutant phenotype with the T-DNA insertion, 500 seeds of the same F2 population were sown on solid MS medium plates supplemented with 50 μg/mL of kanamycin. The S_036291 T3 segregating population was grown on solid medium plates, and mutant phenotype plants were propagated. At this stage, the genetic strategy applied to the xipotl seedlings was repeated for S_036291. For allelism tests, homozygous mutant plants of S_036291 were crossed with homozygous mutant plants of line 287 (xipotl) and vice versa, and plants from the F1 population were analyzed for the root phenotype.
Gene Mapping and Molecular Confirmation
Amplification of the junction T-DNA fragments was performed using the thermal asymmetric interlaced-PCR technique as described by Liu et al. (1995). Fragments adjacent to the right and left T-DNA borders were cloned using the pGem-T Easy Gibco kit (Promega, Madison, WI) and sequenced. The sequences obtained were analyzed using the Blast programs from the National Center for Biotechnology Information, The Arabidopsis Information Resource, and The Institute for Genomic Research databases. To confirm the relation between mutant phenotype and the insertion at the At3g18000 locus, the Salk insertional line S_036291 was requested; it is an insertional mutant at the same locus. Genotyping for S-036291 was performed using the strategy recommended by the T-DNA express link at the Signal web page (http://signal.salk.edu/index.html). DNA from homozygous wild-type plants and homozygous and heterozygous S_036291 plants was used in 20-μL PCR reactions. The gene-specific primers XPL1-LP (5′-TTCTTCAGGACAAACCAGCCTTG-3′) and XPL1-RP (5′-AAACCAAGTAGCATAACACAAATGC-3′) and the T-DNA–specific primer LB1 (5′-GGCAATCAGCTGTTGCCCGTCTCACTGGTG-3′) were included and reactions performed under the following conditions: 94°C for 2 min, followed by 26 cycles of 94°C for 15 s, 52°C for 30 s, and 72°C for 1 min, and by a final extension step at 72°C for 1 min.
Cho and PCho Assays
Seeds were grown on plates with solid MS medium and solid MS medium supplemented with 100 μM Cho chloride or PCho chloride. Both metabolites were purchased from Sigma. Eight-day-old plants were cleared using the method of Malamy and Benfey (1997), and a representative plant was chosen for each treatment and photographed using Nomarski optics in a Leica DMR microscope (Wetzlar, Germany). Seedlings from the same treatments were stained with PI or 2% Evans blue solution according to Mergemann and Sauter (2000) and observed by confocal microscopy or Nomarski optics, respectively.
Analysis of Root Architecture Traits
The Arabidopsis root system was analyzed with an AFX-II-A stereomicroscope (Nikon, Tokyo). All emerged secondary roots that were clearly visible using a 3× objective were taken into account for lateral root number data. Primary root length was determined for each root using a plastic ruler. The length and number of root hairs was measured using a Nikon UW microscope with a micrometer. For all experiments, the overall data was statistically analyzed in the SPSS 10 program as described by López-Bucio et al. (2002).
Expression Analysis in xipotl and S_036291 Seedlings by RT-PCR
Total RNA from 10-d-old wild-type, xipotl, and S_036291 plants was extracted using the Concert Plant RNA reagent from Invitrogen (Carlsbad, CA) according to the manufacturer's protocol. For reverse transcriptase analysis, 200 ng of each RNA was used in a 12.5-μL reaction mixture of the SuperScript one-step RT-PCR with the Platinum Taq kit from Invitrogen, including the XPL1 gene-specific primers XPL1-RTF (5′-CACTCTTTTCGCTCTCAGATC-3′) and XPL1-RTR (5′-CGACCAATACCAGCTCCAAG-3′). The amplification reaction was performed under the following conditions: 50°C for 30 s and 94°C for 2 min, followed by 35 cycles of 94°C for 15 s, and 54°C for 30 s, and by a final extension step at 72°C for 1.5 min.
We followed the same protocol and conditions for control reactions and used specific primers for the Arabidopsis β-tubulin gene TUB-RTF (5′-CTCAAGAGGTTCTCAGCAGTA-3′) and TUB-RTR (5′-TCACCTTCTTCATCCGCAGTT-3′).
Tissue Expression Analysis by RT-PCR
Total RNA from different tissues of 16-d-old wild-type plants was extracted using the Concert Plant RNA reagent from Invitrogen, according to the manufacturer's protocol. For reverse-transcriptase analysis, 200 ng of each RNA was used in a 12.5-μL reaction mixture of the SuperScript one-step RT-PCR with the Platinum Taq kit from Invitrogen, including the XPL1 gene-specific primers XPL1-RTF (5′-CACTCTTTTCGCTCTCAGATC-3′) and XPL1-RTR (5′-CGACCAATACCAGCTCCAAG-3′). The amplification reaction was performed under the same conditions used for the experiment of XPL1 transcript detection previously described.
We followed the same protocol and conditions for control reactions and used specific primers for the Arabidopsis β-tubulin gene TUB-RTF (5′-CTCAAGAGGTTCTCAGCAGTA-3′) and TUB-RTR (5′-TCACCTTCTTCATCCGCAGTT-3′).
PA Inhibition by 1-Butanol
To inhibit PA biosynthesis, we grew wild-type Col 0 ecotype plants on solid MS medium plates, and 5 d after germination plants were transferred to MS plates containing either 0.5% 1-butanol or 0.5% 2-butanol. Five days after transfer, plants were stained with PI and prepared for microscopic analyses.
PA Assays
PA (1,2-diacyl-sn-glycero-3-phosphate sodium salt; Sigma, St. Louis, MO) was dissolved in chloroform, and the solvent was evaporated under a stream of N2. To obtain PA liposomes, we used the method of Potocky et al. (2003). After 30 min of sonication, liposomes were added immediately to solid MS medium at a temperature of 45°C. Plates with concentrations of 28 and 280 μM PA were prepared.
Eight-day-old seedlings from mutant and wild-type plants that were grown on PA plates and MS medium plates with no PA were cleared and observed using the same procedure as previously described for Cho and PCho assays. Plants from each treatment were also stained with PI and Evans blue and observed under the same conditions described for Cho and PCho assays.
Whole Mount in Situ Hybridization
Seven-day-old seedlings were used for whole mount in situ hybridization. The 400-bp cDNA fragment obtained from the RT-PCR reactions was cloned using the pGem-T Easy Gibco kit and sequenced. The construction was digested with SpeI or NcoI to obtain the antisense or sense probe, respectively. Probes were in vitro transcribed using the digoxigenin RNA-labeling kit from Roche (Indianapolis, IN). The in situ hybridization procedure was performed according to Bennett et al. (1996) and considering the protocol for Arabidopsis described in de Almeida et al. (1998).
Determination of PCho and Cho Contents
Fresh tissue (0.5 g) from leaves or roots of 20-d-old plants was pulverized in liquid N2 for PCho and Cho determination. Pulverized tissues were boiled for 5 min in 2 mL of isopropanol, extracted in methanol:chloroform:water (100:100:1), and reextracted with 1 mL of hot 5 mM HCl. The aqueous phase was assayed according to the method described by Nuccio et al. (1998). Cho oxidase, horseradish peroxidase, and aminoantipyrine were purchased from Sigma-Aldrich.
Determination of Total PtdCho Content
The lipid phase from the extraction described above was dried under N2, resuspended in 50 μL of CHCl3, and separated on thin layer chromatography (TLC) using an alkaline solvent system as described by Munnik et al. (1994). PtdCho spots were visualized by exposure to iodide vapor, and individual spots were scraped from the TLC plate and quantified by the method described by Zhou and Arthur (1992).
Confocal Microscopy
Optical sections were obtained after a 15.6-s scan using a Kr-Ar laser scanning confocal microscope (Olympus Fluoview; Tokyo, Japan). For double fluorescent staining, fresh roots were examined using an Olympus microscope (model BX50; Olympus, Japan) and transferred to water containing 5 μg/mL of PI (Sigma) and 2 μg/mL of FDA (Sigma) for 15 to 30 min. Seedlings were rinsed in water and mounted in 50% glycerol on microscope slides. The same sample was recorded separately at wavelengths specific to each stain, after which the two images were merged to produce the final image.
For single staining with PI, fresh roots were transferred to a solution of 10 μg/mL of this dye for 3 min. Imaging was monitored with a 568-nm excitation line and an emission window of 585 to 610 nm for PI.
Image Processing and Root Cell Size Determination
Confocal optical sections were merged and analyzed using Fluoview 2.1 software (Olympus), images were imported as tiff files, and minor modifications were performed using Adobe Photoshop 7.0 software (Adobe Systems, San Jose, CA). Root cell measurements were performed from confocal laser scanning microscopic images with NIH Image 1.63 software (http://rsbweb.nih.gov) and were obtained from a mixture of 50 to 80 epidermal and cortical cells (on average per section) from three sections of the main root with three replicates: apical elongation zone, middle zone, and basal zone of the primary root.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AF367299 (mRNA of At3g18000), AF428454 (mRNA of At1g48600), AY136372 (complete coding sequence of At1g73600), and NM_112553 (mRNA of PLDζ1/At3g16785).
Acknowledgments
The authors wish to thank Marcelina García-Aguilar, Willson Huanca-Mamani, and Anahí Pérez-Torres for their support in the whole mount in situ hybridization experiments and Jorge Molina-Torres for his help with the TLC experiments. We are especially grateful to Teun Munnik and Gert-Jan Deboer for their invaluable advice and comments on phospholipid determination experiments. We also thank June Simpson, John Délano-Frier, and Jean Phillipe Vielle-Calzada for critically reviewing this manuscript. This work was supported in part by the Consejo Nacional de Ciencia y Tecnología, México (Grant 31628-B), the European Commission (Grant ICA-4-CT2000-30017), and by the Howard Hughes Medical Institute (Grant Nbr55003677). A.C.R. was supported by the Consejo Nacional de Ciencia y Tecnología, México (fellowship 137977). We are grateful to the Genomic Analysis Laboratory at the Salk Institute for providing the sequence-indexed Arabidopsis T-DNA insertion mutants.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Luis Herrera-Estrella (lherrera@ira.cinvestav.mx).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.103.018648.
References
- Allan, D. (2000). Lipid metabolic changes caused by short-chain ceramides and the connection with apoptosis. Biochem. J. 345, 603–610. [PMC free article] [PubMed] [Google Scholar]
- Arisz, S.A., Valianpour, F., van Gennip, A.H., and Munnik, T. (2003). Substrate preference of stress-activated phospholipase D in Chlamydomonas and its contribution to PA formation. Plant J. 34, 595–604. [DOI] [PubMed] [Google Scholar]
- Bennett, M.J., Marchant, A., Green, H.G., May, S.T., Ward, S.P., Millner, P.A., Walker, A.R., Schulz, B., and Feldmann, K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273, 948–950. [DOI] [PubMed] [Google Scholar]
- Bolognese, C.P., and McGraw, P. (2000). The isolation and characterization in yeast of a gene for Arabidopsis S-adenosylmethionine:phosphoethanolamine N-methyltransferase. Plant Physiol. 124, 1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza, J.L., Jr., Grisafi, P.L., and Fink, G.R. (1995). A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9, 2131–2142. [DOI] [PubMed] [Google Scholar]
- Chaves, I., Regalado, A.P., Chen, M., Ricardo, C., and Showalter, A.M. (2002). Programmed cell death induced by (β-D-galactosyl)3 Yariv reagent in Nicotiana tabacum BY-2 suspension-cultured cells. Physiol. Plant 116, 548–553. [Google Scholar]
- Cui, Z., Houweling, M., Chen, M.H., Record, M., Chap, H., Vance, D.E., and Terce, F. (1996). A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J. Biol. Chem. 271, 14668–14671. [DOI] [PubMed] [Google Scholar]
- Cui, Z., and Vance, D.E. (1996). Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long-term choline-deficient rats. J. Biol. Chem. 271, 2839–2843. [DOI] [PubMed] [Google Scholar]
- Datko, A.H., and Mudd, S.H. (1988. a). Enzymes of phosphatidylcholine synthesis in Lemna, soybean and carrot. Plant Physiol. 88, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko, A.H., and Mudd, S.H. (1988. b). Phosphatidylcholine synthesis. Plant Physiol. 88, 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida, J., van Montagu, M., and Engler, G. (1998). Whole-mount in situ hybridization in plants. In Methods in Molecular Biology: Arabidopsis Protocols, J.M. Martínez-Sapater and J. Salinas, eds (Totowa, NJ: Humana Press), pp. 373–384. [DOI] [PubMed]
- Den Hartog, M., Musgrave, A., and Munnik, T. (2001). Nod factor-induced phosphatidic acid and diacylglycerol pyrophosphate formation: A role for phospholipase C and D in root hair deformation. Plant J. 25, 55–66. [DOI] [PubMed] [Google Scholar]
- Dhonukshe, P., Laxalt, A.M., Goedhart, J., Gadella, T.W.J., and Munnik, T. (2003). Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 15, 2666–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton, J.H. (1994). Phosphatidylcholine breakdown and signal transduction. Biochim. Biophys. Acta 1212, 26–42. [DOI] [PubMed] [Google Scholar]
- Frank, W., Munnik, T., Kerkmann, K., Salamini, F., and Bartels, D. (2000). Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell 12, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri, T., Takahashi, S., and Shinozaki, K. (2001). Involvement of a novel Arabidopsis phospholipase D, AtPLDδ, in dehydration-inducible accumulation of phosphatidic acid in stress signalling. Plant J. 26, 595–605. [DOI] [PubMed] [Google Scholar]
- Kinney, A.J., and Moore, T.S., Jr. (1987). Phosphatidylcholine synthesis in castor bean endosperm: The localization and control of CTP:choline-phosphate cytidylyltransferase activity. Arch. Biochem. Biophys. 259, 15–21. [DOI] [PubMed] [Google Scholar]
- Kirik, V., Bouyer, D., Schöbinger, U., Bechtold, N., Herzog, M., Boneville, J.M., and Hülskamp, M. (2001). CPR5 is involved in cell proliferation and cell death control and encodes a novel transmembrane protein. Curr. Biol. 11, 1891–1895. [DOI] [PubMed] [Google Scholar]
- Laxalt, A.M., and Munnik, T. (2002). Phospholipid signalling in plant defence. Curr. Opin. Plant Biol. 5, 332–338. [DOI] [PubMed] [Google Scholar]
- Lee, S., Suh, S., Kim, S., Crain, R.C., Kwak, J.M., Nam, H.G., and Lee, Y. (1997). Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J. 12, 547–556. [Google Scholar]
- Liu, Y.G., Mitsukawa, N., Oosumi, T., and Whittier, R. (1995). Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 8, 457–463. [DOI] [PubMed] [Google Scholar]
- López-Bucio, J., Cruz-Ramírez, A., and Herrera-Estrella, L. (2003). The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 6, 280–287. [DOI] [PubMed] [Google Scholar]
- López-Bucio, J., Hernández-Abreu, E., Sánchez-Calderón, L., Nieto-Jacobo, M.F., and Herrera-Estrella, L. (2002). Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol. 129, 144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy, J.E., and Benfey, P.N. (1997). Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124, 33–44. [DOI] [PubMed] [Google Scholar]
- McGraw, P., and Henry, S.A. (1989). Mutations in the Saccharomyces cerevisiae opi3 gene: Effects on phospholipid methylation, growth and cross-pathway regulation of inositol synthesis. Genetics 122, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, S.D., Nuccio, M.L., Rhodes, D., Shachar-Hill, Y., and Hanson, A.D. (2000). Radiotracer and computer modeling evidence that phospho-base methylation is the main route of choline synthesis in tobacco. Plant Physiol. 123, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, S.D., Nuccio, M.L., Ziemak, M.J., and Hanson, A.D. (2001). Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine N-methyltransferase. Proc. Natl. Acad. Sci. USA 98, 10001–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer, H.J.G., and Munnik, T. (2003). Phospholipid-based signaling in plants. Annu. Rev. Plant Biol. 54, 265–306. [DOI] [PubMed] [Google Scholar]
- Mergemann, H., and Sauter, M. (2000). Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 124, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Lee, M., and Schiefelbein, J. (1999). WEREWOLF, a MYB-related protein in Arabidopsis, is a position-dependent regulator of epidermal cell patterning. Cell 99, 473–483. [DOI] [PubMed] [Google Scholar]
- Monks, D.E., Goode, J.H., and Dewey, R.E. (1996). Characterization of soybean choline kinase cDNAs and their expression in yeast and Escherichia coli. Plant Physiol. 110, 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, P., Bessoule, J.J., Mongrand, S., Testet, E., Vincent, P., and Cassagne, C. (1998). Lipid trafficking in plant cells. Prog. Lipid Res. 37, 371–391. [DOI] [PubMed] [Google Scholar]
- Mou, Z., He, Y., Dai, Y., Liu, X., and Li, J. (2000). Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12, 405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou, Z., Wang, X., Fu, Z., Dai, Y., Han, C., Ouyang, J., Bao, F., Hu, Y., and Li, J. (2002). Silencing of phosphoethanolamine N-methyltransferase results in temperature-sensitive male sterility and salt hypersensitivity in Arabidopsis. Plant Cell 14, 2031–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T. (2001). Phosphatidic acid: An emerging plant lipid second messenger. Trends Plant Sci. 6, 227–233. [DOI] [PubMed] [Google Scholar]
- Munnik, T., Irvine, R.F., and Musgrave, A. (1994). Rapid turnover of phosphatidyl inositol 3-phosphate in the green alga Chlamydomonas eugametos: Signs of a phosphatidylinositide 3-kinase signaling pathway in lower plants. Biochem. J. 298, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik, T., Irvine, R.F., and Musgrave, A. (1998). Phospholipid signalling in plants. Biochim. Biophys. Acta 1389, 222–272. [DOI] [PubMed] [Google Scholar]
- Nuccio, M.L., Russell, B.L., Nolte, K.D., Rathinasabapathi, B., Gage, D.A., and Hanson, A.D. (1998). The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J. 16, 487–496. [DOI] [PubMed] [Google Scholar]
- Nuccio, M.L., Ziemak, M.J., Henry, S.A., Weretilnyk, E.A., and Hanson, A.D. (2000). cDNA cloning of phosphoethanolamine N-methyltransferase from spinach by complementation in Schizosaccharomyces pombe and characterization of the recombinant enzyme. J. Biol. Chem. 275, 14095–14101. [DOI] [PubMed] [Google Scholar]
- Obeid, L.M., Linardic, C.M., Karolak, L.A., and Hannun, Y.A. (1993). Programmed cell death induced by ceramide. Science 259, 1769–1771. [DOI] [PubMed] [Google Scholar]
- Ohashi, Y., Oka, A., Rodrigues-Pousada, R., Possenti, M., Ruberti, I., Morelli, G., and Aoyama, T. (2003). Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 300, 1427–1430. [DOI] [PubMed] [Google Scholar]
- Potocky, M., Elias, M., Profotová, B., Novotná, Z., Valentová, O., and Zarsky, V. (2003). Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta 217, 122–130. [DOI] [PubMed] [Google Scholar]
- Prud'homme, M.P., and Moore, T.S. (1992). Phosphatidylcholine synthesis in castor bean endosperm: The occurrence of an S-adenosyl-L-methionine:ethanolamine N-methyltransferase. Plant Physiol. 100, 1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, C., and Wang, X. (2002). The Arabidopsis phospholipase D family. Characterization of a calcium-independent and phosphatidylcholine-selective PLDζ 1 with distinct regulatory domains. Plant Physiol. 128, 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, B., Salido, G.M., Campo, M.L., and Claro, E. (2000). Inhibition of phosphatidylcholine synthesis precedes apoptosis induced by C2-ceramide: Protection by exogenous phosphatidylcholine. Neuroreport 11, 3103–3108. [DOI] [PubMed] [Google Scholar]
- Reire, J., Feldmann, K., and Marks, D. (1994). The GLABRA2 gene encodes a homeodomain protein required for normal trichome development in Arabidopsis. Genes Dev. 8, 1388–1399. [DOI] [PubMed] [Google Scholar]
- Schiefelbein, J. (2000). Constructing a plant cell. The genetic control of root hair development. Plant Physiol. 124, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D.D., Summers, P.S., and Weretilnyk, E.A. (2000). Phosphocholine synthesis in spinach: Characterization of phosphoethanolamine N-methyltransferase. Physiol. Plant 108, 286–294. [Google Scholar]
- Vaux, D.L., and Korsmeyer, S.J. (1999). Cell death in development. Cell 96, 245–254. [DOI] [PubMed] [Google Scholar]
- Wang, C., and Wang, X. (2001). A novel phospholipase D of Arabidopsis that is activated by oleic acid and associated with the plasma membrane. Plant Physiol. 127, 1102–1112. [PMC free article] [PubMed] [Google Scholar]
- Wang, X. (1999). The role of phospholipase D in signaling cascades. Plant Physiol. 120, 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. (2001). Plant phospholipases. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 211–231. [DOI] [PubMed] [Google Scholar]
- Wang, X. (2002). Phospholipase D in hormonal and stress signaling. Curr. Opin. Plant. Biol. 5, 408–414. [DOI] [PubMed] [Google Scholar]
- Yen, C.L., Mar, M.H., and Zeisel, S.H. (1999). Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB J. 13, 135–142. [PubMed] [Google Scholar]
- Zhang, W., Wang, C., Qin, C., Wood, T., Olafsdottir, G., Welti, R., and Wang, X. (2003). The oleate-stimulated phospholipase D, PLDδ, and phosphatidic acid decrease H2O2 induced cell death in Arabidopsis. Plant Cell 15, 2285–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X., and Arthur, G. (1992). Improved procedure for the determination of lipid phosphorous by malachite green. J. Lipid Res. 33, 1233–1236. [PubMed] [Google Scholar]