Abstract
Background
Influenza related morbidity and mortality disproportionately impacts older adults. The serologic response to vaccine is diminished in older adults; however, high dose inactivated influenza vaccine (HD IIV) has shown improved rates of seroconversion compared to standard dose (SD IIV). We hypothesize this may be due to the superior ability of high dose vaccine to activate T follicular helper (Tfh) cells and provide B cell dependent T cell help.
Methods
We measured peripheral Tfh (pTfh) activation in 50 community dwelling adults 65 years or older who were randomly assigned to receive either the HD IIV or SD IIV.
Results
The HD vaccination elicited significantly higher levels of ICOS expression on pTfh cells, at day 7 compared to SD vaccination (p= 0.02). The magnitude of the increase in ICOS+ pTfh cells from baseline to day 7 was predictive of seroconversion for both influenza A and B vaccination.
Conclusion
Strong Tfh activation in response to influenza vaccination forecasts successful seroconversion in older adults, and HD IIV elicits greater Tfh activation than SD IIV. Future vaccine studies should focus on ways to further optimize the Tfh response.
Keywords: Influenza vaccine, high dose influenza vaccine, T follicular helper cells, Tfh, viSNE
Introduction
Influenza vaccination is a mainstay of preventative health; but a large proportion of older adults fail to generate protective immunity following annual vaccination [1]. The rate of seroconversion in adults ≥65 years old is 17–53%, compared with 60–90% in younger adults [2]. The poor seroconversion rate among older adults is an urgent issue as the national and global populations are aging, and 90% of influenza-related deaths occur in adults ≥65 years old [3]. Multiple studies involving older adults have shown high dose inactivated influenza vaccine (HD IIV) elicits higher hemagglutinin inhibition titers and improved rates of seroconversion compared to standard dose vaccine (SD IIV) [4–8]. In 2010 the FDA licensed a trivalent high dose IIV that contains 60ug per vaccine strain, compared to 15ug per strain in the standard dose. A recently published clinical efficacy trial reported HD IIV was 24% more efficacious than SD IIV at preventing influenza and may prevent hospitalizations for influenza related pneumonia [9–11].
The attenuated serologic response in older adults following influenza vaccination is likely multifactorial. Aging is associated with a reduction in the frequency of antibody secreting cells [12, 13] and the number of lymph node germinal centers [14, 15]. Aging can also impair the ability of cognate CD4 T cells to help B cells [16]. The implications for these findings on vaccine responses highlight the need for a better understanding of the changes in cellular immunity with aging.
T follicular helper cells (Tfh) are a subset of CD4+ T cells that provide help to B cells. They were originally identified within germinal centers [17] and defined by surface expression of CXCR5 and PD-1 [18]. When activated they increase expression of co-stimulatory molecules such as ICOS needed to promote B cell differentiation [19, 20]. A circulating counterpart of Tfh cells in the peripheral blood (pTfh cells), express CXCR5 and PD-1, and are able to induce B cell differentiation [20–24]. A direct relationship between the frequency of activated pTfh cells following vaccination and influenza vaccine-induced antibody responses has recently been identified [25–27]. pTfh cells isolated from influenza vaccine recipients are preferentially able to differentiate B cells and stimulate influenza specific antibody secretion over other CD4+ T cell subsets [25].
Aging impacts the function of Tfh cells [28]. A recent study comparing the effects of the standard dose influenza vaccine in young adults and older adults showed that ICOS+pTfh cells significantly increased in young adults following vaccination but not older adults [27]. Vaccination studies in mice have shown that increasing the vaccine dose causes an increase in the frequency of Tfh cells and a corresponding increase in germinal center B cells [29, 30]. Therefore, we hypothesized the higher antigen dose of the influenza vaccine would have superior ability to activate Tfh cells and this would correlate with improve serologic responses in older adults.
Materials and Methods
Study subjects and PBMC preparation
Participants were community dwelling adults in the Nashville area at least 65 years of age and at least 110 pounds. All subjects provided written informed consent prior to enrollment. Exclusion criteria included a history of allergic reaction to the influenza vaccine or vaccine components or a prior history of Guillain-Barre syndrome. Recruitment occurred prior to the 2010–2011 influenza season. The 2010–2011 split-virus trivalent vaccine (Sanofi Pasteur) contained influenza strains A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008. Heparinized blood was drawn to prepare peripheral blood mononuclear cells (PBMC) prior to vaccination and one week following vaccination. PBMC were isolated by Ficoll separation and cryopreserved.
Antibody measurements
Whole blood was obtained for antibody measurements pre-vaccination and at day 28 post-vaccination. Serum was isolated and stored at −80°C until tested for hemagglutination inhibition (HAI) titers. Seroconversion was defined as a 4-fold increase in HAI titer and at least a titer of 1:40. Antibody titers are plotted as the reciprocal of serum dilution.
Flow cytometry staining
PBMCs from pre- and post-vaccination time points for each individual were stained simultaneously to avoid run-to-run variability. Cryopreserved cells were thawed and washed in PBS. Cells were incubated with Live/Dead Fixable Aqua (ThermoFisher) and surface stained with antibodies at room temperature. For Ki-67 staining, cells were fixed and permeabilized with Cytofix/Cytoperm (BD) according to the manufactures protocol followed by incubation with anti-Ki-67. Cytometry was performed on a BD LSRFortessa at the VUMC Flow Cytometry Shared Resource and analyzed using FlowJo v10.0.8 (Tree Star).
Antibodies
Antibodies for fluorescence cytometry included: CD3−BV711 (UCHT1), CD3−Pacific Blue (UCHT1), CD4−BV605 (RPA-T4), CXCR5−AF488 (RF8B2), CD19−BV711 (SJ25C), CD20−APC (L27), HLA-DR-APC (L243) and Ki-67-PE (B56) (BD Biosciences); PD-1-BV421 (EH12.2H7), ICOS-PerCP-Cy5.5 (C398.4A) (BioLegend); CD8−APC-AF750 (3B5) (ThermoFisher) and CD38−PE-Cy7 (HIT2)(eBiosciences).
viSNE analysis
FCS files of pTfh cells gated as live/deadlow CD3+CD8−CD4+CXCR5+PD-1+ (identified as in Figure 1) for day 0 and day 7 of each individual were uploaded to Cytobank [31]. Data were transformed to arcsinh scales with cofactors ranging from 150 to 4,000. The 100 individual FCS files were merged into 4 ‘concatenated’ files based on dose and time point (SD D0, SD D7, HD D0, HD D7) to create representative maps of each group. viSNE maps used 400,000 cellular events proportionally sampled from 100 files of individual subjects/time points and 4 concatenated FCS files. All 11 parameters in the pTfh staining panel were considered for viSNE map creation.
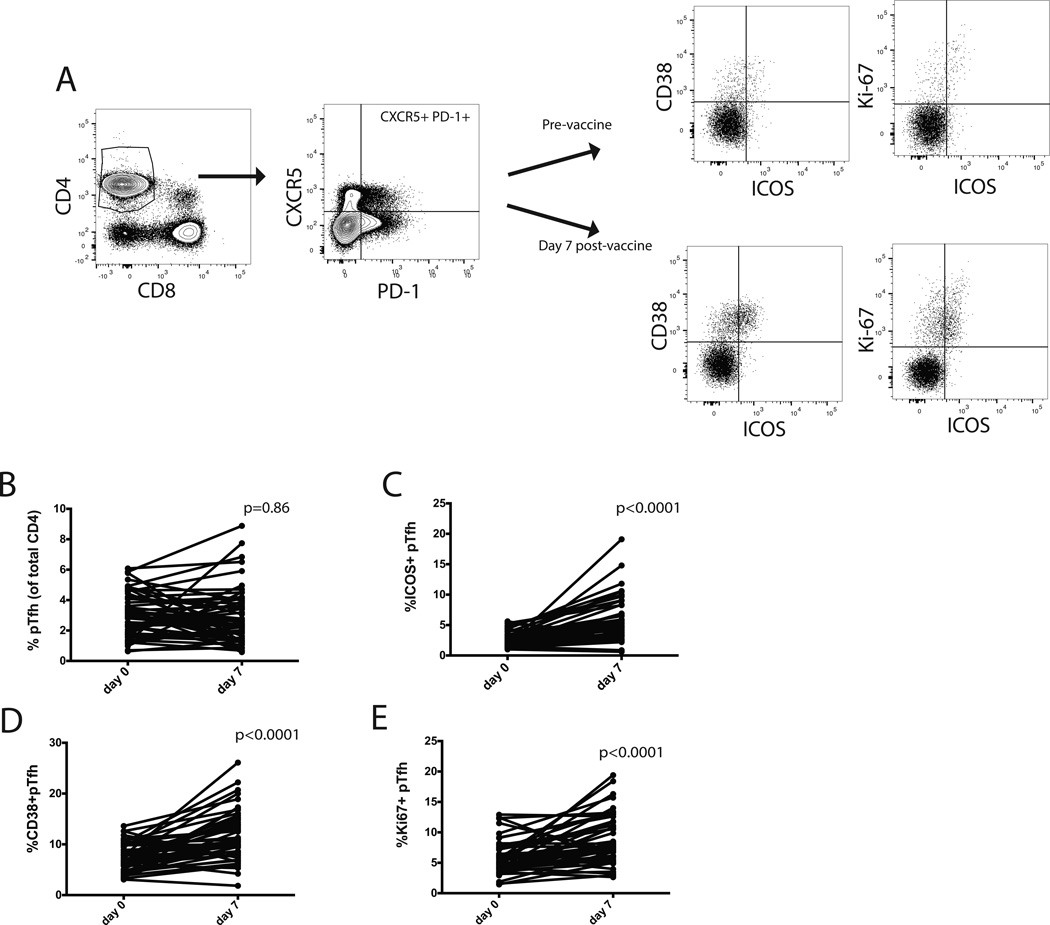
Figure 1. Vaccination increases the frequency of activated pTfh cells.
A) Gating strategy to identify pTfh cells, defined by co-expression of PD-1 and CXCR5 on CD4+ T cells. We measured ICOS, CD38, and Ki-67 on pTfh cells. A representative individual pre and post-vaccination is shown. B) The frequency of CD4+ T cells with the pTfh phenotype did not significantly change pre and post-vaccination for the pooled cohort. C–E) The frequency of ICOS+ pTfh cells (C) CD38+ pTfh cells (D) and Ki67+ pTfh cells increase increased post-vaccination. (Wilcoxon signed rank test).
Statistical analysis
Graphpad Prism (version 7) was used for statistical analyses. Comparisons between paired time points were assessed with Wilcoxon signed rank test. Comparisons between groups were performed with Mann-Whitney tests. All correlation p and rho values were determined by Spearman rank. Age comparison between groups was performed with an unpaired t-test. Comparison of day 28 seroconversion rates between doses was calculated with Fisher’s exact test.
Results
Serologic response to standard dose and high dose vaccine
Healthy adults ≥65 years old were randomized to receive either standard dose inactivated influenza vaccine (SD IIV) or high dose influenza vaccine (HD IIV) (Table 1). Participants in each arm did not differ by age (p= 0.45). Hemagglutination inhibition (HAI) titer to each influenza A (H1N1 and H3N2) and influenza B strain were assessed at day 0 and day 28. Consistent with previous studies [4–8], the HD IIV resulted in a higher level of seroprotection (titer ≥1:40) at day 28 and a higher rate of seroconversion (Table 1).
Table 1.
50 adults at least 65 years old were randomized to receive either high dose or standard dose IIV. Comparison of age was calculated with an unpaired t-test. Comparisons between doses for seroprotection at day 28 and seroconversion were calculated with Fisher’s exact test.
| Standard dose n=24 | High dose n =26 | p | |
|---|---|---|---|
| Age mean (IQR) | 73 (68, 77) | 72 (65, 77) | 0.45 |
| H1N1 | |||
| Seroprotection | 54% | 81% | 0.03 |
| Seroconversion | 33% | 54% | 0.16 |
| H3N2 | |||
| Seroprotection | 46% | 87% | 0.002 |
| Seroconversion | 25% | 81% | <0.001 |
| B | |||
| Seroprotection | 58% | 81% | 0.12 |
| Seroconversion | 4% | 46% | <0.001 |
High dose IIV induces a greater frequency of ICOS+pTfh cells
We evaluated vaccine induced changes in the activation status of CD4+CXCR5+PD-1+ T cells, hereafter referred to as pTfh cells (Fig 1A), on day 0 (pre-vaccination) and day 7 (post-vaccination), since this has been shown to represent peak activation of pTfh cells in response to vaccination [25]. There was no significant change in the overall frequency of pTfh cells from day 0 to day 7 (Fig 1B). We next assessed differences in the activation phenotype of pTfh cells by comparing the frequency of pTfh cells expressing ICOS, CD38 or Ki67 and found significant increases in the expression of each of these markers on pTfh cells after vaccination (Fig 1C–E).
We then compared the groups that received either SD IIV or HD IIV. pTfh frequency did not change following vaccination of the combined cohort (Fig 1B) nor was there a difference in the pTfh frequency pre- and post- vaccination in either group (SD IIV p=0.49; HD IIV p=0.67). ICOS+pTfh cell frequencies in the SD IIV and HD IIV groups were the same at baseline (p=0.4), but both SD IIV and HD IIV vaccination led to increases in ICOS+pTfh cell frequency (Fig 2A). The HD IIV recipients, however, had a significantly greater increase in the frequency of ICOS+pTfh cells with a mean increase of 1.7% for SD IIV and 3.9% for HD IIV recipients (p=0.028).
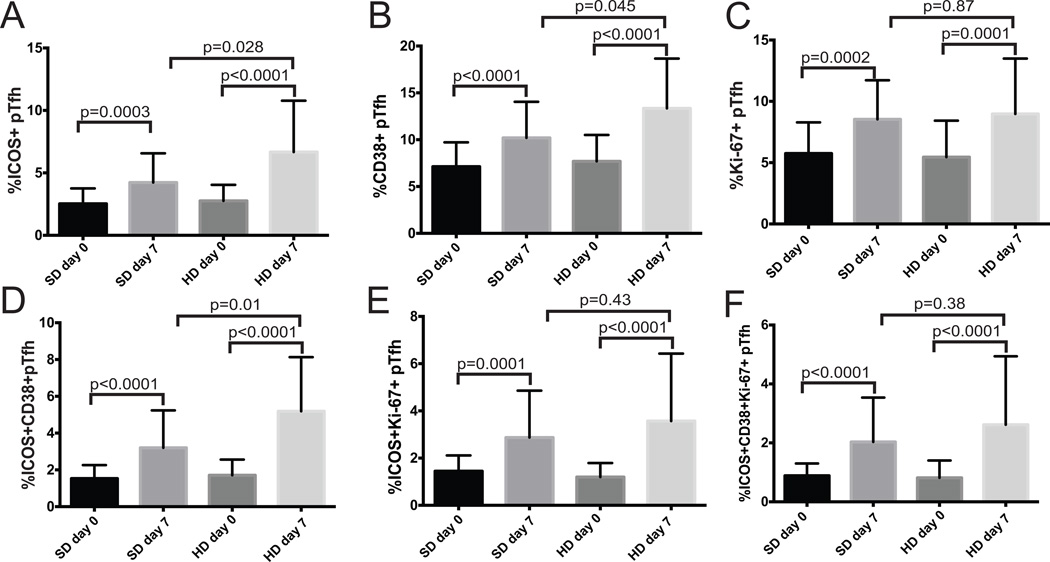
Figure 2. High dose vaccine recipients had a greater increase in activated pTfh cells.
The frequency of pTfh cells with individual activation markers (A–C) or combinations of activation markers (D–F) for standard dose (SD) and high dose (HD) vaccine recipients before (day 0) and after (day 7) vaccination.
CD38 and Ki-67 have previously been shown to increase on pTfh cells after influenza vaccination [25, 27, 32] and in this cohort vaccination induced a significant increase in pTfh cells expressing each of these markers. The change in CD38+pTfh cell frequency was present in both SD IIV and HD IIV recipients, although the post-vaccine CD38+ Tfh frequency was greater in HD IIV recipients (p=0.045, Fig. 2B). While the frequency of Ki67+pTfh cells also increased following vaccination, there was no difference between vaccine doses in the day 7 frequency of Ki67+pTfh cells (p=0.87, Fig 2C).
We then evaluated pTfh cells expressing combinations of these proteins. The magnitude of the change in ICOS+CD38+pTfh cells in HD IIV recipients was significantly higher than that of SD IIV recipients, and the differences were more pronounced between groups than with ICOS or CD38 expression alone (Figure 2D, p=0.01). ICOS+Ki-67+dual expression on pTfh cells also increased in response to vaccination (p<0.001 for both SD IIV and HD IIV), however, there was no vaccine dose dependent difference in day 7 frequencies of ICOS+Ki-67+pTfh cells (p=0.87, Fig 2E) or ICOS+Ki-67+CD38+pTfh cells (p=0.38, Fig 2F). While several markers can be used to identify pTfh activation, HD IIV generated higher levels of ICOS+ and CD38+pTfh cells compared to SD IIV.
High dose IIV induces higher frequencies of circulating plasmablasts
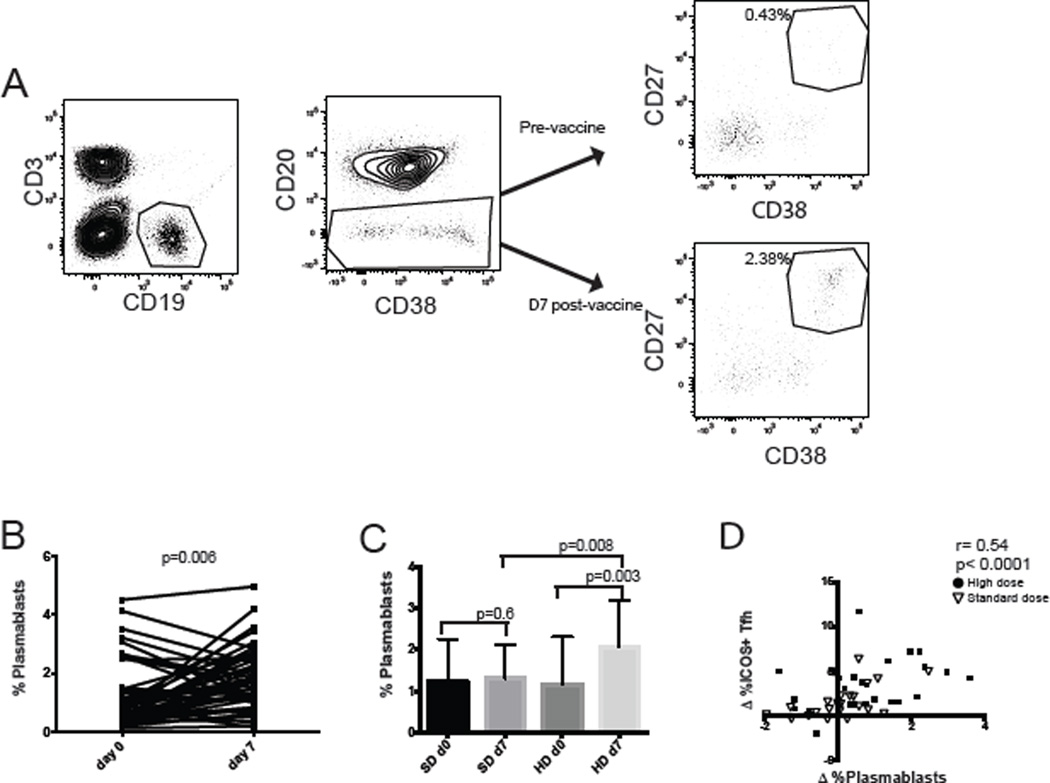
Tfh cells can induce differentiation of B cells [20, 25, 33]; therefore we evaluated the frequencies of circulating plasmablasts before and after vaccination. Plasmablasts were identified as CD19+ B cells that were CD20 negative, and high for CD27 and CD38 expression [34] (Fig 3A). Vaccination induced a significant increase in the frequency of plasmablasts in the cohort (Fig 3B). The increase in plasmablast frequency positively correlated with the increase in ICOS+pTfh frequency when the entire cohort was analyzed (Fig 3C; r=0.45, p<0.001). Baseline plasmablast frequency was the same in each vaccine group, however the HD vaccine group had a significant increase in the frequency of plasmablasts after vaccination (Fig 3D; p=0.003).
Figure 3. Plasmablast frequency increases proportionally to the increase in frequency of ICOS+ pTfh cells after vaccination.
A) Plasmablasts, defined as CD19+ CD20lowCD38highCD27high. cells were measured before and after vaccination. B) The frequency of plasmablasts before and after vaccination for each individual. C) The change in plasmablast frequency from day 0 to day 7 correlated directly with the change in ICOS+ pTfh cells. Spearman’s rank correlation was performed on the whole cohort Subjects who received standard dose or high dose are indicated. D) Plasmablast frequency for either the SD IIV or the HD IIV group before and after vaccination.
Early induction of ICOS+pTfh cells predicts antibody responses
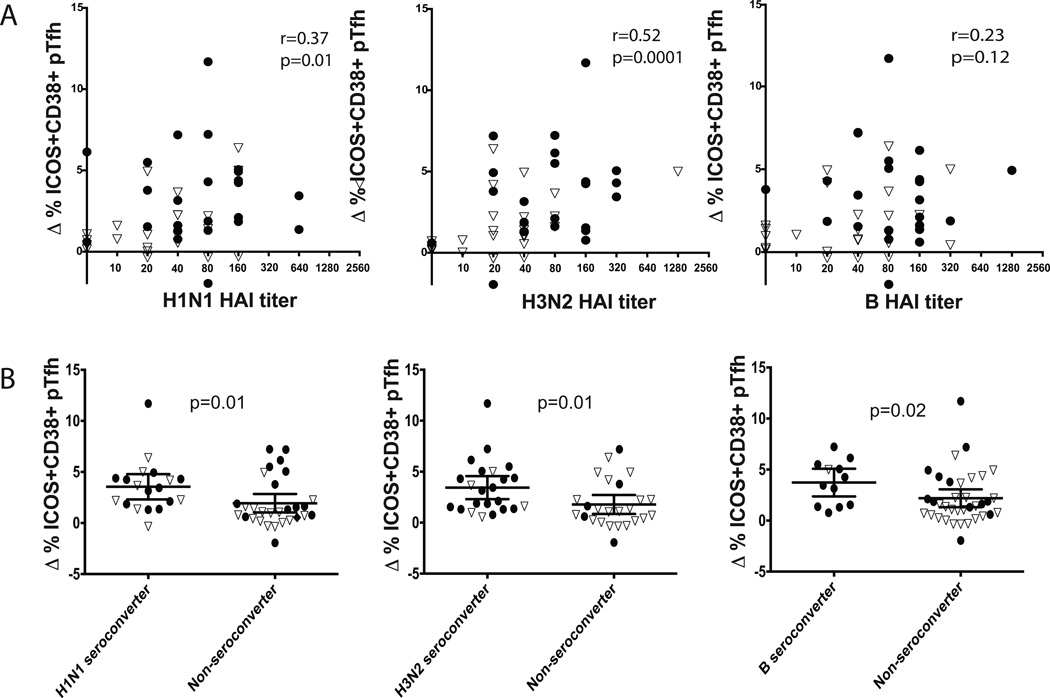
A post-vaccine increase in pTfh cells expressing ICOS has previously been shown to correlate with improved influenza specific antibody responses [25–27]. We evaluated the change in ICOS+pTfh cell frequencies following vaccination with the day 28 HAI tiers. Vaccine-induced increases in the frequencies of ICOS+pTfh cells by day 7 preceded higher HAI titers to H1N1 (r=0.32; p=0.02) and H3N2 (r=0.52; p<.0001) but not influenza B (p=0.27) (Figure 4A).
Figure 4. The change in frequency of ICOS+ pTfh cells day 7 after vaccination correlated with day 28 HAI titers and predicted seroconversion.
A) The change in ICOS+ pTfh cells directly correlated with the day 28 HAI titer for both strains of influenza A but not influenza B (Spearman’s rank). B) The change in ICOS+ pTfh cells was significantly greater in seroconverters compared to non-converters (Mann-Whitney U test). Open triangles indicate standard dose recipients; closed circles are high dose recipients.
We then evaluated seroconversion, which measures the change in titer following vaccination, in relation to the change in frequency of ICOS+pTfh cells. We found significantly greater increases in ICOS+pTfh cells at day 7 in those who seroconverted to both influenza A strains (p=0.004 for both H1N1 and H3N2), as well as influenza B (p=0.02) (Fig 4B). These data show that in healthy older adults, changes in the frequency of vaccine-induced ICOS+pTfh cells at day 7 predict serologic responses 28 days after vaccination. Similar results were found if the analysis was restricted to CD38+ICOS+pTfh cells. However, when restricting the analysis to only changes in ICOS+Ki-67+CD38+ pTh cell frequency, there was only a greater magnitude in H1N1 seroconverters but not H3N2 or influenza B (data not shown).
viSNE analysis reveals multi-dimensional phenotype of activated pTfh cells
pTfh cells are a heterogeneous population [35]. The use of iterative biaxial gating schemes typically used to define cell populations has the potential to introduce bias, as well as to minimize the complex relationships among cellular parameters. We reevaluated our fluorescence cytometry data using viSNE, an analysis tool that preserves sophisticated relationships among multiple phenotypic parameters and plots them on two dimensions [36]. Single cells are arranged on a ‘map’ where distances between cells are based on their phenotypic similarity. We used this tool to generate an unbiased view of pTfh cells before and after vaccination to determine whether differences pTfh cells were present following either SD IIV or HD IIV.
After initial gating to identify pTfh cells, viSNE was used to arrange pTfh cells using all the parameters measured in the fluorescence cytometry panel in an unsupervised fashion. Figure 5A shows a viSNE map of pTfh cells, highlighting the expression patterns of ICOS, CD38, and Ki-67 on cells from HD IIV recipients at day 0 and day 7. A gate was drawn around a discrete population of activated pTfh cells expressing high ICOS, CD38, and variable Ki-67 (Fig 5A, red gate). A similar map was generated simultaneously for the SD vaccination group and the same gate was used to measure frequencies of activated cells.
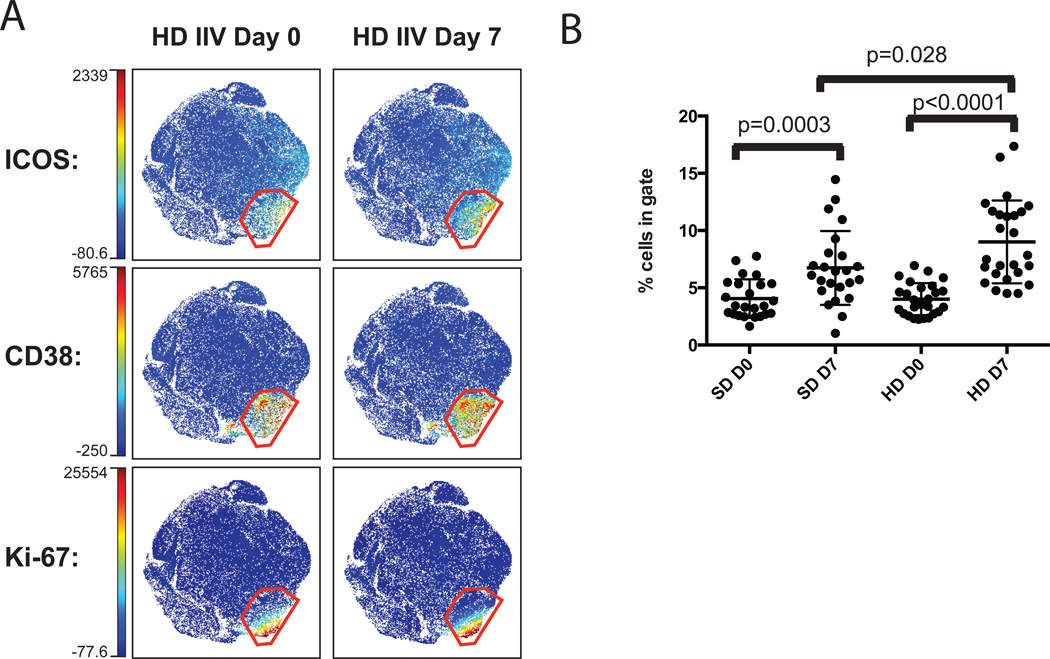
Figure 5. viSNE analysis reveals multi-dimensional phenotype of activated pTfh cells that are significantly more frequent in HD IIV recipients at Day 7.
(A) viSNE maps displaying live pTfh cells highlight expression of ICOS, CD38, and Ki-67 for day 0 and day 7 of HD IIV. Intensity scales indicate the range of expression for each marker. Maps display cells from the concatenated files of groups indicated above the plots. The red gate was drawn based on dual CD38 and ICOS expression. (B) Statistical analysis of the frequency of cells within the red gated population for all individuals. Wilcoxon signed rank test between day 0 and day 7 for paired samples; Mann-Whitney test for comparison between groups
Both groups had higher frequencies of activated pTfh cells post vaccination, however the frequency of the activated population of pTfh cells was significantly greater in HD IIV compared to SD IIV recipients at day 7 post-vaccination (Figure 5B). Analyzing the pTfh response using viSNE allowed for rapid identification of the most phenotypically distinct population simultaneously utilizing all markers in the staining panel. This demonstrates viSNE can be a powerful tool for multiparameter flow cytometry analysis of cellular responses in vaccine studies and is able to identify changes in the phenotype of key cell populations in response to vaccination.
Discussion
Older adults have a blunted response to influenza vaccine compared to young healthy adults. A high dose influenza vaccine increases the rate of seroprotection and seroconversion in older adults [4–8]. Understanding the mechanism behind this improved response will refine our understanding of the correlates of immune protection and help guide the development of future vaccines. While a correlation between the frequency of activated pTfh cells and antibody responses following influenza vaccination has recently been described [25–27, 32], the effect of different vaccine doses on pTfh cell activation has not been previously evaluated. Since greater seroconversion and seroprotection have been demonstrated with high dose IIV in adults ≥65, we felt it was important to evaluate the relationship between vaccine induced pTfh activation and humoral immune responses.
The HD IIV group demonstrated a statistically significant greater increase in ICOS+pTfh cells compared to SD IIV. When we included CD38, an important marker of T cell activation, there was an even stronger effect of HD vaccination on inducing CD38+ICOS+pTfh cells. Expression of Ki-67, a marker of proliferating cells, also increases after vaccination [26, 37, 38] and a recent study showed the frequency of ICOS+CD38+Ki-67+pTfh cells significantly increased in older adults following SD IIV [27]. Consistent with this report, we found that Ki-67 expression increased with vaccination. We did not find, however, a significant difference in Ki-67+pTfh cell frequency between vaccine doses, nor was there a significant difference in the Ki-67+CD38+ICOS+pTfh cells in seroconverters and non-converters. This suggests increases in cellular proliferation may not be sufficient to support seroconversion, and that key functional receptors like ICOS need to be upregulated as well.
We found a direct correlation between the increases in the frequencies of activated pTfh cells and plasmablasts at day 7. This increase in the frequency of activated pTfh cells also directly correlated with the magnitude of the serologic response for the two influenza A strains. When we stratified our cohort by seroconverters and non-seroconverters for each influenza strain, we found the frequency of vaccine-induced ICOS+pTfh cells to be a strong predictor of seroconversion for each of the three vaccine strains.
Two recent reports have compared pTfh responses after influenza vaccination in young and older adults [27, 32] George et al. evaluated the Tfh responses after vaccination in HIV infected and uninfected individuals at different ages. They observed an increase ICOS+pTfh cells in HIV negative older adults who did respond to the vaccine, though the younger HIV negative individuals had the best serologic responses as well as a greater increase in ICOS+pTfh frequency [32]. Another study compared responses of young adults and adults ≥65 years given SD IIV. They reported that while the ICOS+pTfh cell frequency increased in young adults after vaccination, there was no significant increase in this population in older adults [27]. This is in contrast to our study of exclusively older adults in which we identified significant increases in the frequencies of ICOS+pTfh cells with both SD IIV and HD IIV, though with larger increases following HD IIV. Differences between our results in the ICOS+pTfh population may have arisen because of the antibody staining protocols or gating strategies used to identify activated pTfh cells and the method for comparing serologic responses. Herati et al. showed a remarkably high percentage of pTfh cells expressing ICOS ex vivo, even prior to vaccination (mean>40%). Therefore it may have been more difficult to measure subtle changes in ICOS expression after vaccination. They measured total influenza specific IgG or IgM for H1N1 and H3N2 averaged together and expressed it as fold change, while we focused on either the day 28 titer or seroconversion using only HAI antibodies for each strain separately.
Discrepancies in the interpretation of cellular immune responses between studies can arise from differences in biaxial gating used to define cell populations. Our unsupervised analysis of pTfh cells using the multi-dimensional analysis tool viSNE strengthens our findings that activation markers are critical features that distinguish HD and SD IIV recipients. Data continue to emerge regarding the specific parameters of this subset that provides the most efficient help to B cells [22, 25, 35, 39]. Expanded multiparameter panels for mass cytometry is one strategy to address this [22, 25, 35, 39]. However, even with smaller focused fluorescent antibody staining panels, using software for multidimensional analysis, like ViSNE, may help identify changes in distinct pTfh populations. The need to focus on activation of key T cell subsets may explain why previous studies comparing the cellular responses after SD IIV and HD IIV have not shown clear differences [40].
Ours is the first study to describe a dose effect on pTfh responses to influenza vaccination. Since we exclusively studied older adults, the dose response of Tfh activation and the correlation with serologic responses may be unique to older adults, who are known to have attenuation of the immune system at multiple points [12, 13, 41, 42]. Increased antigen delivery through the HD IIV may be one way to overcome some of these defects [29, 43]. Influenza remains one of the leading causes of morbidity and mortality in older adults. The FDA- approved HD IIV is associated with significantly improved serologic responses and improved clinical outcomes [4–11]. This study demonstrates that HD IIV increases the frequency of ICOS+ activated pTfh cells and subsequent serologic responses, and provides insight for vaccine studies targeting improved activation of Tfh populations.
Acknowledgments
COI: H.K.T has received research funding from MedImmune, Sanofi Pasteur, and Gilead and has served as an advisor for Novartis and VaxInnate
Funding: This work was support by Ruth L. Kirschstein National Research Service Award, [T32-AI007474, M.A.P, T32-AI089554, K.J.N]. Flow cytometry was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30CA68485) and the Vanderbilt Digestive Disease Research Center (P30 DK058404). Subject enrollment was supported by the Centers for Disease Control and Prevention (CDC) (1U181P000184-01: Marie Griffin MD, site PI) and was also supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, National Institutes of Health. The funders did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; nor preparation, review, or approval of the manuscript.
Abbreviations
- SD IIV
standard dose inactivated influenza vaccine
- HD IIV
high dose inactivated influenza vaccine
- pTfh
peripheral T follicular helper cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this work was presented as an oral abstract at IDweek 2015.
References
- 1.Fulton RB, Varga SM. Effects of aging on the adaptive immune response to respiratory virus infections. Aging health. 2009;5:775. doi: 10.2217/ahe.09.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA : the journal of the American Medical Association. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine. 2007;25:7656–7663. doi: 10.1016/j.vaccine.2007.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine. 2013;31:861–866. doi: 10.1016/j.vaccine.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. The Journal of infectious diseases. 2009;200:172–180. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 7.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2010;59:1–62. [PubMed] [Google Scholar]
- 8.Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Archives of internal medicine. 2006;166:1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 9.DiazGranados CA, Dunning AJ, Kimmel M, Kirby D, Treanor J, Collins A, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. The New England journal of medicine. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 10.DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: A comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015;33:4988–4993. doi: 10.1016/j.vaccine.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Izurieta HS, Thadani N, Shay DK, Lu Y, Maurer A, Foppa IM, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015;15:293–300. doi: 10.1016/S1473-3099(14)71087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns EA, Lum LG, L'Hommedieu G, Goodwin JS. Specific humoral immunity in the elderly: in vivo and in vitro response to vaccination. Journal of gerontology. 1993;48:B231–B236. doi: 10.1093/geronj/48.6.b231. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki S, Sullivan M, Narvaez CF, Holmes TH, Furman D, Zheng NY, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. The Journal of clinical investigation. 2011;121:3109–3119. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linterman MA. How T follicular helper cells and the germinal centre response change with age. Immunol Cell Biol. 2014;92:72–79. doi: 10.1038/icb.2013.77. [DOI] [PubMed] [Google Scholar]
- 15.Kolar GR, Mehta D, Wilson PC, Capra JD. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scandinavian journal of immunology. 2006;64:314–324. doi: 10.1111/j.1365-3083.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 16.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. The Journal of experimental medicine. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 18.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. The Journal of experimental medicine. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G. Follicular B helper T cell activity is confined to CXCR5(hi)ICOS(hi) CD4 T cells and is independent of CD57 expression. Eur J Immunol. 2006;36:1892–1903. doi: 10.1002/eji.200636136. [DOI] [PubMed] [Google Scholar]
- 20.Chevalier N, Jarrossay D, Ho E, Avery DT, Ma CS, Yu D, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. Journal of immunology. 2011;186:5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 21.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, et al. Human circulating PD-(+)1CXCR3(−)CXCR5(+) memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz BT, Teigler JE, Pissani F, Oster AF, Kranias G, Alter G, et al. Circulating HIV-Specific Interleukin-21(+)CD4(+) T Cells Represent Peripheral Tfh Cells with Antigen-Dependent Helper Functions. Immunity. 2016;44:167–178. doi: 10.1016/j.immuni.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, et al. CXCL13 is a plasma biomarker of germinal center activity. Proc Natl Acad Sci U S A. 2016;113:2702–2707. doi: 10.1073/pnas.1520112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Science translational medicine. 2013;5:176ra32. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallikkuth S, Parmigiani A, Silva SY, George VK, Fischl M, Pahwa R, et al. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood. 2012;120:985–993. doi: 10.1182/blood-2011-12-396648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herati RS, Reuter MA, Dolfi DV, Mansfield KD, Aung H, Badwan OZ, et al. Circulating CXCR5+PD-1+ Response Predicts Influenza Vaccine Antibody Responses in Young Adults but not Elderly Adults. Journal of immunology. 2014;193:3528–3537. doi: 10.4049/jimmunol.1302503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefebvre JS, Masters AR, Hopkins JW, Haynes L. Age-related impairment of humoral response to influenza is associated with changes in antigen specific T follicular helper cell responses. Sci Rep. 2016;6:25051. doi: 10.1038/srep25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, et al. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, et al. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. The Journal of experimental medicine. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotecha N, Krutzik PO, Irish JM. Web-based analysis and publication of flow cytometry experiments. Curr Protoc Cytom. 2010;Chapter 10(Unit10):7. doi: 10.1002/0471142956.cy1017s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George VK, Pallikkuth S, Parmigiani A, Alcaide M, Fischl M, Arheart KL, et al. HIV infection Worsens Age-Associated Defects in Antibody Responses to Influenza Vaccine. The Journal of infectious diseases. 2015;211:1959–1968. doi: 10.1093/infdis/jiu840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Fink K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front Immunol. 2012;3:78. doi: 10.3389/fimmu.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong MT, Chen J, Narayanan S, Lin W, Anicete R, Kiaang HT, et al. Mapping the Diversity of Follicular Helper T Cells in Human Blood and Tonsils Using High-Dimensional Mass Cytometry Analysis. Cell Rep. 2015;11:1822–1833. doi: 10.1016/j.celrep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Amir el AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Miao H, Henn A, Topham DJ, Wu H, Zand MS, et al. Ki-67 expression reveals strong, transient influenza specific CD4 T cell responses after adult vaccination. Vaccine. 2012;30:4581–4584. doi: 10.1016/j.vaccine.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Causi E, Parikh SC, Chudley L, Layfield DM, Ottensmeier CH, Stevenson FK, et al. Vaccination Expands Antigen-Specific CD4+ Memory T Cells and Mobilizes Bystander Central Memory T Cells. PLoS One. 2015;10:e0136717. doi: 10.1371/journal.pone.0136717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014;10:e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen WH, Cross AS, Edelman R, Sztein MB, Blackwelder WC, Pasetti MF. Antibody and Th1-type cell-mediated immune responses in elderly and young adults immunized with the standard or a high dose influenza vaccine. Vaccine. 2011;29:2865–2873. doi: 10.1016/j.vaccine.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130:709–712. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Weng NP, Akbar AN, Goronzy J. CD28(−) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, et al. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]